Synthesis and Photocatalytic sp3 C-H Bond Functionalization of Salen-Ligand-Supported Uranyl(VI) Complexes
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization
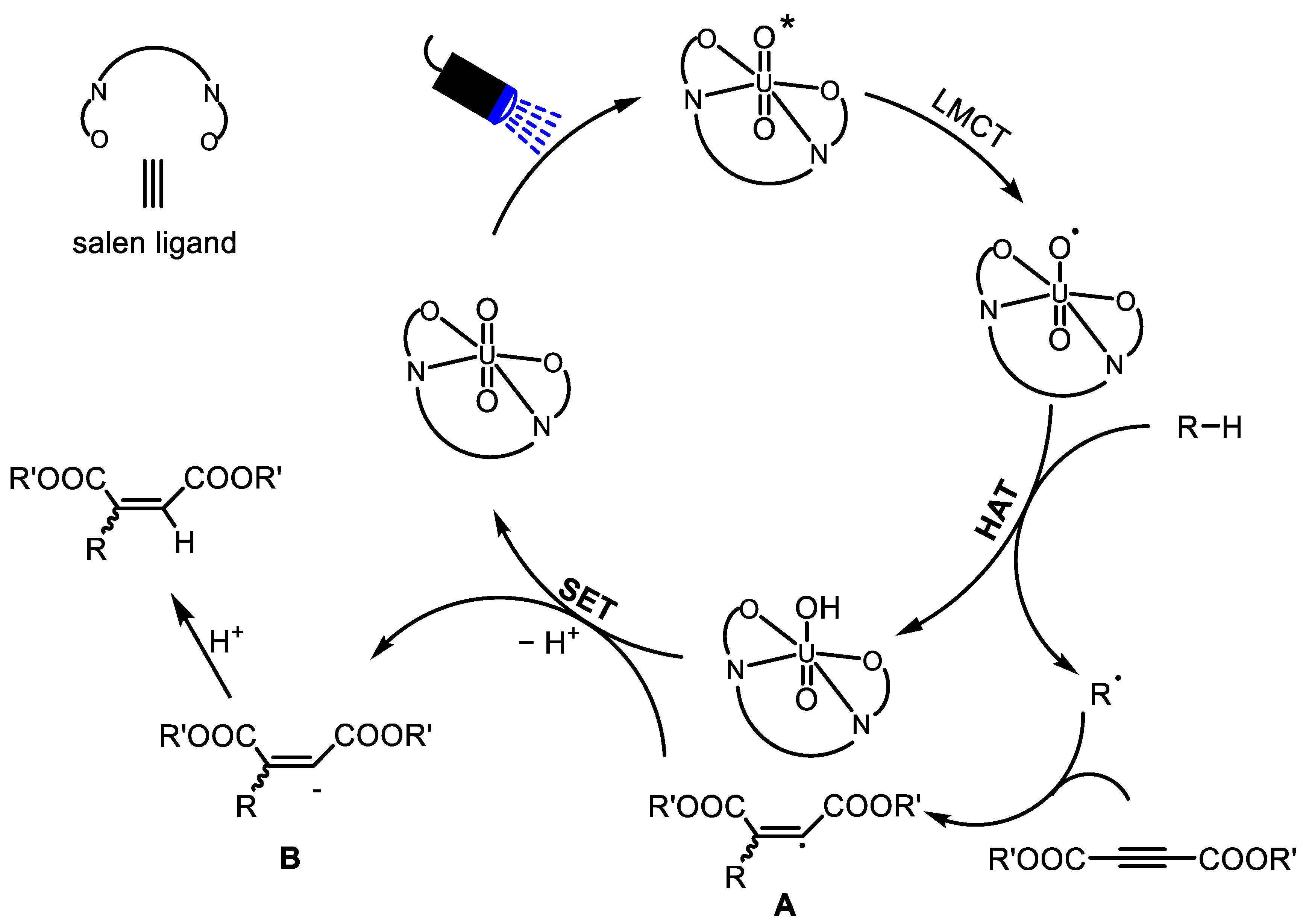
2.2. Photocatalytic Property
3. Materials and Methods
Materials and Instruments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Photocatalysis. A multi-faceted concept for green chemistry. Chem. Soc. Rev. 2009, 38, 1999–2011. [Google Scholar] [CrossRef]
- Qiao, Y.; Schelter, E.J. Lanthanide photocatalysis. Acc. Chem. Res. 2018, 51, 2926–2936. [Google Scholar] [CrossRef]
- Wu, C.; Corrigan, N.; Lim, C.-H.; Liu, W.; Miyake, G.; Boyer, C. Rational design of photocatalysts for controlled polymerization: Effect of structures on photocatalytic activities. Chem. Rev. 2022, 122, 5476–5518. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Exploration of visible-light photocatalysis in heterocycle synthesis and functionalization: Reaction design and beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef]
- Fagnoni, M.; Dondi, D.; Ravelli, D.; Albini, A. Photocatalysis for the formation of the C-C bond. Chem. Rev. 2007, 107, 2725–2756. [Google Scholar] [CrossRef]
- Witzel, S.; Hashmi, A.S.K.; Xie, J. Light in gold catalysis. Chem. Rev. 2021, 121, 8868–8925. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Chen, J.-R.; Xiao, W.-J. Controllable remote C-H bond functionalization by visible-light photocatalysis. Angew. Chem. Int. Ed. 2017, 56, 1960–1962. [Google Scholar] [CrossRef]
- Wang, X.; Luo, N.; Wang, F. Advances and challenges of photocatalytic methane C-C coupling. Chin. J. Chem. 2022, 40, 1492–1505. [Google Scholar] [CrossRef]
- Ye, Z.; Yu, Y.; Lin, Y.-M.; Chen, Y.; Song, S.; Gong, L. Photochemical diversification of strong C(sp3)-H bonds enabled by allyl bromide and sodium fluoride. Nat. Synth. 2023, 2, 766–777. [Google Scholar] [CrossRef]
- Yan, J.; Tang, H.; Kuek, E.J.R.; Shi, X.; Liu, C.; Piper, J.L.; Duan, S.; Zhang, M.; Wu, J. Divergent functionalization of aldehydes photocatalyzed by neutral eosin Y with sulfone reagents. Nat. Commun. 2021, 12, 7214. [Google Scholar] [CrossRef]
- Li, Y.; Lei, M.; Gong, L. Photocatalytic regio-and stereoselective C(sp3)-H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2019, 2, 1016–1026. [Google Scholar] [CrossRef]
- Shen, X.; Huang, C.; Yuan, X.-A.; Yu, S. Diastereoselective and stereodivergent synthesis of 2-cinnamylpyrrolines enabled by photoredox-catalyzed iminoalkenylation of alkenes. Angew. Chem. Int. Ed. 2021, 60, 9672–9679. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xu, Z. Photocatalytic dearomative construction of bridged-ring compounds. Chin. J. Org. Chem. 2024, 44, 670–671. [Google Scholar] [CrossRef]
