Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol
Abstract
1. Introduction
2. Results and Discussion
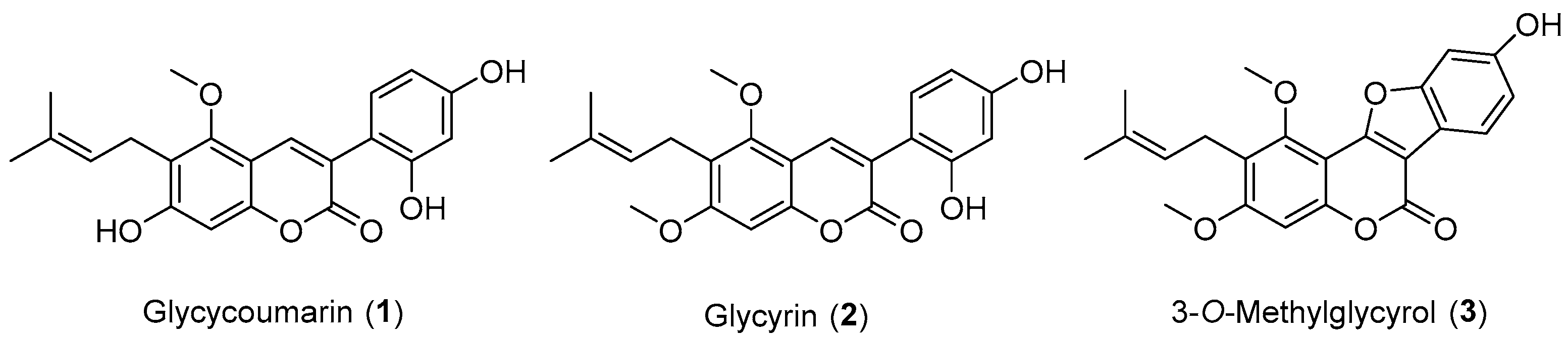
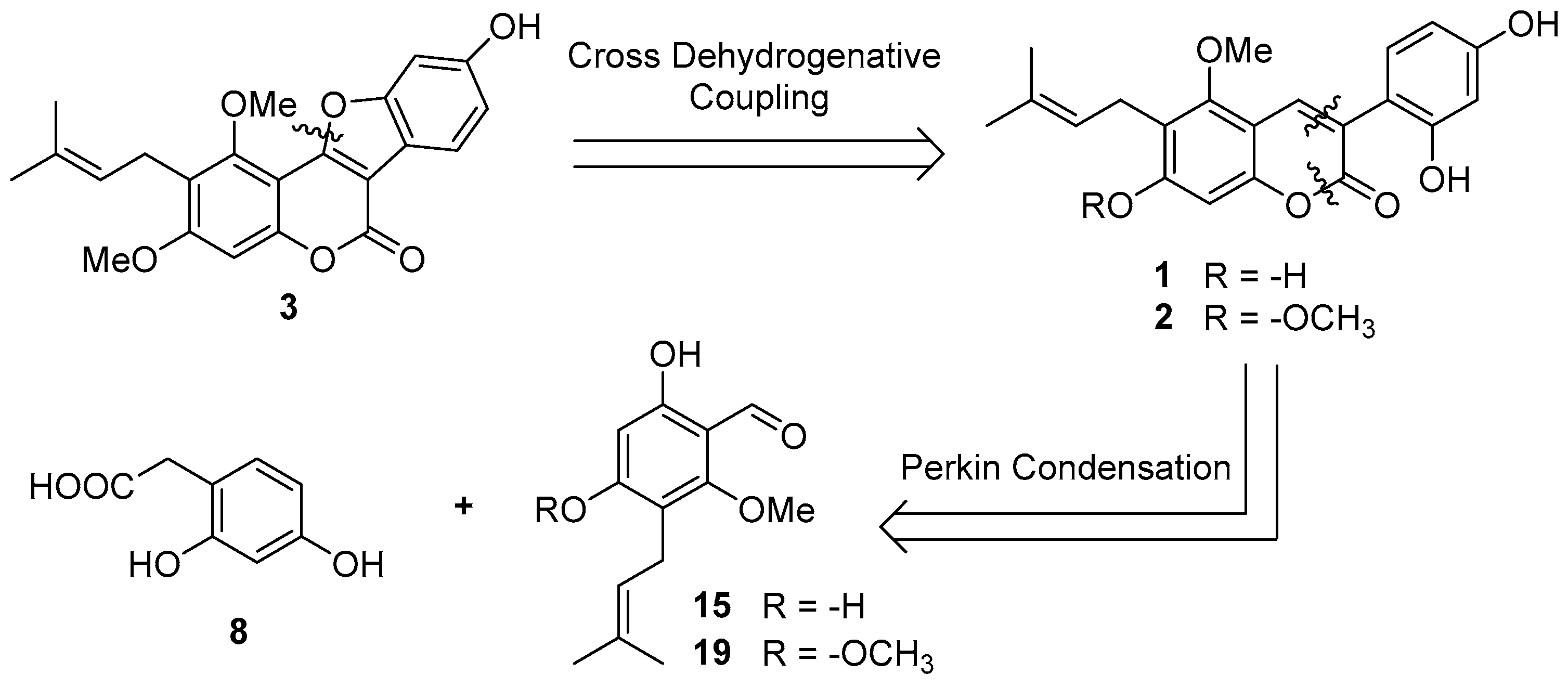
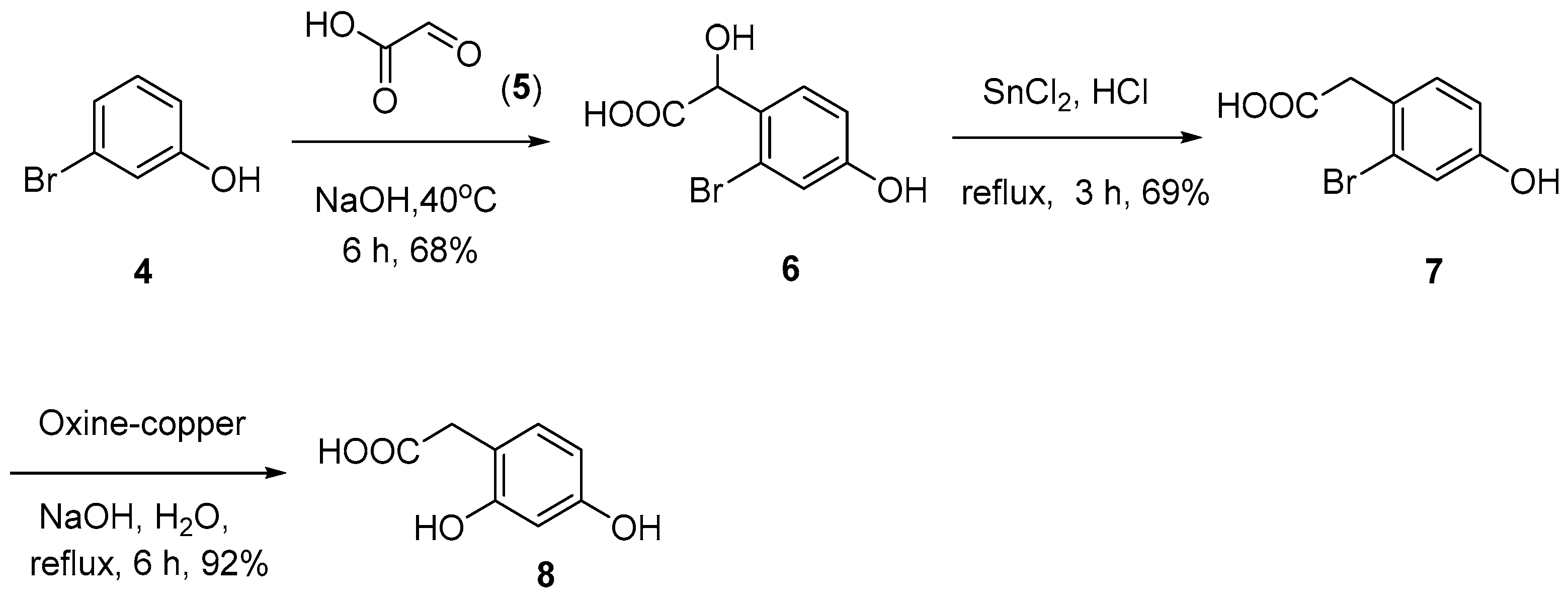
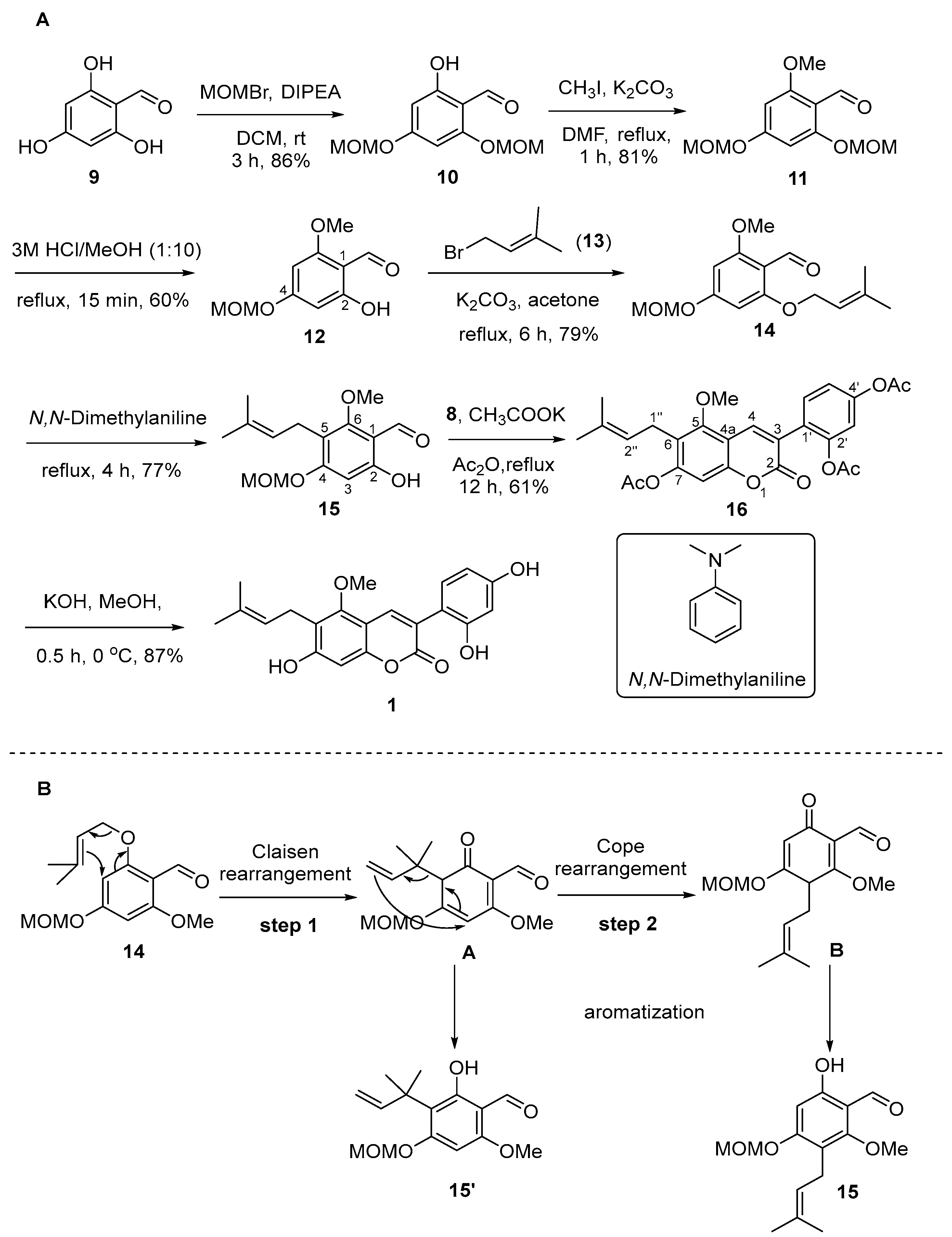
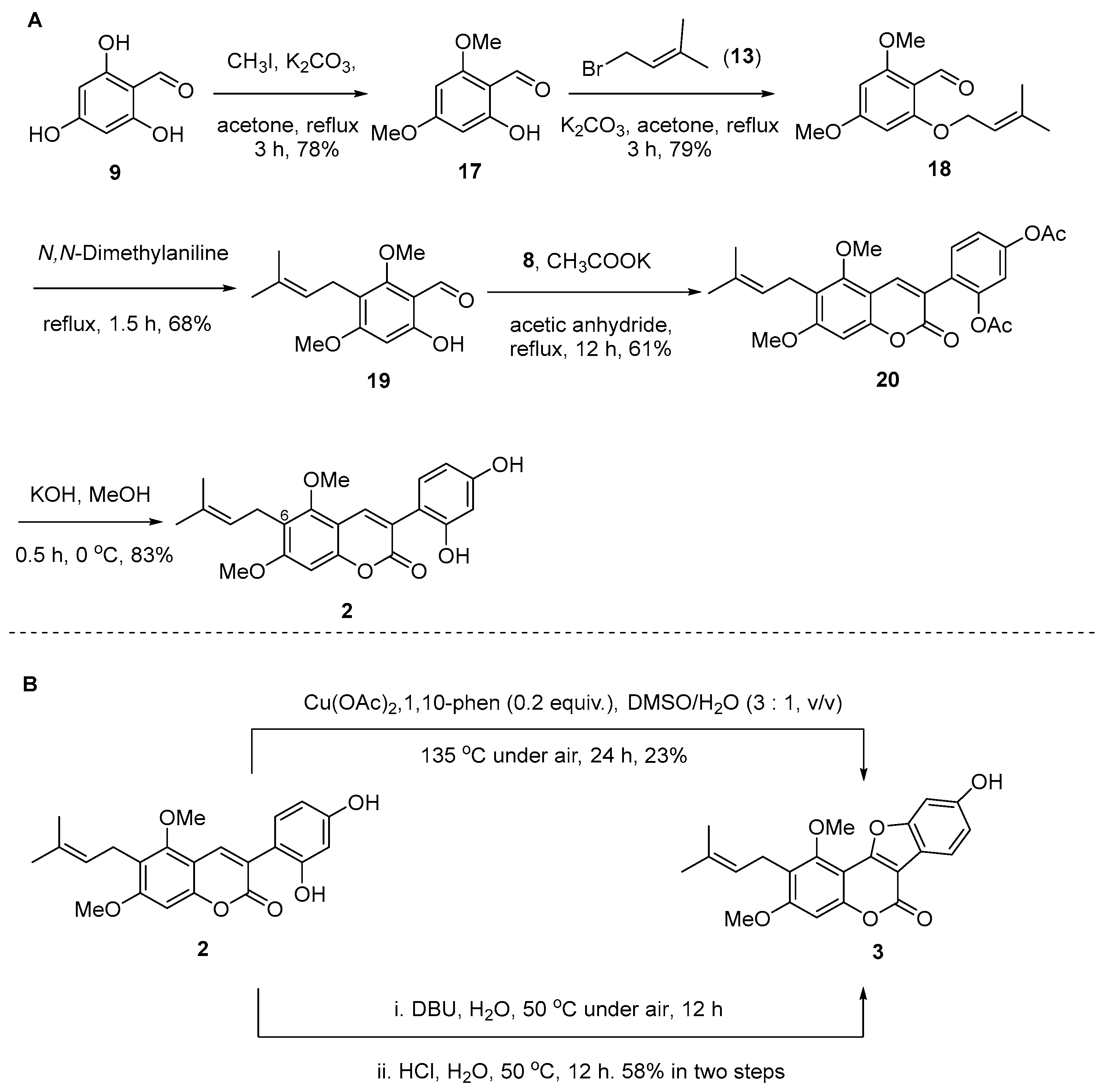
2.1. Synthesis of Natural Coumarins 1–3
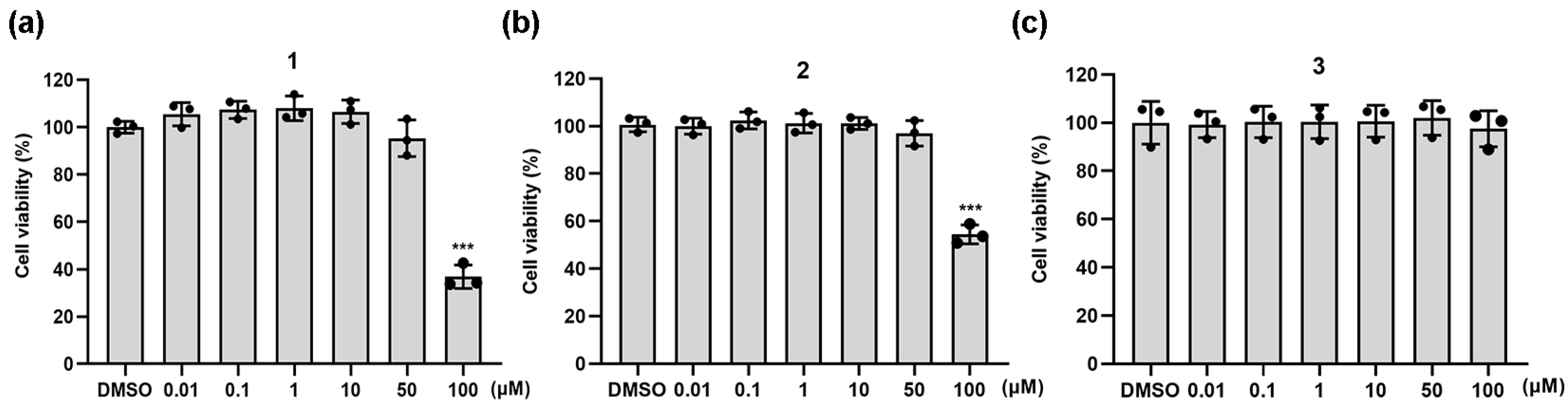
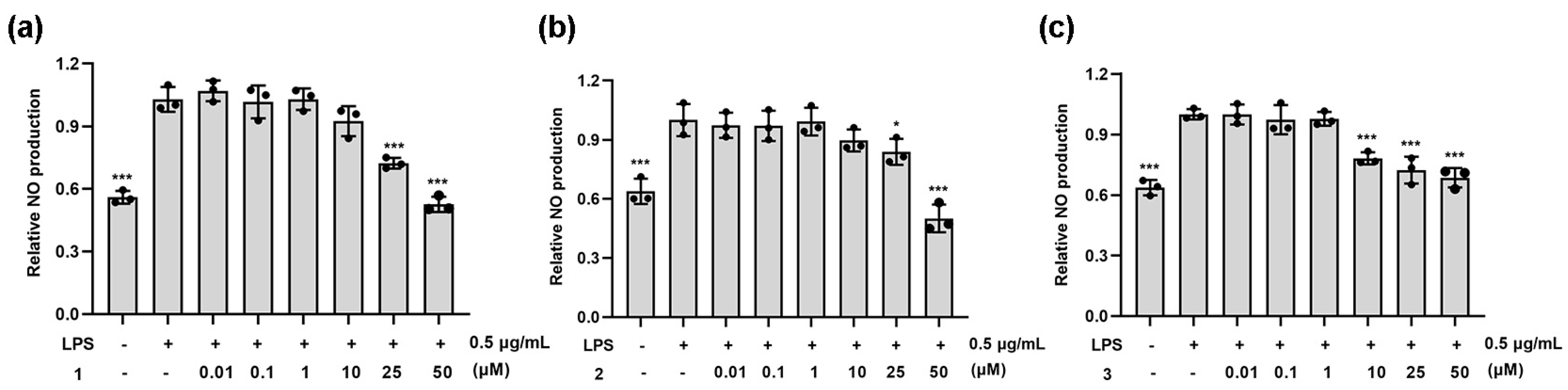
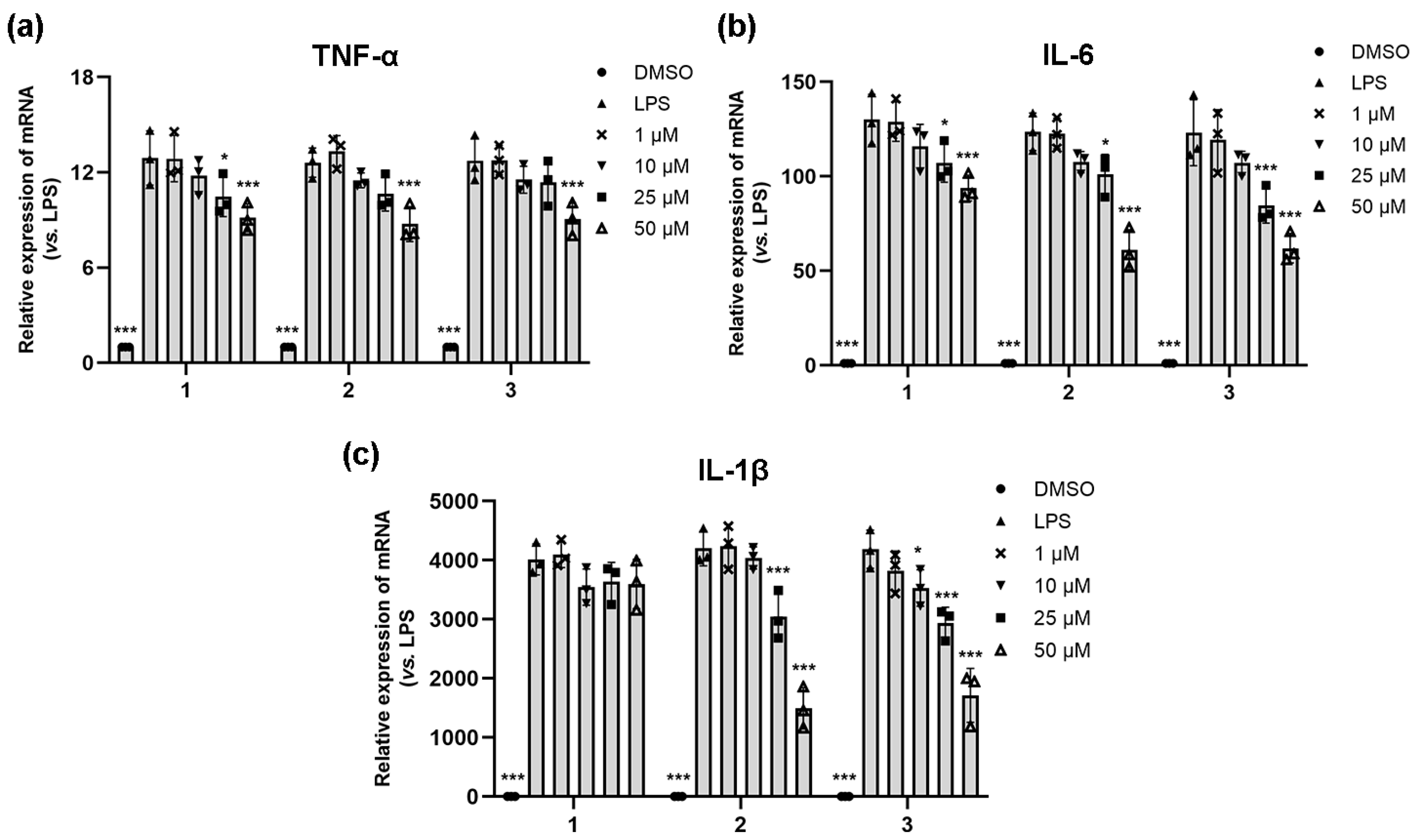
2.2. Anti-Inflammatory Activity of Natural Coumarins 1–3
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Synthesis and Characterization of the Compounds
3.2.1. 2-(2-Bromo-4-hydroxyphenyl)-2-hydroxyacetic Acid (6)
3.2.2. 2-(2-Bromo-4-hydroxyphenyl)acetic Acid (7)
3.2.3. 2-(2,4-Dihydroxyphenyl)acetic Acid (8)
3.2.4. 2-Hydroxy-4,6-bis(methoxymethoxy)benzaldehyde (10)
3.2.5. 2-Methoxy-4,6-bis(methoxymethoxy)benzaldehyde (11)
3.2.6. 2-Hydroxy-6-methoxy-4-(methoxymethoxy)benzaldehyde (12)
3.2.7. 2-Methoxy-4-(methoxymethoxy)-6-((3-methylbut-2-en-1-yl)oxy)benzaldehyde (14)
3.2.8. 6-Hydroxy-2-methoxy-4-(methoxymethoxy)-3-(3-methylbut-2-en-1-yl)benzaldehyde (15)
3.2.9. 4-(7-Acetoxy-5-methoxy-6-(3-methylbut-2-en-1-yl)-2-oxo-2H-chromen-3-yl)-1,3-phenylene Diacetate (16)
3.2.10. Glycycoumarin (1)
3.2.11. 2-Hydroxy-4,6-dimethoxybenzaldehyde (17)
3.2.12. 2,4-Dimethoxy-6-((3-methylbut-2-en-1-yl)oxy)benzaldehyde (18)
3.2.13. 6-Hydroxy-2,4-dimethoxy-3-(3-methylbut-2-en-1-yl)benzaldehyde (19)
