Desmodium intortum (Mill.) Urb. Protein Isolate Aggregates as Pickering Stabilizers: Physicochemical Characteristics and Emulsifying Properties
Abstract
:1. Introduction
2. Results and Discussion
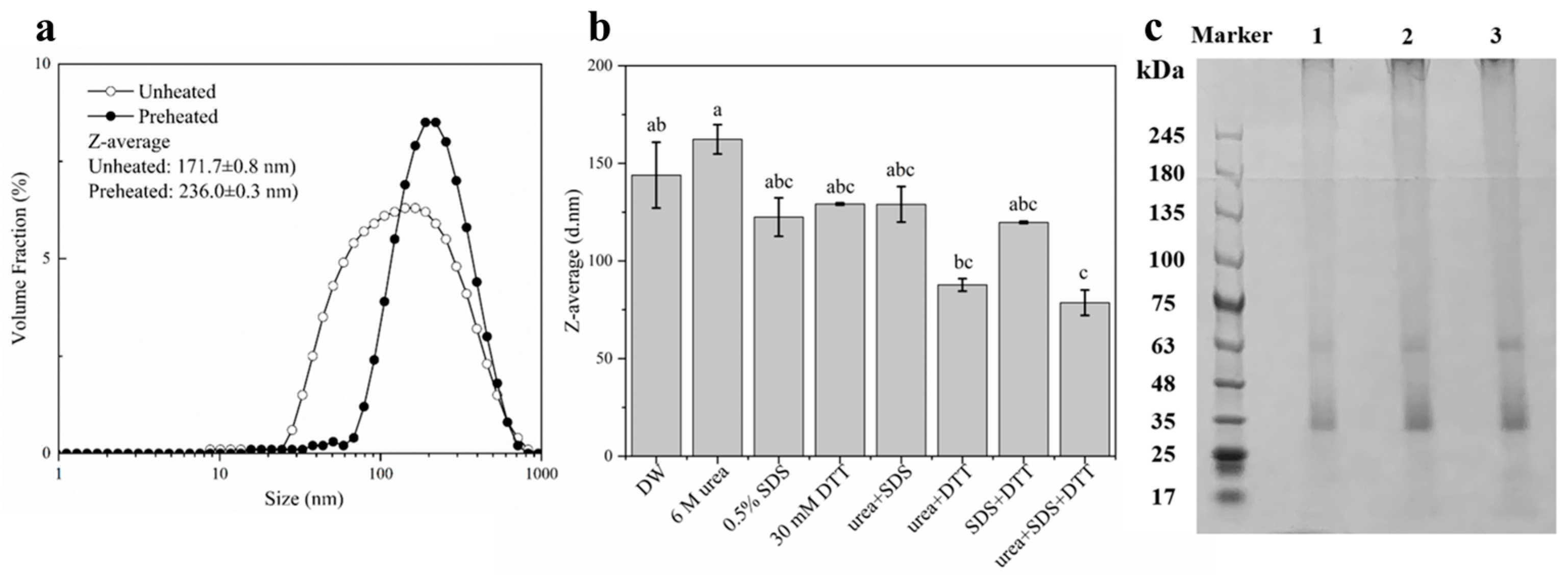
2.1. Characterization of DIPI Aggregates
2.2. Influence of Ionic Strength and pH on the Characteristics of DIPI Aggregates
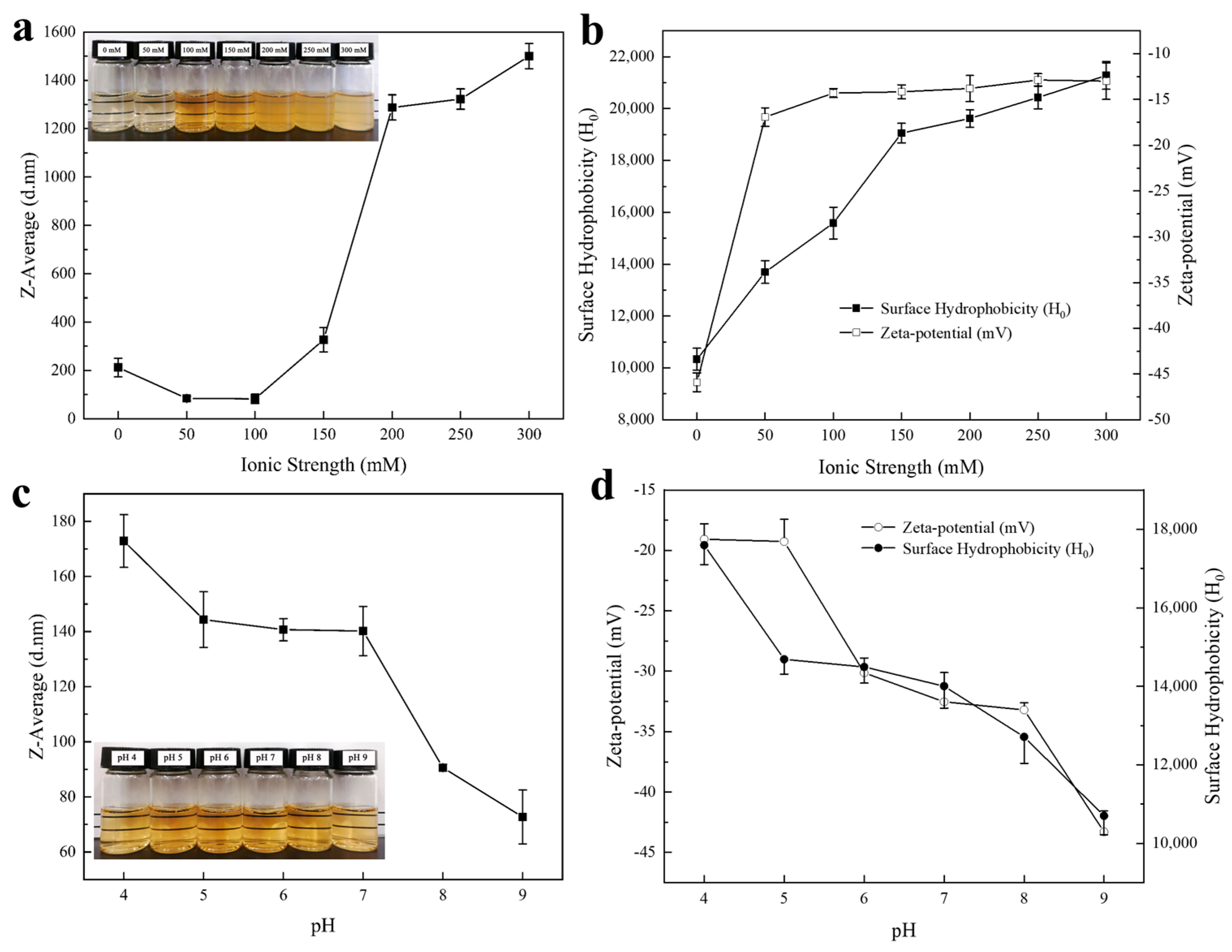
2.2.1. Size, Zeta Potential, and Surface Hydrophobicity: Modulation of Ionic Strength
2.2.2. Size, Zeta Potential, and Surface Hydrophobicity: Modulation of pH
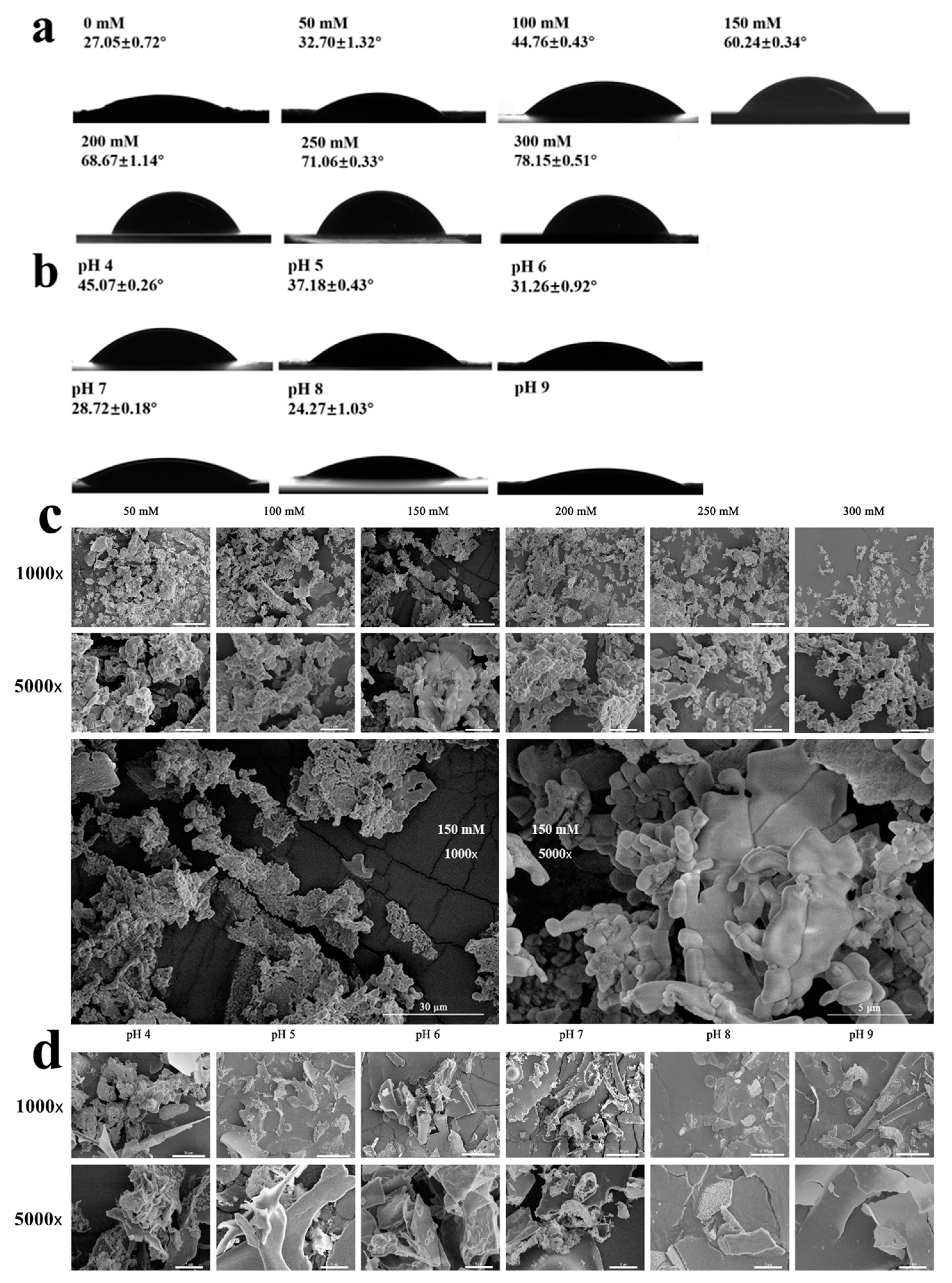
2.2.3. Contact Angle and SEM
2.3. Characteristics of Pickering Emulsions Stabilized by DIPI
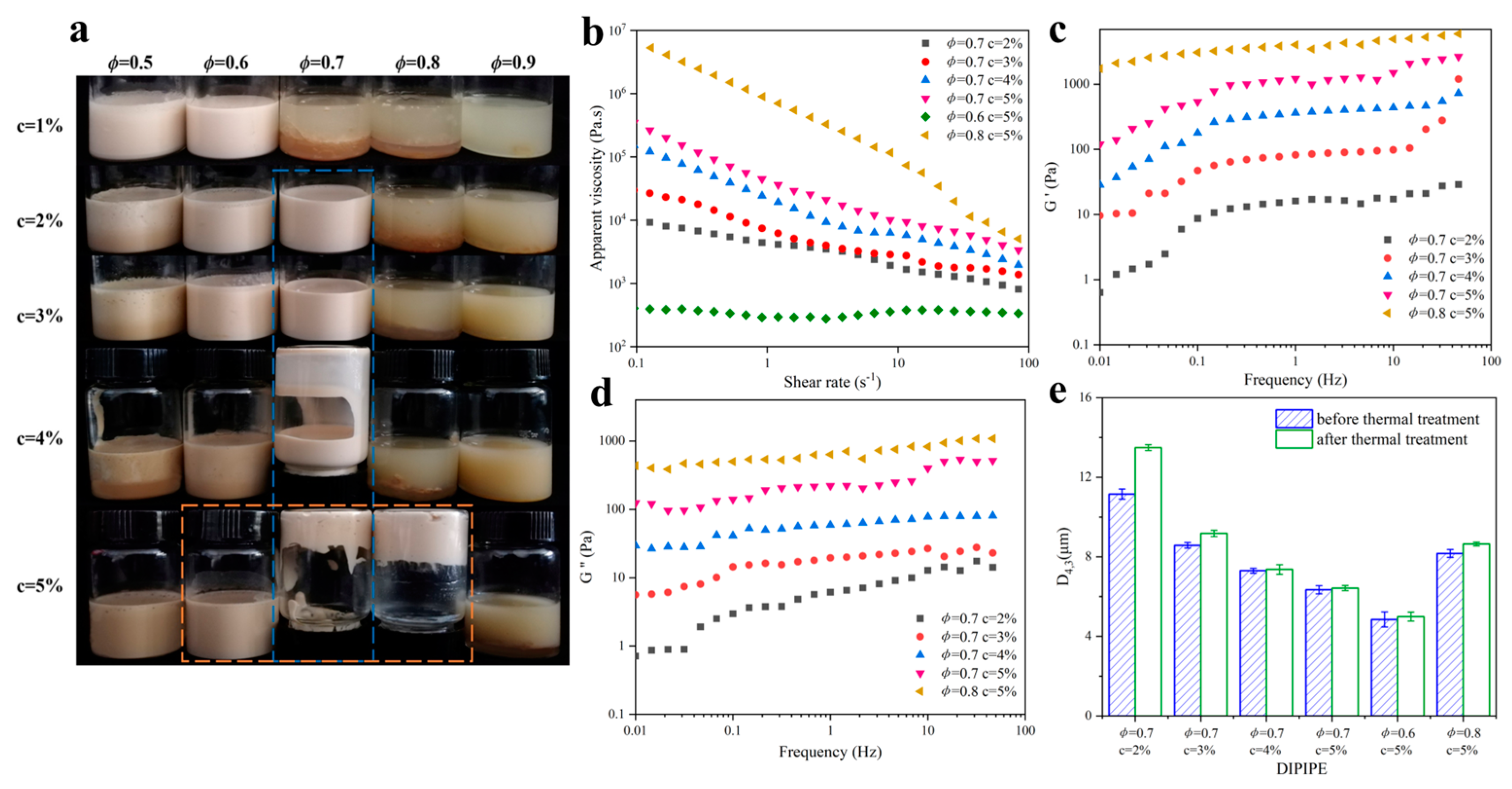
2.3.1. Influence of DIPI Concentration and Oil Fraction on Emulsion Rheological Properties
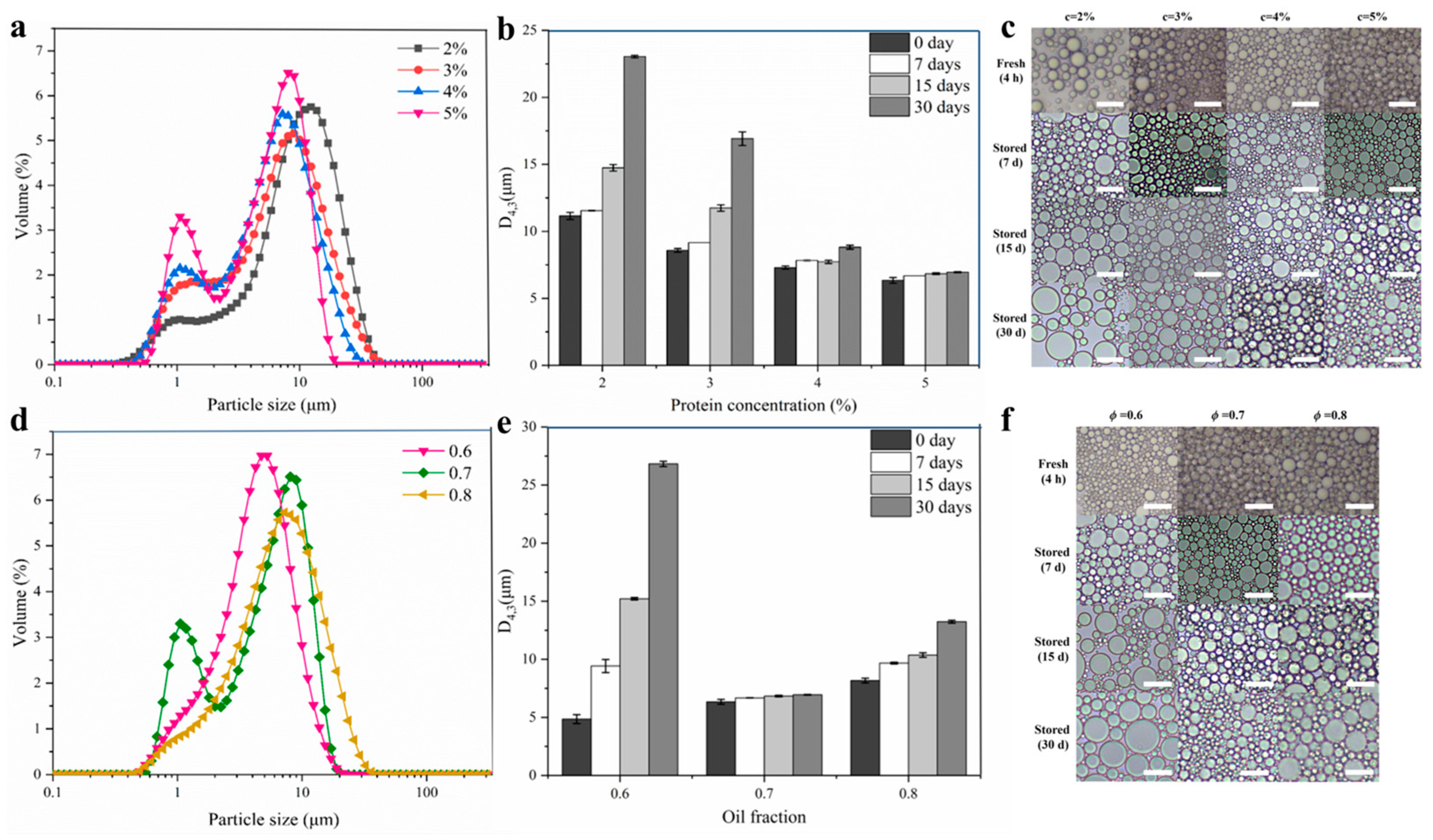
2.3.2. Influence of DIPI Concentration on Emulsion Droplet Size and Microstructure
2.3.3. Influence of Oil Fraction on Emulsion Droplet Size and Microstructure
2.3.4. Influence of DIPI Concentration and Oil Fraction on Emulsion Creaming Index
2.3.5. Confocal Laser Scanning Microscopy Analysis of DIPIPE
3. Materials and Methods
3.1. Materials
3.2. Preparation of DIPI Aggregates
3.3. Characterization of DIPI Aggregate
3.3.1. Size Distribution, Particle Size, and Zeta Potential
3.3.2. Surface Hydrophobicity (H0)
3.3.3. Contact Angle Measurements
3.3.4. Scanning Electron Microscopy (SEM)
