Recent Advances in Food Waste Transformations into Essential Bioplastic Materials
Abstract
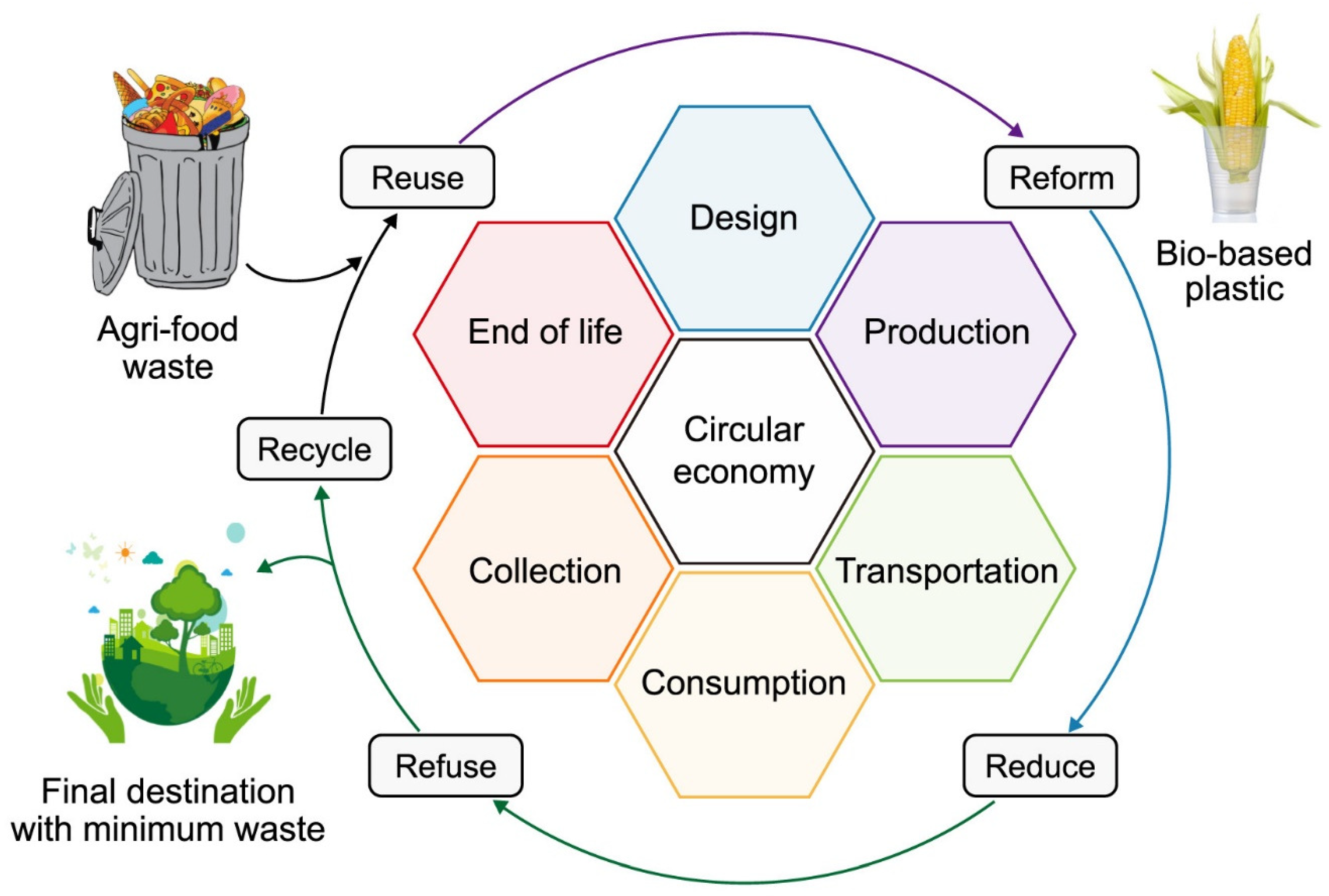
:1. Introduction
2. Present KW Management and Disposal Procedures
3. Ingredients in Kitchen Waste
4. PHAs and the State of PHA Synthesis
| S.No | Substrate | Characteristics of the Substrate | Substrate Pre-treatment | Culture | PHA Production | Extraction/Analysis of the Samples | Fermentation Time (h) | PHA Content (% gPHAs/g Biomass) | Biomass Concentration (g/L) | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Spent coffee grounds (SCG) oil | High amounts of organic fractions like fatty acids and minerals | Supercritical extraction of the SCG oil | Cupriavidus necator DSM 428 | After batch processing, there is an N-limited fed-batch step | Precipitation of the polymer after adding n-hexane to the broth | 48 h | 78.4 | 16.7 | [32,33] |
| 2 | Extraction of the SCG oil with the use of n-hexane in an extractor apparatus | Cupriavidus necator H16 | Fed batch cultivation | Centrifugation of the samples and washing of the cells with 5% (v/v) Triton X and distilled water | 72 h | 90.1 | 49.4 | [33] | ||
| 3 | Used cooking oil | High content of lipid (fat) | nd | C. necator DSM 428 | Batch operation with excess nitrogen | Collection of the broth, washing with hexane, lyophilization, extraction with chloroform | 50 h | 63 | 11.6 | [34] |
| 4 | Waste rapeseed oil | High content of lipid (fat) | nd | Pseudomonas sp. G101 and G106 | Cultivation of the cells in a nitrogen-limited | Dissolving of PHAs in chloroform. Precipitation with methanol and evaporation | 48 h | 21 | nd | [35] |
| 5 | Pure vegetable oil | High content of lipid (fat) | nd | Cupriavidus necator H16 | Batch fermentations | nd | 24 h | n. r | 1.2 | [36] |
| 6 | Composite food waste | Contains easily consumable volatile fatty acids | Mixing of fresh food waste slurry with the inoculum in a 2 L reactor | Cupriavidus necator | Feeding regimes: pulse stepwise (once a day with 7 pulses) and continuous feeding | Analysis with GC | 259 h (8 draw–fill cycles) | 87 | nd | [37] |
| 7 | Restaurant waste | Large proportion of fatty acids, carbohydrate, protein, and fat | Recovering of VFAs by the freezing-thawing method. Centrifugation | E. Coli pnDTM2 | Batch and feed batch culture in the bioreactor | Determination of PHB concentration by HPLC | At 5th day of fermentation | Batch culture 36.4 Fed batch-44 | 39.6 | [38] |
| 8 | Kitchen waste (orange peel and onion peel) | Significant supplies of sugars, lipids, carbs, and mineral acids can be found | nd | Bacterial strains isolated from polluted environments | nd | nd | 48 h | 82 | 12.6 | [39] |
| 9 | Acidogenic fermentation | Cupriavidus necator CCGUG 52238 | Batch and feed batch fermentation | Analysis of the PHB content in the cell mass with HPLC | n. r | 84.5 | 230 | [40] | ||
| 10 | Waste vegetable oil | High content of lipid (fat) | nd | Isolation of corn oil-degrading bacteria from a rice field | nd | nd | 72 h | 37.3 | 0.9 | [41] |
| 11 | Spent palm oil | High content of lipid (fat) | Addition of spent palm oil to the medium and autoclaving. Addition of sterilized 1,4- butanediol. | Cupriavidus necator | nd | nd | n. r | 81 | 12.5 | [42] |
| 12 | Food waste compost (FWC) | Significant supplies of sugars, lipids, carbs, and mineral acids can be found | Acidogenic fermentation in an anaerobic bioreactor. | Mixed microbial culture | Acidogenic fermentation in anaerobic bioreactor | nd | 12 h | 23.7 | nd | [43] |
| 13 | Food waste compost (FWC) Unfermented | Mastication, filtration, oil removal, and dilution in domestic sewage to the required OLR | Mixed microbial culture (aerobic mixed culture) | SBR1: unfermented food waste/aerobic microenvironment | nd | 48 h | 35.2 | nd | [44] |
5. Available Carbon or KW’s Capacity for Biodegradation
