Eco-Friendly Waterborne Polyurethane Coating Modified with Ethylenediamine-Functionalized Graphene Oxide for Enhanced Anticorrosion Performance
Abstract
1. Introduction
2. Results and Discussion
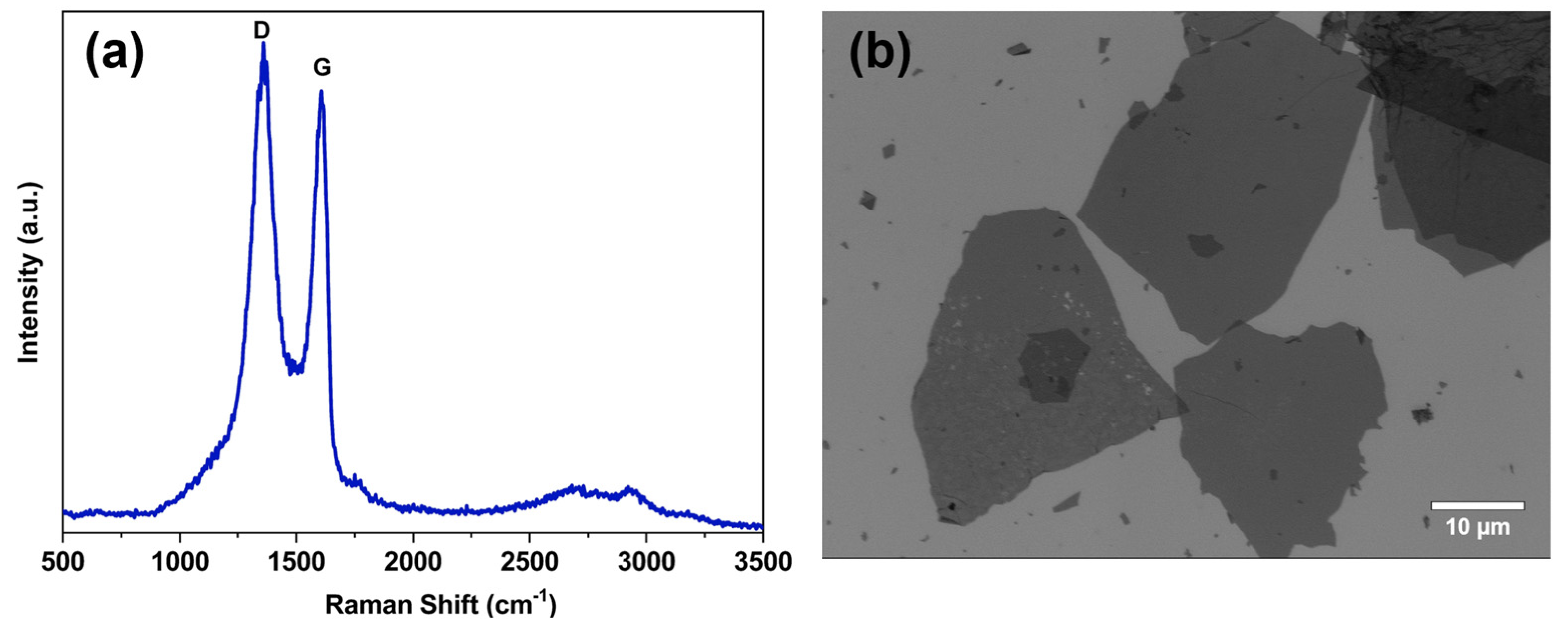
2.1. Characterization of GO
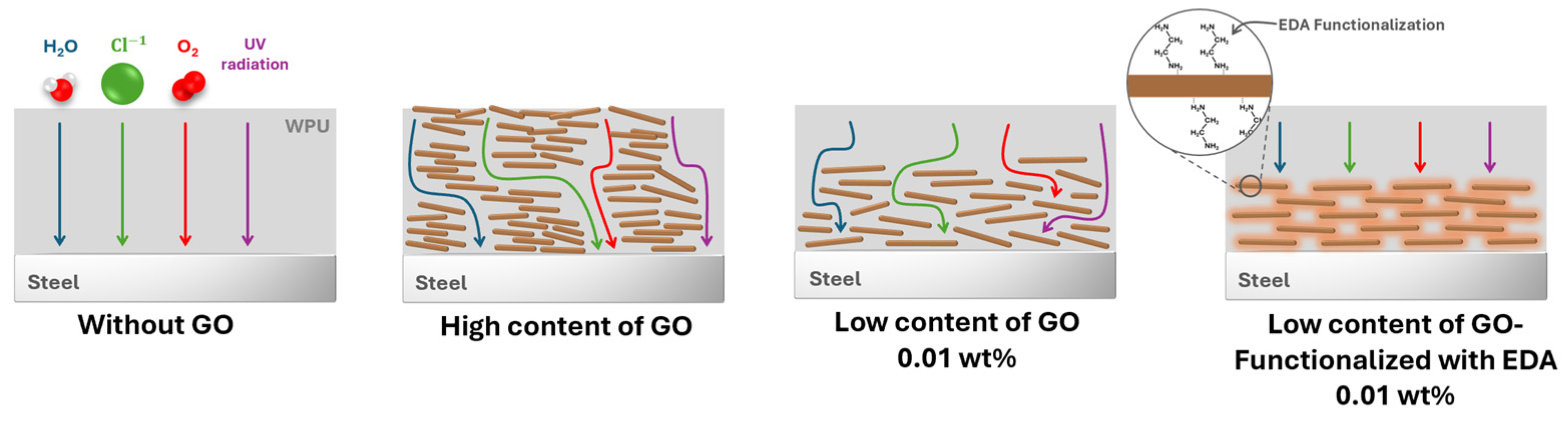
2.2. Dispersion of GO in the WPU Matrix
2.3. Evaluation of Chemical Interaction of GO with WPU Resin
2.4. Hydrophobicity Analysis
2.5. Evaluation of Anticorrosion Performance
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Graphene Oxide
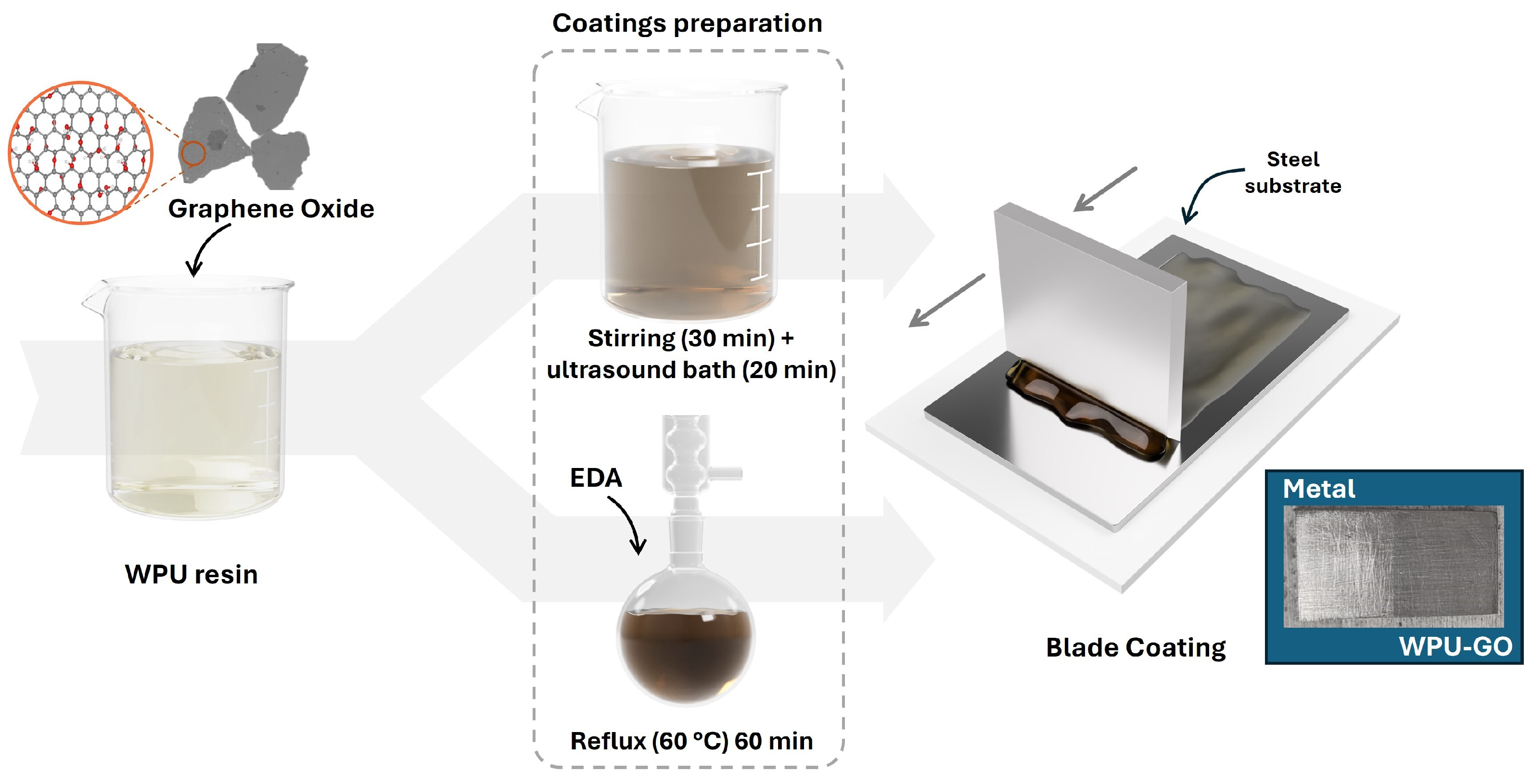
3.2.2. Preparation of GO Waterborne Polyurethane Composite Coatings
3.2.3. Characterization
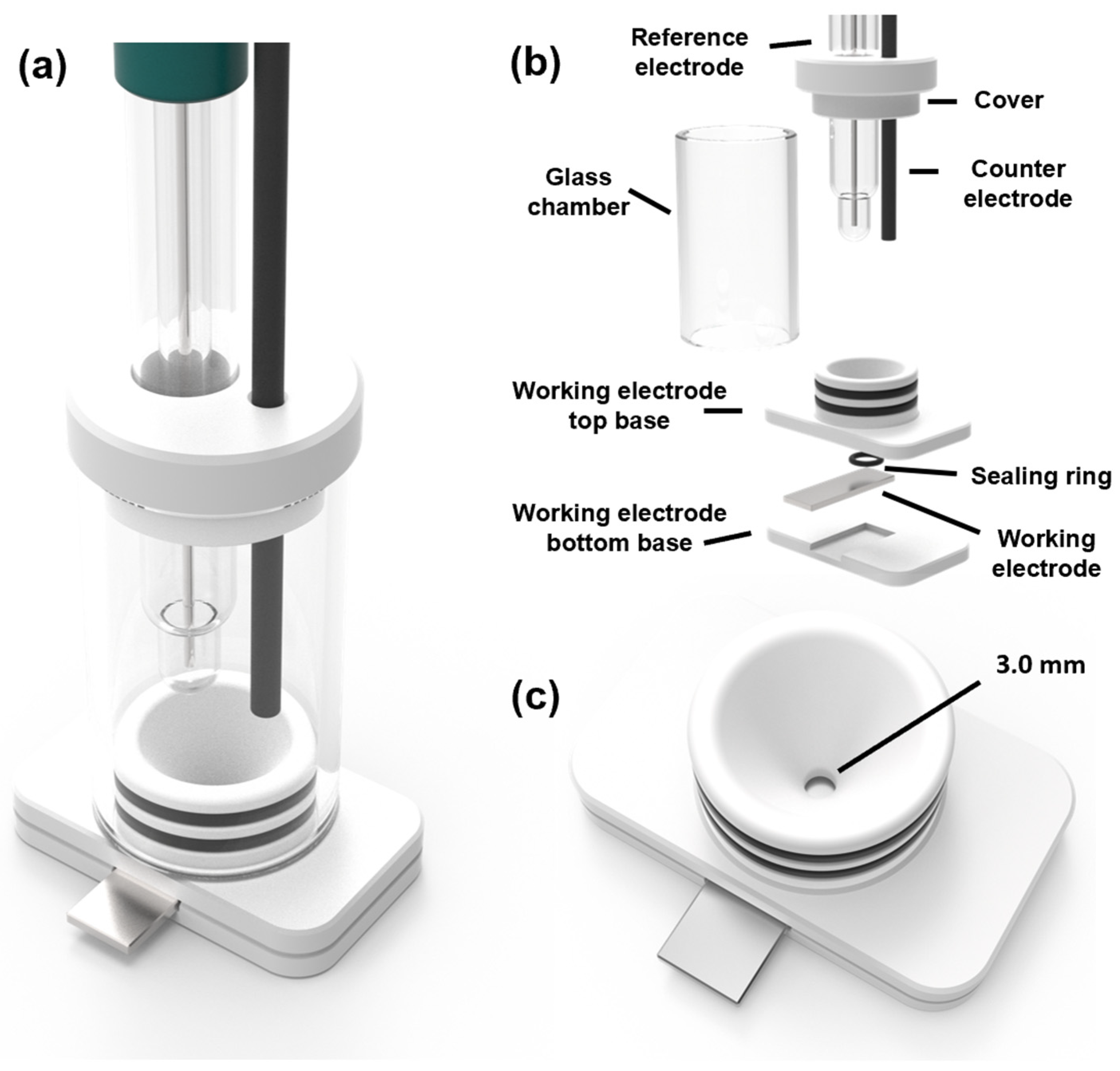
3.2.4. Electrochemical Characterization and UV/Condensation Exposure Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, M.; Wang, B.; Wang, Z. Nature-Inspired Strategy for Anticorrosion. Adv. Eng. Mater. 2019, 21, 1801379. [Google Scholar] [CrossRef]
- Karimi, M. Review of Steel Material Engineering and Its Application in Industry. J. Eng. Ind. Res. 2023, 4, 61–67. [Google Scholar] [CrossRef]
- Amegroud, H.; Boudalia, M.; Elhawary, M.; Garcia, A.J.; Bellaouchou, A.; Amin, H.M.A. Electropolymerized Conducting Polyaniline Coating on Nickel-Aluminum Bronze Alloy for Improved Corrosion Resistance in Marine Environment. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133909. [Google Scholar] [CrossRef]
- Ollik, K.; Lieder, M. Review of the Application of Graphene-Based Coatings as Anticorrosion Layers. Coatings 2020, 10, 883. [Google Scholar] [CrossRef]
- Farzi, G.; Davoodi, A.; Ahmadi, A.; Neisiany, R.E.; Anwer, M.K.; Aboudzadeh, M.A. Encapsulation of Cerium Nitrate within Poly(Urea-Formaldehyde) Microcapsules for the Development of Self-Healing Epoxy-Based Coating. ACS Omega 2021, 6, 31147–31153. [Google Scholar] [CrossRef]
- Aramayo, M.A.F.; Aoki, I.V. Synthesis of Innovative Epoxy Resin and Polyamine Hardener Microcapsules and Their Age Monitoring by Confocal Raman Imaging. J. Appl. Polym. Sci. 2024, 141, e55342. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential Use of Smart Coatings for Corrosion Protection of Metals and Alloys: A Review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Song, H.; Wang, M.; Wang, Y.; Zhang, Y.; Umar, A.; Guo, Z. Waterborne Polyurethane/Graphene Oxide Nanocomposites with Enhanced Properties. Sci. Adv. Mater. 2017, 9, 1895–1904. [Google Scholar] [CrossRef]
