Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria
Abstract
1. Introduction
2. Results and Discussion
2.1. Metal Concentration in Irrigation Water
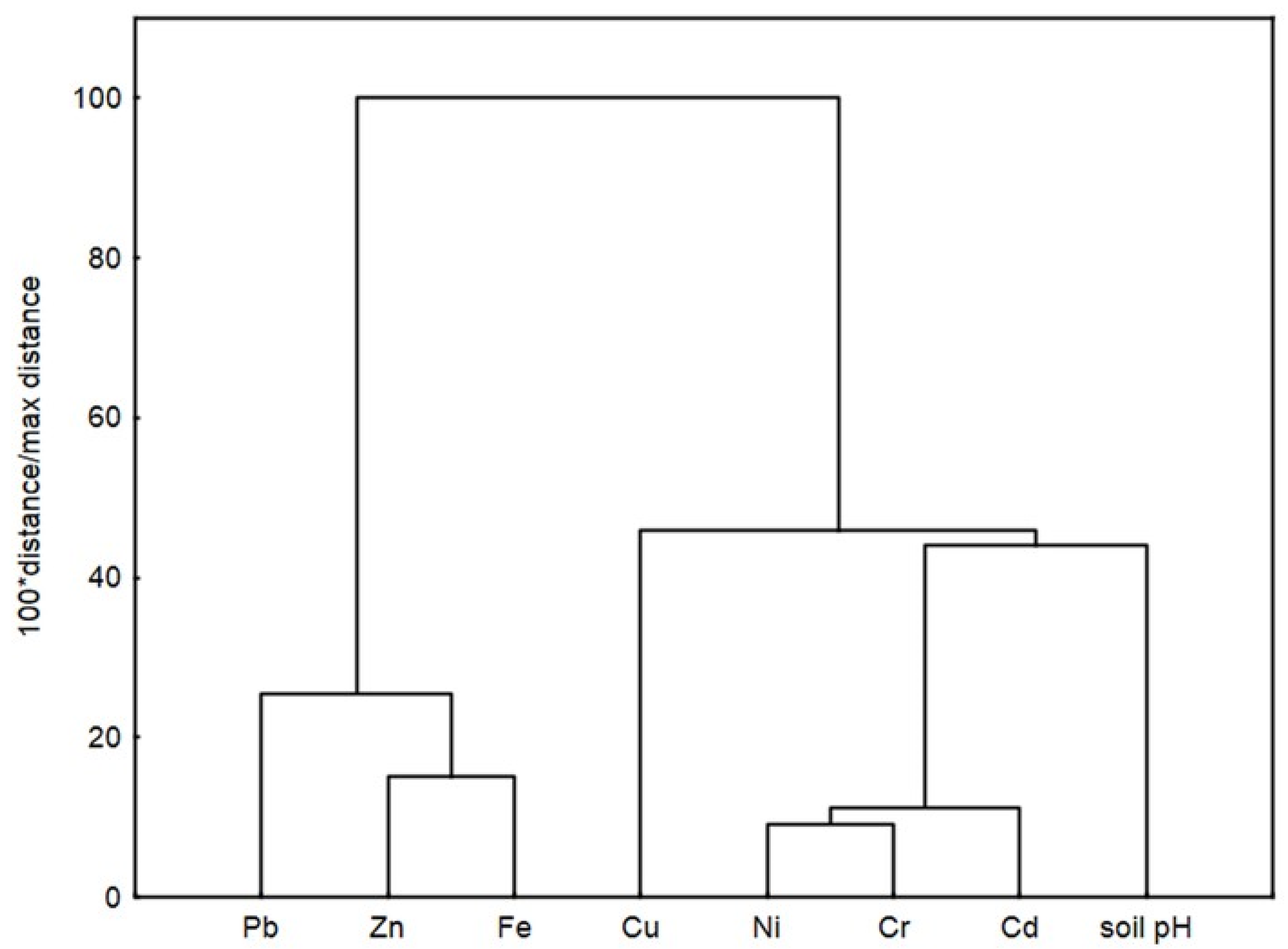
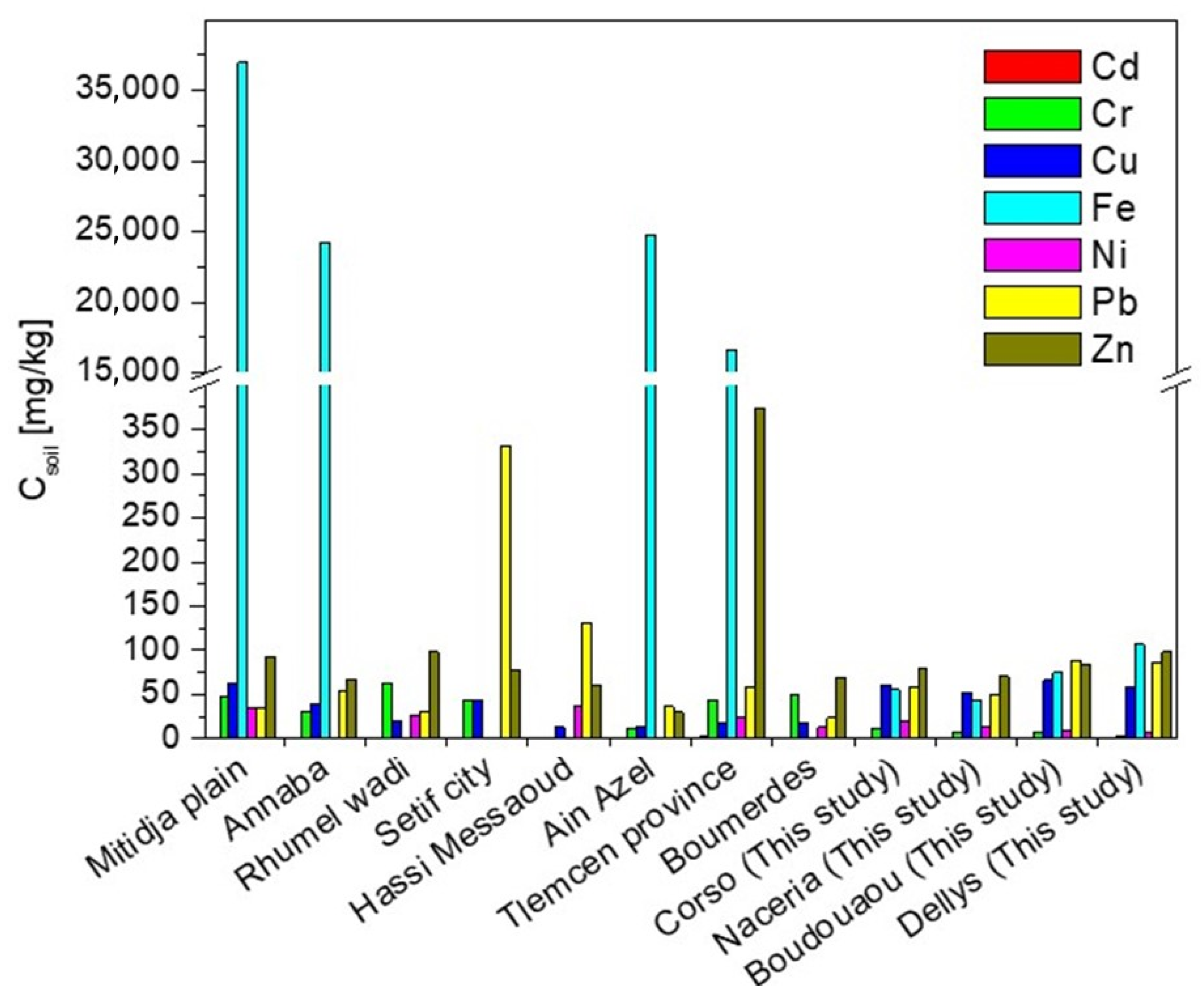
2.2. Metal Concentration in Soils
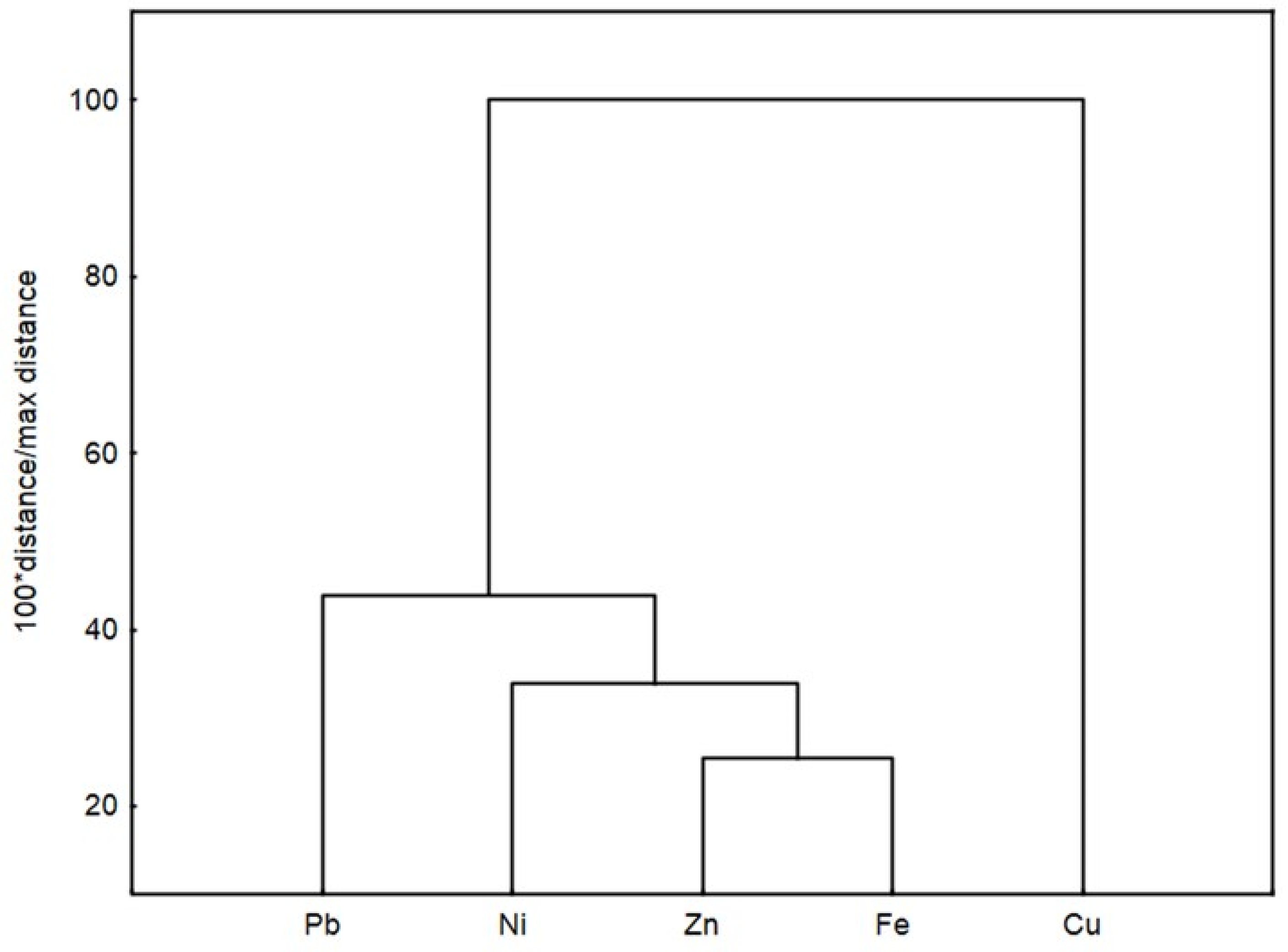
2.3. Metal Concentration in Fruits and Vegetables
2.4. Transfer Factor
2.5. Estimated Daily Intake of Heavy Metals
2.6. Non-Carcinogenic Risk
2.7. Carcinogenic Risk
3. Materials and Methods
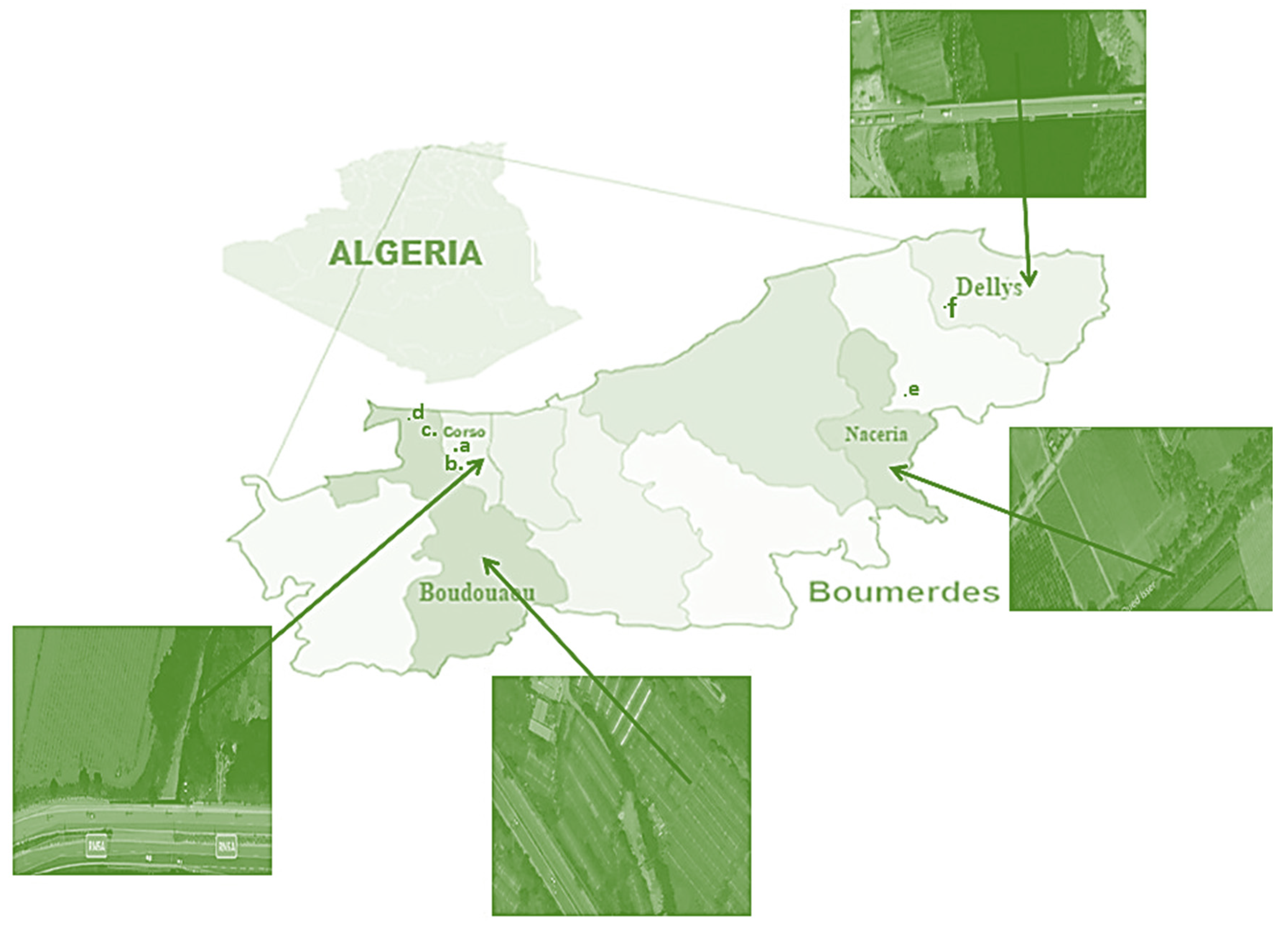
3.1. The Study Area
3.2. Sample Preparation
3.2.1. Water Samples
3.2.2. Soil Samples
3.2.3. Fruit and Vegetable Samples
3.3. Chemicals
3.4. Spectrometric Analysis
3.5. Calculations
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, Z.U.; Khan, S.; Shah, M.T.; Brusseau, M.L.; Khan, S.A.; Mainhagu, J. Transfer of Heavy Metals from Soils to Vegetables and Associated Human Health Risks at Selected Sites in Pakistan. Pedosphere 2018, 28, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Rabadjieva, D.; Tepavitcharova, S.; Kovacheva, A.; Gergulova, R.; Ilieva, R.; Vladov, I.; Nanev, V.; Gabrashanska, M.; Karavoltsos, S. Trace Metals Accumulation in the Eco-System Water-Soil-Vegetation (Agropyron cristatum)-Common Voles (Microtus arvalis)-Parasites (Hymenolepis diminuta) in Radnevo Region, Bulgaria. J. Trace Elem. Med. Biol. 2021, 66, 126750. [Google Scholar] [CrossRef]
- Guerrieri, N.; Mazzini, S.; Borgonovo, G. Food Plants and Environmental Contamination: An Update. Toxics 2024, 12, 365. [Google Scholar] [CrossRef]
