Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods
Abstract
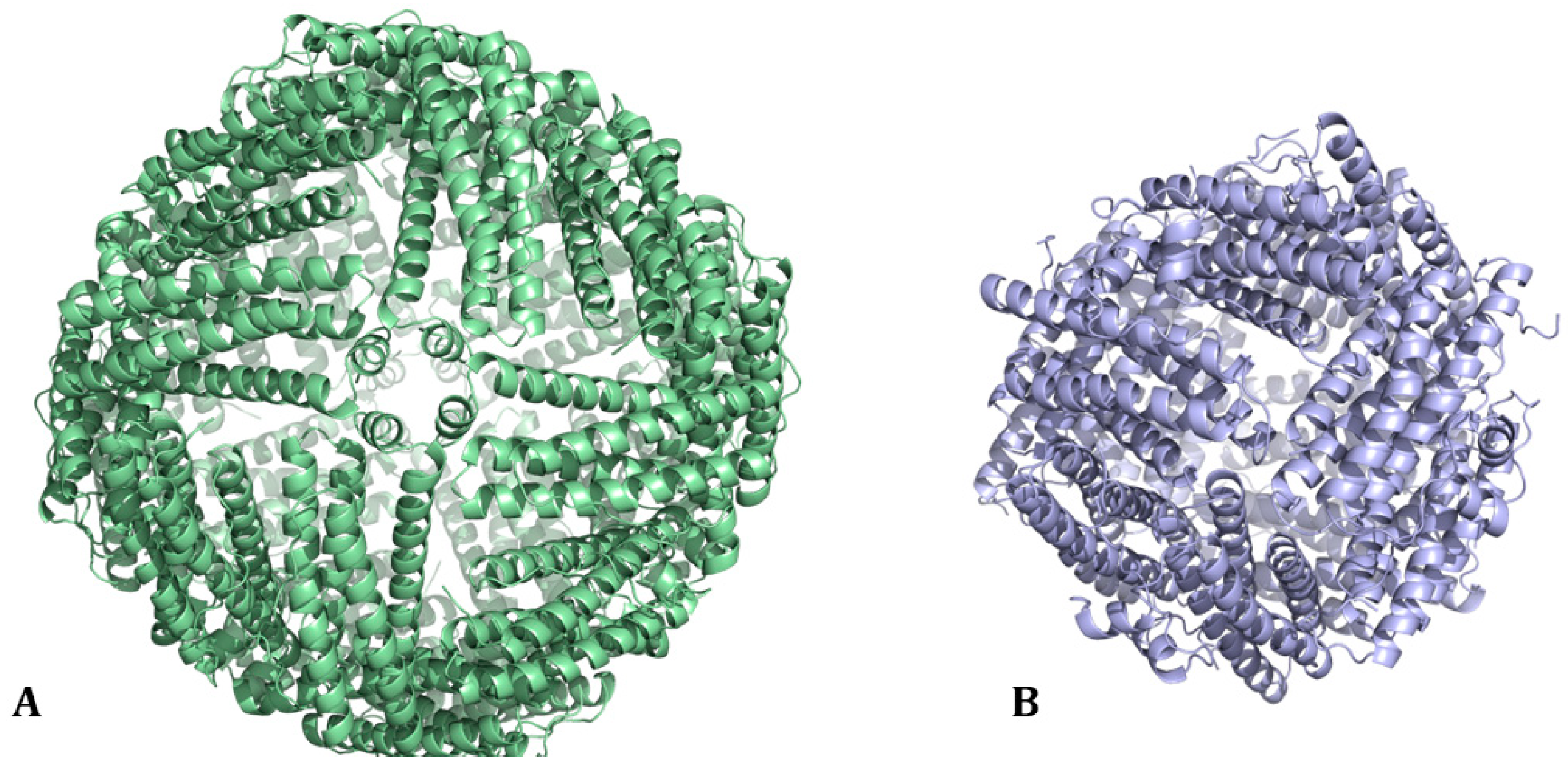
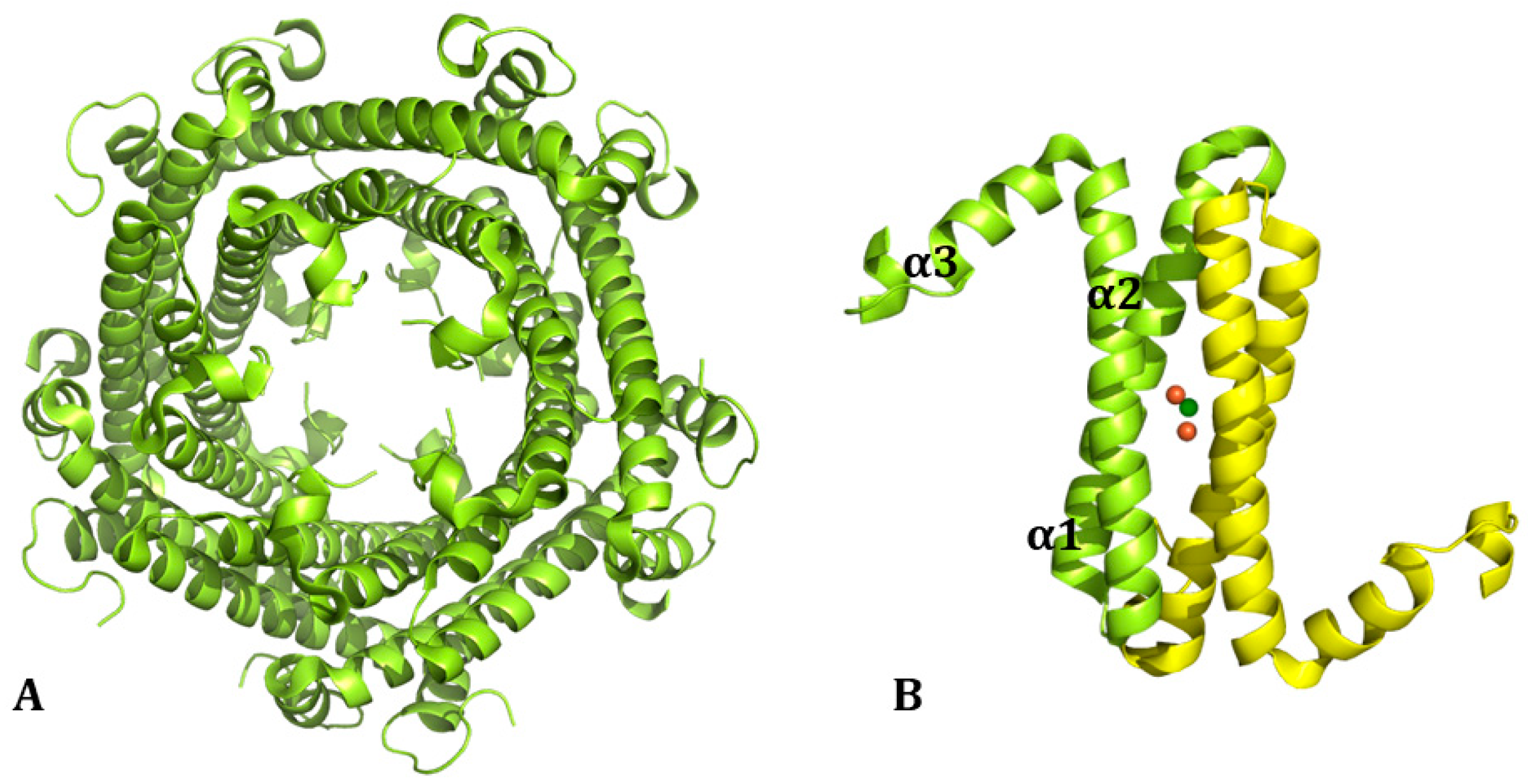
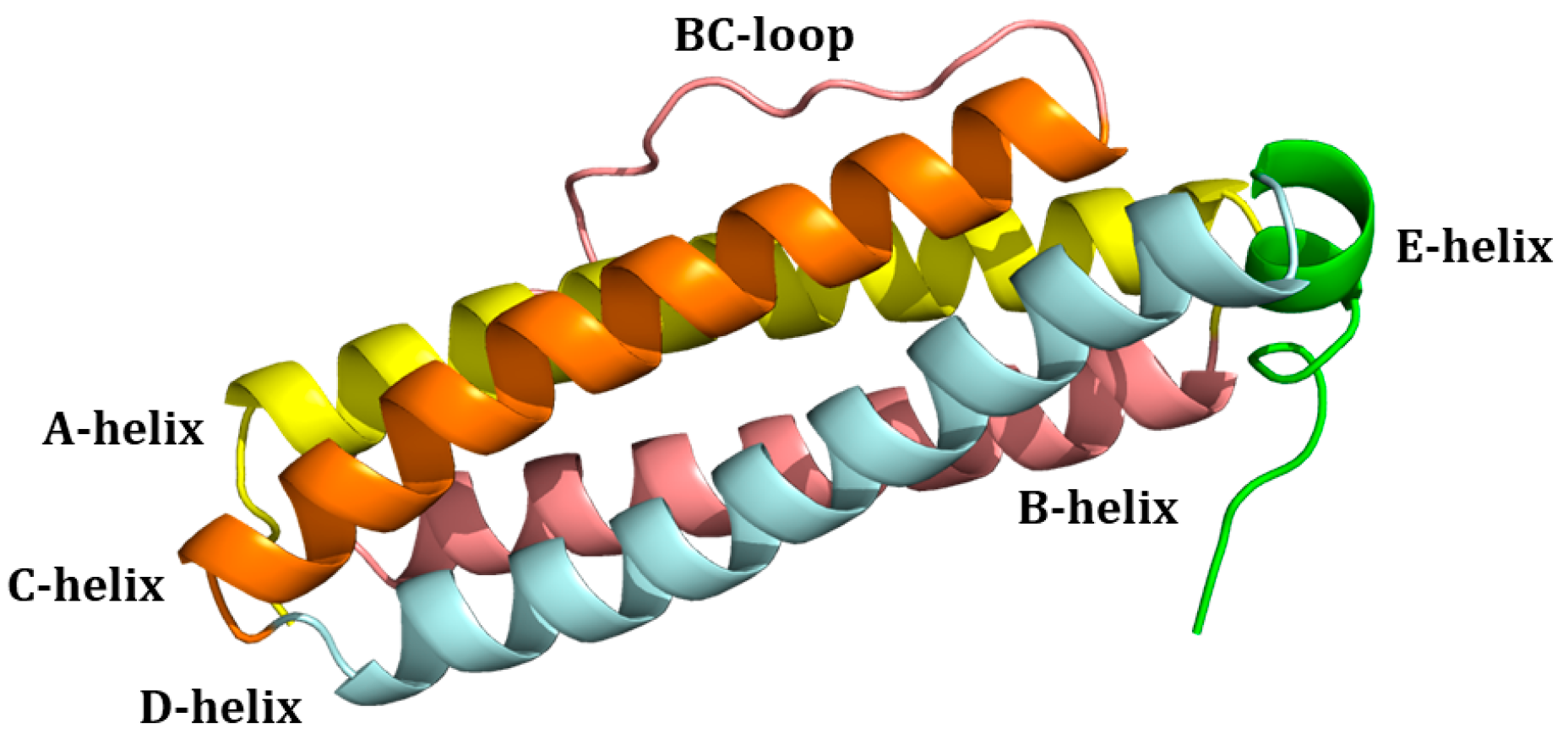
1. Ferritin: Structure and Biological Functions
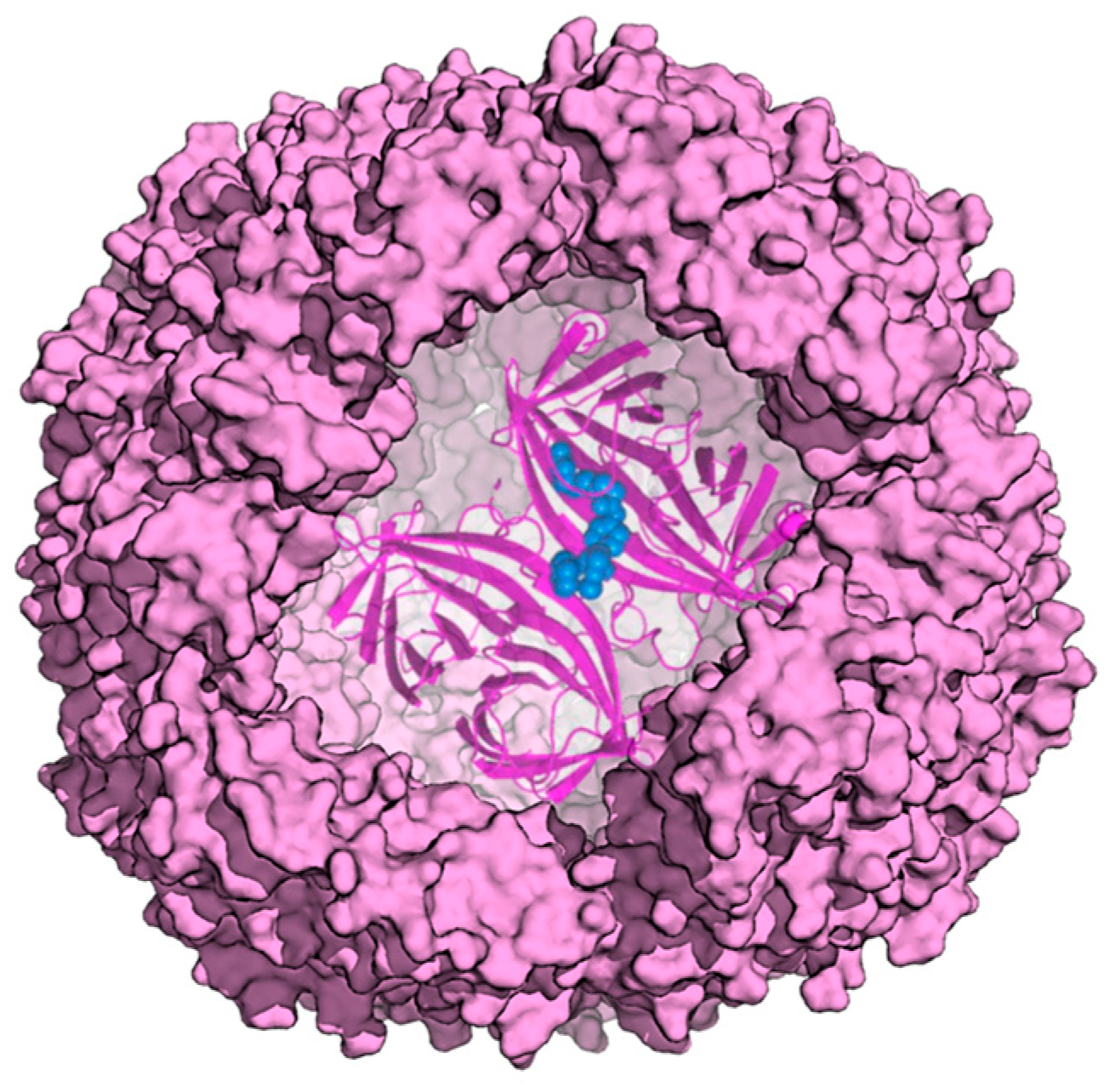
2. Ferritin as Delivery System for Cargo Molecules
3. Bioactive Molecules Loading
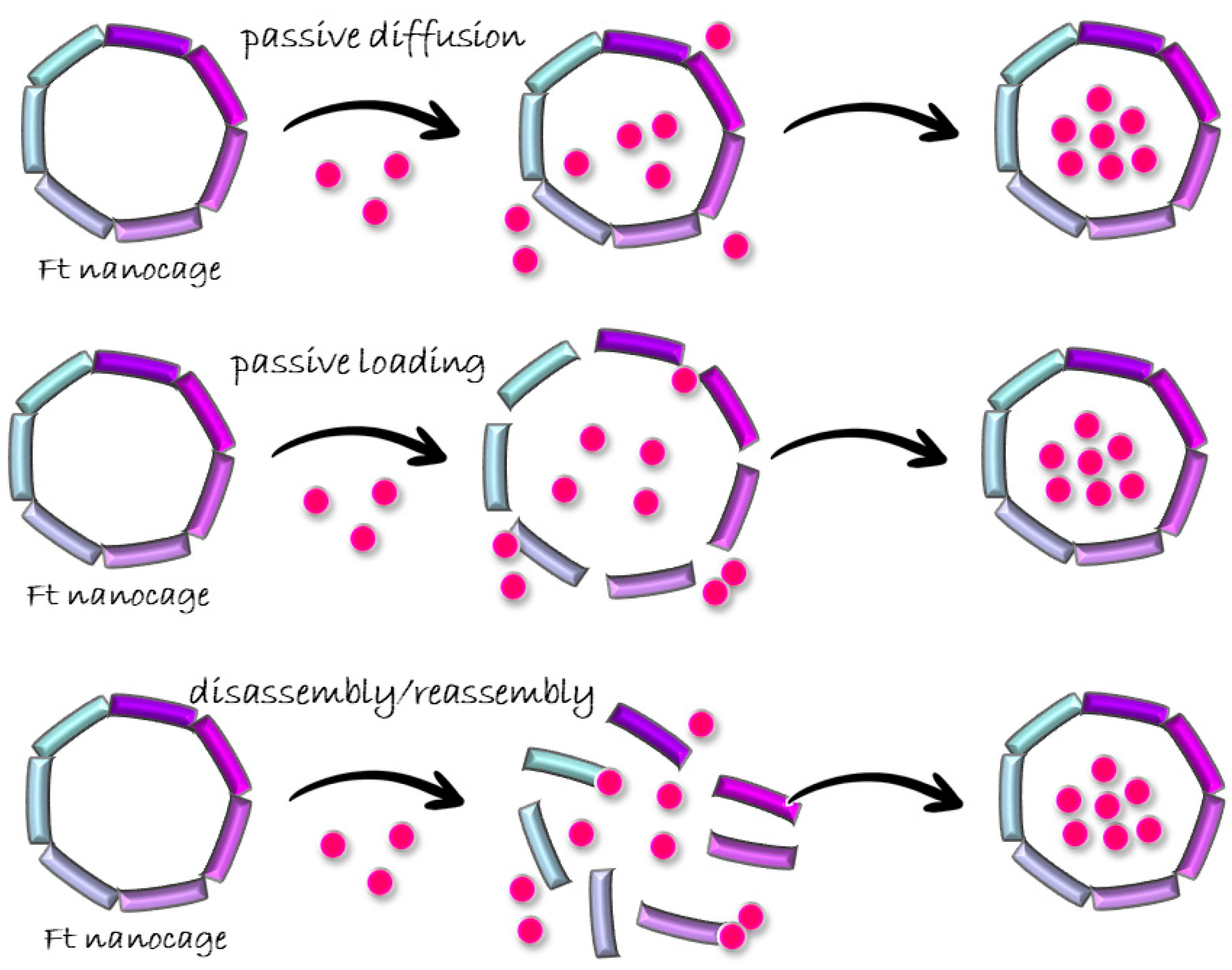
3.1. Passive Diffusion
3.2. Passive Doading
Organic Solvents
3.3. Disassembly/Reassembly
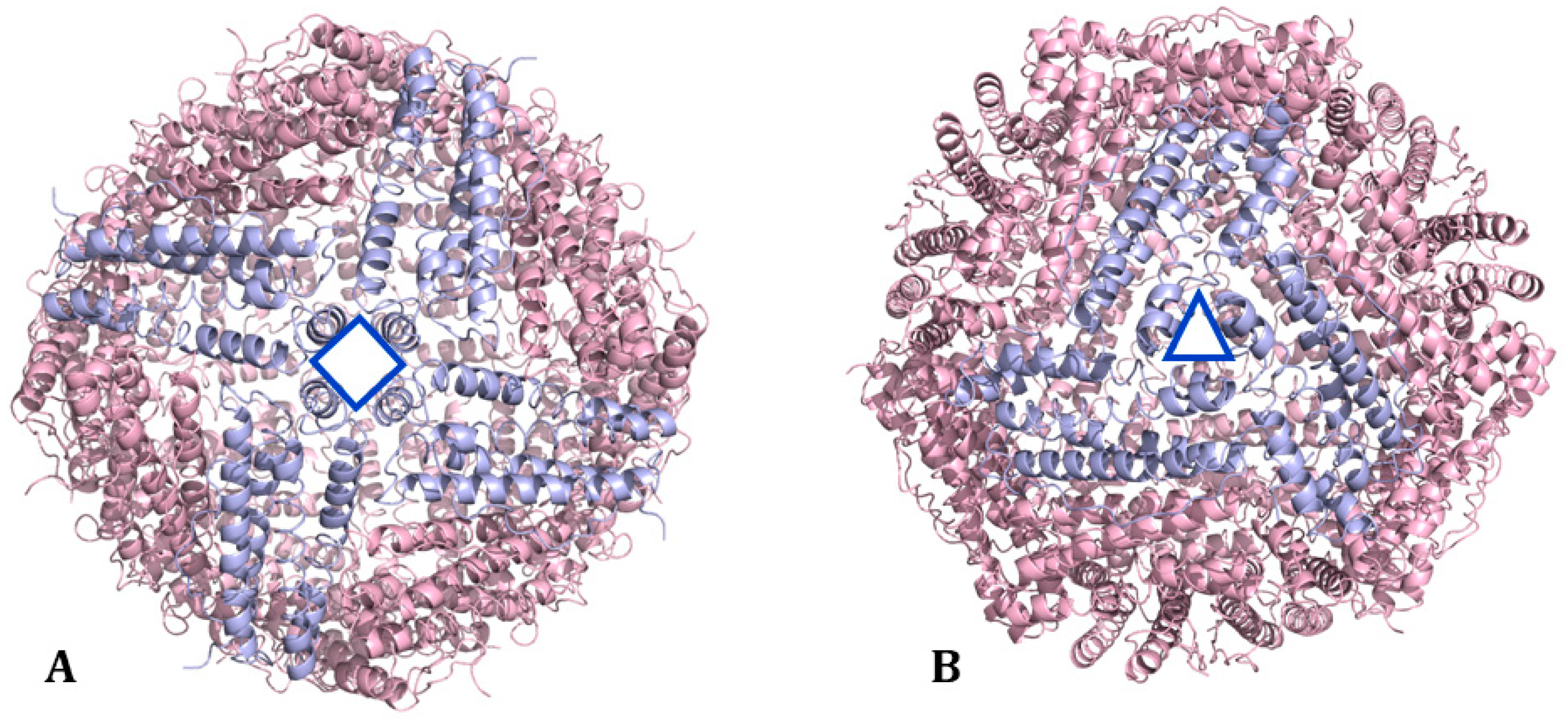
3.3.1. pH Switch Disassembly/Reassembly Protocol
3.3.2. Use of Chemical Denaturants
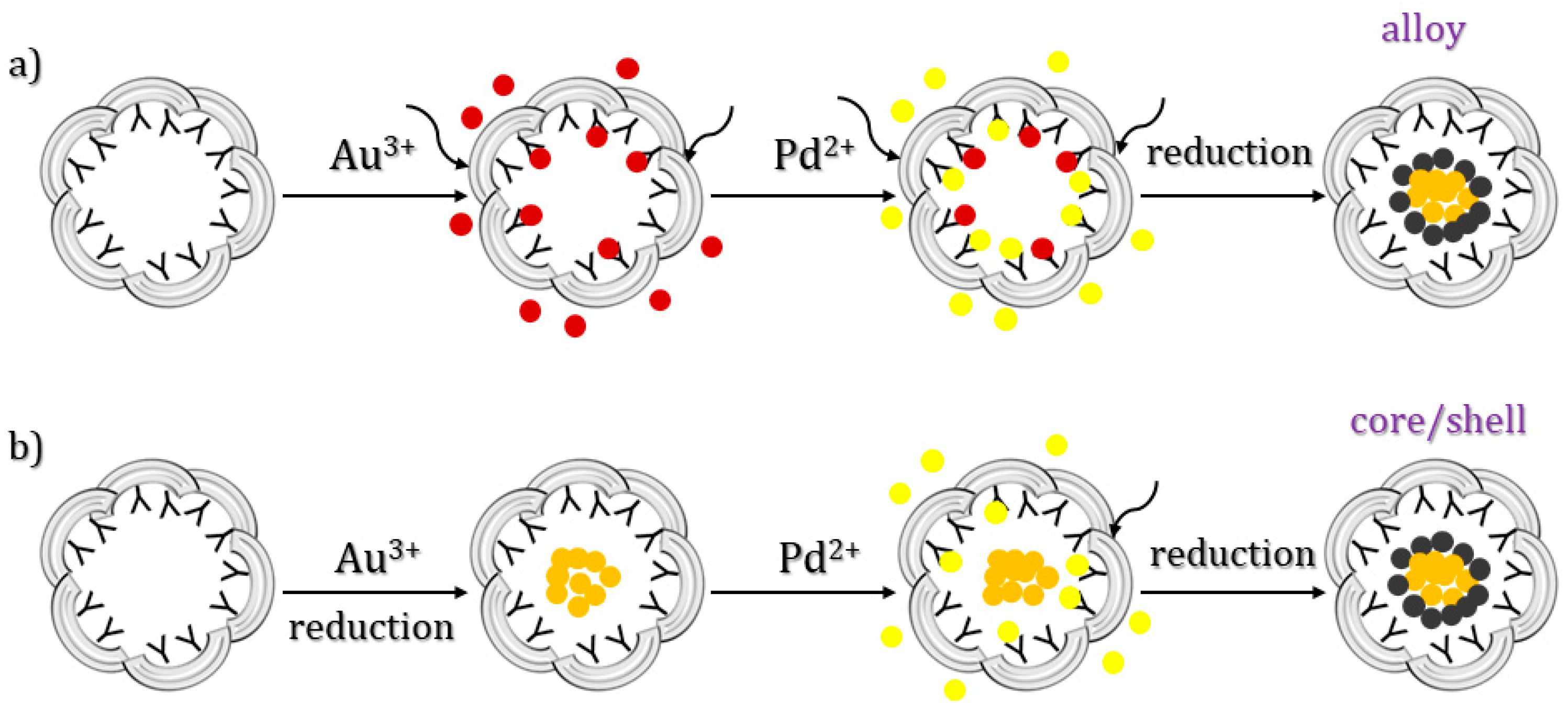
3.4. One-Step Method
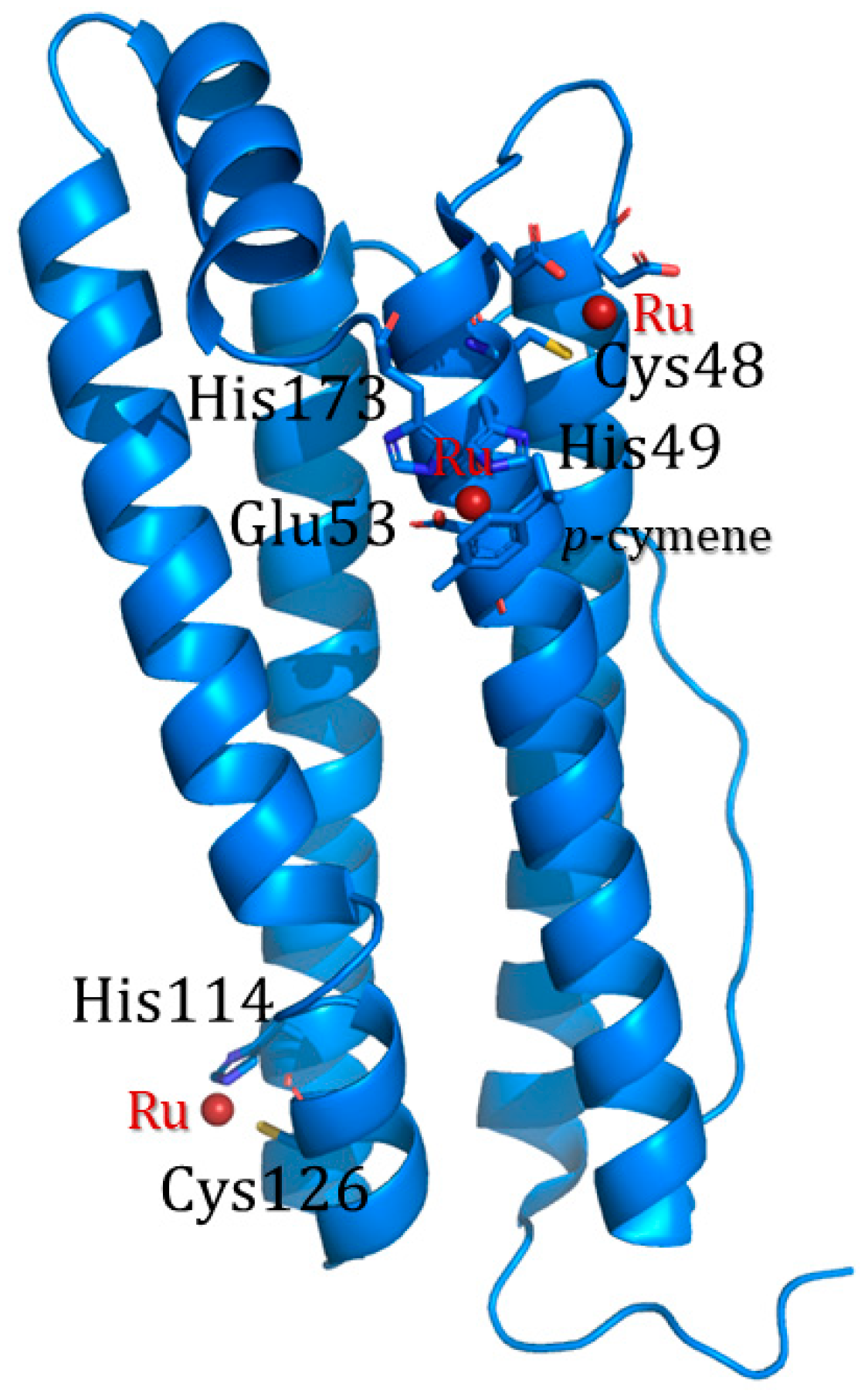
3.5. PINCs Method
4. Conclusions
Funding
Conflicts of Interest
References
- Dautant, A.; Meyer, J.-B.; Yariv, J.; Précigoux, G.; Sweet, R.M.; Kalb, A.J.; Frolow, F. Structure of a Monoclinic Crystal Form of Cytochrome b 1 (Bacterioferritin) from E. Coli. Acta Crystallogr. D Biol. Crystallogr. 1998, 54, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.A.; Filman, D.J.; Finkel, S.E.; Kolter, R.; Hogle, J.M. The Crystal Structure of Dps, a Ferritin Homolog That Binds and Protects DNA. Nat. Struct. Mol. Biol. 1998, 5, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yanatori, I.; Nishina, S.; Kishi, F.; Hino, K. Newly Uncovered Biochemical and Functional Aspects of Ferritin. FASEB J. 2023, 37, e23095. [Google Scholar] [CrossRef]
- He, D.; Hughes, S.; Vanden-Hehir, S.; Georgiev, A.; Altenbach, K.; Tarrant, E.; Mackay, C.L.; Waldron, K.J.; Clarke, D.J.; Marles-Wright, J. Structural Characterization of Encapsulated Ferritin Provides Insight into Iron Storage in Bacterial Nanocompartments. eLife 2016, 5, e18972. [Google Scholar] [CrossRef]
- Yanatori, I.; Kishi, F.; Toyokuni, S. New Iron Export Pathways Acting via Holo-Ferritin Secretion. Arch. Biochem. Biophys. 2023, 746, 109737. [Google Scholar] [CrossRef]
- Hagen, W.R. Maximum Iron Loading of Ferritin: Half a Century of Sustained Citation Distortion. Metallomics 2022, 14, mfac063. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, D.; Arosio, P. Biology of Ferritin in Mammals: An Update on Iron Storage, Oxidative Damage and Neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar] [CrossRef]
- Ross, J.; Lambert, T.; Piergentili, C.; He, D.; Waldron, K.J.; Mackay, C.L.; Marles-Wright, J.; Clarke, D.J. Mass Spectrometry Reveals the Assembly Pathway of Encapsulated Ferritins and Highlights a Dynamic Ferroxidase Interface. Chem. Commun. 2020, 56, 3417–3420. [Google Scholar] [CrossRef]
