Thermodynamic Study of 1,4-Bis(3-methylimidazolium-1-yl)butane Bis(trifluoromethylsulfonyl)imide ([C4(MIm)2][NTf2]2) from 6 to 350 K
Abstract
1. Introduction
2. Results and Discussion
2.1. Heat Capacity Measurements
2.2. Thermodynamic Characteristics of Fusion and the Purity Determination
2.3. Standard Thermodynamic Functions
3. Materials and Methods
3.1. Synthesis of the Bromide Precursor ([C4(MIm)2][Br]2)
3.2. Synthesis of the Test Compound ([C4(MIm)2][NTf2]2)
3.3. Heat Capacity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacFarlane, D.R.; Kar, M.; Pringle, J.M. Fundamentals of Ionic Liquids: From Chemistry to Applications; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Zaitsau, D.H.; Kabo, G.J.; Strechan, A.A.; Paulechka, Y.U.; Tschersich, A.; Verevkin, S.P.; Heintz, A. Experimental vapor pressures of 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imides and a correlation scheme for estimation of vaporization enthalpies of ionic liquids. J. Phys. Chem. A 2006, 110, 7303–7306. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Yermalayeu, A.V.; Schick, C.; Liu, H.; Maginn, E.J.; Bulut, S.; Krossing, I.; Kalb, R. Making sense of enthalpy of vaporization trends for ionic liquids: New experimental and simulation data show a simple linear relationship and help reconcile previous data. J. Phys. Chem. B 2013, 117, 6473–6486. [Google Scholar] [CrossRef]
- Zaitsau, D.H.; Varfolomeev, M.A.; Verevkin, S.P.; Stanton, A.D.; Hindman, M.S.; Bara, J.E. Structure–property relationships in ionic liquids: Influence of branched and cyclic groups on vaporization enthalpies of imidazolium-based ILs. J. Chem. Thermodyn. 2016, 93, 151–156. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide ([P8888]NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Li, Y.; Sun, H.; Wang, H.; Wang, J. Thermal decomposition and volatility of ionic liquids: Factors, evaluation and strategies. J. Mol. Liq. 2022, 366, 120336. [Google Scholar] [CrossRef]
- Brunetti, B.; Ciccioli, A.; Gigli, G.; Lapi, A.; Misceo, N.; Tanzi, L.; Vecchio Ciprioti, S. Vaporization of the prototypical ionic liquid BMImNTf2 under equilibrium conditions: A multi technique study. Phys. Chem. Chem. Phys. 2014, 16, 15653–15661. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and properties of high stability geminal dicationic ionic liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic imidazolium-based ionic liquids: A new strategy for non-toxic and antimicrobial materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Pandolfi, F.; Bortolami, M.; Feroci, M.; Fornari, A.; Scarano, V.; Rocco, D. Recent advances in imidazolium-based dicationic ionic liquids as organocatalysts: A mini-review. Materials 2022, 15, 866. [Google Scholar] [CrossRef]
- Vitorino, J.; Leal, J.P.; Licence, P.; Lovelock, K.R.J.; Gooden, P.N.; Minas da Piedade, M.E.; Shimizu, K.; Rebelo, L.P.N.; Canongia Lopes, J.N. Vaporisation of a dicationic ionic liquid revisited. Chem. Phys. Chem. 2010, 11, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Chilingarov, N.S.; Zhirov, M.S.; Shmykova, A.M.; Martynova, E.A.; Glukhov, L.M.; Chernikova, E.A.; Kustov, L.M.; Markov, V.Y.; Ioutsi, V.A.; Sidorov, L.N. Evaporation study of an ionic liquid with a double-charged cation. J. Phys. Chem. A 2018, 122, 4622–4627. [Google Scholar] [CrossRef]
- Blokhin, A.V.; Paulechka, Y.U.; Strechan, A.A.; Kabo, G.J. Physicochemical properties, structure, and conformations of 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide [C4mim]NTf2 ionic liquid. J. Phys. Chem. B 2008, 112, 4357–4364. [Google Scholar] [CrossRef]
