Analytical Methods for the Determination of Pharmaceuticals and Personal Care Products in Solid and Liquid Environmental Matrices: A Review
Abstract
1. Introduction
2. Environmental Risks Posed by PPCPs
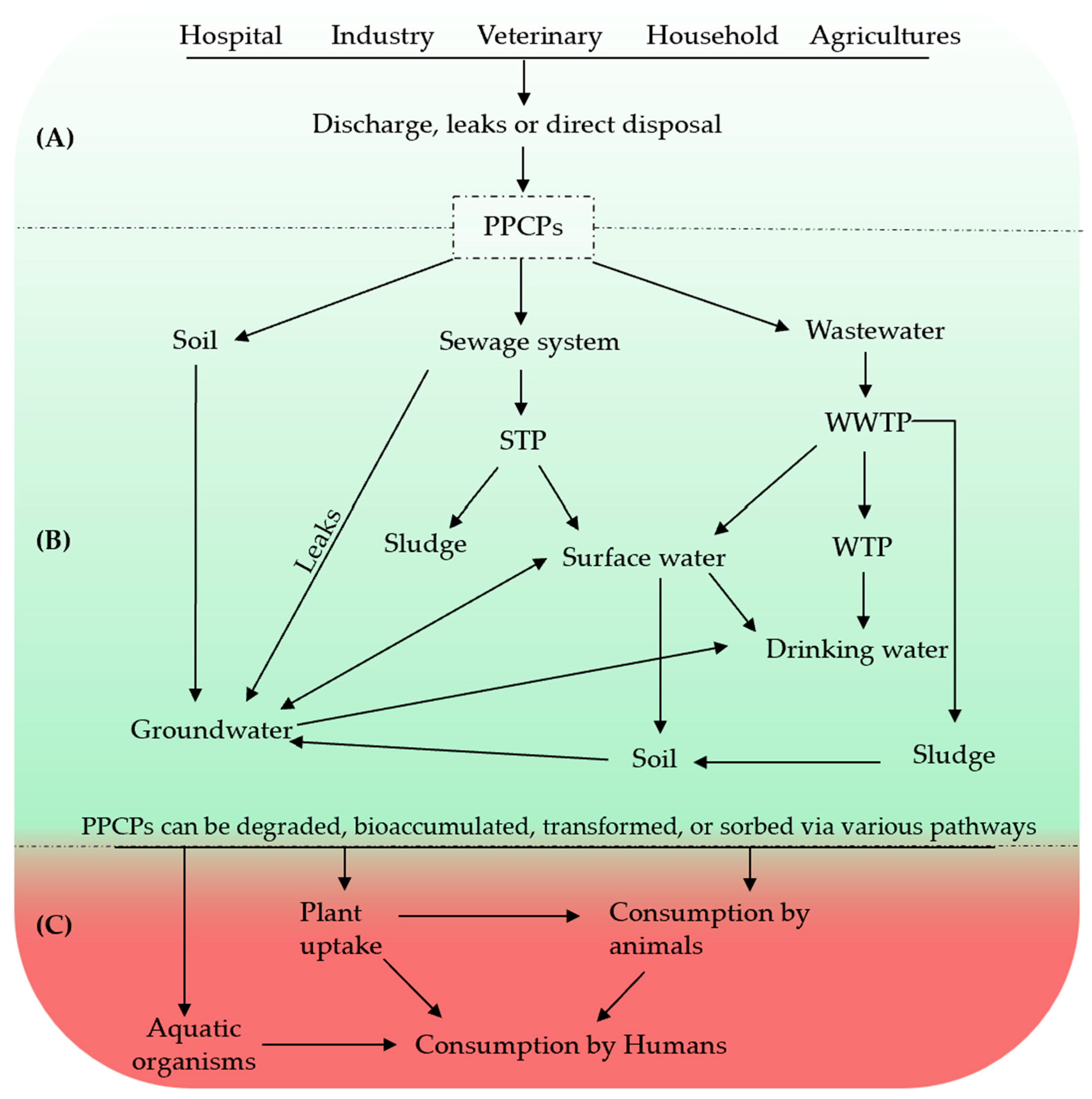
2.1. Sources, Occurrence, and Persistence of PPCPs
2.2. PPCP Exposure and Toxicity
3. Sample Preparation, Extraction, and Clean-Up
3.1. Solid-Phase Extraction (SPE)
3.2. Microwave-Assisted Extraction (MAE)
3.3. Dispersive Liquid–Liquid Microextraction (DLLME)
3.4. Other Extraction Methods
4. Instrumental Analysis
4.1. LC-MS and LC-MS2 Techniques
4.2. GC-MS and GC-MS2 Techniques
4.3. Other Techniques
5. Summary of Analytical Challenges
6. Conclusion and Future Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. Analytical methods for the determination of personal care products in human samples: An overview. Talanta 2014, 129, 448–458. [Google Scholar] [CrossRef]
- Osuoha, J.O.; Anyanwu, B.O.; Ejileugha, C. Pharmaceuticals and personal care products as emerging contaminants: Need for combined treatment strategy. J. Hazard. Mater. Adv. 2023, 9, 100206. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Emerging contaminants: Here today, there tomorrow! Environ. Nanotechnol. Monit. Manag. 2018, 10, 122–126. [Google Scholar] [CrossRef]
- Morales-Caselles, C.; Gao, W.; Ross, P.S.; Fanning, L. Emerging Contaminants of Concern in Canadian Harbours: A Case Study of Halifax Harbour; Marine Affairs Program Technical Report; Dalhousie University: Halifax, NS, Canada, 2016. [Google Scholar]
- Schärer, M.; Bleny, H. Elimination of micropollutants-the Swiss approach. In Proceedings of the Final Conference, Transnational Action Program on Emerging Substances, Brussels, Belgium, 24 September 2015. [Google Scholar]
- Decision, E.U. 495/2015, Commission Implementing Decision (EU) 2015/495 of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union L 2015, 78, 40–42. [Google Scholar]
- Hoenicke, R.; Oros, D.R.; Oram, J.J.; Taberski, K.M. Adapting an ambient monitoring program to the challenge of managing emerging pollutants in the San Francisco Estuary. Environ. Res. 2007, 105, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging environmental contaminants: A global perspective on policies and regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, M.A.; Elsaeidy, A.S.; Ashry, H.; Jobran, A.W.M. Biodegradation method of pharmaceuticals and personal care products. In Handbook of Biodegradable Materials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1093–1131. [Google Scholar]
- Reyes, N.J.D.G.; Geronimo, F.K.F.; Yano, K.A.V.; Guerra, H.B.; Kim, L.-H. Pharmaceutical and personal care products in different matrices: Occurrence, pathways, and treatment processes. Water 2021, 13, 1159. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107 (Suppl. S6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Yang, Y.; Toor, G.S. Contaminants in the urban environment: Pharmaceuticals and personal care products (PPCPs)—Part 2. University of Florida Extension. 2022. Available online: https://edis.ifas.ufl.edu/ (accessed on 12 February 2024).
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Jones-Lepp, T.L.; Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge: The interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2007, 387, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.R.; Kay, P.; Brown, L.E. Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ. Sci. Technol. 2013, 47, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Houtman, C.J.; Van Oostveen, A.M.; Brouwer, A.; Lamoree, M.H.; Legler, J. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environ. Sci. Technol. 2004, 38, 6415–6423. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Löffler, D.; Römbke, J.; Meller, M.; Ternes, T.A. Environmental fate of pharmaceuticals in water/sediment systems. Environ. Sci. Technol. 2005, 39, 5209–5218. [Google Scholar] [CrossRef]
- Klaminder, J.; Brodin, T.; Sundelin, A.; Anderson, N.J.; Fahlman, J.; Jonsson, M.; Fick, J. Long-term persistence of an anxiolytic drug (oxazepam) in a large freshwater lake. Environ. Sci. Technol. 2015, 49, 10406–10412. [Google Scholar] [CrossRef]
- Sultana, S.; Sabir, M.; Ullah, S.; Ahmad, H.R.; Murtaza, G. Contamination of Sewage Water with Active Pharmaceutical Ingredients: An Emerging Threat to Food Products and Human Health. In Emerging Contaminants and Plants: Interactions, Adaptations and Remediation Technologies; Springer: Berlin/Heidelberg, Germany, 2023; pp. 193–231. [Google Scholar]
- La Farre, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Prichard, E.; Granek, E.F. Effects of pharmaceuticals and personal care products on marine organisms: From single-species studies to an ecosystem-based approach. Environ. Sci. Pollut. Res. 2016, 23, 22365–22384. [Google Scholar] [CrossRef] [PubMed]
- Arpin-Pont, L.; Bueno, M.J.M.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the marine environment: A review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Chen, Y.; Yao, B.; Chen, X.; Yu, Y.; Yang, J.; Zhou, Y. Pharmaceuticals and personal care products (PPCPs) in the aquatic environment: Biotoxicity, determination and electrochemical treatment. J. Clean. Prod. 2023, 388, 135923. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci. Total Environ. 2012, 430, 109–118. [Google Scholar] [CrossRef]
- Guerra, P.; Kim, M.; Shah, A.; Alaee, M.; Smyth, S.A. Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci. Total Environ. 2014, 473, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: A case study of emerging pollutant. Desalination Water Treat. 2013, 51, 6158–6164. [Google Scholar] [CrossRef]
- Matsuo, H.; Sakamoto, H.; Arizono, K.; Shinohara, R. Behavior of pharmaceuticals in waste water treatment plant in Japan. Bull. Environ. Contam. Toxicol. 2011, 87, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-f.; Liu, Z.-h.; Yin, H.; Dang, Z.; Wu, P.-x.; Zhu, N.-w.; Lin, Z. Trace determination of sulfonamide antibiotics and their acetylated metabolites via SPE-LC-MS/MS in wastewater and insights from their occurrence in a municipal wastewater treatment plant. Sci. Total Environ. 2019, 653, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, E.M.; Pablos, M.V.; Torija, C.F.; Porcel, M.Á.; González-Doncel, M. Uptake of atenolol, carbamazepine and triclosan by crops irrigated with reclaimed water in a Mediterranean scenario. Ecotoxicol. Environ. Saf. 2020, 191, 110171. [Google Scholar] [CrossRef]
- García-Valverde, M.; Aragonés, A.M.; Andújar, J.A.S.; García, M.D.G.; Martínez-Bueno, M.J.; Fernández-Alba, A.R. Long-term effects on the agroecosystem of using reclaimed water on commercial crops. Sci. Total Environ. 2023, 859, 160462. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Z.-h.; Wang, H.; Dang, Z.; Yin, H.; Zhou, Y.; Liu, Y. Trace determination of eleven natural estrogens and insights from their occurrence in a municipal wastewater treatment plant and river water. Water Res. 2020, 182, 115976. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.; Shaaban, H.; Alqarni, A.; Al-Ansari, R.; Alrashidi, A.; Al-Sultan, F.; Alsulaiman, M.; Alsaif, F.; Aga, O. Multi-class determination of pharmaceuticals as emerging contaminants in wastewater from Eastern Province, Saudi Arabia using eco-friendly SPE-UHPLC-MS/MS: Occurrence, removal and environmental risk assessment. Microchem. J. 2023, 187, 108453. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Beretta, M.; Britto, V.; Tavares, T.M.; da Silva, S.M.T.; Pletsch, A.L. Occurrence of pharmaceutical and personal care products (PPCPs) in marine sediments in the Todos os Santos Bay and the north coast of Salvador, Bahia, Brazil. J. Soils Sediments 2014, 14, 1278–1286. [Google Scholar] [CrossRef]
- Subedi, B.; Balakrishna, K.; Sinha, R.K.; Yamashita, N.; Balasubramanian, V.G.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J. Environ. Chem. Eng. 2015, 3, 2882–2891. [Google Scholar] [CrossRef]
- Ramaswamy, B.R.; Shanmugam, G.; Velu, G.; Rengarajan, B.; Larsson, D.G.J. GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 2011, 186, 1586–1593. [Google Scholar] [CrossRef]
- McClellan, K.; Halden, R.U. Pharmaceuticals and personal care products in archived US biosolids from the 2001 EPA national sewage sludge survey. Water Res. 2010, 44, 658–668. [Google Scholar] [CrossRef]
- Serra-Roig, M.P.; Jurado, A.; Díaz-Cruz, M.S.; Vázquez-Suñé, E.; Pujades, E.; Barceló, D. Occurrence, fate and risk assessment of personal care products in river—Groundwater interface. Sci. Total Environ. 2016, 568, 829–837. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Ames, A.; Czajkowski, K.P. Detection of pharmaceuticals and personal care products in agricultural soils receiving biosolids application. CLEAN-Soil Air Water 2010, 38, 230–237. [Google Scholar] [CrossRef]
- Tran, N.H.; Li, J.; Hu, J.; Ong, S.L. Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4727–4740. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; Ollivier, P.; Togola, A.; Baran, N.; Ghestem, J.-P. Screening of French groundwater for regulated and emerging contaminants. Sci. Total Environ. 2015, 518, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Strauch, G.; Möder, M.; Wennrich, R.; Osenbrück, K.; Gläser, H.-R.; Schladitz, T.; Müller, C.; Schirmer, K.; Reinstorf, F.; Schirmer, M. Indicators for assessing anthropogenic impact on urban surface and groundwater. J. Soils Sediments 2008, 8, 23–33. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sanderson, H.; Roy, K.; Benfenati, E.; Leszczynski, J. Ecotoxicological assessment of pharmaceuticals and personal care products using predictive toxicology approaches. Green Chem. 2020, 22, 1458–1516. [Google Scholar] [CrossRef]
- Saggioro, E.M.; Bila, D.M.; Satyro, S. Ecotoxicology of pharmaceutical and personal care products (PPCPs). Ecotoxicology 2018, 79–110. [Google Scholar] [CrossRef]