6. KW Hydrolysis
7. Various Pre-Treatment Techniques to Enhance Nutrients Available for PHA Production
8. The Effect of KW Pre-Treatment on the Formation of Byproducts and Inhibitors
9. Recent Studies on Using Leftover Food from the Kitchen as a Source of PHA
10. Combined Food Waste and Leftover Meat or Seafood
11. Technical Challenges and Possible Results for Long-Term KW
12. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Chhandama, M.V.L.; Chetia, A.C.; Satyan, K.B.; Ruatpuia, J.V.; Rokhum, S.L. Valorisation of food waste to sustainable energy and other value-added products: A review. Bioresour. Technol. Rep. 2022, 17, 100945. [Google Scholar] [CrossRef]
- Li, C.; Bremer, P.; Harder, M.K.; Lee, M.S.; Parker, K.; Gaugler, E.C.; Mirosa, M. A systematic review of food loss and waste in China: Quantity, impacts and mediators. J. Environ. Manag. 2022, 303, 114092. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental sustainability in the food-energy-water-health nexus: A new methodology and an application to food waste in a circular economy. Waste Manag. 2020, 113, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef] [PubMed]
- Rohini, C.; Geetha, P.; Vijayalakshmi, R.; Mini, M.; Pasupathi, E. Global effects of food waste. J. Pharmacogn. Phytochem. 2020, 9, 690–699. [Google Scholar]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef] [PubMed]
- Hathi, Z.J.; Haque, M.A.; Priya, A.; Qin, Z.-H.; Huang, S.; Lam, C.H.; Ladakis, D.; Pateraki, C.; Mettu, S.; Koutinas, A. Fermentative bioconversion of food waste into biopolymer poly (3-hydroxybutyrate-co-3-hydroxyvalerate) using Cupriavidus necator. Environ. Res. 2022, 215, 114323. [Google Scholar] [CrossRef]
- Priya, A.; Hathi, Z.; Haque, M.A.; Kumar, S.; Kumar, A.; Singh, E.; Lin, C.S. Effect of levulinic acid on production of polyhydroxyalkanoates from food waste by Haloferax mediterranei. Environ. Res. 2022, 214, 114001. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar]
- Vavouraki, A.I.; Volioti, V.; Kornaros, M.E. Optimization of thermo-chemical pretreatment and enzymatic hydrolysis of kitchen wastes. Waste Manag. 2014, 34, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Iacovidou, E.; Ohandja, D.-G.; Gronow, J.; Voulvoulis, N. The household use of food waste disposal units as a waste management option: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1485–1508. [Google Scholar] [CrossRef]
- Hafid, H.S.; Shah, U.K.M.; Baharuddin, A.S.; Ariff, A.B. Feasibility of using kitchen waste as future substrate for bioethanol production: A review. Renew. Sustain. Energy Rev. 2017, 74, 671–686. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, J.; Chen, Y.; Gong, Y.; Li, Y.; Zhang, J. Current situation and development of kitchen waste treatment in China. Procedia Environ. Sci. 2016, 31, 40–49. [Google Scholar] [CrossRef]
- Herszenhorn, E.; Quested, T.; Easteal, S.; Prowse, G.; Lomax, J.; Bucatariu, C. Prevention and Reduction of Food and Drink Waste in Businesses and Households: Guidance for Governments, Local Authorities, Businesses and other Organisations; United Nations Environment Programme: Rome, Italy, 2014. [Google Scholar]
- Canali, M.; Amani, P.; Aramyan, L.; Gheoldus, M.; Moates, G.; Östergren, K.; Silvennoinen, K.; Waldron, K.; Vittuari, M. Food Waste Drivers in Europe, from Identification to Possible Interventions. Sustainability 2017, 9, 37. [Google Scholar] [CrossRef]
- Vavouraki, A.I.; Angelis, E.M.; Kornaros, M. Optimization of thermo-chemical hydrolysis of kitchen wastes. Waste Manag. 2013, 33, 740–745. [Google Scholar] [CrossRef]
- Pleissner, D.; Lin, C.S.K. Valorisation of food waste in biotechnological processes. Sustain. Chem. Process. 2013, 1, 1–6. [Google Scholar] [CrossRef]
