A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives
Abstract
:1. Introduction
2. Natural Resources of the Antibacterial Activity-Guided Derivatives of Cinnamic Acid
3. Classification of the Features of the Organic Modification of Cinnamic Acid Derivatives as an Antibacterial Agent
3.1. Fischer Esterification
3.2. Alkyl Halide Reagent
3.3. Modification via Acid Chloride
3.4. Steglich Reaction
3.5. Mitsunobu Reaction
3.6. Amidation Using Nitrile
4. Utilization of Features in the Modification of Cinnamic Acid Derivatives for Antibacterial Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Cinnamic acid |
| CNF | Cellulose nanofibril |
| CS | Chitosan |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DEAD | Diethyl azodicarboxylate |
| DIC | N,N′-Diisopropylcarbodiimide |
| DMAP | 4-Dimethylaminopyridine |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| Et3N | Triethylamine |
| NHS | N-Hydroxysuccinimide |
| PET | Polyethylene terephthalate |
| Ph3P | Triphenylphosphine |
References
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The Global Threat of Antimicrobial Resistance: Science for Intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Liu, W.T.; Chen, E.Z.; Yang, L.; Peng, C.; Wang, Q.; Xu, Z.; Chen, D.Q. Emerging resistance mechanisms for 4 Types of Common Anti-MRSA Antibiotics in Staphylococcus aureus: A Comprehensive Review. Microb. Pathog. 2021, 156, 104915. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Grare, M.; Demore, B. Fight Against Antimicrobial Resistance: We Always Need New Antibacterials but for Right Bacteria. Molecules 2019, 24, 3152. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Chandra, S.; Roy, A.; Jana, M.; Pahan, K. Cinnamic Acid Activates Pparalpha to Stimulate Lysosomal Biogenesis and Lower Amyloid Plaque Pathology in An Alzheimer’s Disease Mouse Model. Neurobiol. Dis. 2019, 124, 379–395. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric Acid in Cereals: Presence, Antioxidant and Antimicrobial Effects. Int. J. Food Sci. Tech. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates-nature, occurrence, dietary burden, absorption, and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Ghalehshahi, H.G.; Balalaie, S.; Aliahmadi, A. Peptides N-connected to Hydroxycoumarin and Cinnamic Acid Derivatives: Synthesis and Fluorescence Spectroscopic, Antioxidant and Antimicrobial Properties. New J. Chem. 2018, 42, 8831–8842. [Google Scholar] [CrossRef]
- Muhammad, N.; Saeed, M.; Adhikari, A.; Khan, K.M.; Khan, H. Isolation of A New Bioactive Cinnamic Acid Derivative from The Whole Plant of Viola betonicifolia. J. Enzym. Inhib. Med. Chem. 2013, 28, 997–1001. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel Cinnamic Acid Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Modeling Studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Nouni, C.; Theodosis-Nobelos, P.; Rekka, E.A. Antioxidant and Hypolipidemic Activities of Cinnamic Acid Derivatives. Molecules 2023, 28, 6732. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.Z.; Kausar, F.; Hassan, M.; Javaid, S.; Malik, A. Anticancer Activities of Phenolic Compounds from Moringa Oleifera Leaves: In Vitro and In Silico Mechanistic Study. Beni-Suef Univ. J. Basic. Appl. Sci. 2021, 10, 12. [Google Scholar] [CrossRef]