- Ortiz de García, S.A.; Pinto Pinto, G.; García-Encina, P.A.; Irusta-Mata, R. Ecotoxicity and environmental risk assessment of pharmaceuticals and personal care products in aquatic environments and wastewater treatment plants. Ecotoxicology 2014, 23, 1517–1533. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A.; Siegrist, H. Peer reviewed: Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef]
- Cruz, S.; Barceló, D. Personal Care Products in the Aquatic Environment; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Crespo, M.; Solé, M. The use of juvenile Solea solea as sentinel in the marine platform of the Ebre Delta: In Vitro interaction of emerging contaminants with the liver detoxification system. Environ. Sci. Pollut. Res. 2016, 23, 19229–19236. [Google Scholar] [CrossRef]
- Spanier, A.J.; Fausnight, T.; Camacho, T.F.; Braun, J.M. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. In Allergy and Asthma Proceedings; OceanSide Publications: Providence, RI, USA, 2014; Volume 35, p. 475. [Google Scholar]
- Terasaki, M.; Takemura, Y.; Makino, M. Paraben-chlorinated derivatives in river waters. Environ. Chem. Lett. 2012, 10, 401–406. [Google Scholar] [CrossRef]
- Fent, K.; Kunz, P.Y.; Gomez, E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. Chimia 2008, 62, 368. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Ferrari, B.t.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid, and diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, B.; Köllner, B.; Dietrich, D.R.; Hitzfeld, B. Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat. Toxicol. 2005, 75, 53–64. [Google Scholar] [CrossRef]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-blockers in the environment: Part II. Ecotoxicity study. Sci. Total Environ. 2014, 493, 1122–1126. [Google Scholar] [CrossRef]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (B-blockers) on aquatic organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef]
- Ríos, A.L.M.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Oliveira, L.F.S. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef]
- Nilsson, J.R. Methotrexate permits a limited number of cell doublings and affects mitochondrial substructure in Tetrahymena. Protoplasma 1983, 117, 53–61. [Google Scholar] [CrossRef]
- Henschel, K.P.; Wenzel, A.; Diedrich, M.; Fliedner, A. Environmental hazard assessment of pharmaceuticals. Regul. Toxicol. Pharmacol. 1997, 25, 220–225. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Lützhøft, H.C.H.; Andersen, H.R.; Ingerslev, F. Environmental risk assessment of antibiotics: Comparison of mecillinam, trimethoprim and ciprofloxacin. J. Antimicrob. Chemother. 2000, 46 (Suppl. S1), 53–58. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Belden, J.B.; Lydy, M.J. Toxicity of fluoroquinolone antibiotics to aquatic organisms. Environ. Toxicol. Chem. Int. J. 2005, 24, 423–430. [Google Scholar] [CrossRef]
- Sanchez-Prado, L.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Microwave-assisted extraction of emerging pollutants in environmental and biological samples before chromatographic determination. TrAC Trends Anal. Chem. 2015, 71, 119–143. [Google Scholar] [CrossRef]
- Shaaban, H.; Mostafa, A.; Alhajri, W.; Almubarak, L.; AlKhalifah, K. Development and validation of an eco-friendly SPE-HPLC-MS method for simultaneous determination of selected parabens and bisphenol A in personal care products: Evaluation of the greenness profile of the developed method. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 621–628. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Mostafa, A.; Shaaban, H.; Alqarni, A.M.; Alghamdi, M.; Alsultan, S.; Al-Saeed, J.S.; Alsaba, S.; AlMoslem, A.; Alshehry, Y.; Ahmad, R. Vortex-assisted dispersive liquid-liquid microextraction using thymol based natural deep eutectic solvent for trace analysis of sulfonamides in water samples: Assessment of the greenness profile using AGREE metric, GAPI and analytical eco-scale. Microchem. J. 2022, 183, 107976. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Mostafa, A.; Shaaban, H.; Gomaa, M.S.; Albashrayi, D.; Hasheeshi, B.; Bakhashwain, N.; Aseeri, A.; Alqarni, A.; Alamri, A.A. Development and optimization of natural deep eutectic solvent-based dispersive liquid–liquid microextraction coupled with UPLC-UV for simultaneous determination of parabens in personal care products: Evaluation of the eco-friendliness level of the developed method. RSC Adv. 2023, 13, 13183–13194. [Google Scholar] [PubMed]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environ. Monit. Assess. 2014, 186, 541–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hong, Y.; Park, J.-E.; Sharma, V.K.; Cho, S.-I. Sulfonamides and tetracyclines in livestock wastewater. Chemosphere 2013, 91, 888–894. [Google Scholar] [CrossRef]
- Ferguson, P.J.; Bernot, M.J.; Doll, J.C.; Lauer, T.E. Detection of pharmaceuticals and personal care products (PPCPs) in near-shore habitats of southern Lake Michigan. Sci. Total Environ. 2013, 458, 187–196. [Google Scholar] [CrossRef]
- Tran, N.H.; Hu, J.; Ong, S.L. Simultaneous determination of PPCPs, EDCs, and artificial sweeteners in environmental water samples using a single-step SPE coupled with HPLC-MS/MS and isotope dilution. Talanta 2013, 113, 82–92. [Google Scholar] [CrossRef]
- Alqarni, A.M.; Mostafa, A.; Shaaban, H.; Mokhtar, H.I.; Aseeri, A.; Alkarshami, B.; Alonaizi, S.; Alrafidi, R.D.; Aseeri, A.A.; Alrofaidi, M.A. Air-agitated liquid-liquid microextraction method based on solidification of a floating organic droplet (AALLME-SFO) followed by UPLC-MS/MS for trace analysis of steroids in water samples: Assessment of the method environmental impact using Analytical Eco-Scale, green Analytical procedure Index and the Analytical GREEnness metric. Microchem. J. 2024, 200, 110244. [Google Scholar]
- Kim, H.; Homan, M. Evaluation of pharmaceuticals and personal care products (PPCPs) in drinking water originating from Lake Erie. J. Great Lakes Res. 2020, 46, 1321–1330. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Xu, N.; Pan, B.; Ni, J. Pharmaceuticals and personal care products (PPCPs) in water, sediment and freshwater mollusks of the Dongting Lake downstream the Three Gorges Dam. Chemosphere 2022, 301, 134721. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef] [PubMed]
- Giebułtowicz, J.; Nałęcz-Jawecki, G.; Harnisz, M.; Kucharski, D.; Korzeniewska, E.; Płaza, G. Environmental risk and risk of resistance selection due to antimicrobials’ occurrence in two Polish wastewater treatment plants and receiving surface water. Molecules 2020, 25, 1470. [Google Scholar] [CrossRef]
- Pai, C.-W.; Leong, D.; Chen, C.-Y.; Wang, G.-S. Occurrences of pharmaceuticals and personal care products in the drinking water of Taiwan and their removal in conventional water treatment processes. Chemosphere 2020, 256, 127002. [Google Scholar] [CrossRef]
- García-Valverde, M.; Cortes-Corrales, L.; Gómez-Ramos, M.d.M.; Martínez-Bueno, M.J.; Fernández-Alba, A.R. Evaluation of chemical contamination of crops produced in greenhouse by irrigation with reclaimed water. Sci. Total Environ. 2024, 912, 169454. [Google Scholar] [CrossRef]
- Narumiya, M.; Nakada, N.; Yamashita, N.; Tanaka, H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard. Mater. 2013, 260, 305–312. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Karnjanapiboonwong, A.; Suski, J.G.; Shah, A.A.; Cai, Q.; Morse, A.N.; Anderson, T.A. Occurrence of PPCPs at a wastewater treatment plant and in soil and groundwater at a land application site. Water Air Soil Pollut. 2011, 216, 257–273. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, R.; Cui, H.; Liu, D.; Yan, G.; Fan, Y.; Cheng, K.; Cao, Z. Occurrences of typical PPCPs during wastewater treatment and the composting of sewage sludge with micron-sized and nano-sized Fe3O4. Environ. Pollut. 2023, 336, 122386. [Google Scholar] [CrossRef]
- Hanc, A.; Dume, B.; Hrebeckova, T.; Michal, P.; Hrcka, M.; Nemcova, K.; Grasserova, A.; Cajthaml, T. The fate of pharmaceuticals and personal care products during composting of sewage sludge. Sustain. Chem. Pharm. 2024, 38, 101498. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Zhang, Z.; Yang, J.; Chen, W.; Liu, B.; Lu, J. The fate of pharmaceuticals and personal care products (PPCPs) in sewer sediments: Adsorption triggering resistance gene proliferation. J. Hazard. Mater. 2024, 471, 134255. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Occurrence of the main metabolites of the most recurrent pharmaceuticals and personal care products in Mediterranean soils. J. Environ. Manag. 2021, 278, 111584. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Comparison of ultrasound-assisted extraction, QuEChERS and selective pressurized liquid extraction for the determination of metabolites of parabens and pharmaceuticals in sludge. Microchem. J. 2020, 157, 104987. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Hernández, F. Simultaneous determination of acidic, neutral and basic pharmaceuticals in urban wastewater by ultra high-pressure liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 622–632. [Google Scholar] [CrossRef]
- Buchberger, W.W. Current approaches to trace analysis of pharmaceuticals and personal care products in the environment. J. Chromatogr. A 2011, 1218, 603–618. [Google Scholar] [CrossRef]
- Nurmi, J.; Pellinen, J. Multiresidue method for the analysis of emerging contaminants in wastewater by ultra performance liquid chromatography-time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 6712–6719. [Google Scholar] [CrossRef]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef]
- Boles, T.H.; Wells, M.J.M. Analysis of amphetamine and methamphetamine in municipal wastewater influent and effluent using weak cation—Exchange SPE and LC-MS/MS. Electrophoresis 2016, 37, 3101–3108. [Google Scholar] [CrossRef]