3.2.14. 4-(5,7-Dimethoxy-6-(3-methylbut-2-en-1-yl)-2-oxo-2H-chromen-3-yl)-1,3-phenylene Diacetate (20)
3.2.15. Glycyrin (2)
3.2.16. 3-O-Methylglycyrol (3)
3.2.17. Cell Culture
3.2.18. Cell Viability Assay
3.2.19. NO Determination
3.2.20. Real-Time Quantitative PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Kang, S.-M.; Kim, Y.-J.; Lee, I.-J. Exploring the dietary and therapeutic potential of licorice (Glycyrrhiza uralensis Fisch.) sprouts. J. Ethnopharmacol. 2024, 328, 118101. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive constituents of Glycyrrhiza uralensis (licorice): Discovery of the effective components of a traditional herbal medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, K.; Narsaiah, A.V. Total synthesis of natural alkaloids Schwarzinicines A-D. Tetrahedron 2024, 155, 133909. [Google Scholar] [CrossRef]
- Zhu, D.-Y.; Song, G.-Q.; Jian, F.-X.; Chang, X.-R.; Guo, W.-B. Studies on chemical constituents of Glycyrrhiza uralensis Fisch. Acta Chim. Sin. 1984, 42, 1080–1084. [Google Scholar]
- Zang, Y. Pharmacological activities of coumarin compounds in licorice: A review. Nat. Prod. Commun. 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.; Zheng, Y.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food. Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yin, S.; Huo, Y.; Liang, M.; Fan, L.; Ye, M.; Hu, H. Glycycoumarin ameliorates alcohol-induced hepatotoxicity via activation of Nrf2 and autophagy. Free Radic. Biol. Med. 2015, 89, 135–146. [Google Scholar] [CrossRef]