- Garnida, Y.; Rudiansyah, M.; Yasin, G.; Mahmudiono, T.; Kadhim, A.J.; Sharma, S.; Hussein, H.A.; Shichiyakh, R.A.; Abdelbasset, W.K.; Iswanto, A.H. Investigation of parameters in restaurant food waste for use as poultry rations. Food Sci. Technol. 2022, 42, e118621. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastic. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Chavan, S.; Yadav, B.; Tyagi, R.; Drogui, P. A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresour. Technol. 2021, 341, 125900. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Bioconversion of organic wastes into value-added products: A review. Bioresour. Technol. 2021, 344, 126398. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N. Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, M.-K. Strategies for Biosynthesis of C1 Gas-derived Polyhydroxyalkanoates: A review. Bioresour. Technol. 2022, 344, 126307. [Google Scholar] [CrossRef]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipal Wastewater Treatment Plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the functionality of bio-based food packaging films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Sharma, B.; Vaish, B.; Singh, U.K.; Singh, P.; Singh, R.P. Recycling of organic wastes in agriculture: An environmental perspective. Int. J. Environ. Res. 2019, 13, 409–429. [Google Scholar] [CrossRef]
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol. Biofuels 2017, 10, 1–15. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Cruz, M.V.; Paiva, A.; Lisboa, P.; Freitas, F.; Alves, V.D.; Simões, P.; Barreiros, S.; Reis, M.A. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014, 157, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Sarraguça, M.C.; Freitas, F.; Lopes, J.A.; Reis, M.A. Online monitoring of P (3HB) produced from used cooking oil with near-infrared spectroscopy. J. Biotechnol. 2015, 194, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Możejko, J.; Ciesielski, S. Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J. Biosci. Bioeng. 2013, 116, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, R.A.; Hill, D.J.; Kenward, M.A.; Williams, C.D.; Piotrowska-Seget, Z.; Radecka, I.K. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. AMB Express 2011, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hafuka, A.; Sakaida, K.; Satoh, H.; Takahashi, M.; Watanabe, Y.; Okabe, S. Effect of feeding regimens on polyhydroxybutyrate production from food wastes by Cupriavidus necator. Bioresour. Technol. 2011, 102, 3551–3553. [Google Scholar] [CrossRef] [PubMed]
- Eshtaya, M.K.; Nor ‘Aini, A.R.; Hassan, M.A. Bioconversion of restaurant waste into Polyhydroxybutyrate (PHB) by recombinant E. coli through anaerobic digestion. Int. J. Environ. Waste Manag. 2013, 11, 27–37. [Google Scholar] [CrossRef]
- Vijay, R.; Tarika, K. Microbial production of polyhydroxyalkanoates (PHAs) using kitchen waste as an inexpensive carbon source. Biosci. Biotechnol. Res. Asia 2019, 16, 155–166. [Google Scholar] [CrossRef]
- Omar, F.N.; Rahman, A.A.; Hafid, H.S.; Mumtaz, T.; Yee, P.L.; Hassan, M.A. Utilization of kitchen waste for the production of green thermoplastic polyhydroxybutyrate (PHB) by Cupriavidus necator CCGUG 52238. Afr. J. Microbiol. Res. 2011, 5, 2873–2879. [Google Scholar]
- Song, J.-H.; Jeon, C.-O.; Choi, M.-H.; Yoon, S.-C.; Park, W.-J. Polyhydroxyalkanoate (PHA) production using waste vegetable oil by Pseudomonas sp. strain DR2. J. Microbiol. Biotechnol. 2008, 18, 1408–1415. [Google Scholar]
- Nielsen, C.; Rahman, A.; Rehman, A.U.; Walsh, M.K.; Miller, C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017, 10, 1338–1352. [Google Scholar] [CrossRef]