- Saleem, M.; Pierce, D.; Wang, Y.; Sens, D.A.; Somji, S.; Garrett, S.H. Heavy Metal(Oid)s Contamination and Potential Ecological Risk Assessment in Agricultural Soils. J. Xenobiot. 2024, 14, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Osae, R.; Nukpezah, D.; Amoako Darko, D.; Mensah, A. Heavy Metal Mobility, Bioavailability, and Potential Toxicity in Sediments of the Korle Lagoon in Ghana. Int. J. Environ. Stud. 2023, 80, 1556–1572. [Google Scholar] [CrossRef]
- Djarmouni, M.; Sekia, I.; Ameni, D.; Ikessoulen, T.; Baghiani, A. Impact of Toxic Heavy Metals and Their Concentration in Zygophyllum Species, Mentha longifolia, and Thymus vulgaris Traditional Medicinal Plants Consumed in Setif-Algeria. Eur. J. Biol. 2023, 82, 109–123. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar]
- Milanković, V.; Tasić, T.; Leskovac, A.; Petrović, S.; Mitić, M.; Lazarević-Pašti, T.; Novković, M.; Potkonjak, N. Metals on the Menu—Analyzing the Presence, Importance, and Consequences. Foods 2024, 13, 1890. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Yu, G.; Zheng, W.; Wang, W.; Dai, F.; Zhang, Z.; Yuan, Y.; Wang, Q. Health Risk Assessment of Chinese Consumers to Cadmium via Dietary Intake. J. Trace Elem. Med. Biol. 2017, 44, 137–145. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.Y.; Chen, Y.H.; Lin, F.F.; Luo, D.; Gao, S.F. Transfer Characteristics of Cadmium and Lead from Soil to the Edible Parts of Six Vegetable Species in Southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdà, A. The Effect of Soil on Human Health: An Overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef]
