The Role of Fe(III) in Selective Adsorption of Pullulan on Calcite Surfaces: Experimental Investigation and Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Materials and Methods
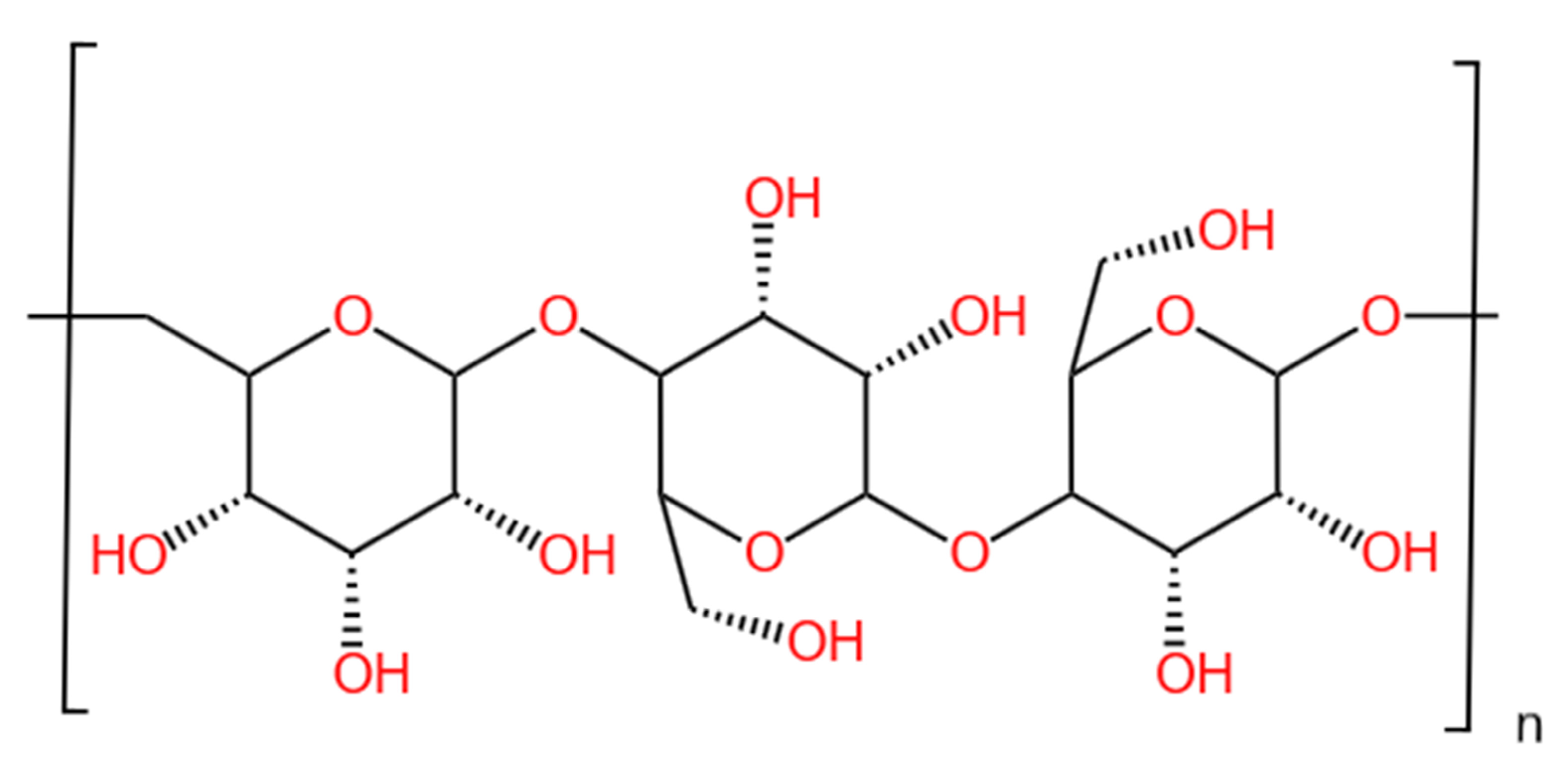
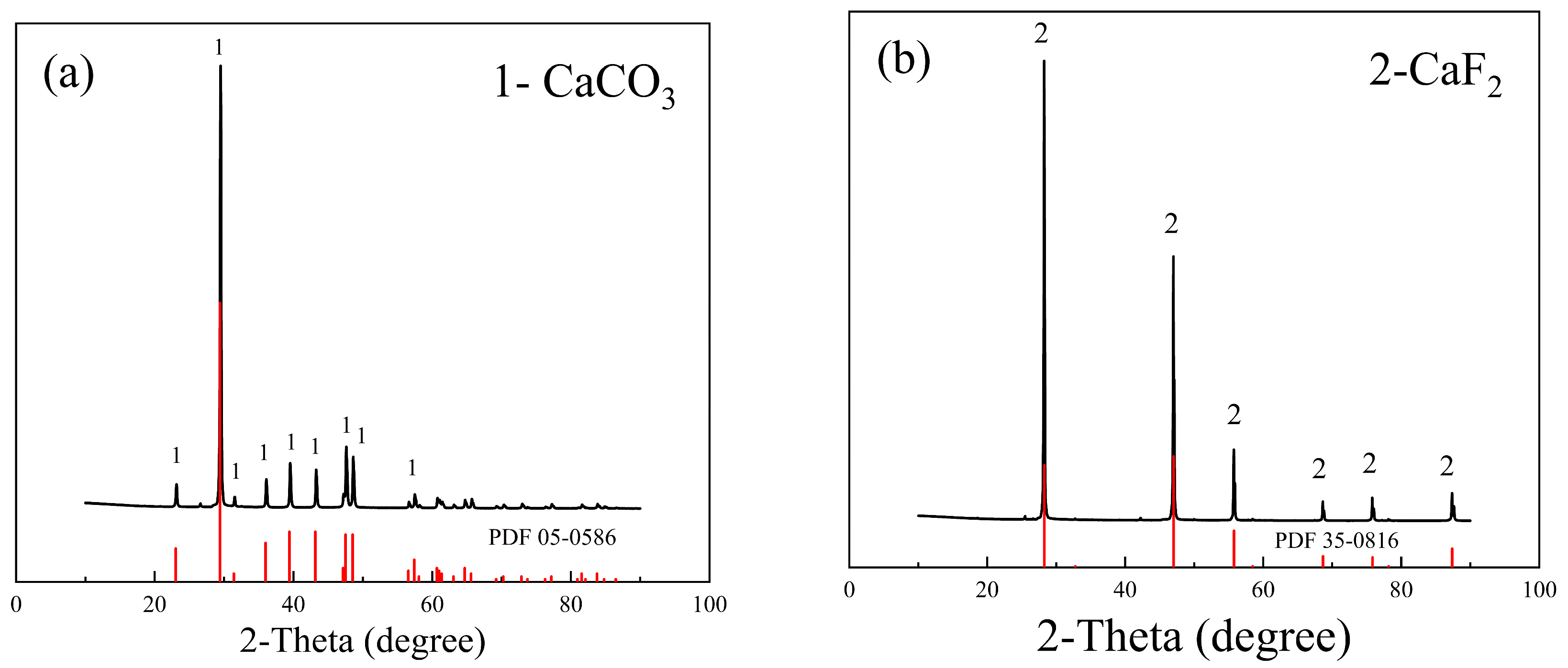
2.1. Samples and Reagents
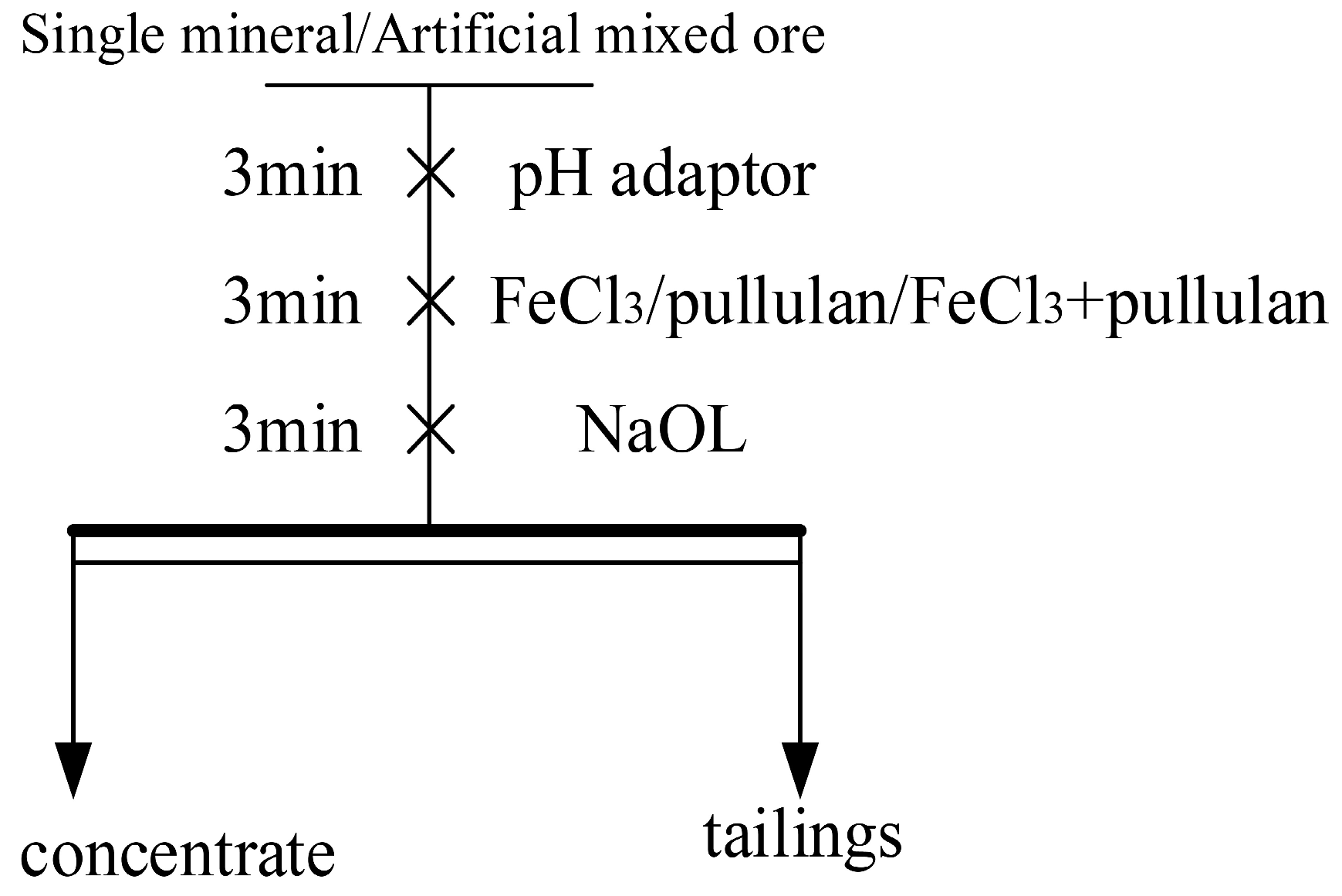
2.2. Flotation Experiments
2.3. Contact Angle Test
2.4. Zeta Potential Measurement
2.5. XPS Analysis
2.6. Molecular Dynamics Simulation
3. Results and Discussion
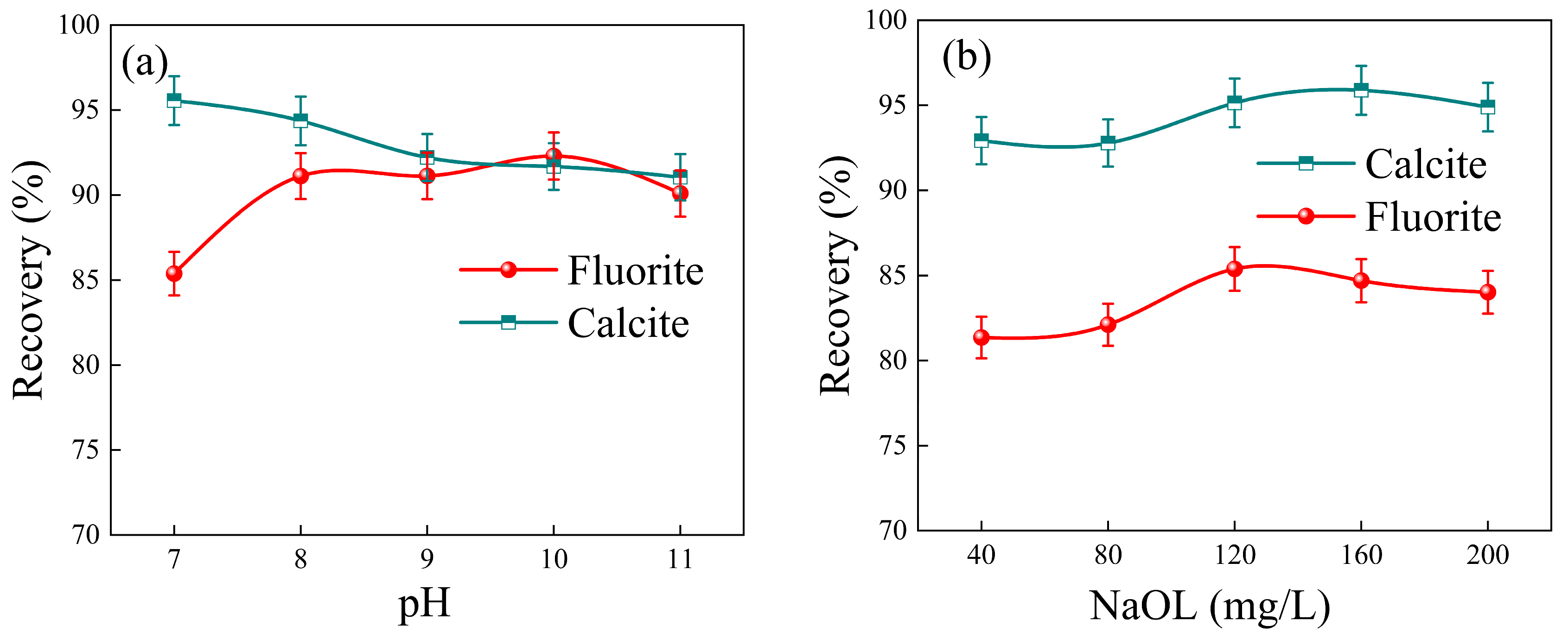
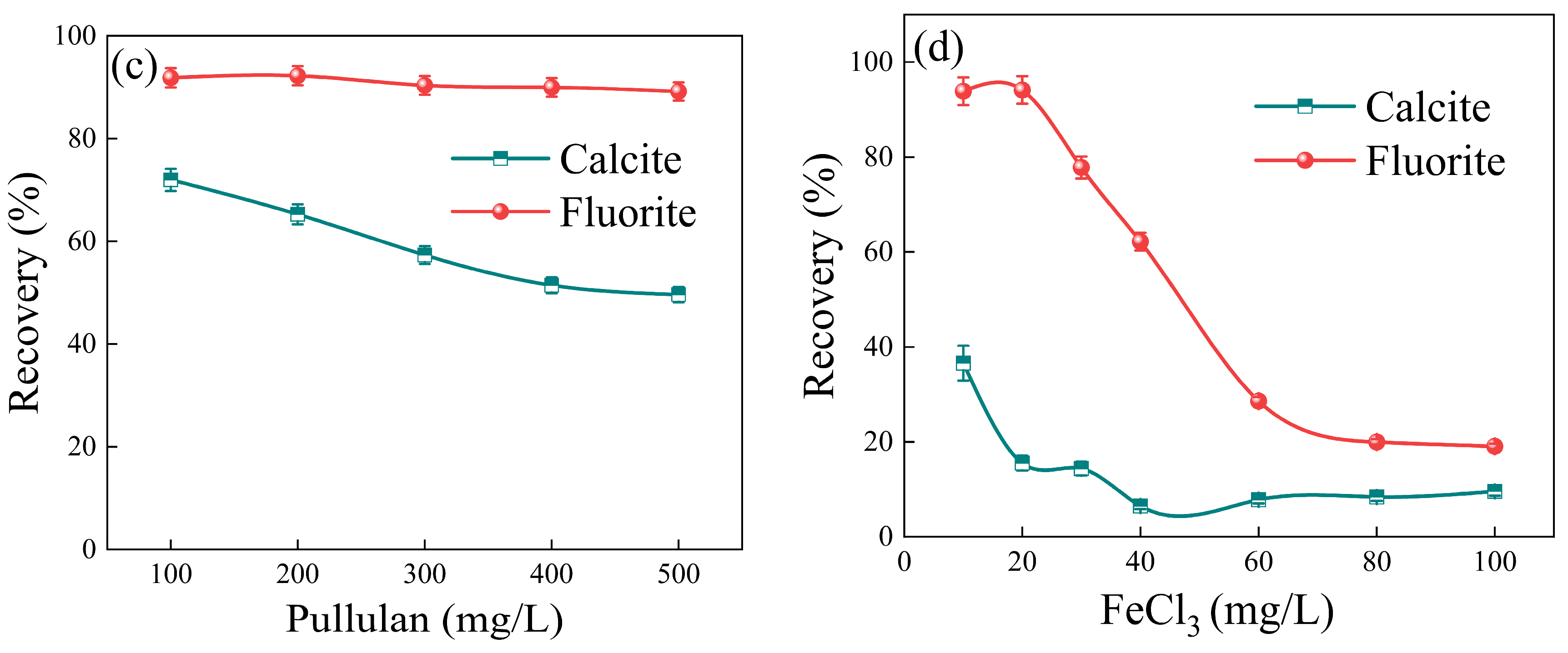
3.1. Flotation Performance
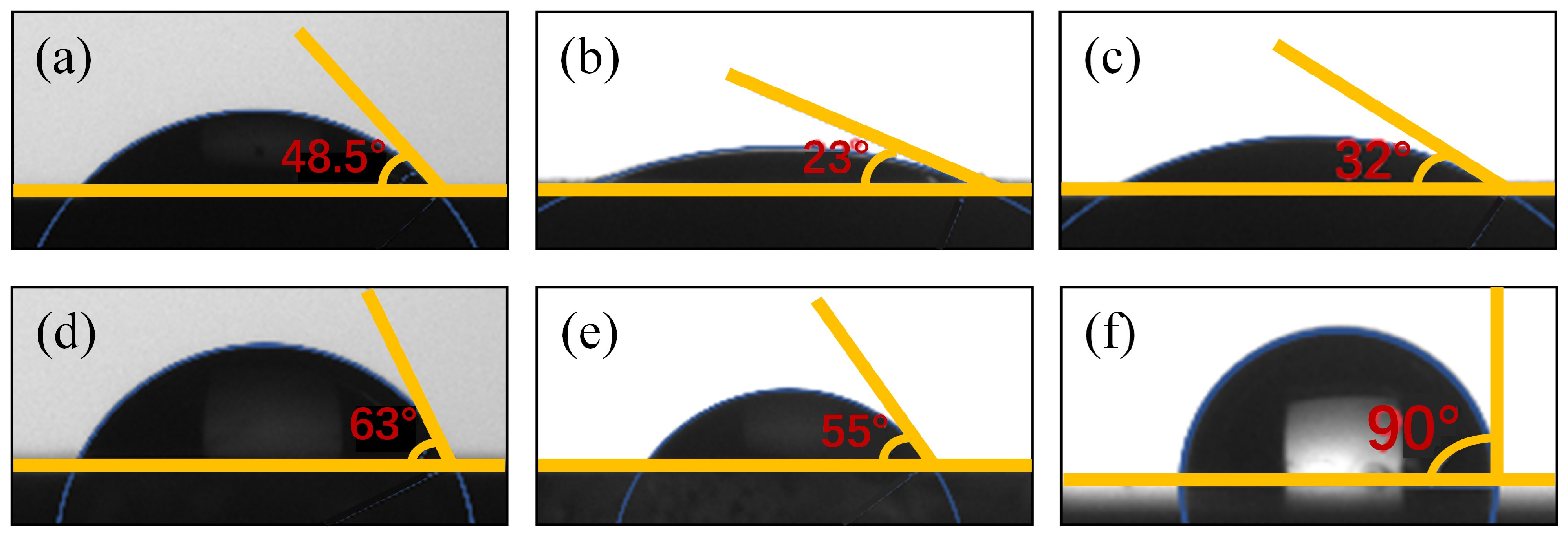
3.2. Contact Angle Results
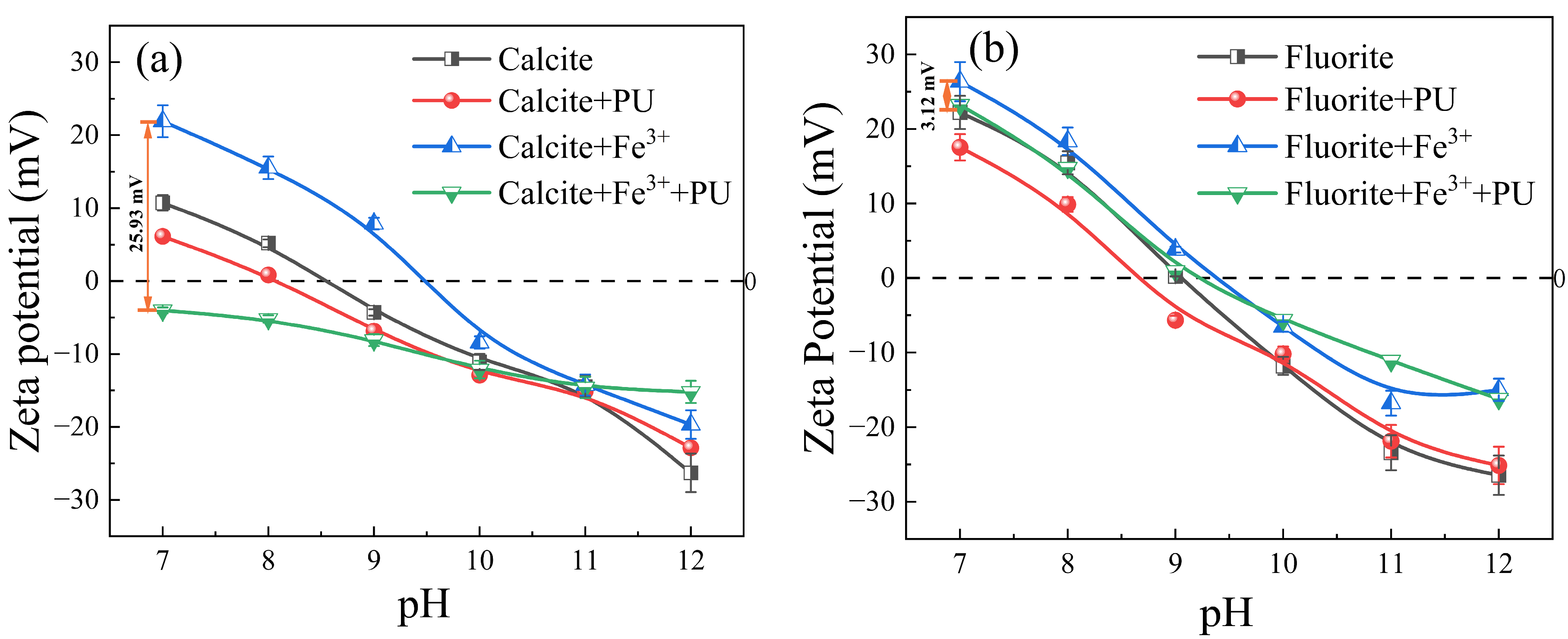
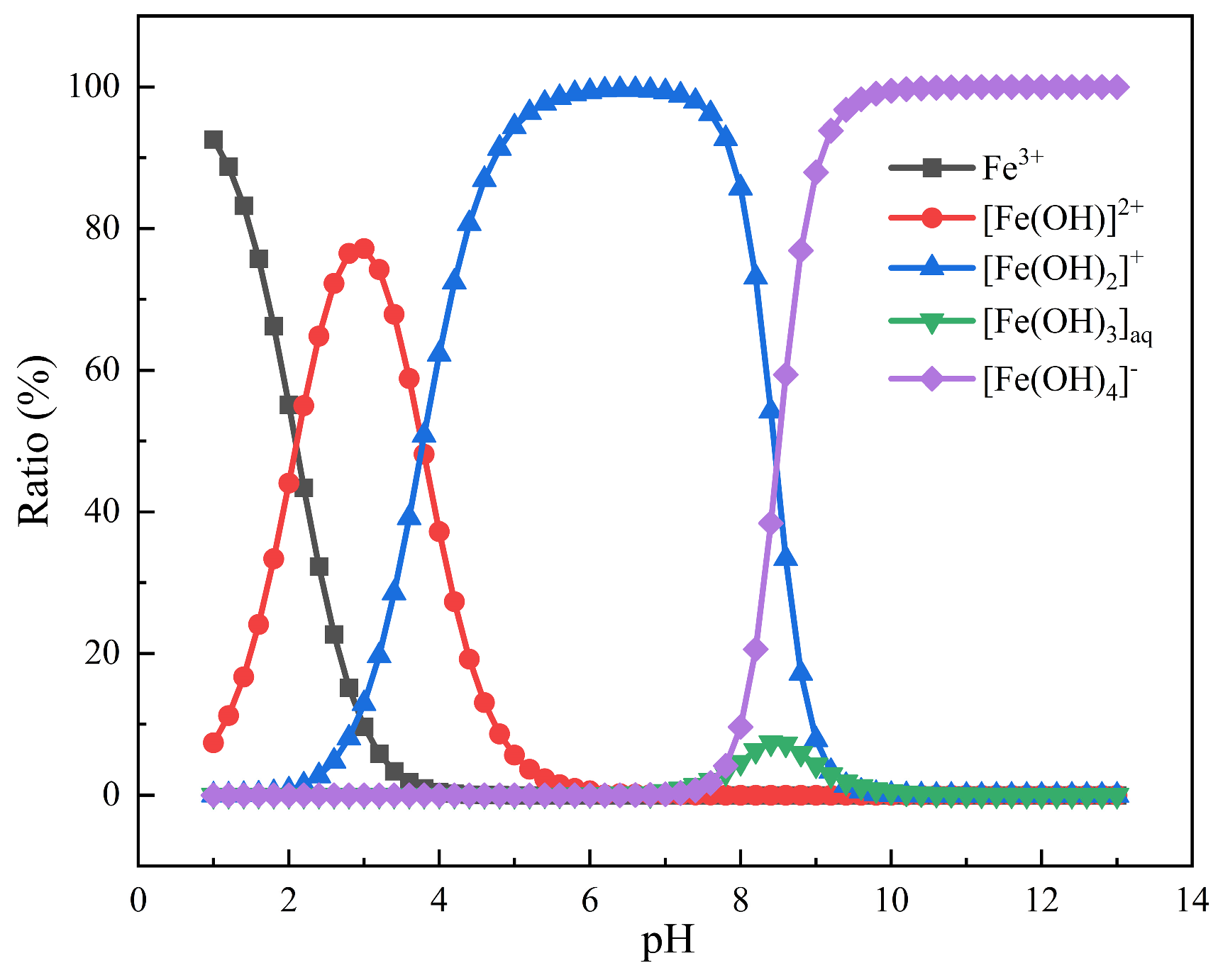
3.3. Zeta Potential Results
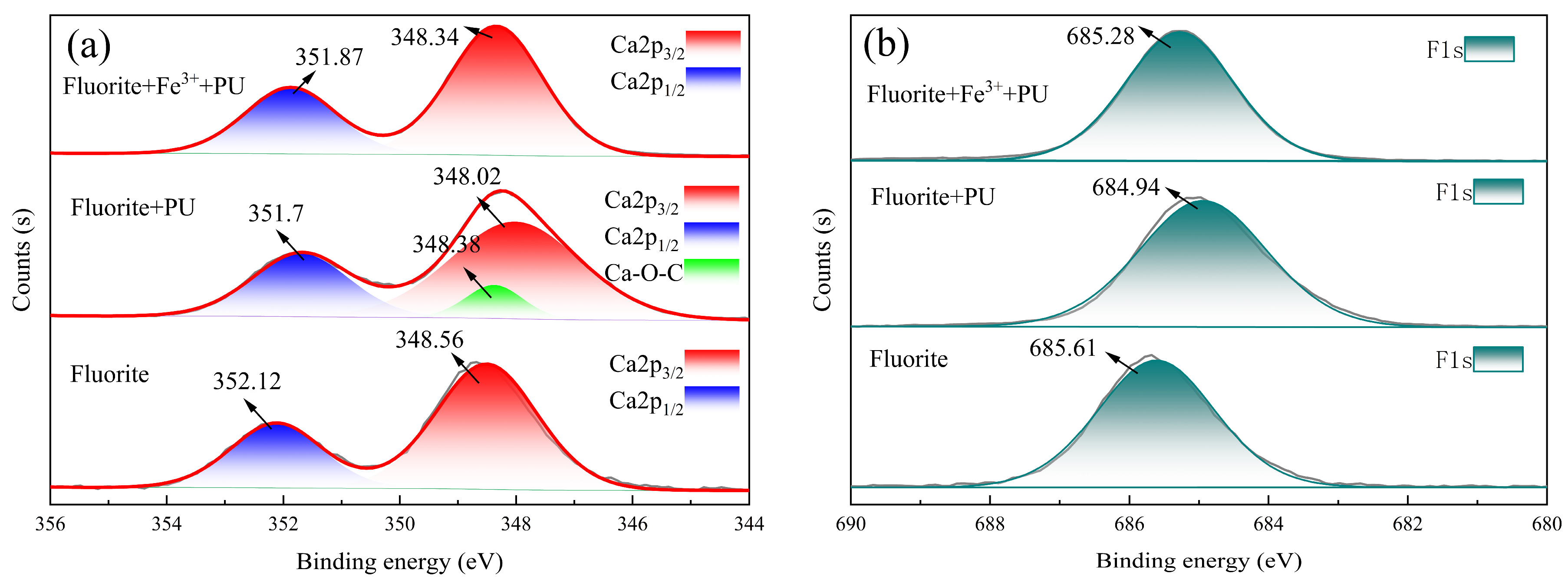
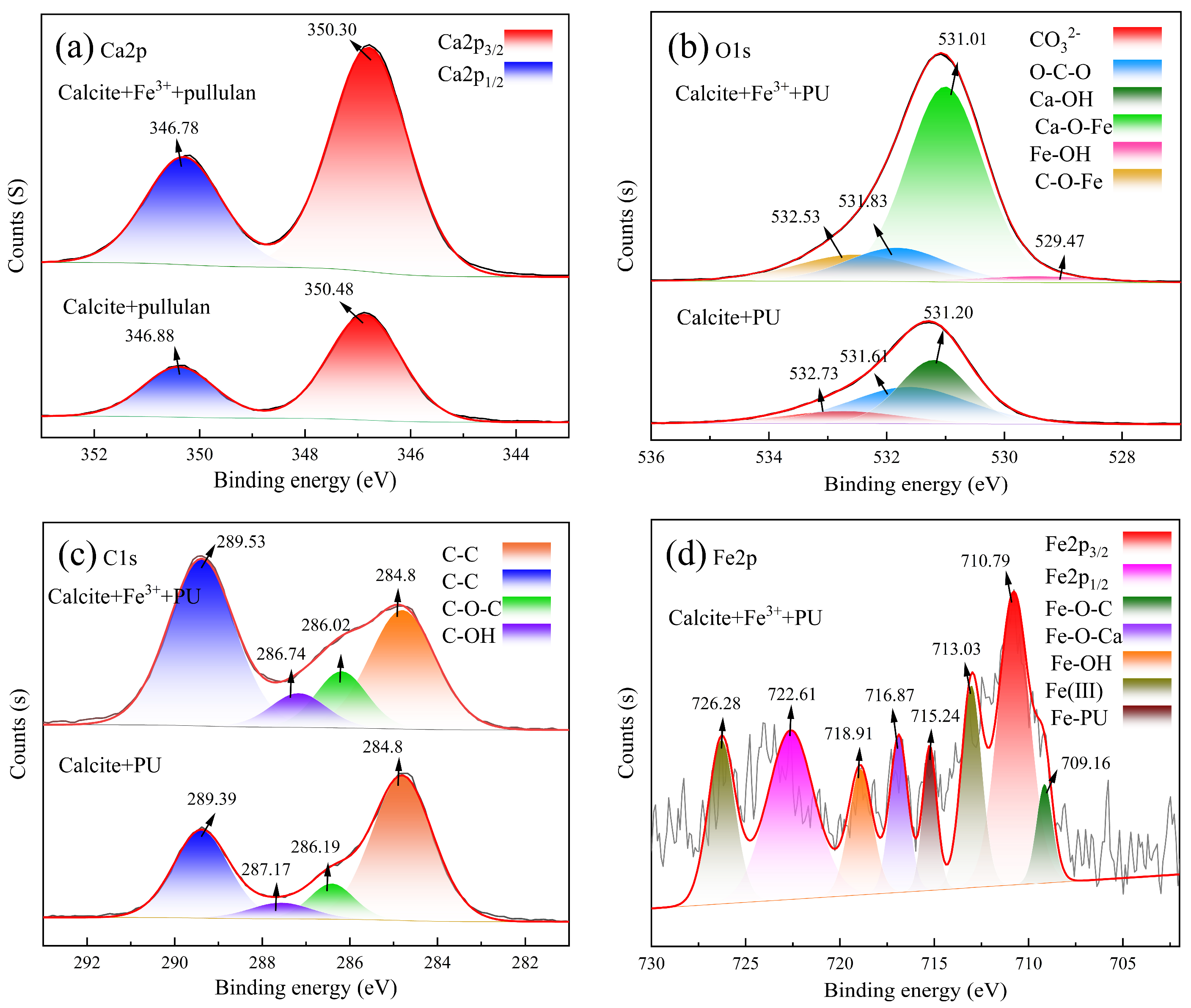
3.4. X-ray Photoelectron Spectroscopy Analyses
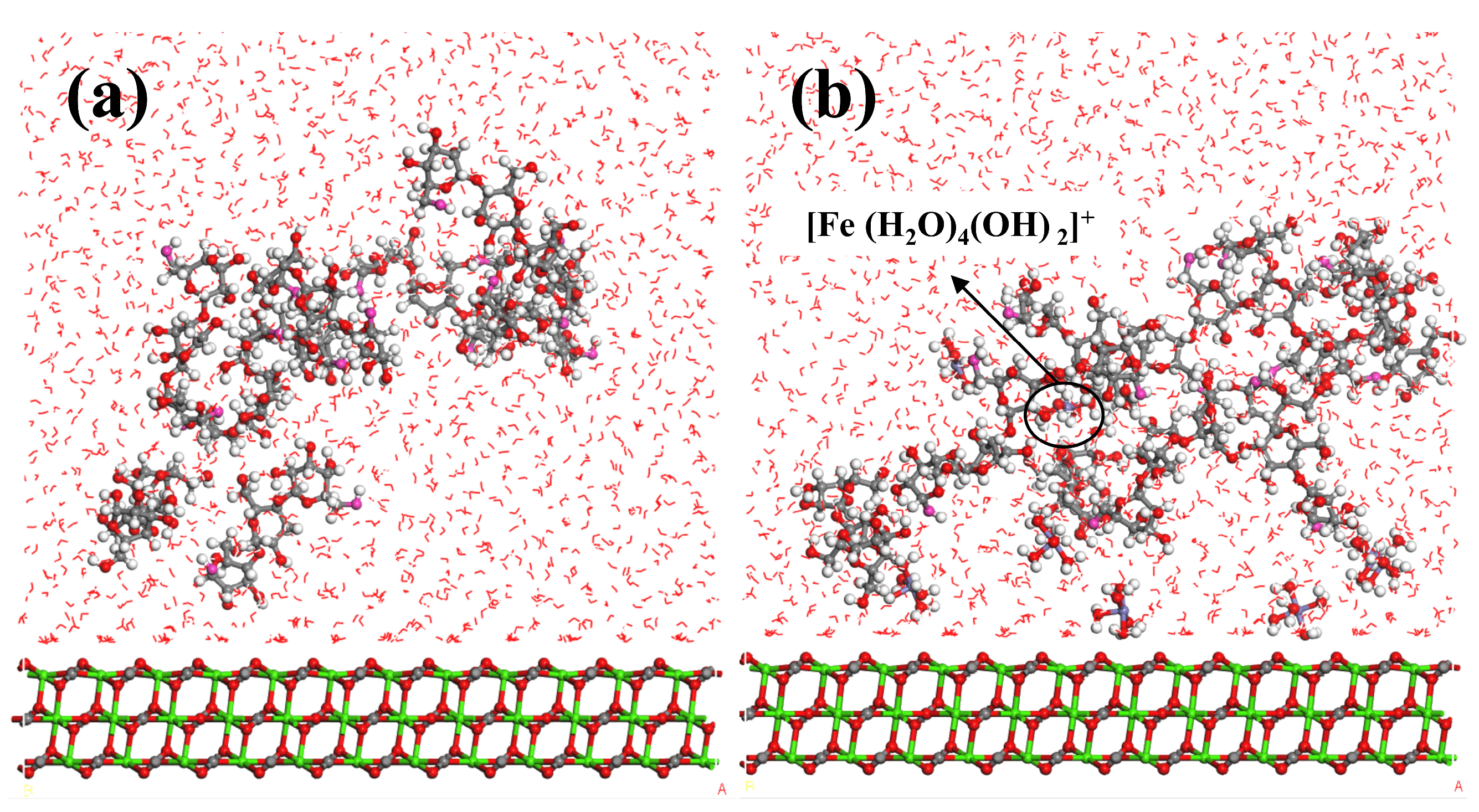
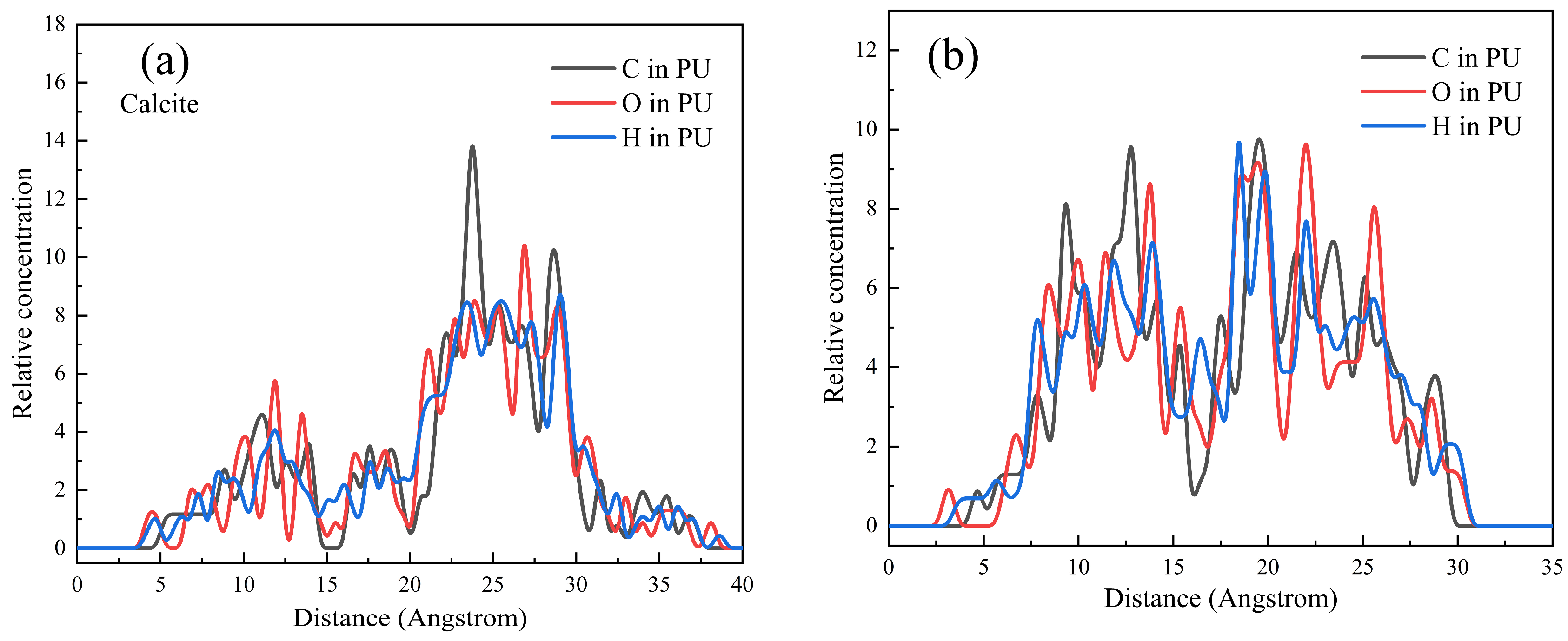
3.5. DFT and MD Simulation Analysis
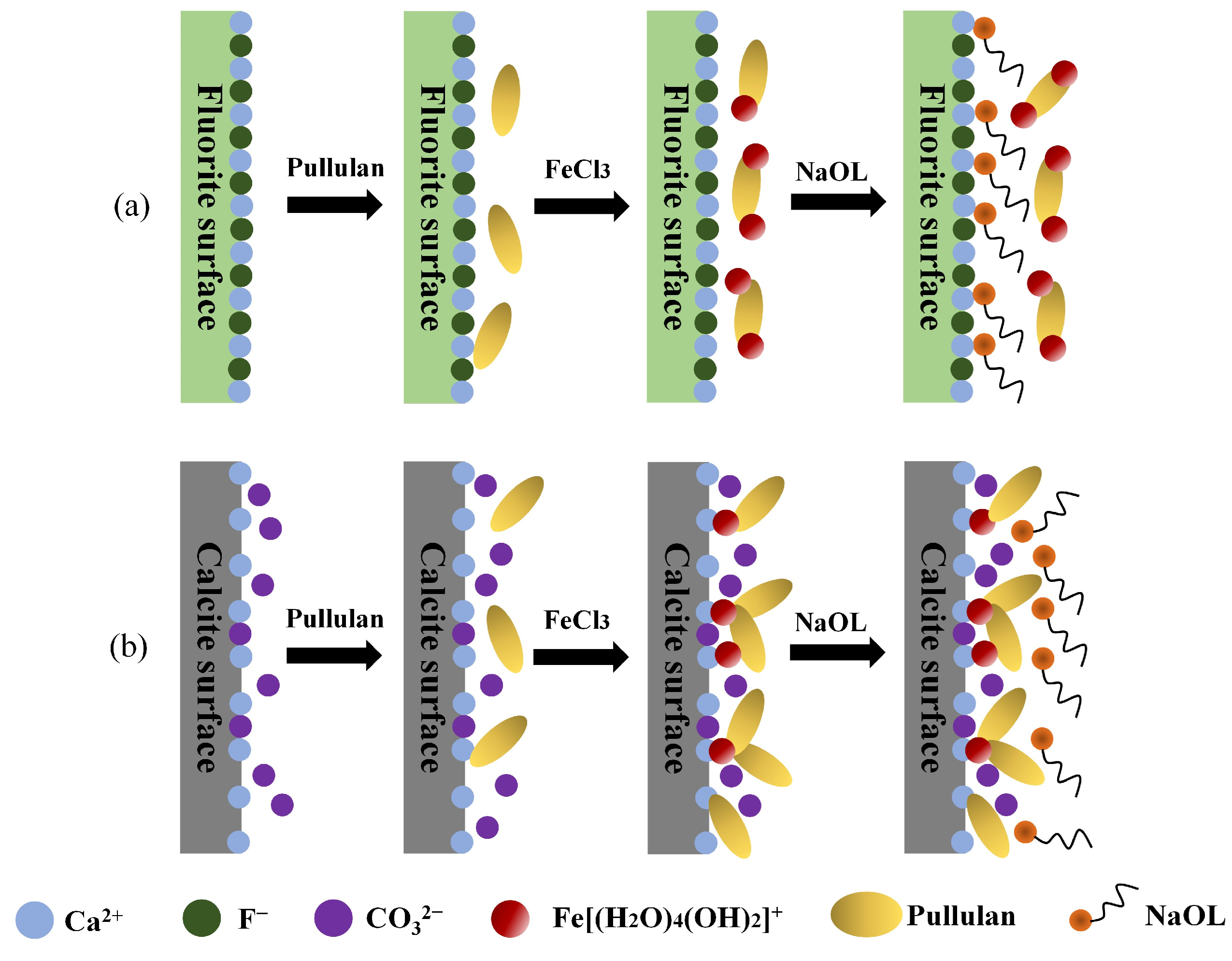
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Song, S. Beneficiation of Fluorite by Flotation in a New Chemical Scheme. Min. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.; Sun, W.; Hu, Y.; Wang, C.; Zhu, S.; Chen, P.; Ding, W.; Ren, L. Selective Adsorption of Trisodium Nitrilotriacetate on Calcite and Its Application for the Separation of Fluorite Minerals. Min. Eng. 2022, 185, 107703. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Yang, J.; Luo, L.; Xu, L. Effect of Dissolved Fluorite and Barite Species on the Flotation and Adsorption Behavior of Bastnaesite. Sep. Purif. Technol. 2020, 237, 116387. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on Flotation Separation of Scheelite from Calcite by Using a Novel Depressant: Sodium Phytate. Min. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Z.; Zhang, Y.; Sun, W.; Gao, Z.; Lei, M. Effect of Artemisia Sphaerocephala Krasch. Gum. Flotat. Sep. Fluorite Calcite. Min. Eng. 2021, 174, 107249. [Google Scholar] [CrossRef]
- Pei, Q.; Zhang, S.; Hayashi, K.; Wang, L.; Cao, H.; Zhao, Y.; Hu, X.; Song, K.; Chao, W. Nature and Genesis of the Xiaobeigou Fluorite Deposit, Inner Mongolia, Northeast China: Evidence from Fluid Inclusions and Stable Isotopes. Resour. Geol. 2019, 69, 148–166. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, C.-Y.; Yin, W.-Z.; Zhu, Y.-M.; Luo, B.-B. Effects of the Crystal Structures of Three Calcium-Containing Minerals on Their Surface Characteristics in Flotation. Sci. Adv. Mater. 2019, 11, 68–73. [Google Scholar] [CrossRef]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption Mechanism of New Mixed Anionic/Cationic Collectors in a Spodumene-Feldspar Flotation System. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Liu, C.; Soraya, D.A.D.; Li, C.; Li, H. Investigations on the Synergistic Effect of Combined NaOl/SPA Collector in Ilmenite Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127267. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, F.; Wei, Q.; Wang, X.; Dong, L. Flotation Separation of Fluorite from Calcite Using Sulfonated Lignite as Depressant. Sep. Purif. Technol. 2020, 242, 116698. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippova, I.V.; Filippov, L.O. Investigation of the Depressants Involved in the Selective Flotation of Scheelite from Apatite, Fluorite, and Calcium Silicates: Focus on the Sodium Silicate/Sodium Carbonate System. Powder Technol. 2019, 352, 501–512. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Xu, Y.; Shu, K.; Fang, S.; Xu, L. The Effect of Dissolved Calcite Species on the Flotation of Bastnaesite Using Sodium Oleate. Min. Eng. 2020, 145, 106095. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. The Mechanism of Fatty Acid Adsorption in the Presence of Fluorite, Calcite and Barite. Int. J. Min. Process 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of Amphoteric Amino Phosphoric Acids with Calcium-Containing Minerals and Selective Flotation. Int. J. Min. Process 2003, 72, 87–94. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Tang, X.; Cao, Y.; Luo, X. Flotation Separation of Fluorite from Calcite by Using Psyllium Seed Gum as Depressant. Min. Eng. 2020, 159, 106514. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, L.; Wu, H.; Tian, J.; Wang, Z.; Gu, X. The Effect of Citric Acid in the Flotation Separation of Bastnaesite from Fluorite and Calcite Using Mixed Collectors. Appl. Surf. Sci. 2020, 529, 147166. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Selective Flotation Separation of Fluorite from Calcite by Using Sesbania Gum as Depressant. Min. Eng. 2021, 174, 107239. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Yan, H.; Zhang, L.; Zhong, C.; Wang, T.; Wang, H.; Xu, L. Adsorption and Depression Mechanism of an Eco-Friendly Depressant Dextrin onto Fluorite and Calcite for the Efficiency Flotation Separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 127987. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Jiao, F.; Qin, W. Use of Citric Acid and Fe(III) Mixture as Depressant in Calcite Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123579. [Google Scholar] [CrossRef]
- Sun, R.; Liu, D.; Tian, X.; Zuo, Q.; Wang, D.; Wen, S. The Role of Copper Ion and Soluble Starch Used as a Combined Depressant in the Flotation Separation of Fluorite from Calcite: New Insights on the Application of Modified Starch in Mineral Processing. Min. Eng. 2022, 181, 107550. [Google Scholar] [CrossRef]
- Ning, S.; Li, G.; Shen, P.; Zhang, X.; Li, J.; Liu, R.; Liu, D. Selective Separation of Chalcopyrite and Talc Using Pullulan as a New Depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126764. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial Sources, Production and Applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Khanda, S.M. Pullulan: An Exopolysaccharide and Its Various Applications. Carbohydr. Polym. 2013, 95, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Wang, J.; Tang, R.; Wei, B.; Jiang, Q. Succinyl Pullulan-Crosslinked Carboxymethyl Chitosan Sponges for Potential Wound Dressing. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 61–70. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Chen, Y.; Huang, G.; Gao, Y. Investigations on the Utilization of Konjac Glucomannan in the Flotation Separation of Chalcopyrite from Pyrite. Min. Eng. 2020, 145, 106098. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, Y.; Wang, J.; Wang, B. Combined Quantum Mechanics and Molecular Dynamics Study on the Calcite Scale Inhibition Mechanism of Carboxymethyl Dextran. Desalination 2023, 553, 116503. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Lyu, W.; Zhu, Y.; Ma, Y.; Li, F. The Role of Gellan Gum in the Selective Flotation Separation of Fluorite from Calcite: An Experimental and Molecular Dynamics Simulation Study. Powder Technol. 2024, 432, 119156. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Zhang, L.; Guo, M.; Feng, B. Flotation Separation of Molybdenite and Talc by Xanthan Gum. Powder Technol. 2021, 388, 158–165. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Sun, W.; Zhai, J.; Yin, Z.; Guan, Q. Effect of Phytic Acid on the Surface Properties of Scheelite and Fluorite for Their Selective Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 80–87. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.; Dou, X.; Zhao, B.; Yang, M. Fluoride Adsorption on an Fe–Al–Ce Trimetal Hydrous Oxide: Characterization of Adsorption Sites and Adsorbed Fluorine Complex Species. Chem. Eng. J. 2013, 223, 364–370. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, C.; Zhu, Y.; Zhu, Y.; Yin, W. Study of the Mechanism of the Fe-BHA Chelates in Scheelite Flotation. Minerals 2022, 12, 484. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Wang, S.; Wang, C.; Chen, M.; Li, H. Efficient Selective Flotation Separation of Fluorite from Calcite Using Ferrous and Ferric Species as Combined Depressant. Min. Eng. 2024, 205, 108451. [Google Scholar] [CrossRef]
- Shi, D.; Li, W.; Han, Y. Fluorite Flotation Separation from Bastnaesite via an Eco-Friendly Polymer as a Depressant and Insight into Its Mechanism of Adsorption. J. Mol. Liq. 2023, 376, 121368. [Google Scholar] [CrossRef]
- Vega-Cantu, Y.I.; Hauge, R.H.; Norman, L.R.; Powell, R.J.; Billups, W.E. Effect of Magnesium and Iron on the Hydration and Hydrolysis of Guar Gum. Biomacromolecules 2006, 7, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, P.; Liu, R.; Deng, Q.; Liu, X.; Li, C.; Xiao, Z. Preparation of Aliphatic Hydroxamic Acid from Litsea Cubeba Kernel Oil and Its Application to Flotation of Fe(III)-Activated Wolframite. Molecules 2023, 29, 217. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, L.; Han, Y.; Zhou, Z. Biosorption of Pb2+ and Zn2+ by Ca-Alginate Immobilized and Free Extracellular Polysaccharides Produced by Leuconostoc Citreum B-2. Int. J. Biol. Macromol. 2021, 193, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Kaisheva, N.S.; Kaishev, A.S. Interaction of Copper(II) Ions with Pectin in Solutions. Pharm. Chem. J. 2023, 57, 218–226. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Pang, Y.; Chen, D.-Y.; Kong, L.-J.; Mehmood, S.; Subbaiah, M.V.; Rao, D.S.; Mouli Pavuluri, C.; Wen, J.-C.; Reddy, G.M. Modification of Chitosan Macromolecule and Its Mechanism for the Removal of Pb(II) Ions from Aqueous Environment. Int. J. Biol. Macromol. 2019, 136, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective Flotation of Scheelite from Calcite Using Xanthan Gum as Depressant. Min. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, Z.; Sun, W.; Pooley, S.G.; Wang, J.; Liu, Y.; Xu, F.; Wang, Q.; Zeng, L.; Wu, Y. New Role of the Conventional Foamer Sodium N-Lauroylsarcosinate as a Selective Collector for the Separation of Calcium Minerals. J. Mol. Liq. 2020, 318, 114031. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, W.; He, Q.; Xing, B.; Wu, Z.; Chen, H.; Wu, D.; Zhang, Q.; Qin, W. Study on Preparation and Physicochemical Properties of Hydroxypropylated Starch with Different Degree of Substitution under Microwave Assistance. Int. J. Biol. Macromol. 2019, 125, 290–299. [Google Scholar] [CrossRef] [PubMed]