3.4. Characterization of DIPIPE
3.4.1. Preparation of DIPIPE
3.4.2. Rheological Properties
3.4.3. Droplet Size Distribution and Volume-Averaged Droplet Size (D4,3)
3.4.4. Microstructure
3.4.5. Creaming Index (CI)
3.4.6. Confocal Laser Scanning Microscopy (CLSM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramsden, W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).—Preliminary Account. Proc. R. Soc. Lond. 1903, 72, 156–164. [Google Scholar]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Yan, B.W.; Jiao, X.D.; Zhu, H.P.; Wang, Q.; Huang, J.L.; Zhao, J.X.; Cao, H.W.; Zhou, W.G.; Zhang, W.H.; Ye, W.J.; et al. Chemical interactions involved in microwave heat-induced surimi gel fortified with fish oil and its formation mechanism. Food Hydrocoll. 2020, 105, 105779. [Google Scholar] [CrossRef]
- Yan, X.J.; Ma, C.C.; Cui, F.Z.; McClements, D.J.; Liu, X.B.; Liu, F.G. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Xia, T.; Xue, C.; Wei, Z. Physicochemical characteristics, applications and research trends of edible Pickering emulsions. Trends Food Sci. Technol. 2021, 107, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C.H. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Zhang, H.; Li, W.; He, Y.; Meng, X.; Liu, B. Bacteriostatic Pickering emulsions stabilized by whey protein isolate–vanillin nanoparticles: Fabrication, characterization and stability in vitro. Food Chem. 2023, 429, 136871. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhao, J.; Zhang, Y.; Jiang, L.; Sui, X. Oil-water interfacial behavior of soy protein nanoparticles in high internal phase Pickering emulsion. Food Hydrocoll. 2024, 152, 109927. [Google Scholar] [CrossRef]
- Huang, G.; Liu, G.; Xu, Z.; Jiang, L.; Zhang, Y.; Sui, X. Stability, rheological behavior and microstructure of Pickering emulsions co-stabilized by soy protein and carboxymethyl chitosan. Food Hydrocoll. 2023, 142, 108773. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, M.; Ettelaie, R.; Sarkar, A. Pea protein microgel particles as Pickering stabilisers of oil-in-water emulsions: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 102, 105583. [Google Scholar] [CrossRef]
- Feng, T.; Fan, C.; Wang, X.; Wang, X.; Xia, S.; Huang, Q. Food-grade Pickering emulsions and high internal phase Pickering emulsions encapsulating cinnamaldehyde based on pea protein-pectin-EGCG complexes for extrusion 3D printing. Food Hydrocoll. 2022, 124, 107265. [Google Scholar] [CrossRef]
- Chen, H.; Dai, H.J.; Zhu, H.K.; Ma, L.; Fu, Y.; Feng, X.; Sun, Y.; Zhang, Y.H. Construction of dual-compartmental micro-droplet via shrimp ferritin nanocages stabilized Pickering emulsions for co-encapsulation of hydrophobic/hydrophilic bioactive compounds. Food Hydrocoll. 2022, 126, 107443. [Google Scholar] [CrossRef]