- Cui, J.; Xu, J.; Li, J.; Qiu, H.; Zheng, S.; Yang, J. A Crosslinkable Graphene Oxide in Waterborne Polyurethane Anticorrosive Coatings: Experiments and Simulation. Compos. B Eng. 2020, 188, 107889. [Google Scholar] [CrossRef]
- Boutoumit, A.; Elhawary, M.; Bellaouchou, A.; Boudalia, M.; Hammani, O.; José Garcia, A.; Amin, H.M.A. Electrochemical, Structural and Thermodynamic Investigations of Methanolic Parsley Extract as a Green Corrosion Inhibitor for C37 Steel in HCl. Coatings 2024, 14, 783. [Google Scholar] [CrossRef]
- Eddahhaoui, F.-Z.; Najem, A.; Elhawary, M.; Boudalia, M.; Campos, O.S.; Tabyaoui, M.; José Garcia, A.; Bellaouchou, A.; Amin, H.M.A. Experimental and Computational Aspects of Green Corrosion Inhibition for Low Carbon Steel in HCl Environment Using Extract of Chamaerops Humilis Fruit Waste. J. Alloys Compd. 2024, 977, 173307. [Google Scholar] [CrossRef]
- Duong, N.T.; An, T.B.; Thao, P.T.; Oanh, V.K.; Truc, T.A.; Vu, P.G.; Hang, T.T.X. Corrosion Protection of Carbon Steel by Polyurethane Coatings Containing Graphene Oxide. Vietnam J. Chem. 2020, 58, 108–112. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wen, S.; Li, S.; Chen, Y.; Wang, J.; Wang, Y.; Wang, C.; Yu, X.; Mao, Y. Waterborne Polyurea Coatings Filled with Sulfonated Graphene Improved Anti-Corrosion Performance. Coatings 2021, 11, 251. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Yuan, X.; Zhang, Y.; Gao, X.; Zhu, B.; Qiao, K. Waterborne Polyurethane Sizing Agent with Excellent Water Resistance and Thermal Stability for Improving the Interfacial Performance of Carbon Fibers/Epoxy Resin Composites. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132817. [Google Scholar] [CrossRef]
- Trovati, G.; Sanches, E.A.; Neto, S.C.; Mascarenhas, Y.P.; Chierice, G.O. Characterization of Polyurethane Resins by FTIR, TGA, and XRD. J. Appl. Polym. Sci. 2010, 115, 263–268. [Google Scholar] [CrossRef]
- Salzano de Luna, M. Recent Trends in Waterborne and Bio-Based Polyurethane Coatings for Corrosion Protection. Adv. Mater. Interfaces 2022, 9, 2101775. [Google Scholar] [CrossRef]
