Design, Synthesis, and Anticancer and Antibacterial Activities of Quinoline-5-Sulfonamides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Screening
2.2.1. In Vitro Cell Viability
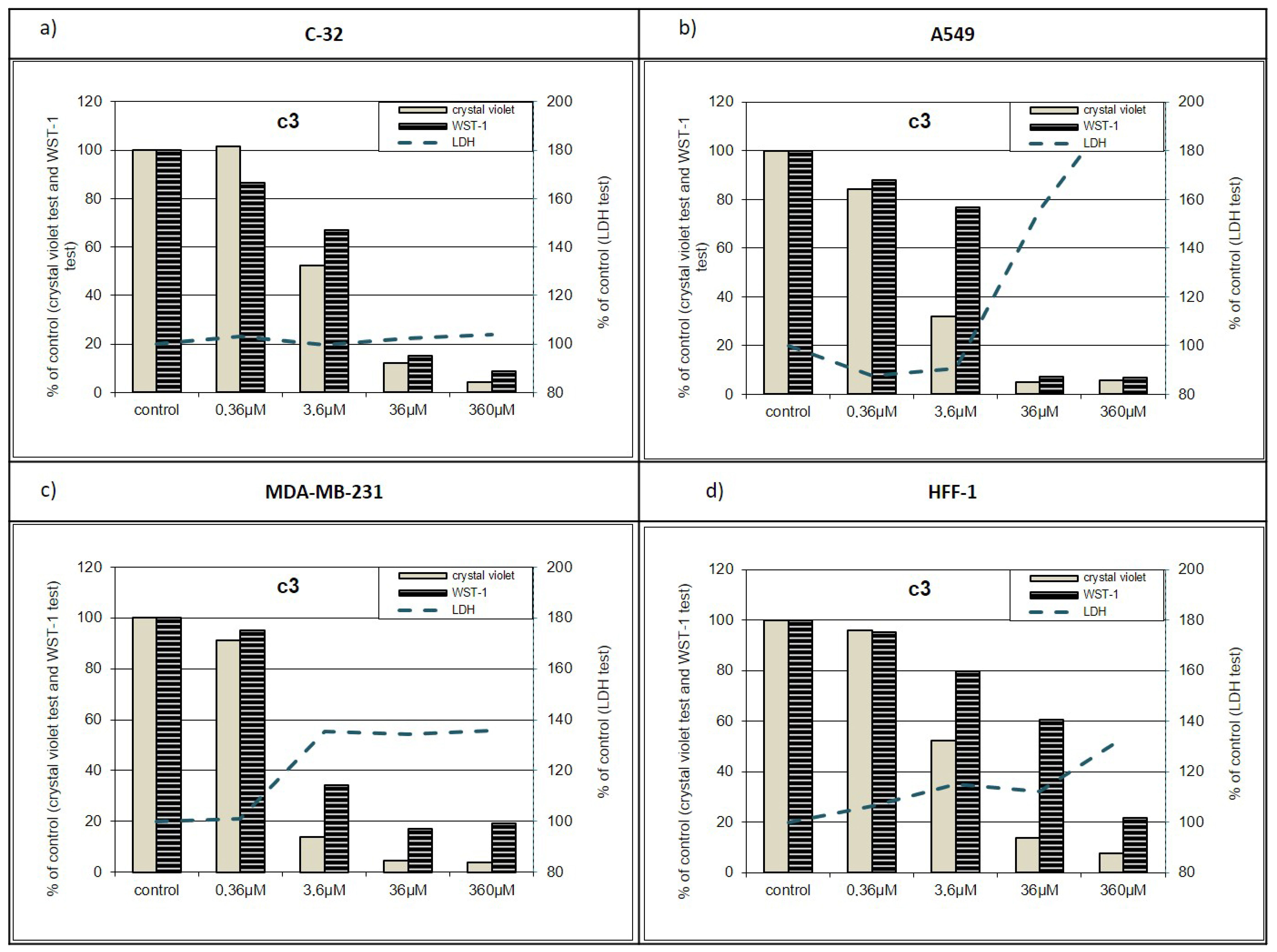
2.2.2. Additional Antiproliferative Tests
Violet-WST-LDH
Wound Healing Assay
Transcriptional Activity
2.2.3. In Vitro Antimicrobial Activity
3. Materials and Methods
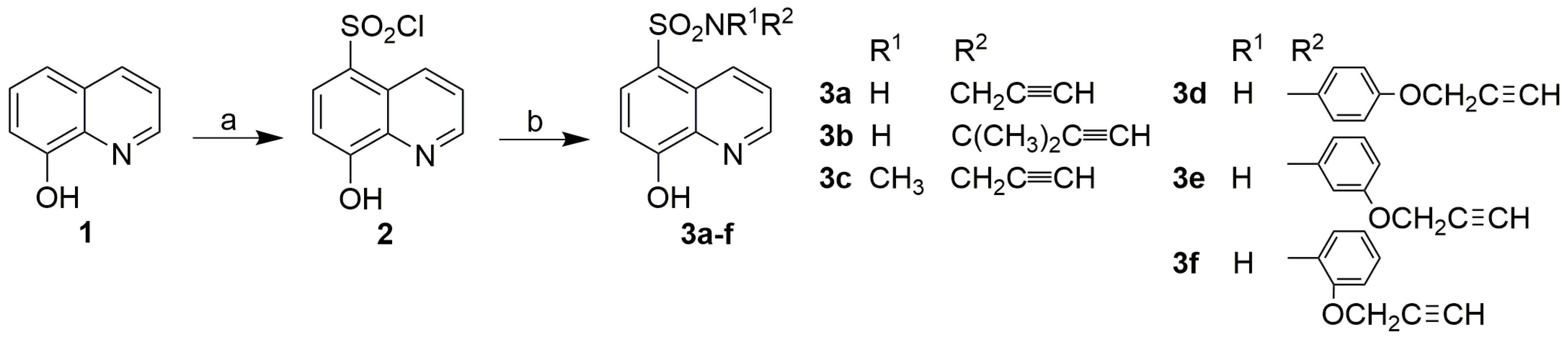
3.1. Chemistry
3.2. Synthesis
3.2.1. Synthesis of 8-Hydroxyquinoline-5-Sulfonyl Chloride (2)
3.2.2. Synthesis of 8-Hydroxyquinoline-5-Sulfonamides (3)
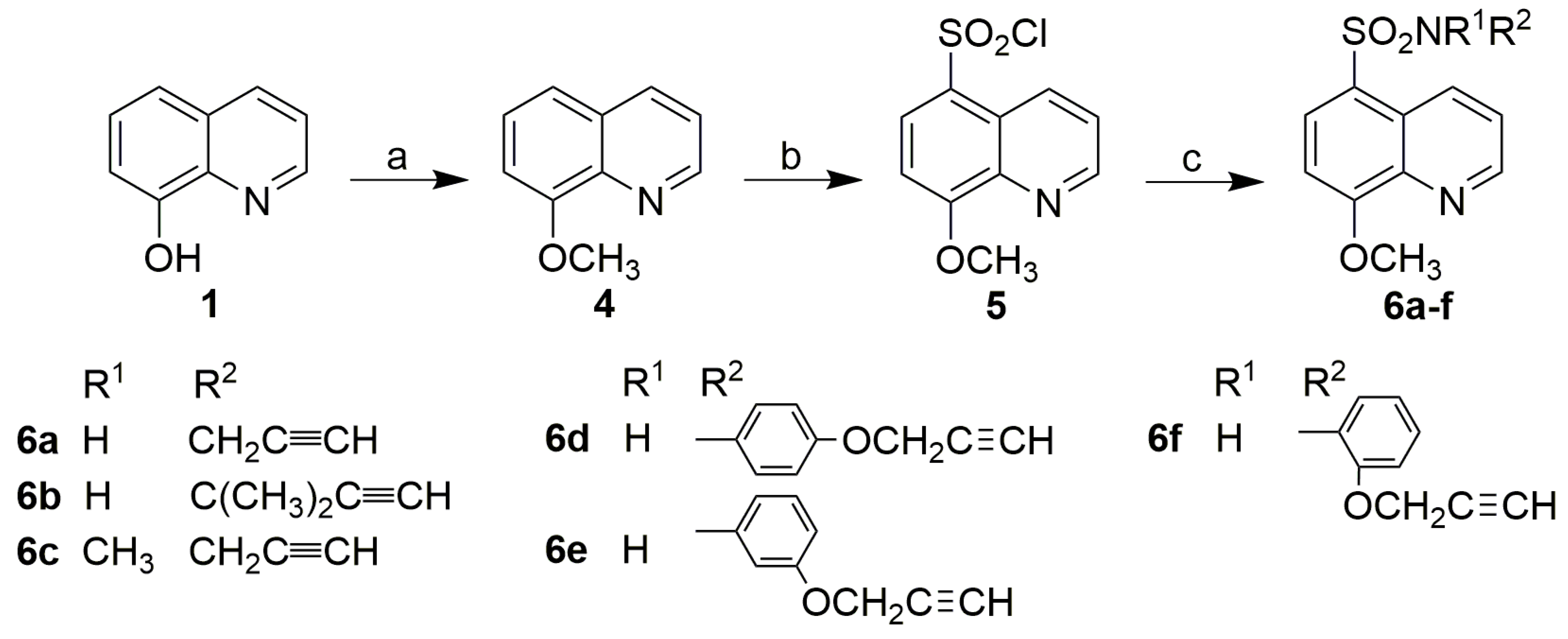
3.2.3. Synthesis of 8-Methoxyquinoline (4)
3.2.4. Synthesis of 8-Methoxyquinoline-5-Sulfochloride (5)
3.2.5. Synthesis of 8-Methoxyquinoline-5-Sulfonamides (6)
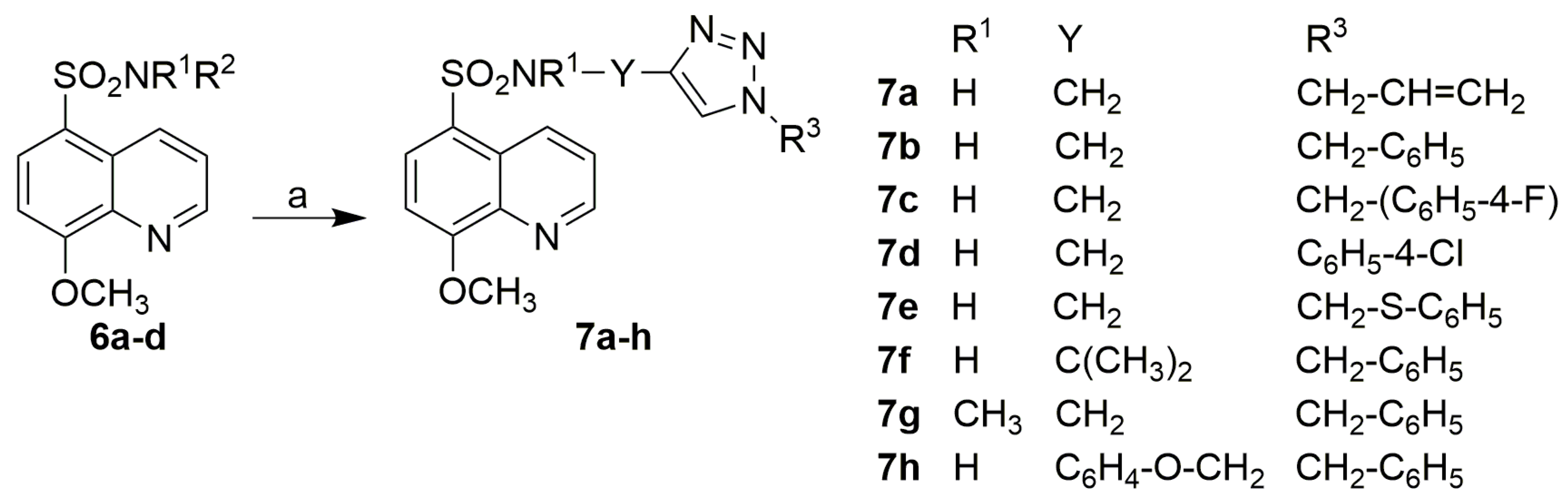
3.2.6. Synthesis of 1,2,3-Triazole Derivatives of 8-Methoxyquinoline-5-Sulfonamide (7)
Procedure A: Preparation of Derivative 7a
Procedure B: Preparation of Derivatives 7b, c, and 7e–h
Procedure C: Preparation of Derivative 7d
3.3. Biological Evaluation
3.3.1. Cell Culture
3.3.2. Effect of Compounds on Number and Viability of Cells
3.3.3. Wound Healing Assay
3.3.4. Transcriptional Activity of H3, BCL-2, BAX, P21, and P53 Genes
3.3.5. In Vitro Antibacterial Evaluation
3.3.6. Determination of Minimum Bactericidal Concentrations
3.3.7. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polanski, J.; Kurczyk, A.; Bak, A.; Musiol, R. Privileged structures-dream or reality: Preferential organization of azanaphthalene scaffold. Curr. Med. Chem. 2012, 19, 1921–1945. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorg. Chem. 2021, 108, 104633. [Google Scholar] [CrossRef]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent advances in the synthesis and biological activity of 8-hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Dorababu, A. Recent update on antibacterial and antifungal activity of quinoline scaffolds. Arch. Pharm. 2021, 354, e2000232. [Google Scholar] [CrossRef]
- Czaplinska, B.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Slodek, A.; Korzec, M.; Musiol, R. Theoretical and experimental investigations of large stokes shift fluorophores based on a quinoline scaffold. Molecules 2020, 25, 2488. [Google Scholar] [CrossRef]
- Chotsiri, P.; Mahamar, A.; Hoglund, R.M.; Koita, F.; Sanogo, K.; Diawara, H.; Dicko, A.; Simpson, J.A.; Bousema, T.; White, N.J.; et al. Mechanistic modeling of primaquine pharmacokinetics, gametocytocidal activity, and mosquito infectivity. Clin. Pharmacol. Ther. 2022, 111, 676–685. [Google Scholar] [CrossRef]
- Musiol, R.; Mrozek-Wilczkiewicz, A.; Polanski, J. Synergy against fungal pathogens: Working together is better than working alone. Curr. Med. Chem. 2014, 21, 870–893. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, S. 8-Hydroxyquinolines: Are view of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [PubMed]