- Abu Almaaty, A.H.; Elgrahy, N.A.; Fayad, E.; Abu Ali, O.A.; Mahdy, A.R.E.; Barakat, L.A.A.; El Behery, M. Design, Synthesis and Anticancer Evaluation of Substituted Cinnamic Acid Bearing 2-Quinolone Hybrid Derivatives. Molecules 2021, 26, 4724. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Cheng, J.B.; Su, W.Q.; Li, H.Z.; Xiao, T.; Chen, D.A.; Zhang, Z.L. Cinnamic Acid Hybrids as Anticancer Agents: A Mini-Review. Arch. Pharm. 2022, 355, e2200052. [Google Scholar] [CrossRef]
- Zenta, F.; Soekamto, N.H.; Dali, S.; Firdausiah, S.; Rasyid, H.; Bahriah, B.; Agustan, A.; Tahir, D. Development trans-N-benzyl Hydroxyl Cinnamamide Based Compounds from Cinnamic Acids and characteristics Anticancer Potency. J. Iran. Chem. Soc. 2022, 19, 2845–2853. [Google Scholar] [CrossRef]
- Alarcón-López, A.; Hernández-Serda, M.; Aguirre-Vidal, P.; Cárdenas-Granados, L.; Vázquez-Valadez, V.; Martínez-Soriano, P.; Briseño-Lugo, P.; Velázquez-Sánchez, A.; Rul-Ramírez, E.; Jiménez-Jiménez, M.L.; et al. Computational Insight and Anticancer Effect of Cinnamic Acid-Derivative Amide Compounds. J. Braz. Chem. Soc. 2024, 35, e-20230195. [Google Scholar] [CrossRef]
- Savych, A. Cinnamic Acid and Its Derivatives in the Herbal Mixtures and Their Antidiabetic Activity. Farmacia 2021, 69, 595–601. [Google Scholar] [CrossRef]
- Anthony, P.C.; Eseyin, O.A.; Attih, E.; Johnson, E.; Ebong, A.; Effiong, A.E. Asanga Edet Effiong, Synthesis of Some Esters of Cinnamic Acid and Evaluation of Their In Vitro Antidiabetic and Antioxidant Properties. Trop. J. Pharm. Life Sci. 2022, 21, 131–136. [Google Scholar]
- Nair, A.; Preetha Rani, M.R.; Salin Raj, P.; Ranjit, S.; Rajankutty, K.; Raghu, K.G. Cinnamic Acid is Beneficial to Diabetic Cardiomyopathy Via Its Cardioprotective, Anti-Inflammatory, Anti-Dyslipidemia, and Antidiabetic Properties. J. Biochem. Mol. Toxicol. 2022, 36, e23215. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Proenca, C.; Varela, C.; Janela, J.; Tavares da Silva, E.J.; Fernandes, E.; Roleira, F.M.F. New Phenolic Cinnamic Acid Derivatives as Selective COX-2 Inhibitors. Design, Synthesis, Biological Activity, and Structure-Activity Relationships. Bioorg. Chem. 2019, 91, 103179. [Google Scholar] [CrossRef]
- Jacob, B.B.; Baba, H.; Oluwadiya, J.O. Synthesis, Characterization and evaluation of Anti-inflammatory and Antimicrobial Properties of Some Cinnamic Acid Derivatives. Nig. J. Pharm. Res. 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Chen, P.; Xu, Z.; Wang, X.; He, J.; Yang, J.; Wang, J.; Chattipakorn, N.; Wu, D.; Tang, Q.; Liang, G.; et al. Discovery of New Cinnamic Derivatives as Anti-Inflammatory Agents for Treating Acute Lung Injury in Mice. Arch. Pharm. 2023, 356, e2200191. [Google Scholar] [CrossRef]
- Wu, F.-S.; Huang, C.-M.; Chen, L.-C.; Chang, T.-H.; Shu, C.-W.; Sung, P.-J.; Chu, Y.-C.; Cheng, M.-J.; Hsiao, J.-W.; Chen, J.-J. A New Cinnamic Acid Derivative and Anti-Inflammatory Constituents from Capparis lanceolaris. Chem. Nat. Comp. 2023, 59, 493–496. [Google Scholar] [CrossRef]
- Habboby, M.; Munaf Hashim, A. Evaluation of Anti-inflammatory Effects of Cinnamic Acid Against Dextran Sodium Sulfate Induced Ulcerative Colitis in Male Mice. Iraqi J. Pharm. Sci. 2023, 32, 33–40. [Google Scholar] [CrossRef]