- Dong, Z.; MacMillan, D.W.C. Metallaphotoredox-enabled deoxygenative arylation of alcohols. Nature 2021, 598, 451–456. [Google Scholar] [CrossRef]
- Wang, P.-Z.; Xiao, W.-J.; Chen, J.-R. Electrochemical reactors enable divergent site selectivity in the C-H carboxylation of N-heteroarenes. Angew. Chem. Int. Ed. 2023, 62, e202302227. [Google Scholar]
- Bay, A.V.; Farnam, E.J.; Scheidt, K.A. Synthesis of cyclohexanones by a tandem photocatalyzed annulation. J. Am. Chem. Soc. 2022, 144, 7030–7037. [Google Scholar] [CrossRef]
- Wang, Z.; Hisahiro, E. Recent trends in phenol synthesis by photocatalytic oxidation of benzene. Dalton Trans. 2023, 52, 9525–9540. [Google Scholar] [CrossRef]
- Henry Blackwell, J.; Harris, G.R.; Smith, M.A.; Gaunt, M.J. Modular photocatalytic synthesis of α-trialkyl-α-tertiary amines. J. Am. Chem. Soc. 2021, 143, 15946–15959. [Google Scholar] [CrossRef]
- Hsieh, S.-Y.; Bode, J.W. Silicon amine reagents for the photocatalytic synthesis of piperazines from aldehydes and ketones. Org. Lett. 2016, 18, 2098–2101. [Google Scholar] [CrossRef]
- Shaw, M.H.; Shurtleff, V.W.; Terrett, J.A.; Cuthbertson, J.D.; MacMillan, D.W.C. Native functionality in triple catalytic cross-coupling: sp3 C-H bonds as latent nucleophiles. Science 2016, 352, 1304–1308. [Google Scholar] [CrossRef]
- Ding, M.; Zhou, S.; Yao, S.; Zhu, C.; Li, W.; Xie, J. Decarboxylative amination with nitroarenes via synergistic catalysis. Chin. J. Chem. 2024, 42, 351–355. [Google Scholar] [CrossRef]
- Heck, R.F. Acylation, methylation, and carboxyalkylation of olefins by group VIII metal derivatives. J. Am. Chem. Soc. 1968, 90, 5518–5526. [Google Scholar] [CrossRef]
- Song, G.; Xue, D. Research progress on light-promoted transition metal-catalyzed C-Heteroatom bond coupling reactions. Chin. J. Org. Chem. 2022, 42, 2275. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Decatungstate anion for photocatalyzed “window ledge” reactions. Acc. Chem. Res. 2016, 49, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Catalyst-controlled site-selective bond activation. Acc. Chem. Res. 2017, 50, 549–555. [Google Scholar] [CrossRef]
- Britton, L.; Docherty, J.H.; Nichol, G.S.; Dominey, A.P.; Thomas, S.P. Iron-catalysed C(sp2)-H borylation with expanded functional group tolerance. Chin. J. Chem. 2022, 40, 2875–2881. [Google Scholar] [CrossRef]