- Medeiros, G.S.; Nisar, M.; Peter, J.; Andrade, R.J.E.; Fechine, G.J.M. Different Aspects of Polymer Films Based on Low-density Polyethylene Using Graphene as Filler. J. Appl. Polym. Sci. 2023, 140, 1–12. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Liang, L.; Wang, S.; Shi, H.; Xie, Y.; Yang, M.; Pi, K. The Effect of Functional Graphene Oxide Nanoparticles on Corrosion Resistance of Waterborne Polyurethane. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124565. [Google Scholar] [CrossRef]
- Pinto, G.M.; Cremonezzi, J.M.O.; Ribeiro, H.; Andrade, R.J.E.; Demarquette, N.R.; Fechine, G.J.M. From two-dimensional Materials to Polymer Nanocomposites with Emerging Multifunctional Applications: A Critical Review. Polym. Compos. 2023, 44, 1438–1470. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas Barrier Performance of Graphene/Polymer Nanocomposites. Carbon N. Y. 2016, 98, 313–333. [Google Scholar] [CrossRef]
- Nine, M.J.; Cole, M.A.; Johnson, L.; Tran, D.N.H.; Losic, D. Robust Superhydrophobic Graphene-Based Composite Coatings with Self-Cleaning and Corrosion Barrier Properties. ACS Appl. Mater. Interfaces 2015, 7, 28482–28493. [Google Scholar] [CrossRef]
- Tan, B.; Thomas, N.L. A Review of the Water Barrier Properties of Polymer/Clay and Polymer/Graphene Nanocomposites. J. Memb. Sci. 2016, 514, 595–612. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Chen, J.; Huo, D.; Gao, X.; Dong, J.; Yin, Y.; Liu, J.; Nan, D. Melamine-Modified Graphene Oxide as a Corrosion Resistance Enhancing Additive for Waterborne Epoxy Resin Coatings. Coatings 2024, 14, 488. [Google Scholar] [CrossRef]
- Jena, G.; Philip, J. A Review on Recent Advances in Graphene Oxide-Based Composite Coatings for Anticorrosion Applications. Prog. Org. Coat. 2022, 173, 107208. [Google Scholar] [CrossRef]
- Nayak, S.R.; Mohana, K.N.S. Corrosion Protection Performance of Functionalized Graphene Oxide Nanocomposite Coating on Mild Steel. Surf. Interfaces 2018, 11, 63–73. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Shao, N.; Dong, Z. Modification of Electrochemical Exfoliation of Graphene Oxide with Dopamine and Tannic to Enhance Anticorrosion Performance of Epoxy Coatings. Coatings 2023, 13, 1809. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A Comprehensive Review on Graphene-Based Anti-Corrosive Coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Liang, A.; Jiang, X.; Hong, X.; Jiang, Y.; Shao, Z.; Zhu, D. Recent Developments Concerning the Dispersion Methods and Mechanisms of Graphene. Coatings 2018, 8, 33. [Google Scholar] [CrossRef]
- Pu, N.W.; Wang, C.A.; Liu, Y.M.; Sung, Y.; Wang, D.S.; Ger, M. Der Dispersion of Graphene in Aqueous Solutions with Different Types of Surfactants and the Production of Graphene Films by Spray or Drop Coating. J. Taiwan Inst. Chem. Eng. 2012, 43, 140–146. [Google Scholar] [CrossRef]
- Wen, J.G.; Geng, W.; Geng, H.Z.; Zhao, H.; Jing, L.C.; Yuan, X.T.; Tian, Y.; Wang, T.; Ning, Y.J.; Wu, L. Improvement of Corrosion Resistance of Waterborne Polyurethane Coatings by Covalent and Noncovalent Grafted Graphene Oxide Nanosheets. ACS Omega 2019, 4, 20265–20274. [Google Scholar] [CrossRef]
- Ning, Y.J.; Zhu, Z.R.; Cao, W.W.; Wu, L.; Jing, L.C.; Wang, T.; Yuan, X.T.; Teng, L.H.; Bin, P.S.; Geng, H.Z. Anti-Corrosion Reinforcement of Waterborne Polyurethane Coating with Polymerized Graphene Oxide by the One-Pot Method. J. Mater. Sci. 2021, 56, 337–350. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Li, J.; Yang, J. Self-Aligned Graphene as Anticorrosive Barrier in Waterborne Polyurethane Composite Coatings. J. Mater. Chem. A Mater. 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Maslekar, N.; Zetterlund, P.B.; Kumar, P.V.; Agarwal, V. Mechanistic Aspects of the Functionalization of Graphene Oxide with Ethylene Diamine: Implications for Energy Storage Applications. ACS Appl. Nano Mater. 2021, 4, 3232–3240. [Google Scholar] [CrossRef]
- Fan, X.; Xia, Y.; Wu, S.; Zhang, D.; Oliver, S.; Chen, X.; Lei, L.; Shi, S. Covalently Immobilization of Modified Graphene Oxide with Waterborne Hydroxyl Acrylic Resin for Anticorrosive Reinforcement of Its Coatings. Prog. Org. Coat. 2022, 163, 106685. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Wu, J.; Hong, R.; Xu, J. Preparation and Characterization of Graphene Oxide/Polyaniline/Polydopamine Nanocomposites towards Long-Term Anticorrosive Performance of Epoxy Coatings. Polymers 2022, 14, 3355. [Google Scholar] [CrossRef]
