Investigation of the Structural Properties and Antioxidant Potency of Pectic Polysaccharides Derived from Rohdea japonica (Thunb.) Roth
Abstract
:1. Introduction
2. Results and Discussion
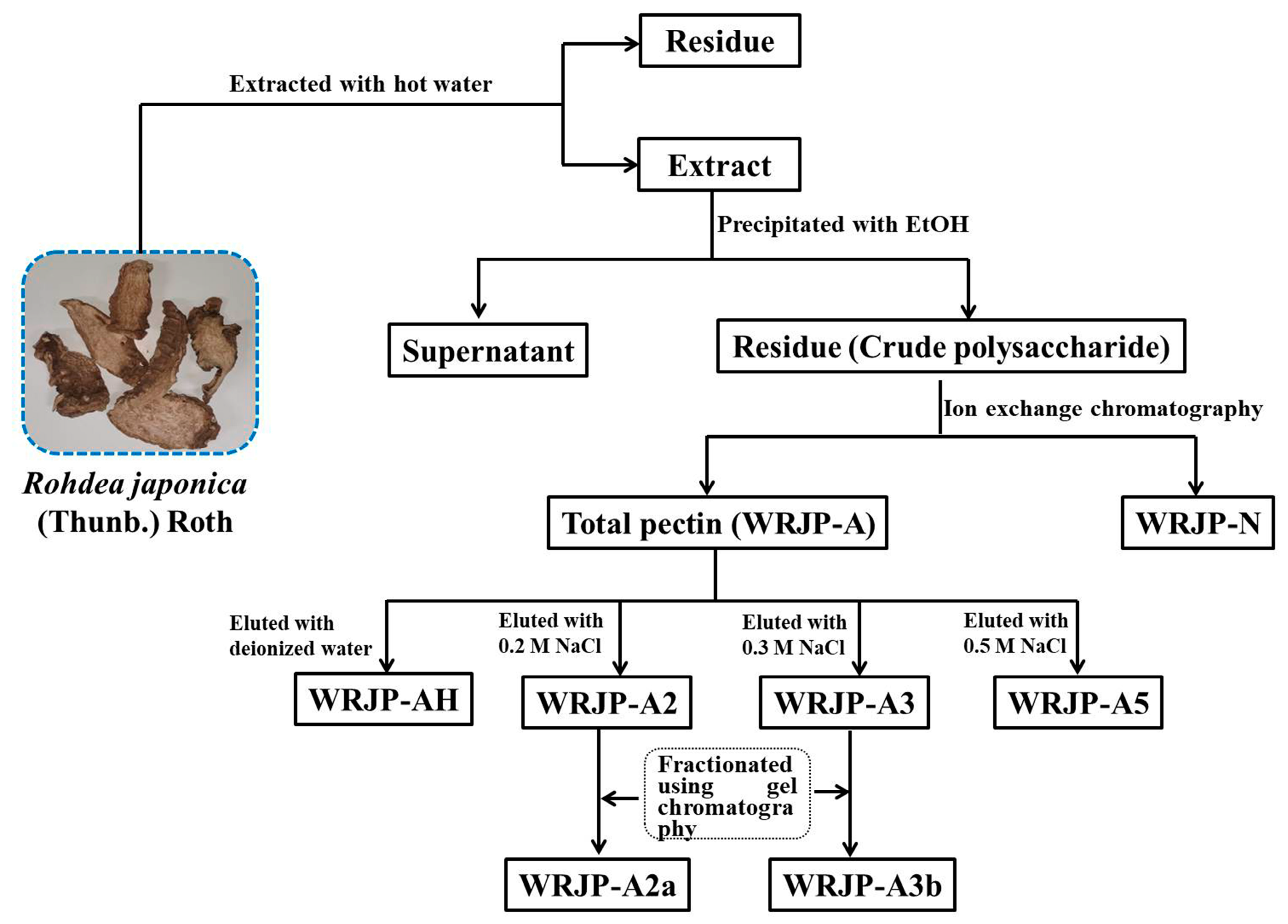
2.1. Extraction of Pectic Polysaccharides from R. japonica
2.2. Purity, Homogeneity, and Molecular Weight of WRJP-A2a and WRJP-A3b
2.3. FT-IR Analysis of WRJP-A2a and WRJP-A3b
2.4. NMR Analysis of WRJP-A2a and WRJP-A3b
2.4.1. NMR Analysis of WRJP-A2a
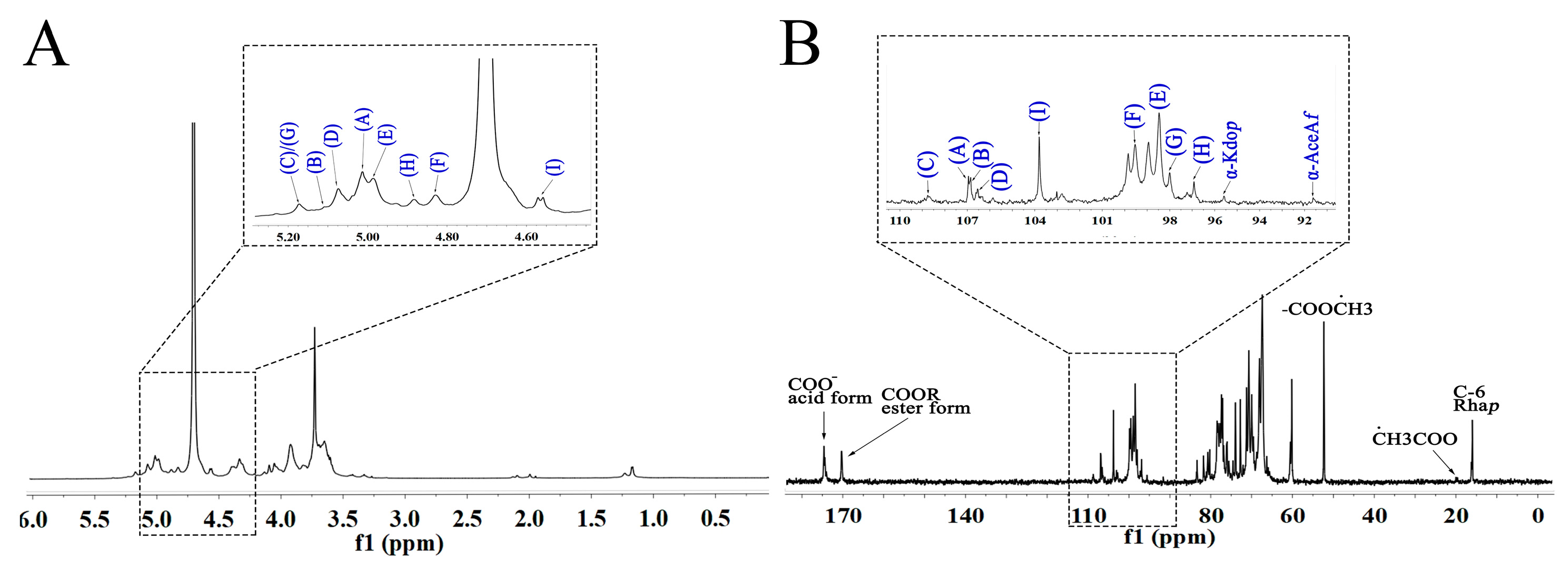

| Residues | Glycosidic Linkage | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| A | 4)-α-GalAp-(1→ | H | 4.98 | 3.67 | 3.93 | 4.05 | 4.72 | |
| C | 98.56 | 67.43 | 68.05 | 81.57 | 70.57 | 174.15 | ||
| B | 4)-α-GalAp6Me-(1→ | H | 4.83 | 3.67 | 3.93 | 4.05 | 4.72 | |
| C | 99.59 | 67.43 | 68.05 | 81.57 | 70.57 | 170.34 |
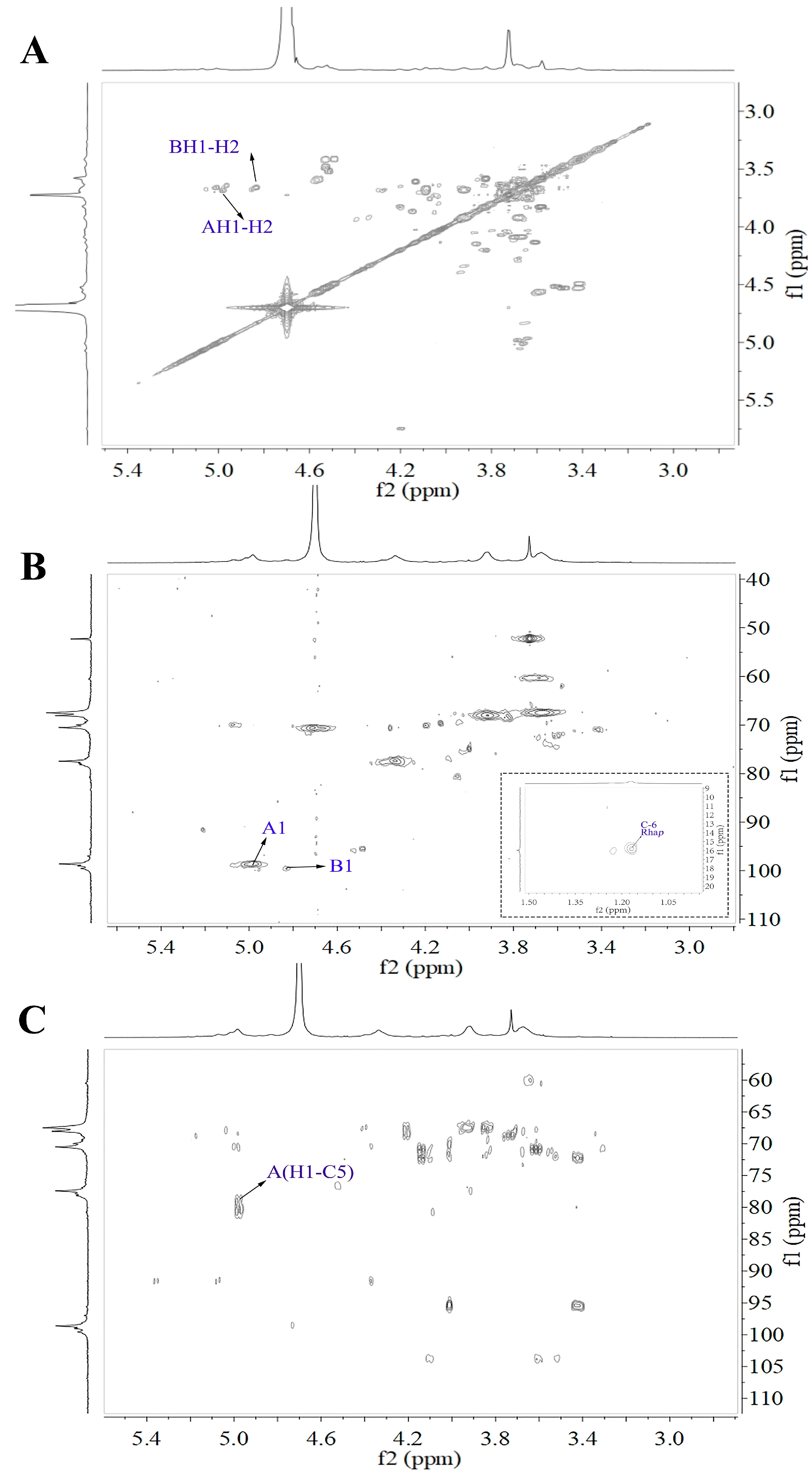
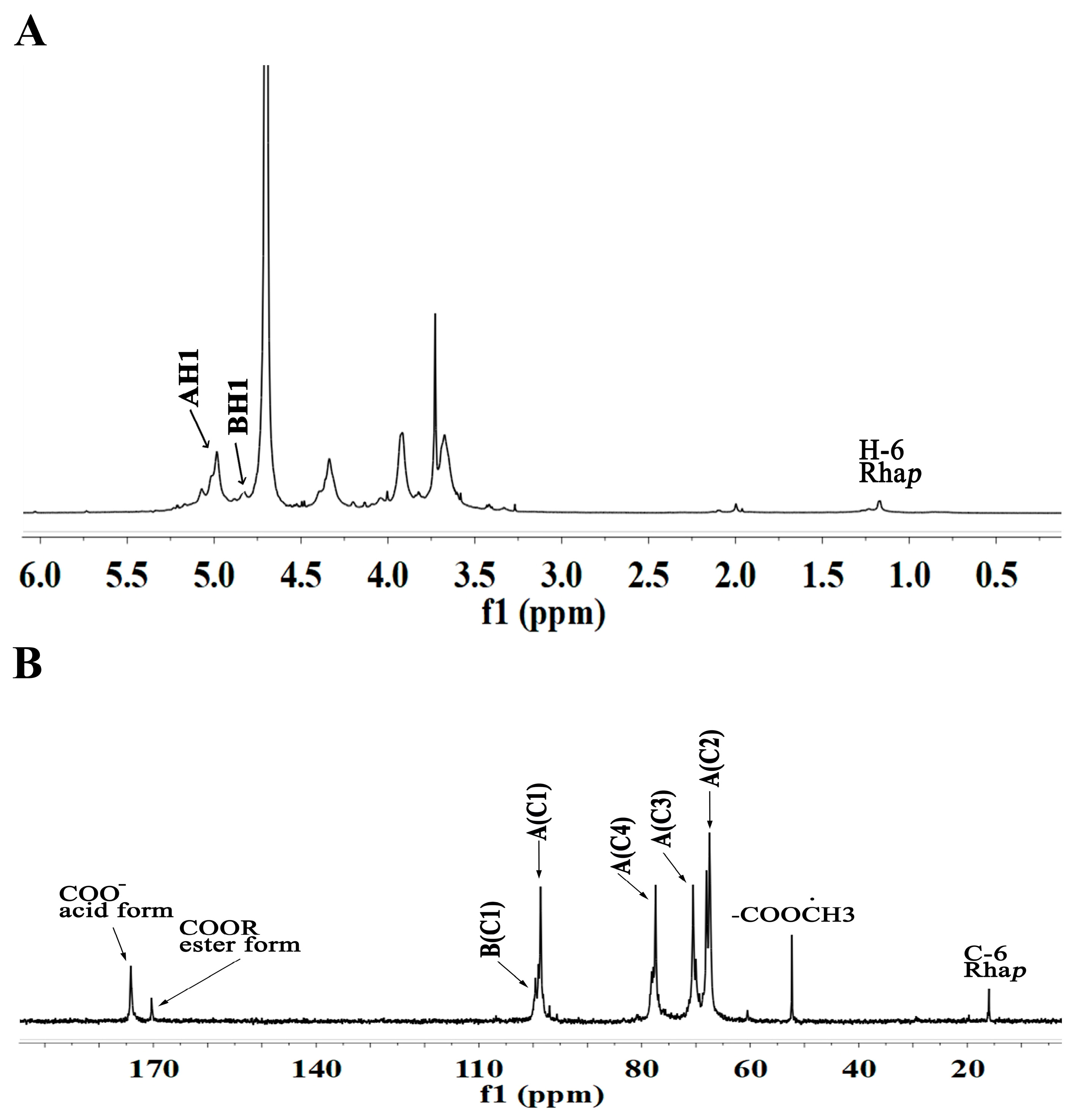
2.4.2. NMR Analysis of WRJP-A3b
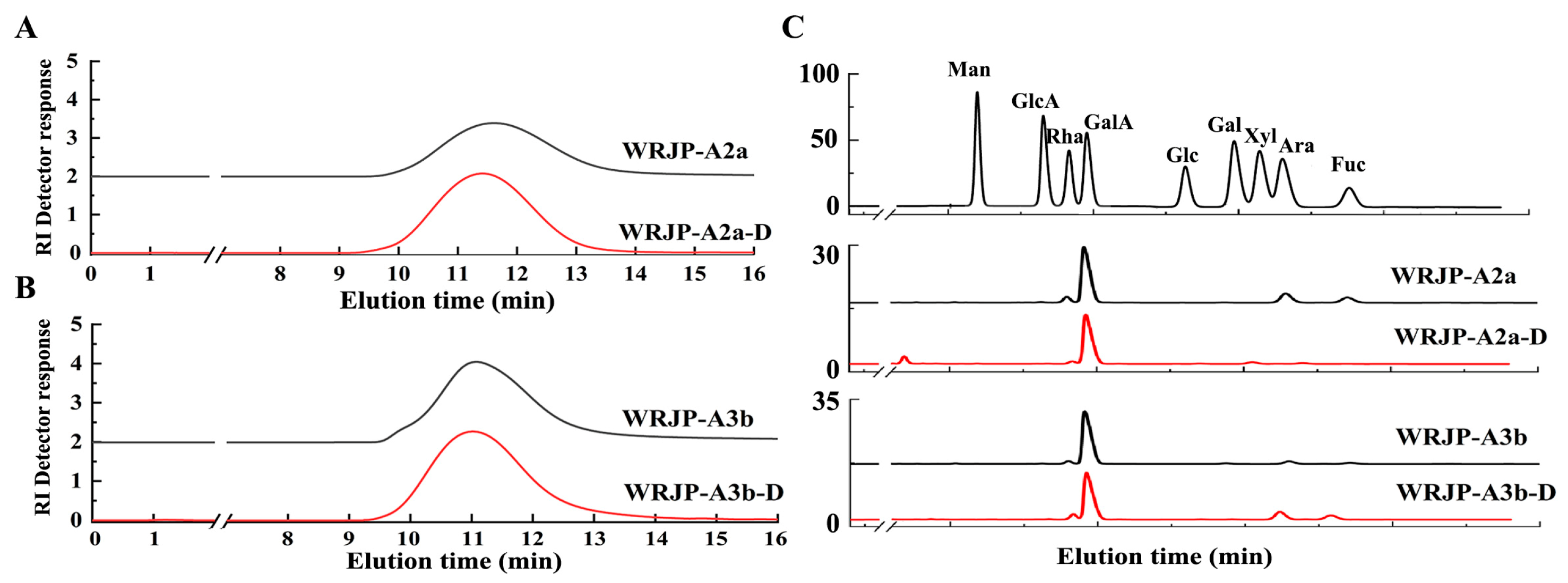
2.5. Enzymatic Analysis of WRJP-A2a and WRJP-A3b
2.5.1. Preparation of De-Esterified Pectin
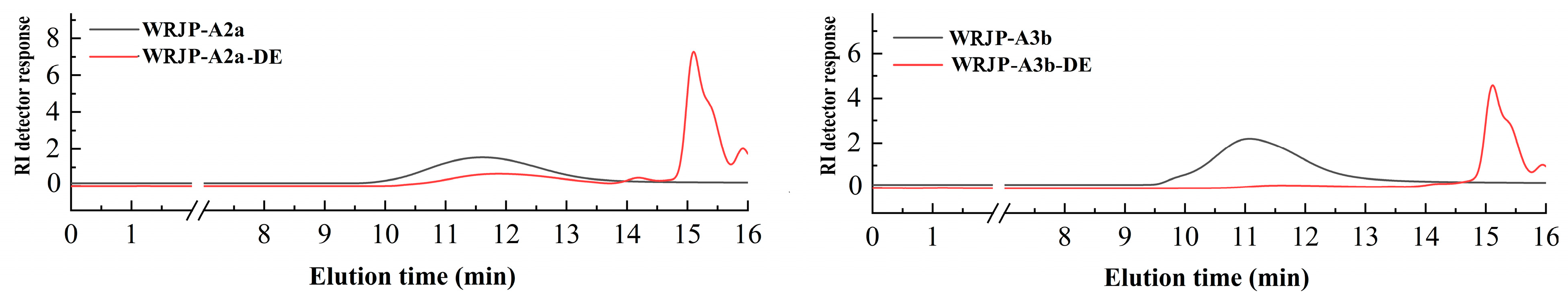
2.5.2. Analysis of Enzymatic Hydrolysates
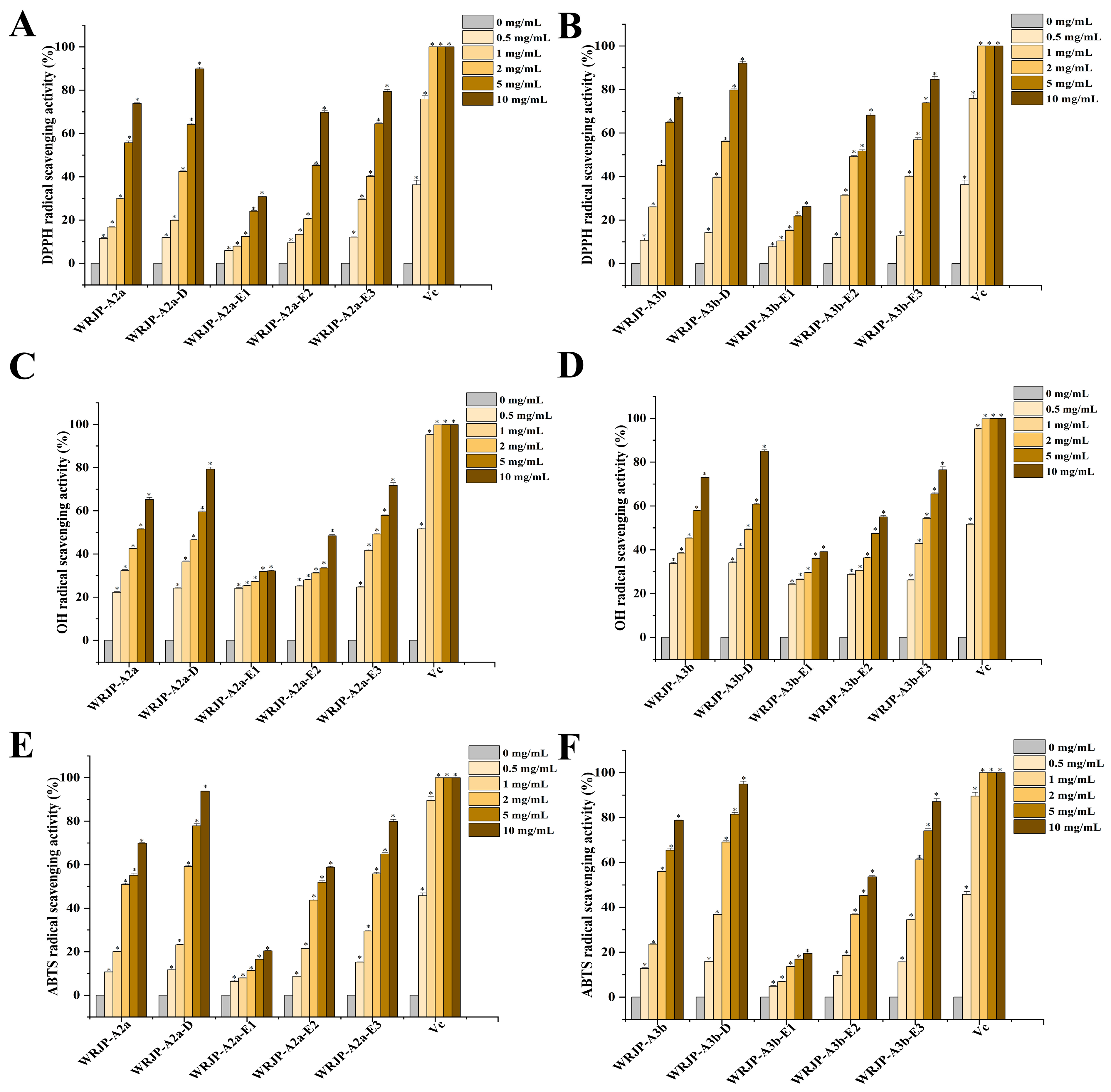
2.6. Antioxidant Activity Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Preparation of Pectin from R. japonica