- Xu, M.; Huang, H.; Li, N.; Li, F.; Wang, D.; Luo, Q. Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol. Environ. Saf. 2019, 175, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Lis, H.; Kobylis, P.; Stepnowski, P. The triple-sorbents solid-phase extraction for pharmaceuticals and estrogens determination in wastewater samples. Microchem. J. 2019, 149, 103965. [Google Scholar] [CrossRef]
- Salas, D.; Borrull, F.; Fontanals, N.; Marcé, R.M. Combining cationic and anionic mixed-mode sorbents in a single cartridge to extract basic and acidic pharmaceuticals simultaneously from environmental waters. Anal. Bioanal. Chem. 2018, 410, 459–469. [Google Scholar] [CrossRef]
- Sadutto, D.; Picó, Y. Sample preparation to determine pharmaceutical and personal care products in an all-water matrix: Solid phase extraction. Molecules 2020, 25, 5204. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, H.; Li, H.; Wang, M.; Qiu, B.; Yang, Z. Influence of filtration during sample pretreatment on the detection of antibiotics and non-steroidal anti-inflammatory drugs in natural surface waters. Sci. Total Environ. 2019, 650, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Barceló, D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography-mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J. Chromatogr. A 2007, 1152, 97–115. [Google Scholar] [CrossRef] [PubMed]
- de la Serna Calleja, M.Á.; Bolado, S.; Jiménez, J.J.; López-Serna, R. Performance critical comparison of offline SPE, online SPE, and direct injection for the determination of CECs in complex liquid environmental matrices. Microchem. J. 2023, 187, 108395. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, T.; Qi, C.; Peng, G.; Lu, M.; Huang, J.; Blaney, L.; Yu, G. Automated online solid-phase extraction liquid chromatography tandem mass spectrometry investigation for simultaneous quantification of per-and polyfluoroalkyl substances, pharmaceuticals and personal care products, and organophosphorus flame retardants in environmental waters. J. Chromatogr. A 2019, 1602, 350–358. [Google Scholar]
- Rozaini, M.N.H.; Kiatkittipong, W.; Saad, B.; Yahaya, N.; Shaharun, M.S.; Sangu, S.S.; Saheed, M.S.M.; Wong, Y.F.; Mohamad, M.; Sambudi, N.S. Green adsorption–desorption of mixed triclosan, triclocarban, 2-phenylphenol, bisphenol A and 4-tert-octylphenol using MXene encapsulated polypropylene membrane protected micro-solid-phase extraction device in amplifying the HPLC analysis. Microchem. J. 2021, 170, 106695. [Google Scholar] [CrossRef]
- González-Hernández, P.; Lago, A.B.; Pasán, J.; Ruiz-Pérez, C.; Ayala, J.H.; Afonso, A.M.; Pino, V. Application of a pillared-layer Zn-triazolate metal-organic framework in the dispersive miniaturized solid-phase extraction of personal care products from wastewater samples. Molecules 2019, 24, 690. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, L.; He, Q.; Sun, Q.; Yin, D.; Zhang, Y. A review on recent developments and applications of green sorbents-based solid phase extraction techniques. Adv. Sample Prep. 2023, 6, 100065. [Google Scholar] [CrossRef]
- Huang, X.-C.; Ma, J.-K.; Wei, S.-L. Preparation and application of a novel magnetic molecularly imprinted polymer for simultaneous and rapid determination of three trace endocrine disrupting chemicals in lake water and milk samples. Anal. Bioanal. Chem. 2020, 412, 1835–1846. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Chen, Y.; Wang, J.; Ping, H.; Lu, A. Graphene-derivatized silica composite as solid-phase extraction sorbent combined with GC-MS/MS for the determination of polycyclic musks in aqueous samples. Molecules 2018, 23, 318. [Google Scholar] [CrossRef]
- Tang, H.-z.; Wang, Y.-h.; Li, S.; Wu, J.; Li, J.-w.; Zhou, H.-y.; Gao, Z.-x. Graphene oxide composites for magnetic solid-phase extraction of twelve quinolones in water samples followed by MALDI-TOF MS. Anal. Bioanal. Chem. 2019, 411, 7039–7049. [Google Scholar] [CrossRef]
- Jakubus, A.; Godlewska, K.; Gromelski, M.; Jagiello, K.; Puzyn, T.; Stepnowski, P.; Paszkiewicz, M. The possibility to use multi-walled carbon nanotubes as a sorbent for dispersive solid phase extraction of selected pharmaceuticals and their metabolites: Effect of extraction condition. Microchem. J. 2019, 146, 1113–1125. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Huang, X. One-pot preparation of magnetic carbon adsorbent derived from pomelo peel for magnetic solid-phase extraction of pollutants in environmental waters. J. Chromatogr. A 2018, 1546, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, N.C.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Palomino, G.T.; Cabello, C.P. Carbon composite membrane derived from MIL-125-NH2 MOF for the enhanced extraction of emerging pollutants. Chemosphere 2019, 231, 510–517. [Google Scholar] [CrossRef]
- Abu-Samra, A.; Morris, J.S.; Koirtyohann, S.R. Wet ashing of some biological samples in a microwave oven. Anal. Chem. 1975, 47, 1475–1477. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Mechlińska, A.; Zygmunt, B.; Namieśnik, J. Green analytical chemistry in sample preparation for determination of trace organic pollutants. TrAC Trends Anal. Chem. 2009, 28, 943–951. [Google Scholar] [CrossRef]
- Delgado, B.; Pino, V.; Anderson, J.L.; Ayala, J.H.; Afonso, A.M.; Gonzalez, V. An in-situ extraction—Preconcentration method using ionic liquid-based surfactants for the determination of organic contaminants contained in marine sediments. Talanta 2012, 99, 972–983. [Google Scholar] [CrossRef]
- Madej, K. Microwave-assisted and cloud-point extraction in determination of drugs and other bioactive compounds. TrAC Trends Anal. Chem. 2009, 28, 436–446. [Google Scholar] [CrossRef]
- Bélanger, J.M.R.; Paré, J.R.J. Applications of microwave-assisted processes (MAP™) to environmental analysis. Anal. Bioanal. Chem. 2006, 386, 1049–1058. [Google Scholar] [CrossRef]
- Song, Y.; Wu, L.; Lu, C.; Li, N.; Hu, M.; Wang, Z. Microwave-assisted liquid-liquid microextraction based on solidification of ionic liquid for the determination of sulfonamides in environmental water samples. J. Sep. Sci. 2014, 37, 3533–3538. [Google Scholar] [CrossRef]
- Pérez, R.A.; Albero, B.; Miguel, E.; Sánchez-Brunete, C. Determination of parabens and endocrine-disrupting alkylphenols in soil by gas chromatography-mass spectrometry following matrix solid-phase dispersion or in-column microwave-assisted extraction: A comparative study. Anal. Bioanal. Chem. 2012, 402, 2347–2357. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Maraschi, F.; Porta, A.; Profumo, A. Fast low-pressurized microwave-assisted extraction of benzotriazole, benzothiazole and benezenesulfonamide compounds from soil samples. Talanta 2016, 147, 322–327. [Google Scholar] [CrossRef]
- Kotnik, K.; Kosjek, T.; Krajnc, U.; Heath, E. Trace analysis of benzophenone-derived compounds in surface waters and sediments using solid-phase extraction and microwave-assisted extraction followed by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 3179–3190. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Microwave-assisted extraction combined with on-line solid phase extraction followed by ultra-high-performance liquid chromatography with tandem mass spectrometric determination of benzotriazole UV stabilizers in marine sediments and sewage sludges. J. Sep. Sci. 2013, 36, 781–788. [Google Scholar] [CrossRef]
- Wu, S.-F.; Liu, L.-L.; Ding, W.-H. One-step microwave-assisted headspace solid-phase microextraction for the rapid determination of synthetic polycyclic musks in oyster by gas chromatography-mass spectrometry. Food Chem. 2012, 133, 513–517. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, P.; Dai, X.; Hou, X.; Zhao, L.; Liang, N. Trace determination of antibacterial pharmaceuticals in fishes by microwave-assisted extraction and solid-phase purification combined with dispersive liquid-liquid microextraction followed by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2016, 1011, 136–144. [Google Scholar]
- Zhang, Y.; Guo, W.; Yue, Z.; Lin, L.; Zhao, F.; Chen, P.; Wu, W.; Zhu, H.; Yang, B.; Kuang, Y. Rapid determination of 54 pharmaceutical and personal care products in fish samples using microwave-assisted extraction—Hollow fiber—Liquid/solid phase microextraction. J. Chromatogr. B 2017, 1051, 41–53. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, Y.C.; Ding, W.H. Rapid determination of alkylphenols in aqueous samples by in situ acetylation and microwave-assisted headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. J. Sep. Sci. 2012, 35, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ballesteros, E. Determination of 13 endocrine disrupting chemicals in environmental solid samples using microwave-assisted solvent extraction and continuous solid-phase extraction followed by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lopardo, L.; Rydevik, A.; Kasprzyk-Hordern, B. A new analytical framework for multi-residue analysis of chemically diverse endocrine disruptors in complex environmental matrices utilising ultra-performance liquid chromatography coupled with high-resolution tandem quadrupole time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 689–704. [Google Scholar]
- Rezaei Motlagh, S.; Harun, R.; Awang Biak, D.R.; Hussain, S.A.; Omar, R.; Khezri, R.; Elgharbawy, A.A. Ionic liquid-based microwave-assisted extraction of lipid and eicosapentaenoic acid from Nannochloropsis oceanica biomass: Experimental optimization approach. J. Appl. Phycol. 2021, 33, 2015–2029. [Google Scholar] [CrossRef]
- Wang, J.; Jing, W.; Tian, H.; Liu, M.; Yan, H.; Bi, W.; Chen, D.D.Y. Investigation of deep eutectic solvent-based microwave-assisted extraction and efficient recovery of natural products. ACS Sustain. Chem. Eng. 2020, 8, 12080–12088. [Google Scholar] [CrossRef]
- Djozan, D.; Assadi, Y.; Haddadi, S.H. Anodized aluminum wire as a solid-phase microextraction fiber. Anal. Chem. 2001, 73, 4054–4058. [Google Scholar] [CrossRef]