- Liu, K.T.-C.; Haines, R.S.; Harper, J.B. The effect of bisimidazolium-based ionic liquids on a bimolecular substitution process. Are two head(group)s better than one? Org. Biomol. Chem. 2020, 18, 7388–7395. [Google Scholar] [CrossRef]
- Ciccioli, A.; Latini, A.; Luongo, A.; Smirnova, N.N.; Markin, A.V.; Vecchio Ciprioti, S. Thermodynamic study of formamidinium lead iodide (CH5N2PbI3) from 5 to 357 K. Entropy 2022, 24, 145. [Google Scholar] [CrossRef]
- Westrum, E.F. Determination of purity and phase behavior by adiabatic calorimetry. In Analytical Calorimetry; Porter, R.S., Johnson, J.F., Eds.; Springer: Boston, MA, USA, 1968. [Google Scholar] [CrossRef]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between dicationic and monocationic ionic liquids: Liquid density, thermal properties, surface tension, and shear viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Endo, T.; Sakaguchi, K.; Higashihara, K.; Kimura, Y. Structure–property relationship for 1-Isopropyl-3-methylimidazolium- and 1-tert-butyl-3-methylimidazolium-based ionic liquids: Thermal properties, densities, viscosities, and quantum chemical calculations. J. Chem. Eng. Data 2019, 64, 5857–5868. [Google Scholar] [CrossRef]
- Kazakov, A.; Magee, J.W.; Chirico, R.D.; Paulechka, E.; Diky, V.; Muzny, C.D.; Kroenlein, K.; Frenkel, M. NIST Standard Reference Database 147: Ionic Liquids Database—ILThermo (Version 2.0); National Institute of Standards and Technology: Gaithersburg, MD, USA, 2006. Available online: http://ilthermo.boulder.nist.gov (accessed on 28 August 2024).
- Liu, J.; Yang, W.; Li, Z.; Ren, F.; Hao, H. Experimental investigation of thermo-physical properties of geminal dicationic ionic compounds for latent thermal energy storage. J. Mol. Liq. 2020, 307, 112994. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Kabo, G.J.; Blokhin, A.V.; Shaplov, A.S.; Lozinskaya, E.I.; Vygodskii, Y.S. Thermodynamic properties of 1-alkyl-3-methylimidazolium bromide ionic liquids. J. Chem. Thermodyn. 2007, 39, 158–166. [Google Scholar] [CrossRef]
- Debye, P. Zur Theorie der spezifischen Wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar] [CrossRef]
- Paulechka, Y.U.; Blokhin, A.V.; Kabo, G.J.; Strechan, A.A. Thermodynamic properties and polymorphism of 1-alkyl-3-methylimidazolium bis(triflamides). J. Chem. Thermodyn. 2007, 39, 866–877. [Google Scholar] [CrossRef]
- Zábranský, M.; Růžička, V., Jr. Estimation of the heat capacity of organic liquids as a function of temperature using group additivity: An amendment. J. Phys. Chem. Ref. Data 2004, 33, 1071–1081. [Google Scholar] [CrossRef]
- Prohaska, T.; Irrgeher, J.; Benefield, J.; Böhlke, J.; Chesson, L.; Coplen, T.; Ding, T.; Dunn, P.; Gröning, M.; Holden, N.; et al. Standard atomic weights of the elements 2021 (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 573–600. [Google Scholar] [CrossRef]
- Varushchenko, R.M.; Druzhinina, A.I.; Sorkin, E.L. Low-temperature heat capacity of 1-bromoperfluorooctane. J. Chem. Thermodyn. 1997, 29, 623–637. [Google Scholar] [CrossRef]
- Preston-Thomas, H. The international temperature scale of 1990 (ITS-90). Metrologia 1990, 27, 3–10. [Google Scholar] [CrossRef]
- Strouse, G.F.; Tew, W.L. Assessment of Uncertainties of Calibration of Resistance Thermometers at the National Institute of Standards and Technology NISTIR 5319, 1994. Available online: https://www.nist.gov/sites/default/files/documents/calibrations/5319.pdf (accessed on 28 August 2024).