- Shi, H.; Liu, W.; Xie, Y.; Yang, M.; Liu, C.; Zhang, F.; Wang, S.; Liang, L.; Pi, K. Synthesis of Carboxymethyl Chitosan-Functionalized Graphene Nanomaterial for Anticorrosive Reinforcement of Waterborne Epoxy Coating. Carbohydr. Polym. 2021, 252, 117249. [Google Scholar] [CrossRef]
- Yu, S.; Yang, Y.; Ma, L.; Jia, W.; Zhou, Q.; Zhu, J.; Wang, J. SiC Nanowires Enhanced Graphene Composite Coatings with Excellent Tribological and Anticorrosive Properties. Tribol. Int. 2023, 188, 108894. [Google Scholar] [CrossRef]
- Guo, X.; Xu, H.; Pu, J.; Yao, C.; Yang, J.; Liu, S. Corrosion Performance and Rust Conversion Mechanism of Graphene Modified Epoxy Surface Tolerant Coating. Front. Mater. 2021, 8, 767776. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman Spectroscopy of Graphene and Graphite: Disorder, Electron–Phonon Coupling, Doping and Nonadiabatic Effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion Behaviour of Graphene Oxide and Reduced Graphene Oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon N. Y. 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Parvin, N.; Kumar, V.; Joo, S.W.; Park, S.S.; Mandal, T.K. Recent Advances in the Characterized Identification of Mono-to-Multi-Layer Graphene and Its Biomedical Applications: A Review. Electronics 2022, 11, 3345. [Google Scholar] [CrossRef]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of Graphene Oxide: Variations in Reported Approaches. Carbon N. Y. 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Otsuka, H.; Urita, K.; Honma, N.; Kimuro, T.; Amako, Y.; Kukobat, R.; Bandosz, T.J.; Ukai, J.; Moriguchi, I.; Kaneko, K. Transient Chemical and Structural Changes in Graphene Oxide during Ripening. Nat. Commun. 2024, 15, 1708. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, I.; Chee, S.-S.; Song, J.-H.; Kang, Y.; Lee, C.; Lee, W.; Ham, M.-H.; Kim, I.S. Graphene Oxide Nanocomposite Membrane Cooperatively Cross-Linked by Monomer and Polymer Overcoming the Trade-off between Flux and Rejection in Forward Osmosis. J. Memb. Sci. 2020, 598, 117684. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Sun, Q.; Shi, B. Enhanced Mechanical Property, Chemical Resistance and Abrasion Durability of Waterborne Polyurethane Based Coating by Incorporating Highly Dispersed Polyacrylic Acid Modified Graphene Oxide. Prog. Org. Coat. 2022, 170, 106949. [Google Scholar] [CrossRef]