- Badar, I.H.; Wang, Z.; Chen, Q.; Liu, Q.; Ma, J.; Liu, H.; Kong, B. Ultrasonic enhancement of structural and emulsifying properties of heat-treated soy protein isolate nanoparticles to fabricate flaxseed-derived diglyceride-based pickering emulsions. Food Chem. 2024, 442, 138469. [Google Scholar] [CrossRef]
- Hei, X.; Liu, Z.; Li, S.; Wu, C.; Jiao, B.; Hu, H.; Ma, X.; Zhu, J.; Adhikari, B.; Wang, Q. Freeze-thaw stability of Pickering emulsion stabilized by modified soy protein particles and its application in plant-based ice cream. Int. J. Biol. Macromol. 2024, 257, 128183. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Bi, S.; Zhou, Y.; Liu, Y.; Wan, J.; Zeng, L.; Zhu, Q.; Pang, J.; Huang, X. Studies on stabilized mechanism of high internal phase Pickering emulsions from the collaboration of low dose konjac glucomannan and myofibrillar protein. Food Hydrocoll. 2023, 143, 108862. [Google Scholar] [CrossRef]
- Zheng, X.H.; Ren, C.; Wei, Y.X.; Wang, J.M.; Xu, X.B.; Du, M.; Wu, C. Soy protein particles with enhanced anti-aggregation behaviors under various heating temperatures, pH, and ionic strengths. Food Res. Int. 2023, 170, 112924. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Cao, C.; Kong, B.; Wang, H.; Zhang, H.; Liu, Q. Effects of different pH conditions on interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Hydrocoll. 2022, 131, 107752. [Google Scholar] [CrossRef]