- Gramage-Doria, R.; Bruneau, C. Carbon-carbon bond forming reactions in diazines via transition-metal-catalyzed C-H bond activation. Synthesis 2023, 55, 3470–3486. [Google Scholar] [CrossRef]
- Chen, Z.; Rong, M.-Y.; Nie, J.; Zhu, X.-F.; Shi, B.-F.; Ma, J.-A. Catalytic alkylation of unactivated C(sp3)-H bonds for C(sp3)-C(sp3) bond formation. Chem. Soc. Rev. 2019, 48, 4921–4942. [Google Scholar] [CrossRef]
- Burrows, H.D.; Kemp, T.J. The photochemistry of the uranyl ion. Chem. Soc. Rev. 1974, 3, 139–165. [Google Scholar] [CrossRef]
- Fox, A.R.; Bart, S.C.; Meyer, K.; Cummins, C.C. Towards uranium catalysts. Nature 2008, 455, 341–349. [Google Scholar] [CrossRef]
- Li, Y.; Su, J.; Mitchell, E.; Zhang, G.; Li, J. Photocatalysis with visible-light-active uranyl complexes. Sci. China Chem. 2013, 56, 1671–1681. [Google Scholar] [CrossRef]
- Liddle, S.T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 2015, 54, 8604–8641. [Google Scholar] [CrossRef]
- Sytko, V.V.; Umreiko, D.S. Spectroscopic properties and electronic structure of uranyl complex compounds. J. Appl. Spectrosc. 1998, 65, 857–870. [Google Scholar] [CrossRef]
- Coughlin, E.J.; Qiao, Y.; Lapsheva, E.; Zeller, M.; Schelter, E.J.; Bart, S.C. Uranyl functionalization mediated by redox-active ligands: Generation of O-C bonds via acylation. J. Am. Chem. Soc. 2018, 141, 1016–1026. [Google Scholar] [CrossRef]
- Arnold, P.L.; Turner, Z.R. Carbon oxygenate transformations by actinide compounds and catalysts. Nat. Rev. Chem. 2017, 1, 0002. [Google Scholar] [CrossRef]
- Tang, S.-B.; Zhang, S.-Y.; Li, W.-J.; Jiang, Y.-X.; Wang, Z.-X.; Long, B.; Su, J. Photocatalytic oxidative cleavage of aryl alkene C=C bonds using a uranyl cation. Org. Chem. Front. 2023, 10, 5130–5137. [Google Scholar] [CrossRef]
- Kannan, S.; Vaughn, A.E.; Weis, E.M.; Barnes, C.L.; Duval, P.B. Anhydrous photochemical uranyl(VI) reduction: Unprecedented retention of equatorial coordination accompanying reversible axial oxo/alkoxide exchange. J. Am. Chem. Soc. 2006, 128, 14024–14025. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Tang, S.-B.; Jiang, Y.-X.; Zhu, R.-Y.; Wang, Z.-X.; Long, B.; Su, J. Mechanism of the visible-light-promoted C(sp3)-H oxidation via uranyl photocatalysis. Inorg. Chem. 2024, 63, 2418–2430. [Google Scholar] [CrossRef]
- Liu, H.; Ghatak, T.; Eisen, M.S. Organoactinides in catalytic transformations: Scope, mechanisms and Quo Vadis. Chem. Commun. 2017, 53, 11278–11297. [Google Scholar] [CrossRef]