- Lima, T.C.; Ferreira, A.R.; Silva, D.F.; Lima, E.O.; de Sousa, D.P. Antifungal Activity of Cinnamic Acid and Benzoic Acid Esters Against Candida albicans Strains. Nat. Prod. Res. 2018, 32, 572–575. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, J.; Zhou, M.; Wang, M.; Feng, J. Antifungal Activity and Action Mechanism of the Natural Product Cinnamic Acid Against Sclerotinia sclerotiorum. Plant Dis. 2019, 103, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Perez-Castillo, Y.; Lima, T.C.; Ferreira, A.R.; Silva, C.R.; Campos, R.S.; Neto, J.B.A.; Magalhaes, H.I.F.; Cavalcanti, B.C.; Junior, H.V.N.; de Sousa, D.P. Bioactivity and Molecular Docking Studies of Derivatives from Cinnamic and Benzoic Acids. Biomed. Res. Int. 2020, 2020, 6345429. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jing, Y.; Cheng, L.; Si, Z.; Mou, Z.; Niu, D.; Ma, F.; Liu, C. The Antifungal Activity of Trans-Cinnamic Acid and Its Priming Effect in Apple in response to Valsa mali. Plant Pathol. 2023, 72, 1595–1603. [Google Scholar] [CrossRef]
- Hong, S.; Cha, K.H.; Park, J.H.; Jung, D.S.; Choi, J.H.; Yoo, G.; Nho, C.W. Cinnamic Acid Suppresses Bone Loss Via Induction of Osteoblast Differentiation with Alteration of Gut Microbiota. J. Nutr. Biochem. 2022, 101, 108900. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Q.; Sun, P.; Qin, L.; Zhang, L. Effect of Cinnamon and Aconite on Bone Formation-Bone Absorption Coupling in Bone Microenvironment. Rev. Bras. De Farmacogn. 2023, 34, 511–512. [Google Scholar] [CrossRef]
- Wiesner, J.; Kettler, K.; Jomaa, H.; Schlitzer, M. Structure-Activity Relationships of Novel Anti-Malarial Agents. Bioorg. Med. Chem. Lett. 2001, 11, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Seck, R.; Malamine, M.; Gassama, A.; Christian, C.; Cojean, S. Synthesis and Antimalarial Activity of Cinnamic Acid Derivatives. Eur. J. Biomed. Pharm. Sci. 2019, 6, 450–454. [Google Scholar]
- Silva, A.T.; Bento, C.M.; Pena, A.C.; Figueiredo, L.M.; Prudencio, C.; Aguiar, L.; Silva, T.; Ferraz, R.; Gomes, M.S.; Teixeira, C.; et al. Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs. Molecules 2020, 25, 66. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M. Pharmacological and Therapeutic Applications of Sinapic Acid-An Updated Review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Thakar, S.R.; Dhamade, P.N.; Bansode, D.A. Cinnamic Acid and Its Derivatives as Potential Anti-Tubercular Agents. Indian Drugs 2023, 60, 7–19. [Google Scholar] [CrossRef]
- Alifah, L.H.; Jatmika, C.; Hayun, H. Exploration of Ferulic Acid and Its Derivatives as Potent Anti-Tyrosinase: A Systematic Review. Egypt J. Chem. 2024, 67, 257–271. [Google Scholar] [CrossRef]
- De, P.; Baltas, M.; Bedos-Belval, F. Cinnamic Acid Derivatives as Anticancer Agents—A Review. Curr. Med. Chem. 2011, 18, 1672–1703. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- De, P.; Bedos-Belval, F.; Vanucci-Bacque, C.; Baltas, M. Cinnamic Acid Derivatives in Tuberculosis, Malaria and Cardiovascular Diseases—A Review. Curr. Org. Chem. 2012, 16, 747–768. [Google Scholar]
- Guzman, J. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Gaikwad, N.; Nanduri, S.; Madhavi, Y.V. Cinnamamide: An insight into the pharmacological advances and structure-activity relationships. Eur. J. Med. Chem. 2019, 181, 111561. [Google Scholar] [CrossRef] [PubMed]
- Ernawati, T.; Radji, M.; Hanafi, M.; Mun’im, A.; Yanuar, A. Cinnamic Acid Derivatives as α-Glucosidase Inhibitor Agents. Indones. J. Chem. 2017, 17, 151–160. [Google Scholar] [CrossRef]