- Sutter, M.; Boehringer, D.; Gutmann, S.; Günther, S.; Prangishvili, D.; Loessner, M.J.; Stetter, K.O.; Weber-Ban, E.; Ban, N. Structural Basis of Enzyme Encapsulation into a Bacterial Nanocompartment. Nat. Struct. Mol. Biol. 2008, 15, 939–947. [Google Scholar] [CrossRef]
- Jones, J.A.; Giessen, T.W. Advances in Encapsulin Nanocompartment Biology and Engineering. Biotechnol. Bioeng. 2021, 118, 491–505. [Google Scholar] [CrossRef]
- Andrews, S.C. The Ferritin-like Superfamily: Evolution of the Biological Iron Storeman from a Rubrerythrin-like Ancestor. BBA Gen. Subj. 2010, 1800, 691–705. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: The Protein Nanocage and Iron Biomineral in Health and in Disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Powell, L.W.; Alpert, E.; Isselbacher, K.J.; Drysdale, J.W. Human Isoferritins: Organ Specific Iron and Apoferritin Distribution. Br. J. Haematol. 1975, 30, 47–55. [Google Scholar] [CrossRef]
- Hintze, K.J.; Theil, E.C. Cellular Regulation and Molecular Interactions of the Ferritins. Cell Mol. Life Sci. 2006, 63, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.M.; Janney, D.E.; Gerkin, R.C.; Buseck, P.R. The Structure of Ferritin Cores Determined by Electron Nanodiffraction. J. Struct. Biol. 2000, 131, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Koralewski, M.; Balejčíková, L.; Mitróová, Z.; Pochylski, M.; Baranowski, M.; Kopčanský, P. Morphology and Magnetic Structure of the Ferritin Core during Iron Loading and Release by Magnetooptical and NMR Methods. ACS Appl. Mater. Interfaces 2018, 10, 7777–7787. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, G.; Ciambellotti, S.; Messori, L.; Merlino, A. Cisplatin Binding Sites in Human H-Chain Ferritin. Inorg. Chem. 2017, 56, 9064–9070. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Balogh, L.P. Nanomedicine in Cancer; Nanomedicine’s most cited; Pan Stanford Publishing: Singapore, 2017; ISBN 978-981-4745-80-2. [Google Scholar]
- Balogh, L.P. Nano-Enabled Medical Applications; Jenny Stanford Publishing Pte. Ltd.: Singapore, 2021; ISBN 978-0-429-67789-2. [Google Scholar]
- Xia, D.; Tao, J.; He, Y.; Zhu, Q.; Chen, D.; Yu, M.; Cui, F.; Gan, Y. Enhanced Transport of Nanocage Stabilized Pure Nanodrug across Intestinal Epithelial Barrier Mimicking Listeria Monocytogenes. Biomaterials 2015, 37, 320–332. [Google Scholar] [CrossRef]