- Sessler, J.L.; Melfi, P.J.; Pantos, G.D. Urnanium complexes of multidentate N-donor ligands. Coord. Chem. Rev. 2006, 250, 816–843. [Google Scholar] [CrossRef]
- Fonseca, S.M.; Burrows, H.D.; Miguel, M.G.; Sarakha, M.; Bolte, M. Photooxidation of cellulose acetate and cellobiose by the uranyl ion. Photochem. Photobiol. Sci. 2004, 3, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Behera, N.; Sethi, S. Unprecedented catalytic behavior of uranyl(VI) compounds in chemical reactions. Eur. J. Inorg. Chem. 2021, 95–111. [Google Scholar] [CrossRef]
- Vallet, V.; Wahlgren, U.; Schimmelpfennig, B.; Szabó, Z.; Grenthe, I. The mechanism for water exchange in [UO2(H2O)5]2+ and [UO2(Oxalate)2(H2O)]2−, as studied by quantum chemical methods. J. Am. Chem. Soc. 2001, 123, 11999–12008. [Google Scholar] [CrossRef]
- Mao, Y.; Bakac, A. Uranyl-sensitized photochemical oxidation of naphthalene by molecular oxygen. Role of electron transfer. J. Phys. Chem. 1997, 101, 7929–7933. [Google Scholar] [CrossRef]
- Sarakha, M.; Bolte, M.; Burrows, H.D. Electron-transfer oxidation of chlorophenols by uranyl ion excited state in aqueous solution: Steady-state and nanosecond flash photolysis studies. J. Phys. Chem. 2000, 104, 3142–3149. [Google Scholar] [CrossRef][Green Version]
- Mao, Y.; Bakac, A. Photocatalytic oxidation of toluene to benzaldehyde by molecular oxygen. J. Phys. Chem. 1996, 100, 4219–4223. [Google Scholar] [CrossRef]
- West, J.G.; Bedell, T.A.; Sorensen, E.J. The uranyl cation as a visible-light photocatalyst for C(sp3)-H fluorination. Angew. Chem. Int. Ed. 2016, 55, 8923–8927. [Google Scholar] [CrossRef]
- Capaldo, L.; Merli, D.; Fagnoni, M.; Ravelli, D. Visible light uranyl photocatalysis: Direct C-H to C-C bond conversion. ACS Catal. 2019, 9, 3054–3058. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, Y.; Yu, L.; Ni, S.; Wang, Y.; Pan, Y. Uranyl-catalysed C-H alkynylation and olefination. Org. Chem. Front. 2021, 8, 5968–5974. [Google Scholar] [CrossRef]
- Hu, D.; Zhou, Y.; Jiang, X. From aniline to phenol: Carbon-nitrogen bond activation via uranyl photoredox catalysis. Natl. Sci. Rev. 2022, 9, nwab156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rizvi, S.A.; Hu, D.; Sun, D.; Gao, A.; Zhou, Y.; Li, J.; Jiang, X. Selective late-stage oxygenation of sulfides with ground-state oxygen by uranyl photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 13499–13506. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, D.; Li, D.; Jiang, X. Uranyl-photocatalyzed hydrolysis of diaryl ethers at ambient environment for the directional degradation of 4-O-5 lignin. JACS Au 2021, 1, 1141–1146. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, X. Stepwise benzylic oxygenation via uranyl-photocatalysis. Green Chem. 2022, 24, 124–129. [Google Scholar] [CrossRef]