- Ning, F.; Ge, Z.; Qiu, L.; Wang, X.; Luo, L.; Xiong, H.; Huang, Q. Double-induced se-enriched peanut protein nanoparticles preparation, characterization and stabilized food-grade pickering emulsions. Food Hydrocoll. 2020, 99, 105308. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. Pea protein exhibits a novel Pickering stabilization for oil-in-water emulsions at pH 3.0. LWT-Food Sci. Technol. 2014, 58, 463–469. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy protein nanoparticle aggregates as pickering stabilizers for oil-in-water emulsions. J. Agric. Food. Chem. 2013, 61, 8888–8898. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.Q.; Li, C.Z.; Chen, X.C.; Cui, H.Y.; Lin, L. Pickering emulsion stabilized by gliadin/soybean polysaccharide composite colloidal nanoparticle: Physicochemical properties and its application on washing of fresh-cut cabbage. Food Res. Int. 2022, 161, 111886. [Google Scholar] [CrossRef]
- Lv, P.F.; Wang, D.; Dai, L.; Wu, X.J.; Gao, Y.X.; Yuan, F. Pickering emulsion gels stabilized by high hydrostatic pressure-induced whey protein isolate gel particles: Characterization and encapsulation of curcumin. Food Res. Int. 2020, 132, 109032. [Google Scholar] [CrossRef] [PubMed]
- Ridella, F.; Marcet, I.; Gutiérrez, G.; Rendueles, M.; Díaz, M. Characterization of Pickering emulsions stabilized by delipidated egg yolk granular protein nanoparticles crosslinked with ultraviolet radiation. Food Chem. 2024, 433, 137330. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, J.; Chen, F. Heat-induced changes in the physicochemical properties and in vitro digestibility of rice protein fractions. J. Food Sci. Technol. 2021, 58, 1368–1377. [Google Scholar] [CrossRef]