- Deng, H.; Xu, Q.; Guo, H.Y.; Huang, X.; Chen, F.; Jin, L.; Quan, Z.S.; Shen, Q.K. Application of Cinnamic Acid in The Structural Modification of Natural Products: A Review. Phytochemistry 2023, 206, 113532. [Google Scholar] [CrossRef]
- Kernou, O.-N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, L.; Yan, G.; Huang, D. Recent Advances of Cinnamic Acids in Organic Synthesis. Asian J. Org. Chem. 2020, 9, 842–862. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Stepanovic, S.; Antic, N.; Dakic, I.; Svabic-Vlahovic, M. In Vitro Antimicrobial Activity of Propolis and Synergism between Propolis and Antimicrobial Drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, S.; Shariatpanahi, M.; Hamedi, M.; Ahmadkhaniha, R.; Samadi, N.; Ostad, S.N. Chemical Composition, Oral Toxicity and Antimicrobial Activity of Iranian propolis. Food Chem. 2007, 103, 1097–1103. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sova, M.; Ergun, S. Antimicrobial Activity of Trans-Cinnamic Acid and Commonly Used Antibiotics Against Important Fish Pathogens and Nonpathogenic Isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Huang, S.T.; Sun, F.M.; Chiang, Y.L.; Chiang, C.J.; Tsai, C.M.; Weng, C.J. Transformation of Cinnamic Acid from Trans- to Cis-Form Raises a Notable Bactericidal and Synergistic Activity Against Multiple-Drug Resistant Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2011, 43, 188–194. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-Function Relationships of The Antibacterial Activity of Phenolic Acids and Their Metabolism by Lactic Acid Bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Mark, S.; Barber, V.S.M.; Brian, S. DeCaux, Antimicrobial Intermediates of The General Phenylpropanoid and Lignin Specific Pathways. Phytochemistry 2000, 54, 53–56. [Google Scholar]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R.; et al. Antimicrobial Activity of Ferulic Acid Against Cronobacter sakazakii and Possible Mechanism of Action. Foodborne Pathog. Dis. 2016, 13, 196–204. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antimicrobial Activity of Pomegranate Fruit Constituents Against Drug-Resistant Mycobacterium tuberculosis and Beta-Lactamase Producing Klebsiella pneumoniae. Pharm. Biol. 2015, 53, 1474–1480. [Google Scholar] [CrossRef]
- Malheiro, J.F.; Maillard, J.Y.; Borges, F.; Simões, M. Evaluation of Cinnamaldehyde and Cinnamic Acid Derivatives in Microbial Growth Control. Int. Biodeter. Biodegr. 2019, 141, 71–78. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial Mechanisms of Cinnamon and Its Constituents: A Review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Liefferinge, E.V.; Forte, C.; Degroote, J.; Ovyn, A.; Van Noten, N.; Mangelinckx, S.; Michiels, J. In Vitro and In Vivo Antimicrobial Activity of Cinnamaldehyde and Derivatives Towards The Intestinal Bacteria of The Weaned Piglet. Ital. J. Anim. Sci. 2022, 21, 493–506. [Google Scholar] [CrossRef]
- Falbo, F.; Gemma, S.; Koch, A.; Mazzotta, S.; Carullo, G.; Ramunno, A.; Butini, S.; Schneider-Stock, R.; Campiani, G.; Aiello, F. Synthetic Derivatives of Natural Cinnamic Acids as Potential Anti-Colorectal Cancer Agents. Chem. Biol. Drug Des. 2024, 103, e14415. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.C.; Medeiros, G.A.; Almeida, F.S.; Rocha, J.D.C.; Perez-Castillo, Y.; Keesen, T.S.L.; de Sousa, D.P. Antileishmanial Activity of Cinnamic Acid Derivatives against Leishmania infantum. Molecules 2023, 28, 2844. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial Effect and Mode of Action of Chlorogenic Acid on Staphylococcus aureus. Eur. Food Res. Technol. 2013, 238, 589–596. [Google Scholar] [CrossRef]