3.3.1. Extraction of Pectin
3.3.2. Fractionation of the Pectin
3.4. Chemical Characterization Analysis
3.5. FT-IR Spectroscopy
3.6. Nuclear Magnetic Resonance Analysis
3.7. De-Esterification and Enzymatic Hydrolysis
3.8. Antioxidant Activity Assay
3.8.1. DPPH Radical-Scavenging Activity
3.8.2. ABTS Radical-Scavenging Activity
3.8.3. Hydroxyl Radical-Scavenging Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Yu, W.; Li, Q. The preparation and potential bioactivities of modified pectins: A review. Foods 2023, 12, 1016. [Google Scholar] [CrossRef]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Goellner, E.M.; Utermoehlen, J.; Kramer, R.; Classen, B. Structure of arabinanogalactan from Larix laricina and its reactivity with antibodies directed against type-II-arabinanogalactans. Carbohydr. Polym. 2011, 86, 1739–1744. [Google Scholar] [CrossRef]
- Wang, J.Q.; Hu, S.Z.; Nie, S.P.; Yu, Q.; Xie, M.Y. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Sultana, N. Biological properties and biomedical applications of pectin and pectin-based composites: A review. Molecules 2023, 28, 7974. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Zhao, C.Y.; Feng, L.P.; Han, Y.H.; Du, H.J.; Xiao, H.; Zheng, J.K. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci. Technol. 