- Rerknimitr, P.; Kantikosum, K.; Chottawornsak, N.; Tangkijngamvong, N.; Kerr, S.J.; Prueksapanich, P.; Sithisarankul, P.; Kumtornrut, C.; Asawanonda, P.; Sutheparuk, S.; et al. Chronic Occupational Exposure to Lead Leads to Significant Mucocutaneous Changes in Lead Factory Workers. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1993–2000. [Google Scholar] [CrossRef]
- Huang, J.L.; Mo, Z.Y.; Li, Z.Y.; Liang, G.Y.; Liu, H.L.; Aschner, M.; Ou, S.Y.; Zhou, B.; Chen, Z.M.; Jiang, Y.M. Association of Lead and Cadmium Exposure with Kidney Stone Incidence: A Study on the Non-Occupational Population in Nandan of China. J. Trace Elem. Med. Biol. 2021, 68, 126852. [Google Scholar] [CrossRef] [PubMed]
- Kawatra, B.L.; Bakhetia, P. Consumption of Heavy Metal and Minerals by Adult Women through Food in Sewage and Tube-Well Irrigated Area around Ludhiana City (Punjab, India). J. Hum. Ecol. 2008, 23, 351–354. [Google Scholar] [CrossRef]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Kazimi, S.G.T.; Karami, A.M.; Al Suliman, N.M.S.; Ouladsmane, M. Wastewater-Irrigated Vegetables Are a Significant Source of Heavy Metal Contaminants: Toxicity and Health Risks. Molecules 2023, 28, 1371. [Google Scholar] [CrossRef] [PubMed]