- Meilawati, L.; Saepudin, E.; Ernawati, T. Antibacterial Activity of Methyl Cinnamate, Cinnamic Acid and Cinnamic Acid Derivatives Derived from Alpinia malaccensis Rhizomes. Int. J. Agric. Sci. 2023, 30, 449–454. [Google Scholar]
- Starkey, L.S. Introduction to Strategies for Organic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- de Morais, M.C.; de Oliveira Lima, E.; Perez-Castillo, Y.; de Sousa, D.P. Synthetic Cinnamides and Cinnamates: Antimicrobial Activity, Mechanism of Action, and In Silico Study. Molecules 2023, 28, 1918. [Google Scholar] [CrossRef]
- Araújo, M.O.; Freire Pessoa, H.L.; Lira, A.B.; Castillo, Y.P.; de Sousa, D.P. Synthesis, Antibacterial Evaluation, and QSAR of Caffeic Acid Derivatives. J. Chem. 2019, 2019, 3408315. [Google Scholar] [CrossRef]
- Silva, R.H.N.; Andrade, A.C.M.; Nobrega, D.F.; de Castro, R.D.; Pessoa, H.L.F.; Rani, N.; de Sousa, D.P. Antimicrobial Activity of 4-Chlorocinnamic Acid Derivatives. Biomed. Res. Int. 2019, 2019, 3941242. [Google Scholar] [CrossRef]
- Sun, C.; Zhi, H.; Li, H.; Li, J.; Shao, K.; Lin, Y. Synthesis, Characterization and Antibacterial Study of Cinnamic Acid Derivatives. Malays. J. Chem. 2020, 22, 58–63. [Google Scholar]
- Nimse, S.B.; Pal, D.; Mazumder, A.; Mazumder, R. Synthesis of Cinnamanilide Derivatives and Their Antioxidant and Antimicrobial Activity. J. Chem. 2015, 2015, 208910. [Google Scholar] [CrossRef]
- Fregnan, A.M.; Brancaglion, G.A.; Galvão, A.F.C.; D’Sousa Costa, C.O.; Moreira, D.R.M.; Soares, M.B.P.; Bezerra, D.P.; Silva, N.C.; de Souza Morais, S.M.; Oliver, J.C.; et al. Synthesis of Piplartine Analogs and Preliminary Findings on Structure–Antimicrobial Activity Relationship. Med. Chem. Res. 2017, 26, 603–614. [Google Scholar] [CrossRef]
- Li, X.; Sheng, J.; Huang, G.; Ma, R.; Yin, F.; Song, D.; Zhao, C.; Ma, S. Design, Synthesis and Antibacterial Activity of Cinnamaldehyde Derivatives as Inhibitors of The Bacterial Cell Division Protein FtsZ. Eur. J. Med. Chem. 2015, 97, 32–41. [Google Scholar] [CrossRef]
- List, B.; Mitschke, B. The Steglich Esterification. Synfacts 2019, 15, 522–524. [Google Scholar]
- Zala, A.R.; Rajani, D.P.; Kumari, P. Design, Synthesis, Molecular Docking and In Silico ADMET Investigations of Novel Piperidine-Bearing Cinnamic Acid Hybrids as Potent Antimicrobial Agents. J. Iran. Chem. Soc. 2023, 20, 1843–1856. [Google Scholar] [CrossRef]
- Mingoia, M.; Conte, C.; Di Rienzo, A.; Dimmito, M.P.; Marinucci, L.; Magi, G.; Turkez, H.; Cufaro, M.C.; Del Boccio, P.; Di Stefano, A.; et al. Synthesis and Biological Evaluation of Novel Cinnamic Acid-Based Antimicrobials. Pharmacy 2022, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Zala, A.R.; Rajani, D.P.; Kumari, P. Design, Synthesis, Molecular Docking and Antimicrobial and Antimycobacterial Activities of Novel Hybrid of Coumarin-Cinnamic Acids. Chem. Data Coll. 2022, 39, 100862. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhu, D.D.; Li, Z.L.; Sun, J.; Zhu, H.L. Synthesis, Molecular Modeling and Biological Evaluation of Beta-Ketoacyl-Acyl Carrier Protein Synthase III (FabH) as Novel Antibacterial Agents. Bioorg. Med. Chem. 2011, 19, 4513–4519. [Google Scholar] [CrossRef]