- Yan, M.; Ye, L.; Yin, S.; Lu, X.; Liu, X.; Lu, S.; Cui, J.; Fan, L.; Kaplowitz, N.; Hu, H. Glycycoumarin protects mice against acetaminophen-induced liver injury predominantly via activating sustained autophagy. Brit. J. Pharmacol. 2018, 175, 3747–3757. [Google Scholar] [CrossRef]
- Kinoshita, T.; Saitoh, T.; Shibata, S. A new 3-Arylcoumarin from licorice root. Chem. Pharm. Bull. 1978, 26, 135–140. [Google Scholar] [CrossRef]
- Shiozawa, T.; Urata, S.; Kinoshita, T.; Saitoh, T. Revised structures of glycyrol and isoglycyrol, constituents of the root of Glycyrrhiza uralensis. Chem. Pharm. Bull. 1989, 39, 2239–2240. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Sashida, Y.; Mae, T.; Kishida, H.; Nishiyama, T.; Tsukagawa, M.; Konishi, E.; Takahashi, K.; Kawada, T.; et al. Phenolics with PPAR-gamma ligand-binding activity obtained from licorice (Glycyrrhiza uralensis roots) and ameliorative effects of glycyrin on genetically diabetic KK-A(y) mice. Bioorg. Med. Chem. Lett. 2003, 13, 4267–4272. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kikuzaki, H.; Fukuda, S.; Nakatani, N. Antibacterial compounds of licorice against upper airway respiratory tract pathogens. J. Nutr. Sci. Vitaminol. 2001, 47, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xu, Y.; Duan, X.; Qin, X.; Huang, L.; Zhang, L. Method for Preparing 7,3(2′,4′)-trihydroxy-5-methoxy-6-isopentenyl coumarin. CN 116903570A, 20 October 2023. [Google Scholar]