- Djozan, D.; Assadi, Y. Modified pencil lead as a new fiber for solid-phase microextraction. Chromatographia 2004, 60, 313–317. [Google Scholar] [CrossRef]
- Cacho, J.; Ferreira, V.; Fernandez, P. Microextraction by demixing for the determination of volatile compounds in aqueous solutions. Anal. Chim. Acta 1992, 264, 311–317. [Google Scholar] [CrossRef]
- He, Y.; Lee, H.K. Liquid-phase microextraction in a single drop of organic solvent by using a conventional microsyringe. Anal. Chem. 1997, 69, 4634–4640. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Sun, D.; Yang, S.; Liu, H.; Chen, L. Strategies of dispersive liquid-liquid microextraction for coastal zone environmental pollutant determination. J. Chromatogr. A 2021, 1658, 462615. [Google Scholar] [CrossRef] [PubMed]
- Primel, E.G.; Caldas, S.S.; Marube, L.C.; Escarrone, A.L.V. An overview of advances in dispersive liquid-liquid microextraction for the extraction of pesticides and emerging contaminants from environmental samples. Trends Environ. Anal. Chem. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Marube, L.C.; Caldas, S.S.; Soares, K.L.; Primel, E.G. Dispersive liquid-liquid microextraction with solidification of floating organic droplets for simultaneous extraction of pesticides, pharmaceuticals and personal care products. Microchim. Acta 2015, 182, 1765–1774. [Google Scholar] [CrossRef]
- Rozaini, M.N.H.; Saad, B.; Yahaya, N.; Lim, J.W.; Mohd Aris, M.N.; Ramachandran, M.R. Determination of Three Endocrine Disruptors in Water Samples by Ultrasound-Assisted Salt-Induced Liquid-Liquid Microextraction (UA-SI-LLME) and High-Performance Liquid Chromatography—Diode Array Detection (HPLC-DAD). Anal. Lett. 2022, 55, 132–145. [Google Scholar] [CrossRef]
- Pawliszyn, J. Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 2014, 119, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Razavi, N.; Yazdinejad, S.R. Separation and determination of amitriptyline and nortriptyline by dispersive liquid-liquid microextraction combined with gas chromatography flame ionization detection. Talanta 2008, 75, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Zgoła-Grześkowiak, A. Application of DLLME to isolation and concentration of non-steroidal anti-inflammatory drugs in environmental water samples. Chromatographia 2010, 72, 671–678. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Ding, W.-H. Determination of synthetic polycyclic musks in aqueous samples by ultrasound-assisted dispersive liquid–liquid microextraction and gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1723–1730. [Google Scholar] [CrossRef]
- Zhang, P.-P.; Shi, Z.-G.; Yu, Q.-W.; Feng, Y.-Q. A new device for magnetic stirring-assisted dispersive liquid-liquid microextraction of UV filters in environmental water samples. Talanta 2011, 83, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Ohoro, C.R.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Distribution and chemical analysis of pharmaceuticals and personal care products (PPCPs) in the environmental systems: A review. Int. J. Environ. Res. Public Health 2019, 16, 3026. [Google Scholar] [CrossRef]
- de Sousa, D.N.R.; Grosseli, G.M.; Mozeto, A.A.; Carneiro, R.L.; Fadini, P.S. Ultrasound-assisted extraction method for the simultaneous determination of emerging contaminants in freshwater sediments. J. Sep. Sci. 2015, 38, 3454–3460. [Google Scholar] [CrossRef]
- Montemurro, N.; Postigo, C.; Chirón, S.; Barcelò, D.; Pérez, S. Analysis and fate of 14 relevant wastewater-derived organic pollutants in long-term exposed soil. Anal. Bioanal. Chem. 2019, 411, 2687–2696. [Google Scholar] [CrossRef]
- Okuda, T.; Yamashita, N.; Tanaka, H.; Matsukawa, H.; Tanabe, K. Development of extraction method of pharmaceuticals and their occurrences found in Japanese wastewater treatment plants. Environ. Int. 2009, 35, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Tobin, J.; Paull, B. Multi-residue determination of pharmaceuticals in sludge and sludge enriched soils using pressurized liquid extraction, solid phase extraction and liquid chromatography with tandem mass spectrometry. J. Environ. Monit. 2008, 10, 353–361. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; de Alda, M.J.L.; Barceló, D. Determination of antimicrobials in sludge from infiltration basins at two artificial recharge plants by pressurized liquid extraction–liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1130, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Bin, L.K.; Janakiraman, A.K.; Abd Razak, F.S.; Uddin, A.B.M.H.; Sarker, M.Z.I.; Ming, L.C.; Goh, B.H. Supercritical fluid technology and its pharmaceutical applications: A revisit with two decades of progress. Indian J. Pharm. Educ. Res. 2020, 54, s1–s11. [Google Scholar] [CrossRef]
- Baduel, C.; Mueller, J.F.; Tsai, H.; Ramos, M.J.G. Development of sample extraction and clean-up strategies for target and non-target analysis of environmental contaminants in biological matrices. J. Chromatogr. A 2015, 1426, 33–47. [Google Scholar] [CrossRef]
- Osemwengie, L.I. Determination of synthetic musk compounds in sewage biosolids by gas chromatography/mass spectrometry. J. Environ. Monit. 2006, 8, 897–903. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Alder, A.C.; Giger, W.; Theiß, N.; Löffler, D.; Ternes, T.A. Extraction and determination of sulfonamides, macrolides, and trimethoprim in sewage sludge. J. Chromatogr. A 2005, 1085, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, N.S.; Asimakopoulos, A.G.; Bletsou, A.A. Emerging contaminants: A tutorial mini-review. Glob. NEST J. 2012, 14, 72–79. [Google Scholar]
- Barceló, D. Applications of LC-MS in Environmental Chemistry; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Gao, P.; Ding, Y.; Li, H.; Xagoraraki, I. Occurrence of pharmaceuticals in a municipal wastewater treatment plant: Mass balance and removal processes. Chemosphere 2012, 88, 17–24. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Lonappan, L.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Diclofenac in municipal wastewater treatment plant: Quantification using laser diode thermal desorption—Atmospheric pressure chemical ionization—Tandem mass spectrometry approach in comparison with an established liquid chromatography-electrospray ionization-tandem mass spectrometry method. J. Chromatogr. A 2016, 1433, 106–113. [Google Scholar]
- Pérez-Lemus, N.; López-Serna, R.; Pérez-Elvira, S.I.; Barrado, E. Analytical methodologies for the determination of pharmaceuticals and personal care products (PPCPs) in sewage sludge: A critical review. Anal. Chim. Acta 2019, 1083, 19–40. [Google Scholar] [CrossRef]
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceuticals and personal-care products in environmental matrices. TrAC Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Wang, C.; Gardinali, P.R. Comparison of multiple API techniques for the simultaneous detection of microconstituents in water by on-line SPE-LC-MS/MS. J. Mass Spectrom. 2012, 47, 1255–1268. [Google Scholar] [CrossRef]
- Benedetti, B.; Baglietto, M.; MacKeown, H.; Scapuzzi, C.; Di Carro, M.; Magi, E. An optimized processing method for polar organic chemical integrative samplers deployed in seawater: Toward a maximization of the analysis accuracy for trace emerging contaminants. J. Chromatogr. A 2022, 1677, 463309. [Google Scholar] [CrossRef]
- Pfeifer, T.; Tuerk, J.; Bester, K.; Spiteller, M. Determination of selected sulfonamide antibiotics and trimethoprim in manure by electrospray and atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Palmgrén, J.J.; Töyräs, A.; Mauriala, T.; Mönkkönen, J.; Auriola, S. Quantitative determination of cholesterol, sitosterol, and sitostanol in cultured Caco-2 cells by liquid chromatography—Atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. B 2005, 821, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lien, G.-W.; Chen, C.-Y.; Wang, G.-S. Comparison of electrospray ionization, atmospheric pressure chemical ionization and atmospheric pressure photoionization for determining estrogenic chemicals in water by liquid chromatography tandem mass spectrometry with chemical derivatizations. J. Chromatogr. A 2009, 1216, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cruz, M.S.; Barceló, D. LC-MS2 trace analysis of antimicrobials in water, sediment and soil. TrAC Trends Anal. Chem. 2005, 24, 645–657. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Hu, L.-X.; Zhao, J.-H.; Han, Y.; Liu, Y.-S.; Zhao, J.-L.; Yang, B.; Ying, G.-G. Suspect, non-target and target screening of pharmaceuticals and personal care products (PPCPs) in a drinking water system. Sci. Total Environ. 2022, 808, 151866. [Google Scholar] [CrossRef] [PubMed]
- López-Roldán, R.; de Alda, M.L.; Gros, M.; Petrovic, M.; Martín-Alonso, J.; Barceló, D. Advanced monitoring of pharmaceuticals and estrogens in the Llobregat River basin (Spain) by liquid chromatography-triple quadrupole-tandem mass spectrometry in combination with ultra performance liquid chromatography—Time of flight-mass spectrometry. Chemosphere 2010, 80, 1337–1344. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC—High resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef]
- Mhuka, V.; Dube, S.; Nindi, M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-Orbitrap™ MS. Emerg. Contam. 2020, 6, 250–258. [Google Scholar] [CrossRef]
- Abdallah, M.A.-E.; Nguyen, K.-H.; Ebele, A.J.; Atia, N.N.; Ali, H.R.H.; Harrad, S. A single run, rapid polarity switching method for determination of 30 pharmaceuticals and personal care products in waste water using Q-Exactive Orbitrap high resolution accurate mass spectrometry. J. Chromatogr. A 2019, 1588, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Scientific, T. Q Exactive Plus Orbitrap LC-MS/MS System: Characterize, Quantify and Confirm with Unmatched Confidence. 2015. Available online: https://assets.thermofisher.com/ (accessed on 12 February 2024).