- Bulakowska, A.; Slawinski, J.; Halasa, R.; Hering, A.; Gucwa, M.; Ochocka, J.R.; Stefanowicz-Hajduk, J. An In Vitro Antimicrobial, Anticancer and Antioxidant Activity of N-[(2-Arylmethylthio) phenylsulfonyl]cinnamamide Derivatives. Molecules 2023, 28, 3087. [Google Scholar] [CrossRef]
- De, P.; Koumba Yoya, G.; Constant, P.; Bedos-Belval, F.; Duran, H.; Saffon, N.; Daffe, M.; Baltas, M. Design, Synthesis, and Biological Evaluation of New Cinnamic Derivatives as Antituberculosis Agents. J. Med. Chem. 2011, 54, 1449–1461. [Google Scholar] [CrossRef]
- Nishikido, J. Esterification: Methods, Reactions, and Application, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 3–157. [Google Scholar]
- Zolnowska, B.; Slawinski, J.; Garbacz, K.; Jarosiewicz, M.; Kawiak, A. N-(2-Arylmethylthio-4-Chloro-5-Methylbenzenesulfonyl) amide Derivatives as Potential Antimicrobial Agents-Synthesis and Biological Studies. Int. J. Mol. Sci. 2019, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, M.; Xu, C.; Yam, K.L. Critical Review of Controlled Release Packaging to Improve Food Safety and Quality. Crit. Rev. Food Sci. Nutr. 2018, 59, 2386–2399. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Mahfoudh, R.; Moundanga, S.; Brachais, C.H.; Chambin, O.; Debeaufort, F. Modeling of The Release Kinetics of Phenolic Acids Embedded in Gelatin/Chitosan Bioactive-Packaging Films: Influence of Both Water Activity and Viscosity of The Food Simulant on The Film Structure and Antioxidant Activity. Int. J. Biol. Macromol. 2020, 160, 780–794. [Google Scholar] [CrossRef]
- Wen, Y.; Ye, F.; Zhu, J.; Zhao, G. Corn Starch Ferulates with Antioxidant Properties Prepared by N,N′-Carbonyldiimidazole-Mediated Grafting Procedure. Food Chem. 2016, 208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Properties of PLA Films with Cinnamic Acid: Effect of The Processing Method. Food Bioprod. Process 2022, 133, 25–33. [Google Scholar] [CrossRef]
- Hazer, B.; Karahaliloglu, Z.; Ashby, R.D. Poly (vinyl chloride) Derived Food Packaging Applications with Antioxidative and Anticancer Properties. ACS Food Sci. Technol. 2023, 3, 761–771. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Tsagkalias, I.; Vouvoudi, E.C.; Achilias, D.S. Development of Bio-Composites with Enhanced Antioxidant Activity Based on Poly (lactic acid) with Thymol, Carvacrol, Limonene, or Cinnamaldehyde for Active Food Packaging. Polymers 2021, 13, 3652. [Google Scholar] [CrossRef]
- Nedved, E.; Kalatskajakaja, J.N.; Ovchinnikov, I.A.; Rybinskaya, E.I.; Kraskouski, A.N.; Nikalaichuk, V.V.; Hileuskaya, K.S.; Kulikouskaya, V.I.; Agabekov, V.E.; Laman, N.A. Growth Parameters and Antioxidant Activity in Cucumber Seedlings with the Application of Chitosan and Hydroxycinnamic Acids Conjugates under Salt Stress. Appl. Biochem. Microbiol. 2022, 58, 69–76. [Google Scholar] [CrossRef]
- Nagy, V.; Sahirah, P.; Hjalmarsdottir, M.A.; Masson, M. Chitosan-Hydroxycinnamic Acid Conjugates: Optimization of The Synthesis and Investigation of The Structure Activity Relationship. Carbohydr. Polym. 2022, 277, 118896. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Gao, X.; Chen, G.; Wang, Z. Probing the Structure-Antioxidant Activity Relationships of Four Cinnamic Acids Porous Starch Esters. Carbohydr. Polym. 2021, 256, 117428. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Physicochemical and Antimicrobial Properties of Cassava Starch Films with Ferulic or Cinnamic Acid. LWT 2021, 144, 111242. [Google Scholar] [CrossRef]