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Ning, X.; Liu, Y.; Jia, M.; Wang, Q.; Sun, Z.; Ji, L.; Mayo, K.H.; Zhou, Y.; Sun, L. Pectic polysaccharides from Radix Sophorae Tonkinensis exhibit significant antioxidant effects. Carbohydr. Polym. 2021, 262, 117925. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, K.; Li, J.; Ye, X.; Chen, S. An optimize adaptable method for determining the monosaccharide composition of pectic polysaccharides. Int. J. Biol. Macromol. 2024, 277, 133591. [Google Scholar] [CrossRef]
- Wen, H.; Kuang, Y.; Lian, X.; Li, H.; Zhou, M.; Tan, Y.; Zhang, X.; Pan, Y.; Zhang, J.; Xu, J. Physicochemical characterization, antioxidant and anticancer activity evaluation of an acidic polysaccharide from Alpinia officinarum Hance. Molecules 2024, 29, 1810. [Google Scholar] [CrossRef]
- Li, J.; Hsiung, S.Y.; Kao, M.R.; Xing, X.; Chang, S.C.; Wang, D.; Hsieh, P.Y.; Liang, P.H.; Zhu, Z.; Cheng, T.R.; et al. Structural compositions and biological activities of cell wall polysaccharides in the rhizome, stem, and leaf of Polygonatum odoratum (Mill.) Druce. Carbohydr. Res. 2022, 521, 108662. [Google Scholar] [CrossRef]
- Zhang, S.H.; Ma, M.H.; Liu, S.C.; Jin, G.C.; Li, W.C.; Zhao, Y.; Li, Y.Y.; Li, X.Y.; Zhu, Z.X.; Liu, C.X.; et al. Structure characterization and anti-tumor activity of polysaccharides from Rohdea chinensis. Rec. Nat. Prod. 2024, 18, 339–346. [Google Scholar] [CrossRef]