- Dong, H.; Wu, M.; Wang, Y.; Du, W.; He, Y.; Shi, Z. Total syntheses and anti-inflammatory activities of syringin and its natural analogues. J. Nat. Prod. 2021, 84, 2866–2874. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wu, M.; Li, Y.; Lu, L.; Qin, J.; He, Y.; Shi, Z. Total syntheses and anti-inflammatory evaluations of pongamosides A−C, natural furanoflavonoid glucosides from fruit of Pongamia pinnata (L.) Pierre. J. Nat. Prod. 2022, 85, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Long, B.; Feng, C.; Yang, T.; Jiang, X.; He, Y.; Dong, H. Total syntheses of pongaflavone and its natural analogues. J. Asian Nat. Prod. Res. 2023, 25, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Jiang, Y.; Song, X.; Lu, G.; Zhang, Q.; Du, Z.; Chan, A.S.C.; Zou, Y. Synthesis of phenolic coumestans via a sequential Dehydrogenation/Oxa-Michael addition reaction of 2′,4′-dihydroxyl-3-arylcoumarins. J. Org. Chem. 2022, 87, 5785–5794. [Google Scholar] [CrossRef]
- Dong, H.; Liao, L.; Long, B.; Che, Y.; Peng, T.; He, Y.; Mei, L.; Xu, B. Total synthesis and antibacterial evaluation of lupinifolin and its natural analogues. J. Nat. Prod. 2024, 87, 1044–1058. [Google Scholar] [CrossRef]
- Dong, H.; Che, Y.; Zhu, X.; Zhong, Y.; Lin, J.; Wang, J.; Du, W.; Song, T. Total syntheses and antibacterial studies of natural isoflavones: Scandenone, osajin, and 6,8-diprenylgenistein. Molecules 2024, 29, 2574. [Google Scholar] [CrossRef]