| Sample | CaCO3 | CaF2 |
|---|---|---|
| Calcite | 99.2 | / |
| Fluorite | / | 98.5 |
| Fluorite: Calcite | Product | Fluorite (CaF2) | Fluorite | |
|---|---|---|---|---|
| Yield (%) | Grade (%) | Recovery (%) | ||
| 1:1 | Concentrate | 49.82 | 85.43 | 87.21 |
| Products in cell | 50.18 | 12.43 | 12.79 | |
| Feed | 100.00 | 48.80 | 100.00 | |
| Mineral | Surface | Adsorption Energy ∆E/(kJ/mol) |
|---|---|---|
| Calcite | {104} | −102.8358 |
| Fluorite | {111} | −1.1718 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, K.; Qiu, T.; Qiu, X.; Zhao, G.; Jiao, Q.; Fang, J.; Lai, R.; Yang, W. The Role of Fe(III) in Selective Adsorption of Pullulan on Calcite Surfaces: Experimental Investigation and Molecular Dynamics Simulation. Molecules 2024, 29, 4194. https://doi.org/10.3390/molecules29174194
Ding K, Qiu T, Qiu X, Zhao G, Jiao Q, Fang J, Lai R, Yang W. The Role of Fe(III) in Selective Adsorption of Pullulan on Calcite Surfaces: Experimental Investigation and Molecular Dynamics Simulation. Molecules. 2024; 29(17):4194. https://doi.org/10.3390/molecules29174194
Chicago/Turabian StyleDing, Kaiwei, Tingsheng Qiu, Xianhui Qiu, Guanfei Zhao, Qinghao Jiao, Jiangjie Fang, Ruisen Lai, and Wenhui Yang. 2024. "The Role of Fe(III) in Selective Adsorption of Pullulan on Calcite Surfaces: Experimental Investigation and Molecular Dynamics Simulation" Molecules 29, no. 17: 4194. https://doi.org/10.3390/molecules29174194