- Sparasci, F.; Pitre, L.; Rouillé, G.; Thermeau, J.-P.; Truong, D.; Galet, F.; Hermier, Y. An adiabatic calorimeter for the realization of the ITS-90 in the cryogenic range at the LNE-CNAM. Int. J. Thermophys. 2011, 32, 201–214. [Google Scholar] [CrossRef]
- Sabbah, R.; Xu-wu, A.; Chickos, J.S.; Planas Leitão, M.L.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Archer, D.G. Thermodynamic properties of synthetic sapphire (α-Al2O3), standard reference material 720 and the effect of temperature-scale differences on thermodynamic properties. J. Phys. Chem. Ref. Data 1993, 22, 1441–1453. [Google Scholar] [CrossRef]
- Douglas, T.B.; Furukawa, G.T.; McCoskey, R.E.; Ball, A.F. Calorimetric properties of normal heptane from 0 to 520 K. J. Res. Natl. Bur. Stand. 1954, 53, 139–153. [Google Scholar] [CrossRef]
- Furukawa, G.T.; McCoskey, R.E.; King, G.J. Calorimetric properties of benzoic acid from 0 to 410 K. J. Res. Natl. Bur. Stand. 1951, 47, 256–261. [Google Scholar] [CrossRef]
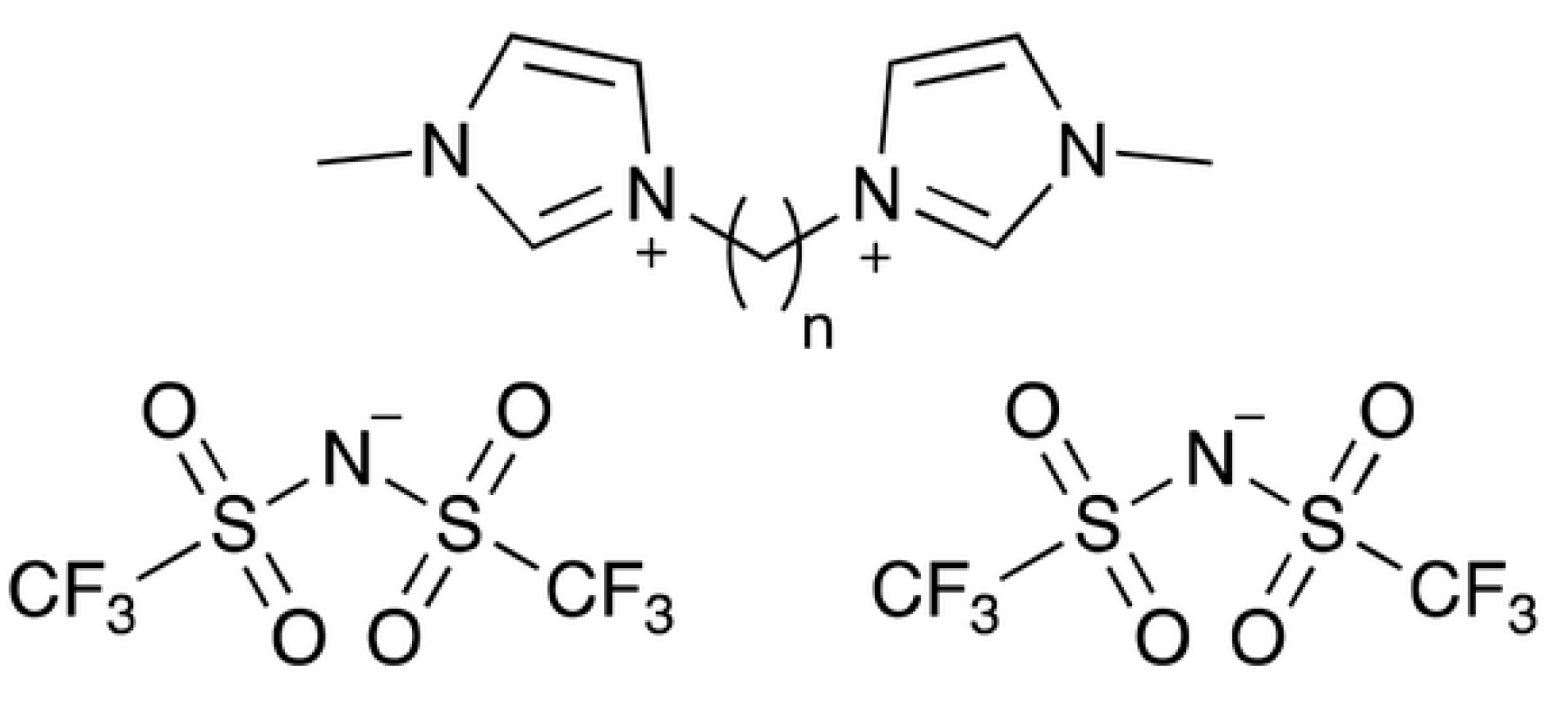

| T/K | Cp | T/K | Cp | T/K | Cp |
|---|---|---|---|---|---|
| Series 1 | |||||
| 6.52 | 14.1 | 17.14 | 67.49 | 47.73 | 220.5 |
| 6.85 | 14.7 | 17.61 | 70.68 | 49.37 | 226.6 |
| 7.22 | 16.1 | 18.09 | 73.60 | 51.01 | 232.6 |
| 7.52 | 17.1 | 18.57 | 76.49 | 52.66 | 238.5 |
| 7.81 | 18.3 | 19.05 | 79.79 | 54.31 | 244.5 |
| 8.21 | 19.6 | 19.53 | 83.09 | 56.40 | 251.7 |
| 8.74 | 21.4 | 20.01 | 86.29 | 58.11 | 257.3 |
| 9.21 | 23.7 | 20.99 | 92.56 | 60.12 | 263.9 |
| 9.78 | 25.8 | 22.45 | 102.1 | 61.81 | 269.1 |
| 10.33 | 28.2 | 23.94 | 110.9 | 63.62 | 274.8 |
| 10.89 | 30.3 | 25.45 | 120.0 | 65.11 | 279.4 |