- Nchiozem-Ngnitedem, V.; Sperlich, E.; Matieta, V.Y.; Kuete, J.R.N.; Kuete, V.; Omer, E.A.; Efferth, T.; Schmidt, B. Synthesis and bioactivity of isoflavones from Ficus carica and some non-natural analogues. J. Nat. Prod. 2023, 86, 1520–1528. [Google Scholar] [CrossRef]
- Song, X.; Luo, X.; Sheng, J.; Li, J.; Zhu, Z.; Du, Z.; Miao, H.; Yan, M.; Li, M.; Zou, Y. Copper-catalyzed intramolecular cross dehydrogenative coupling approach to coumestans from 2′-hydroxyl-3-arylcoumarins. Rsc. Adv. 2019, 9, 17391–17398. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xu, T.; Zhang, E.; Zhang, X.; Wei, W.; Zou, Y. Synthesis of coumestrol and aureol. J. Nat. Prod. 2016, 79, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- Robledo-O’Ryan, N.; Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Moncada-Basualto, M.; Mura, F.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis, antioxidant and antichagasic properties of a selected series of hydroxy-3-arylcoumarins. Bioorgan. Med. Chem. 2017, 25, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.; Liu, J.; He, M.; Qiang, Y.; Ni, J. Quantification and bio-assay of α-glucosidase inhibitors from the roots of Glycyrrhiza uralensis Fisch. Nat. Prod. Res. 2016, 30, 2130–2134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, D.; Gao, Z.; Kan, H.; Qiu, F.; Chen, L.; Li, H. Licocoumarone induces BxPC-3 pancreatic adenocarcinoma cell death by inhibiting DYRK1A. Chem-Biol. Interact. 