- Mrozek-Wilczkiewicz, A.; Kalinowski, D.S.; Musiol, R.; Finster, J.; Szurko, A.; Serafin, K.; Knas, M.; Kamalapuram, S.K.; Kovacevic, Z.; Jampilek, J.; et al. Investigating the anti-proliferative activity of styrylazanaphthalenes and azanaphthalenediones. Bioorg. Med. Chem. 2010, 18, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Bissani, C.; Gasparin, D.; Pilger, A. 8-Hydroxyquinoline, derivatives and metal-complexes: A review of antileukemia activities. ChemistrySelect 2023, 8, e202204219. [Google Scholar] [CrossRef]
- Abeydeera, N.; Benin, B.M.; Mudarmah, K.; Pant, B.D.; Chen, G.; Shin, W.S.; Kim, M.-H.; Huang, S.D. Harnessing the dual antimicrobial mechanism of action with Fe(8-hydroxyquinoline)3 to develop a topical ointment for mupirocin-resistant MRSA infections. Antibiotics 2023, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.D.; Ma, K.Y.; Wang, Y.L.; Sun, Y.; Shang, X.F.; Zhao, Z.M.; Wang, R.X.; Chen, Y.J.; Zhu, J.K.; Liu, Y.Q. Design, synthesis, and antifungal evaluation of 8-hydroxyquinoline metal complexes against phytopathogenic fungi. J. Agric. Food Chem. 2020, 68, 11096–11104. [Google Scholar] [CrossRef]
- Pivarcsik, T.; Posa, V.; Kovacs, H.; May, N.V.; Spengler, G.; Posa, S.P.; Toth, S.; Nezafat Yazdi, Z.; Ozvegy-Laczka, C.; Ugrai, I.; et al. Metal complexes of a 5-nitro-8-hydroxyquinoline-proline hybrid with enhanced water solubility targeting multidrug resistant cancer cells. Int. J. Mol. Sci. 2023, 24, 593. [Google Scholar] [CrossRef]
- Pape, V.F.S.; Palko, R.; Toth, S.; Szabo, M.J.; Sessler, J.; Dorman, G.; Enyedy, E.A.; Soos, T.; Szatmari, I.; Szakacs, G. Structure-activity relationships of 8-hydroxyquinoline-derived Mannich bases with tertiary amines targeting multidrug-resistant cancer. J. Med. Chem. 2022, 65, 7729–7745. [Google Scholar] [CrossRef]
- Joaquim, A.R.; Gionbelli, M.P.; Gosmann, G.; Fuentefria, A.M.; Lopes, M.S.; Fernandes de Andrade, S. Novel antimicrobial 8-hydroxyquinoline-based agents: Current development, structure-activity relationships, and perspectives. J. Med. Chem. 2021, 64, 16349–16379. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.E.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Czaplinska, B.; Maron, A.; Malecki, J.G.; Szafraniec-Gorol, G.; Matussek, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Danikiewicz, W.; Musiol, R.; Slodek, A. Comprehensive exploration of the optical and biological properties of new quinoline based cellular probes. Dyes Pigment. 2017, 144, 119–132. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Cieslik, W.; Romanowicz, A.; Musiol, R.; Krasowska, A. Blocking and dislocation of Candida albicans Cdr1p transporter by styrylquinolines. Int. J. Antimicrob. Agents 2017, 50, 171–176. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Nevin, E.; Soral, M.; Gonec, T.; Kollar, P.; Oravec, M.; Coffey, A.; O’Mahony, J.; Liptaj, T.; et al. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as potential antimycobacterial agents. Bioorg. Med. Chem. 2015, 23, 4188–4196. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Vitezova, M.; Kos, J.; Kollar, P.; Jampilek, J. Effect of selected 8-hydroxyquinoline-2-carboxanilides on viability and sulfate metabolism of Desulfovibrio piger. J. Appl. Biomed. 2018, 16, 241–246. [Google Scholar] [CrossRef]
- Conan, P.; Leon, A.; Gourdel, M.; Rollet, C.; Chair, L.; Caroff, N.; Le Goux, N.; Le Jossic-Corcos, C.; Sinane, M.; Gentile, L.; et al. Identification of 8-hydroxyquinoline derivatives that decrease cystathionine beta synthase (CBS) activity. Int. J. Mol. Sci. 2022, 23, 6769. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, M.A.; Al-Kaabi, A.; Samadi, A.; Antony, P.; Vijayan, R.; Al-Keridis, L.A.; Saadeh, H.A.; Abutaha, N. Synthesis and biological applications of some novel 8-hydroxyquinoline urea and thiourea derivatives. Arab. J. Chem. 2022, 15, 103905. [Google Scholar] [CrossRef]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B. Quinoline–based hybrid compounds with antimalarial activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef]
- Teng, P.; Li, C.; Peng, Z.; Anne Marie, V.; Nimmagadda, A.; Su, M.; Li, Y.; Sun, X.; Cai, J. Facilely accessible quinoline derivatives as potent antibacterial agents. Bioorg. Med. Chem. 2018, 26, 3573–3579. [Google Scholar] [CrossRef]
- Zieba, A.; Wojtyczka, R.D.; Kepa, M.; Idzik, D. Antimicrobial activity of novel 1-methyl-3-thio-4-aminoquinolinium salts. Folia Microbiol. 2010, 55, 3–9. [Google Scholar] [CrossRef]
- Zieba, A.; Wojtyczka, R.D.; Kepa, M.; Idzik, D. Synthesis and in vitro antimicrobial activity of 1-methyl-3-sulfothio-4-aminoquinolinium chlorides. Acta Pol. Pharm. Drug Res. 2013, 70, 163–166. [Google Scholar]
- Wojtyczka, R.D.; Zieba, A.; Dziedzic, A.; Kepa, M.; Idzik, D. An activity of thioacyl derivativesof 4-aminoquinolinium salts towards biofilm producing and planktonic forms of coagulase-negative staphylococci. BioMed Res. Int. 2015, 2015, 725939. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kos, J.; Kollar, P.; Kralova, K.; Jampilek, J. Activity of ring-substituted 8-hydroxyquinoline-2-carboxanilides against intestinal sulfate-reducing bacteria Desulfovibrio piger. Med. Chem. Res. 2018, 27, 278–284. [Google Scholar] [CrossRef]
- Empel, A.; Kisiel, E.; Wojtyczka, R.D.; Kepa, M.; Idzik, D.; Sochanik, A.; Wasik, T.J.; Zieba, A. Synthesis and antimicrobial activity of sulfur derivatives of quinolinium salts. Molecules 2018, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Al-Matarneh, C.M.; Nicolescu, A.; Marinaş, I.C.; Gaboreanu, M.D.; Shova, S.; Dascalu, A.; Silion, M.; Pinteala, M. New library of iodo-quinoline derivatives obtained by an alternative synthetic pathway and their antimicrobial activity. Molecules 2024, 29, 772. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, K. Synthetic and medicinal perspective of quinolines as antiviral agents. Eur. J. Med. Chem. 2021, 215, 113220. [Google Scholar] [CrossRef]