- Yin, S.; Davey, K.; Dai, S.; Liu, Y.; Bi, J. A Critical Review of Ferritin as a Drug Nanocarrier: Structure, Properties, Comparative Advantages and Challenges. Particuology 2022, 64, 65–84. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin Protein Nanocages Use Ion Channels, Catalytic Sites, and Nucleation Channels to Manage Iron/Oxygen Chemistry. Curr. Opin. Chem. Biol. 2011, 15, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Declercq, J.-P. X-ray Structures of Ferritins and Related Proteins. BBA Gen. Subj. 2010, 1800, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and Uptake of H-Ferritin Are Mediated by Human Transferrin Receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Kidane, T.Z.; Sauble, E.; Linder, M.C. Release of Iron from Ferritin Requires Lysosomal Activity. Am. J. Physiol. Cell Physiol. 2006, 291, C445–C455. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhai, M.; Xie, X.; Zhang, Y.; Ma, S.; Li, Z.; Yu, F.; Zhao, B.; Zhang, M.; Yang, Y.; et al. Apoferritin Nanocage for Brain Targeted Doxorubicin Delivery. Mol. Pharm. 2017, 14, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Fan, K.; Wang, L.; Ying, X.; Sanders, A.J.; Guo, T.; Xing, X.; Zhou, M.; Du, H.; Hu, Y.; et al. TfR1 Binding with H-Ferritin Nanocarrier Achieves Prognostic Diagnosis and Enhances the Therapeutic Efficacy in Clinical Gastric Cancer. Cell Death Dis. 2020, 11, 92. [Google Scholar] [CrossRef]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef]
- Li, J.Y.; Paragas, N.; Ned, R.M.; Qiu, A.; Viltard, M.; Leete, T.; Drexler, I.R.; Chen, X.; Sanna-Cherchi, S.; Mohammed, F.; et al. Scara5 Is a Ferritin Receptor Mediating Non-Transferrin Iron Delivery. Dev. Cell 2009, 16, 35–46. [Google Scholar] [CrossRef]
- Turino, L.N.; Ruggiero, M.R.; Stefanìa, R.; Cutrin, J.C.; Aime, S.; Geninatti Crich, S. Ferritin Decorated PLGA/Paclitaxel Loaded Nanoparticles Endowed with an Enhanced Toxicity Toward MCF-7 Breast Tumor Cells. Bioconj. Chem. 2017, 28, 1283–1290. [Google Scholar] [CrossRef]
- Zang, J.; Chen, H.; Zhao, G.; Wang, F.; Ren, F. Ferritin Cage for Encapsulation and Delivery of Bioactive Nutrients: From Structure, Property to Applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Tang, W.; Guo, C.; Chen, H.; Lin, X.; Liu, G.; Fei, B.; Chen, X.; Xu, B.; Xie, J. Ferritin Nanocages To Encapsulate and Deliver Photosensitizers for Efficient Photodynamic Therapy against Cancer. ACS Nano 2013, 7, 6988–6996. [Google Scholar] [CrossRef] [PubMed]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-Based Nanomedicine Platforms for Drug Delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin Nanoparticles for Targeting and Visualizing Tumour Tissues. Nat. Nanotech. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.; Dafni, H.; Meir, G.; Harmelin, A.; Neeman, M. Ferritin as an Endogenous MRI Reporter for Noninvasive Imaging of Gene Expression in C6 Glioma Tumors. Neoplasia 2005, 7, 109–117. [Google Scholar] [CrossRef]