- Amulya, K.; Jukuri, S.; Mohan, S.V. Sustainable multistage process for enhanced productivity of bioplastics from waste remediation through aerobic dynamic feeding strategy: Process integration for up-scaling. Bioresour. Technol. 2015, 188, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Mohan, S.V. Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour. Technol. 2012, 103, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, A.; Sasidharan, S. Microbial production of polyhydroxy alkanotes (PHA) from Alcaligens spp. and Pseudomonas oleovorans using different carbon sources. Afr. J. Biotechnol. 2010, 9, 3144–3150. [Google Scholar]
- Yan, S.; Yao, J.; Yao, L.; Zhi, Z.; Chen, X.; Wu, J. Fed batch enzymatic saccharification of food waste improves the sugar concentration in the hydrolysates and eventually the ethanol fermentation by Saccharomyces cerevisiae H058. Braz. Arch. Biol. Technol. 2012, 55, 183–192. [Google Scholar] [CrossRef]
- Malakahmad, A.; Basri, N.E.A.; Zain, S.M. Production of renewable energy by transformation of kitchen waste to biogas, case study of Malaysia. In Proceedings of the 2011 IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Langkawi, Malaysia, 25–28 September 2011; pp. 219–223. [Google Scholar]
- Cheng, S.-S.; Chao, Y.-C.; Wong, S.-C.; Chen, C.-C.; Yang, K.-H.; Yang, Y.-F. Study on hydrogen production potential utilizing leachate from aerobic bio-leaching bed fed with napiergrass and kitchen waste. Energy Procedia 2012, 29, 72–81. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, X.; Ma, H. Glucoamylase production from food waste by Aspergillus niger under submerged fermentation. Process Biochem. 2008, 43, 280–286. [Google Scholar] [CrossRef]
- WHO. Food Systems for Health: Information Brief; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Preethi, R.; Sasikala, P.; Aravind, J. Microbial production of polyhydroxyalkanoate (PHA) utilizing fruit waste as a substrate. Res. Biotechnol. 2012, 3, 61–69. [Google Scholar]
- Follonier, S.; Goyder, M.S.; Silvestri, A.-C.; Crelier, S.; Kalman, F.; Riesen, R.; Zinn, M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 42–52. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwiecień, M.; Adamus, G.; Kowalczuk, M.; Strohmeier, K.; Schober, S. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by Ps. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Albuquerque, M.; Torres, C.; Reis, M. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: Effect of the influent substrate concentration on culture selection. Water Res. 2010, 44, 3419–3433. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Kim, M.; Nam, K. Increased 3HV concentration in the bacterial production of 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV) copolymer with acid-digested rice straw waste. J. Polym. Environ. 2016, 24, 98–103. [Google Scholar] [CrossRef]
- Ryu, B.-G.; Kim, J.; Kim, K.; Choi, Y.-E.; Han, J.-I.; Yang, J.-W. High-cell-density cultivation of oleaginous yeast Cryptococcus curvatus for biodiesel production using organic waste from the brewery industry. Bioresour. Technol. 2013, 135, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hua, F.; Tsang, Y.F.; Chan, S.; Sin, S.; Chua, H.; Yu, P.; Ren, N. Synthesis of PHAs from waster under various C: N ratios. Bioresour. Technol. 2007, 98, 1690–1693. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Lustres, R.; Torres, M.D.; Piñeiro, B.; Enjamio, C.; Domínguez, H. Intensification and biorefinery approaches for the valorization of kitchen wastes–A review. Bioresour. Technol. 2022, 360, 127652. [Google Scholar] [CrossRef]
- Melikoglu, M. Solid-state fermentation of wheat pieces by Aspergillus oryzae: Effects of microwave pretreatment on enzyme production in a Biorefinery. Int. J. Green Energy 2012, 9, 529–539. [Google Scholar] [CrossRef]
- Shana, A.D.; Ouki, S.; Asaadi, M.; Pearce, P. Influence of an intermediate thermal hydrolysis process (ITHP) on the kinetics of anaerobic digestion of sewage sludge. Chem. Eng. 2012, 29. [Google Scholar]