| 11.35 | 32.7 | 26.98 | 128.7 | 66.31 | 282.8 |
| 11.84 | 35.0 | 28.51 | 137.8 | 67.63 | 286.7 |
| 12.14 | 36.4 | 30.07 | 145.0 | 69.30 | 291.2 |
| 12.61 | 38.8 | 31.64 | 153.3 | 71.17 | 296.8 |
| 13.08 | 41.9 | 33.21 | 161.1 | 73.23 | 302.3 |
| 13.44 | 43.5 | 34.80 | 167.8 | 75.42 | 307.6 |
| 13.90 | 46.1 | 36.39 | 175.0 | 78.25 | 315.9 |
| 14.35 | 49.0 | 37.99 | 182.2 | 81.23 | 323.8 |
| 14.81 | 52.0 | 39.60 | 188.5 | 83.84 | 330.3 |
| 15.27 | 55.41 | 41.21 | 195.0 | 87.31 | 338.1 |
| 15.73 | 57.90 | 42.84 | 201.1 | 90.88 | 347.3 |
| 16.20 | 60.93 | 44.46 | 207.8 | 93.85 | 355.0 |
| 16.67 | 64.26 | 46.35 | 215.2 | 96.81 | 362.0 |
| Series 2 | |||||
| 83.12 | 328.0 | 149.78 | 484.5 | 236.12 | 669.0 |
| 85.67 | 334.8 | 151.97 | 489.5 | 240.86 | 678.9 |
| 87.77 | 340.0 | 154.14 | 493.8 | 245.59 | 688.3 |
| 89.88 | 344.9 | 156.31 | 498.1 | 250.33 | 700.1 |
| 91.99 | 350.4 | 158.47 | 503.9 | 255.07 | 711.4 |
| 94.10 | 355.4 | 160.64 | 509.6 | 260.91 | 725.5 |
| 96.21 | 360.6 | 162.82 | 513.8 | 265.13 | 736.3 |
| 98.33 | 365.9 | 164.99 | 517.5 | 269.88 | 747.5 |
| 100.45 | 371.2 | 167.17 | 522.9 | 274.63 | 760.7 |
| 102.57 | 376.5 | 169.34 | 527.7 | 279.39 | 773.3 |
| 104.69 | 380.8 | 171.52 | 532.1 | 284.15 | 787.4 |
| 106.81 | 386.3 | 173.70 | 537.0 | 288.89 | 804.2 |
| 108.94 | 390.9 | 175.88 | 541.1 | 293.65 | 817.5 |
| 111.07 | 395.9 | 178.06 | 545.3 | 298.38 | 834.1 |
| 113.20 | 401.3 | 180.24 | 550.0 | 303.10 | 850.9 |
| 115.34 | 405.8 | 182.42 | 554.7 | 307.81 | 870.5 |
| 117.49 | 411.0 | 184.61 | 560.0 | 312.50 | 890.8 |
| 119.63 | 415.6 | 186.82 | 566.0 | 317.18 | (917.5) |
| 121.77 | 421.4 | 189.01 | 569.8 | 321.84 | (958.9) |
