Batch Preparation and Performance Study of Boehmite-Based Electrospun Nanofiber Separators for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results
2.1. Properties of Spinning Solutions
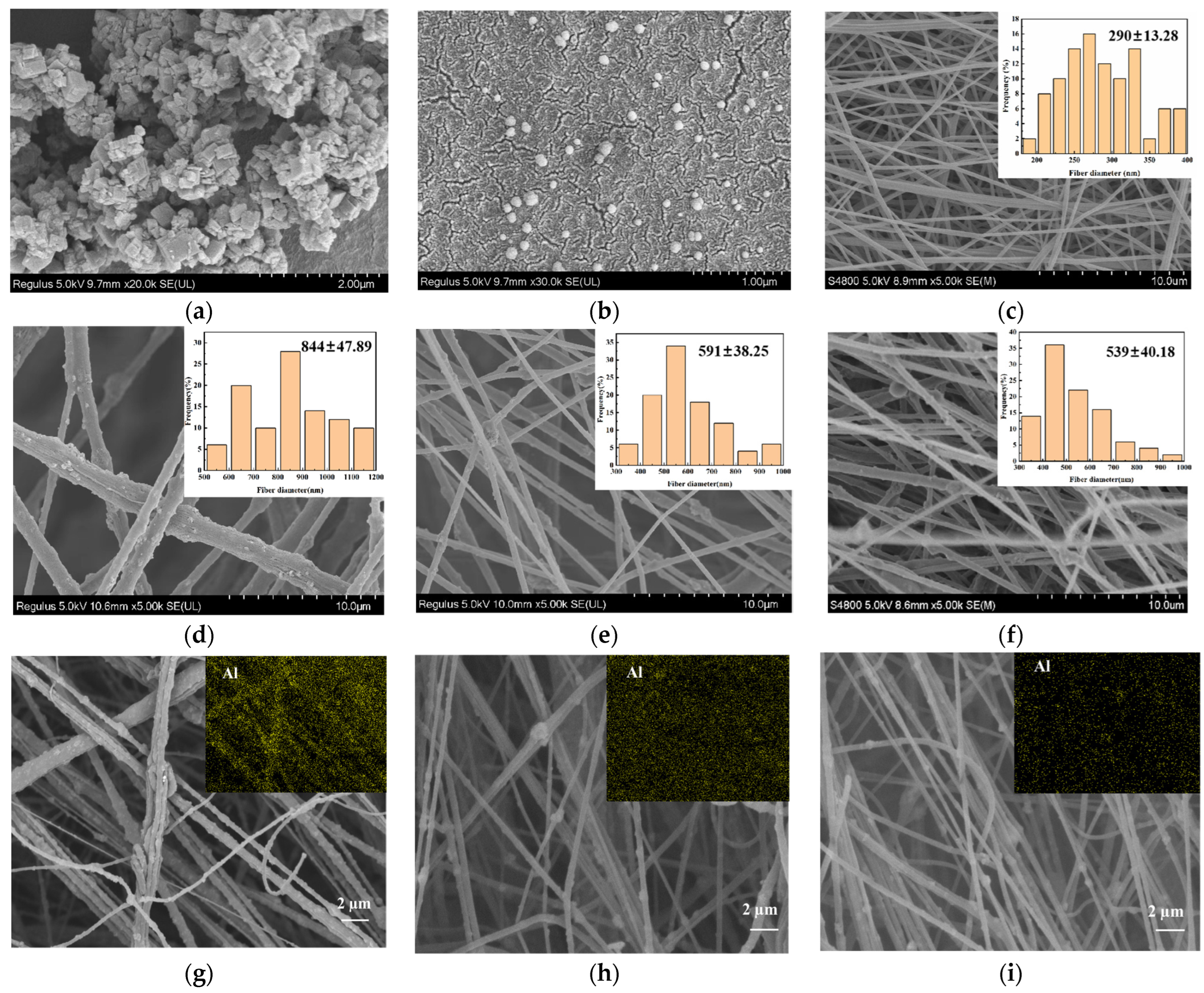
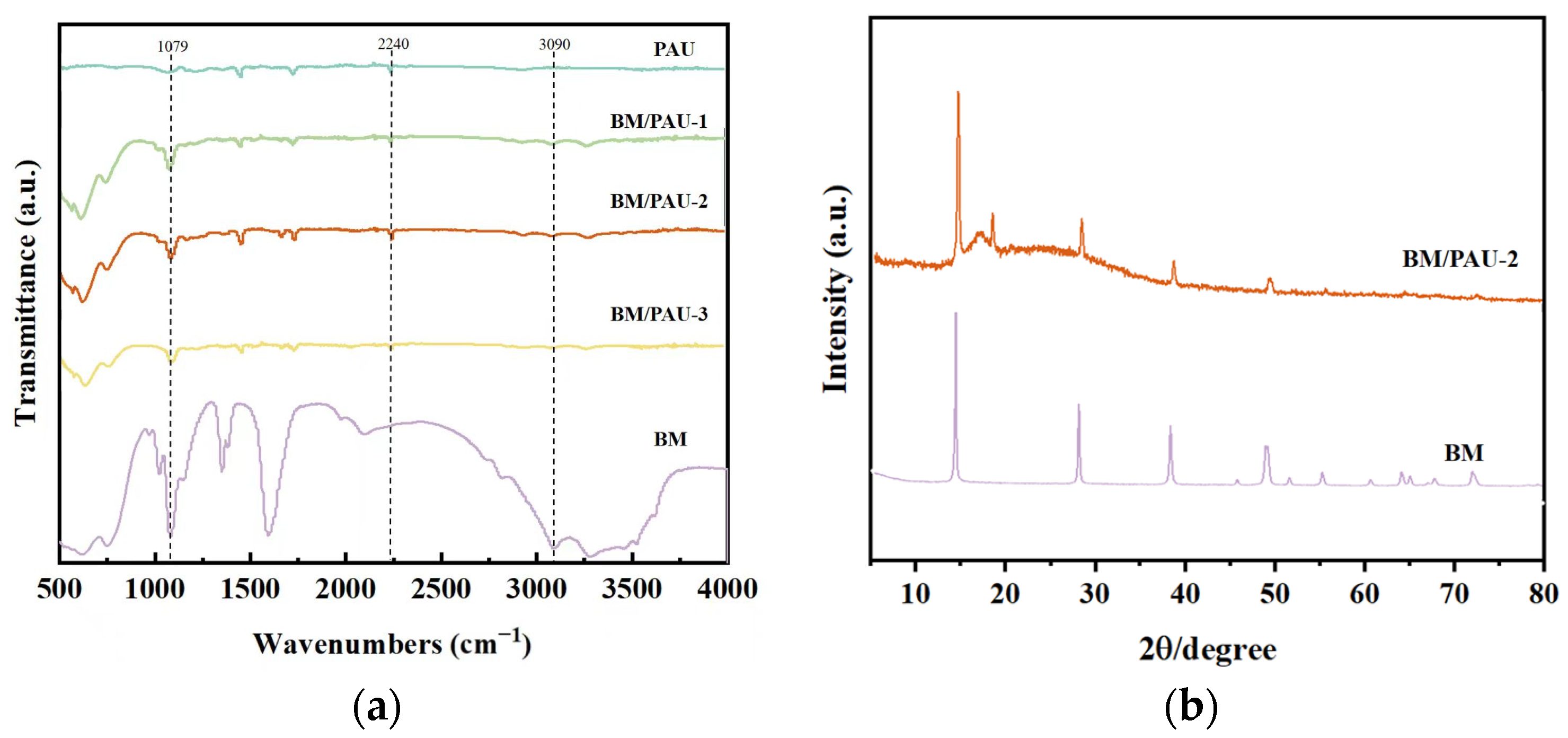
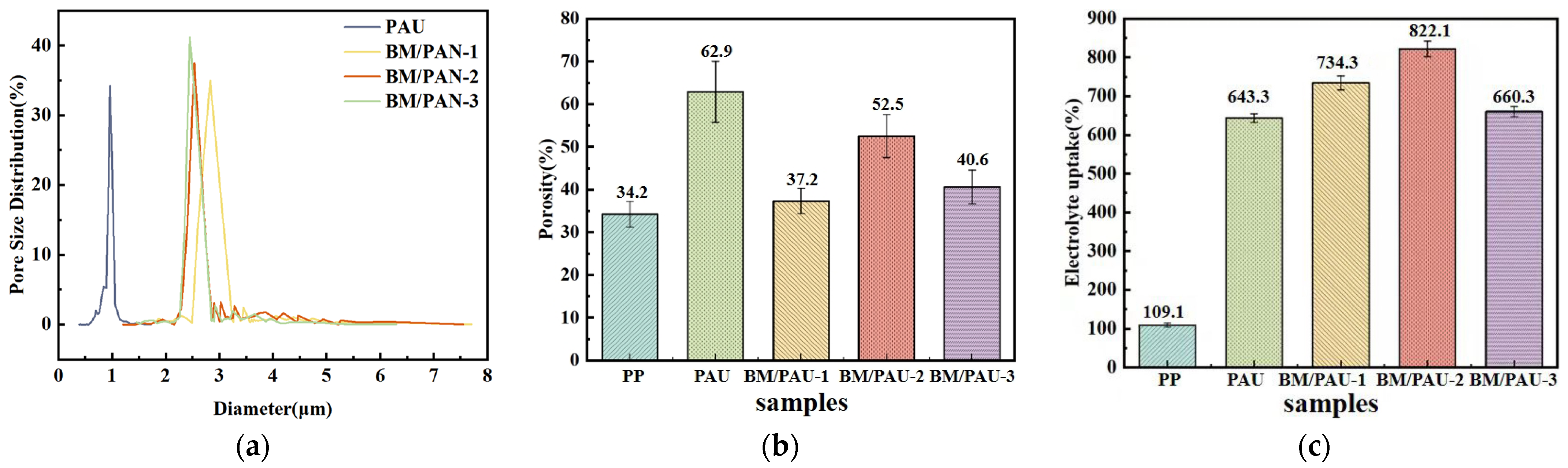
2.2. Morphology and Structure of ENSs
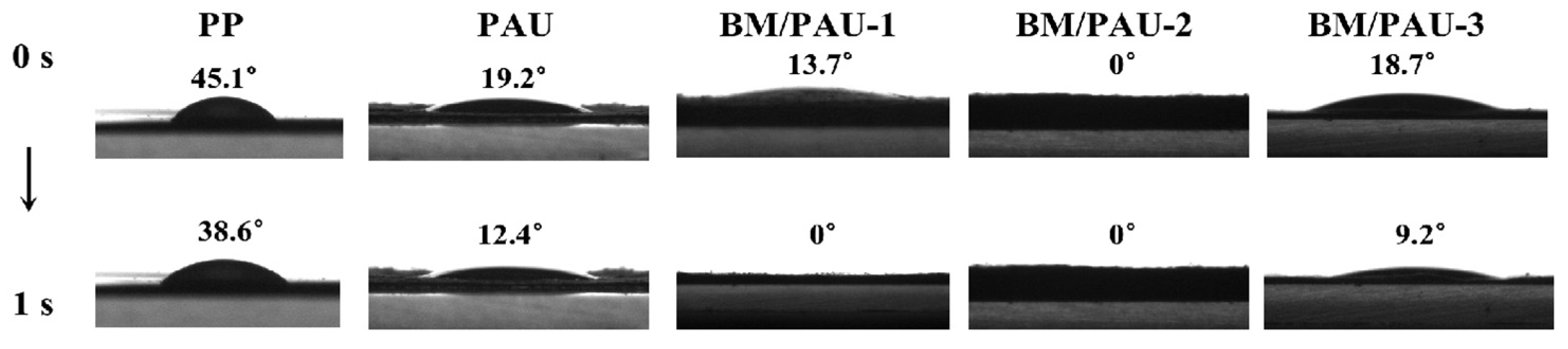
2.3. Electrolyte Affinity of ENSs
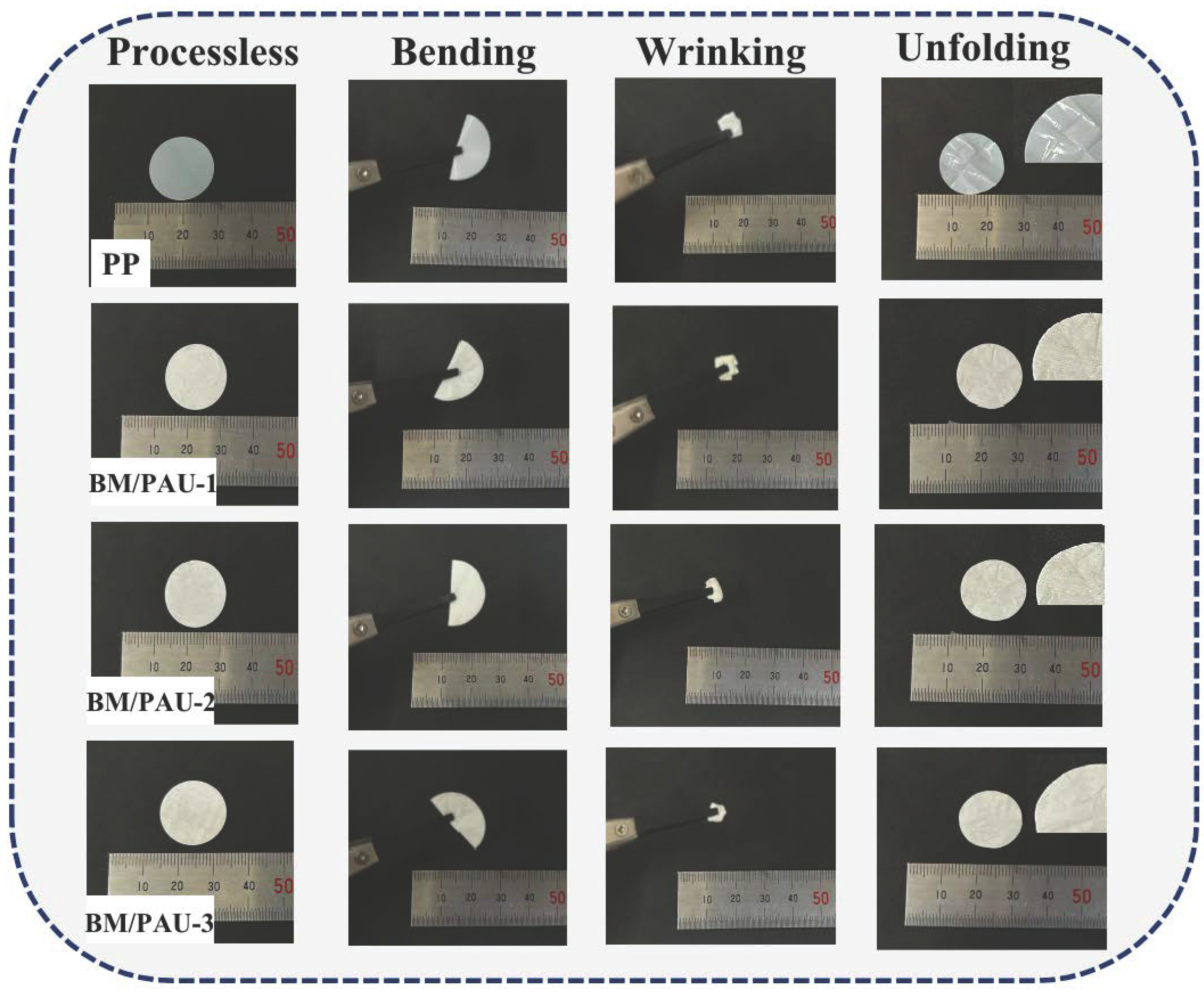
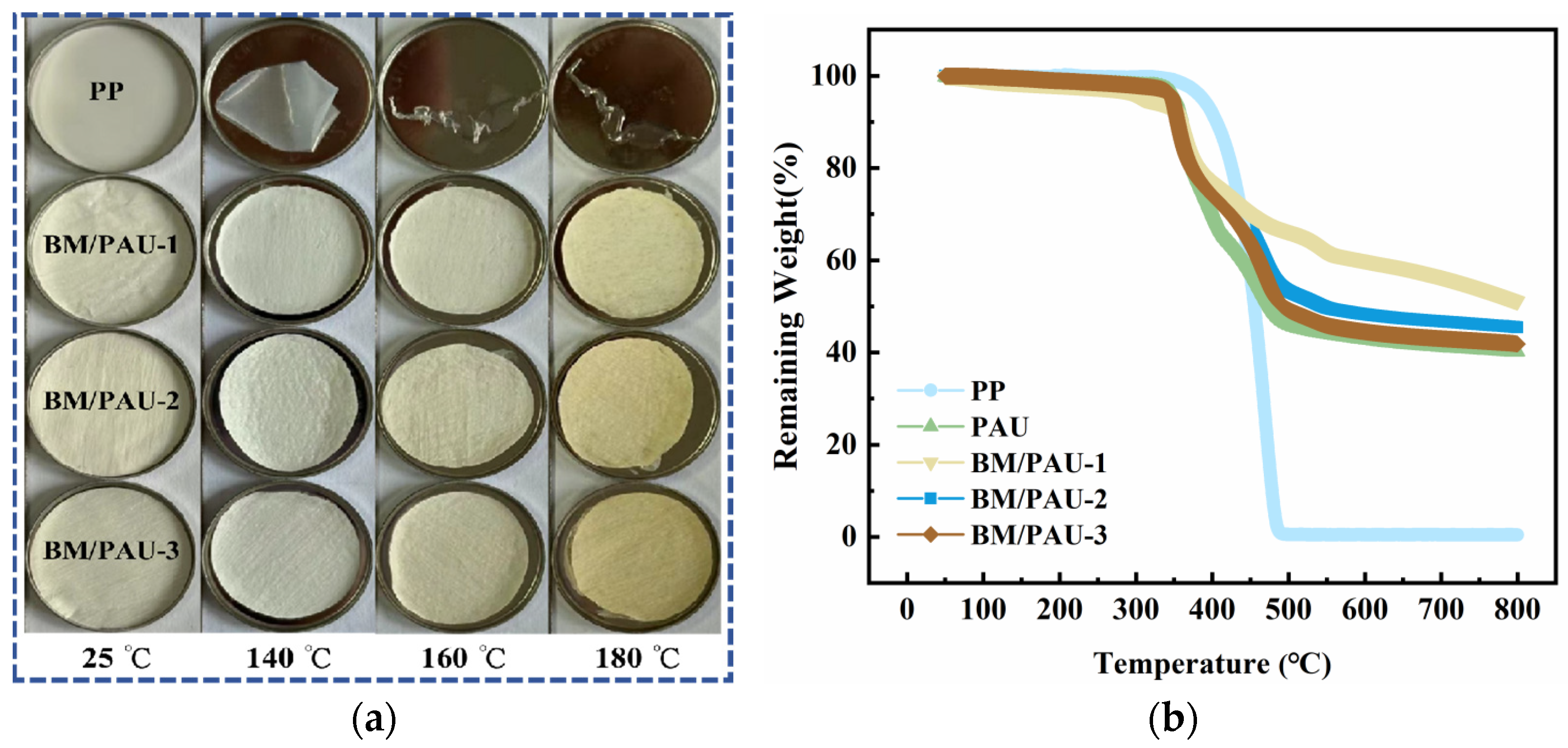
2.4. Flexibility and Thermal Stability of ENSs
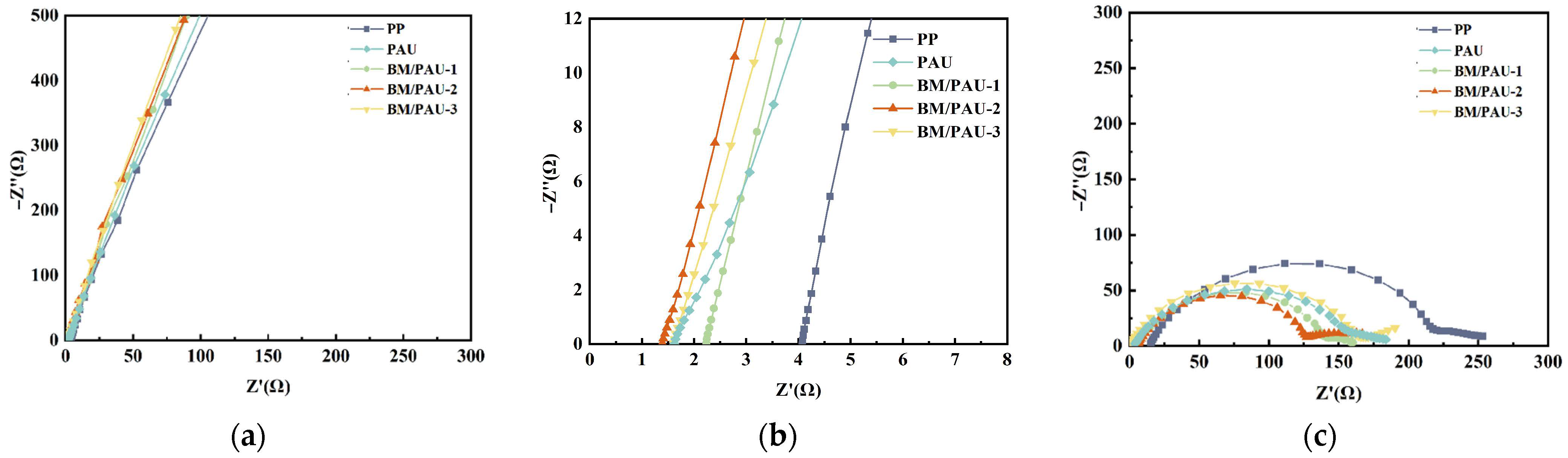
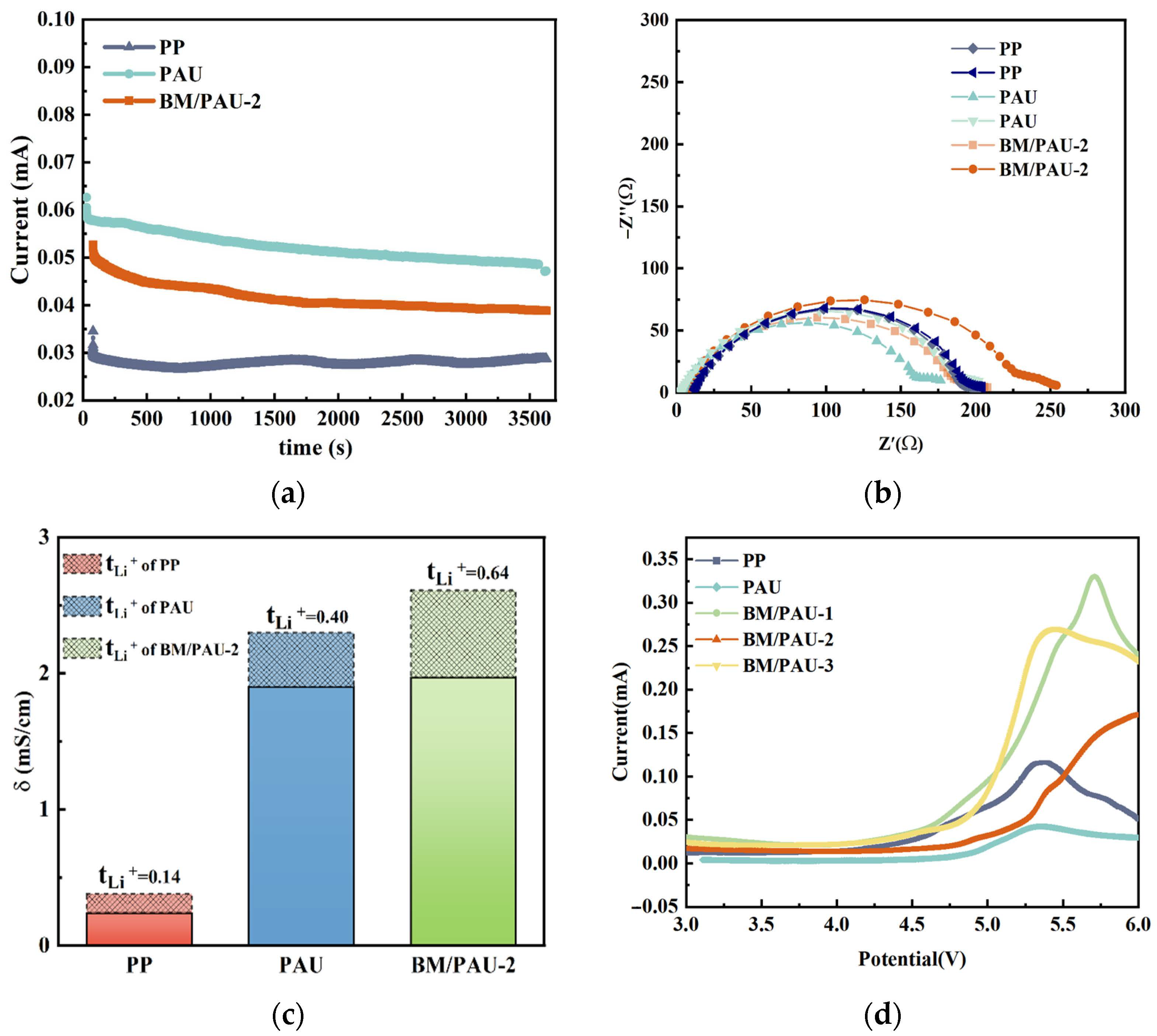
2.5. Electrochemical Performances
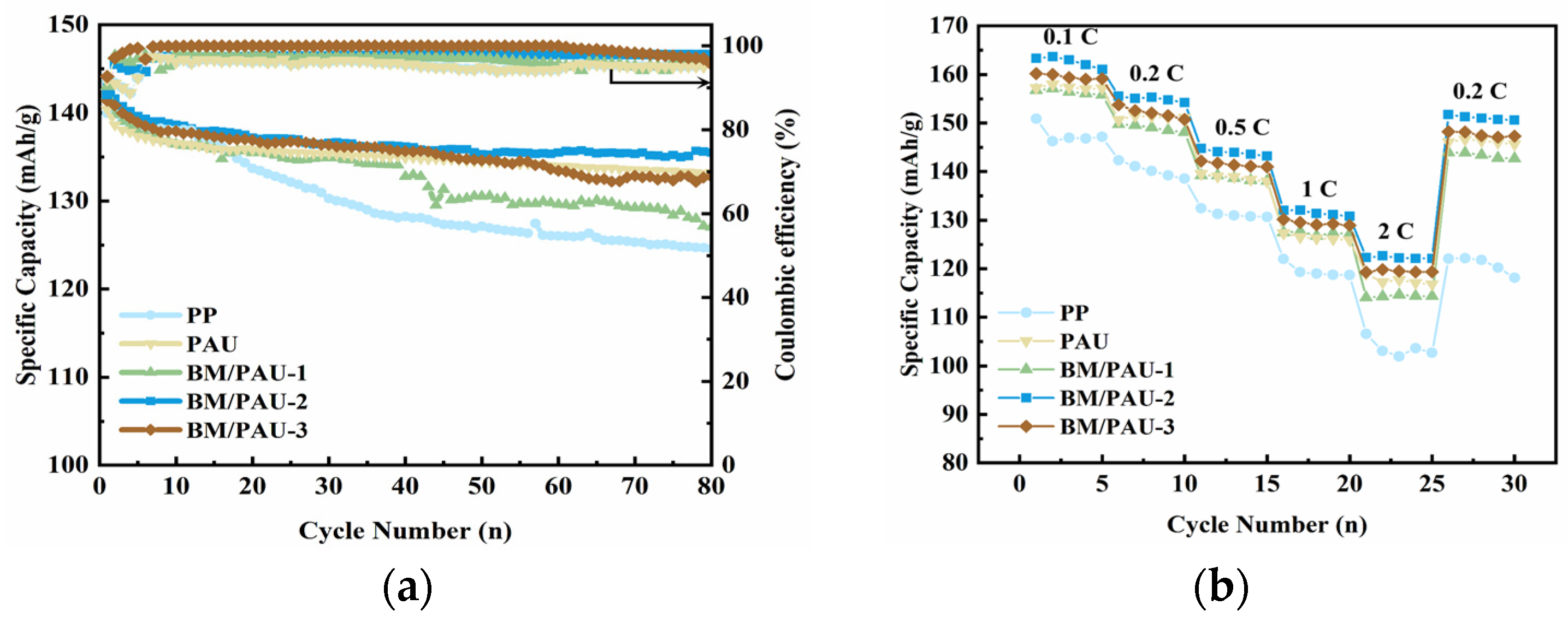
2.6. Cycling and Rate Performances
3. Materials and Methods
3.1. Preparation of BM Particles
3.2. Preparation of Spinning Solution
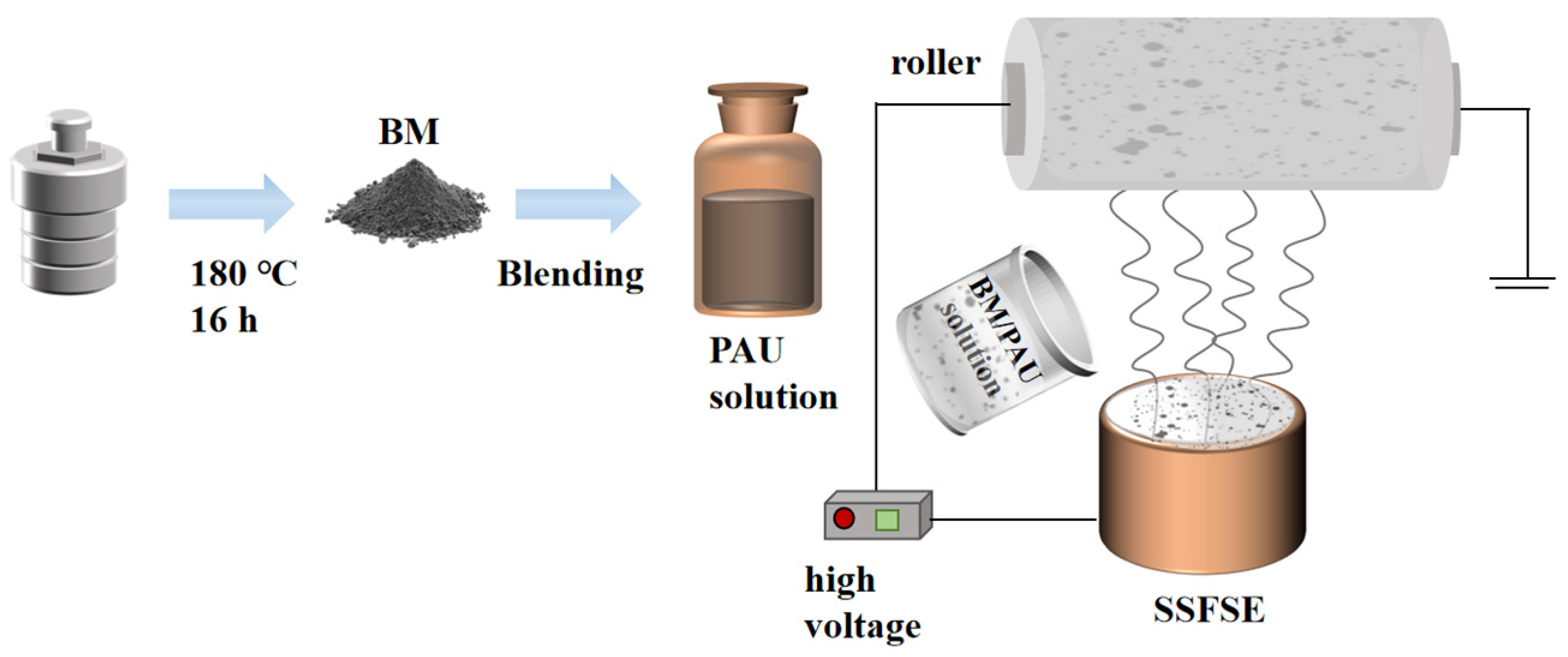
3.3. Batch Fabrication of Nanofiber Separators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Wang, Q.S.; Jiang, L.H.; Yu, Y.; Sun, J.H. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.; Jeong, H.; Kang, W.; Jo, C. A Review of Functional Separators for Lithium Metal Battery Applications. Materials 2020, 13, 4625. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.F.; Yuan, S.; Wang, Z.Y.; Shi, L.Y.; Jo, J.H.; Myung, S.T.; Zhu, J.F. Construction of silica-oxygen-borate hybrid networks on Al2O3-coated polyethylene separators realizing multifunction for high-performance lithium ion batteries. J. Power Sources 2020, 472, 228445. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xiang, Y.K.; Jamil, M.I.; Lu, J.G.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Polymers/zeolite nanocomposite membranes with enhanced thermal and electrochemical performances for lithium-ion batteries. J. Membr. Sci. 2018, 564, 753–761. [Google Scholar] [CrossRef]
- Xu, G.J.; Chen, X.; Zhu, Z.M.; Wu, P.X.; Wang, H.; Chen, X.D.; Gao, W.; Liu, Z. Pulse gas-assisted multi-needle electrospinning of nanofibers. Adv. Compos. Hybrid Mater. 2020, 3, 98–113. [Google Scholar]
- Wei, L.; Yu, H.N.; Sun, R.J.; Liu, C.K.; Chen, M.Y.; Liu, H.J.; Xiong, J.; Qin, X.H. Experimental investigation of process parameters for the filtration property of nanofiber membrane fabricated by needleless electrospinning apparatus. J. Ind. Text. 2021, 50, 1528–1541. [Google Scholar] [CrossRef]
- Ng, J.J.; Supaphol, P. Rotating-disk electrospinning: Needleless electrospinning of poly(caprolactone), poly(lactic acid) and poly(vinyl alcohol) nanofiber mats with controlled morphology. J. Polym. Sci. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Chen, R.X.; Wan, Y.Q.; Si, N.; He, J.H.; Ko, F.; Wang, S.Q. BUBBLE RUPTURE IN BUBBLE ELECTROSPINNING. Therm. Sci. 2015, 19, 1141–1149. [Google Scholar] [CrossRef]
- Yin, J.; Ahmed, A.; Xu, L. High-Throughput Free Surface Electrospinning Using Solution Reservoirs with Different Depths and Its Preparation Mechanism Study. Adv. Fiber Mater. 2021, 3, 251–264. [Google Scholar] [CrossRef]
- Ding, W.F.; Xu, L. Batch Fabrication of Electrospun PAN/PU Composite Separators for Safe Lithium-Ion Batteries. Batteries 2024, 10, 6. [Google Scholar] [CrossRef]