- Rahman, M.M. Synthesis and Properties of Waterborne Polyurethane Adhesives: Effect of Chain Extender of Ethylene Diamine, Butanediol, and Fluoro-Butanediol. J. Adhes. Sci. Technol. 2013, 27, 2592–2602. [Google Scholar] [CrossRef]
- Lei, L.; Zhong, L.; Lin, X.; Li, Y.; Xia, Z. Synthesis and Characterization of Waterborne Polyurethane Dispersions with Different Chain Extenders for Potential Application in Waterborne Ink. Chem. Eng. J. 2014, 253, 518–525. [Google Scholar] [CrossRef]
- Bruckmoser, K.; Resch, K. Investigation of Ageing Mechanisms in Thermoplastic Polyurethanes by Means of IR and Raman Spectroscopy. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2014; Volume 339, pp. 70–83. [Google Scholar]
- Christopher, G.; Anbu Kulandainathan, M.; Harichandran, G. Comparative Study of Effect of Corrosion on Mild Steel with Waterborne Polyurethane Dispersion Containing Graphene Oxide versus Carbon Black Nanocomposites. Prog. Org. Coat. 2015, 89, 199–211. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a Green Graphene Oxide Reduction with Reusable Potassium Carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Guo, W.; Chen, J.; Sun, S.; Zhou, Q. In Situ Monitoring the Molecular Diffusion Process in Graphene Oxide Membranes by ATR-FTIR Spectroscopy. J. Phys. Chem. C 2016, 120, 7451–7456. [Google Scholar] [CrossRef]
- Hu, Y.; Cao, K.; Ci, L.; Mizaikoff, B. Selective Chemical Enhancement via Graphene Oxide in Infrared Attenuated Total Reflection Spectroscopy. J. Phys. Chem. C 2019, 123, 25286–25293. [Google Scholar] [CrossRef]
- Surekha, G.; Krishnaiah, K.V.; Ravi, N.; Padma Suvarna, R. FTIR, Raman and XRD Analysis of Graphene Oxide Films Prepared by Modified Hummers Method. J. Phys. Conf. Ser. 2020, 1495, 012012. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Ahmad, S. Covalently Functionalized Ethylene Diamine Modified Graphene Oxide Poly-Paraphenylene Diamine Dispersed Polyurethane Anticorrosive Nanocomposite Coatings. Prog. Org. Coat. 2021, 150, 105966. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Saeed, A.; Iqbal, S. FT-IR Spectroscopic and Thermal Study of Waterborne Polyurethane-Acrylate Leather Coatings Using Tartaric Acid as an Ionomer. e-Polymers 2016, 16, 463–474. [Google Scholar] [CrossRef]