| 123.91 | 425.8 | 191.20 | 574.4 | 326.35 | (1190) |
| 126.06 | 430.4 | 193.39 | 579.2 | 329.23 | (3998) |
| 128.21 | 435.7 | 196.12 | 586.2 | 331.24 | (9457) |
| 130.36 | 441.0 | 199.21 | 592.6 | 333.21 | (12,079) |
| 132.51 | 446.0 | 202.88 | 598.2 | 334.03 | (15,155) |
| 134.66 | 450.6 | 206.10 | 603.3 | 334.15 | (23,042) |
| 136.82 | 455.5 | 209.67 | 610.2 | 334.37 | (9651) |
| 138.98 | 460.0 | 213.71 | 619.0 | 336.21 | 1011 |
| 141.14 | 465.4 | 217.81 | 628.1 | 339.24 | 1013 |
| 143.29 | 469.6 | 222.02 | 637.8 | 342.21 | 1017 |
| 145.45 | 475.1 | 226.73 | 651.0 | ||
| 147.62 | 480.1 | 231.41 | 659.7 | ||
| Series 3 | |||||
| 316.69 | 994 | 330.21 | 1007 | 345.41 | 1021 |
| 318.74 | 997 | 332.90 | 1008 | 347.51 | 1025 |
| 321.12 | 1000 | 335.42 | 1010 | 350.12 | 1032 |
| 323.49 | 1000 | 338.21 | 1013 | ||
| 327.52 | 1006 | 342.42 | 1017 | ||
| Series 4 | |||||
| 266.13 | 738.0 | 327.28 | 1060 | 337.42 | 70,215 |
| 269.33 | 749.0 | 330.73 | 1280 | 337.48 | 72,541 |
| 271.92 | 754.0 | 331.80 | 1351 | 337.58 | 85,145 |
| 275.02 | 763.0 | 332.80 | 1460 | 337.58 | 120,451 |
| 278.42 | 771.0 | 334.65 | 1740 | 337.59 | 200,471 |
| 281.52 | 780.0 | 335.78 | 2011 | 337.60 | 243,321 |
| 284.34 | 788.0 | 336.52 | 4655 | 337.61 | 281,541 |
| 287.91 | 800.2 | 336.72 | 8858 | 337.64 | 180,451 |
| 290.31 | 808.0 | 336.82 | 9501 | 337.67 | 92,514 |
| 294.12 | 820.0 | 336.99 | 14,214 | 337.74 | 10,012 |
| 297.01 | 836.0 | 337.13 | 15,111 | 337.78 | 1010 |
| 308.54 | 880.0 | 337.24 | 22,154 | 338.82 | 1014 |
| 311.90 | 888.9 | 337.29 | 27,581 | 340.52 | 1015 |
| 316.27 | 915.9 | 337.37 | 30,145 | ||
| 320.16 | 956.7 | 337.40 | 35,214 | ||