- Palazolo, G.; Sorgentini, D.; Wagner, J. Coalescence and flocculation in o/w emulsions of native and denatured whey soy proteins in comparison with soy protein isolates. Food Hydrocoll. 2005, 19, 595–604. [Google Scholar] [CrossRef]
- Wen, J.Y.; Zhao, J.R.; Jiang, L.Z.; Sui, X.A. Oil-water interfacial behavior of zein-soy protein composite nanoparticles in high internal phase Pickering emulsion. Food Hydrocoll. 2024, 149, 109659. [Google Scholar] [CrossRef]
- Matulis, D.; Lovrien, R. 1-Anilino-8-Naphthalene sulfonate anion-protein binding depends primarily on ion pair formation. Biophys. J. 1998, 74, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.X.; Hou, Y.N.; Xue, Z.Y.; Bai, L.J.; Wang, W.X.; Chen, H.; Yang, H.W.; Yang, L.X.; Wei, D.L. Soy protein isolate/genipin-based nanoparticles for the stabilization of Pickering emulsion to design self-healing guar gum-based hydrogels. Biomacromolecules 2023, 24, 2087–2099. [Google Scholar] [CrossRef]
- Zeng, X.; Li, Y.; Li, P.; Zhao, J.; Li, X.; Wang, X.; Liu, B.; Ni, L.; Li, H.; Xi, Y. Encapsulation of roast beef flavor by soy protein isolate/chitosan complex Pickering emulsions to improve its releasing properties during the processing of plant-based meat analogues. Food Chem. 2024, 450, 139313. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Dai, X.; Zhang, Y.; Yang, Y.; Jiang, B.; Li, D.; Feng, Z. Post-self-assemble of whey protein isolation nanofibrils and its contribution to the stability of pickering emulsion. Food Hydrocoll. 2024, 151, 109766. [Google Scholar] [CrossRef]