- Aime, S.; Frullano, L.; Geninatti Crich, S. Compartmentalization of a Gadolinium Complex in the Apoferritin Cavity: A Route To Obtain High Relaxivity Contrast Agents for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2002, 41, 1017–1019. [Google Scholar] [CrossRef]
- Ueno, T.; Abe, M.; Hirata, K.; Abe, S.; Suzuki, M.; Shimizu, N.; Yamamoto, M.; Takata, M.; Watanabe, Y. Process of Accumulation of Metal Ions on the Interior Surface of Apo-Ferritin: Crystal Structures of a Series of Apo-Ferritins Containing Variable Quantities of Pd(II) Ions. J. Am. Chem. Soc. 2009, 131, 5094–5100. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, Y.; Yuan, H.; Gregas, M.K.; Vo-Dinh, T. Apoferritin Protein Cages: A Novel Drug Nanocarrier for Photodynamic Therapy. Chem. Commun. 2008, 14, 4579. [Google Scholar] [CrossRef]
- Sun, C.; Yang, H.; Yuan, Y.; Tian, X.; Wang, L.; Guo, Y.; Xu, L.; Lei, J.; Gao, N.; Anderson, G.J.; et al. Controlling Assembly of Paired Gold Clusters within Apoferritin Nanoreactor for in Vivo Kidney Targeting and Biomedical Imaging. J. Am. Chem. Soc. 2011, 133, 8617–8624. [Google Scholar] [CrossRef]
- Iwahori, K.; Yamashita, I. Fabrication of CdS Nanoparticles in the Bio-Template, Apoferritin Cavity by a Slow Chemical Reaction System. J. Phys. Conf. Ser. 2007, 61, 492–496. [Google Scholar] [CrossRef]
- Butts, C.A.; Swift, J.; Kang, S.; Di Costanzo, L.; Christianson, D.W.; Saven, J.G.; Dmochowski, I.J. Directing Noble Metal Ion Chemistry within a Designed Ferritin Protein. Biochemistry 2008, 47, 12729–12739. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F. Uranium-Loaded Apoferritin with Antibodies Attached: Molecular Design for Uranium Neutron-Capture Therapy. Proc. Natl. Acad. Sci. USA 1992, 89, 11064–11068. [Google Scholar] [CrossRef] [PubMed]
- Hestericová, M.; Heinisch, T.; Lenz, M.; Ward, T.R. Ferritin Encapsulation of Artificial Metalloenzymes: Engineering a Tertiary Coordination Sphere for an Artificial Transfer Hydrogenase. Dalton Trans. 2018, 47, 10837–10841. [Google Scholar] [CrossRef]
- Chasteen, N.D.; Harrison, P.M. Mineralization in Ferritin: An Efficient Means of Iron Storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef]
- Zhu, B.; Lin, Q.; Ke, C.-H.; Huang, H.-Q. Single Subunit Type of Ferritin from Visceral Mass of Saccostrea Cucullata: Cloning, Expression and Cisplatin-Subunit Analysis. Fish Shellfish Immunol. 2011, 31, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Huang, L.; Huang, H.-Q. Cloning Analysis of Ferritin and the Cisplatin-Subunit for Cancer Cell Apoptosis in Aplysia Juliana Hepatopancreas. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 156, 95–103. [Google Scholar] [CrossRef]
