Abstract
The emergence of natural products has provided extremely valuable references for the treatment of various diseases. Cucurbitacin B, a tetracyclic triterpenoid compound isolated from cucurbitaceae and other plants, is the most abundant member of the cucurbitin family and exhibits a wide range of biological activities, including anti-inflammatory, anti-cancer, and even agricultural applications. Due to its high toxicity and narrow therapeutic window, structural modification and dosage form development are necessary to address these issues with cucurbitacin B. This paper reviews recent research progress in the pharmacological action, structural modification, and application of cucurbitacin B. This review aims to enhance understanding of advancements in this field and provide constructive suggestions for further research on cucurbitacin B.
1. Introduction
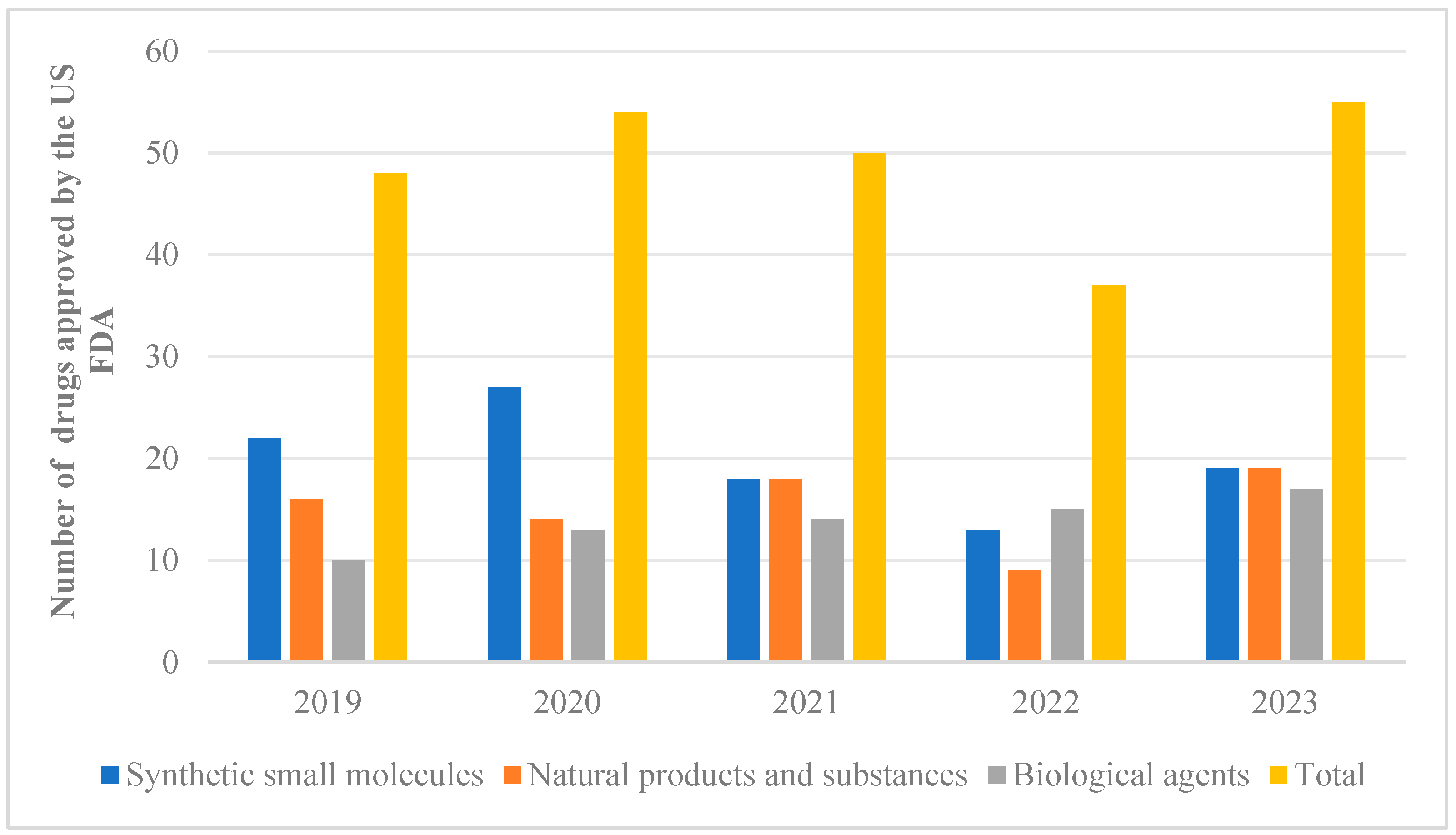
Natural products extracted from animals, plants, and microorganisms possess distinct structural characteristics and unique biological activities. Studies have demonstrated that these natural products can be utilized in the treatment of various diseases such as neurodegenerative disorders, cancer, and diabetes, among others [1,2,3,4]. These studies provide valuable references for the development and application of novel drugs. Out of the 2191 drugs and small-molecule new chemical entities approved for sale between 1981 and 2023, approximately 23.5 percent were directly or indirectly derived from natural products [5,6]. From 2019 to 2023, the US FDA approved a total of 243 new drugs, which were classified and counted. Among them, 76 drugs were developed based on natural pharmacophores (Figure 1). What is exciting is that a high proportion of natural products could be observed in approved synthetic drugs starting from 2019. However, there was a downward trend in 2022, followed by a significant increase in the number of natural products occurring in 2023. These findings suggest that natural products are regaining their appeal for the treatment of diseases. Notably, artemisinin [7], which was awarded the Nobel Prize in 2015, plays an extremely important role in combating parasitic diseases and holds significant value for the advancement of human medicine.
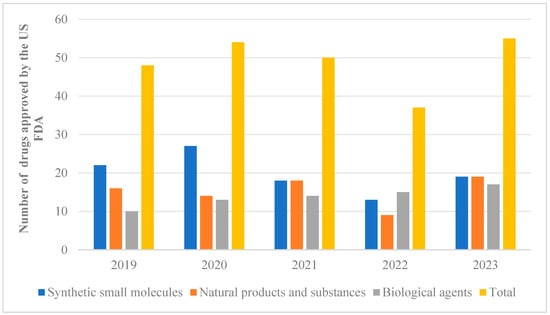
Figure 1.
From 2019 to 2023, the US FDA approved 243 new drug classifications and statistics.
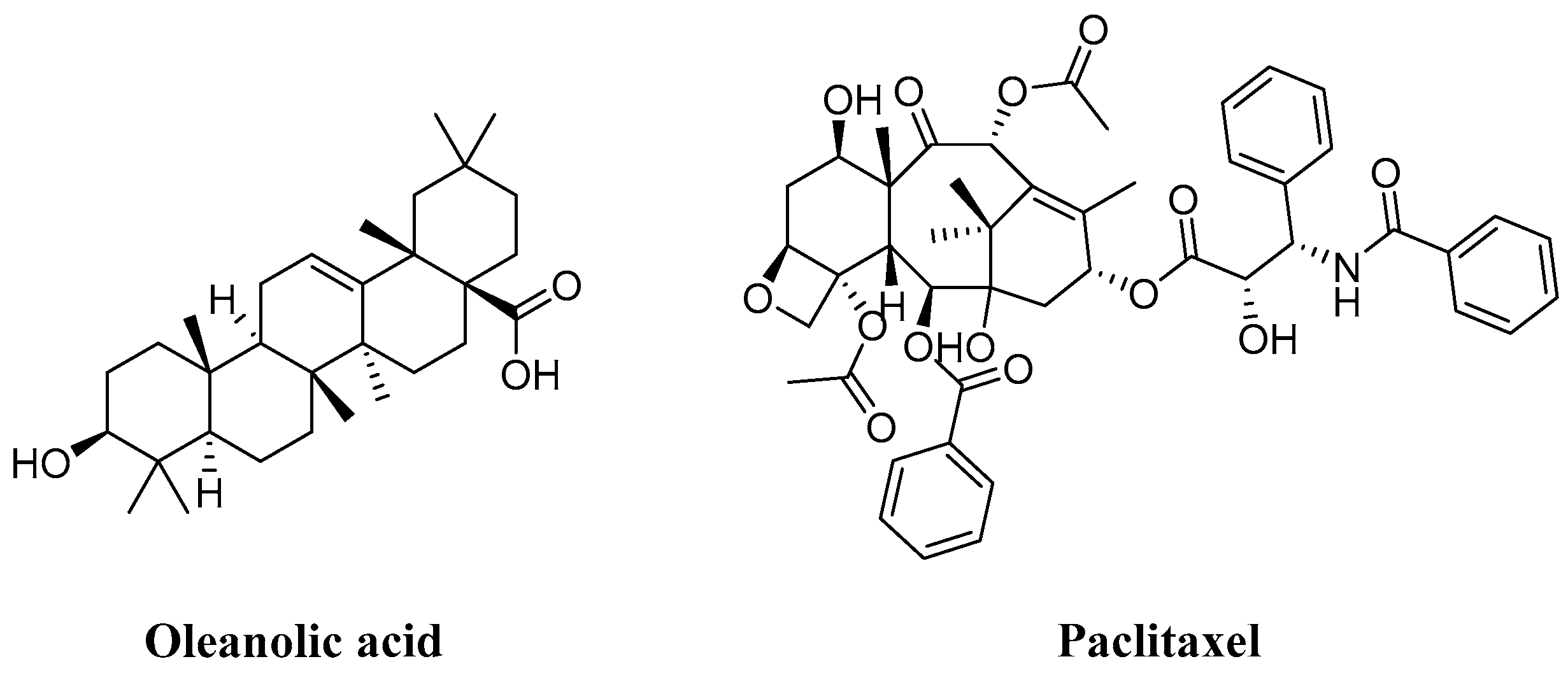
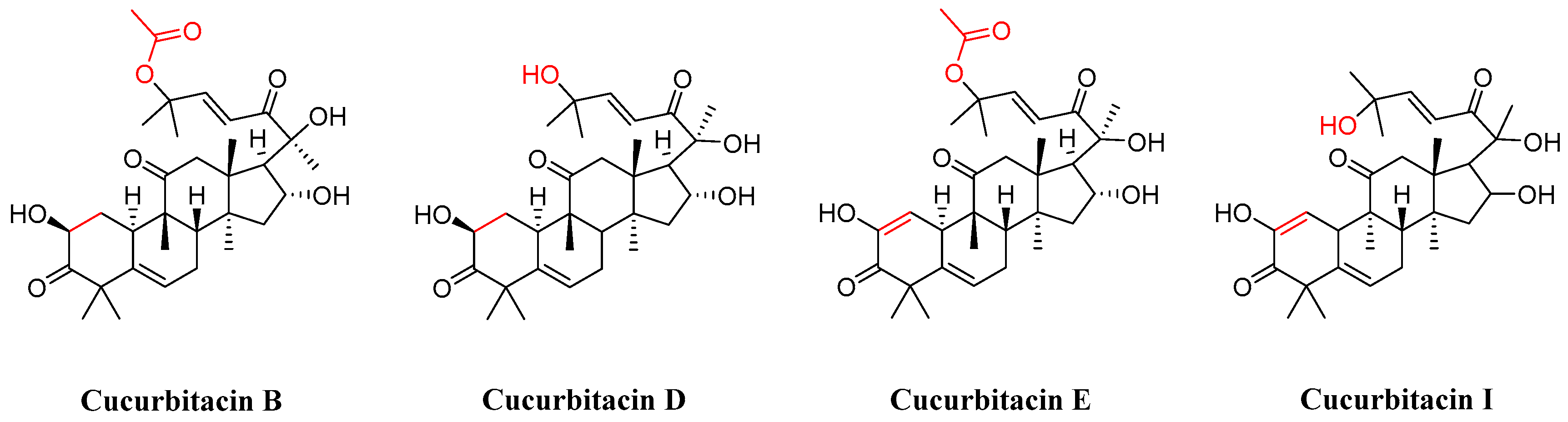
In the realm of natural products, triterpenoids are widely found in plants, exhibiting a variety of biological activities and possessing complex structures. Among them, tetracyclic triterpenoids and pentacyclic triterpenoids have been extensively studied [8]. Among pentacyclic triterpenes, oleanolic acid is particularly renowned for its excellent anti-inflammatory, antibacterial, antiviral properties [9,10]. Among tetracyclic triterpenes, paclitaxel stands out as the most famous one due to its significant anti-tumor activity and widespread use in cancer treatment (Figure 2) [11]. Cucurbitacins in the widely existing plant family also belong to tetracyclic triterpenoids, which are classified into 12 classes from cucurbitacin A to cucurbitacin T. Among these, cucurbitacins B and D appear more frequently, and researchers have analyzed and explored cucurbitacins B, D, E, as well as I (Figure 3). The key distinction among these four cucurbitacins lies in whether there is a double bond between C-1 and C-2 and whether C-25 is attached to a hydroxyl group or an acetyl group. Taking cucurbitacin B as an illustration, in cucurbitacin B, there is a single bond between C-1 and C-2, and C-25 is connected to an acetyl group. The difference is that in cucurbitacin D, C-25 is linked to a hydroxyl group, while in cucurbitacin E, there is a double bond between C-1 and C-2. Cucurbitacin I mainly features the connection of a hydroxyl group at position C-1 and C-2. Additionally, both cucurbitacins B and E inhibit the Wnt and STAT3 signaling pathways. Cucurbitacins B and I increase ROS levels. Cucurbitacin D inhibits STAT3 activation. Furthermore, cucurbitacins B, D, and E down-regulate the expression of key cell cycle regulators such as cyclin B1 [12]. Due to the high content of cucurbitacin B, significant pharmacological activity, and great development potential, numerous researchers have conducted research on it [13]. Cucurbitacin B is primarily found in cucurbitaceae and cruciferous plants. The fruit stalk of the Chinese medicine melon, which belongs to the cucurbitaceae family, is widely used in traditional Chinese medicine for treating abdominal distention and constipation due to its abundant presence of cucurbitin [14]. It can also be found in various other plants such as Cucumis melo, Cucurbita andreana, Ecballium elaterium, Wilbrandia ebracteata, and Trichosanthes cucumerina.