- Cui, S.; McClements, D.J.; Shi, J.L.; Xu, X.F.; Ning, F.J.; Liu, C.R.; Zhou, L.Y.; Sun, Q.J.; Dai, L. Fabrication and characterization of low-fat Pickering emulsion gels stabilized by zein/phytic acid complex nanoparticles. Food Chem. 2023, 402, 134179. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.A.; Yang, X.Q.; Scholten, E. Tuning particle properties to control rheological behavior of high internal phase emulsion gels stabilized by zein/tannic acid complex particles. Food Hydrocoll. 2019, 89, 163–170. [Google Scholar] [CrossRef]
- Wu, J.J.; Xi, J.F.; Chen, H.B.; Li, S.J.; Zhang, L.; Li, P.; Wu, W.B. Flexible 2D nanocellulose-based SERS substrate for pesticide residue detection. Carbohydr. Polym. 2022, 277, 118890. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.H.; Li, C.C.; Shi, X.T.; Xiao, H.N. Naturally occurring protein/polysaccharide hybrid nanoparticles for stabilizing oil-in-water Pickering emulsions and the formation mechanism. Food Chem. 2022, 395, 133641. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhan, X.Y.; Wei, Y.; Sun, C.X.; Mao, L.K.; McClements, D.J.; Gao, Y.X. Composite zein-propylene glycol alginate particles prepared using solvent evaporation: Characterization and application as Pickering emulsion stabilizers. Food Hydrocoll. 2018, 85, 281–290. [Google Scholar] [CrossRef]