- Letsididi, K.S.; Lou, Z.; Latridiid, R.; Mohammed, K.; Maguy, B.L. Antimicrobial and Antibiofilm Effects of Trans-Cinnamic Acid Nanoemulsion and Its Potential Application on Lettuce. LWT 2018, 94, 25–32. [Google Scholar] [CrossRef]
- Han, Y.; Ding, J.; Zhang, J.; Li, Q.; Yang, H.; Sun, T.; Li, H. Fabrication and Characterization of Polylactic Acid Coaxial Antibacterial Nanofibers Embedded with Cinnamaldehyde/Tea Polyphenol with Food Packaging Potential. Int. J. Biol. Macromol. 2021, 184, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial Activity in Gelatin-Bacterial Cellulose Composite Film by Thermally Crosslinking with Cinnamaldehyde Towards Food Packaging Application. Food Packag. Shelf Life 2022, 31, 100766. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Antibacterial Properties of Cinnamic and Ferulic Acids Incorporated to Starch and PLA Monolayer and Multilayer Films. Food Control 2022, 136, 108878. [Google Scholar] [CrossRef]
- Wang, M.; Yue, L.; Niazi, S.; Khan, I.M.; Zhang, Y.; Wang, Z. Synthesis and Characterization of Cinnamic Acid Conjugated N-(2-Hydroxy)-Propyl-3-Trimethylammonium Chitosan Chloride Derivatives: A Hybrid Flocculant with Antibacterial Activity. Int. J. Biol. Macromol. 2022, 206, 886–895. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Multilayer Antimicrobial Films Based on Starch and PLA With Superficially Incorporated Ferulic or Cinnamic Acids for Active Food Packaging Purposes. Food Chem. Adv. 2023, 2, 100250. [Google Scholar] [CrossRef]
- Mastoor, S.; Nazim, F.; Rizwan-Ul-Hasan, S.; Ahmed, K.; Khan, S.; Ali, S.N.; Abidi, S.H. Analysis of the Antimicrobial and Anti-Biofilm Activity of Natural Compounds and Their Analogues against Staphylococcus aureus Isolates. Molecules 2022, 27, 6874. [Google Scholar] [CrossRef]
- LakshmiBalasubramaniam, S.; Patel, A.S.; Tajvidi, M.; Skonberg, D. Biodegradable Cellulose Nanofibril Films with Active Functionality for Food Packaging Applications. ACS Food Sci. Technol. 2023, 3, 1323–1333. [Google Scholar] [CrossRef]
- LakshmiBalasubramaniam, S.; Patel, A.S.; Nayak, B.; Howell, C.; Skonberg, D. Antioxidant and Antimicrobial Modified Cellulose Nanofibers for Food Applications. Food Biosci. 2021, 44, 101421. [Google Scholar] [CrossRef]
- Yue, L.; Wang, M.; Khan, I.M.; Xu, J.; Peng, C.; Wang, Z. Preparation, Characterization, and Antibiofilm Activity of Cinnamic Acid Conjugated Hydroxypropyl Chitosan Derivatives. Int. J. Biol. Macromol. 2021, 189, 657–667. [Google Scholar] [CrossRef]
- Skaro Bogojevic, S.; Perminova, D.; Jaksic, J.; Milcic, M.; Medakovic, V.; Milovanovic, J.; Nikodinovic-Runic, J.; Maslak, V. Novel Cinnamic Acid-Based PET Derivatives as Quorum Sensing Modulators. J. Mol. Struct. 2024, 1300, 137291. [Google Scholar] [CrossRef]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan-Hydroxycinnamic Acid Conjugates: Preparation, Antioxidant and Antimicrobial Activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.K.; Anandan, R.; Mathew, S. Vanillic acid and Coumaric Acid Grafted Chitosan Derivatives: Improved Grafting Ratio and Potential Application in Functional Food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
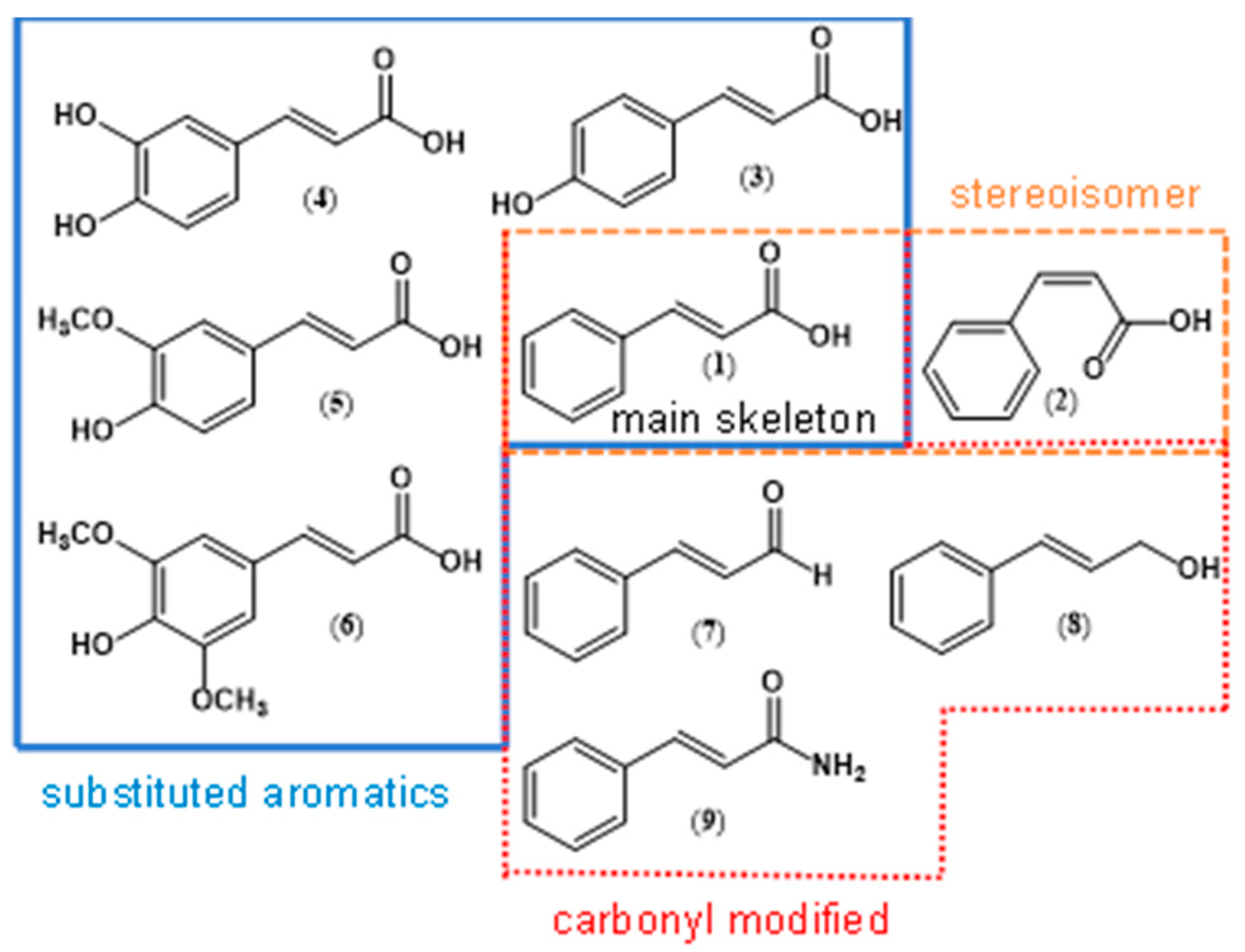
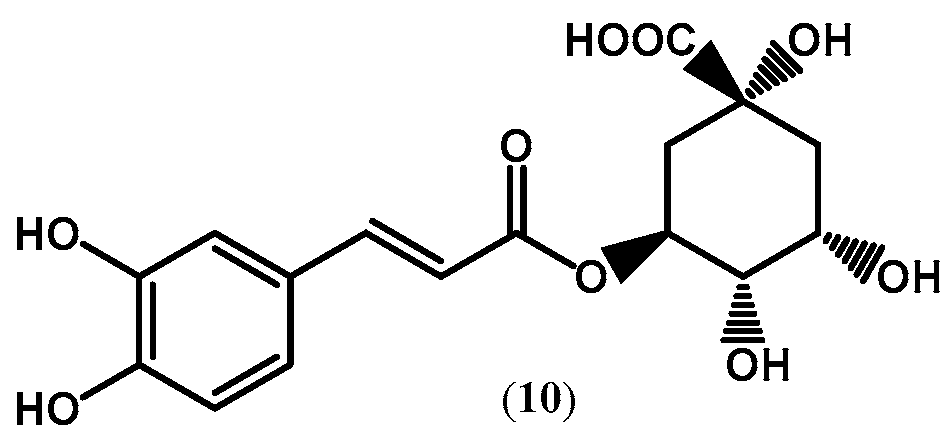
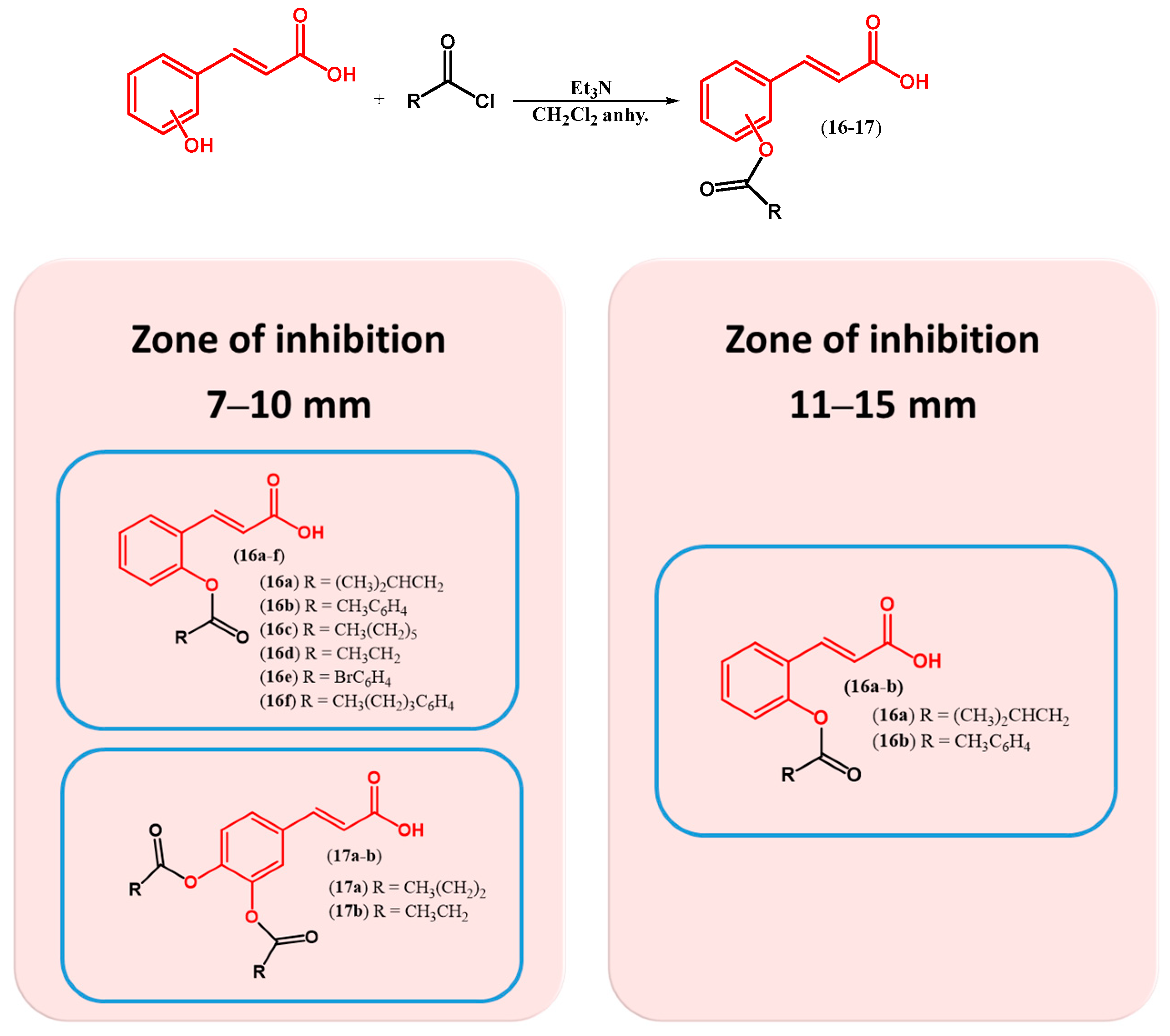

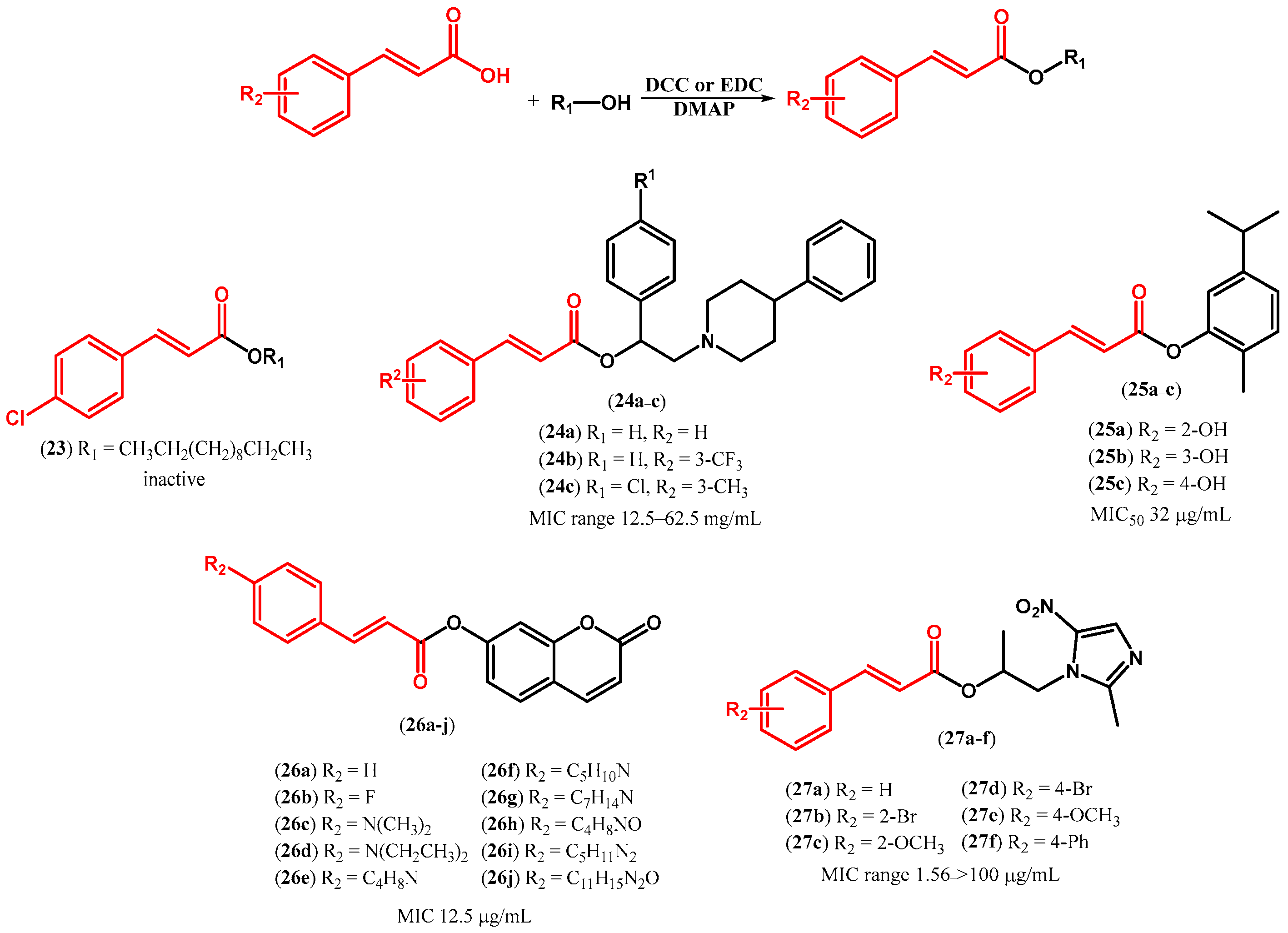
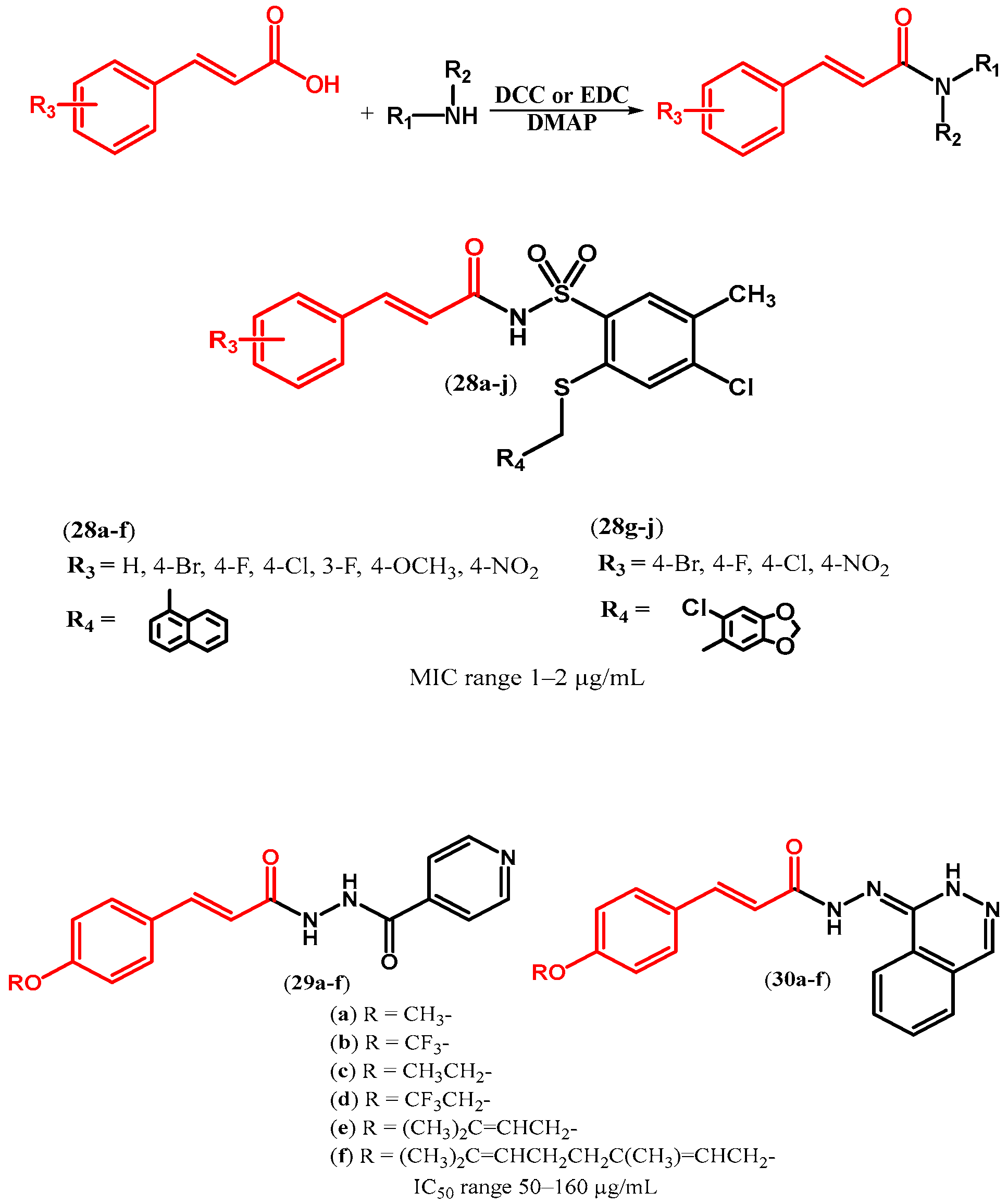