- Flora of China Editorial Committee of Chinese Academy of Sciences. The Flora of China; Science Press: Beijing, China, 1978; Volume 15, pp. 16–18. [Google Scholar]
- Fu, L.G.; Chen, T.Q.; Lang, K.Y.; Hong, T.; Lin, Q. Higher Plants of China; Qingdao Publishing House: Qingdao, China, 2002; Volume 13. [Google Scholar]
- Umebayashi, C.; Yamamoto, N.; Nakao, H.; Toi, Y.; Chikahisa-Muramatsu, L.; Kanemaru, K.; Masuda, T.; Oyama, Y. Flow cytometric estimation of cytotoxic activity of rhodexin A isolated from Rhodea japonica in human leukemia K562 cells. Biol. Pharm. Bull. 2003, 26, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Boulet, J.C.; Williams, P.; Doco, T. A Fourier transform infrared spectroscopy study of wine polysaccharides. Carbohydr. Polym. 2007, 69, 79–85. [Google Scholar] [CrossRef]
- Singthong, J.; Cui, S.W.S.; Ningsanond, H.; Goff, D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of ultrasound on the properties and antioxidant activity of hawthorn pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; An, F.P.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Wikiera, A.; Kozioł, A.; Mika, M.; Bozena, S. Structure and bioactivity of apple pectin isolated with Arabinananase and mannanase. Food Chem. 2022, 388, 133020. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Li, X.; Shen, W.; Wang, J.; Zhao, D.; Sun, H.; Xu, X.; Li, C.; Zha, X. Chemical structure, antioxidant and anti-inflammatory activities of two novel pectin polysaccharides from purple passion fruit (Passiflora edulia Sims) peel. J. Mol. Struct. 2022, 1264, 133309. [Google Scholar] [CrossRef]
- Li, Y.; Lin, D.; Jiao, B.; Xu, C.; Qin, J.; Ye, G.; Su, G. Purification, antioxidant and hepatoprotective activities of polysaccharide from Cissus pteroclada Hayata. Int. J. Biol. Macromol. 2015, 77, 307–313. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Song, X.Y.; Jiang, Y.Z.; Yao, J.R.; Jiang, Y.; Li, Z.; Dai, F. Chemical structure and antioxidant activity of a neutral polysaccharide from Asteris Radix et Rhizoma. Carbohydr. Polym. 2022, 286, 119309. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.H.; Yan, J.M.; Zhang, X.; Song, C.C.; Zhang, M.S.; Mayo, K.H.; Sun, L.; Cheng, H.R.; Zhou, Y.F. Structure and antioxidant activity of six mushroom-derived heterogalactans. Int. J. Biol. Macromol. 2022, 209, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocoll. 2021, 113, 106392. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity ofpolysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetricmethod for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef] [PubMed]
