Differences in the Renal Accumulation of Radiogallium-Labeled (Glu)14 Peptides Containing Different Optical Isomers of Glutamic Acid
Abstract
:1. Introduction
2. Results
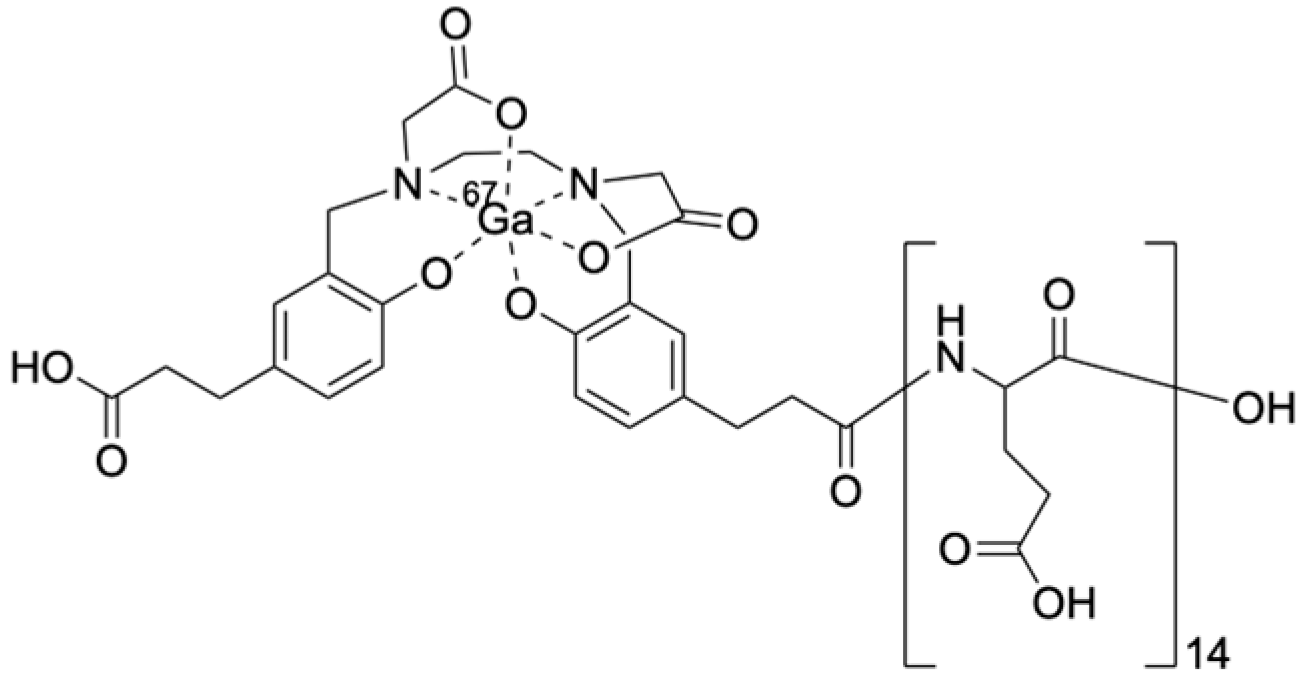
2.1. Synthesis of [67Ga]Ga-HBED-CC-(d-Glu)14, [67Ga]Ga-HBED-CC-(dl-Glu)14, and [67Ga]Ga-HBED-CC-(d-Glu-l-Glu)7
2.2. Hydroxyapatite-Binding Assay
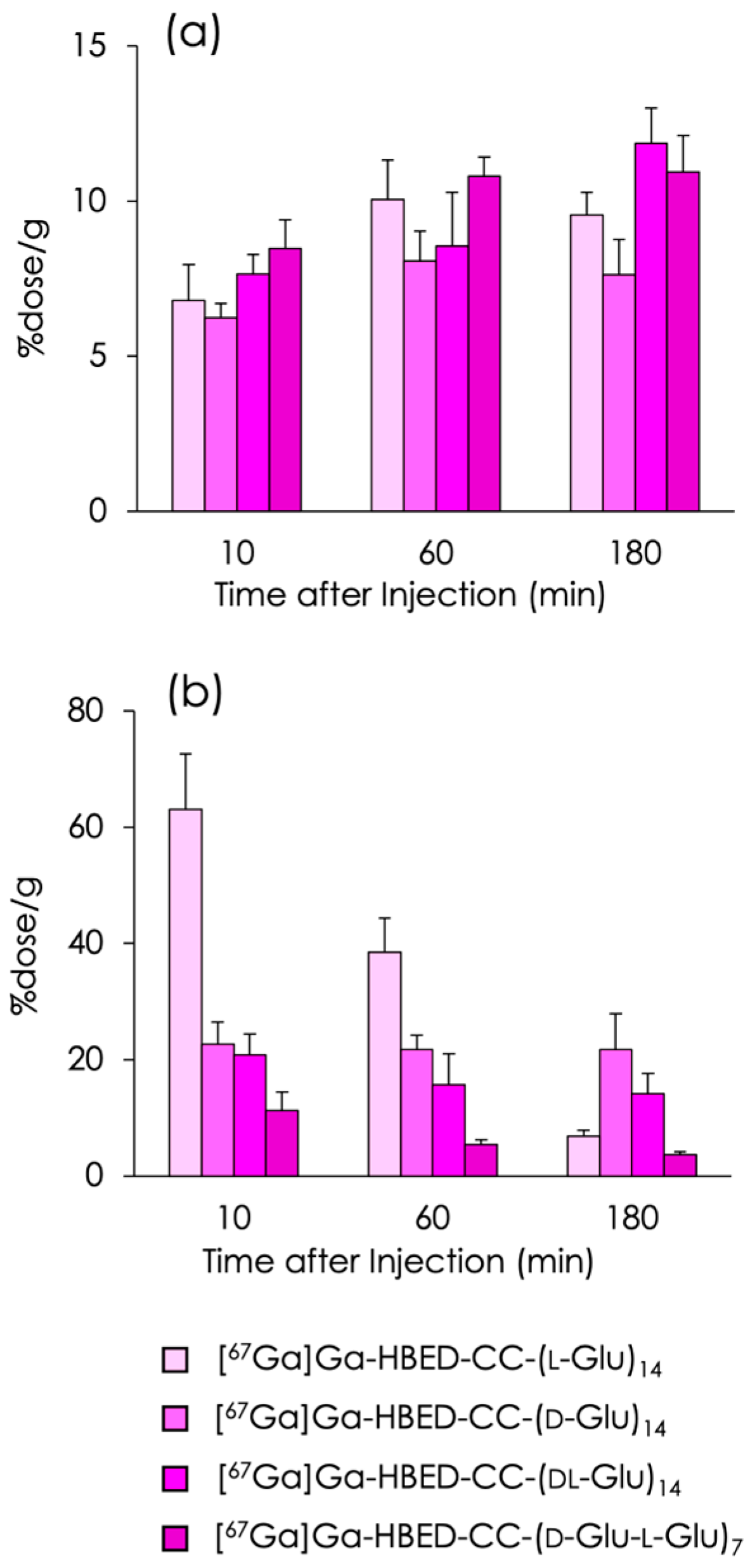
2.3. Biodistribution Experiments
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HBED-CC-(d-Glu)14, HBED-CC-(dl-Glu)14, and HBED-CC-(d-Glu-l-Glu)7
- MS (ESI+) analysis of HBED-CC-(d-Glu)14 calcd for C96H132N16O52 [M + 2H]2+: m/z = 1170.9 found 1170.8, yield: 3%.
- MS (ESI+) analysis of HBED-CC-(dl-Glu)14 calcd for C96H132N16O52 [M + 2H]2+: m/z = 1170.9 found 1170.9, yield: 3%.
- MS (ESI+) analysis of HBED-CC-(d-Glu-l-Glu)7 calcd for C96H132N16O52 [M + 2H]2+: m/z = 1170.9 found 1170.7, yield: 1%.