- Fytianos, K.; Katsianis, G.; Triantafyllou, P.; Zachariadis, G. Accumulation of Heavy Metals in Vegetables Grown in an Industrial Area in Relation to Soil. Bull. Environ. Contam. Toxicol. 2001, 67, 423–430. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F.M. Atmospheric Deposition of Heavy Metals (Cu, Zn, Cd and Pb) in Varanasi City, India. Environ. Monit. Assess. 2008, 142, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kachenko, A.G.; Singh, B. Heavy Metals Contamination In Vegetables Grown In Urban And Metal Smelter Contaminated Sites In Australia. Water Air Soil Pollut. 2006, 169, 101–123. [Google Scholar] [CrossRef]
- Cherfouh, R.; Lucas, Y.; Derridj, A.; Merdy, P. Long-Term, Low Technicality Sewage Sludge Amendment and Irrigation with Treated Wastewater under Mediterranean Climate: Impact on Agronomical Soil Quality. Environ. Sci. Pollut. Res. 2018, 25, 35571–35581. [Google Scholar] [CrossRef] [PubMed]
- Baziz, D.A.; Maazouzi, A.; Lachache, S. Physical-Chemical Characterisation of the Urban Wastewater—Case Study of the Boumerdes Region, North-Algeria. J. Water Land Dev. 2022, 54, 243–250. [Google Scholar] [CrossRef]
- Ghemmit-Doulache, N. Heavy Metals Detection in Soil Irrigated by STEP of Boumerdes-Algeria. J. Res. Green Chem. 2018, 1, 1–6. [Google Scholar]
- Drechsel, P.; Marjani Zadeh, S.; Pedrero, F. Water Quality in Agriculture: Risks and Risk Mitigation; The Food and Agriculture Organization of the United Nations and International Water Management Institute: Rome, Italy, 2023. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Guidelines for Water Reuse 600/R-12/618; USEPA: Washington, DC, USA, 2012.