- Ganesan, B.; Seefeldt, K.; Weimer, B.C. Fatty acid production from amino acids and α-keto acids by Brevibacterium linens BL2. Appl. Environ. Microbiol. 2004, 70, 6385–6393. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Tam, V.W.; Xue, C. Advanced progress in recycling municipal and construction solid wastes for manufacturing sustainable construction materials. Resour. Conserv. Recycl. X 2020, 6, 100036. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y. Effects of thermal pretreatment on acidification phase during twophase batch anaerobic digestion of kitchen waste. Renew. Energy 2015, 77, 550–557. [Google Scholar] [CrossRef]
- Dors, G.; Mendes, A.A.; Pereira, E.B.; de Castro, H.F.; Furigo, A. Simultaneous enzymatic hydrolysis and anaerobic biodegradation of lipid-rich wastewater from poultry industry. Appl. Water Sci. 2013, 3, 343–349. [Google Scholar] [CrossRef]
- Thongdumyu, P.; Intrasungkha, N.; Sompong, O. Optimization of ethanol production from food waste hydrolysate by co-culture of Zymomonas mobilis and Candida shehatae under non-sterile condition. Afr. J. Biotechnol. 2014, 13, 866–873. [Google Scholar]
- Kumar, M.; Turner, S. Plant cellulose synthesis: CESA proteins crossing kingdoms. Phytochemistry 2015, 112, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Yoon, H.H. Ethanol production from food residues. Biomass Bioenergy 2011, 35, 3271–3275. [Google Scholar] [CrossRef]
- Yang, X.; Lee, J.H.; Yoo, H.Y.; Shin, H.Y.; Thapa, L.P.; Park, C.; Kim, S.W. Production of bioethanol and biodiesel using instant noodle waste. Bioprocess Biosyst. Eng. 2014, 37, 1627–1635. [Google Scholar] [CrossRef]
- Moon, H.C.; Song, I.S.; Kim, J.C.; Shirai, Y.; Lee, D.H.; Kim, J.K.; Chung, S.O.; Kim, D.H.; Oh, K.K.; Cho, Y.S. Enzymatic hydrolysis of food waste and ethanol fermentation. Int. J. Energy Res. 2009, 33, 164–172. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.-K.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Kuo, W.-C.; Cheng, K.-Y. Use of respirometer in evaluation of process and toxicity of thermophilic anaerobic digestion for treating kitchen waste. Bioresour. Technol. 2007, 98, 1805–1811. [Google Scholar] [CrossRef]
- Ballesteros, M.; Sáez, F.; Ballesteros, I.; Manzanares, P.; Negro, M.J.; Martínez, J.M.; Castañeda, R.; Oliva Dominguez, J.M. Ethanol production from the organic fraction obtained after thermal pretreatment of municipal solid waste. Appl. Biochem. Biotechnol. 2010, 161, 423–431. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of bioplastic through food waste valorization. Environ. Int. 2019, 127, 625–644. [Google Scholar] [CrossRef]
- Kover, A.; Kraljić, D.; Marinaro, R.; Rene, E.R. Processes for the valorization of food and agricultural wastes to value-added products: Recent practices and perspectives. Syst. Microbiol. Biomanuf. 2021, 2, 50–66. [Google Scholar] [CrossRef]
- Banu, J.R.; Merrylin, J.; Usman, T.M.; Kannah, R.Y.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. Impact of pretreatment on food waste for biohydrogen production: A review. Int. J. Hydrogen Energy 2020, 45, 18211–18225. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, Y.; Liu, Y. New insights into co-digestion of activated sludge and food waste: Biogas versus biofertilizer. Bioresour. Technol. 2017, 241, 448–453. [Google Scholar] [CrossRef]
- Zhou, S.; Runge, T.M. Validation of lignocellulosic biomass carbohydrates determination via acid hydrolysis. Carbohydr. Polym. 2014, 112, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bishnoi, N.R. Ethanol production from pretreated wheat straw hydrolyzate by Saccharomyces cerevisiae via sequential statistical optimization. Ind. Crop. Prod. 2013, 41, 221–226. [Google Scholar] [CrossRef]