4.3. Preparation of [67Ga]Ga-HBED-CC-(d-Glu)14, [67Ga]Ga-HBED-CC-(dl-Glu)14, and [67Ga]Ga-HBED-CC-(d-Glu-l-Glu)7
4.4. Hydroxyapatite-Binding Assays
4.5. Animals
4.6. Biodistribution Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogawa, K.; Ishizaki, A. Well-designed bone-seeking radiolabeled compounds for diagnosis and therapy of bone metastases. BioMed Res. Int. 2015, 2015, 676053. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Saji, H. Advances in drug design of radiometal-based imaging agents for bone disorders. Int. J. Mol. Imaging 2011, 2011, 537687. [Google Scholar] [CrossRef]
- Cook, G.J.R. Imaging of Bone Metastases in Breast Cancer. Semin. Nucl. Med. 2022, 52, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.M.; Pinkerton, T.C. Determination of charge and size of technetium diphosphonate complexes by anion-exchange liquid chromatography. Anal. Chem. 1985, 57, 246–253. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Hanaoka, H.; Hashimoto, K.; Nishimura, H.; Saji, H. Design of a radiopharmaceutical for the palliation of painful bone metastases: Rhenium-186-labeled bisphosphonate derivative. J. Labelled Cmp. Radiopharm. 2004, 47, 753–761. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Ono, M.; Hanaoka, H.; Ishino, S.; Hashimoto, K.; Nishimura, H.; Saji, H. Development of a rhenium-186-labeled MAG3-conjugated bisphosphonate for the palliation of metastatic bone pain based on the concept of bifunctional radiopharmaceuticals. Bioconjug. Chem. 2005, 16, 751–757. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Arano, Y.; Otaka, A.; Ueda, M.; Uehara, T.; Magata, Y.; Hashimoto, K.; Saji, H. Rhenium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation. Nucl. Med. Biol. 2006, 33, 513–520. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Inoue, Y.; Ono, M.; Saji, H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. J. Nucl. Med. 2006, 47, 2042–2047. [Google Scholar]
- Ogawa, K.; Mukai, T.; Asano, D.; Kawashima, H.; Kinuya, S.; Shiba, K.; Hashimoto, K.; Mori, H.; Saji, H. Therapeutic effects of a 186Re-complex-conjugated bisphosphonate for the palliation of metastatic bone pain in an animal model. J. Nucl. Med. 2007, 48, 122–127. [Google Scholar]
- Ogawa, K.; Kawashima, H.; Shiba, K.; Washiyama, K.; Yoshimoto, M.; Kiyono, Y.; Ueda, M.; Mori, H.; Saji, H. Development of [90Y]DOTA-conjugated bisphosphonate for treatment of painful bone metastases. Nucl. Med. Biol. 2009, 36, 129–135. [Google Scholar] [CrossRef]
- Ogawa, K.; Mukai, T.; Kawai, K.; Takamura, N.; Hanaoka, H.; Hashimoto, K.; Shiba, K.; Mori, H.; Saji, H. Usefulness of competitive inhibitors of protein binding for improving the pharmacokinetics of 186Re-MAG3-conjugated bisphosphonate (186Re-MAG3-HBP), an agent for treatment of painful bone metastases. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Takai, K.; Kanbara, H.; Kiwada, T.; Kitamura, Y.; Shiba, K.; Odani, A. Preparation and evaluation of a radiogallium complex-conjugated bisphosphonate as a bone scintigraphy agent. Nucl. Med. Biol. 2011, 38, 631–636. [Google Scholar] [CrossRef]
- Uehara, T.; Jin, Z.L.; Ogawa, K.; Akizawa, H.; Hashimoto, K.; Nakayama, M.; Arano, Y. Assessment of 186Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bones. Nucl. Med. Biol. 2007, 34, 79–87. [Google Scholar] [CrossRef]