- Khadidja, B.; Nadjiba, C. Biochemical Approach to Assess Groundwater Pollution by Heavy Metals Pollutants and Organics (Case Reghaia Lake, Algeria). Highlights Bioinform. 2020, 3, 20225. [Google Scholar] [CrossRef]
- Belkhiri, L.; Tiri, A.; Mouni, L. Assessment of Heavy Metals Contamination in Groundwater: A Case Study of the South of Setif Area, East Algeria. In Achievements and Challenges of Integrated River Basin Management; InTech: Hyogo, Japan, 2018. [Google Scholar]
- Boukhalfa, C. Heavy Metals in the Water and Sediments of Oued Es-Souk, Algeria, a River Receiving Acid Effluents from an Abandoned Mine. Afr. J. Aquat. Sci. 2007, 32, 245–249. [Google Scholar] [CrossRef]
- Bouragba, S.; Komai, K.; Nakayama, K. Assessment of Distributed Hydrological Model Performance for Simulation of Multi-Heavy-Metal Transport in Harrach River, Algeria. Water Sci. Technol. 2019, 80, 11–24. [Google Scholar] [CrossRef]
- Chaouch, N.; Birech, S.; Messaoudi, I. Detection of Toxic Heavy Metals in the Water of Chott Ain El Beida (Bowl of Ouargla, South-East of Algeria). Mater. Biomater. Sci. 2019, 2, 005–008. [Google Scholar]
- Guezgouz, N.; Parisi, C.; Boubsil, S.; Grieco, G.; Hana, S.A.; Guerriero, G. Heavy Metals Assessment in the Medjerda River Basin (Northeastern Algeria): A Preliminary Water Analysis and Toad Skin Biopsy. Proc. Zool. Soc. 2021, 74, 104–113. [Google Scholar] [CrossRef]
- Krika, A. Assessment of Heavy Metals Pollution in Water and Sediments of Djendjen River, North Eastern Algeria. Pollution 2018, 4, 495–502. [Google Scholar] [CrossRef]
- Kaddour, K.; Smail, M. Assessment of Heavy Metal Pollution Due to the Lead—Zinc Mine at the Ain Azel Area (Northeast of Algeria). J. Environ. Res. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in Soil PH and Mobility of Heavy Metals in Contaminated Soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Environmental Chemistry for a Sustainable World, Vol.10: Soil Pollution—An Emerging to Agriculture; Springer Nature: Singapore, 2017; p. 175. ISBN 978-981-10-4273-7. [Google Scholar]
- GB15618-2018; State Administration of Market Regulation Soil Environmental Quality—Risk Control Standard for Soil of Agricultural Land. Ministry of Ecology and Environment of the PRC: Beijing, China, 2018.
- Singh, S.; Kumar, M. Heavy Metal Load of Soil, Water and Vegetables in Peri-Urban Delhi. Environ. Monit. Assess. 2006, 120, 79–91. [Google Scholar] [CrossRef]
- Laribi, A.; Shand, C.; Wendler, R.; Mouhouche, B.; Colinet, G. Concentrations and Sources of Cd, Cr, Cu, Fe, Ni, Pb and Zn in Soil of the Mitidja Plain, Algeria. Toxicol. Environ. Chem. 2019, 101, 59–74. [Google Scholar] [CrossRef]
- Maas, S.; Scheifler, R.; Benslama, M.; Crini, N.; Lucot, E.; Brahmia, Z.; Benyacoub, S.; Giraudoux, P. Spatial Distribution of Heavy Metal Concentrations in Urban, Suburban and Agricultural Soils in a Mediterranean City of Algeria. Environ. Pollut. 2010, 158, 2294–2301. [Google Scholar] [CrossRef]
- El-Hadef El-Okki, M.; Sahli, L.; Bentellis, A.; Azzoug, R.; Laing, G.D.; Rached, O. Assessment of Metal Contamination in Soil Banks of the Rhumel Wadi (Northeast Algeria). Toxicol. Environ. Chem. 2016, 98, 53–63. [Google Scholar] [CrossRef]
- Sellami, S.; Zeghouan, O.; Dhahri, F.; Mechi, L.; Moussaoui, Y.; Kebabi, B. Assessment of Heavy Metal Pollution in Urban and Peri-Urban Soil of Setif City (High Plains, Eastern Algeria). Environ. Monit. Assess. 2022, 194, 126. [Google Scholar] [CrossRef] [PubMed]
- Benhaddya, M.L.; Hadjel, M. Spatial Distribution and Contamination Assessment of Heavy Metals in Surface Soils of Hassi Messaoud, Algeria. Environ. Earth Sci. 2014, 71, 1473–1486. [Google Scholar] [CrossRef]
- Ramdani, S.; Amar, A.; Belhsaien, K.; El Hajjaji, S.; Ghalem, S.; Zouahri, A.; Douaik, A. Assessment of Heavy Metal Pollution and Ecological Risk of Roadside Soils in Tlemcen (Algeria) Using Flame-Atomic Absorption Spectrometry. Anal. Lett. 2018, 51, 2468–2487. [Google Scholar] [CrossRef]
- ALI-NORM 01/12A; Food Additives and Contaminants. Codex Alimentarius Commission. Joint FAO/WHO Food Standards Program. FAO/WHO: Rome, Italy, 2001; Volume 12.