- Campbell-Platt, G. Food Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Girisuta, B.; Dussan, K.; Haverty, D.; Leahy, J.; Hayes, M. A kinetic study of acid catalysed hydrolysis of sugar cane bagasse to levulinic acid. Chem. Eng. J. 2013, 217, 61–70. [Google Scholar] [CrossRef]
- Hoseinpour, H.; Karimi, K.; Zilouei, H.; Taherzadeh, M.J. Simultaneous pretreatment of lignocellulose and hydrolysis of starch in mixtures to sugars. BioResources 2010, 5, 2457–2469. [Google Scholar] [CrossRef]
- El-Tayeb, T.; Abdelhafez, A.; Ali, S.; Ramadan, E. Effect of acid hydrolysis and fungal biotreatment on agro-industrial wastes for obtainment of free sugars for bioethanol production. Braz. J. Microbiol. 2012, 43, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Millati, R.; Syamsiyah, S.; Muriana, R.; Ayuningsih, Y. Effect of furfural, hydroxymethylfurfural and acetic acid on indigeneous microbial isolate for bioethanol production. Agric. J. 2010, 5, 105–109. [Google Scholar] [CrossRef]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef]
- Iwaki, A.; Kawai, T.; Yamamoto, Y.; Izawa, S. Biomass conversion inhibitors furfural and 5-hydroxymethylfurfural induce formation of messenger RNP granules and attenuate translation activity in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2013, 79, 1661–1667. [Google Scholar] [CrossRef]
- Yang, S.; Franden, M.A.; Yang, Q.; Chou, Y.-C.; Zhang, M.; Pienkos, P.T. Identification of inhibitors in lignocellulosic slurries and determination of their effect on hydrocarbon-producing microorganisms. Front. Bioeng. Biotechnol. 2018, 6, 23. [Google Scholar] [CrossRef]
- Lyra Colombi, B.; Silva Zanoni, P.R.; Tavares, B.B.L. Effect of phenolic compounds on bioconversion of glucose to ethanol by yeast Saccharomyces cerevisiae PE-2. Can. J. Chem. Eng. 2018, 96, 1444–1450. [Google Scholar] [CrossRef]
- Radhika, D.; Murugesan, A. Bioproduction, statistical optimization and characterization of microbial plastic (poly 3-hydroxy butyrate) employing various hydrolysates of water hyacinth (Eichhornia crassipes) as sole carbon source. Bioresour. Technol. 2012, 121, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Loan, T.T.; Trang, D.T.Q.; Huy, P.Q.; Ninh, P.X.; Van Thuoc, D. A fermentation process for the production of poly (3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol. Rep. 2022, 33, e00700. [Google Scholar] [CrossRef]
- Vastano, M.; Corrado, I.; Sannia, G.; Solaiman, D.K.; Pezzella, C. Conversion of no/low value waste frying oils into biodiesel and polyhydroxyalkanoates. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Yadav, B.; Pandey, A.; Kumar, L.R.; Tyagi, R.D. Bioconversion of waste (water)/residues to bioplastics-A circular bioeconomy approach. Bioresour. Technol. 2020, 298, 122584. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P. Biosynthesis and biocompatibility of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Tsuge, T.; Saito, Y.; Kikkawa, Y.; Hiraishi, T.; Doi, Y. Biosynthesis and compositional regulation of poly [(3-hydroxybutyrate)-co-(3-hydroxyhexanoate)] in recombinant Ralstonia eutropha expressing mutated polyhydroxyalkanoate synthase genes. Macromol. Biosci. 2004, 4, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Kamilah, H.; Tsuge, T.; Yang, T.A.; Sudesh, K. Waste cooking oil as substrate for biosynthesis of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate): Turning waste into a value-added product. Malays. J. Microbiol. 2013, 9, 51–59. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kucera, D.; Nebesarova, J.; Kalina, M.; Novackova, I.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates on waste frying oil employing selected Halomonas strains. Bioresour. Technol. 2019, 292, 122028. [Google Scholar] [CrossRef] [PubMed]
- Andler, R.; Valdées, C.; Urtuvia, V.; Andreeßen, C.; Díaz-Barrera, A. Fruit residues as a sustainable feedstock for the production of bacterial polyhydroxyalkanoates. J. Cleaner Prod. 2021, 307, 127236. [Google Scholar] [CrossRef]