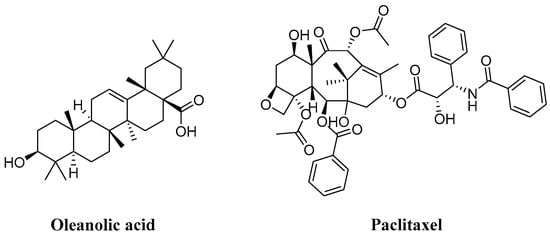
Figure 2.
Chemical structure of oleanic acid and paclitaxel.
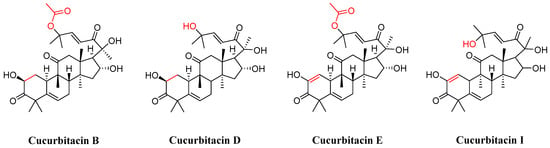
Figure 3.
Chemical structure of cucurbitacin B, D, E, and I.
Since the isolation and extraction of cucurbitacin B, it has garnered extensive attention from researchers due to its high toxicity and narrow therapeutic window, necessitating structural modification and improvement in dosage form. Numerous derivatives have been designed and synthesized, with studies conducted on the effects of different dosage forms. From the past to the present, significant progress has been made in understanding its related molecular mechanism and target research. It has been reported that a research team used cucurbitacin B as a probe to directly target IGF2BP1 in liver cancer cells. These findings will provide valuable references for further development and research on cucurbitacin B [15]. Furthermore, considering the adverse effects of conventional pesticides and the beneficial impact of cucurbitacin B on various pests, the investigation into cucurbitacin B aims to offer valuable insights for the development of novel pesticides. According to the investigation and research, cucurbitacin B has been used as a listed drug in China, such as cucurbitacin tablets approved by the State Food and Drug Administration in 1998 for the adjuvant treatment of persistent hepatitis, chronic hepatitis, and primary liver cancer caused by dampness and heat toxicity. In China, there are 18 pharmaceutical companies that produce and sell cucurbitacin tablets or capsules for the treatment of chronic hepatitis and primary liver cancer. This paper reviews the biological and pharmacological research on cucurbitacin B and summarizes the latest progress on its structurally modified derivatives. The purpose of this study is to understand the therapeutic potential and value of cucurbitacin B as a drug discovery platform, aiming to provide valuable information for its development.
Cucurbitacin B exhibits a variety of pharmacological effects, including antioxidant and anti-inflammatory activities, protective effects on the nervous system, and excellent anti-tumor effects [16,17,18,19]. In this paper, based on numerous studies, the mechanism of action of cucurbitacin B in terms of antioxidant, anti-inflammatory, neuroprotective, cardiovascular, and anti-tumor properties, as well as its applications in agronomy, are introduced.
2. Pharmacological Activity
2.1. Antioxidant Activity
Oxidative stress is a negative effect produced by free radicals in the body and is thought to be an important factor in aging and disease [20]. Cucurbitacin B can reduce CCl4-induced oxidative stress in hepatocytes, and cucurbitacin B induces the intracellular accumulation of lipid ROS and the lipid peroxides MDA and Fe2+, while decreasing GSH levels [21,22].
Yang et al. [23] found that cucurbitacin B can also significantly reduce the mitochondrial volume and increase the mitochondrial membrane density, and cucurbitacin B can activate the SLC7A11 mitochondrial oxidative stress pathway to induce iron apoptosis, demonstrating its potential as a lead compound for the development of some anti-inflammatory drugs.
Previous studies have also shown that cucurbitacin B and cucurbitacin I have antioxidant properties, which can inhibit lipid peroxides [24]. In addition, Tehila et al. [25] found that cucurbitacin glycosides (cucurbitacin B and cucurbitacin E) have antioxidant and free radical scavenging capabilities, suggesting that cucurbitacins are expected to provide a preventive and therapeutic option for oxidation-damaged diseases.
2.2. Anti-Inflammatory Activity
Inflammation is a basic pathological process in which biological tissues are stimulated by certain stimuli such as trauma, infection, and other injury factors [26]. Inflammation can exacerbate the development of many complex diseases, such as diabetes, Alzheimer’s disease, cardiovascular disease, and age-related diseases such as cancer [27,28,29,30].
In recent years, many studies have shown that inflammation is closely related to the pathophysiological process of brain I/R injury. However, few studies have focused on the role of cucurbitacin B. Chu et al. [31] found that cucurbitacin B decreased OGD/R-induced cytotoxicity, ROS production, and levels of pro-inflammatory cytokines (including TNF-α, IL-1β, and IL-6) in OGD/R-induced cell damage models. These results suggest that cucurbitacin B can inhibit the activation of NLRP3 inflammasome to inhibit the inflammatory response and cell damage in brain I/R injury.
Sun et al. [18] found that cucurbitacins B, E, and I can significantly reduce the activation activity of NF-κB induced by TLR 2/4 agonists in cells. Pretreatment with these three elements can block the phosphorylation of JAK1, STAT1, and STAT3. At the same time, Nrf2/ARE signals can be up-regulated to protect cells from neuroinflammatory damage. Cucurbitacins B, E, and I can significantly inhibit neuroinflammatory response by inhibiting neuroinflammatory mediators in microglia stimulated by TLR 2/4 agonists.
Xue et al. [32] discovered that cucurbitacin B effectively inhibits the production of pro-inflammatory cytokines IL-1β, TNF-α, and IL-18 in ATP-stimulated macrophages. This inhibition is achieved by regulating TLR4 signaling to reduce the expression of NLRP3 inflammasome and suppress the generation of pro-IL-1β. Additionally, cucurbitacin B exhibits its inhibitory effects on NLRP3 activation and the subsequent formation of the NLRP3 inflammasome complex by downregulating ACS expression, thereby leading to a decrease in both IL-1β production and cleaved caspase-1 activity. Cucurbitacin B can also down-regulate key enzymes in the HIF-1α signaling pathway and glycolysis pathway. In addition, the data demonstrated that cucurbitacin B effectively mitigated MSU-induced arthritis in a murine model of gout. These findings suggest that cucurbitacin B functions as an inhibitor of the NLRP3 inflammasome, suppressing inflammation induced by various stimuli and exhibiting potential as an anti-inflammatory agent for diseases associated with the NLRP3 inflammasome.
Zhong et al. [33] conducted a study that demonstrated cucurbitacin B can reduce the influx of inflammatory cells and improve histology. Additionally, it regulated the expression of RANK/RANKL and OPG following the induction of periodontitis, and it decreased the levels of inflammatory mediators TNF-α and IL-1β in the gum tissue.
Liu et al. [34] validated cucurbitacin B as a possible anti-inflammatory component of Trichosanthis Pericarpium (TP)–Trichosanthis Radix (TR) through network pharmacology and intestinal microbiota sequencing.
Omar et al. [35] demonstrated experimentally that cucurbitacin B, the main component isolated from Cucumis prophetarum, exhibits the strongest anti-inflammatory activity, and they demonstrated its anti-inflammatory activity by slowing the elevated levels of TNF-a, IL-6, COX-2, and iNOS in carrageenan-induced prostates.
2.3. Neuroprotection
Mental illness, which accounts for 7 percent of China’s population, has overtaken heart disease and cancer to become the biggest burden on the country’s healthcare system, according to the World Health Organization [36]. Cucurbitacin B is worthy of further study for the treatment of such diseases. Ge et al. [37] discovered that a single administration of cucurbitacin B exhibited potential antidepressant effects in the forced swim test (FST) and tail suspension test (TST). Repeated administration of cucurbitacin B prevented depression-like behavior induced by chronic and unpredictable mild stress (CUMS). The repeated use of cucurbitacin B counteracted the downregulation of the hippocampal BDNF system caused by CUMS.
Li et al. [38] have demonstrated that cucurbitacin B potently promotes neurite outgrowth and lengthening in PC12 cells and primary neurons. Furthermore, the administration of cucurbitacin B has been shown to enhance the generation of new neurons in the hippocampus of ICR and APP/PS1 mice, thereby ameliorating the working memory deficits observed in these mice models. Notably, this compound does not appear to influence long-term memory. Collectively, these findings suggest that cucurbitacin B may represent a promising novel therapeutic candidate for the treatment of Alzheimer’s disease.
Liu et al. [39] demonstrated that cucurbitacin B mitigates the memory impairment induced by STZ-ICV in rats. The compound effectively reduced nitrous oxide stress in the STZ-ICV AD prototype brain, diminished the apoptosis of neuronal cells, downregulated the activity of acetylcholinesterase, and decreased the level of glutamate in the brain of STZ-ICV treated rats. Concurrently, cucurbitacin B enhanced the levels of GABA.
2.4. Cardiovascular Protection
Cardiovascular diseases encompass a spectrum of pathologies affecting the circulatory system, which can be categorized into acute and chronic conditions, predominantly associated with arteriosclerosis. Cardiovascular disease constitutes a significant proportion of annual mortality rates [40].
Xiao et al. [41] have demonstrated that cucurbitacin B exerts protective effects on the heart, including the prevention of hypertrophy, the amelioration of compromised cardiac function following myocardial infarction, the mitigation of fibrosis under pressure overload conditions, and the modulation of autophagic activity. This compound upregulated the expression of key autophagic markers such as LC3-II, Atg5, Atg7, Atg12, and Beclin1 while downregulating p62 expression. The study suggests that the cardioprotective mechanisms of cucurbitacin B may be mediated through the activation of autophagy, potentially via the inhibition of the Akt signaling pathway. Collectively, these findings indicate that cucurbitacin B may affect the autophagic process in cardiac tissue and cardiomyocytes, which may offer valuable insights for the therapeutic management of myocardial hypertrophy and heart failure.
Chen et al. [42] have demonstrated that cucurbitacin B exhibits a concentration-dependent inhibitory effect on lactate dehydrogenase (LDH) release induced by oxygen–glucose deprivation/reperfusion (OGD/R) in cardiomyocytes. Notably, cucurbitacin B potently reversed the inhibition of superoxide dismutase (SOD) products caused by OGD/R. Furthermore, this compound effectively attenuated apoptosis in cardiomyocytes subjected to OGD/R exposure. Importantly, cucurbitacin B has been shown to enhance cardiac function and promote fibrotic scar repair following ischemia/reperfusion (I/R) injury. Additionally, it significantly reduced the size of myocardial scars and enhanced fibrotic scar repair. These beneficial effects of cucurbitacin B are mediated through the JAK2/STAT3 signaling pathway, which not only facilitates cardiac function and myocardial repair following I/R injury but also mitigates LDH release, oxidative damage, and apoptosis in cardiomyocytes, thereby offering cardiomyocyte protection through modulation of the JAK2/STAT3 signaling pathway. Given the protective effect of cucurbitacin B on cardiac I/R injury, it provides a new route for the protection and treatment of I/R injury.