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniatti-Brazaca, S.G. Heavy Metals in Food Chain Heavy Metals in Vegetables and Potential Risk for Human Health. Sci. Agric. 2012, 69, 54–60. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Marzaioli, R.; De Crescenzo, S.; Trifuoggi, M. Human Health Risk from Consumption of Two Common Crops Grown in Polluted Soils. Sci. Total Environ. 2019, 691, 195–204. [Google Scholar] [CrossRef]
- Bounar, A.; Boukaka, K.; Leghouchi, E. Determination of Heavy Metals in Tomatoes Cultivated under Green Houses and Human Health Risk Assessment. Qual. Assur. Saf. Crops Foods 2020, 12, 78–86. [Google Scholar] [CrossRef]
- de Sousa, F.F.; do Carmo, M.G.F.; Lima, E.S.A.; da Costa Barros de Souza, C.; do Amaral Sobrinho, N.M.B. Lead and Cadmium Transfer Factors and the Contamination of Tomato Fruits (Solanum lycopersicum) in a Tropical Mountain Agroecosystem. Bull. Environ. Contam. Toxicol. 2020, 105, 325–331. [Google Scholar] [CrossRef]
- Grochowska-Niedworok, E.; Nieć, J.; Baranowska, R. Assessment of Cadmium and Lead Content in Tomatoes and Tomato Products. Rocz. Panstw. Zakl. Hig. Ann. Natl. Inst. Hyg. 2020, 71, 313–319. [Google Scholar] [CrossRef]
- Ametepey, S.T.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Health Risk Assessment and Heavy Metal Contamination Levels in Vegetables from Tamale Metropolis, Ghana. Int. J. Food Contam. 2018, 5, 5. [Google Scholar] [CrossRef]
- Cheshmazar, E.; Arfaeinia, H.; Karimyan, K.; Sharafi, H.; Hashemi, S.E. Dataset for Effect Comparison of Irrigation by Wastewater and Ground Water on Amount of Heavy Metals in Soil and Vegetables: Accumulation, Transfer Factor and Health Risk Assessment. Data Brief 2018, 18, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Thien, B.N.; Ba, V.N.; Man, M.T.; Hong Loan, T.T. Analysis of the Soil to Food Crops Transfer Factor and Risk Assessment of Multi-Elements at the Suburban Area of Ho Chi Minh City, Vietnam Using Instrumental Neutron Activation Analysis (INAA). J. Environ. Manag. 2021, 291, 112637. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population Health Risk Due to Dietary Intake of Heavy Metals in the Industrial Area of Huludao City, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiao, M.; Liu, Y.; Zhu, Y. Health Risk Assessment of Heavy Metals in Soils and Vegetables from Wastewater Irrigated Area, Beijing-Tianjin City Cluster, China. J. Environ. Sci. 2012, 24, 690–698. [Google Scholar] [CrossRef]
- Mapanda, F.; Mangwayana, E.N.; Nyamangara, J.; Giller, K.E. Uptake of Heavy Metals by Vegetables Irrigated Using Wastewater and the Subsequent Risks in Harare, Zimbabwe. Phys. Chem. Earth 2007, 32, 1399–1405. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.M.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of Heavy Metals in Fruits and Vegetables: Health Risk Implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- Chowdhury, A.I.; Shill, L.C.; Raihan, M.M.; Rashid, R.; Bhuiyan, M.N.H.; Reza, S.; Alam, M.R. Human Health Risk Assessment of Heavy Metals in Vegetables of Bangladesh. Sci. Rep. 2024, 14, 15616. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health Risk Assessment of Heavy Metals via Dietary Intake of Foodstuffs from the Wastewater Irrigated Site of a Dry Tropical Area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Moulouel, H.; Bensalem, R.; Machane, D.; Bendaoud, A.; Gharbi, S.; Oubaiche, E.H.; Ousalem, H.; Skendri, W. High Resistant Sand Injected Marl and Low Resistant Damaged Marl to Locate and Characterize the Thénia Fault Zone in Boumerdes City (North-Central Algeria). Pure Appl. Geophys. 2017, 174, 103–115. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). Manual of Methods in Aquatic Environment Research. Part 9. Analysis of Metals and Organochlorine in Fish. FAO Fish Technical Paper 212. Section 2; FAO: Rome, Italy, 1983. [Google Scholar]
- Eliku, T.; Leta, S. Heavy Metals Bioconcentration from Soil to Vegetables and Appraisal of Health Risk in Koka and Wonji Farms, Ethiopia. Environ. Sci. Pollut. Res. 2017, 24, 11807–11815. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, G.; Khan, A.; Zishan Ahmad, M.; Irshad, U. Determination of Concentration of Heavy Metals in Fruits, Vegetables, Groundwater, and Soil Samples of the Cement Industry and Nearby Communities and Assessment of Associated Health Risks. J. Food Qual. 2021, 2021, 3354867. [Google Scholar] [CrossRef]