- Kovalcik, A.; Pernicova, I.; Obruca, S.; Szotkowski, M.; Enev, V.; Kalina, M.; Marova, I. Grape winery waste as a promising feedstock for the production of polyhydroxyalkanoates and other value-added products. Food Bioprod. Process. 2020, 124, 1–10. [Google Scholar] [CrossRef]
- Pereira, J.R.; Araujo, D.; Freitas, P.; Marques, A.C.; Alves, V.D.; Sevrin, C.; Grandfils, C.; Fortunato, E.; Reis, M.A.; Freitas, F. Production of medium-chain-length polyhydroxyalkanoates by Pseudomonas chlororaphis subsp. aurantiaca: Cultivation on fruit pulp waste and polymer characterization. Int. J. Biol. Macromol. 2021, 167, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Santimano, M.; Prabhu, N.N.; Garg, S. PHA production using low-cost agroindustrial wastes by Bacillus sp. strain COL1/A6. 2009. Res. J. Microbiol. 2009, 4, 89–96. [Google Scholar] [CrossRef]
- Low, T.J.; Mohammad, S.; Sudesh, K.; Baidurah, S. Utilization of banana (Musa sp.) fronds extract as an alternative carbon source for poly (3-hydroxybutyrate) production by Cupriavidus necator H16. Biocatal. Agric. Biotechnol. 2021, 34, 102048. [Google Scholar] [CrossRef]
- Hidayat, N.; Alamsyah, R.; Roslan, A.M.; Hermansyah, H.; Gozan, M. Production of polyhydroxybutyrate from oil palm empty fruit bunch (OPEFB) hydrolysates by Bacillus cereus suaeda B-001. Biocatal. Agric. Biotechnol. 2019, 18, 101019. [Google Scholar]
- Alsafadi, D.; Ibrahim, M.I.; Alamry, K.A.; Hussein, M.A.; Mansour, A. Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci. Total. Environ. 2020, 737, 139716. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Suriyamoorthy, P.; Moses, J.; Anandharamakrishnan, C. Biocomposites from food wastes. In Composites for Environmental Engineering; Wiley: Hoboken, NJ, USA, 2019; pp. 319–345. [Google Scholar]
- Alcaraz-Zapata, W.; Acosta-Cárdenas, A.; Villa-Restrepo, A.F. Evaluation of polyhydroxyalkanoate (PHAs) production with a bacterial isolate using cassava flour hydrolysates as an alternative substrate. Dyna 2019, 86, 75–81. [Google Scholar] [CrossRef]
- Mohapatra, S.; Sarkar, B.; Samantaray, D.; Daware, A.; Maity, S.; Pattnaik, S.; Bhattacharjee, S. Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ. Technol. 2017, 38, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A.; Investigators, D.; De Micheli, V. Comparison between different D-D imer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Modig, T.; Liden, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Aditiya, H.; Mahlia, T.; Chong, W.; Nur, H.; Sebayang, A. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Karmee, S.K. Liquid biofuels from food waste: Current trends, prospect and limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Woon, K.S.; Lo, I.M. A proposed framework of food waste collection and recycling for renewable biogas fuel production in Hong Kong. Waste Manag. 2016, 47, 3–10. [Google Scholar] [CrossRef]
- Osman, N.B.; Othman, H.T.; Karim, R.A.; Mazlan, M.A.F. Biomass in Malaysia: Forestry-based residues. Int. J. Biomass Renew. 2014, 3, 7–14. [Google Scholar] [CrossRef]
- Moh, Y.C.; Abd Manaf, L. Overview of household solid waste recycling policy status and challenges in Malaysia. Resour. Conserv. Recycl. 2014, 82, 50–61. [Google Scholar] [CrossRef]
- Giwa, A.S.; Ali, N.; Akhter, M.S. Cellulose Degradation Enzymes in Filamentous Fungi, A Bioprocessing Approach Towards Biorefinery. Mol. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.S.; Nasir, A.; Izhar, A.; Muhammad, A.; Rong-Bo, G.; Fu-Li, L.; Ming, L. Prospects of China’s biogas: Fundamentals, challenges and considerations. Energy Rep. 2020, 6, 2973–2987. [Google Scholar] [CrossRef]