- Gao, T.T.; Tian, P.; Xu, Q.J.; Pang, H.C.; Ye, J.W.; Ning, G.L. Class of Boehmite/Polyacrylonitrile Membranes with Different Thermal Shutdown Temperatures for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 2112–2123. [Google Scholar] [CrossRef]
- Feng, K.Z.; Rong, D.Q.; Ren, W.A.; Wen, X.G. Hierarchical flower-like γ-AlOOH and γ-Al2O3 microspheres: Synthesis and adsorption properties. Mater. Express 2015, 5, 371–375. [Google Scholar] [CrossRef]
- Shayapat, J.; Chung, O.H.; Park, J.S. Electrospun polyimide-composite separator for lithium-ion batteries. Electrochim. Acta 2015, 170, 110–121. [Google Scholar] [CrossRef]
- Fu, W.J.; Wei, C.D.; Zuo, J.; Zhang, J.P.; Zhang, J.Y.; Xu, S.N. A Facile Temperature-Controlled “Green” Method to Prepare Multi-kinds of High-Quality Alumina Hydrates via a Ga-In-Sn-Alloyed Aluminum-Water Interface Reaction. ACS Omega 2022, 7, 19775–19783. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.M.; Young, C.J.; Wang, Y.W.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, S.; Huh, H. PU-RGO Composite; Effect of Chain Extender’s Structure on Properties. J. Nanosci. Nanotechnol. 