- York, W.S.; Darvill, A.G.; McNeil, M.; Albersheim, P. 3-deoxy-D-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 1985, 138, 109–126. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bi, H.; Li, X.; Ni, W.; Han, H.; Li, N.; Wang, B.; Zhou, Y.; Tai, G. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides: Structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.Z.; Wu, Z.P.; Kuang, C.T. Antioxidant activity of polysaccharide from Camellia cake against ABTS and DPPH free radicals. Adv. Mater. Res. 2012, 550–553, 1545–1549. [Google Scholar] [CrossRef]
- Yang, X.; Wang, R.; Zhang, S.; Zhu, W.; Tang, J.; Liu, J.; Chen, P.; Zhang, D.; Ye, W.; Zheng, Y. Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohydr. Polym. 2014, 101, 386–391. [Google Scholar] [CrossRef] [PubMed]


| WRJP | WRJP-N | WRJP-A | WRJP-A2a | WRJP-A3b | |
|---|---|---|---|---|---|
| Yield (w%) | 8.1 a | 49.7 b | 26.7 b | 40.4 c | 6.6 c |
| Molecular weight (kDa) | ND | ND | 42.7 | 64.1 | |
| Monosaccharide Composition | |||||
| GalA | 29.9 | NONE | 55.2 | 60.9 | 82.4 |
| Rha | 4.7 | NONE | 5.9 | 6.7 | 4.8 |
| Gal | 39.9 | 72.6 | 24.4 | 19.9 | 5.1 |
| Ara | 7.4 | 4.0 | 10.9 | 10.7 | 2.9 |
| Glc | 15.2 | 21.4 | 1.0 | 0.3 | 1.1 |
| GlcA | 1.1 | NONE | 0.4 | 0.8 | 1.5 |
| Xyl | NONE | NONE | 0.9 | NONE | 1.0 |
| Man | 1.7 | 2.0 | 0.3 | 0.6 | 1.2 |
| Residues | Glycosidic Linkage | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| A | α-Araf-(1→ AtI | H | 5.01 | 4.00 | 3.86 | 4.06 | 3.65 | |