- Ciambellotti, S.; Pratesi, A.; Severi, M.; Ferraro, G.; Alessio, E.; Merlino, A.; Messori, L. The NAMI A—Human Ferritin System: A Biophysical Characterization. Dalton Trans. 2018, 47, 11429–11437. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Diao, H.; Zhang, J.; Li, H.; Sun, H.; Guo, Z. Encapsulation of Platinum Anticancer Drugs by Apoferritin. Chem. Commun. 2007, 7, 3453. [Google Scholar] [CrossRef]
- Suzuki, M.; Abe, M.; Ueno, T.; Abe, S.; Goto, T.; Toda, Y.; Akita, T.; Yamada, Y.; Watanabe, Y. Preparation and Catalytic Reaction of Au/Pd Bimetallic Nanoparticles in Apo-Ferritin. Chem. Commun. 2009, 28, 4871. [Google Scholar] [CrossRef]
- Niemeyer, J.; Abe, S.; Hikage, T.; Ueno, T.; Erker, G.; Watanabe, Y. Noncovalent Insertion of Ferrocenes into the Protein Shell of Apo-Ferritin. Chem. Commun. 2008, 28, 6519. [Google Scholar] [CrossRef]
- Takezawa, Y.; Böckmann, P.; Sugi, N.; Wang, Z.; Abe, S.; Murakami, T.; Hikage, T.; Erker, G.; Watanabe, Y.; Kitagawa, S.; et al. Incorporation of Organometallic Ru Complexes into Apo-Ferritin Cage. Dalton Trans. 2011, 40, 2190–2195. [Google Scholar] [CrossRef]
- Abe, S.; Hirata, K.; Ueno, T.; Morino, K.; Shimizu, N.; Yamamoto, M.; Takata, M.; Yashima, E.; Watanabe, Y. Polymerization of Phenylacetylene by Rhodium Complexes within a Discrete Space of Apo-Ferritin. J. Am. Chem. Soc. 2009, 131, 6958–6960. [Google Scholar] [CrossRef]
- Abe, S.; Niemeyer, J.; Abe, M.; Takezawa, Y.; Ueno, T.; Hikage, T.; Erker, G.; Watanabe, Y. Control of the Coordination Structure of Organometallic Palladium Complexes in an Apo-Ferritin Cage. J. Am. Chem. Soc. 2008, 130, 10512–10514. [Google Scholar] [CrossRef]
- Cohen, M.H.; Williams, G.A.; Sridhara, R.; Chen, G.; McGuinn, W.D.; Morse, D.; Abraham, S.; Rahman, A.; Liang, C.; Lostritto, R.; et al. United States Food and Drug Administration Drug Approval Summary. Clin. Cancer Res. 2004, 10, 1212–1218. [Google Scholar] [CrossRef]
- Kuruppu, A.I.; Zhang, L.; Collins, H.; Turyanska, L.; Thomas, N.R.; Bradshaw, T.D. An Apoferritin-based Drug Delivery System for the Tyrosine Kinase Inhibitor Gefitinib. Adv. Health Mater. 2015, 4, 2816–2821. [Google Scholar] [CrossRef]
- Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. pH-Dependent Structures of Ferritin and Apoferritin in Solution: Disassembly and Reassembly. Biomacromolecules 2011, 12, 1629–1640. [Google Scholar] [CrossRef]
- Webb, B.; Frame, J.; Zhao, Z.; Lee, M.L.; Watt, G.D. Molecular Entrapment of Small Molecules within the Interior of Horse Spleen Ferritin. Arch. Biochem. Biophys. 1994, 309, 178–183. [Google Scholar] [CrossRef]