2.5. Antitumor Activity
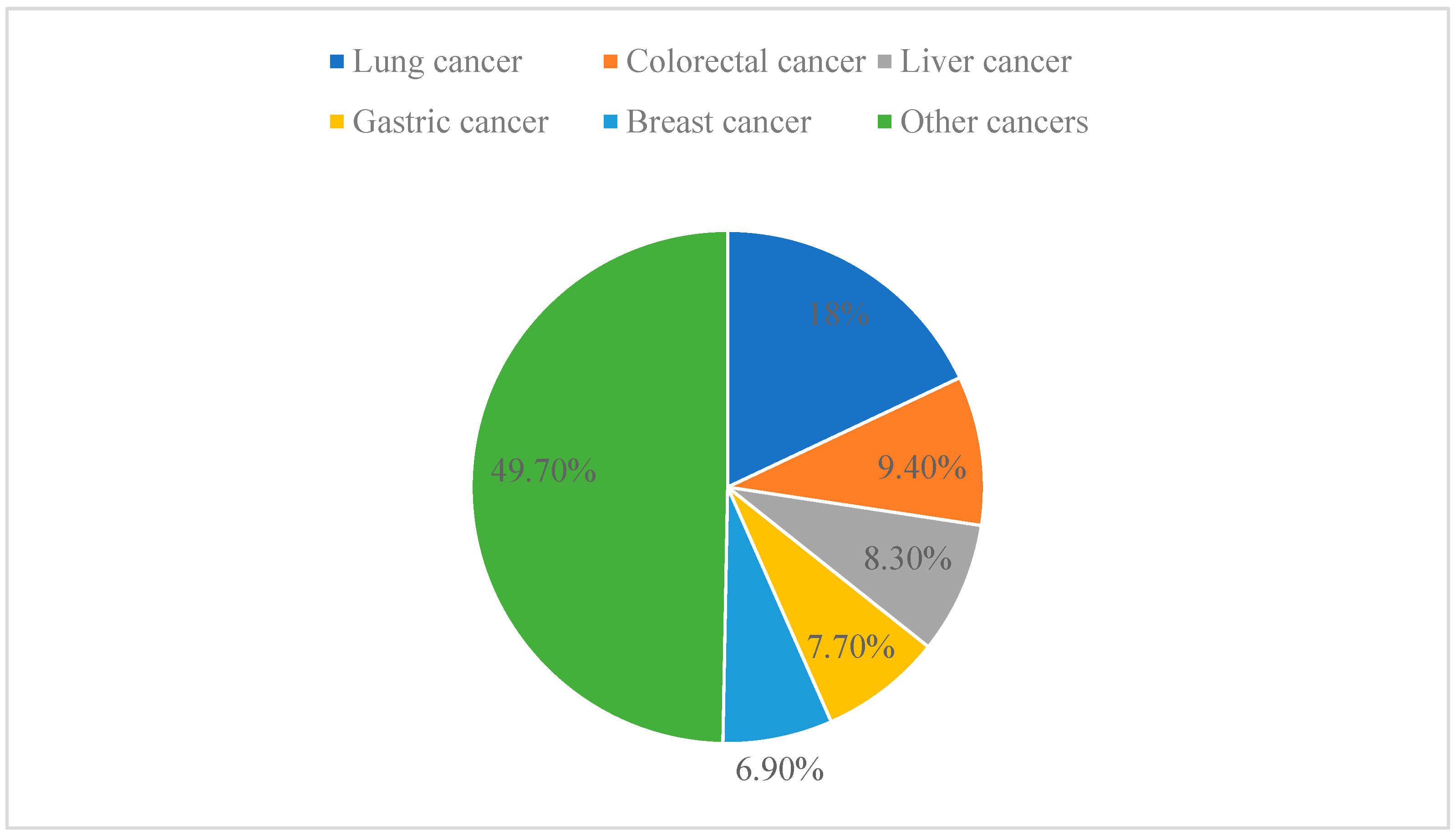
Cancer is one of the leading causes of death worldwide and has even surpassed cardiovascular disease as the leading cause of premature death at this stage [43]. In 2020, the latest estimate from the World Health Organization (WHO) is that 9.95 million people will die of cancer worldwide. Among the different types of cancer, lung cancer is the leading cause of cancer death, accounting for 18.0% of all cancer deaths, followed by colorectal cancer (9.4%), liver cancer (8.3%), stomach cancer (7.7%), and breast cancer (6.9%) (Figure 4) [44]. Anticancer drugs aim to stop tumor growth by killing cancer cells and preventing tumor discovery and metastasis [45,46]. Traditional molecular sources are natural products that have a lot of potential in the field of pharmacology. The lead compounds of modern anticancer drugs are in part low-molecular-weight metabolites derived from terrestrial and marine organisms. Despite significant advances in cancer research over recent decades, anticancer therapy still faces serious challenges. Therefore, efforts have been made to explore new molecular mechanisms and develop new therapeutic strategies or drugs to treat cancer [47,48].
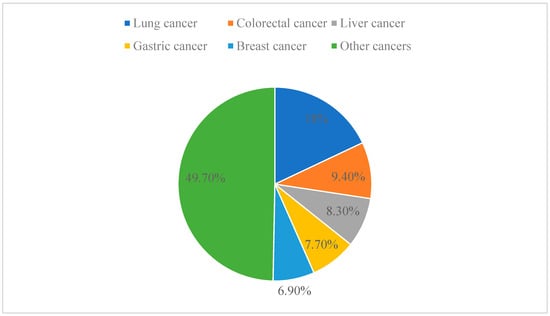
Figure 4.
Different types of cancer as a proportion of total cancer.
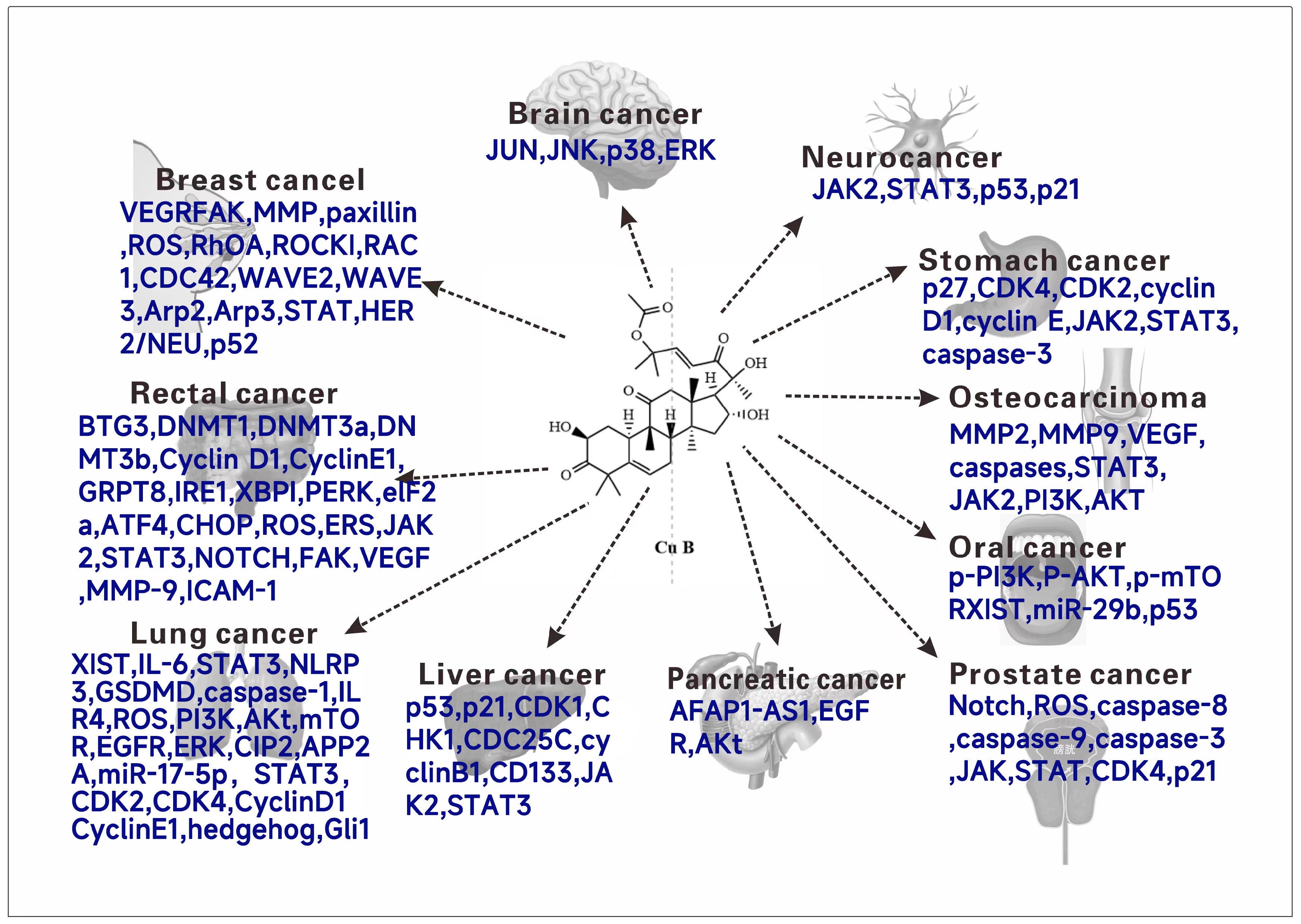
The natural product cucurbitacin B and its synthetic analogs have been studied and talked about, conducting experiments to explore cytotoxic activity against different human tumor cell lines. Various experiments and related reviews have also discussed or suggested possible mechanisms of action of cucurbitacin B. Cucurbitacin B has cytotoxic activity on a variety of tumor cells (Figure 5), and it has a good clinical application prospect as an antitumor drug. In this section, the antitumor activity of cucurbitacin B and its mechanism will be systematically introduced.
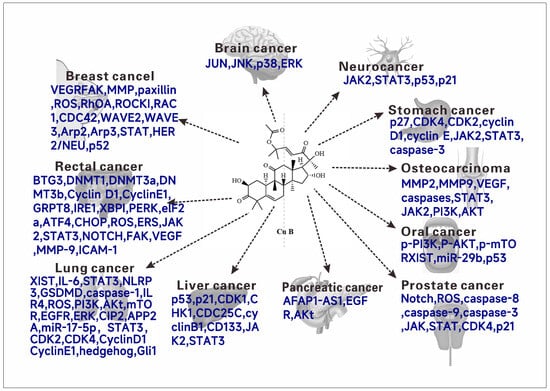
Figure 5.
The anticancer molecular mechanism (Targets) of cucurbitacin B.
2.5.1. Breast Cancer
Luo et al. [49] studied the effect of cucurbitacin B on MDA-MB-231 cells and found that cucurbitacin B could induce morphological changes in the cells, but it had no effect on their viability. It inhibited the migration and invasion of the cells, as well as cell adhesion to the matrix, type I collagen, fibronectin, and endothelial cells. Additionally, cucurbitacin B inhibited the phosphorylation of FAK and paxillin, and it could also induce the production of reactive oxygen species (ROS), which is helpful for the anti-metastatic potential of the cells.
Liang et al. [50] studied the effects of cucurbitine BMDA-MB-231 and SKBR-3 cells. Cucurbitacin B could significantly inhibit cell adhesion. It could decrease the deformability of cells. The expression levels of RAC1, CDC42, WAVE2/3, and ARP2/3 proteins in RhoA and ROCK1 and the RAC1/CDC42 pathways can be decreased, and the migration and invasion of cells in vitro can be inhibited.
2.5.2. Glial Tumor
Yin et al. [51] studied the effects of cucurbitacin B on glioblastoma U87, T98G, U118, U343, and U373, and they found that cucurbitacin B inhibited the proliferation and clonal growth of glioblastoma. It can block the G2/M phase of the cell cycle, reduce cell migration, and induce cell apoptosis. Furthermore, it affects GBM cells through the JNK/c-Jun signaling pathway by increasing the levels of p-p38, p-JNK, and p-JUN.
2.5.3. Neuroblastoma
Zheng et al. [52] studied the effect of cucurbitacin B on the neuroblastoma SH-SY5Y cell line. They found that cucurbitacin B induced early apoptosis in these cells and altered the expression of proteins involved in proliferation and apoptosis, such as up-regulating the p53 and p21 genes.
2.5.4. Gastric Cancer
Xie et al. [53] studied the effect of cucurbitacin B on gastric cancer cells MKN-45 and found that cucurbitacin B inhibited the proliferation of these cells. It was observed that cucurbitacin B affected the cell cycle transition from the G0/G1 phase to the S phase. Additionally, cucurbitacin B upregulated the expression of p27 and downregulated the expression of CDK4, CDK2, cyclin D1, and cyclin E mRNA.
Xu et al. [54] studied the effects of cucurbitacin B on SGC7901, BGC823, MGC803, and MKN74 gastric cancer cells. Cucurbitacin B showed dose-dependent inhibition on gastric cancer cells. By inhibiting the STAT3 signaling pathway, it can decrease the phosphorylation of TYR-705 in STAT3 and inhibit the expression of the STAT3 target gene, thus inhibiting the growth and invasion of gastric cancer cells and inducing the apoptosis of cancer cells. The combination of cucurbitacin B and cisplatin (DDP) enhanced the activation of caspase-3 and the cleavage of caspase-3 substrate PARP, and it decreased the expression level of pSTAT3.
2.5.5. Osteosarcoma
Zhang et al. [55] studied the effect of cucurbitacin B on human osteosarcoma cells (U-2OS). They found that cucurbitacin B could inhibit the proliferation of U-2OS cells. It significantly reduced the viability of U-2OS cells and affected the viability count of U-2OS cells. Additionally, cucurbitacin B induced apoptosis in U-2OS cells and inhibited the migration of these cells. It may down-regulate the expression of MMP2, MMP9, and VEGF, which could significantly inhibit cell migration and angiogenesis. Furthermore, cucurbitacin B promoted an increase in caspase levels and significantly inhibited the STAT3/JAK2 signaling pathway.