- Giwa, A.S.; Ali, N.; Asif, M. Swine manure valorization in fabrication of nutrition and energy. Appl. Microbiol. Biotechnol. 2020, 104, 9921–9933. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hamed, I.H.; Hang, S.; Jie, F.; Zi-Yong, L.; Ming, L.; Fu-Li, L. A two-stage anaerobic bioconversion of corn stover: Impact of pure bacterial pretreatment on methane production. Environ. Technol. Innov. 2020, 20, 101141. [Google Scholar] [CrossRef]
- Hamouda, H.I.; Ali, N.; Su, H.; Feng, J.; Lu, M.; Li, F.L. Exploration of Two Pectate Lyases from Caldicellulosiruptor Bescii Reveals that the CBM66 Module Has a Crucial Role in Pectic Biomass Degradation. Appl. Environ. Microbiol. 2020, 86, 16. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Hamed, I.H.; Hang, S.; Fu-Li, L.; Ming, L. Combinations of alkaline hydrogen peroxide and lithium chloride/N,N-dimethylacetamide pretreatments of corn stalk for improved biomethanation. Environ. Res. 2020, 186, 109563. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zhang, Q.; Liu, Z.Y. Emerging technologies for the pretreatment of lignocellulosic materials for bio-based products. Appl. Microbiol. Biotechnol. 2020, 104, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Gong, H.; Liu, X.; Giwa, A.S.; Wang, K. Evaluation of bacterial association in methane generation pathways of an anaerobic digesting sludge via metagenomic sequencing. Arch. Microbiol. 2020, 202, 31–41. [Google Scholar] [CrossRef]
- Ali, N.; Gong, H.; Giwa, A.S.; Yuan QWang, K.J. Metagenomic analysis and characterization of acidogenic microbiome and effect of pH on organic acid production. Arch. Microbiol. 2019, 201, 1163–1171. [Google Scholar] [CrossRef]
- Ali, N.; Giwa, A.S.; Abdalla MLiu, X. Alkaline hydrogen peroxide pretreatment of bamboo culm for improved enzymatic release of reducing sugars using recombinant cellulases. Cellulose 2020, 27, 769–779. [Google Scholar] [CrossRef]
- Ali, N.; Xue, Y.; Gan, L.; Liu, J.; Long, M. Purification, characterization, gene cloning and sequencing of a new β-glucosidase from Aspergillus niger BE-2. Appl. Biochem. Microbiol. 2016, 52, 564–571. [Google Scholar] [CrossRef]
- Ali, N.; Ting, Z.; Li, H.; Xue, Y.; Gan, L.; Liu, J.; Long, M. Heterogeneous Expression and Functional Characterization of Cellulose-Degrading Enzymes from Aspergillus niger for Enzymatic Hydrolysis of Alkali Pretreated Bamboo Biomass. Mol. Biotechnol. 2015, 57, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Jiarui, X.; Quan, Y.; Qibin, W.; Nasir, A.; Kaijun, W. Carbon-nitrogen nexus was changed by improved organic carbon pre-concentration and autotrophic deammonification to improve energy self-sufficiency for wastewater treatment. J. Water Process Eng. 2021, 44, 102432. [Google Scholar] [CrossRef]
- Yuan, Q.; Hui, G.; Hao, X.; Heng, X.; Zhengyu, J.; Nasir, A.; Kaijun, W. Strategies to improve aerobic granular sludge stability and nitrogen removal based on feeding mode and substrate. Environ. Sci. 2021, 84, 144–154. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giwa, A.S.; Shafique, E.; Ali, N.; Vakili, M. Recent Advances in Food Waste Transformations into Essential Bioplastic Materials. Molecules 2024, 29, 3838. https://doi.org/10.3390/molecules29163838
Giwa AS, Shafique E, Ali N, Vakili M. Recent Advances in Food Waste Transformations into Essential Bioplastic Materials. Molecules. 2024; 29(16):3838. https://doi.org/10.3390/molecules29163838
Chicago/Turabian StyleGiwa, Abdulmoseen Segun, Ehtisham Shafique, Nasir Ali, and Mohammadtaghi Vakili. 2024. "Recent Advances in Food Waste Transformations into Essential Bioplastic Materials" Molecules 29, no. 16: 3838. https://doi.org/10.3390/molecules29163838
APA StyleGiwa, A. S., Shafique, E., Ali, N., & Vakili, M. (2024). Recent Advances in Food Waste Transformations into Essential Bioplastic Materials. Molecules, 29(16), 3838. https://doi.org/10.3390/molecules29163838