- Simsek, E.; Akif Kilic, M. Magic Ferritin: A Novel Chemotherapeutic Encapsulation Bullet. J. Magn. Magn. Mater. 2005, 293, 509–513. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Chen, H.; Sun, J.; Feng, F. Protein Nanocages for Delivery and Release of Luminescent Ruthenium(II) Polypyridyl Complexes. ACS Appl. Mater. Interfaces 2016, 8, 22756–22761. [Google Scholar] [CrossRef]
- Li, L.; Muñoz-Culla, M.; Carmona, U.; Lopez, M.P.; Yang, F.; Trigueros, C.; Otaegui, D.; Zhang, L.; Knez, M. Ferritin-Mediated siRNA Delivery and Gene Silencing in Human Tumor and Primary Cells. Biomaterials 2016, 98, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, F.; Digiacomo, L.; Marchini, C.; Amici, A.; Salomone, F.; Fiume, G.; Rossetta, A.; Gratton, E.; Pozzi, D.; Caracciolo, G. The Intracellular Trafficking Mechanism of Lipofectamine-Based Transfection Reagents and Its Implication for Gene Delivery. Sci. Rep. 2016, 6, 25879. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, N.; Pane, F.; Messori, L.; Amoresano, A.; Merlino, A. Cisplatin Encapsulation within a Ferritin Nanocage: A High-Resolution Crystallographic Study. Chem. Commun. 2016, 52, 4136–4139. [Google Scholar] [CrossRef]
- Ferraro, G.; Monti, D.M.; Amoresano, A.; Pontillo, N.; Petruk, G.; Pane, F.; Cinellu, M.A.; Merlino, A. Gold-Based Drug Encapsulation within a Ferritin Nanocage: X-ray Structure and Biological Evaluation as a Potential Anticancer Agent of the Auoxo3-Loaded Protein. Chem. Commun. 2016, 52, 9518–9521. [Google Scholar] [CrossRef]
- Petruk, G.; Monti, D.M.; Ferraro, G.; Pica, A.; D’Elia, L.; Pane, F.; Amoresano, A.; Furrer, J.; Kowalski, K.; Merlino, A. Encapsulation of the Dinuclear Trithiolato-Bridged Arene Ruthenium Complex Diruthenium-1 in an Apoferritin Nanocage: Structure and Cytotoxicity. ChemMedChem 2019, 14, 594–602. [Google Scholar] [CrossRef]
- Ferraro, G.; Petruk, G.; Maiore, L.; Pane, F.; Amoresano, A.; Cinellu, M.A.; Monti, D.M.; Merlino, A. Caged Noble Metals: Encapsulation of a Cytotoxic Platinum(II)-Gold(I) Compound within the Ferritin Nanocage. Int. J. Biol. Macromol. 2018, 115, 1116–1121. [Google Scholar] [CrossRef]
- Ferraro, G.; Pratesi, A.; Cirri, D.; Imbimbo, P.; Maria Monti, D.; Messori, L.; Merlino, A. Arsenoplatin-Ferritin Nanocage: Structure and Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 1874. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Petruk, G.; Maiore, L.; Pane, F.; Amoresano, A.; Cinellu, M.A.; Merlino, A. Ferritin Nanocages Loaded with Gold Ions Induce Oxidative Stress and Apoptosis in MCF-7 Human Breast Cancer Cells. Dalton Trans. 2017, 46, 15354–15362. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, N.; Ferraro, G.; Helliwell, J.R.; Amoresano, A.; Merlino, A. X-ray Structure of the Carboplatin-Loaded Apo-Ferritin Nanocage. ACS Med. Chem. Lett. 2017, 8, 433–437. [Google Scholar] [CrossRef]