2017, 17, 7480–7484. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Q.Q.; Luo, N.; Chen, F.; Fu, Q. High safety and electrochemical performance electrospun para-aramid nanofiber composite separator for lithium-ion battery. Compos. Sci. Technol. 2022, 225, 109479. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, M.D.; Cheng, Y.W.; Li, H.; Ji, G.J.; Chen, H.; Fu, X.G.; Awuye, D.E.; Zhu, Y.B.; Yin, X.C.; et al. Boehmite-enhanced poly(vinylidene fluoride-co-hexafluoropropylene)/polyacrylonitrile (PVDF-HFP/PAN) coaxial electrospun nanofiber hybrid membrane: A superior separator for lithium-ion batteries. Adv. Compos. Hybrid Mater. 2023, 6, 219. [Google Scholar] [CrossRef]
- Sun, X.L.; Xu, J.H.; Zhi, X.K.; Zhang, J.P.; Hou, K.W.; Bian, Y.H.; Li, X.L.; Wang, L.; Liang, G.C. Electrospun organically modified sepiolite/PVDF coating on polypropylene separator to improve electrochemical performance of lithium-ion battery. Express Polym. Lett. 2024, 18, 575–591. [Google Scholar] [CrossRef]
- Huang, J.N.; Cao, Y.H.; Huang, Z.Y.; Imbraguglio, S.A.; Wang, Z.; Peng, X.F.; Guo, Z. Comparatively Thermal and Crystalline Study of Poly(methyl-methacrylate)/Polyacrylonitrile Hybrids: Core-Shell Hollow Fibers, Porous Fibers, and Thin Films. Macromol. Mater. Eng. 2016, 301, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Feng, T.T.; Liang, Y.F.; Wu, M.Q. Construction of PMIA@PAN/PVDF-HFP/TiO2 coaxial fibrous separator with enhanced mechanical strength and electrolyte affinity for lithium-ion batteries. Chin. Chem. Lett. 2023, 34, 108350. [Google Scholar] [CrossRef]
- Surianarayanan, M.; Vijayaraghavan, R.; Raghavan, K.V. Spectroscopic investigations of polyacrylonitrile thermal degradation. J. Polym. Sci. A Polym. Chem. 1998, 36, 2503–2512. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Yang, K.S.; Aminabhavi, T.M. Polyacrylonitrile-based nanofibers A state-of-the-art review. Prog. Polym. Sci. 2012, 37, 487–513. [Google Scholar] [CrossRef]
- Pozyczka, K.; Marzantowicz, M.; Dygas, J.R.; Krok, F. Ionic conductivity and lithium transference number of poly (ethylene oxide): LiTFSI system. Electrochim. Acta. 2017, 227, 127–135. [Google Scholar] [CrossRef]