| T°i1/K | T°f1/K | ΔH°1/J | ΔH°2/J | ΔH°3/J g−1 | ΔH°4/J g−1 | ΔfusH°/J mol−1 |
|---|---|---|---|---|---|---|
| 281.21 | 342.58 | 155.52 | 78.35 | 63.21 | 6.108 | 52,520 |
| 280.34 | 345.24 | 162.98 | 82.91 | 64.08 | 9.466 | 53,220 |
| 285.32 | 347.15 | 157.51 | 79.19 | 59.08 | 12.09 | 52,655 |
| Mean value: | ||||||
| (52.79 ± 0.28) kJ mol−1 | ||||||
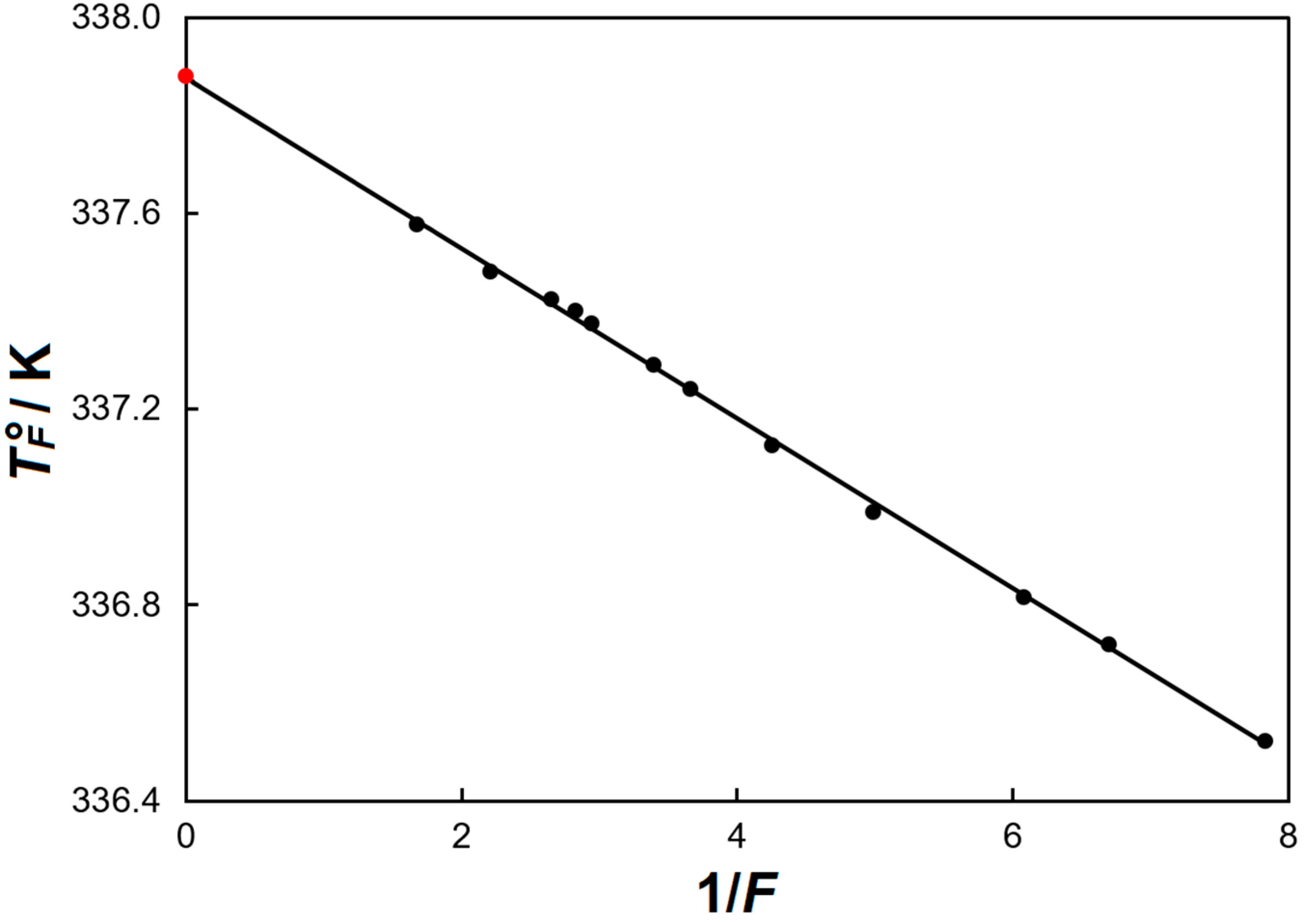
| T°F (exp.)/K | F | F−1 | T°F (calc.)/K |
|---|---|---|---|
| 336.52 | 0.1276 | 7.835 | 336.52 |
| 336.72 | 0.1494 | 6.696 | 336.72 |
| 336.82 | 0.1645 | 6.078 | 336.82 |
| 336.99 | 0.2004 | 4.989 | 337.01 |
| 337.13 | 0.2350 | 4.255 | 337.14 |
| 337.24 | 0.2728 | 3.666 | 337.24 |
| 337.29 | 0.2943 | 3.398 | 337.29 |
| 337.37 | 0.3393 | 2.947 | 337.37 |
| 337.40 | 0.3538 | 2.826 | 337.39 |
| 337.42 | 0.3768 | 2.654 | 337.42 |
| 337.48 | 0.4528 | 2.209 | 337.50 |
| 337.58 | 0.5966 | 1.676 | 337.59 |
| 1 | 1.000 | 337.70 | |
| ∞ | 0.000 | 337.88 |
| T/K | C°p(T)/J K−1 mol−1 | [H°(T) − H°(0)]/kJ mol−1 | S°(T)/J K−1 mol−1 | −[G°(T) − H°(0)]/kJ mol−1 |
|---|---|---|---|---|
| Solid | ||||
| 5 | 7.46 | 0.00995 | 2.68 | 0.00342 |
| 15 | 53.2 | 0.290 | 29.20 | 0.148 |
| 20 | 86.12 | 0.6383 | 48.99 | 0.3415 |
| 25 | 117.5 | 1.149 | 71.64 | 0.6424 |
| 30 | 145.1 | 1.807 | 95.56 | 1.060 |
| 35 | 168.9 | 2.593 | 119.8 | 1.598 |
| 40 | 190.1 | 3.491 | 143.7 | 2.257 |
| 45 | 210.0 | 4.492 | 167.3 | 3.035 |
| 50 | 228.9 | 5.590 | 190.4 | 3.929 |
| 60 | 263.6 | 8.056 | 235.3 | 6.059 |
| 70 | 293.2 | 10.84 | 278.2 | 8.628 |
| 80 | 320.2 | 13.91 | 319.1 | 11.62 |
| 90 | 345.4 | 17.24 | 358.3 | 15.00 |
| 100 | 370.0 | 20.82 | 396.0 | 18.78 |