- Lucignano, R.; Pratesi, A.; Imbimbo, P.; Monti, D.M.; Picone, D.; Messori, L.; Ferraro, G.; Merlino, A. Evaluation of Auranofin Loading within Ferritin Nanocages. Int. J. Mol. Sci. 2022, 23, 14162. [Google Scholar] [CrossRef]
- Monti, D.M.; Ferraro, G.; Merlino, A. Ferritin-Based Anticancer Metallodrug Delivery: Crystallographic, Analytical and Cytotoxicity Studies. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101997. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Auranofin: Past to Present, and Repurposing. Int. Immunopharmacol. 2021, 101, 108272. [Google Scholar] [CrossRef]
- Conti, L.; Ciambellotti, S.; Giacomazzo, G.E.; Ghini, V.; Cosottini, L.; Puliti, E.; Severi, M.; Fratini, E.; Cencetti, F.; Bruni, P.; et al. Ferritin Nanocomposites for the Selective Delivery of Photosensitizing Ruthenium-Polypyridyl Compounds to Cancer Cells. Inorg. Chem. Front. 2022, 9, 1070–1081. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, R.; Zang, J.; Zhou, T.; Zhao, G. Encapsulation of β-Carotene within Ferritin Nanocages Greatly Increases Its Water-Solubility and Thermal Stability. Food Chem. 2014, 149, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-Y.; Chen, B.H. Analysis and Stability of Carotenoids in the Flowers of Daylily (Hemerocallis d Isticha) as Affected by Various Treatments. J. Agric. Food Chem. 2000, 48, 5962–5968. [Google Scholar] [CrossRef]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-Ferritin–Nanocaged Doxorubicin Nanoparticles Specifically Target and Kill Tumors with a Single-Dose Injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef]
- Lucignano, R.; Stanzione, I.; Ferraro, G.; Di Girolamo, R.; Cané, C.; Di Somma, A.; Duilio, A.; Merlino, A.; Picone, D. A New and Efficient Procedure to Load Bioactive Molecules within the Human Heavy-Chain Ferritin Nanocage. Front. Mol. Biosci. 2023, 10, 1008985. [Google Scholar] [CrossRef]
- Inoue, I.; Chiba, M.; Ito, K.; Okamatsu, Y.; Suga, Y.; Kitahara, Y.; Nakahara, Y.; Endo, Y.; Takahashi, K.; Tagami, U.; et al. One-Step Construction of Ferritin Encapsulation Drugs for Cancer Chemotherapy. Nanoscale 2021, 13, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Chen, Z.; Cherrier, M.V.; Martin, L.; Bui, T.T.T.; Li, W.; Lynham, S.; Nicolet, Y.; Ebrahimi, K.H. A Versatile Virus-Mimetic Engineering Approach for Concurrent Protein Nanocage Surface-Functionalization and Cargo Encapsulation. Small 2024, 20, e2310913. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [Google Scholar] [CrossRef]