Wu et al. [56] studied the effects of cucurbitacin B on HOS and 143B cells. Cucurbitacin B inhibited the differentiation of M0 into M2 macrophages, resulting in a decrease in the number of M2 macrophages in vivo, and did not affect the polarization of M2-M1 macrophages. It can inhibit the differentiation of M2 macrophages by inhibiting the PI3K/AKT signaling pathway and then inhibiting the proliferation, migration, and invasion of osteosarcoma cells. The progression of osteosarcoma can be effectively slowed down by regulating the differentiation of M2 macrophages.
2.5.6. Oral Squamous Cancer
Yu et al. [57] studied the effects of cucurbitacin B on CAL27 and SCC25 cells. They found that cucurbitacin B can regulate the expression of the PI3K-AKT pathway and reduce the expression levels of p-PI3K, p-AKT, and p-mTOR, thus inhibiting the migration of human tongue squamous cancer cells, blocking the cell cycle in the G2 phase, and inducing cell apoptosis, which inhibits the growth of oral squamous cell carcinoma.
Tao et al. [58] investigated the effects of cucurbitacin B on CAL27 and SCC9 cells. Cucurbitacin B plays an anticancer role by inhibiting the migration and invasion potential of oral squamous cell carcinoma cells. It can decrease the expression of XIST and increase the expression of miR-29b by inducing apoptosis and inhibiting cell growth through the p53 protein.
2.5.7. Prostate Cancer
Ahmed et al. [59] studied the effect of cucurbitacin B on LNCaP cells and found that cucurbitacin B could inhibit the proliferation of these cells, alter their morphology, promote the activation of caspase, increase intracellular reactive oxygen species (ROS), and induce apoptosis; furthermore, the Notch signaling pathway in LNCaP cells was down-regulated.
Ahmed et al. [60] continued to study the effect of cucurbitacin B on PC-3 cells, and found that cucurbitacin B can reduce the activity of these cells. It promotes the activation of caspase-8, -9, and -3. Additionally, cucurbitacin B induces ROS-mediated oxidative stress, increases ROS levels, and triggers apoptosis. When acting on PC-3 cells, ROS can downregulate the expression of cyclin D1 and cyclin-dependent kinase 4 (CDK4) while enhancing the mRNA expression of the CDK inhibitor p21 Cip1. Furthermore, it significantly reduces the expression of the JAK/STAT signaling pathway.
2.5.8. Pancreatic Cancer
Zhou et al. [61] studied the effects of cucurbitacin B on four types of cells: ASPC-1, BxPC-3, HPAC, and MiaPaCa-2. They found that cucurbitacin B can down-regulate the expression of AFAP1-AS1, thereby inhibiting cell proliferation. Additionally, by inhibiting the expression of AFAP1-AS1, it can block the cell cycle and inhibit the EGFR/Akt signaling pathway. Furthermore, cucurbitacin B regulates the mirNA-146B-5p/EGFR axis by modulating AFAP1-AS1, affecting cell proliferation.
2.5.9. Hepatocellular Carcinoma
Li et al. [62] investigated the impact of cucurbitacin B on the growth of liver cancer cells, including the Huh7, Hep3B, and Hepa1/6 lines, demonstrating a significant inhibitory effect. Treatment with cucurbitacin B suppressed cell viability and proliferation, triggered the enrichment of differentially expressed proteins within the DNA damage repair pathway, and interrupted the cell cycle through the activation of DNA damage response. This activation led to the activation of the ATM-dependent p53-CDK1 and CHK1-CDC25C pathways, resulting in the increased expression of p53 and p21 and a concomitant decrease in cyclin-dependent kinase 1 (CDK1) levels. Additionally, cucurbitacin B enhanced the phosphorylation of checkpoint kinase 1 (p-CHK1) and inhibited the protein levels of cell division cycle 25C (CDC25C).
Wang et al. [63] investigated the impact of cucurbitacin B on HepG2 cells, revealing its broad anti-tumor properties in CD133+ HepG2 cells, including enhanced sensitivity to sorafenib. The synergistic combination of cucurbitacin B with sorafenib markedly suppressed the viability of CD133+ HepG2 cells. Additionally, cucurbitacin B inhibited the expression levels of cyclin B1, CDK1, and the JAK2/STAT3 signaling pathway in CD133+ HepG2 cells.
2.5.10. Lung Cancer
Liu et al. [64] studied the effect of cucurbitacin B on A549 cells. Cucurbitacin B can inhibit the proliferation of lung cancer cells and promote apoptosis of lung cancer cells. It can inhibit the expression of XIST and the IL-6/STAT3 signaling pathway in cells, thus promoting the expression of miR-let-7c, and the overexpression of miR-let-7c can promote the regulation of lung cancer cells through the IL-6/STAT3 axis. In addition, the knockdown of XIST can enhance its influence on cell proliferation and apoptosis.
Yuan et al. [65] further explored the impact of cucurbitacin B on A549 cell behavior. Their findings indicate that cucurbitacin B effectively suppresses the morphological alterations, migration, and invasive capabilities triggered by TGF-β1 in these cells. Additionally, it inhibits the epithelial–mesenchymal transition (EMT) mediated by TGF-β1 and counteracts TGF-β1-induced EMT by suppressing reactive oxygen species production through the PI3K/Akt/mTOR signaling pathway.
Liu et al. [66] studied the effects of cucurbitacin B on human gefitinib-resistant NSCLC cell lines A549, NCI-H1299 (H1299), NCI-H1975 (H1975), and NCI-H820 (H820). Cucurbitacin B can inhibit the invasion and migration of gefitinib-resistant NSCLC cells and induce caspase-dependent apoptosis. It can induce lysosomal degradation of EGFR, thereby inhibiting the phosphorylation of ERK and Akt, and inhibit the CIP2A/PP2A signaling axis. Cisplatin (DDP) can enhance calucinin-B-dependent apoptosis and enhance its inhibition of the EGFR and CIP2A/Akt pathways.
Yu et al. [67] studied the effect of cucurbitacin B on PC9/GR cells and found that cucurbitacin B could improve the down-regulation of miR-17-5p expression induced by gefitinib, regulate the miR-17-5p/STAT3 axis, decrease the level of STAT3 protein, and inhibit phosphorylation. The suppression of cell proliferation and the enhancement of apoptosis conferred resistance to gefitinib in non-small cell lung cancer (NSCLC), a synergistic combination of cucurbitacin and gefitinib markedly potentiated apoptosis in PC9/GR cells.
Yu et al. [68] studied the effect of cucurbitacin B on A549/DDP cells and found that cucurbitacin B had a strong inhibitory effect on the proliferation of A549/DDP cells. It can block G0 and G1 phase cells, up-regulate the expression of p53 and cycle-related gene p21, and then inhibit the expressions of CDK2, CDK4, CyclinD1, and CyclinE1. In A549/DDP cells, the treatment significantly downregulates the expression levels of SMO and GLI1 RNA and proteins, which are pivotal molecules within the hedgehog signaling pathway.
2.5.11. Rectal Cancer
Mao et al. [69] studied the effects of cucurbitacin B on SW480, Caco-2, HCT116, and Col205. Cucurbitacin B can reduce the levels of DNA methyltransferase (DNMT1, DNMT3a, and DNMT3b) through the demethylation of the BTG3 promoter in rectal cancer cells and re-activate BTG3.The cell cycle of cancer cells can be blocked in the G1 phase in vitro, and the levels of Cyclin D1 and Cyclin E1 in cancer cells can be significantly reduced.
Huang et al. [70] studied the effect of cucurbitin B on HT-29 and SW620 cells. Cucurbitacin B can inhibit the viability and proliferation of colorectal cancer cells. Furthermore, cucurbitacin B can increase the expression of GRP78 and activate the IRE1/XBP1 and PERK/eIF2α/ATF4/CHOP signaling pathways in UPR, demonstrating that it can produce ROS and induce ERS, which can induce apoptosis through ERS and its signal transduction pathway.
Zhang et al. [71] investigated the effect of cucurbitacin B on HCT116 cells and found that it inhibits the proliferation of colorectal cancer cells. Additionally, cucurbitacin B can inhibit the JAK2/STAT3 signaling pathway, reduce the migration and invasion ability of M2-like TAM polarization-induced colon cancer cells, enhance the anti-tumor response, and increase the expression of CD4 and CD8 in the tumor microenvironment.
Prasad et al. [72] studied the effects of cucurbitacin B on HCT116, SW480, and DLD1 cells and found that the combination of cucurbitacin B and cucurbitacin I could significantly inhibit the viability of the HCT116, SW480, and DLD1 cell lines. Both of them inhibit the G2/M cell cycle. They down-regulate the Notch signaling pathway and reduce the expression of CSC markers and the Notch signaling protein in tumor tissue.
2.5.12. Bile Duct Cancer
Putthaporn et al. [73] studied the effect of cucurbitacin B on KKU-452 cells and found that cucurbitacin B significantly inhibited the activation of FAK and reduced the level of phosphorylated FAK protein. It could significantly decrease the amount of MMP-9 and inhibit the expression of ICAM-1 and VEGF. Cucurbitacin B can inhibit the migration, invasion, and adhesion of biliary duct cancer cells in a dose-dependent manner.
2.5.13. Bladder Cancer
Yener et al. [74] explored the impact of cucurbitacin B on MB49 cell viability, documenting a reduction in cell proliferation in the presence of cucurbitacin B combined with cisplatin. Furthermore, cucurbitacin B induced apoptosis and autophagy within the tumor tissue of MB49 mice. The study also demonstrated that cucurbitacin B could enhance autophagy, as evidenced by increased levels of LC3II and Beclin-1 proteins, while concurrently decreasing the phosphorylation of p27, PRAS40, and raf-1. Additionally, the combination therapy attenuated the phosphorylation of AKT, ERK1/ERK2, mTOR, and BAD, while AMPKα levels were upregulated.
2.5.14. Lymphoma
Mikinori et al. [75] studied the effect of cucurbitacin B on the primary invasive lymphoma cell lines BCBL-1, BC-1, GTO, and TY-1. Cucurbitacin B showed a dose-dependent effect on cell proliferation of PEL cell lines, which could be activated by caspase and then induce apoptosis. In addition, it can aggregate actin, inhibit p-cofilin levels, and then destroy cell shape, resulting in BBCL-1 cell stasis in the G2/M phase.
3. Research Progress of Cucurbitacin B Derivatives
The natural product exhibits good biological activity but also has certain limitations, necessitating structural modifications to enhance its therapeutic effect and overcome these limitations in order to ultimately develop a safe and more effective drug. For cucurbitacin B, it was first isolated from the bitter principle of cucurbitaceae in 1957 [76,77]. Until now, no studies on the total synthesis of cucurbitacin B have been found. The most recent study is that of Michae et al. [78], focusing on the synthesis of the cucurbitacin core by the Diels–Alder reaction, which has a guiding effect on the total synthesis of cucurbitacin B.
The high toxicity of cucurbitacin B poses a significant challenge that necessitates immediate resolution, rendering it an urgent concern in drug development. Therefore, it is imperative to design and synthesize a series of derivatives based on cucurbitacin B as a lead compound in order to elucidate its structure–activity relationship and identify compounds with reduced toxicity and enhanced anticancer efficacy. Presented herein is a review highlighting recent advancements in the synthesis of novel cucurbitacin B derivatives.