| C | 106.98 | 83.36 | 76.00 | 80.47 | 60.46 | |||
| B | α-Araf-(1→ At Ⅱ | H | 5.10 | 4.03 | 3.93 | 4.06 | 3.74 | |
| C | 106.64 | 83.24 | 76.18 | 80.47 | 60.34 | |||
| C | → 5)-α-Araf-(1 → | H | 5.16 | 4.10 | 4.08 | 4.22 | 3.72 | |
| C | 108.91 | 81.02 | 77.11 | 81.02 | 66.12 | |||
| D | → 3,5)-α-Araf-(1 → | H | 5.07 | 4.21 | 4.03 | 4.00 | 3.80 | |
| C | 106.60 | 78.55 | 83.24 | 83.46 | 66.11 | |||
| E | → 4)-α-GalAp-(1 → | H | 4.98 | 3.65 | 4.03 | 4.02 | 4.64 | |
| C | 98.57 | 67.40 | 69.59 | 81.67 | 70.67 | 174.62 | ||
| F | → 4)-α-GalAp6Me-(1 → | H | 4.83 | 3.72 | 3.91 | 4.01 | 4.70 | |
| C | 99.61 | 66.12 | 68.10 | 81.75 | 69.67 | 170.32 | ||
| G | → 2)-α-Rhap-(1 → | H | 5.17 | 4.33 | 4.03 | 3.33 | 3.65 | 1.16 |
| C | 98.06 | 76.12 | 69.59 | 71.37 | 67.40 | 15.97 | ||
| H | → 2,4)-α-Rhap-(1 → | H | 4.92 | 4.39 | 3.99 | 3.93 | 3.65 | 1.23 |
| C | 96.98 | 76.12 | 73.64 | 76.96 | 67.40 | 16.12 | ||
| I | → 3,6)-β-Galp-(1 → | H | 4.56 | 3.60 | 4.00 | 4.03 | 3.63 | 3.91 |
| C | 103.87 | 72.40 | 83.06 | 69.59 | 74.00 | 68.10 |
| Fractions | Yield a (%) | TBA Test | Molecular Weight (kDa) | Monosaccahride Composition (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GalA | Rha | Gal | Ara | Glc | GlcA | Man | Xyl | ||||