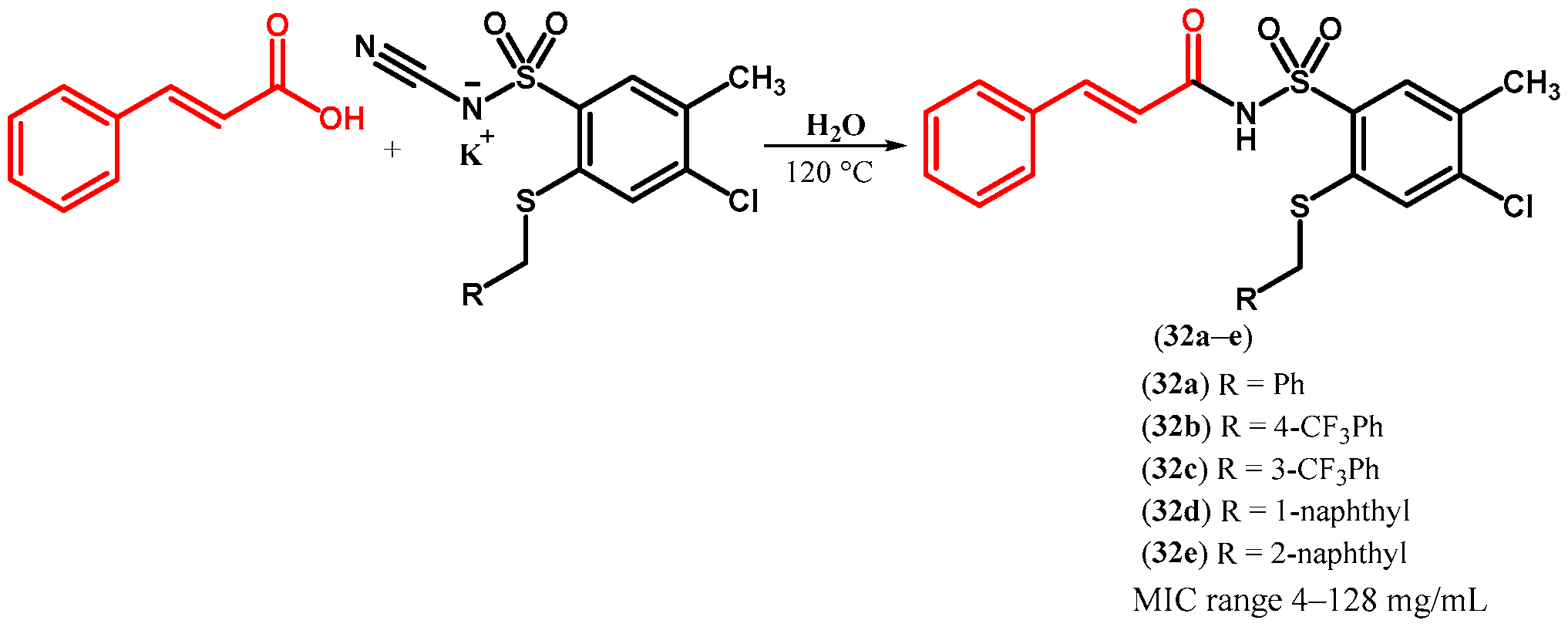
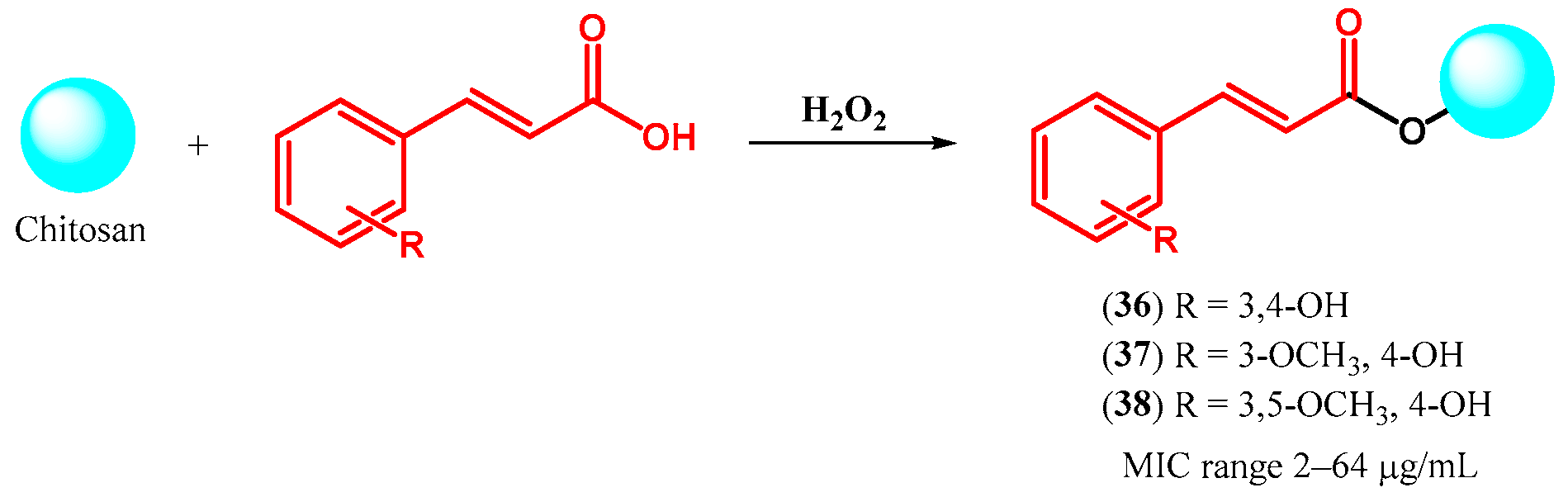

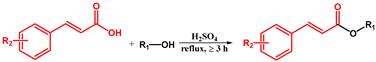 | ||||||
|---|---|---|---|---|---|---|
| Entry | Compound | Ref. | R1 | R2 | Bacteria | MIC Range (μg/mL) |
| 1 | 11a–c | [69] | CH3; CH2CH3; or (CH2)2CH3 | H | S. aureus, S. epidermidis, and P. aeruginosa. | 0.98–512 |
| 2 | 12a–c | [70] | 3,4-OH | S. aureus and E. coli | ||
| 3 | 13a–c | [71] | 4-Cl | S. aureus and P. aeruginosa | ||
| 4 | 11d | [69] | (CH2)3CH3 | H | S. aureus, S. epidermidis, and P. aeruginosa. | 0.89–128 |
| 5 | 12d | [70] | 3,4-OH | S. aureus and E. coli | ||
| 6 | 13d | [71] | 4-Cl | S. aureus and P. aeruginosa | ||
| 7 | 11e | [69] | (CH2)4CH3 | H | S. aureus, S. epidermidis, and P. aeruginosa. | 0.80–128 |
| 8 | 12e | [70] | 3,4-OH | S. aureus and E. coli | ||
| 9 | 11f | [69] | CH(CH3)2 | H | S. aureus, S. epidermidis, and P. aeruginosa. | 2.02–128 |
| 10 | 12f | [70] | 3,4-OH | S. aureus and E. coli | ||
| 11 | 13e | [71] | 4-Cl | S. aureus and P. aeruginosa | ||
| 12 | 11g | [69] | (CH2)2CH(CH3)2 | H | S. aureus, S. epidermidis, and P. aeruginosa. | 1.60–3.17 |
| 13 | 12g | [70] | 3,4-OH | S. aureus and E. coli | ||
| 14 | 12h | [70] | (CH2)2OCH3 | 3,4-OH | S. aureus and E. coli | 3.52 |
| 15 | 13f | [71] | 4-Cl | S. aureus and P. aeruginosa | ||
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Compound | Ref. | R1 | R2 | Bacteria | MIC (μg/mL) |
| 1 | 14a | [71] | (CH2)4CH3 | Cl | S. aureus and P. aeruginosa | Inactive |
| 2 | 14b | (CH2)8CH3 | Cl | |||
| 3 | 15a | [69] | (CH2)9CH3 | H | S. aureus, S. epidermidis, and P. aeruginosa | 128 |
| 4 | 14c | [71] | CH2Ph(4-Cl) | Cl | S. aureus and P. aeruginosa | Inactive |
| 5 | 14d | CH2Ph(4-OCH3) | Cl | |||
| 6 | 15b | [69] | CH2Ph(4-Cl) | H | S. aureus, S. epidermidis, and P. aeruginosa | |
 | ||||
|---|---|---|---|---|
| Entry | Compound | Ref. | Condition | Antibacterial Activity |
| 1 | 33 | [99] | DIC, DMAP, at 24 °C for 24 h | |
| 2 | [100] | a. SOCl2, pyridine, at rt for 24 h | Bacteria load range 6.9–7.1 log CFU/mL of S. epidermidis a | |
| b. EDC, DMAP, at rt for 24 h | ||||
| 3 | 34 | [101] | EDC, at rt for 24 h | MIC range 256–2048 µg/mL against E. coli and S. aureus |
| 4 | 35a | [102] | DCC, DMAP, at rt for 24 h | Inactive against S. aureus, E. coli, and P. aeruginosa |
| 35b | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annuur, R.M.; Triana, D.; Ernawati, T.; Murai, Y.; Aswad, M.; Hashimoto, M.; Tachrim, Z.P. A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives. Molecules 2024, 29, 3929. https://doi.org/10.3390/molecules29163929
Annuur RM, Triana D, Ernawati T, Murai Y, Aswad M, Hashimoto M, Tachrim ZP. A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives. Molecules. 2024; 29(16):3929. https://doi.org/10.3390/molecules29163929
Chicago/Turabian StyleAnnuur, Rose Malina, Desita Triana, Teni Ernawati, Yuta Murai, Muhammad Aswad, Makoto Hashimoto, and Zetryana Puteri Tachrim. 2024. "A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives" Molecules 29, no. 16: 3929. https://doi.org/10.3390/molecules29163929
APA StyleAnnuur, R. M., Triana, D., Ernawati, T., Murai, Y., Aswad, M., Hashimoto, M., & Tachrim, Z. P. (2024). A Review of Cinnamic Acid’s Skeleton Modification: Features for Antibacterial-Agent-Guided Derivatives. Molecules, 29(16), 3929. https://doi.org/10.3390/molecules29163929