3.1. Modification and Pharmacological Activity of 2-Hydroxyl Group
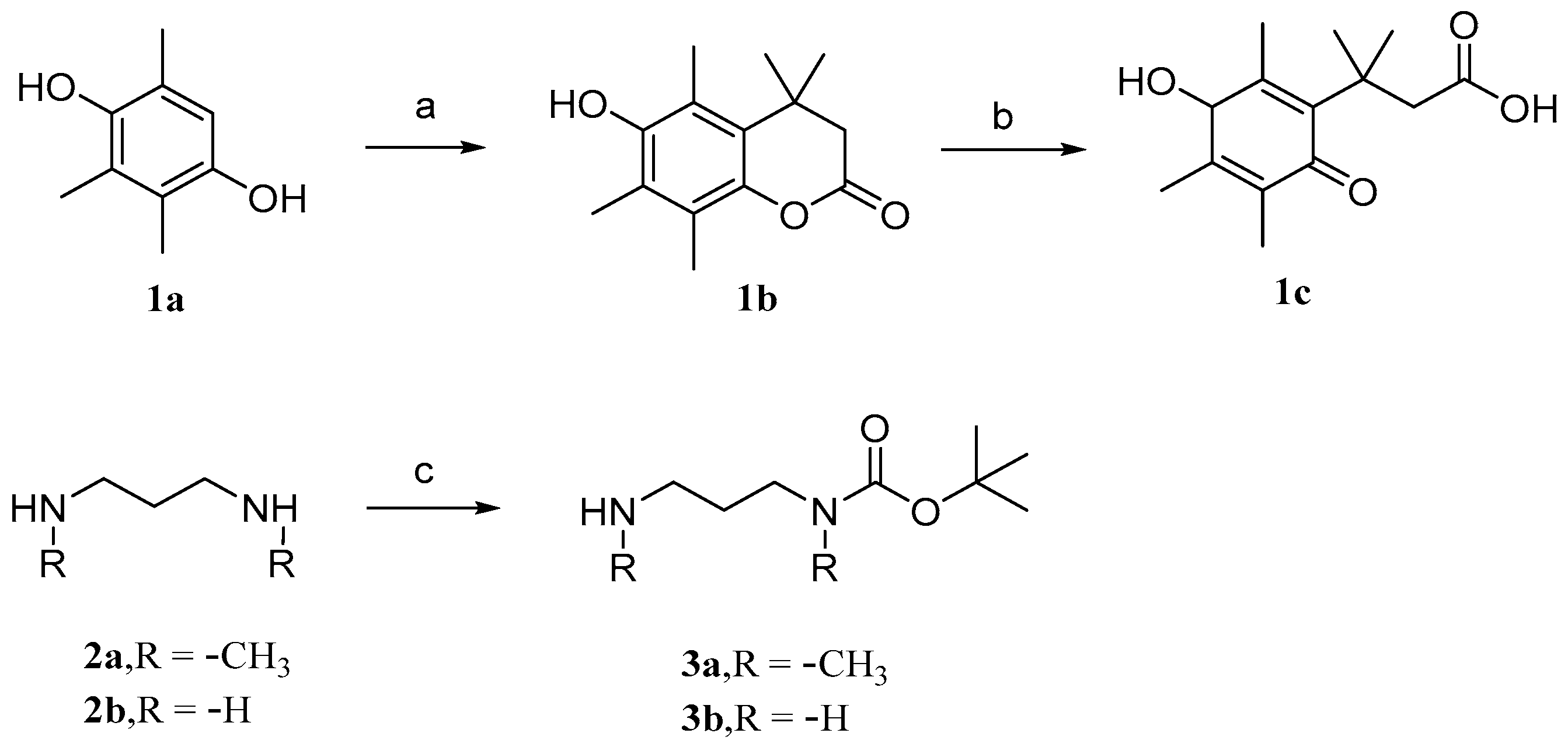
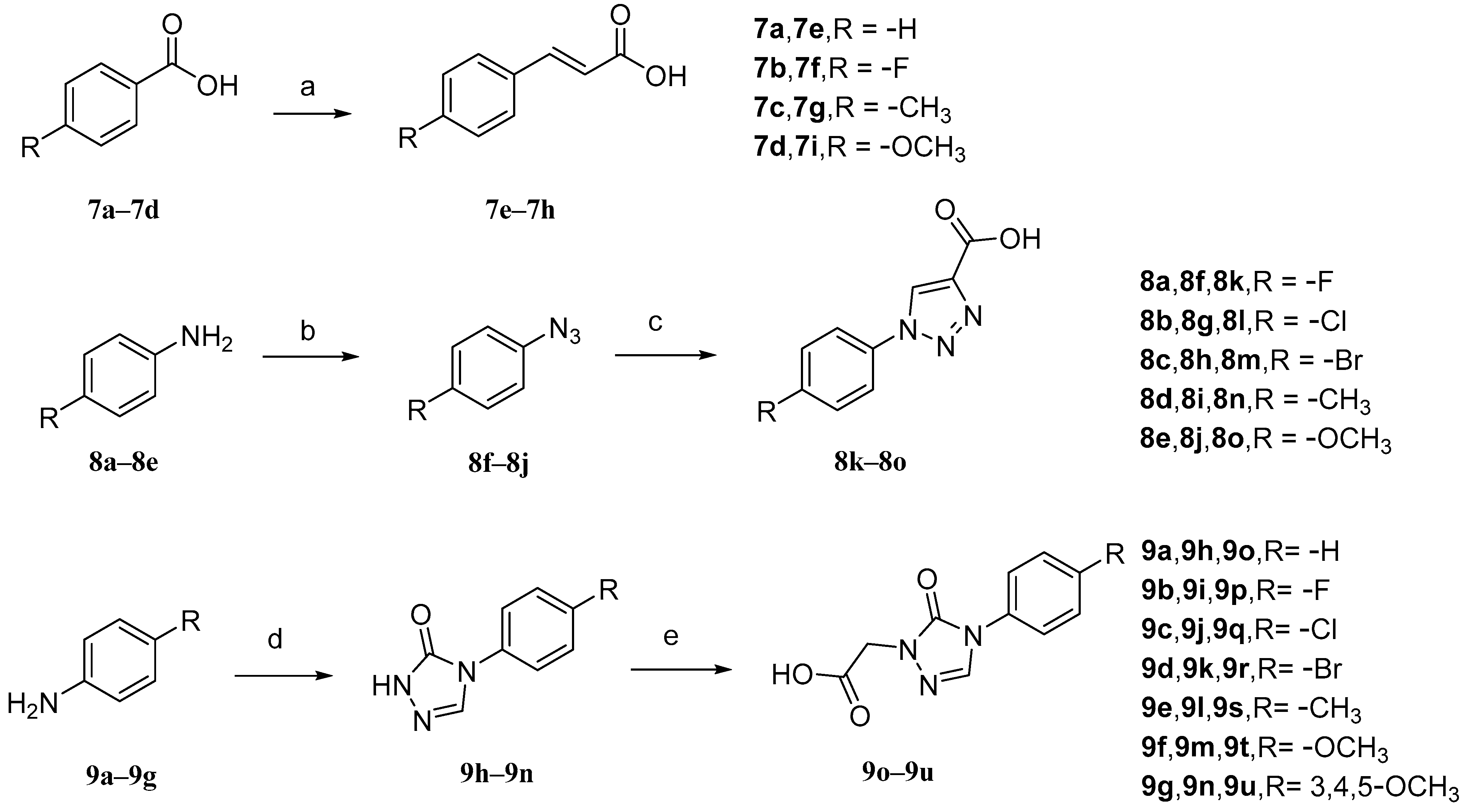
Parichat et al. [79] designed a prodrug based on cucurbitacin B to reduce its toxicity to normal cells while maintaining or enhancing its anticancer activity. Figure 6 and Figure 7 depict the synthetic pathways for compounds 1, 2, and 3. Firstly, trimethylhydroquinone reacts with 3,3-dimethacrylic acid in the presence of anhydrous methanesulfonic acid at a controlled temperature of 70 °C. Then, it is treated with N-bromosuccinimide (NBS) in an acetone aqueous solution to obtain compound 1c. In DCM, the addition of dibutyl dicarbonate (Boc2O) to 1,3-diaminopropane produces monoBOC-protected intermediates 3a and 3b.
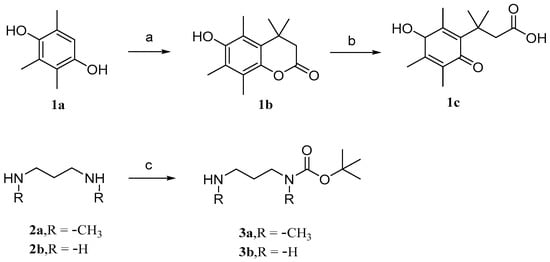
Figure 6.
Synthetic routes of compounds 1a–1c, 2a–2b, and 3a–3b. Reagents and conditions: (a) 3,3-dimethylacrylic acid, CH3SO3H, 70 °C; (b) NBS, acetone, H2O; (c) Boc2O, DCM.
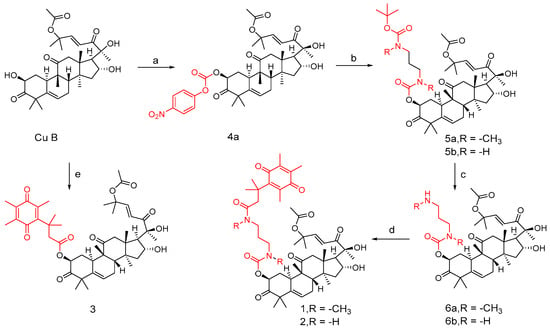
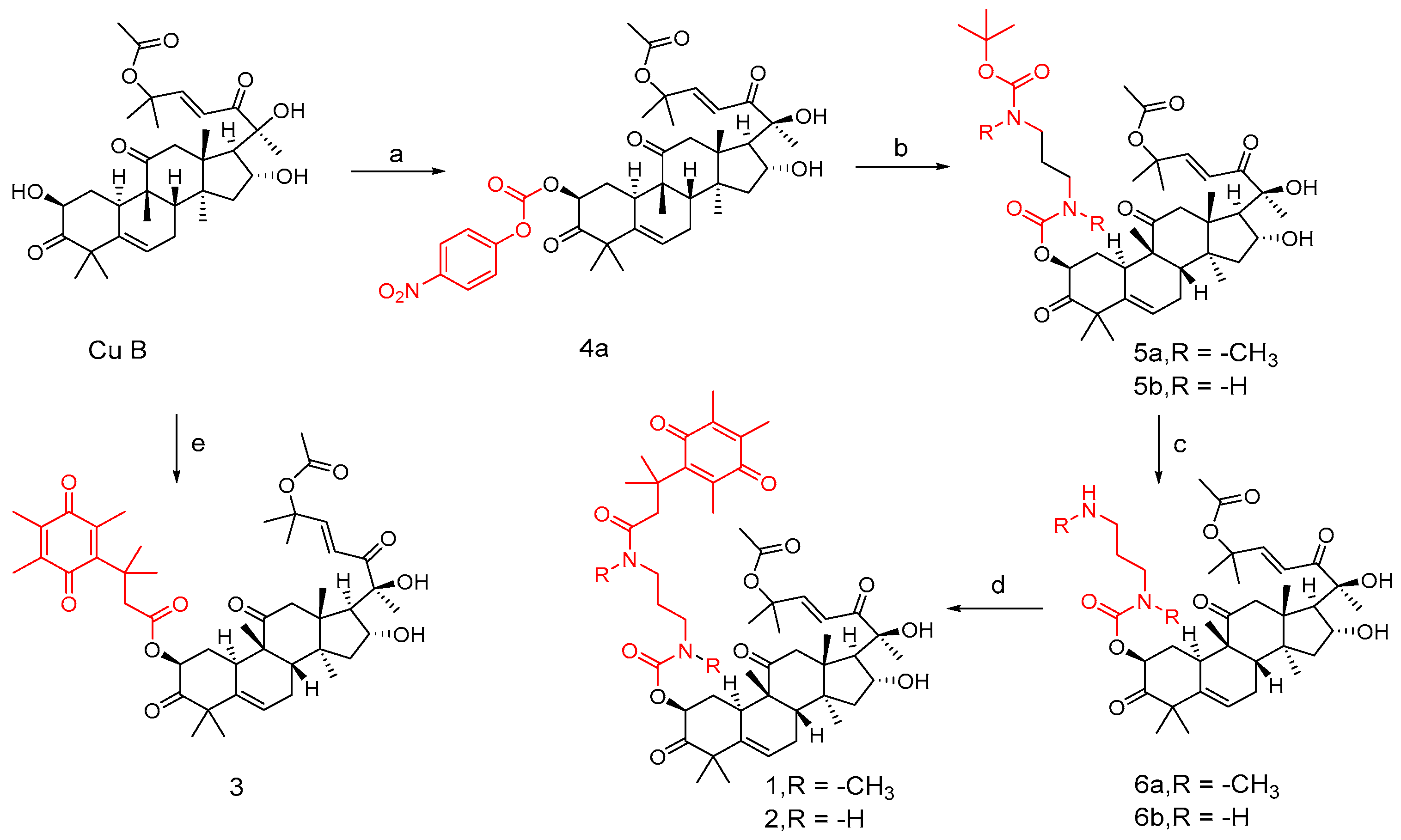
Figure 7.
Synthetic routes of compounds 1, 2, and 3. Reagents and conditions: (a) 4-nitrophenyl chloroformate, Et3N, DCM, 0 °C; (b) compounds 3a and 3b, Et3N, DCM; (c) 10%TFA, DCM; (d) compound 1c, EDCI, DMAP, DCM; (e) 1c, EDCI, DMAP, DCM.
In DCM, cucurbitacin B was added to triethylamine and 4-nitrophenylchloroformate at 0 °C to obtain compound 4a. Similarly, compounds 3a and 3b were obtained by adding triethylamine to cucurbitacin B. To obtain intermediates 5a and 5b, add them in the same way as before. Add 10% TFA to solutions of compounds 5a and 5b in DCM medium to obtain intermediates 6a and 6b. Compounds 1 and 2 can be obtained by adding compound 1c, EDC, and DMAP to intermediates 6a and 6b, respectively. Similarly, compound 3 can be obtained by adding the mixed system to cucurbitacin B.