| WRJP-A2a-DE1 | 27.6 | - | 50.3 | 15.7 | 15.1 | 43.1 | 23.2 | 1.1 | 0.8 | 0.8 | 0.2 |
| WRJP-A2a-DE2 | 4.4 | + | 4.9 | 35.0 | 14.3 | 17.5 | 21.4 | 3.5 | 5.4 | 1.2 | 1.7 |
| WRJP-A2a-DE3 | 67.0 | - | <2.0 | 98.4 | 0.3 | 0.3 | - | 0.6 | - | - | 0.4 |
| WRJP-A3b-DE1 | 11.2 | - | 66.1 | 27.4 | 27.2 | 21.4 | 15.3 | 5.1 | 1.8 | 1.8 | - |
| WRJP-A3b-DE2 | 3.2 | + | 4.8 | 38.3 | 15.4 | 17.5 | 13.2 | 5.9 | 4.4 | 2.4 | 2.9 |
| WRJP-A3b-DE3 | 84.6 | - | <2.0 | 97.1 | - | - | 0.5 | 0.6 | - | 1.4 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Lin, Z.; Cui, K.; Zang, H.; Zhou, Y.; Zhang, L.; Liu, D. Investigation of the Structural Properties and Antioxidant Potency of Pectic Polysaccharides Derived from Rohdea japonica (Thunb.) Roth. Molecules 2024, 29, 4135. https://doi.org/10.3390/molecules29174135
Yan S, Lin Z, Cui K, Zang H, Zhou Y, Zhang L, Liu D. Investigation of the Structural Properties and Antioxidant Potency of Pectic Polysaccharides Derived from Rohdea japonica (Thunb.) Roth. Molecules. 2024; 29(17):4135. https://doi.org/10.3390/molecules29174135
Chicago/Turabian StyleYan, Su, Zhiying Lin, Kuo Cui, Hao Zang, Yifa Zhou, Lihui Zhang, and Duo Liu. 2024. "Investigation of the Structural Properties and Antioxidant Potency of Pectic Polysaccharides Derived from Rohdea japonica (Thunb.) Roth" Molecules 29, no. 17: 4135. https://doi.org/10.3390/molecules29174135