- Kos, J.; Ku, C.F.; Kapustikova, I.; Oravec, M.; Zhang, H.J.; Jampilek, J. 8-Hydroxyquinoline-2-carboxanilides as antiviral agents against avian influenza virus. ChemistrySelect 2019, 4, 4582–4587. [Google Scholar] [CrossRef]
- Afzal, O.; Kuma, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Van de Walle, T.; Cools, L.; Mangelinckx, S.; D’Hooghe, M. Recent contributions of quinolines to antimalarial and anticancer drug discovery research. Eur. J. Med. Chem. 2021, 226, 113865. [Google Scholar] [CrossRef]
- Sharma, V.; Mehta, D.K.; Das, R. Synthetic methods of quinoline derivatives as potent anticancer agents. Mini Rev. Med. Chem. 2017, 17, 1557–1572. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Wang, T.; Xiao, J. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019, 165, 59–79. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Tea Kaceli, M.; Arijit Mondal, A.; Farzaei, M.H.; Anupam Bishayee, A. Recent advances in improved anticancer efficacies of camptothecin nano-formulations: A systematic review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef]
- Hongmanee, P.; Rukseree, K.; Buabut, B.; Somsri, B.; Palittapongarnpim, P. In vitro activities of cloxyquin (5-chloroquinolin-8-ol) against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1105–1106. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Taggart, J.E.; Zhang, X.; Benbrook, D.M.; Lind, S.E.; Ding, W.Q. Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline). Cancer Lett. 2011, 312, 11–17. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Nycz, J.E.; Pesko, M.; Carroll, J.; Kralova, K.; Vejsova, M.; O’Mahony, J.; Coffey, A.; Mrozek, A.; et al. Investigating the activity spectrum for ring-substituted 8-hydroxyquinolines. Molecules 2010, 15, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, W.; Musiol, R.; Nycz, J.E.; Jampilek, J.; Vejsova, M.; Wolff, M.; Machura, B.; Polanski, J. Contribution to investigation of antimicrobial activity of styrylquinolines. Bioorg. Med. Chem. 2012, 20, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Ahmad, S.; Alam, M.S. Bioactive triazoles: A potential review. J. Chem. Pharm. Res. 2012, 4, 5157–5164. [Google Scholar]
- Lengerli, D.; Ibis, K.; Nural, Y.; Banoglu, E. The 1,2,3-triazole ‘all-in-one’ ring system in drug discovery: A good bioisostere, a good pharmacophore, a good linker, and a versatile synthetic tool. Expert Opin. Drug Discov. 2022, 17, 1209–1236. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Ben Hadda, T.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Khan, S.A.; Akhtar, M.J.; Gogoi, U.; Meenakshi, D.U.; Das, A. An overview of 1,2,3-triazole-containing hybrids and their potential anticholinesterase activities. Pharmaceuticals 2023, 16, 179. [Google Scholar] [CrossRef]