- Dong, R.S.; Lu, F.; Liu, P.D.; Li, X.Y.; Huang, R.; Liu, G.D.; Chen, J. Preparation of nanocellulose-polyvinyl alcohol composite hydrogels from Desmodium intortum (Mill.) Urb.: Chemical property characterization. Ind. Crops Prod. 2021, 176, 11437. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Chang, H.; Yao, G.; Chen, J.; Dong, R. Desmodium intortum (Mill.) Urb. Protein Isolate Aggregates as Pickering Stabilizers: Physicochemical Characteristics and Emulsifying Properties. Molecules 2024, 29, 3923. https://doi.org/10.3390/molecules29163923
Tang X, Chang H, Yao G, Chen J, Dong R. Desmodium intortum (Mill.) Urb. Protein Isolate Aggregates as Pickering Stabilizers: Physicochemical Characteristics and Emulsifying Properties. Molecules. 2024; 29(16):3923. https://doi.org/10.3390/molecules29163923
Chicago/Turabian StyleTang, Xuemei, Hui Chang, Guanglong Yao, Jian Chen, and Rongshu Dong. 2024. "Desmodium intortum (Mill.) Urb. Protein Isolate Aggregates as Pickering Stabilizers: Physicochemical Characteristics and Emulsifying Properties" Molecules 29, no. 16: 3923. https://doi.org/10.3390/molecules29163923
APA StyleTang, X., Chang, H., Yao, G., Chen, J., & Dong, R. (2024). Desmodium intortum (Mill.) Urb. Protein Isolate Aggregates as Pickering Stabilizers: Physicochemical Characteristics and Emulsifying Properties. Molecules, 29(16), 3923. https://doi.org/10.3390/molecules29163923