- Renew, J.E.; Huang, C.-H. Simultaneous determination of fluoroquinolone, sulfonamide, and trimethoprim antibiotics in wastewater using tandem solid phase extraction and liquid chromatography—Electrospray mass spectrometry. J. Chromatogr. A 2004, 1042, 113–121. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The effect of signal suppression and mobile phase composition on the simultaneous analysis of multiple classes of acidic/neutral pharmaceuticals and personal care products in surface water by solid-phase extraction and ultra performance liquid chromatography-negative electrospray tandem mass spectrometry. Talanta 2008, 74, 1299–1312. [Google Scholar] [PubMed]
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography-tandem mass spectrometry (LC-MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Shaaban, H.; Górecki, T. Fused core particles as an alternative to fully porous sub-2 μm particles in pharmaceutical analysis using coupled columns at elevated temperature. Anal. Methods 2012, 4, 2735–2743. [Google Scholar] [CrossRef]
- Shaaban, H.; Gorecki, T. Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta 2015, 132, 739–752. [Google Scholar] [CrossRef]
- Shaaban, H. New insights into liquid chromatography for more eco-friendly analysis of pharmaceuticals. Anal. Bioanal. Chem. 2016, 408, 6929–6944. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)—A powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Li, B.; Zhang, T. Direct rapid analysis of multiple PPCPs in municipal wastewater using ultrahigh performance liquid chromatography-tandem mass spectrometry without SPE pre-concentration. Anal. Chim. Acta 2012, 738, 59–68. [Google Scholar] [CrossRef] [PubMed]
- López-Serna, R.; Marín-de-Jesús, D.; Irusta-Mata, R.; García-Encina, P.A.; Lebrero, R.; Fdez-Polanco, M.; Muñoz, R. Multiresidue analytical method for pharmaceuticals and personal care products in sewage and sewage sludge by online direct immersion SPME on-fiber derivatization—GCMS. Talanta 2018, 186, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Kopperi, M.; Parshintsev, J.; Ruiz-Jiménez, J.; Riekkola, M.L. Nontargeted evaluation of the fate of steroids during wastewater treatment by comprehensive two-dimensional gas chromatography—Time-of-flight mass spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 17008–17017. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, L. Analysis of endocrine disrupting compounds, pharmaceuticals and personal care products in sewage sludge by gas chromatography-mass spectrometry. Talanta 2012, 89, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, A.; Ballesteros, E. Combined microwave-assisted extraction and continuous solid-phase extraction prior to gas chromatography—Mass spectrometry determination of pharmaceuticals, personal care products and hormones in soils, sediments and sludge. Sci. Total Environ. 2012, 419, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; de Alarcón-Gómez, B.; Rodríguez-Gómez, R.; García-Córcoles, M.T.; Çipa, M.; Zafra-Gómez, A. Analytical methods for the determination of emerging contaminants in sewage sludge samples. A review. Talanta 2019, 192, 508–533. [Google Scholar] [CrossRef] [PubMed]
- Milinovic, J.; Vidal, M.; Lacorte, S.; Rigol, A. Leaching of heavy metals and alkylphenolic compounds from fresh and dried sewage sludge. Environ. Sci. Pollut. Res. 2014, 21, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Vakondios, N.; Mazioti, A.A.; Koukouraki, E.E.; Diamadopoulos, E. An analytical method for measuring specific endocrine disruptors in activated sludge (biosolids) using solid phase microextraction-gas chromatography. J. Environ. Chem. Eng. 2016, 4, 1910–1917. [Google Scholar] [CrossRef]
- Vallecillos, L.; Pocurull, E.; Borrull, F. A simple and automated method to determine macrocyclic musk fragrances in sewage sludge samples by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1314, 38–43. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.-F.; Xu, L.; Liu, L.-Y.; Song, W.-W.; Zhu, F.-J.; Li, Y.-F.; Ma, W.-L. Occurrence and fate of benzotriazoles UV filters in a typical residential wastewater treatment plant in Harbin, China. Environ. Pollut. 2017, 227, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Krogh, K.A.; Halling-Sørensen, B.; Björklund, E. Determination of ten steroid hormones in animal waste manure and agricultural soil using inverse and integrated clean-up pressurized liquid extraction and gas chromatography-tandem mass spectrometry. Anal. Methods 2011, 3, 1087–1095. [Google Scholar] [CrossRef]
- Basaglia, G.; Pietrogrande, M.C. Optimization of a SPME/GC/MS method for the simultaneous determination of pharmaceuticals and personal care products in waters. Chromatographia 2012, 75, 361–370. [Google Scholar] [CrossRef]
- Selim, M.I.; Shawky, M.; Wooten, A.; Rushing, B. Comparison of LC-MS and GC-MS for the analysis of pharmaceuticals and personal care products in surface water and treated wastewaters. Curr. Trends Mass Spectrom. 2016, 14, 8–14. Available online: https://www.spectroscopyonline.com/ (accessed on 12 February 2024).
- Tsizin, S.; Bokka, R.; Keshet, U.; Alon, T.; Fialkov, A.B.; Tal, N.; Amirav, A. Comparison of electrospray LC-MS, LC-MS with Cold EI and GC-MS with Cold EI for sample identification. Int. J. Mass Spectrom. 2017, 422, 119–125. [Google Scholar] [CrossRef]
- Chauhan, A.; Goyal, M.K.; Chauhan, P. GC-MS technique and its analytical applications in science and technology. J. Anal. Bioanal. Tech 2014, 5, 222. [Google Scholar] [CrossRef]
- Matamoros, V.; Jover, E.; Bayona, J.M. Part-per-trillion determination of pharmaceuticals, pesticides, and related organic contaminants in river water by solid-phase extraction followed by comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. Anal. Chem. 2010, 82, 699–706. [Google Scholar] [CrossRef]
- Jover, E.; Matamoros, V.; Bayona, J.M. Characterization of benzothiazoles, benzotriazoles and benzosulfonamides in aqueous matrixes by solid-phase extraction followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 4013–4019. [Google Scholar] [CrossRef]
- Muscalu, A.M.; Górecki, T. Comprehensive two-dimensional gas chromatography in environmental analysis. TrAC Trends Anal. Chem. 2018, 106, 225–245. [Google Scholar] [CrossRef]
- Prebihalo, S.; Brockman, A.; Cochran, J.; Dorman, F.L. Determination of emerging contaminants in wastewater utilizing comprehensive two-dimensional gas-chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2015, 1419, 109–115. [Google Scholar] [CrossRef]
- Wardencki, W.; Namieśnik, J. Some remarks on gas chromatographic challenges in the context of green analytical chemistry. Pol. J. Environ. Stud 2002, 11, 185–187. [Google Scholar]
- Biswas, D.; Mitra, D. Green techniques in gas chromatography. In Green Chromatographic Techniques: Separation and Purification of Organic and Inorganic Analytes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 103–121. [Google Scholar]
- Bartram, R.; Froehlich, P. Considerations on switching from helium to hydrogen. LCGC N. Am. 2010, 28, 890–900. [Google Scholar]
- Frost, N.W.; Jing, M.; Bowser, M.T. Capillary electrophoresis. Anal. Chem. 2010, 82, 4682–4698. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.E.; Wang, C.; Ma, Y. Determination of pharmaceutical and personal care products in wastewater by capillary electrophoresis with UV detection. Talanta 2011, 84, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Rao, Q.; Zhu, K.; Jiang, Z.; Ding, S. Simultaneous determination of five tetracycline and macrolide antibiotics in feeds using HPCE. J. Sep. Sci. 2009, 32, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Soto-Chinchilla, J.J.; García-Campaña, A.M.; Gámiz-Gracia, L. Analytical methods for multiresidue determination of sulfonamides and trimethoprim in meat and ground water samples by CE-MS and CE-MS/MS. Electrophoresis 2007, 28, 4164–4172. [Google Scholar] [CrossRef]
- Ahrer, W.; Scherwenk, E.; Buchberger, W. Determination of drug residues in water by the combination of liquid chromatography or capillary electrophoresis with electrospray mass spectrometry. J. Chromatogr. A 2001, 910, 69–78. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Zhang, X. Simultaneous determination of pharmaceutical and personal care products in wastewater by capillary electrophoresis with head-column field-amplified sample stacking. Anal. Methods 2014, 6, 7978–7983. [Google Scholar] [CrossRef]
- Espina-Benitez, M.; Araujo, L.; Prieto, A.; Navalón, A.; Vílchez, J.L.; Valera, P.; Zambrano, A.; Dugas, V. Development of a new microextraction fiber combined to on-line sample stacking capillary electrophoresis UV detection for acidic drugs determination in real water samples. Int. J. Environ. Res. Public Health 2017, 14, 739. [Google Scholar] [CrossRef]