- Vaishnani, M.J.; Bijani, S.; Rahamathulla, M.; Baldaniya, L.; Jain, V.; Thajudeen, K.Y.; Ahmed, M.M.; Farhana, S.A.; Pasha, I. Biological importance and synthesis of 1,2,3-triazole derivatives: A review. Green Chem. Lett. Rev. 2024, 17, 2307989. [Google Scholar] [CrossRef]
- Arafa, F.M.; Said, H.; Osman, D.; Rezki, N.; Aouad, M.R.; Hagar, M.; Osman, M.; Elwakil, B.H.; Jaremko, M.; Tolba, M.M. Nanoformulation-based 1,2,3-triazole sulfonamides for anti-toxoplasma in vitro study. Trop. Med. Infect. Dis. 2023, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Swaina, B.; Angelib, A.; Angapellya, S.; Thackera, P.S.; Singha, P.; Supuranb, C.T.; Arifuddina, M. Synthesis of a new series of 3-functionalised-1-phenyl-1,2,3-triazole sulfamoylbenzamides as carbonic anhydrase I, II, IV and IX inhibitors. J Enzym. Inhib. Med. Chem. 2019, 34, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Kumar, A.; Kumar, V.; Yadav, M.; Lal, K. Recent progress in 1H-1,2,3-triazoles as potential antifungal agents. Curr. Top. Med. Chem. 2021, 21, 2109–2133. [Google Scholar] [CrossRef] [PubMed]
- Marzi, M.; Farjam, M.; Kazeminejad, Z.; Shiroudi, A.; Kouhpayeh, A.; Zarenezhad, E. A recent overview of 1,2,3-triazole-containing hybrids as novel antifungal agents: Focusing on synthesis, mechanism of action, and structure-activity relationship (SAR). J. Chem. 2022, 2022, 7884316. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma, A.; Joshi, K.; Singh, B.; Sharma, S.; Batra, K.; Kaur, K.; Singh, D.; Chadha, R.; et al. 1,2,3-Triazole derivatives as an emerging scaffold for antifungal drug development against Candida albicans: A comprehensive review. Chem. Biodivers. 2023, 20, e202300024. [Google Scholar] [CrossRef]
- El Malah, T.; Nour, H.F.; Satti, A.A.E.; Hemdan, B.A.; El-Sayed, W.A. Design, synthesis, and antimicrobial activities of 1,2,3-triazole glycoside clickamers. Molecules 2020, 25, 790. [Google Scholar] [CrossRef]
- Hryhoriv, H.; Mariutsa, I.; Kovalenko, S.M.; Georgiyants, V.; Perekhoda, L.; Filimonova, N.; Geyderikh, O.; Sidorenko, L. The search for new antibacterial agents among 1,2,3-triazole functionalized ciprofloxacin and norfloxacin hybrids: Synthesis, docking studies, and biological activity evaluation. Sci. Pharm. 2022, 90, 2. [Google Scholar] [CrossRef]
- Todorov, L.; Kostova, I. 1,2,3-Triazoles and their metal chelates with antimicrobial activity. Front. Chem. 2023, 11, 1247805. [Google Scholar] [CrossRef]
- Al-Taweel, S.; Al-Saraireh, Y.; Al-Trawneh, S.; Alshahateet, S.; Al-Tarawneh, R.; Ayed, N.; Alkhojah, M.; Al-Khaboori, W.; Zereini, W.; Al-Qaralleh, O. Synthesis and biological evaluation of ciprofloxacin-1,2,3-triazole hybrids as antitumor, antibacterial, and antioxidant agents. Heliyon 2023, 9, e22592. [Google Scholar] [CrossRef]