- Njuguna, S.M.; Makokha, V.A.; Yan, X.; Gituru, R.W.; Wang, Q.; Wang, J. Health Risk Assessment by Consumption of Vegetables Irrigated with Reclaimed Waste Water: A Case Study in Thika (Kenya). J. Environ. Manag. 2019, 231, 576–581. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures Risk Assessment Forum Technical Panel; USEPA: Washington, DC, USA, 2000.
- Javed, M.; Usmani, N. Accumulation of Heavy Metals and Human Health Risk Assessment via the Consumption of Freshwater Fish Mastacembelus Armatus Inhabiting, Thermal Power Plant Effluent Loaded Canal. SpringerPlus 2016, 5, 776. [Google Scholar] [CrossRef]
- USEPA (United State Environmental Protection Agency). Risk Based Screening Table. Composite Tables; USEPA: Washington, DC, USA, 2024.
- Battsengel, E.; Murayama, T.; Fukushi, K.; Nishikizawa, S.; Chonokhuu, S.; Ochir, A.; Tsetsgee, S.; Davaasuren, D. Ecological and Human Health Risk Assessment of Heavy Metal Pollution in the Soil of the Ger District in Ulaanbaatar, Mongolia. Int. J. Environ. Res. Public Health 2020, 17, 4668. [Google Scholar] [CrossRef]



| Sampling Site/Crop | Cwater ± SD (mg/L) | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Ni | Pb | Zn | |
| Corso/ grape | <LOD | <LOD | 0.82 ± 0.01 | 0.13 ± 0.02 | 0.11 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| Corso/ melon | <LOD | <LOD | 0.88 ± 0.01 | 0.11 ± 0.02 | 0.09 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 |
| Naceria/ watermelon | <LOD | <LOD | 0.52 ±0.01 | 0.04 ± 0.01 | 0.14 ± 0.02 | 0.03 ± 0.01 | 0.13 ± 0.01 |
| Boudouaou/ tomato | <LOD | 0.09 ± 0.01 | 1.17 ± 0.02 | 0.67 ± 0.02 | 1.98 ± 0.04 | 0.09 ± 0.01 | 0.31 ± 0.02 |
| Boudouaou/ zucchini | <LOD | <LOD | 0.79 ± 0.02 | 0.54 ± 0.02 | 0.87 ± 0.02 | 0.07 ± 0.02 | 1.02 ± 0.04 |
| Boudouaou/ carrot | <LOD | 0.19 ± 0.01 | 0.31 ± 0.01 | 0.59 ± 0.01 | 0.11 ± 0.02 | 0.07 ± 0.02 | 0.47 ± 0.02 |
| Dellys/lettuce | <LOD | 0.11 ± 0.02 | 0.70 ± 0.03 | 1.10 ± 0.02 | 0.02 ± 0.01 | 0.82 ± 0.05 | 0.62 ± 0.01 |
| Dellys/potato | <LOD | 0.09 ± 0.01 | 0.56 ± 0.01 | 0.97 ± 0.04 | 0.91 ± 0.03 | 0.42 ± 0.03 | 0.88 ± 0.04 |
| Dellys/green pepper | <LOD | 0.26 ± 0.01 | 0.87 ± 0.02 | 0.87 ± 0.03 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.54 ± 0.02 |
| ML (mg/L) [24,25] | 0.01 | 0.10 | 0.20 | 5.00 | 0.20 | 5.00 | 2.00 |
| Sampling Site/Crop | pH | Csoil ± SD (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Ni | Pb | Zn | |||
| Corso/ grape | 6.99 ± 0.03 | 0.09 ± 0.02 | 12.17 ± 0.85 | 57.12 ± 6.28 | 53.21 ± 8.47 | 19.52 ±1.85 | 61.11 ±3.24 | 76.12 ± 6.43 | |
| Corso/ melon | 7.13 ± 0.05 | 0.07 ± 0.02 | 11.39 ± 0.35 | 63.23 ± 9.56 | 57.64 ± 7.65 | 21.24 ± 2.27 | 54.22 ± 5.68 | 83.27 ± 8.67 | |
| Naceria/ watermelon | 6.80 ± 0.08 | 0.02 ± 0.00 | 6.91 ± 0.60 | 52.10 ± 4.71 | 42.74 ± 7.85 | 13.55 ± 3.11 | 49.22 ± 2.29 | 70.12 ± 4.78 | |
| Boudouaou/ tomato | 7.11 ± 0.07 | 0.04 ± 0.00 | 11.20 ± 0.50 | 66.25 ± 1.05 | 62.33 ± 1.02 | 15.60 ± 2.28 | 72.35 ± 1.20 | 81.24 ± 1.50 | |
| Boudouaou/ zucchini | 6.57 ± 0.07 | 0.02 ± 0.01 | 8.92 ± 0.29 | 78.00 ± 1.32 | 72.97 ± 1.04 | 10.82 ± 4.34 | 92.82 ± 1.63 | 71.9 ± 5.32 | |
| Boudouaou/ carrot | 6.92 ± 0.09 | <LOD | 2.24 ± 0.80 | 52.64 ± 6.51 | 89.11 ± 10.48 | 3.25 ± 0.89 | 101.20 ± 8.43 | 95.12 ± 1.33 | |
| Dellys/ lettuce | 6.32 ± 0.13 | <LOD | 2.31 ± 0.20 | 74.64 ± 8.68 | 105.35 ± 1.63 | 5.23 ± 1.87 | 68.90 ± 1.31 | 108.21 ± 1.87 | |
| Dellys/ potato | 7.13 ± 0.08 | <LOD | 3.50 ± 0.11 | 38.25 ± 1.34 | 120.42 ± 10.95 | 4.94 ± 0.85 | 110.25 ± 10.84 | 102.32 ± 1.89 | |
| Dellys/ green pepper | 7.31 ± 0.09 | <LOD | 2.61 ± 0.2 | 60.20 ± 10.57 | 95.43 ± 1.74 | 7.27 ± 0.35 | 79.52 ± 1.11 | 82.40 ± 1.05 | |
| ML (mg/kg) [35,36] | - | 3 | 150 | 140 | 5000 | 75 | 300 | 300 | |
| Metal | pHsoil | Cd | Cr | Cu | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| pHsoil | 1 | |||||||
| Cd | 0.244 | 1 | ||||||
| Cr | 0.169 | 0.880 ** | 1 | |||||
| Cu | −0.559 | 0.090 | 0.250 | 1 | ||||
| Fe | −0.056 | −0.680 * | −0.784 * | −0.197 | 1 | |||
| Ni | 0.250 | 0.916 ** | 0.939 ** | 0.182 | −0.827 ** | 1 | ||
| Pb | 0.073 | −0.578 | −0.523 | −0.277 | 0.750 * | −0.730 * | 1 | |
| Zn | −0.199 | −0.459 | −0.658 | −0.180 | 0.833 ** | −0.644 | 0.456 | 1 |
| Food | Cfood ± SD (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Ni | Pb | Zn | |
| Fruits | |||||||
| Grape | <LOD | <LOD | 2.74 ± 0.16 | 0.94 ± 0.02 | 0.21 ± 0.01 | 0.43 ± 0.01 | 1.33 ± 0.12 |
| Melon | <LOD | <LOD | 3.45 ± 0.05 | 1.32 ± 0.02 | 0.42 ± 0.03 | 0.31 ± 0.06 | 9.01 ± 0.03 |