- Himmelsbach, M.; Buchberger, W.; Klampfl, C.W. Determination of antidepressants in surface and waste water samples by capillary electrophoresis with electrospray ionization mass spectrometric detection after preconcentration using off-line solid-phase extraction. Electrophoresis 2006, 27, 1220–1226. [Google Scholar] [CrossRef]
- Dodds, J.N.; Baker, E.S. Ion mobility spectrometry: Fundamental concepts, instrumentation, applications, and the road ahead. J. Am. Soc. Mass Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef]
- Michelmann, K.; Silveira, J.A.; Ridgeway, M.E.; Park, M.A. Fundamentals of trapped ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 2014, 26, 14–24. [Google Scholar] [CrossRef]
- Celma, A.; Sancho, J.V.; Schymanski, E.L.; Fabregat-Safont, D.; Ibanez, M.; Goshawk, J.; Barknowitz, G.; Hernandez, F.; Bijlsma, L. Improving target and suspect screening high-resolution mass spectrometry workflows in environmental analysis by ion mobility separation. Environ. Sci. Technol. 2020, 54, 15120–15131. [Google Scholar] [CrossRef]
- Castellanos, A.; Benigni, P.; Hernandez, D.R.; DeBord, J.D.; Ridgeway, M.E.; Park, M.A.; Fernandez-Lima, F. Fast screening of polycyclic aromatic hydrocarbons using trapped ion mobility spectrometry-mass spectrometry. Anal. Methods 2014, 6, 9328–9332. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Grabanski, C.B.; Martin, E.; Miller, D.J. Comparisons of Soxhlet extraction, pressurized liquid extraction, supercritical fluid extraction and subcritical water extraction for environmental solids: Recovery, selectivity and effects on sample matrix. J. Chromatogr. A 2000, 892, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Ene, A.; Bogdevich, O.; Sion, A. Levels and distribution of organochlorine pesticides (OCPs) and polycyclic aromatic hydrocarbons (PAHs) in topsoils from SE Romania. Sci. Total Environ. 2012, 439, 76–86. [Google Scholar] [CrossRef]
- Mallek, M. Analytical Methodology Based on a Silicone Rod (SR) Micro Extraction Combined with HPLC-DAD Method for the Determination of Pharmaceuticals and Antibacterial Products in Effluent Wastewaters: Characterization of the Sorption Removal Processes by Cork. Doctoral Thesis, University of Girona, Girona, Spain, 2018. [Google Scholar]
- Tegegne, B.; Chandravanshi, B.S.; Zewge, F.; Chimuka, L. Solid-phase optimisation for simultaneous determination of thirteen pharmaceuticals in Ethiopian water samples with HPLC-DAD detection: An initial assessment. Environ. Monit. Assess. 2021, 193, 310. [Google Scholar] [CrossRef]
- Salgado, R.; Noronha, J.P.; Oehmen, A.; Carvalho, G.; Reis, M.A.M. Analysis of 65 pharmaceuticals and personal care products in 5 wastewater treatment plants in Portugal using a simplified analytical methodology. Water Sci. Technol. 2010, 62, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, S.; Abrell, L.; Wickramasekara, S.; Chefetz, B.; Chorover, J. Quantifying PPCP interaction with dissolved organic matter in aqueous solution: Combined use of fluorescence quenching and tandem mass spectrometry. Water Res. 2012, 46, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Wasswa, J.; Mladenov, N.; Pearce, W. Assessing the potential of fluorescence spectroscopy to monitor contaminants in source waters and water reuse systems. Environ. Sci. Water Res. Technol. 2019, 5, 370–382. [Google Scholar] [CrossRef]
- Wicker, A.P.; Carlton, D.D., Jr.; Tanaka, K.; Nishimura, M.; Chen, V.; Ogura, T.; Hedgepeth, W.; Schug, K.A. On-line supercritical fluid extraction—Supercritical fluid chromatography-mass spectrometry of polycyclic aromatic hydrocarbons in soil. J. Chromatogr. B 2018, 1086, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Primel, E.G.; Caldas, S.S.; Escarrone, A.L.V. Multi-residue analytical methods for the determination of pesticides and PPCPs in water by LC-MS/MS: A review. Cent. Eur. J. Chem. 2012, 10, 876–899. [Google Scholar] [CrossRef]
- Guo, K.; Ji, C.; Li, L. Stable-isotope dimethylation labeling combined with LC-ESI MS for quantification of amine-containing metabolites in biological samples. Anal. Chem. 2007, 79, 8631–8638. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gao, J.; Li, W.; Huang, J.; Yu, G. Determination of 27 pharmaceuticals and personal care products (PPCPs) in water: The benefit of isotope dilution. Front. Environ. Sci. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Kang, J.; Hick, L.A.; Price, W.E. Using calibration approaches to compensate for remaining matrix effects in quantitative liquid chromatography/electrospray ionization multistage mass spectrometric analysis of phytoestrogens in aqueous environmental samples. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2007, 21, 4065–4072. [Google Scholar] [CrossRef]
- Gopal, C.M.; Bhat, K.; Praveenkumarreddy, Y.; Kumar, V.; Basu, H.; Joshua, D.I.; Singhal, R.K.; Balakrishna, K. Evaluation of selected pharmaceuticals and personal care products in water matrix using ion trap mass spectrometry: A simple weighted calibration curve approach. J. Pharm. Biomed. Anal. 2020, 185, 113214. [Google Scholar] [CrossRef]
- Cortese, M.; Gigliobianco, M.R.; Magnoni, F.; Censi, R.; Di Martino, P. Compensate for or minimize matrix effects? Strategies for overcoming matrix effects in liquid chromatography-mass spectrometry technique: A tutorial review. Molecules 2020, 25, 3047. [Google Scholar] [CrossRef]
- Ngumba, E.; Kosunen, P.; Gachanja, A.; Tuhkanen, T. A multiresidue analytical method for trace level determination of antibiotics and antiretroviral drugs in wastewater and surface water using SPE-LC-MS/MS and matrix-matched standards. Anal. Methods 2016, 8, 6720–6729. [Google Scholar]
| Chemical structure | 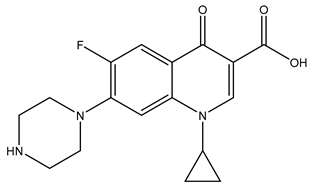 | 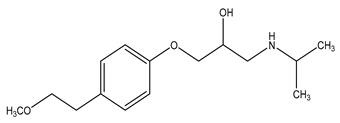 | 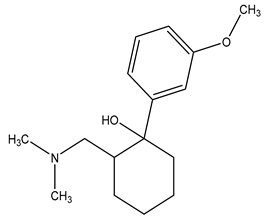 |
| Name | Ciprofloxacin | Metoprolol | Tramadol |
| Formula | C17H18FN3O3 | C15H25NO3 | C16H25NO2 |
| CAS | 85721-33-1 | 51384-51-1 | 123154-38-1 |
| Molecular Weight | 331.34 g/mol | 267.36 g/mol | 263.37 g/mol |
| Water solubility at 25 °C (mg/mL) a | 36 | >1000 | 0.036 |
| pKa a | 6.09; 8.74 | 9.7 | 9.23 |
| Log Kow a | 0.28 | 1.88 | 3.01 |
| Log Koc a | 4.78 | 1.79 | 2.79 |
| Chemical structure | 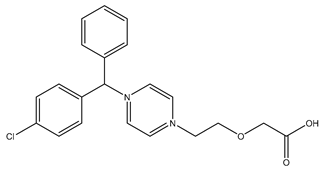 | 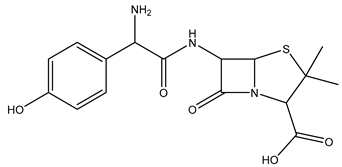 | 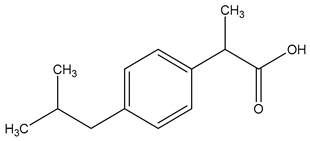 |
| Name | Cetirizine | Amoxicillin | Ibuprofen |
| Formula | C21H25ClN2O3 | C16H19N3O5S | C13H18O2 |
| CAS | 83881-51-0 | 26787-78-0 | 15687-27-1 |
| Molecular Weight | 388.9 g/mol | 365.4 g/mol | 206.28 g/mol |
| Water solubility at 25 °C (mg/mL) a | 0.101 | 0.011 | 0.021 |
| pKa a | 1.52; 2.92; 8.27 | 2.6 | 4.91 |
| Log Kow a | 1.70 | 0.87 | 3.97 |
| Log Koc a | 2.30 | 2 | Log 3400 |
| Chemical structure | 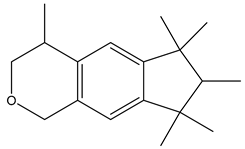 | 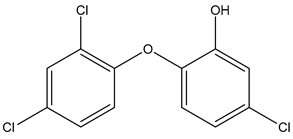 | 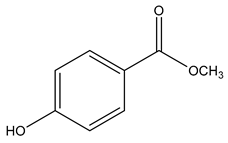 |
| Name | Galaxolide | Triclosan | Methylparabens |
| Formula | C18H26O | C12H7Cl3O2 | C8H8O3 |
| CAS | 1222-05-5 | 3380-34-5 | 99-76-3 |
| Molecular Weight | 258.4 g/mol | 289.5 g/mol | 152.15 g/mol |
| Water solubility at 25 °C (mg/mL) a | 0.00175 | 0.01 | 2.5 |
| pKa a | −6.9 | 7.9 | 8.5 |
| Log Kow a | 5.90 | 4.76 | 1.96 |
| Log Koc a | Log 2.0 × 104 | 3.54 | Log 87 |
| PPCPs | Classification | Occurrence | Concentration | Country | Ref. |
|---|---|---|---|---|---|
| Pharmaceuticals | |||||
| Ofloxacin and ciprofloxacin | Antibiotics | WWTP influent | 1000–2200 ng/L | Italy | [31] |
| Ciprofloxacin, clarithromycin and sulfamethoxazole | Antibiotics | Wastewater influent | 570–1200 ng/L | Canada | [32] |
| Ciprofloxacin | Antibiotic | Biosolids | 6500 ng/g | Canada | [32] |
| Amoxicillin | Antibiotics | STP | 172 ng/L | India | [33] |
| Ketoprofen, diclofenac, and indomethacin | Analgesic/NSAIDs | Sludge | 4.4–77 ng/g | Japan | [34] |
| Sulfapyridine, sulfadiazine, sulfamethoxazole, sulfamethazine, and three sulfonamide acetylated metabolites | Antibiotics | WWTP (effluent and influent) | 3–124 ng/L | China | [35] |
| Metoprolol, atenolol, betaxolol, Sotalol, Pindolol, and Nadolol | β-blocker | Hospital wastewater | 3.4–6700 ng/L | Italy | [31] |
| Atenolol and carbamazepine | β-blocker, antibiotics | Soil | 0.1–64.6 ng/g | Spain | [36] |
| Tramadol, ofloxacin, gemfibrozil, atenolol, caffeine, and cetirizine | Analgesic, antibiotics, lipid regulator, β-blocker, stimulant and antihistamine | Irrigation water | 1100–4400 ng/L | Spain | [37] |
| Tramadol, cetirizine, and clarithromycin | Analgesic, antihistamine and antibiotics | Soil | 12.7–14.6 ng/g | Spain | [37] |