- Al-Humaidi, J.Y.; Shaaban, M.M.; Rezki, N.; Aouad, M.R.; Zakaria, M.; Jaremko, M.; Hagar, M.; Elwakil, B.H. 1,2,3-Triazole-benzofused molecular conjugates as potential antiviral agents against SARS-CoV-2 virus variants. Life 2022, 12, 1341. [Google Scholar] [CrossRef]
- Agouram, N. 1,2,3-Triazole derivatives as antiviral agents. Med. Chem. Res. 2023, 32, 2458–2472. [Google Scholar] [CrossRef]
- Awolade, P.; Cele, N.; Ebenezer, O.; Kerru, N.; Gummidi, L.; Gu, L.; Palma, G.; Kaur, M.; Singh, P. Synthesis of 1H-1,2,3-triazole-linked quinoline-isatin molecular hybrids as anti-breast cancer and anti-methicillin-resistant Staphylococcus aureus (MRSA) agents. Anticancer Agents Med. Chem. 2021, 21, 1228–1239. [Google Scholar] [CrossRef]
- Krstulovic, L.; Miskovic Spoljaric, K.; Rastija, V.; Filipovic, N.; Bajic, M.; Glavas-Obrovac, L. Novel 1,2,3-triazole-containing quinoline–benzimidazole hybrids: Synthesis, antiproliferative activity, in silico ADME predictions, and docking. Molecules 2023, 28, 6950. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perea, M.; Suarez-Castro, A.; Fraire-Soto, I.; Sifuentes-Padilla, J.L.; Gutierrez-Hernandez, R.; Reyes-Estrada, C.A.; Lopez-Hernandez, Y.; Cortes-Garcia, C.J.; Chacon-Garcia, L.; Granados-Lopez, A.J.; et al. Proliferation, migration and invasion of breast cancer cell lines are inhibited by 1,5-disubstituted tetrazol-1,2,3-triazole hybrids through interaction with p53. Molecules 2023, 28, 7600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ali, I.; Abbas, F.; Shafiq, F.; Yadav, A.K.; Ghate, M.D.; Kumar, D. In-silico identification and exploration of small molecule coumarin-1,2,3-triazole hybrids as potential EGFR inhibitors for targeting lung cancer. Mol. Divers. 2024. [Google Scholar] [CrossRef] [PubMed]
- Varani, T.; Abdouss, M.; Azerang, P.; Tahghighi, A. Acetylenic sulfones and acetylenic sulfonamide analogs: A novel and preferable antimicrobial drugs based on computational strategies. J. Comput. Biophys. Chem. 2022, 21, 115–122. [Google Scholar] [CrossRef]
- Kisiel-Nawrot, E.; Pindjakova, D.; Latocha, M.; Bak, A.; Kozik, V.; Suwinska, K.; Sochanik, A.; Cizek, A.; Jampilek, J.; Zieba, A. Design, synthesis and antimicrobial properties of new tetracyclic quinobenzothiazine derivatives. Int. J. Mol. Sci. 2022, 23, 15078. [Google Scholar] [CrossRef]
- Kisiel-Nawrot, E.; Latocha, M.; Bak, A.; Kozik, V.; Jampilek, J.; Zieba, A. Anticancer efficacy of antibacterial quinobenzothiazines. Appl. Sci. 2023, 13, 2886. [Google Scholar] [CrossRef]
- Kisiel-Nawrot, E.; Pindjakova, D.; Latocha, M.; Bak, A.; Kozik, V.; Suwinska, K.; Cizek, A.; Jampilek, J.; Zieba, A. Towards anticancer and antibacterial agents: Design and synthesis of 1,2,3-triazol-quinobenzothiazine derivatives. Int. J. Mol. Sci. 2023, 24, 13250. [Google Scholar] [CrossRef]