- Jia, Y.; Meng, J.; Hu, D.; Kang, H.; Jiang, X. Hydroxylation of organoborons via uranyl photocatalysis. Org. Chem. Front. 2023, 10, 2688–2694. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, Y.; Li, D.; Jiang, X. Degradation of plastic wastes to commercial chemicals and monomers under visible light. Sci. Bull. 2023, 68, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- McGrail, B.T.; Pianowski, L.S.; Burns, P.C. Photochemical water oxidation and origin of nonaqueous uranyl peroxide complexes. J. Am. Chem. Soc. 2014, 136, 4797–4800. [Google Scholar] [CrossRef]
- Meng, J.; Ji, L.; Jiang, X. Deprotection of benzyl-derived groups via uranyl-photocatalysis. Organometallics 2024, 43, 1682–1686. [Google Scholar] [CrossRef]
- Zhao, X.; Bai, L.; Li, J.; Jiang, X. Photouranium-catalyzed C-F activation hydroxylation via water splitting. J. Am. Chem. Soc. 2024, 146, 11173–11180. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, C.; Zhou, R.; Gao, W.; Wang, S.; Liu, K.; Chen, S.; Hu, K.; Mei, L.; Yuan, L.; et al. Visible-light-enabled C-H functionalization by a direct hydrogen atom transfer uranyl photocatalyst. Chem.-Eur. J. 2020, 26, 16521–16529. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, S.; Liu, K.; Yuan, L.; Zhao, Y.; Chai, Z.; Mei, L. Facile construction of diverse diarylmethane scaffolds via uranyl-catalyzed 1,6-addition reaction. Tetrahedron Lett. 2020, 61, 152076. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Chai, Z.; Shi, W.; Yuan, L. Is the sacrificial agent really just a sacrificial agent? A case study on the photocatalytic reduction of U(VI) by alcohols. Chem. Eng. J. 2023, 460, 141742. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.I.; Trzesowska-Kruszynska, A.; Kruszynski, R.; Kumar, P.; Jain, S.L. Seven-coordinated chiral uranyl(VI) salen complex as effective catalyst for C-H bond activation of dialkylanilines under visible light. Polyhedron 2017, 124, 177–183. [Google Scholar] [CrossRef]
- Arnold, P.L.; Purkis, J.M.; Rutkauskaite, R.; Kovacs, D.; Love, J.B.; Austin, J. Controlled photocatalytic hydrocarbon oxidation by uranyl complexes. ChemCatChem 2019, 11, 3786–3790. [Google Scholar] [CrossRef]
- Takao, K.; Tsushima, S. The oxidation of borohydrides by photoexcited [UO2(CO3)3]4−. Dalton Trans. 2018, 47, 5149–5152. [Google Scholar] [CrossRef] [PubMed]