| Estrone, 17b-estradiol, estriol, 2-hydroxyestrone, 16α-hydroxyestrone, 4-hydroxyestrone, 2-hydroxyestradiol, 4- hydroxyestradiol, 17-epiestriol, 16keto-estradiol, and 16-epiestriol | Steroidal hormones | WWTP (effluent and influent) | n.d.–62.9 ng/L | China | [38] |
| River water | n.d.–51.7 ng/L | ||||
| Atenolol, ciprofloxacin, sulfamethoxazole, ranitidine | β-blocker, Antibiotics and antihistamine | WWTP (effluent and influent) | 34–3585 ng/L | Saudi Arabia | [39] |
| Metformin and acetaminophen | Antihyperglycemic and analgesic | Sea water | <LOQ–>3000 ng/L | Saudi Arabia | [40] |
| Personal care products (PCPs) | |||||
| 3-Benzophenone galaxolide and tonalide | UVA/UVB filters, Synthetic fragrances | Irrigation water | 1200–18,900 ng/L | Spain | [37] |
| Galaxolide | Fragrances | Soil | 2.8 ng/g | Spain | [37] |
| Galaxolide and tonalide | Fragrances | Sediment | 2.39–52.5 ng/g | Brazil | [41] |
| Oxybenzone, triclosan, and triclocarban | UV filters and disinfectants | Wastewater (effluent and influent) and sludge | n.d.–8880 ng/L | India | [42] |
| Triclosan and parabens | Disinfectants and preservatives | Surface water and sediment | n.d.–5160 ng/L | India | [43] |
| Triclocarban and triclosan | Disinfectants | Biosolids | 12,640–36,060 ng/g | USA | [44] |
| Triclosan and triclocarban | Disinfectants | Biosolids | 2900–6800 ng/g | Canada | [32] |
| Benzotriazole and methyl benzotriazol | UV filters | Groundwater | 267–1980 ng/L | Spain | [45] |
| Triclosan (TCS) and triclocarban (TCC) | Disinfectants | Biosolids | 760–22,588 ng/g | USA | [46] |
| Triclosan (TCS) and triclocarban (TCC) | Disinfectants | Soils | 2.8–221 ng/g | ||
| Diethyltoluamide | Insect repellent | Surface water | 1.4–527 ng/L | Singapore | [47] |
| Tonalide, galaxolide | Fragrances | Groundwater | 1–50 ng/L | France | [48,49] |
| Musk ambrette, musk ketone, and musk xylen | 209–1304 ng/L | ||||
| Analyte | Matrix | Extraction Method | Analytical Technique | Analytical Conditions | Analysis Time (min) | Analytical Parameters | Ref. |
|---|---|---|---|---|---|---|---|
| Ofloxacin and ciprofloxacin | WWTP influent | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (125 mm× 2.0 mm, particle size 5 μm) MP: acetonitrile (A) and HPLC grade water with 0.1% formic acid (B) Flow rate= 0.2 mL/min | 45 min | Recovery = 93–107% LOD = 1–3 ng/L | [31] |
| Ibuprofen, carbamazepine, and triclosan | WWTP effluent | SPE Oasis HLB | LC-ESI-MS/MS | C18 Column (100 mm× 2.1 mm, particle size 3 μm). MP: acetonitrile/methanol (A) and HPLC grade water with 0.1% formic acid (B), Flow rate = 0.25 mL/min | 35 min | Recovery = >80% LOQ = 1–2 ng/L | [79] |
| Amoxicillin | STP | SPE Oasis HLB | HPLC-PDA | λ: 228 nm C18 Column (250 mm × 4.6 mm, 5 μm) MP: isocratic program of 0.45% Na2HPO4 in water (A) and Methanol (B) in the fixed ratio of 2:1 Flow rate = 1.5 mL/min | 4.9 min | Recovery = 64–88% LOD = 1 ng/L LOQ =10 ng/L R2 = 0.998 | [33] |
| Ampicillin, ciprofloxacin, gatifloxacin, sparfloxacin and cefuroxime | STP | SPE Oasis HLB | HPLC-PDA | λ: 215 and 280 nm C18 Column (250 mm × 4.6 mm, 5 μm) MP: 0.1% aqueous TFA (A) and acetonitrile (B) Flow rate = 1.0 mL/min | 30 min | Recovery = 25–108% LOD = 10 ng/L LOQ =30 ng/L R2 = 0.9806–0.9957 | [80] |
| Sulfapyridine, sulfadiazine, sulfamethoxazole, sulfamethazine, and three sulfonamide acetylated metabolites | WWTP (effluent and influent) | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (100 mm × 2.1 mm, particle size 2.6 μm) MP: methanol (A) and distilled water (B) Flow rate = 0.3 mL/min | 6 min | Recovery = 77.7–148.1% LOD = 0.01–0.23 ng/L LOQ = 0.03–0.78 ng/L R2 = 0.995–0.999 | [35] |
| Sulfathiazole, sulfamethazine, sulfamethoxazole, oxytetracycline, and chlortetracycline | WWTP effluent | SPE Oasis HLB and MCX | LC-ESI-IT–TOF/MS | C18 column (75 mm× 3.0 mm, particle size 3.0 μm). MP: 0.3% formic acid and 0.1% ammonium acetate in water (A) and methanol and Acetonitrile (1:1) (B). | 6 min | Recovery = 63–118% LOD = 9.8–25.8 ng/L R2 = 0.9964–0.9996 | [81] |
| Estrone, 17β-Estradiol, estriol, 2-Hydroxyestrone, 16α-Hydroxyestrone, 4-Hydroxyestrone, 2-Hydroxyestradiol, 4- Hydroxyestradiol, 17-Epiestriol, 16keto-estradiol, and 16-Epiestriol | WWTP (effluent and influent) and river water | SPE Oasis HLB Derivatization | GC-MS | HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm) Flow rate = 0.85 mL/min Temperature program: start with 100 °C, rising at 10 °C/min to 200 °C, then 8 °C/min to 260 °C, then 3 °C/min to 310 °C, and finally held at 310 °C for 2 min | 37 min | LOD =1.4–6.0 ng/L LOQ =4.8–19.8 ng/L R2 = 0.9838–0.9995 | [38] |
| Atenolol, bisoprolol, ciprofloxacin, ofloxacin, sulfamethoxazole, clarithromycin, trimethoprim, ketoprofen, diphenhydramine HCl, ranitidine, and carbamazepine | WWTP (effluent and influent) | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (30 mm × 2.1 mm, particle size 1.9 μm) MP: 0.1% formic acid in water (A) and ethanol (B) Flow rate = 0.5 mL/min | 7.2 min | Recovery = 70.4–112.2% LOD = 0.06–16.0 ng/L LOQ = 0.20–52.8 ng/L R2 = 0.9921–0.9992 | [39] |
| Sulfapyridine, sulfamethazine, sulfamethoxazole and sulfaphenazole | Surface water | Deep eutectic solvent (DES) and liquid–liquid microextraction | UHPLC-PDA | λ: 270 nm. C18 column (30 mm × 2.1 mm, particle size 1.7 μm) MP: 0.1% formic acid in water (A) and 90% acetonitrile in ethanol (B) Flow rate = 1 mL/min | 3.5 min | Recovery = 78.2–105.2% LOD = 0.78–3.42 ng/L LOQ = 2.38–10.37 ng/L R2 = 0.9991–0.9997 | [77] |
| Lincomycin, ibuprofen, paraxanthin, and naproxen | Lake | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (150 mm × 2.1 mm, particle size 3.5 μm) MP: ammonium formate/formic acid buffer (A) and acetonitrile (B) Flow rate = 0.2 mL/min | 50 min | Recovery = 78–118% LOD = 1.7–25 ng/L LOQ = 2.5–25 ng/L R2 ≥ 0.99 | [82] |
| Acetaminophen, caffeine, carbamazepine, ibuprofen, ketoprofen, fenoprofen, naproxen, propyphenazone, clofibric acid, gemfibrozil, diclofenac, indomethacin, salicylic acid, crotamiton, and trimethoprim | Raw wastewater, surface water and groundwater samples | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (150 mm × 2.1 mm, particle size 3.5 μm). MP: ammonium acetate in water (A) and ammonium acetate in methanol (B) Flow rate = 0.25 mL/min | 18 min | Recovery = 66.5–102% LOD = 0.1–5 ng/L LOQ = 0.3–10 ng/L R2 ≥ 0.996 | [83] |
| Estrone, 17 α-estradiol, estriol, progesterone, pquiline, diethylstilbestrol, and ethinylestradiol | Sea water, mineral water, and tap water | Air-agitated liquid–liquid microextraction method based on solidification of a floating organic droplet (AALLME-SFO) | LC-ESI-MS/MS | C18 column (50 mm × 2.1 mm, particle size 1.7 μm) MP: 0.1% formic acid in water (A) and ethanol (B) Flow rate = 0.4 mL/min Flow rate = 0.05 mL/min | 6.7 min | Recovery = 90.14–99.60% LOD = 0.021–0.034 ng/L LOQ = 0.046–0.103 ng/L R2 = 0.9990–0.9998 | [84] |
| Atrazine, bisphenol-A, caffeine, carbamazepine, gemfibrozil, ibuprofen, naproxen, simazine, sulfamethoxazole, and trimethoprim | Raw and treated water | SPE Oasis HLB | LC-MS/MS | N.R. | N.R. | Recovery = 70–128% LOD = 1–2.5 ng/L | [85] |
| Sulfadiazine, sulfapyridine, sulfathiazole, trimethoprim, sulfamerazine, sulfamethazine, sulfamethoxazole, naproxen, paracetamol, diclofenac acid, ibuprofen, ketoprofen, flumequine, ciprofloxacin, norfloxacin, ofloxacin, macrolides, clarithromycin, roxithromycin, erythromycin, tetracyclines, oxytetracycline, isochlortetracycline, tetracycline, cephalexin monohydrate, cefotaxime sodium, penicillin G, amphenicols, chloramphenicol, thiamphenicol, triclocarban, triclosan, carbamazepine, aminoglycoside, spectinomycin, antihyperlipidemic, gemfibrozil, diltiazem, diphenhydramine, and bisphenol A. | Lake | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (50 mm × 2.1 mm, particle size 1.8 μm). MP: Water (A) and Acetonitril (B) fro −ESI and 0.1% formic acid in water (A) and acetonitrile (B) for +ESI. Flow rate = 0.25 mL/min | N.R. | Recovery = 70–125% LOD = 0.01–1.1 ng/L LOQ = 0.03–3.3 ng/L R2 = 0.9937–0.9999 | [86] |
| Ibuprofen, diclofenac, fexofenadine, atorvastatin, irbesartan, triclosan, sulfasalazine, benzophenone 1, 2, and 4, methylparaben, diazepam, and oxazepam | WWTP (effluent and influent) and surface water | SPE Oasis HLB | LC-ESI-MS/MS | Separation of acidic analytes: C18 column (150 mm × 1.0 mm, particle size 1.7 μm). MP: (80:20) Water:Methanol conatin 1 mM Ammonium fluoride (A) and (5:95) Water:Methanol conatin 1 mM Ammonium fluoride (B) Flow rate = 0.04 mL/min Separation of basicanalytes: CHIRALPAK CBH HPLC column. MP: 1 mM ammonium acetate/methanol 85:15 (v/v) (Isocratic). Flow rate = 0.1 mL/min | N.R. | Recovery = 44.6–120% LOQ = 0.31–41.43 ng/L | [87] |