- Talele, T.T. Acetylene group, friend or foe in medicinal chemistry. J. Med. Chem. 2020, 63, 5625–5663. [Google Scholar] [CrossRef]
- Ertl, P.; Altmann, E.; Racine, S. The most common linkers in bioactive molecules and their bioisosteric replacement network. Bioorg. Med. Chem. 2023, 81, 117194. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide bond bioisosteres: Strategies, synthesis, and successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Applications of bioisosteres in the design of biologically active compounds. J. Agric. Food Chem. 2023, 71, 18087–18122. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Qu, B.; Yuan, J.; Liu, X.; Zhang, S.; Ma, X.; Lu, L. Anticancer activities of natural antimicrobial peptides from animals. Front. Microbiol. 2024, 14, 1321386. [Google Scholar] [CrossRef]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef]
- Nubel, U.; Dordel, J.; Kurt, K.; Strommenger, B.; Westh, H.; Shukla, S.K.; Zemlickova, H.; Leblois, R.; Wirth, T.; Jombart, T.; et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010, 6, e1000855. [Google Scholar] [CrossRef]
- Oravcova, V.; Zurek, L.; Townsend, A.; Clark, A.B.; Ellis, J.C.; Cizek, A. American crows as carriers of vancomycin-resistant enterococci with van A gene. Environ. Microbiol. 2014, 16, 939–949. [Google Scholar] [CrossRef]
- Global Laboratory Standards for a Healthier World. Available online: https://clsi.org/ (accessed on 18 June 2024).
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Measuring Cell Viability/Cytotoxicity. Dojindo EU GmbH, Munich, Germany. Available online: https://www.dojindo.eu.com/Protocol/Dojindo-Cell-Proliferation-Protocol.pdf (accessed on 18 June 2024).
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07; NCCLS: Wayne, PA, USA, 2018. [Google Scholar]
- Schwalbe, R.; Steele-Moore, L.; Goodwin, A.C. Antimicrobial Susceptibility Testing Protocols; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Scandorieiro, S.; de Camargo, L.C.; Lancheros, C.A.; Yamada-Ogatta, S.F.; Nakamura, C.V.; de Oliveira, A.G.; Andrade, C.G.; Duran, N.; Nakazato, G.; Kobayashi, R.K. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.C.; Meireles, L.M.; Lemos, M.F.; Guimaraes, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
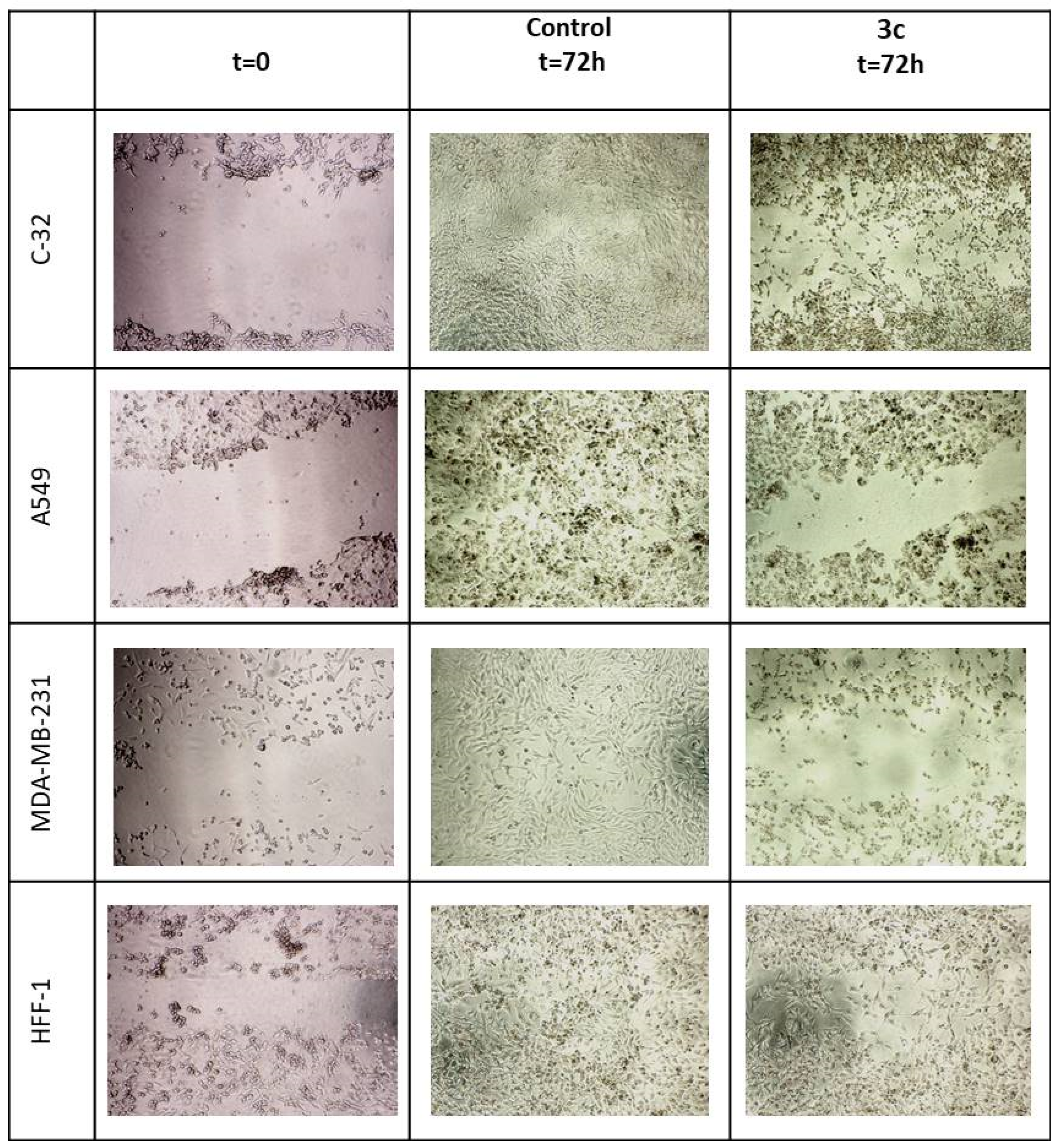
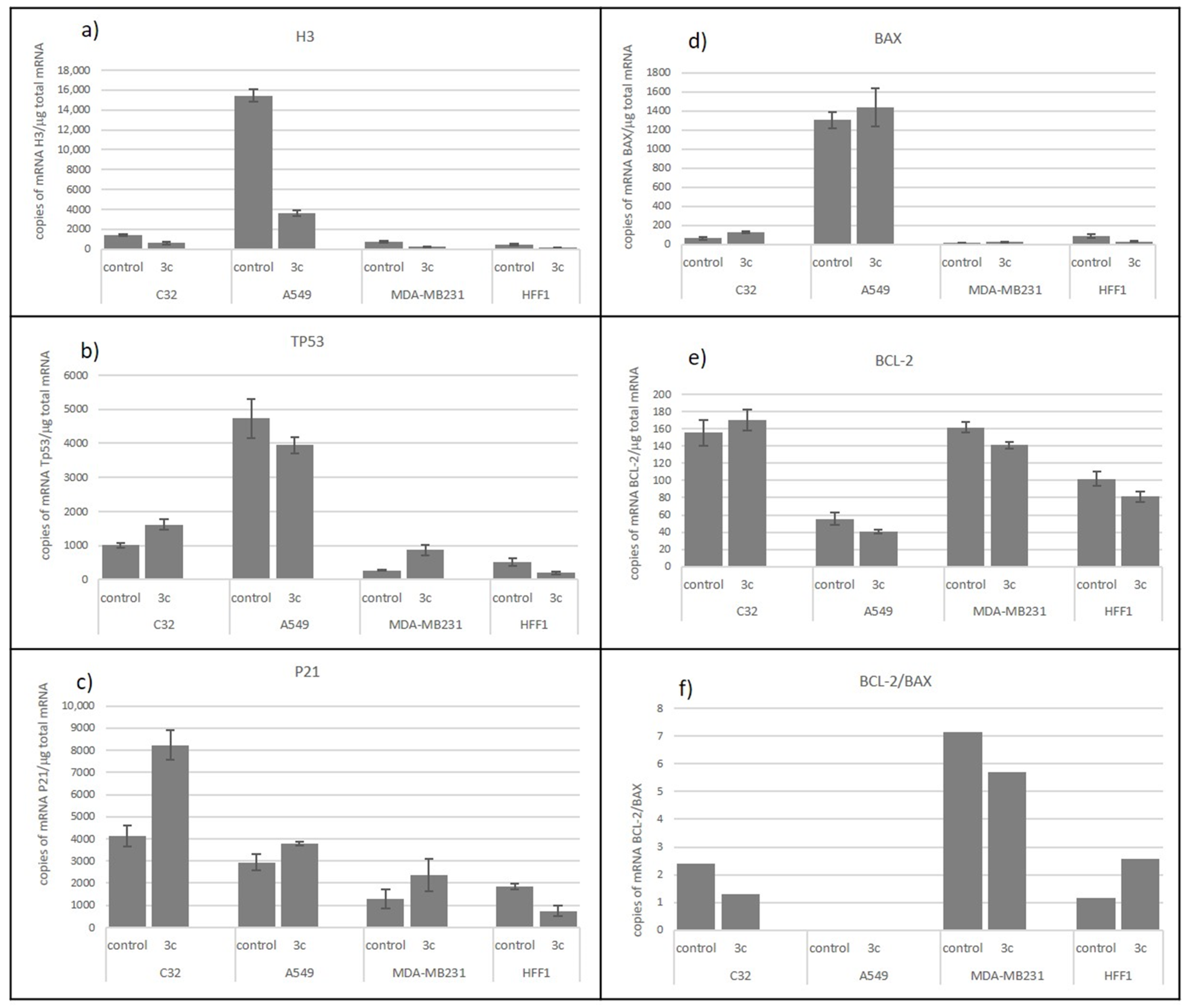
| Comp. | IC50 [µM] | |||
|---|---|---|---|---|
| C-32 | MDA-MB-231 | A549 | HFF-1 | |
| 3a | 19.8 ±1.1 | 23.3 ± 1.2 | 23.1 ± 0.7 | ˃100 |
| 3b | 22.1 ±1.5 | 55.8 ± 1.9 | 17.2 ± 1.5 | ˃100 |
| 3c | 9.8 ± 0.6 | 2.2 ± 0.1 | 13.9 ± 0.6 | ˃100 |
| 3d | 10.4 ± 0.5 | 45.3 ± 5.4 | 12.9 ± 1.1 | ˃100 |
| 3e | 8.6 ± 0.9 | 15.2 ± 0.8 | 11.5 ± 0.7 | ˃100 |