To evaluate the cytotoxicity of cucurbitacin B and compounds 1, 2, and 3 against breast cancer (MCF-7) and non-cancer Vero (African green monkey kidney) cells. Tamoxifen (TAM), a known anticancer drug, was used as a positive control (Table 1). Cucurbitacin B itself exhibited good activity against MCF-7 cells (IC50 = 12.0 µM), while compounds 1, 2, and 3 demonstrated IC50 values of 18.1, 15.4, and 16.6 µM, respectively, indicating better cytotoxicity against MCF-7 cells compared to TAM. Cucurbitine B exhibited high toxicity towards non-cancerous Vero cells (IC50 = 0.04 µM), while compounds 1, 2, and 3 showed low cytotoxic activity against Vero cells, being 310, 47, and 2 times less toxic than cucurbitine B, respectively [79]. To some extent, the introduction of quinone fragments at the 2-hydroxyl group of cucurbitacin B adversely affects its anti-tumor activity.

Table 1.
Cytotoxicity of cucurbitacin B and compounds 1, 2, and 3 against MCF-7 and Vero cells.
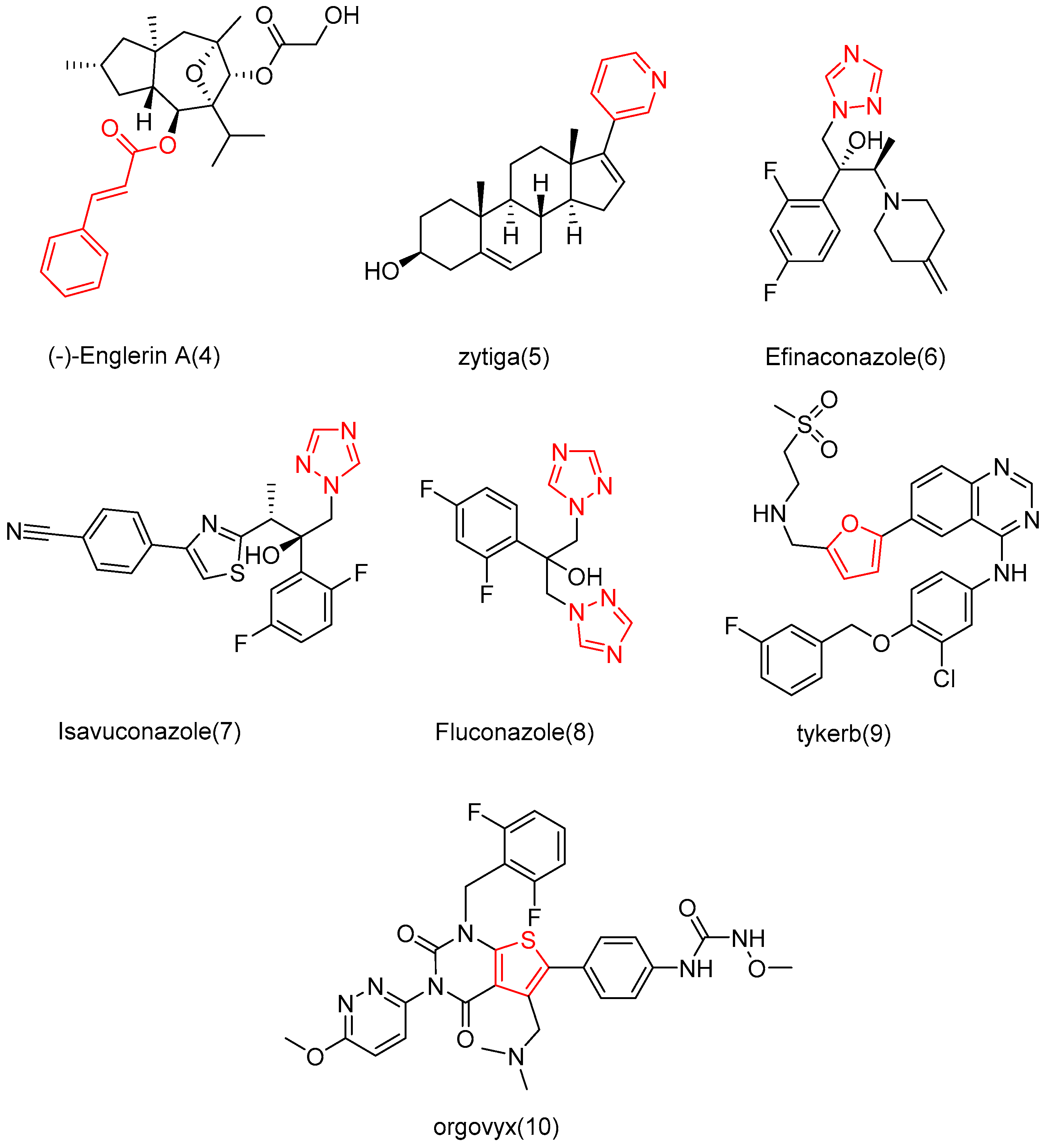
Drugs are often designed and synthesized using cinnamic acid, pyridine/furan, and triazolyl [80,81,82,83]. For example, cinnamic acid is found in many active molecules with good efficacy, such as (−)-Englerin A (4) and zytiga (5), which contains the pyridine part, and has been approved for marketing by the FDA. In addition, triazole has been successfully applied in the development of Efinaconazole (6), Isavuconazole (7), Fluconazole (8), Tykerb (9), and Orgovyx (10), which contain furan or thiophene fragments (Figure 8). Since these fragments are commonly used in drug research, Shang et al. [84] utilized them to modify the 2-site OH of cucurbitacin B with the hope of enhancing anti-tumor activity and reducing cytotoxicity.
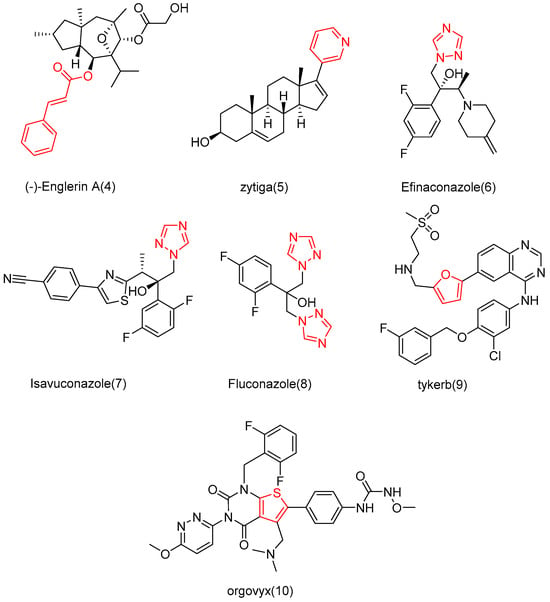
Figure 8.
Chemical structure of marketed drugs.
The intermediates 7e–7h (Figure 9) were initially prepared. Malonic acid and pyridine were added to benzaldehyde (7a–7d) with different para-substituents, and the solvent used was DMF. The reaction temperature was maintained at 90 °C. Intermediates 8k–8o, aniline with different para-substituents (8a–8e), NaNO2, 10% hydrochloric acid, and solvent (H2O) were used. Sodium azide was added after the reaction for 30 min to obtain intermediates 8f–8j. Then, sodium ascorbate, propionic acid, and copper sulfate pentahydrate were added. The solvents that were used were n-BuOH and H2O. Intermediates 9o–9u, aniline with various substituents (9a–9g), triethyl orthoacetate, methyl hydrazyl formate, and sodium methanol were added to MeOH to form 9h–9n. Then, chloroacetic acid and K2CO3 were added to react with 9h–9n in acetonitrile at a controlled temperature of 60 °C.
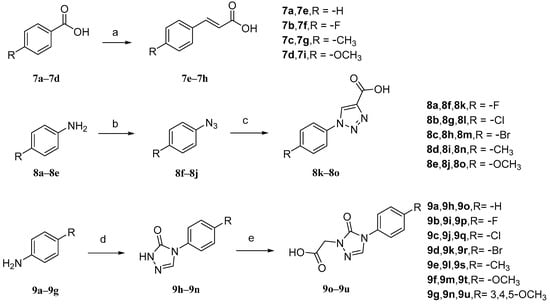
Figure 9.
Synthetic routes of intermediates 7e–7h, 8k–8o, and 9o–9u. Reagents and conditions: (a) malonic acid, pyridine, DMF, 90 °C, 6 h; (b) (i) 10% HCl, NaNO2, H2O, 0–5 °C, 30 min; (ii) NaN3, H2O, 0–5 °C, 2–4 h; (c) propiolic acid, L-ascorbic acid sodium salt, CuSO4·5H2O, n-BuOH/H2O, r.t., 24 h; (d) triethyl orthoacetate, methyl carbazate, methanol, 78 °C, reflux, CH3ONa; (e) chloroacetic acid, CH3CN, K2CO3, 60 °C.
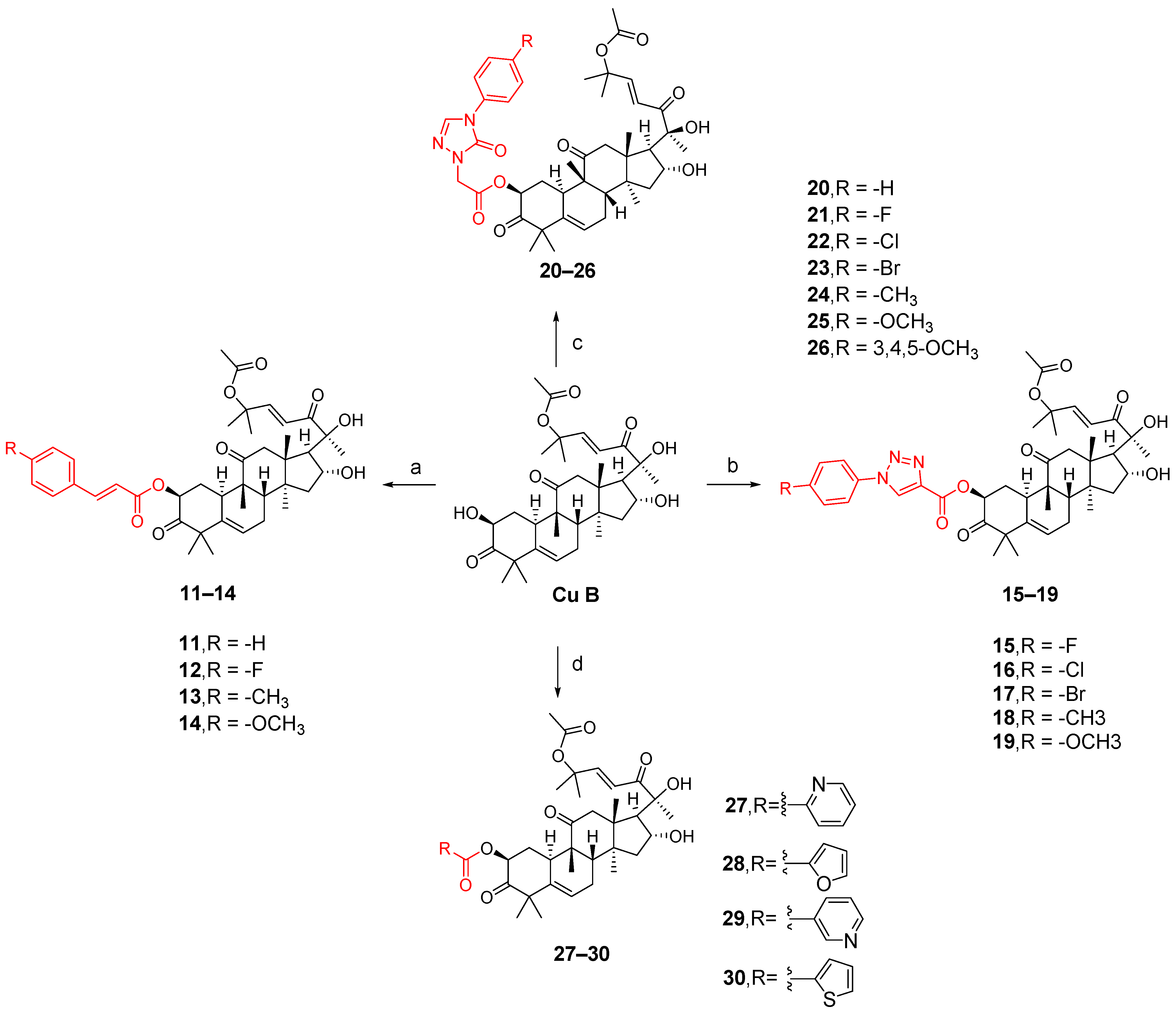
The synthesis of compounds 11–14 involved the addition of 1,2-dichloroethane (DCE), EDCI, DMAP, and cucurbitacin B to intermediates 7e–7h for reaction at a temperature of 37 °C. Compounds 15–19 were synthesized by adding EDCI, DMAP, cucurbitacin B, and intermediates 8k–8o to DCE at a temperature of 60 °C. For compounds 20–26, all other conditions remained the same except that the temperature was controlled at 80 °C. Compounds 27–30 involved reacting four carboxylic acid intermediates (2-pyridinic acid, 2-furanoic acid, niacin or 2-thiophenic carboxylic acid) with cucurbitinol B. All other conditions remained the same, but the reaction took place at a temperature of 65 °C (Figure 10). Except for compound 18, compounds 15 and 20–27 exhibited comparable or slightly reduced anti-proliferative activity against HCT-116 (Table 2). Furthermore, these compounds demonstrated a weak inhibitory effect on the growth of normal L02 cells, indicating their good safety profile. Compound 21 exhibited the highest selectivity index for anti-proliferative activity, with an SI value of 2.94, which was five times higher than that of cucurbitacin B. To further investigate the anticancer potential of compound 21, ten different cancer cell lines, including A549, HCT116, MDA-MB-231, MCF-7, SK-OV-3, HeLa, HepG-2, BXPC-3,PANC-1, and CFPAC-1, were tested. As shown in Table 3, compound 21 demonstrated comparable or slightly improved anticancer activity compared to cucurbitacin B while exhibiting superior tumor specificity. Among these cancer cells, A549 was the most sensitive to compound 21, with an IC50 value of 0.009 μM and an SI value of 11.11, which was superior to cucurbitacin B, suggesting that compound 21 has potential for cancer treatment [84].