| Watermelon | <LOD | <LOD | 0.94 ± 0.02 | 1.12 ± 0.05 | 0.12 ± 0.02 | 0.31 ± 0.02 | 3.12 ± 0.06 |
| ML [44] | 0.05 | 1.0 | 4.5 | n.a. | 0.8 | 0.1 | n.a. |
| Vegetables | |||||||
| Tomato | <LOD | <LOD | 13.01 ± 0.45 | 1.75 ± 0.05 | 0.24 ± 0.05 | 1.11 ± 0.04 | 8.04 ± 0.52 |
| Zucchini | <LOD | <LOD | 9.36 ± 0.82 | 0.94 ± 0.02 | < LOD | 0.43 ± 0.01 | 6.54 ± 0.51 |
| Carrot | <LOD | 0.09 ± 0.01 | 6.75 ± 0.29 | 17.87 ± 0.22 | 0.62 ± 0.02 | 2.91 ± 0.10 | 12.21 ± 0.41 |
| Potato | <LOD | <LOD | 4.86 ± 0.13 | 30.72 ± 0.16 | 0.82 ± 0.02 | 0.77 ± 0.02 | 13.14 ± 0.13 |
| Lettuce | <LOD | <LOD | 5.62 ± 0.26 | 54.20 ± 0.64 | 1.01 ± 0.19 | 3.45 ± 0.26 | 19.52 ± 0.67 |
| Green pepper | <LOD | <LOD | 3.44 ± 0.15 | 21.00 ± 0.88 | 0.11 ± 0.01 | 1.19 ± 0.08 | 9.84 ± 0.18 |
| ML [44] | 0.05 | 1.0 | 40 | 425 | 10 | 0.1 | 100 |
| Metal | Cu | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|
| Cu | 1 | ||||
| Fe | −0.068 | 1 | |||
| Ni | −0.023 | 0.832 *** | 1 | ||
| Pb | 0.203 | 0.767 ** | 0.693 * | 1 | |
| Zn | 0.197 | 0.896 *** | 0.841 *** | 0.791 ** | 1 |
| Food | FIR (g/Day/Person) | EDI (mg/Day) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Fe | Ni | Pb | Zn | |||
| Fruits | |||||||||
| Grape | 80 | <1.39 × 10−6 | <8.02 × 10−6 | 2.93 × 10−4 | 1.01 × 10−4 | 2.25 × 10−5 | 4.60 × 10−5 | 1.09 × 10−4 | |
| Melon | 150 | <2.61 × 10−6 | <1.50 × 10−5 | 6.92 × 10−4 | 2.65 × 10−4 | 8.42 × 10−5 | 6.21 × 10−5 | 1.81 × 10−3 | |
| Watermelon | 150 | <2.61 × 10−6 | <1.50 × 10−5 | 1.88 × 10−4 | 2.25 × 10−4 | 2.41 × 10−5 | 6.21 × 10−5 | 6.25 × 10−3 | |
| All fruits | <6.60 × 10−6 | <3.81 × 10−5 | 1.17 × 10−3 | 5.91 × 10−4 | 1.31 × 10−4 | 1.70 × 10−4 | 2.54 × 10−3 | ||
| Vegetables | |||||||||
| Tomato | 100 | <1.74 × 10−6 | <1.00 × 10−5 | 1.74 × 10−4 | 2.34 × 10−4 | 3.21 × 10−5 | 1.48 × 10−4 | 1.07 × 10−3 | |
| Zucchini | 30 | <5.21 × 10−7 | <3.01 × 10−6 | 3.75 × 10−4 | 6.70 × 10−4 | <1.00 × 10−6 | 3.93 × 10−5 | 2.62 × 10−4 | |
| Carrot | 40 | <6.95 × 10−7 | 4.01 × 10−6 | 3.61 × 10−4 | 9.55 × 10−4 | 3.31 × 10−5 | 1.56 × 10−4 | 6.53 × 10−4 | |
| Potato | 200 | <3.47 × 10−6 | <2.00 × 10−5 | 1.30 × 10−3 | 8.21 × 10−4 | 2.19 × 10−4 | 2.06 × 10−4 | 3.51 × 10−3 | |
| Lettuce | 50 | <8.69 × 10−7 | <5.01 × 10−6 | 3.76 × 10−4 | 3.89 × 10−3 | 7.42 × 10−5 | 2.87 × 10−4 | 1.30 × 10−3 | |
| Green pepper | 120 | <2.08 × 10−6 | <1.20 × 10−5 | 5.52 × 10−4 | 3.40 × 10−3 | 1.76 × 10−5 | 2.36 × 10−4 | 1.57 × 10−3 | |
| All vegetables | <9.38 × 10−6 | <5.41 × 10−5 | 3.14 × 10−3 | 1.68 × 10−2 | 3.76 × 10−5 | 1.07 × 10−3 | 8.37 × 10−3 | ||
| All food | <1.60 × 10−5 | <9.22 × 10−5 | 4.31 × 10−3 | 1.73 × 10−2 | 5.07 × 10−4 | 1.24 × 10−3 | 1.09 × 10−2 | ||
| MTDI [56] | 0.021 | 0.2 | 30 | n.a. | 0.3 | 0.21 | 60 | ||
| Element | λ [nm] | Concentration Range [mg/L] | Correlation Coefficient (R2) | Limit of Detection | ||
|---|---|---|---|---|---|---|
| For Water [mg/L] | For Soil [mg/kg] | For Food [mg/kg] | ||||
| Cd | 228.8 | 0–2.0 | 0.9990 | 0.0005 | 0.005 | 0.013 |
| Cr | 357.9 | 0–2.0 | 0.9982 | 0.003 | 0.03 | 0.075 |
| Cu | 324.8 | 0–2.0 | 0.9980 | 0.001 | 0.01 | 0.025 |
| Fe | 248.3 | 0–2.0 | 0.9984 | 0.002 | 0.02 | 0.050 |
| Ni | 232.0 | 0–2.0 | 0.9974 | 0.001 | 0.01 | 0.025 |
| Pb | 217.0 | 0–2.0 | 0.9996 | 0.006 | 0.06 | 0.150 |
| Zn | 213.9 | 0–20.0 | 0.9950 | 0.004 | 0.04 | 0.100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksouh, M.Y.; Boudieb, N.; Benosmane, N.; Moussaoui, Y.; Michalski, R.; Klyta, J.; Kończyk, J. Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria. Molecules 2024, 29, 4187. https://doi.org/10.3390/molecules29174187
Aksouh MY, Boudieb N, Benosmane N, Moussaoui Y, Michalski R, Klyta J, Kończyk J. Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria. Molecules. 2024; 29(17):4187. https://doi.org/10.3390/molecules29174187
Chicago/Turabian StyleAksouh, Mohamed Younes, Naima Boudieb, Nadjib Benosmane, Yacine Moussaoui, Rajmund Michalski, Justyna Klyta, and Joanna Kończyk. 2024. "Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria" Molecules 29, no. 17: 4187. https://doi.org/10.3390/molecules29174187
APA StyleAksouh, M. Y., Boudieb, N., Benosmane, N., Moussaoui, Y., Michalski, R., Klyta, J., & Kończyk, J. (2024). Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria. Molecules, 29(17), 4187. https://doi.org/10.3390/molecules29174187