2020, 316, 108913. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-L.; Raja, A.; Hong, B.-C.; Lee, O.-H. Organocatalytic Enantioselective Michael–Acetalization–Reduction–Nef Reaction for a One-Pot Entry to the Functionalized Aflatoxin System. Total Synthesis of (−)-Dihydroaflatoxin D2 and (−)-and (+)-Microminutinin. Org. Lett. 2017, 19, 3494–3497. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Riemer, M. Synthesis of allyl- and prenylcoumarins via microwave-promoted tandem claisen rearrangement/wittig olefination. Synthesis 2016, 48, 141–149. [Google Scholar] [CrossRef]
- Mali, R.S.; Joshi, P.P.; Sandhua, P.K.; Manekar-Tilve, A. Efficient syntheses of 6-prenylcoumarins and linear pyranocoumarins: Total synthesis of suberosin, toddaculin, O-methylapigravin (O-methylbrosiperin), O-methylbalsamiferone, dihydroxanthyletin, xanthyletin and luvangetin. J. Chem. Soc. Perkin Trans. 2002, 1, 371–376. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
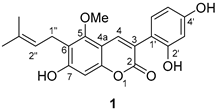 | |||
|---|---|---|---|
| No. | Natural Product (δ1) 1 | Synthetic Sample (δ1) 1 | ∆ = δ1 − δ2 |
| 2 | 159.9 | 160.0 | −0.1 |
| 3 | 120.3 | 120.3 | 0 |
| 4 | 136.3 | 136.4 | −0.1 |
| 4a | 106.1 | 106.2 | −0.1 |
| 5 | 158.3 | 158.3 | 0 |
| 6 | 113.4 | 113.4 | 0 |
| 7 | 159.2 | 159.2 | 0 |
| 8 | 97.8 | 97.9 | −0.1 |
| 8a | 152.9 | 152.9 | 0 |
| 1′ | 118.3 | 118.3 | 0 |
| 2′ | 155.9 | 156.0 | −0.1 |