- Mashita, T.; Tsushima, S.; Takao, K. Photocatalytic oxygenation of cyclohexene initiated by excitation of [UO2(OPCyPh2)4]2+ under visible light. ACS Omega 2019, 4, 7194–7199. [Google Scholar] [CrossRef]
- Deka, H.; Fridman, N.; Eisen, M.S. A sacrificial iminato ligand in the catalytic cyanosilylation of ketones promoted by organoactinide complexes. Inorg. Chem. 2022, 61, 3598–3606. [Google Scholar] [CrossRef]
- Sun, X.; Gong, X.; Xie, Z.; Zhu, C. A uranium(IV) alkyl complex: Synthesis and catalytic property in carbonyl hydroboration. Chin. J. Chem. 2022, 40, 2047–2053. [Google Scholar] [CrossRef]
- Makarov, K.; Ritacco, I.; Fridman, N.; Caporaso, L.; Eisen, M.S. Against all odds, uranium and thorium iminato complexes enable the cleavage of C=O bonds in isocyanates. ACS Catal. 2023, 13, 11798–11814. [Google Scholar] [CrossRef]
- Makarov, K.; Kaushansky, A.; Eisen, M.S. Catalytic hydroboration of esters by versatile thorium and uranium amide complexes. ACS Catal. 2022, 12, 273–284. [Google Scholar] [CrossRef]
- Yu, J.; Liu, K.; Wu, Q.; Li, B.; Kong, X.; Hu, K.; Mei, L.; Yuan, L.; Chai, Z.; Shi, W. Facile access to uranium and thorium phosphaethynolate complexes supported by tren: Experimental and theoretical study. Chin. J. Chem. 2021, 39, 2125–2131. [Google Scholar] [CrossRef]
- Makarov, K.; Saha, S.; Ghatak, T.; Fridman, N.; Eisen, M.S. Remodeling of N-Heterocyclic iminato ligand frameworks for the facile synthesis of isoureas from alcohols and carbodiimides promoted by organoactinide (Th, U) complexes. ACS Omega 2021, 6, 14692–14700. [Google Scholar] [CrossRef]
- Saha, S.; Eisen, M.S. Mild catalytic deoxygenation of amides promoted by thorium metallocene. Dalton Trans. 2020, 49, 12835–12841. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Kato, M.; Takao, S.; Nagasawa, A.; Bernhard, G.; Hennig, C.; Ikeda, Y. Molecular structure and electrochemical behavior of uranyl(VI) complex with pentadentate schiff base ligand: Prevention of uranyl(V) cation-cation interaction by fully chelating equatorial coordination sites. Inorg. Chem. 2010, 49, 2349–2359. [Google Scholar] [CrossRef]
- Azam, M.; Al-Resayes, S.I.; Velmurugan, G.; Venuvanalingam, P.; Wagler, J.; Kroke, E. Novel uranyl(VI) complexes incorporating propylene-bridged salen-type N2O2-ligands: A structural and computational approach. Dalton Trans. 2015, 44, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Nocton, G.; Horeglad, P.; Vetere, V.; Pécaut, J.; Dubois, L.; Maldivi, P.; Edelstein, N.M.; Mazzanti, M. Synthesis, structure, and bonding of stable complexes of pentavalent uranyl. J. Am. Chem. Soc. 2010, 132, 495–508. [Google Scholar] [CrossRef]