- Suthar, V.; Asare, M.A.; de Souza, F.M.; Gupta, R.K. Effect of Graphene Oxide and Reduced Graphene Oxide on the Properties of Sunflower Oil-Based Polyurethane Films. Polymers 2022, 14, 4974. [Google Scholar] [CrossRef]
- Cui, L.; Xiang, T.; Hu, B.; Lv, Y.; Rong, H.; Liu, D.; Zhang, S.; Guo, M.; Lv, Z.; Chen, D. Design of Monolithic Superhydrophobic Concrete with Excellent Anti-Corrosion and Self-Cleaning Properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133345. [Google Scholar] [CrossRef]
- Xu, H.; Hu, H.; Wang, H.; Li, Y.; Li, Y. Corrosion Resistance of Graphene/Waterborne Epoxy Composite Coatings in CO2-Satarated NaCl Solution. R. Soc. Open Sci. 2020, 7, 191943. [Google Scholar] [CrossRef]
- Najem, A.; Campos, O.S.; Girst, G.; Raji, M.; Hunyadi, A.; García-Antón, J.; Bellaouchou, A.; Amin, H.M.A.; Boudalia, M. Experimental and DFT Atomistic Insights into the Mechanism of Corrosion Protection of Low-Carbon Steel in an Acidic Medium by Polymethoxyflavones from Citrus Peel Waste. J. Electrochem. Soc. 2023, 170, 093512. [Google Scholar] [CrossRef]
- Heiba, A.R.; Taher, F.A.; Abou Shahba, R.M.; Abdel Ghany, N.A. Corrosion Mitigation of Carbon Steel in Acidic and Salty Solutions Using Electrophoretically Deposited Graphene Coatings. J. Coat. Technol. Res. 2021, 18, 501–510. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, R.; Du, A.; Zhang, X.; Yang, H.; Fan, Y.; Zhao, X.; Cao, X. Investigation of the Anticorrosion Properties of Graphene Oxide Doped Thin Organic Anticorrosion Films for Hot-Dip Galvanized Steel. Appl. Surf. Sci. 2019, 480, 646–654. [Google Scholar] [CrossRef]
- Chen, L.; Song, R.G.; Li, X.W.; Guo, Y.Q.; Wang, C.; Jiang, Y. The Improvement of Corrosion Resistance of Fluoropolymer Coatings by SiO2/Poly(Styrene-Co-Butyl Acrylate) Nanocomposite Particles. Appl. Surf. Sci. 2015, 353, 254–262. [Google Scholar] [CrossRef]
- ISO 4628-8; Paints and Varnishes—Evaluation of Degradation of Coatings—Designation of Quantity and Size of Defects, and of Intensity of Uniform Changes in Appearance—Part 8: Assessment of Degree of Delamination and Corrosion around a Scribe or Other 2012. ISO: Geneva, Switzerland, 2012.