| 3′ | 102.6 | 102.6 | 0 |
| 4′ | 155.2 | 155.2 | 0 |
| 5′ | 105.8 | 106.1 | −0.3 |
| 6′ | 131.5 | 131.5 | 0 |
| 1″ | 22.2 | 22.3 | −0.1 |
| 2″ | 122.6 | 122.6 | 0 |
| 3″ | 130.6 | 130.7 | −0.1 |
| 4″ | 17.7 | 17.7 | 0 |
| 5″ | 25.4 | 25.4 | 0 |
| -OCH3 | 62.7 | 62.8 | −0.1 |
 | 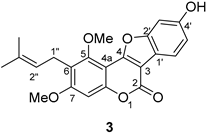 | |||||
|---|---|---|---|---|---|---|
| No. | Natural Product (δ1) 1 | Synthetic Sample (δ1) 1 | ∆ = δ1 − δ2 | Natural Product (δ1) 1 | Synthetic Sample (δ1) 1 | ∆ = δ1 − δ2 |
| 2 | 160.9 | 160.9 | 0 | 157.9 | 157.9 | 0 |
| 3 | 121.8 | 121.9 | −0.1 | 103.4 | 103.4 | 0 |
| 4 | 136.4 | 136.6 | −0.2 | 158.3 | 158.3 | 0 |
| 4a | 107.5 | 107.5 | 0 | 101.3 | 101.3 | 0 |
| 5 | 155.0 | 155.0 | 0 | 153.6 | 153.6 | 0 |
| 6 | 119.6 | 119.6 | 0 | 121.0 | 121.0 | 0 |
| 7 | 158.9 | 159.0 | −0.1 | 161.0 | 161.0 | 0 |
| 8 | 95.6 | 95.7 | −0.1 | 97.0 | 96.9 | −0.1 |
| 8a | 153.7 | 153.8 | −0.1 | 153.8 | 153.8 | 0 |
| 1′ | 113.7 | 113.7 | 0 | 114.6 | 114.6 | 0 |
| 2′ | 156.5 | 156.6 | −0.1 | 156.7 | 156.7 | 0 |
| 3′ | 103.1 | 103.2 | −0.1 | 99.0 | 99.0 | 0 |
| 4′ | 160.3 | 160.4 | −0.1 | 157.6 | 157.6 | 0 |
| 5′ | 106.7 | 106.7 | 0 | 114.6 | 114.6 | 0 |
| 6′ | 132.0 | 132.1 | −0.1 | 121.1 | 121.1 | 0 |
| 1″ | 22.7 | 22.7 | 0 | 22.5 | 22.4 | 0.1 |
| 2″ | 122.7 | 122.8 | −0.1 | 122.6 | 122.6 | 0 |
| 3″ | 131.5 | 131.6 | −0.1 | 131.8 | 131.8 | 0 |
| 4″ | 18.1 | 18.2 | −0.1 | 18.1 | 18.2 | −0.1 |
| 5″ | 25.8 | 25.9 | −0.1 | 25.9 | 25.9 | 0 |