- Pal, M.K.; Kushwah, N.P.; Wadawale, A.P.; Jain, V.K. Diorganogallium complexes containing tripodal schiff bases: Synthesis and structure of [N{Me2GaO(C6H4)CH=N–CH2–CH2-}3]. J. Chem. Res. 2010, 34, 485–488. [Google Scholar] [CrossRef]
- Panattoni, C.; Graziani, R.; Bandoli, G.; Zarli, B.; Bombieri, G. Chemistry of the uranyl group.II. preparation and properties of triphenylphosphine oxide and triphenylarsine oxide complexes of uranyl acetate and the structure of (UO2(CH3COO)2(C6H5)3PO)2. Inorg. Chem. 1969, 8, 320–325. [Google Scholar] [CrossRef]
- Adão, P.; Pessoa, J.C.; Henriques, R.T.; Kuznetsov, M.L.; Avecilla, F.; Maurya, M.R.; Kumar, U.; Correia, I. Synthesis, characterization, and application of vanadium-salan complexes in oxygen transfer reactions. Inorg. Chem. 2009, 48, 3542–3561. [Google Scholar] [CrossRef]
- Bi, W.-Y.; Lü, X.-Q.; Chai, W.-L.; Song, J.-R.; Wong, W.-Y.; Wong, W.-K.; Jones, R.A. Construction and NIR luminescent property of hetero-bimetallic Zn-Nd complexes from two chiral salen-type schiff-base ligands. J. Mol. Struct. 2008, 891, 450–455. [Google Scholar] [CrossRef]
- Lucaccini, E.; Baldoví, J.J.; Chelazzi, L.; Barra, A.-L.; Grepioni, F.; Costes, J.-P.; Sorace, L. Electronic structure and magnetic anisotropy in lanthanoid single-ion magnets with C3 symmetry: The Ln(trenovan) series. Inorg. Chem. 2017, 56, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- You, Z.-L. Solvent-controlled syntheses and crystal structures of a pair of novel thiocyanate-bridged copper(II) complexes constructed from 4-bromo-2-(cyclopropyliminomethyl) phenolate. Z. Anorg. Allg. Chem. 2006, 632, 669–674. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Ni, Z.-H.; Li, X.; Tian, L.; Jiang, J. Synthesis, crystal structures, and luminescent properties of phenoxo-bridged heterometallic trinuclear propeller-and sandwich-like Schiff-base complexes. Inorg. Chem. 2009, 48, 5946–5956. [Google Scholar] [CrossRef]
- CCDC-2350338 (1), 2350339 (2), 2350340 (3), 2350342 (4), 2350341 (5), and 2350343 (6) Contain the Crystallographic Data for This Paper. These Data Can be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/structures (accessed on 22 July 2024).

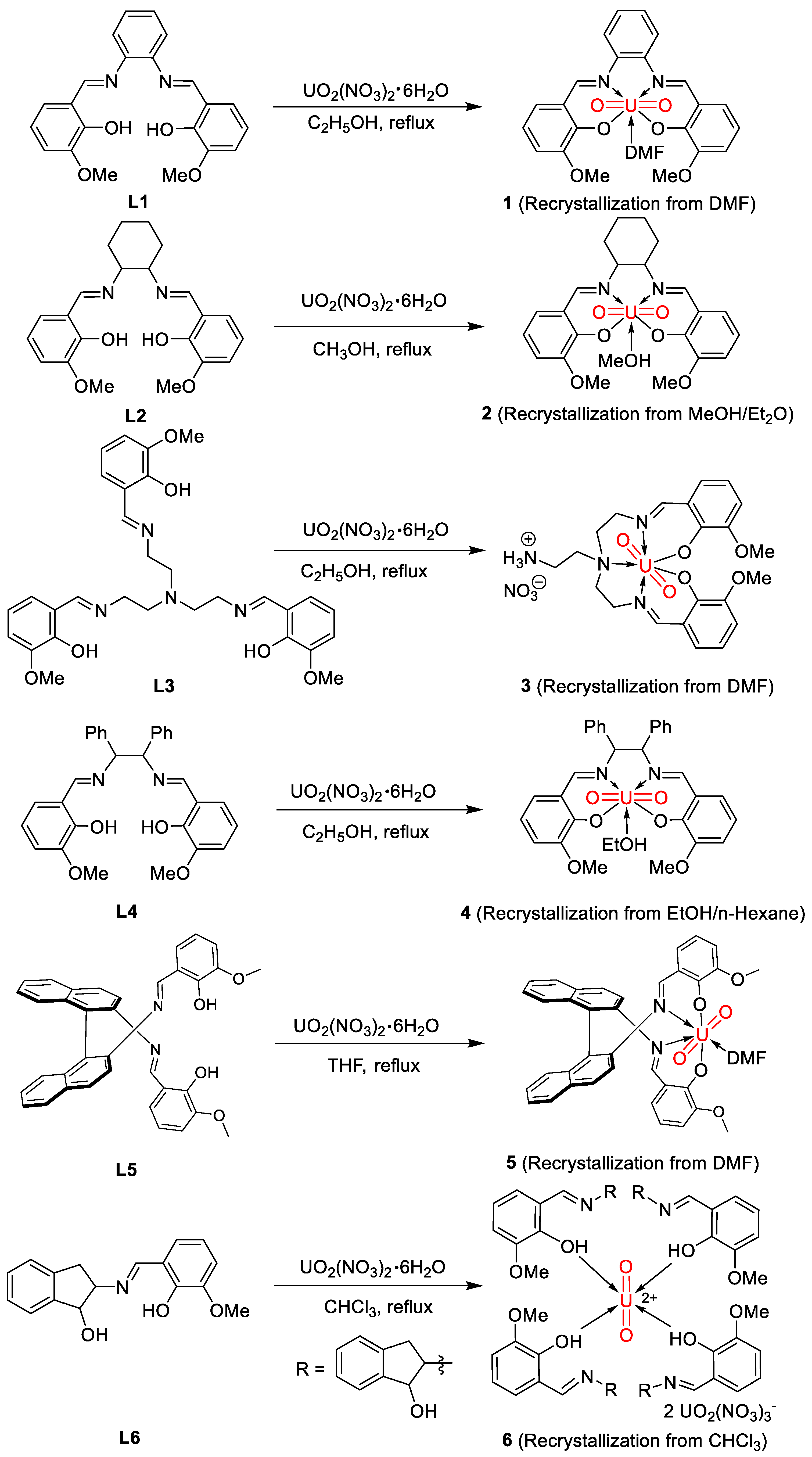
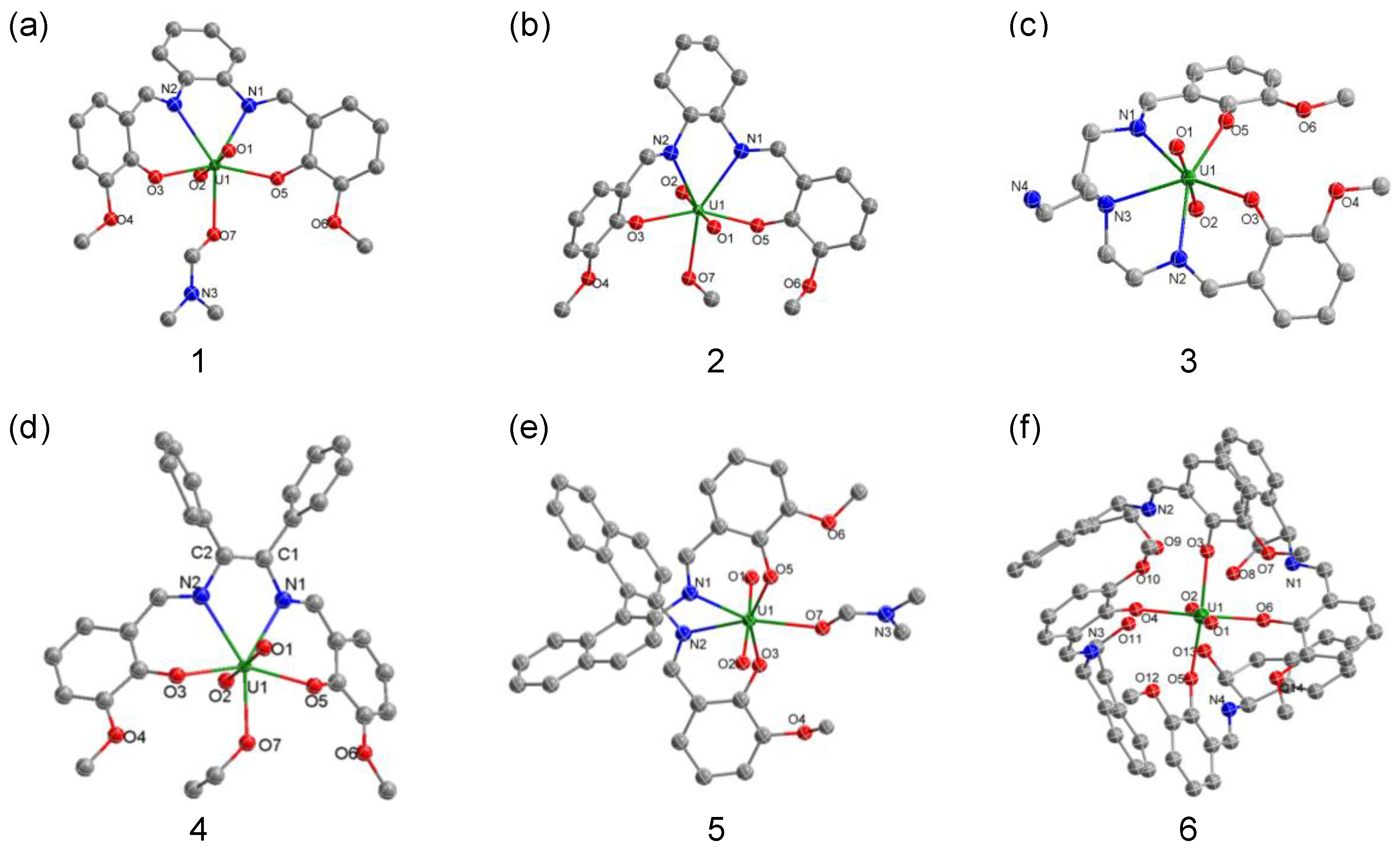

| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| U1-O1 | 1.793(3) | 1.795(8) | 1.776(3) | 1.767(10) | 1.783(4) |
| U1-O2 | 1.782(3) | 1.791(8) | 1.765(3) | 1.776(11) | 1.782(4) |