| Azithromycin, clarithromycin, metronidazole, ciprofloxacin, norfloxacin, vancomycin, and sulfamethoxazole | WWTP (effluent and influent) and surface water | SPE Oasis HLB | LC-coupled to hybrid MS/MS/Ion trap | C18 column (100 mm × 4.6 mm, particle size 2.6 μm). MP: 0.2% formic acid (A) and acetonitrile with 0.2% formic acid (B) Flow rate = 0.5 mL/min | 35 min | LOD = 0.03–15 ng/L LOQ = 0.9–50 ng/L | [88] |
| Acetylsalicylic acid, naproxen, methyl and ethyl paraben, estrone, 17β-estradiol | Drinking water | SPE-DEX | LC-ESI-MS/MS | In −ESI: C18 column (50 mm × 2.1 mm, particle size 2.7 μm). MP: 10 mM N-methylmorpholine (A) and methanol (B). Flow rate = 0.5 mL/min In +ESI: Kinetex PFP HPLC column column (50 mm × 2.1 mm, particle size 2.6 μm). MP: 5 mM ammonium acetate (A) and methanol (B). Flow rate = 0.8 mL/min | 5.5 min (−ESI) 6.5 min (+ESI) | Recovery = 32.9–130.1% LOD = 0.2–0.9 ng/L LOQ = 1.0–2.3 ng/L R2 = 0.9976–0.9996 | [89] |
| Chloroform, tribromomethane, and dichloroacetonitrile | Drinking water | SPE-DEX | GC-ECD | DB1701 column | N.R. | LOD = 0.01–0.03 μg/L LOQ = 0.2 μg/L R2 = 0.9926–0.9992 | [89] |
| Analyte | Matrix | Extraction Method | Analytical Technique | Analytical Conditions | Analysis Time (min) | Analytical Parameters | Ref. |
|---|---|---|---|---|---|---|---|
| Ciprofloxacin, clarithromycin, indomethacin, ketoprofen, tramadol, diazepam, carbamazepine, ranitidine, and furosemide | Plant (root and stem/leaf) | QuEChERS | LC-ESI-MS/MS | C18 column (2.1 mm × 100 mm, particle size 1.8 μm) MP: 0.1% formic acid in ultrapure water (A) and acetonitrile (B) Flow rate = 0.3 mL/min | 18 min | Recovery = 41.0–120.0% LOQ = 0.05–50 ng/g R2 = 0.9980–1.000 | [90] |
| Tramadol, cetirizine, and clarithromycin | Soil | QuEChERS | LC-ESI- MS/MS | C18 column (2.1 mm × 100 mm, particle size 1.8 μm) MP: 0.1% formic acid in ultrapure water (A) and acetonitrile (B) Flow rate = 0.3 mL/min | 18 min | Recovery = 70–120% LOQ < 0.5 ng/g R2 > 0.99 | [37] |
| Acetaminophen, azithromycin, diclofenac, furosemide, norfloxacin, salbutamol, and sulfamerazine | Sludge | Ultrasonication and SPE Oasis HLB | Micro LC- MS/MS | C18 column (2.1 mm × 100 mm, particle size 1.7 μm) | N.R. | Recovery = 86.2–114.5% RSD = 2.9–29.8% | [91] |
| Ibuprofen, atenolol, carbamazepine, erythromycin, diclofenac, and diazepam | Sediment | Samples extracted with methanol and evaporated until dry, then diluted with methanol | LC-ESI- MS/MS | C18 column (150 mm × 4.6 mm, particle size 3 μm) MP: 5 mM ammonium acetate in water (A) and 5 mM ammonium acetate in methanol (B) Flow rate = 1 mL/min | N.R. | Recovery = 87–97% LOQ = 0.1 ng/g Reproducibility > 90% | [41] |
| Tonalide, galaxolide, and caffeine | Sediment | Samples extracted with methanol and evaporated until dry, then diluted with methanol | GC-MS/MS | DB-XLB column (60 m × 0.25 mm × 0.25 μm) Flow rate = 1 mL/min | N.R. | ||
| Ketoprofen, diclofenac, and indomethacin | Sludge | SPE Oasis HLB | LC-ESI- MS/MS | C8 column (150 mm × 2.0 mm, particle size 3 μm) MP: aqueous NH4H2O2/CH2O2 buffer; 10 mM, pH 3.7 (A) and acetonitrile (B) | N.R. | Recovery = 52–137% (at pH-7) LOD = 0.16–0.5 ng/g LOQ = 0.49–1.53 ng/g | [34,92] |
| Estrogens, caffeine, triclosan, and ciprofloxacin | Sludge and soil | SPE Oasis HLB | HPLC-UV | λ: 200, 254 and 279 nm. C18 column (25 × 4.6 mm, 5 μm) MP: Acetonitrile (A) and Water (B) Flow rate = 0.8–1.0 mL/min | 21 min | Recovery = 28–104% LOD = 0.30–6.42 ng/g | [93] |
| Carbamazepine, clarithromycin, carbamazepine-10,11-epoxide, clindamycin, diclofenac, diltiazem, diphenhydramine, erythromycin, fluoxetine, paroxetine, norfluoxetine, gemfibrozil, josamycin, triclosan, and triclocarban | Biosolids and soils | SPE | LC-ESI- MS/MS | C8 column (100 × 4.6 mm, 3 μm) MP: 0.1% formic acid (A) and Acetonitrile (B) Flow rate = 0.3 mL/min | 30 min | Recovery = 26–146% LOQ = 0.1–27 ng/g R2 > 0.99 | [46] |
| Quetiapine, fumarate, aripiprazole, and oxybenzone. | Sludge | SPE Oasis HLB | LC-ESI- MS/MS | C8 column (150 × 2.1 mm, 3 μm) MP: 0.1% formic acid in water (A) and methanol (B) | N.R. | Recovery = 100 ± 30% LOQ = 0.5–20 ng/L R2 ≥ 0.99 | [42] |
| Triclosan | Sediment | SPE | GC-MS | HP-5 MS capillary column (30 m × 0.32 mm × 0.25 μm) Flow rate = 2.25 mL/min Temperature program: 50 °C/min for 1 min, 30 °C/min to 200 °C, then 2 °C/min to 230 °C, then 15 °C/min to 320 °C (10 min hold) | 11 min | Recovery = 84.6 ± 5.7% LOD = 1.5 ng/g R2 = 0.999 | [43] |
| Sulfadiazine, sulfapyridine, sulfathiazole, trimethoprim, sulfamerazine, sulfamethazine, sulfamethoxazole, naproxen, paracetamol, diclofenac acid, ibuprofen, ketoprofen, flumequine, ciprofloxacin, norfloxacin, ofloxacin, macrolides, clarithromycin, roxithromycin, erythromycin, tetracyclines, oxytetracycline, isochlortetracycline, tetracycline, cephalexin monohydrate, cefotaxime sodium, penicillin G, amphenicols, chloramphenicol, thiamphenicol, triclocarban, triclosan, carbamazepine, aminoglycoside, spectinomycin, antihyperlipidemic, gemfibrozil, diltiazem, diphenhydramine, and bisphenol A. | Sediment | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (50 mm × 2.1 mm, particle size 1.8 μm). MP: Water (A) and Acetonitril (B) fro −ESI and 0.1% formic acid in water (A) and acetonitrile (B) for +ESI. Flow rate = 0.25 mL/min | N.R. | Recovery = 75–118% LOD = 0.01–0.6 ng/g LOQ = 0.03–1.7 ng/g R2 = 0.9937–0.9999 | [86] |
| Ibuprofen, diclofenac, ketoprofen, bezafibrate, benzophenone 1, 2, and 4, methylparaben, morphine, and nomorphine | Sludge | MAE | LC-ESI-MS/MS | Separation of acidic analytes: C18 column (150 mm × 1.0 mm, particle size 1.7 μm). MP: (80:20) Water:Methanol conatin 1 mM Ammonium fluoride (A) and (5:95) Water:Methanol conatin 1 mM Ammonium fluoride (B) Flow rate = 0.04 mL/min Separation of basicanalytes: CHIRALPAK CBH HPLC column. MP: 1 mM ammonium acetate/methanol 85:15 (v/v) (Isocratic). Flow rate = 0.1 mL/min | N.R. | Recovery = 40.8–119.4% LOQ = 0.36–13.22 ng/g | [87] |
| Azithromycin, clarithromycin, metronidazole, ciprofloxacin, norfloxacin, vancomycin, and sulfamethoxazole | Sewage sludge | QuEChERS | LC- coupled to hybrid MS/MS/Ion trap | C18 column (100 mm × 4.6 mm, particle size 2.6 μm). MP: 0.2% formic acid (A) and 0.2% formic acid in acetonitrile (B) Flow rate = 0.5 mL/min | 35 min | LOD = 2.2–490 ng/g LOQ = 7.4–1670 ng/g | [88] |
| Carbamazepine, triclosan, ibuprofen, and galaxolide | Sewage sludge | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (100 mm × 2.1 mm, particle size 1.7 μm). MP: 0.1% formic acid in water (A) and 0.1% formic acid in methanol (B) Flow rate = 0.3 mL/min | 16 min | Recovery = 89.7–93.6% LOQ = 0.6–1.2 ng/g R2 > 0.99 | [94] |
| Telmisartan, airtazapine, amitriptyline, caffeine, carbamazepine, cetirizine, citalopram, venlafaxine, and triclosan. | Sewage sludge | Accelerated solvent extractor | LC-MS/MS | C18 column (100 mm × 3 mm, particle size 2.7 m). MP: 0.5 mM ammonium fluoride in water with 0.01% formic acid (A) and methanol (B) Flow rate = 0.4 mL/min | 23.5 min | LOQ = 300–600 ng/g | [95] |
| Tetracycline, sulfamethoxazole, and triclocarban | Sediment | SPE Oasis HLB | LC-ESI-MS/MS | C18 column (100 mm × 2.1 mm, particle size 1.7 m). MP: Water (A) and 0.1% formic acid (B) Flow rate = 0.2 mL/min | 7.5 min | Recovery = 81.3–100% LOD = 2–3 ng/g LOQ = 18.2–27.8 ng/g | [96] |
| Carbamazepin, ibuprofen, caffeine, sulfamethoxazole, methylparaben, and propylparaben, | Soil | Ultrasonic-assisted extraction and dispersive SPE | LC-ESI-MS/MS | C18 column (50 mm × 3 mm, particle size 2.6 μm). MP: 0.1% formic acid (A) and 0.1% formic acid in methanol (B) Flow rate = 0.6 mL/min | 30 min | Recovery = 26–102% LOD = 0.07–1.34 ng/g LOQ = 0.24–4.46 ng/g | [97,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, A.M. Analytical Methods for the Determination of Pharmaceuticals and Personal Care Products in Solid and Liquid Environmental Matrices: A Review. Molecules 2024, 29, 3900. https://doi.org/10.3390/molecules29163900
Alqarni AM. Analytical Methods for the Determination of Pharmaceuticals and Personal Care Products in Solid and Liquid Environmental Matrices: A Review. Molecules. 2024; 29(16):3900. https://doi.org/10.3390/molecules29163900
Chicago/Turabian StyleAlqarni, Abdulmalik M. 2024. "Analytical Methods for the Determination of Pharmaceuticals and Personal Care Products in Solid and Liquid Environmental Matrices: A Review" Molecules 29, no. 16: 3900. https://doi.org/10.3390/molecules29163900
APA StyleAlqarni, A. M. (2024). Analytical Methods for the Determination of Pharmaceuticals and Personal Care Products in Solid and Liquid Environmental Matrices: A Review. Molecules, 29(16), 3900. https://doi.org/10.3390/molecules29163900