- Suzuki, K.; Satake, M.; Suwada, J.; Oshikiri, S.; Ashino, H.; Dozono, H.; Hino, A.; Kasahara, H.; Minamizawa, T. Synthesis and evaluation of a novel 68Ga-chelate-conjugated bisphosphonate as a bone-seeking agent for PET imaging. Nucl. Med. Biol. 2011, 38, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Fellner, M.; Baum, R.P.; Kubicek, V.; Hermann, P.; Lukes, I.; Prasad, V.; Rosch, F. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: First human study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 834. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Wu, Z.; Choi, S.R.; Ploessl, K.; Smith, M.; Alexoff, D.; Zhu, L.; Kung, H.F. A New [68Ga]Ga-HBED-CC-Bisphosphonate as a Bone Imaging Agent. Mol. Pharm. 2020, 17, 1674–1684. [Google Scholar] [CrossRef]
- Jin, W.; Zhao, R.; Wang, R.; Choi, S.R.; Ploessl, K.; Alexoff, D.; Wu, Z.; Zhu, L.; Kung, H.F. Theranostic Agent Targeting Bone Metastasis: A Novel [68Ga]Ga/[177Lu]Lu-DOTA-HBED-bisphosphonate. J. Med. Chem. 2024, 67, 4793–4803. [Google Scholar] [CrossRef]
- Kasugai, S.; Fujisawa, R.; Waki, Y.; Miyamoto, K.; Ohya, K. Selective drug delivery system to bone: Small peptide (Asp)6 conjugation. J. Bone Miner. Res. 2000, 15, 936–943. [Google Scholar] [CrossRef]
- Ishizaki, J.; Waki, Y.; Takahashi-Nishioka, T.; Yokogawa, K.; Miyamoto, K. Selective drug delivery to bone using acidic oligopeptides. J. Bone Miner. Metab. 2009, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Ishizaki, A.; Takai, K.; Kitamura, Y.; Kiwada, T.; Shiba, K.; Odani, A. Development of novel radiogallium-labeled bone imaging agents using oligo-aspartic acid peptides as carriers. PLoS ONE 2013, 8, e84335. [Google Scholar] [CrossRef]
- Ogawa, K.; Ishizaki, A.; Takai, K.; Kitamura, Y.; Makino, A.; Kozaka, T.; Kiyono, Y.; Shiba, K.; Odani, A. Evaluation of Ga-DOTA-(d-Asp)n as bone imaging agents: D-aspartic acid peptides as carriers to bone. Sci. Rep. 2017, 7, 13971. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Nishizawa, K.; Mishiro, K.; Effendi, N.; Fuchigami, T.; Munekane, M.; Wakabayashi, H.; Kinuya, S. Synthesis and Evaluation of Radiogallium Labeled Bone-Imaging Probes Using Oligo-gamma-Carboxy Glutamic Acid Peptides as Carriers to Bone. Mol. Pharm. 2024, 21, 2375–2382. [Google Scholar] [CrossRef]
- Hirata, S.; Mishiro, K.; Higashi, T.; Fuchigami, T.; Munekane, M.; Arano, Y.; Kinuya, S.; Ogawa, K. Synthesis and evaluation of a multifunctional probe with a high affinity for prostate-specific membrane antigen (PSMA) and bone. Nucl. Med. Biol. 2022, 114-115, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, A.; Mishiro, K.; Shiba, K.; Hanaoka, H.; Kinuya, S.; Odani, A.; Ogawa, K. Fundamental study of radiogallium-labeled aspartic acid peptides introducing octreotate derivatives. Ann. Nucl. Med. 2019, 33, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Yu, J.; Ishizaki, A.; Yokokawa, M.; Kitamura, M.; Kitamura, Y.; Shiba, K.; Odani, A. Radiogallium complex-conjugated bifunctional peptides for detecting primary cancer and bone metastases simultaneously. Bioconjug. Chem. 2015, 26, 1561–1570. [Google Scholar] [CrossRef]
- Obaha, A.; Novinec, M. Regulation of Peptidase Activity beyond the Active Site in Human Health and Disease. Int. J. Mol. Sci. 2023, 24, 17120. [Google Scholar] [CrossRef]
- Lee, M.F.; Poh, C.L. Strategies to improve the physicochemical properties of peptide-based drugs. Pharm. Res. 2023, 40, 617–632. [Google Scholar] [CrossRef]