| 110 | 393.6 | 24.64 | 432.3 | 22.92 |
| 120 | 416.7 | 28.69 | 467.6 | 27.42 |
| 130 | 439.9 | 32.97 | 501.8 | 32.27 |
| 140 | 462.8 | 37.49 | 535.3 | 37.45 |
| 150 | 485.1 | 42.23 | 568.0 | 42.97 |
| 160 | 507.0 | 47.19 | 600.0 | 48.82 |
| 170 | 528.7 | 52.37 | 631.4 | 54.97 |
| 180 | 550.3 | 57.76 | 662.2 | 61.44 |
| 190 | 572.0 | 63.37 | 692.5 | 68.21 |
| 200 | 593.3 | 69.20 | 722.4 | 75.29 |
| 210 | 611.6 | 75.22 | 751.8 | 82.66 |
| 220 | 633.7 | 81.45 | 780.8 | 90.32 |
| 230 | 655.9 | 87.89 | 809.4 | 98.27 |
| 240 | 677.6 | 94.56 | 837.8 | 106.5 |
| 250 | 699.6 | 101.4 | 865.9 | 115.0 |
| 260 | 722.7 | 108.6 | 893.8 | 123.8 |
| 270 | 748.0 | 115.9 | 921.5 | 132.9 |
| 280 | 775.8 | 123.6 | 949.2 | 142.3 |
| 290 | 806.3 | 131.5 | 977.0 | 151.9 |
| 298.15 | 836.0 | 138.1 | 999.7 | 159.9 |
| 300 | 841.8 | 139.7 | 1005 | 161.8 |
| 310 | 874.4 | 148.3 | 1033 | 172.0 |
| 320 | 907.0 | 157.2 | 1061 | 182.5 |
| 330 | 939.6 | 166.4 | 1090 | 193.2 |
| 337.88 | 963.8 | 173.9 | 1112 | 201.9 |
| Liquid | ||||
| 337.88 | 1012 | 226.7 | 1269 | 201.9 |
| 340 | 1014 | 228.9 | 1275 | 204.6 |
| 350 | 1032 | 239.1 | 1304 | 217.5 |
| Chemical Name | Source | Purification Method | Purity/% | Analysis Method |
|---|---|---|---|---|
| 1,4-bis(3-methylimidazolium-1-yl)butane bis(trifluoromethylsulfonyl)imide [C4(MIm)2][NTf2]2 | Synthesis | Repeated washing with water; drying in vacuum | >99.0 (mole fraction) | 1H NMR spectroscopy, TG analysis, adiabatic calorimetry |
| 1,4-bis(3-methylimidazolium-1-yl)butane dibromide [C4(MIm)2][Br]2 | Repeated washing with ethyl acetate; drying in vacuum | 1H NMR spectroscopy | ||