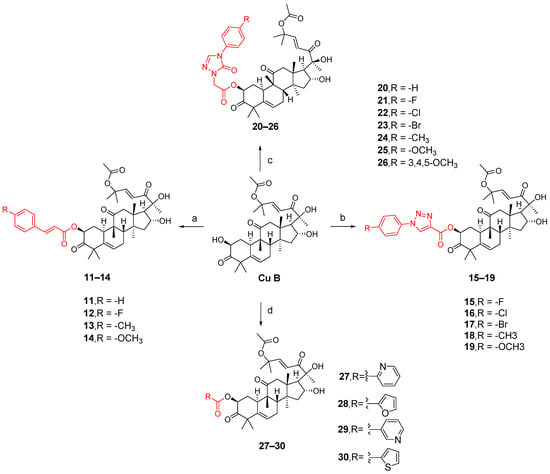
Figure 10.
Synthetic routes of compounds 11–30. Reagents and conditions: (a) 1,2-dichloroethane, 7e–7h, EDCI, DMAP, 37 °C, 16 h; (b) 1,2-dichloroethane, 8k–8o, EDCI, DMAP, 60 °C, 20 h; (c) 1,2-dichloroethane, 9o–9u, EDCI, DMAP, 80 °C, 24 h; (d) 1,2-dichloroethane, 0.5 equiv 2-picolinic acid, 2-furoic acid, nicotinic acid or 2-thiophene carboxylic acid, EDCI, DMAP, 65 °C, 18 h.

Table 2.
Antiproliferative activity of cucurbitacin B and compounds 15 and 20–27.

Table 3.
Antiproliferative activity of compound 21 on various cell lines.
The structure–activity relationship of this series of compounds demonstrates that the activity of the 1,2,4-triazolone fragment introduced into the 2-hydroxyl group of cucurbitacin B is superior to that of cinnamic acid, 1,2,3-triazole, and other five-membered heterocyclic fragments. Furthermore, among compounds 20–26, the preliminary structure–activity relationship indicates that the introduction of stronger electron-withdrawing groups in the para-position of the phenyl group or more electron-donating groups on the phenyl group is advantageous for inhibiting the proliferation of HCT116.
3.2. Modification and Pharmacological Activity of 16-Hydroxyl Group
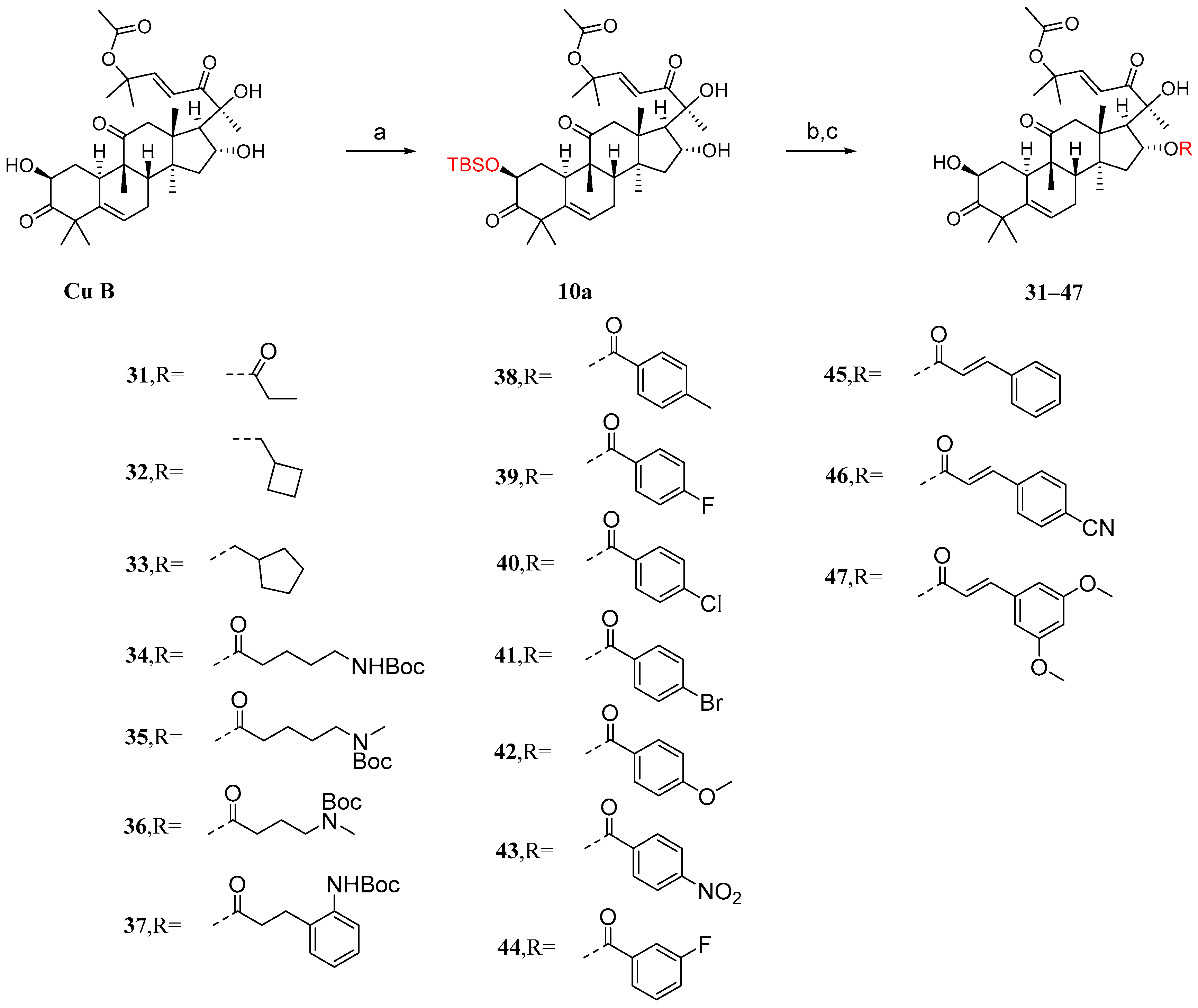
It has been reported that the introduction of an acyl group in the 2-hydroxyl moiety can significantly reduce anticancer activity [85]. Ge et al. [86] introduced acyl groups into the 16-hydroxyl segment to investigate their impact on anticancer activity. Due to the higher reactivity of the 2-hydroxyl group compared to the 16-hydroxyl group, it is easier to introduce acyl groups there. Therefore, protection of the 2-hydroxyl group is necessary. As shown in Figure 11, in the presence of imidazole, cucurbitacin B was treated with T-butyldimethylsilyl chloride (TBSCl) to obtain the TBS-protected intermediate 10a. Since the hydroxyl group of C-20 is a tertiary hydroxyl group, the steric hindrance at C-20 is higher than that at C-16, making the esterification reaction difficult. Intermediate 10a was added to a dichloromethane solution along with EDCI, DMAP, and TEA to reverse the esterification reaction using different carboxylic acids. Then, in a THF solution, TBAF or acetic acid was added to remove the TBS protection. This resulted in compounds 31–47 with yields ranging from 48% to 86%.
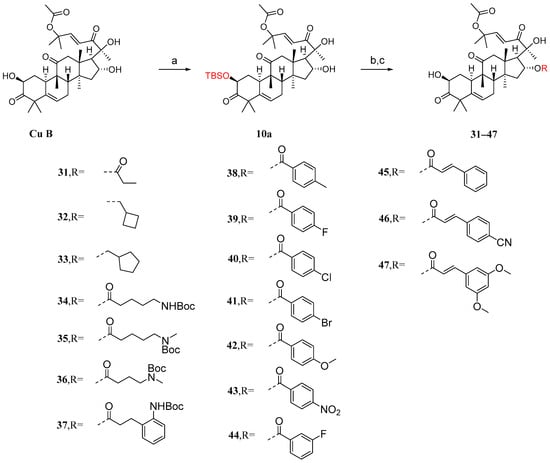
Figure 11.
Synthetic routes of compounds 31–47. Reagents and conditions: (a) TBSCl, imidazole, DCM; (b) RCOOH, EDCI, TEA, DMAP, DCM; (c) TBAF/AcOH, THF.
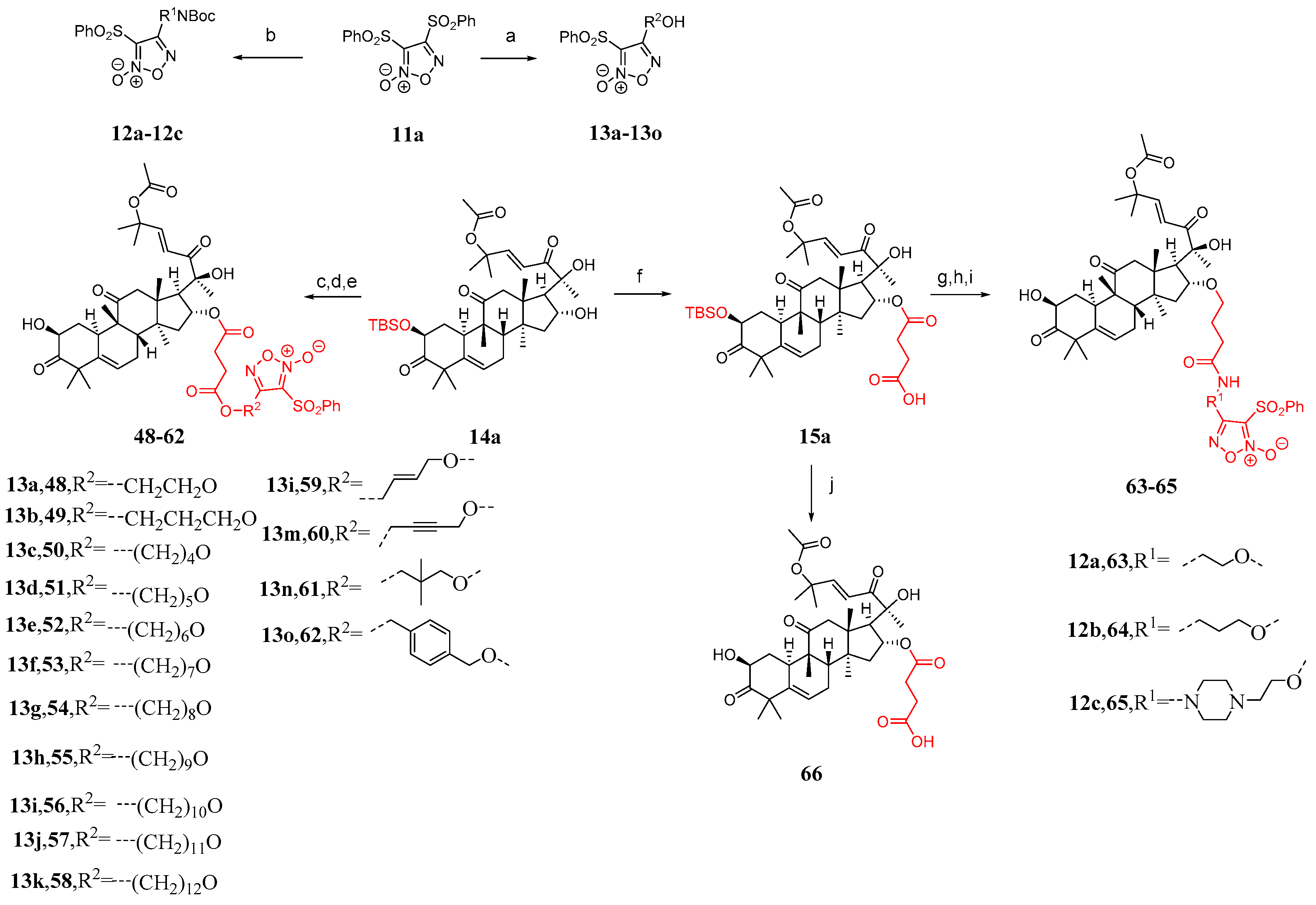
Some studies have shown that the toxicity of bruceopitrol can be reduced by introducing a phenylsulfonyl-substituted furacil NO release fragment [87,88,89,90,91]. Therefore, Ge et al. [86] designed 18 cucurbitacin B derivatives (Figure 12) and introduced phenylsulfonyl-substituted furapyridine NO-releasing fragments. Compound 11a reacted with different diols or alkanols to yield 12a–12c or 13a–13o, respectively. The desired carboxylic acid was synthesized by adding DMAP and succinic anhydride to compounds 13a–13o, followed by esterification with compound 14a and removal of the TBS group, resulting in the formation of compounds 48–62 in two steps. Compound 15a was obtained from the reaction between compound 14a and succinic anhydride. Compounds 2a–2c were esterified with compounds 12a–12c by removing the Boc group using TFA, followed by the addition of HATU and DIPEA and the removal of the TBS group to yield compounds 63–65. Compound 66 was obtained by directly removing the TBS group from compound 15a.