| 3f | 29.6 ± 2.6 | 14.7 ± 1.2 | 19.1 ± 0.8 | 28.1 ± 2.2 |
| 6a | ˃100 | ˃100 | ˃100 | – |
| 6b | 40.6 ± 4.9 | ˃100 | ˃100 | – |
| 6c | 70.1 ± 8.4 | ˃100 | ˃100 | – |
| 6d | 86.4 ± 3.9 | ˃100 | ˃100 | – |
| 6e | 32.2 ± 4.8 | ˃100 | ˃100 | – |
| 6f | 27.7 ± 2.2 | ˃100 | ˃100 | – |
| CPT | 10.6 ± 0.5 | 10.3 ± 0.7 | 17.3 ± 1.3 | 26.0 ± 2.0 |
| DOX | 0.3 ± 0.02 | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 |
| Comp. | IC50 [µM] | ||
|---|---|---|---|
| C-32 | MDA-MB-231 | A549 | |
| 7a | ˃100 | ˃100 | ˃100 |
| 7b | ˃100 | ˃100 | ˃100 |
| 7c | 89.0 ± 3.5 | ˃100 | ˃100 |
| 7d | ˃100 | ˃100 | ˃100 |
| 7e | ˃100 | 72.2 ± 3.6 | ˃100 |
| 7f | ˃100 | ˃100 | ˃100 |
| 7g | 42.6 ± 3.4 | ˃100 | ˃100 |
| 7h | ˃100 | ˃100 | ˃100 |
| CPT | 10.6 ± 0.5 | 10.3 ± 0.7 | 17.3 ± 1.3 |
| DOX | 0.3 ± 0.02 | 0.6 ± 0.1 | 1.0 ± 0.1 |
| Comp. | MIC/MBC [µM] | ||||||
|---|---|---|---|---|---|---|---|
| SA | MRSA1 | MRSA2 | EF | VRE1 | VRE2 | VRE3 | |
| 3a | 122/122 | 122/122 | 244/244 | 977/>977 | 977/NT | 977/NT | 977/NT |
| 3b | 55.2/110 | 55.2/110 | 110/110 | 883/>883 | 883/NT | 883/NT | 883/NT |
| 3c | 29.0/29.0 | 29.0/29.0 | 29.0/29.0 | 464/>464 | 464/NT | 464/NT | 464/NT |
| 3d | 45.2/45.2 | 45.2/45.2 | 90.4/90.4 | 362/362 | 362/NT | 362/NT | 362/NT |
| 3e | 45.2/45.2 | 45.2/90.4 | 90.4/90.4 | 362/362 | 362/NT | 362/NT | 362/NT |
| 3f | 45.2/45.2 | 45.2/90.4 | 90.4/90.4 | 362/362 | 362/NT | 362/NT | 362/NT |
| AMP | 5.72/5.72 | >45.8/>45.8 | >45.8/>45.8 | 2.81/2.81 | 11.5/11.5 | 11.5/11.5 | 11.5/11.5 |
| OXA | 1.25/1.25 | 79.8/79.8 | 29.7/29.7 | – | – | – | – |
| CPX | 1.51/3.02 | 48.3/96.6 | 193/386 | 1.51/3.02 | 1.51/3.02 | 3.02/3.02 | 193/386 |
| Comp. | Conc. | S. aureus Respiration Inhibition [%] |
|---|---|---|
| 3a | 1× MIC (1× MBC) | 92.5 |
| 3b | 2× MIC (2× MBC) | 94.4 |
| 3c | 2× MIC (2× MBC) | 95.2 |
| 3d | 1× MIC (1× MBC) | 73.0 |
| 3e | 1× MIC (1× MBC) | 73.7 |
| 3f | 1× MIC (1× MBC) | 81.6 |
| APM | 16× MIC (>16× MBC) | 81.9 |
| CPX | 32× MIC (16× MBC) | 95.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieba, A.; Pindjakova, D.; Latocha, M.; Plonka-Czerw, J.; Kusmierz, D.; Cizek, A.; Jampilek, J. Design, Synthesis, and Anticancer and Antibacterial Activities of Quinoline-5-Sulfonamides. Molecules 2024, 29, 4044. https://doi.org/10.3390/molecules29174044
Zieba A, Pindjakova D, Latocha M, Plonka-Czerw J, Kusmierz D, Cizek A, Jampilek J. Design, Synthesis, and Anticancer and Antibacterial Activities of Quinoline-5-Sulfonamides. Molecules. 2024; 29(17):4044. https://doi.org/10.3390/molecules29174044
Chicago/Turabian StyleZieba, Andrzej, Dominika Pindjakova, Malgorzata Latocha, Justyna Plonka-Czerw, Dariusz Kusmierz, Alois Cizek, and Josef Jampilek. 2024. "Design, Synthesis, and Anticancer and Antibacterial Activities of Quinoline-5-Sulfonamides" Molecules 29, no. 17: 4044. https://doi.org/10.3390/molecules29174044