- Domhan, C.; Uhl, P.; Kleist, C.; Zimmermann, S.; Umstatter, F.; Leotta, K.; Mier, W.; Wink, M. Replacement of l-Amino Acids by d-Amino Acids in the Antimicrobial Peptide Ranalexin and Its Consequences for Antimicrobial Activity and Biodistribution. Molecules 2019, 24, 2987. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef]
- Gooding, E.A.; Sharma, S.; Petty, S.A.; Fouts, E.A.; Palmer, C.J.; Nolan, B.E.; Volk, M. pH-dependent helix folding dynamics of poly-glutamic acid. Chem. Phys. 2013, 422, 115–123. [Google Scholar] [CrossRef]
- Yongye, A.B.; Li, Y.; Giulianotti, M.A.; Yu, Y.; Houghten, R.A.; Martínez-Mayorga, K. Modeling of peptides containing d-amino acids: Implications on cyclization. J. Comput. Aided Mol. Des. 2009, 23, 677–689. [Google Scholar] [CrossRef]
- Rolleman, E.J.; Valkema, R.; de Jong, M.; Kooij, P.P.; Krenning, E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Singla, S.; Thakral, P.; Bal, C.S. Dosimetric analyses of kidneys, liver, spleen, pituitary gland, and neuroendocrine tumors of patients treated with 177Lu-DOTATATE. Clin. Nucl. Med. 2013, 38, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Arano, Y.; Fujioka, Y.; Akizawa, H.; Ono, M.; Uehara, T.; Wakisaka, K.; Nakayama, M.; Sakahara, H.; Konishi, J.; Saji, H. Chemical design of radiolabeled antibody fragments for low renal radioactivity levels. Cancer Res. 1999, 59, 128–134. [Google Scholar]
- Suzuki, H.; Araki, M.; Tatsugi, K.; Ichinohe, K.; Uehara, T.; Arano, Y. Reduction of the Renal Radioactivity of 111In-DOTA-Labeled Antibody Fragments with a Linkage Cleaved by the Renal Brush Border Membrane Enzymes. J. Med. Chem. 2023, 66, 8600–8613. [Google Scholar] [CrossRef]
- Makarem, A.; Konrad, M.; Liolios, C.; Kopka, K. A Convenient Synthesis for HBED-CC-tris(tert-butyl ester). Synlett 2018, 29, 1239–1243. [Google Scholar] [CrossRef]
- Echigo, H.; Munekane, M.; Fuchigami, T.; Washiyama, K.; Mishiro, K.; Wakabayashi, H.; Takahashi, K.; Kinuya, S.; Ogawa, K. Optimizing the pharmacokinetics of an 211At-labeled RGD peptide with an albumin-binding moiety via the administration of an albumin-binding inhibitor. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2663–2671. [Google Scholar] [CrossRef]
- Ben Azzouna, R.; Guez, A.; Benali, K.; Al-Shoukr, F.; Gonzalez, W.; Karoyan, P.; Rouzet, F.; Le Guludec, D. Synthesis, gallium labelling and characterization of P04087, a functionalized phosphatidylserine-binding peptide. EJNMMI Radiopharm. Chem. 2017, 2, 3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, K.; Nishizawa, K.; Mishiro, K.; Munekane, M.; Fuchigami, T.; Echigo, H.; Wakabayashi, H.; Kinuya, S. Differences in the Renal Accumulation of Radiogallium-Labeled (Glu)14 Peptides Containing Different Optical Isomers of Glutamic Acid. Molecules 2024, 29, 3993. https://doi.org/10.3390/molecules29173993
Ogawa K, Nishizawa K, Mishiro K, Munekane M, Fuchigami T, Echigo H, Wakabayashi H, Kinuya S. Differences in the Renal Accumulation of Radiogallium-Labeled (Glu)14 Peptides Containing Different Optical Isomers of Glutamic Acid. Molecules. 2024; 29(17):3993. https://doi.org/10.3390/molecules29173993
Chicago/Turabian StyleOgawa, Kazuma, Kota Nishizawa, Kenji Mishiro, Masayuki Munekane, Takeshi Fuchigami, Hiroaki Echigo, Hiroshi Wakabayashi, and Seigo Kinuya. 2024. "Differences in the Renal Accumulation of Radiogallium-Labeled (Glu)14 Peptides Containing Different Optical Isomers of Glutamic Acid" Molecules 29, no. 17: 3993. https://doi.org/10.3390/molecules29173993