| U1-O3 | 2.246(3) | 2.250(8) | 2.222(2) | 2.289(10) | 2.267(4) |
| U1-O5 | 2.272(3) | 2.248(8) | 2.261(3) | 2.272(9) | 2.272(4) |
| U1-O7 | 2.434(3) | 2.513(8) | - | 2.44(4) | 2.419(4) |
| U1-N1 | 2.538(3) | 2.542(10) | 2.571(3) | 2.498(13) | 2.578(5) |
| U1-N2 | 2.535(3) | 2.575(9) | 2.544(3) | 2.552(12) | 2.562(4) |
| O1-U1-O2 | 175.34(12) | 178.3(4) | 176.01(14) | 176.8(5) | 176.10(19) |
| O3-U1-O5 | 154.44(9) | 154.9(3) | 85.28(11) | 156.1(4) | 153.51(16) |
| N1-U1-N2 | 63.89(9) | 64.7(3) | 131.17(12) | 63.2(4) | 70.09(14) |
| ν(C=N) | ν(O-H) | ν(C=O)DMF | ν(C-O) | σ(U-O) | ν(NO3−) | |
|---|---|---|---|---|---|---|
| L1 | 1612 | 3420 | 1248 | |||
| 1 | 1580 | 1620 | 1240 | 497 | ||
| L2 | 1630 | 3500 | 1270 | |||
| 2 | 1620 | 1243 | 494 | |||
| L3 | 1645 | 3500 | 1276 | |||
| 3 | 1625 | 1245 | 500 | 1460, 1380, 1295 | ||
| L4 | 1630 | 3400 | 1255 | |||
| 4 | 1608 | 1246 | 499 | |||
| L5 | 1603 | 3400 | 1223 | |||
| 5 | 1588 | 1620 | 1250 | 487 | ||
| L6 | 1635 | 3500 | 1282 | |||
| 6 | 1618 | 3374 | 1251 | 473 |
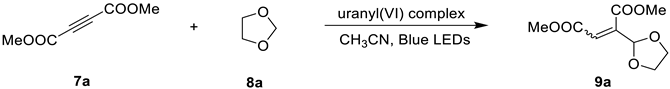 | ||||
|---|---|---|---|---|
| Entry | Catalyst (5 mol%) | Solvent | Yield (%) | Ratio of Z/E |
| 1 | UO2(NO3)2·6H2O | (CH3)2CO | 18 | 2:1 |
| 2 | UO2(NO3)2·6H2O | CH3CN | 39 | 2:1 |
| 3 | Complex 1 | CH3CN | 41 | 6:1 |
| 4 | Complex 2 | CH3CN | 59 | 6:1 |
| 5 | Complex 3 | CH3CN | 61 | 5:1 |
| 6 | Complex 4 | CH3CN | 79 | 10:1 |
| 7 | Complex 5 | CH3CN | 67 | 10:1 |
| 8 | Complex 6 | CH3CN | 51 | 4:1 |
| 9 b | Complex 4 | CH3CN | N.D | - |
| 10 c | Complex 4 | CH3CN | N.D | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Gong, X.; Li, Y.; Zhao, Q.; Zhu, C. Synthesis and Photocatalytic sp3 C-H Bond Functionalization of Salen-Ligand-Supported Uranyl(VI) Complexes. Molecules 2024, 29, 4077. https://doi.org/10.3390/molecules29174077
He J, Gong X, Li Y, Zhao Q, Zhu C. Synthesis and Photocatalytic sp3 C-H Bond Functionalization of Salen-Ligand-Supported Uranyl(VI) Complexes. Molecules. 2024; 29(17):4077. https://doi.org/10.3390/molecules29174077
Chicago/Turabian StyleHe, Jialu, Xingxing Gong, Yafei Li, Qianyi Zhao, and Congqing Zhu. 2024. "Synthesis and Photocatalytic sp3 C-H Bond Functionalization of Salen-Ligand-Supported Uranyl(VI) Complexes" Molecules 29, no. 17: 4077. https://doi.org/10.3390/molecules29174077
APA StyleHe, J., Gong, X., Li, Y., Zhao, Q., & Zhu, C. (2024). Synthesis and Photocatalytic sp3 C-H Bond Functionalization of Salen-Ligand-Supported Uranyl(VI) Complexes. Molecules, 29(17), 4077. https://doi.org/10.3390/molecules29174077