| -OCH3 | 63.4 | 63.4 | 0 | 57.0 | 57.0 | 0 |
| -OCH3 | 56.8 | 56.9 | −0.1 | 63.0 | 63.0 | 0 |
| Name | Sequence (5′-3′) |
|---|---|
| TNF | Forward 5′- GCCTCTTCTCATTCCTGCTTGTGG -3′ |
| Reverse 5′- GTGGTTTGTGAGTGTGAGGGTCTG -3′ | |
| IL-6 | Forward 5′- CTTCTTGGGACTGATGCTGGTGAC -3′ |
| Reverse 5′- AGGTCTGTTGGGAGTGGTATCCTC -3′ | |
| IL-1β | Forward 5′- TCGCAGCAGCACATCAACAAGAG -3′ |
| Reverse 5′- AGGTCCACGGGAAAGACACAGG -3′ | |
| GAPDH | Forward 5′- GGCAAATTCAACGGCACAGTCAAG -3′ |
| Reverse 5′- TCGCTCCTGGAAGATGGTGATGG -3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.; Long, B.; Yang, X.; Wang, N.; Wang, X.; He, Y.; Dong, H. Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol. Molecules 2024, 29, 3942. https://doi.org/10.3390/molecules29163942
Peng T, Long B, Yang X, Wang N, Wang X, He Y, Dong H. Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol. Molecules. 2024; 29(16):3942. https://doi.org/10.3390/molecules29163942
Chicago/Turabian StylePeng, Ting, Bin Long, Xiuli Yang, Na Wang, Ximeng Wang, Yujiao He, and Hongbo Dong. 2024. "Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol" Molecules 29, no. 16: 3942. https://doi.org/10.3390/molecules29163942
APA StylePeng, T., Long, B., Yang, X., Wang, N., Wang, X., He, Y., & Dong, H. (2024). Total Syntheses and Anti-Inflammatory Studies of Three Natural Coumarins: Glycycoumarin, Glycyrin, and 3-O-Methylglycyrol. Molecules, 29(16), 3942. https://doi.org/10.3390/molecules29163942