- Sundquist, W.I.; Kräusslich, H.-G. HIV-1 assembly, budding, and maturation. Cold Spring Harb. Perspect. Med. 2012, 2, a006924. [Google Scholar] [CrossRef] [PubMed]
- Tatur, J.; Hagedoorn, P.-L.; Overeijnder, M.L.; Hagen, W.R. A highly thermostable ferritin from the hyperthermophilic archaeal anaerobe Pyrococcus furiosus. Extremophiles 2006, 10, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; Bian, Y.; Wang, S.; Chai, Q.; Guo, Z.; Wang, Z.; Zhu, P.; Peng, P.; Yan, X.; et al. Dual-targeting nanoparticle vaccine elicits a therapeutic antibody response against chronic hepatitis B. Nat. Nanotechnol. 2020, 15, 406–416. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, D.; He, J.; Hong, J.; Yuan, C.; Liang, M. Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery. Nat. Protoc. 2021, 16, 4878–4896. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Zhang, S.; Chen, X.; Sun, G.; Zhang, B.; Jiang, B.; Yang, Y.; Yan, X.; Fan, K. Re-engineering the inner surface of ferritin nanocage enables dual drug payloads for synergistic tumor therapy. Theranostics 2022, 12, 1800–1815. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zhang, R.; Chen, X.; Sun, G.; Zhou, M.; Han, Q.; Zhang, B.; Zhao, Y.; Jiang, B.; et al. Bioengineered Dual-Targeting Protein Nanocage for Stereoscopical Loading of Synergistic Hydrophilic/Hydrophobic Drugs to Enhance Anticancer Efficacy. Adv. Funct. Mater. 2021, 31, 2102004. [Google Scholar] [CrossRef]


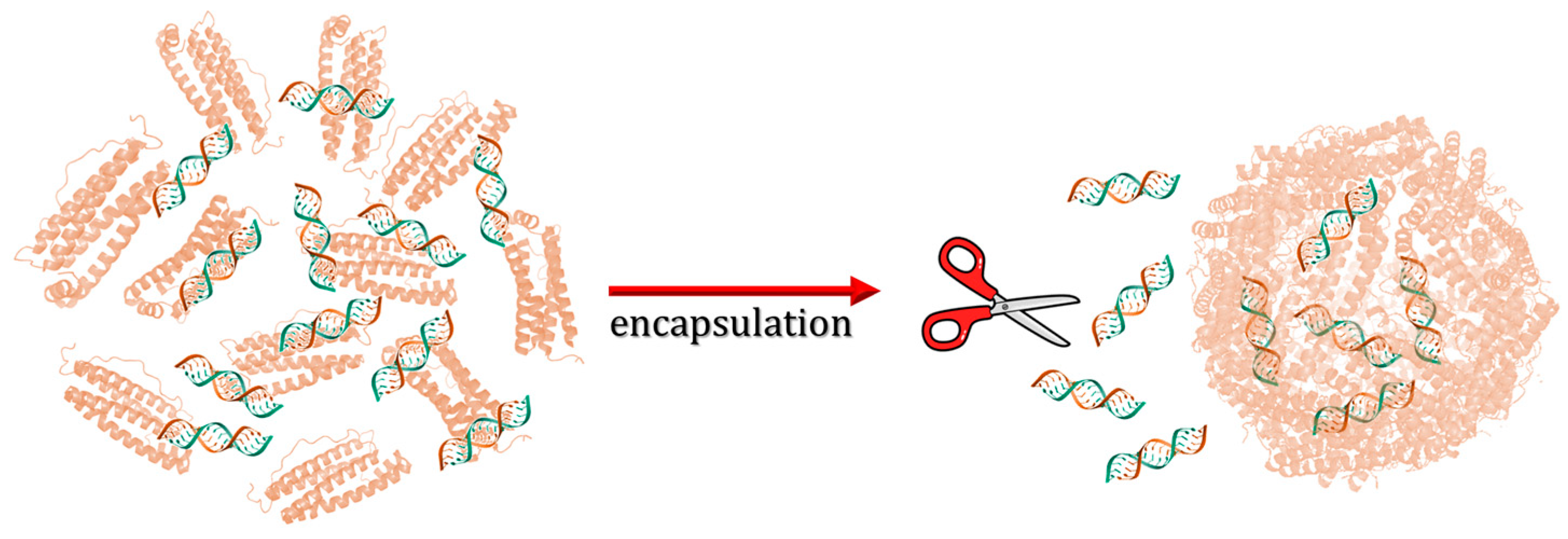
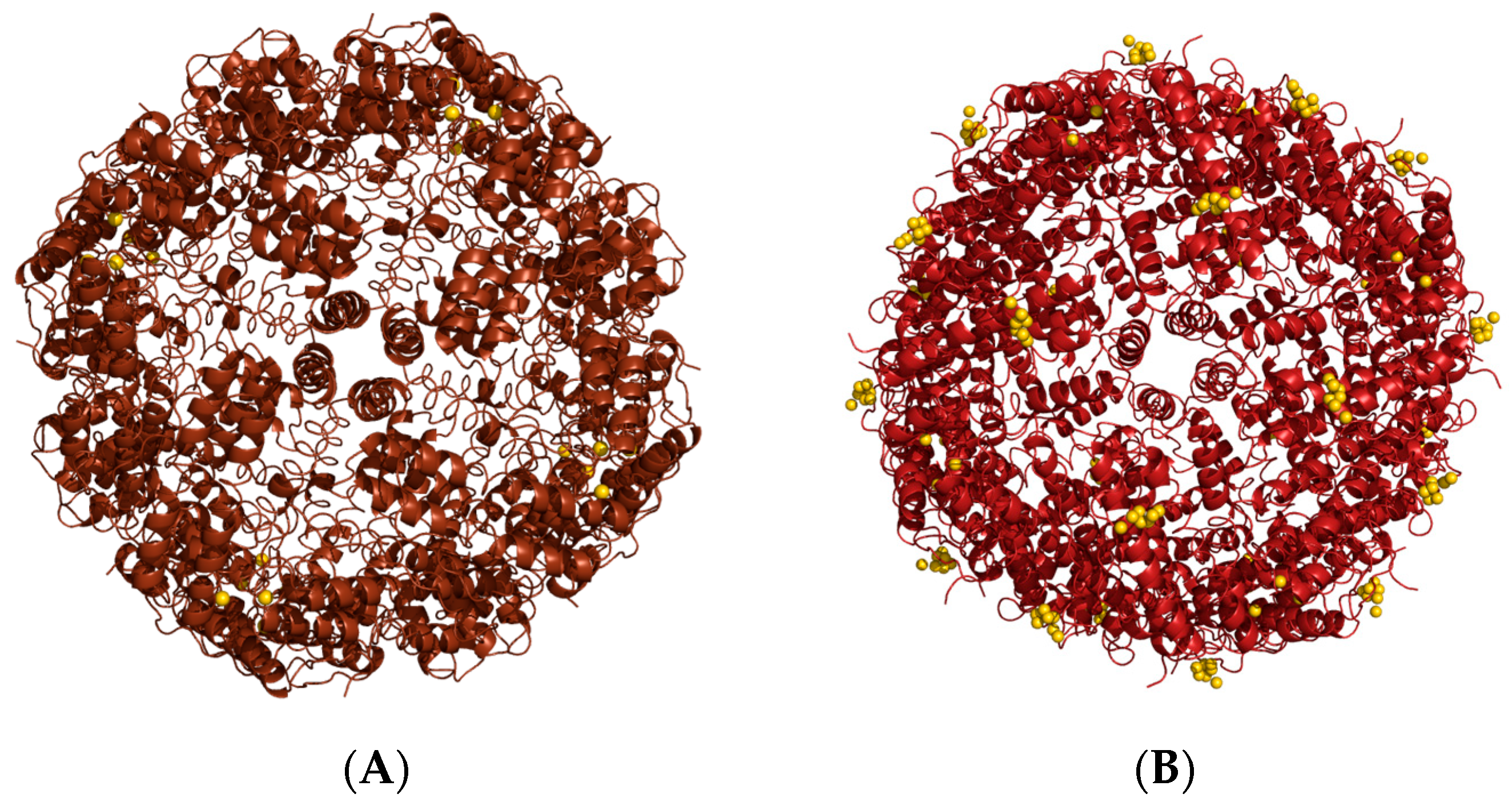
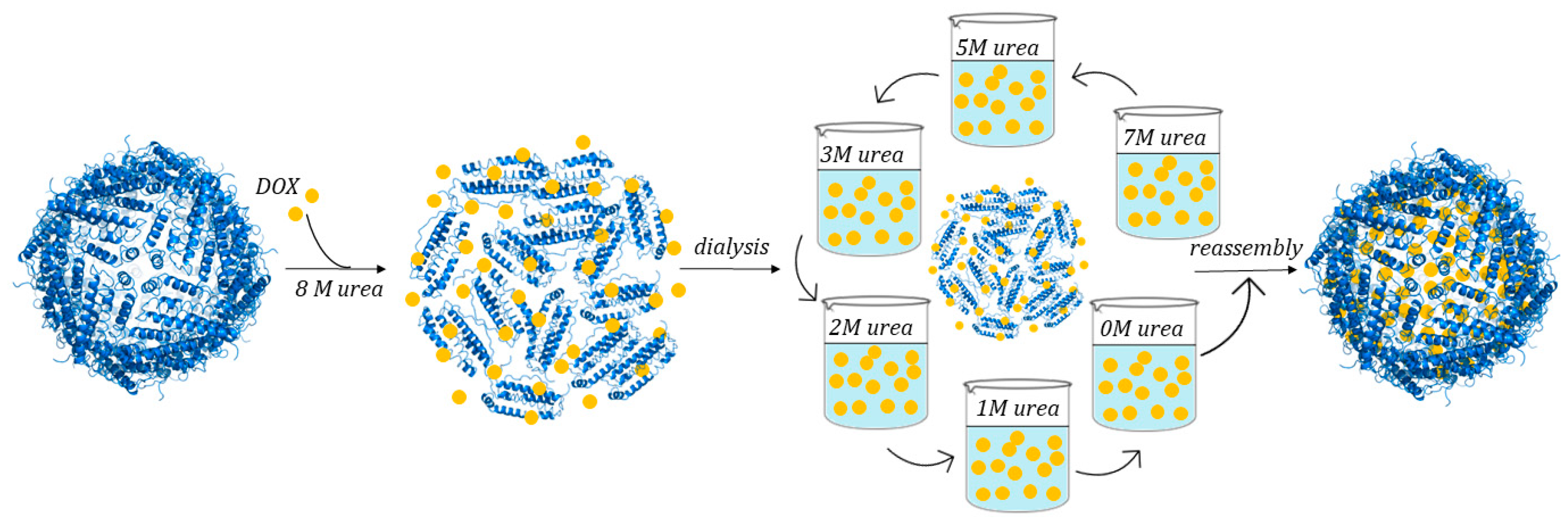

| Samples | Initial Concentration (μM) | Final Concentration (μM) | Percentage Lost (%) |
|---|---|---|---|
| FR1 | 2.5 | 0.8 | 68 |
| FR8 | 2.5 | 1.6 | 36 |
| FR17 | 2.5 | 1.5 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucignano, R.; Ferraro, G. Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods. Molecules 2024, 29, 4045. https://doi.org/10.3390/molecules29174045
Lucignano R, Ferraro G. Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods. Molecules. 2024; 29(17):4045. https://doi.org/10.3390/molecules29174045
Chicago/Turabian StyleLucignano, Rosanna, and Giarita Ferraro. 2024. "Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods" Molecules 29, no. 17: 4045. https://doi.org/10.3390/molecules29174045
APA StyleLucignano, R., & Ferraro, G. (2024). Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods. Molecules, 29(17), 4045. https://doi.org/10.3390/molecules29174045