- Idrees, M.; Abbas, S.M.; Ata, U.R.; Ahmad, N.; Mushtaq, M.W.; Naqvi, R.A.; Nam, K.W.; Muhammad, B.; Iqbal, Z. Mechanistic insights into high lithium storage performance of mesoporous chromium nitride anchored on nitrogen-doped carbon nanotubes. Chem. Eng. J. 2017, 7, 361–370. [Google Scholar] [CrossRef]

| Spinning Solutions of Samples | Mass Ratio of BM and PAU | Spinning Solution Property | |
|---|---|---|---|
| Viscosity (mPa·s) | Conductivity (µS·cm−1) | ||
| PAU | / | 844 ± 7 | 678 ± 8 |
| BM/PAU-1 | 12:15 | 869 ± 5 | 649 ± 2 |
| BM/PAU-2 | 6:15 | 768 ± 2 | 550 ± 8 |
| BM/PAU-3 | 4:15 | 702 ± 7 | 526 ± 5 |
| Separators | PAU | BM/PAU-1 | BM/PAU-2 | BM/PAU-3 |
|---|---|---|---|---|
| Pore size (µm) | 0.79–1.04 | 2.61–3.71 | 2.45–3.41 | 2.38–3.18 |
| Average pore size (µm) | 0.98 | 2.90 | 2.72 | 2.60 |
| Pore count (/cm2) | 2373 | 1068 | 1580 | 1864 |
| Separators | PP | PAU | BM/PAU-1 | BM/PAU-2 | BM/PAU-3 |
|---|---|---|---|---|---|
| Thickness (mm) | 0.025 ± 0.002 | 0.083 ± 0.007 | 0.068 ± 0.005 | 0.070 ± 0.008 | 0.074 ± 0.010 |