| 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [C4MIm][NTf2] CAS No.: 174899-83-3 | Sigma-Aldrich | Without any purification | >99.0 (mass fraction) | — |
| lithium bis(trifluoromethylsulfonyl)imide Li[NTf2] CAS No.: 90076-65-6 | ||||
| 1,4-dibromobutane C4H8Br2 CAS No.: 110-52-1 | ||||
| 1-methylimidazole C4H6N2 CAS No.: 616-47-7 | Purified by distillation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markin, A.V.; Ciccioli, A.; Lapi, A.; Sologubov, S.S.; Smirnova, N.N.; Vecchio Ciprioti, S. Thermodynamic Study of 1,4-Bis(3-methylimidazolium-1-yl)butane Bis(trifluoromethylsulfonyl)imide ([C4(MIm)2][NTf2]2) from 6 to 350 K. Molecules 2024, 29, 4180. https://doi.org/10.3390/molecules29174180
Markin AV, Ciccioli A, Lapi A, Sologubov SS, Smirnova NN, Vecchio Ciprioti S. Thermodynamic Study of 1,4-Bis(3-methylimidazolium-1-yl)butane Bis(trifluoromethylsulfonyl)imide ([C4(MIm)2][NTf2]2) from 6 to 350 K. Molecules. 2024; 29(17):4180. https://doi.org/10.3390/molecules29174180
Chicago/Turabian StyleMarkin, Alexey V., Andrea Ciccioli, Andrea Lapi, Semen S. Sologubov, Natalia N. Smirnova, and Stefano Vecchio Ciprioti. 2024. "Thermodynamic Study of 1,4-Bis(3-methylimidazolium-1-yl)butane Bis(trifluoromethylsulfonyl)imide ([C4(MIm)2][NTf2]2) from 6 to 350 K" Molecules 29, no. 17: 4180. https://doi.org/10.3390/molecules29174180
APA StyleMarkin, A. V., Ciccioli, A., Lapi, A., Sologubov, S. S., Smirnova, N. N., & Vecchio Ciprioti, S. (2024). Thermodynamic Study of 1,4-Bis(3-methylimidazolium-1-yl)butane Bis(trifluoromethylsulfonyl)imide ([C4(MIm)2][NTf2]2) from 6 to 350 K. Molecules, 29(17), 4180. https://doi.org/10.3390/molecules29174180