- Wood, K.A. Optimizing the Exterior Durability of New Fluoropolymer Coatings. In Progress in Organic Coatings; Elsevier: Amsterdam, The Netherlands, 2001; Volume 43. [Google Scholar]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-Film Particles of Graphite Oxide 1: High-Yield Synthesis and Flexibility of the Particles. Carbon N. Y. 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Rocha, J.F.; Hostert, L.; Bejarano, M.L.M.; Cardoso, R.M.; Santos, M.D.; Maroneze, C.M.; Gongora-Rubio, M.R.; Silva, C.D.C.C. Graphene Oxide Fibers by Microfluidics Assembly: A Strategy for Structural and Dimensional Control. Nanoscale 2021, 13, 6752–6758. [Google Scholar] [CrossRef] [PubMed]
- ASTM G154; Standard Practice for Operating Fluorescent Ultraviolet (UV) Lamp Apparatus for Exposure of Materials. ASTM: West Conshohocken, PA, USA, 2023.







| Sample | Ecorr/(V) vs. Ag|AgCl | icorr/(A cm−2) | Corrosion Rate (mm/Year) | η |
|---|---|---|---|---|
| WPU | −0.811 | 9.03 × 10−7 | 1.01 × 10−2 | - |
| 1.3-GO | −0.379 | 2.57 × 10−6 | 2.88 × 10−2 | - |
| 0.1-GO | −0.361 | 1.02 × 10−7 | 1.15 × 10−3 | 88.70% |
| 0.01-GO | −0.126 | 9.34 × 10−9 | 1.05 × 10−4 | 99.00% |
| 0.01-GO-EDA | −0.118 | 3.70 × 10−9 | 4.15 × 10−5 | 99.60% |
| Ref. | Coating | Graphene Derivative | Concentration (wt%) | Application Method | Ecorr (V) | icorr (A cm−2) |
|---|---|---|---|---|---|---|
| [34] | Waterborne hydroxyl acrylic | WHAR MGO | 0.50 | Bar coater | −0.27 | 0.90 × 10−6 |
| [35] | Epoxy | GO-PANI-PDA | - | Wire bar coater | −0.59 | 3.83 × 10−8 |
| [36] | Waterborne epoxy | CMCS-rGO | 0.05 | Bar coater | −0.63 | 3.05 × 10−10 |
| [18] | WPU | GO-PNNG | 0.05 | - | −0.06 | 4.98 × 10−10 |
| [37] | Polyvinyl alcohol | GO-PVA-SiC | 10.00 | Spray | −0.45 | 1.22 × 10−6 |
| [38] | Epoxy | PA-G-EP | 1.00 | Bar coater | −0.62 | 3.10 × 10−8 |
| This work | WPU | GO-EDA | 0.01 | Blade coater | −0.12 | 3.70 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aramayo, M.A.F.; Ferreira Fernandes, R.; Santos Dias, M.; Bozzo, S.; Steinberg, D.; Rocha Diniz da Silva, M.; Maroneze, C.M.; de Carvalho Castro Silva, C. Eco-Friendly Waterborne Polyurethane Coating Modified with Ethylenediamine-Functionalized Graphene Oxide for Enhanced Anticorrosion Performance. Molecules 2024, 29, 4163. https://doi.org/10.3390/molecules29174163
Aramayo MAF, Ferreira Fernandes R, Santos Dias M, Bozzo S, Steinberg D, Rocha Diniz da Silva M, Maroneze CM, de Carvalho Castro Silva C. Eco-Friendly Waterborne Polyurethane Coating Modified with Ethylenediamine-Functionalized Graphene Oxide for Enhanced Anticorrosion Performance. Molecules. 2024; 29(17):4163. https://doi.org/10.3390/molecules29174163
Chicago/Turabian StyleAramayo, Mariel Amparo Fernandez, Rafael Ferreira Fernandes, Matheus Santos Dias, Stella Bozzo, David Steinberg, Marcos Rocha Diniz da Silva, Camila Marchetti Maroneze, and Cecilia de Carvalho Castro Silva. 2024. "Eco-Friendly Waterborne Polyurethane Coating Modified with Ethylenediamine-Functionalized Graphene Oxide for Enhanced Anticorrosion Performance" Molecules 29, no. 17: 4163. https://doi.org/10.3390/molecules29174163
APA StyleAramayo, M. A. F., Ferreira Fernandes, R., Santos Dias, M., Bozzo, S., Steinberg, D., Rocha Diniz da Silva, M., Maroneze, C. M., & de Carvalho Castro Silva, C. (2024). Eco-Friendly Waterborne Polyurethane Coating Modified with Ethylenediamine-Functionalized Graphene Oxide for Enhanced Anticorrosion Performance. Molecules, 29(17), 4163. https://doi.org/10.3390/molecules29174163