| Rb (Ω) | 4.00 | 1.70 | 2.24 | 1.39 | 1.61 |
| δ (mS/cm) | 0.24 | 1.90 | 1.19 | 1.97 | 1.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Liu, Y.; Xu, L. Batch Preparation and Performance Study of Boehmite-Based Electrospun Nanofiber Separators for Lithium-Ion Batteries. Molecules 2024, 29, 3938. https://doi.org/10.3390/molecules29163938
Ding W, Liu Y, Xu L. Batch Preparation and Performance Study of Boehmite-Based Electrospun Nanofiber Separators for Lithium-Ion Batteries. Molecules. 2024; 29(16):3938. https://doi.org/10.3390/molecules29163938
Chicago/Turabian StyleDing, Wenfei, Yuxing Liu, and Lan Xu. 2024. "Batch Preparation and Performance Study of Boehmite-Based Electrospun Nanofiber Separators for Lithium-Ion Batteries" Molecules 29, no. 16: 3938. https://doi.org/10.3390/molecules29163938
APA StyleDing, W., Liu, Y., & Xu, L. (2024). Batch Preparation and Performance Study of Boehmite-Based Electrospun Nanofiber Separators for Lithium-Ion Batteries. Molecules, 29(16), 3938. https://doi.org/10.3390/molecules29163938