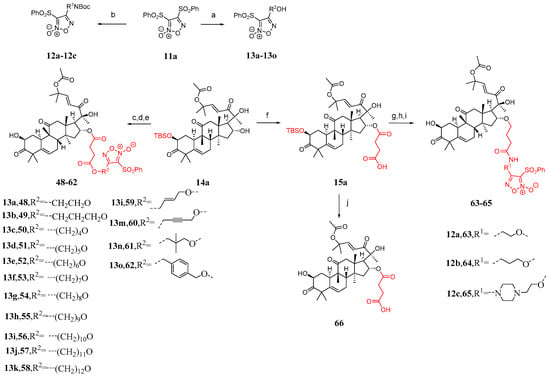
Figure 12.
Synthetic routes of compounds 48–66. Reagents and conditions: (a) corresponding alcohol amine, NaOH, THF; (b) corresponding diol, NaOH, THF; (c) 13a–13o, succinic anhydride, DMAP, DCM; (d) EDCI, TEA, DMAP, DCM; (e) TBAF, AcOH, THF; (f) succinic anhydride, DMAP, DCM; (g) 12a–12c, TFA, DCM; (h) HATU, DIPEA, DMF; (i) TBAF, AcOH, THF; (j) TBAF, AcOH, THF.
The activity against the proliferation of the human hepatocellular carcinoma cell line HepG2 and the normal hepatocellular cell line L02 was evaluated [85]. Cucurbitacin B exhibits good activity against HepG2 cells, but its high toxicity results in a low therapeutic index (TI). The compounds in the 31–47 subseries were found to be less cytotoxic to L02 cells compared to cucurbitacin B, but their antihepatoma activity against HepG2 cells was also significantly reduced. Among the compounds in the 31–47 subseries, compound 45 exhibited the highest TI value (Table 4). Furthermore, the introduction of electron-withdrawing groups in the phenyl para-position of cinnamic acid appears to be beneficial against HepG2 proliferation; however, it simultaneously increases cytotoxicity towards L02 cells.

Table 4.
Activity and TI of cucurbitacin B derivatives 31–66 against HepG2 and L02 cell lines.
The compounds 48–62 exhibited effective activity against HepG2 cells, with a significant decrease in their cytotoxicity against L02 compared to cucurbitacin B, as shown in Table 5. Compounds 63–65 showed lower toxicity compared to cucurbitacin B, resulting in a slight increase in TI values. Compound 49 demonstrated a significantly higher TI value, showing a 14.7-fold increase compared to cucurbitacin B, suggesting its potential as a treatment for liver cancer [86]. The structure–activity relationship analysis of compounds 48–58 suggests that a carbon chain length ranging from 3 to 5 is optimal.

Table 5.
Cytotoxic activity of compounds 48–65 against HepG2 cells and L02.
3.3. Modification and Pharmacological Activity of C-25 Acetyl Group
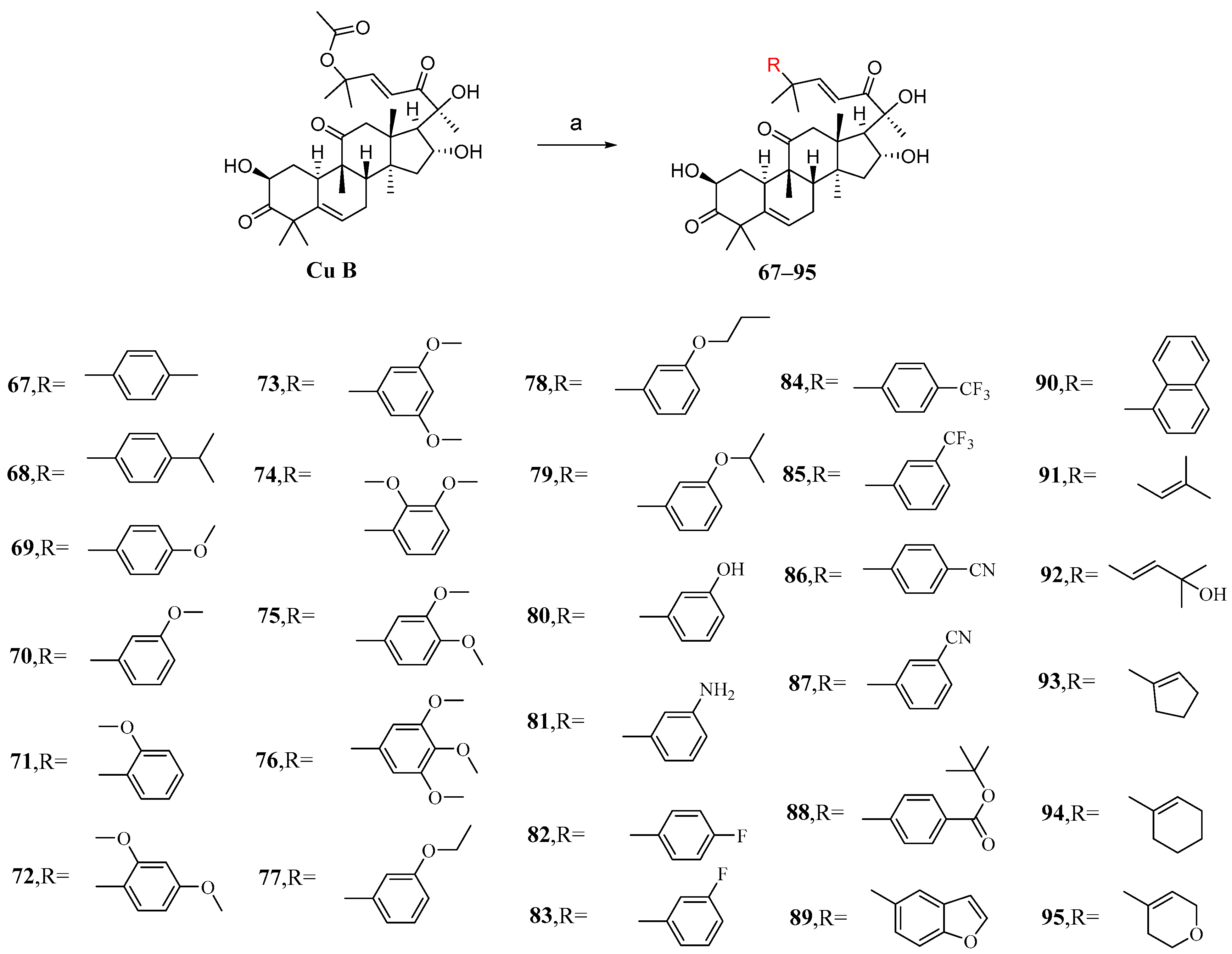
The main purpose of modifying C-25 acetoxy in Zhuo et al.’s study was to enhance the stability and pharmacokinetic properties of cucurbitacin B while maintaining or improving its antiproliferative activity [92]. After conducting numerous experiments to determine the optimal conditions, it was found that Pd2(dba)3 exhibited the highest catalytic yield as a palladium catalyst. Cucurbitacin B was introduced into a DCM solution containing KF and Pd2(dba)3 to react with various intermediates, resulting in the production of compounds 67–95 (Figure 13).
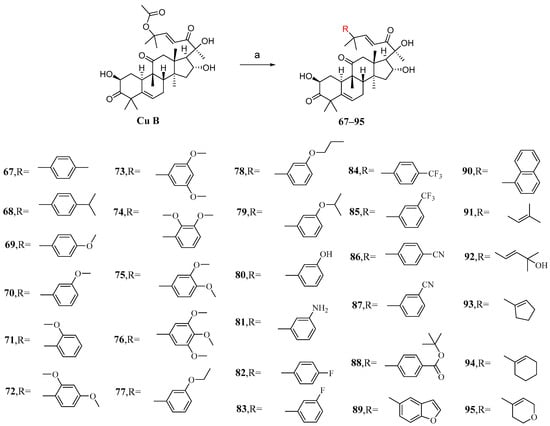
Figure 13.
Synthetic routes of compounds 67–95. Reagents and conditions: (a) RB(OH)2, KF, Pd2(dba)3, DCM, rt.
The synthesized compounds 67–95 were all evaluated in vitro for their cytotoxic activity against A549 cells. Compound 87, with an IC50 value of 6.8 nM, exhibited stronger cytotoxicity than cucurbitacin B, being twice as potent (Table 6) [92]. The structure–activity relationship reveals that the introduction of substituents with reduced steric hindrance at the meta-position of the phenyl group enhances antiproliferative activity.

Table 6.
Cytotoxic activity of compounds 67–95 against A549 cells.
3.4. Co-Modification of 2-OH and 16-OH
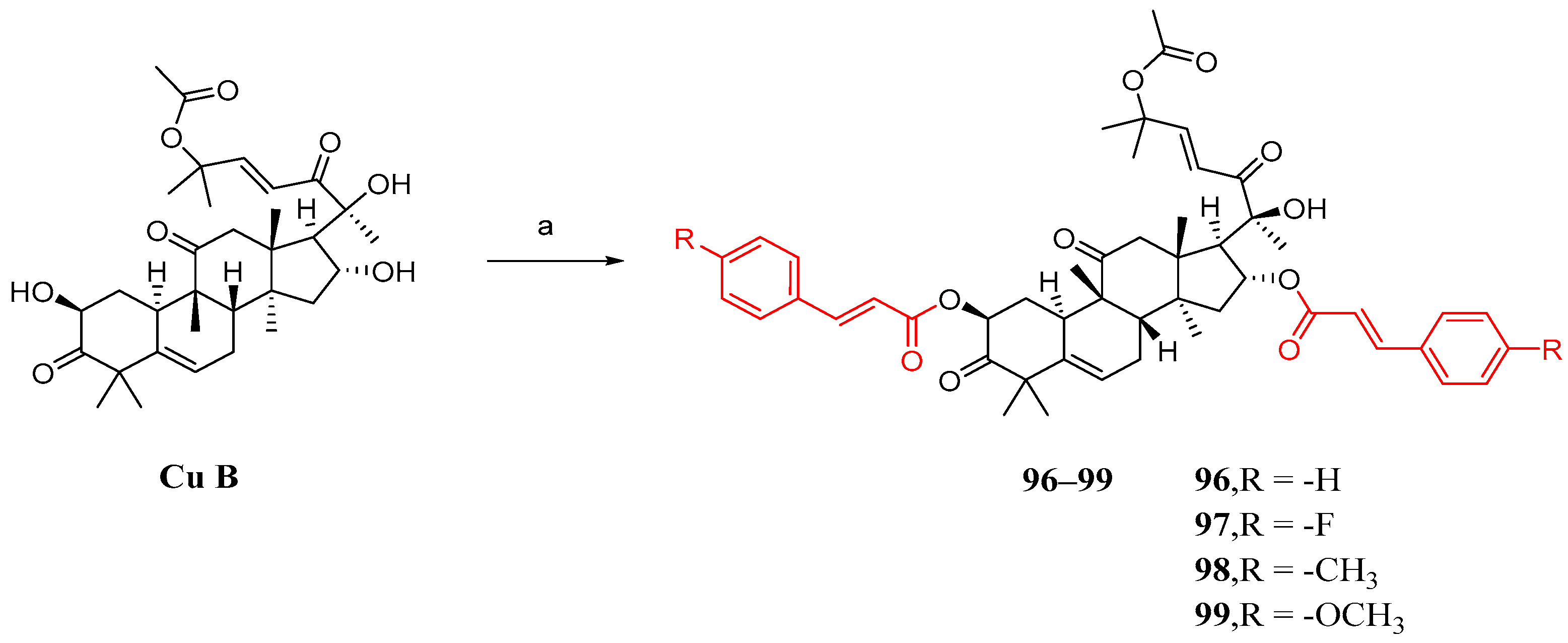
Shang et al. synthesized cucurbitacin B derivatives using the same method as described previously. However, the results indicated that the simultaneous introduction of substituted cinnamic acid fragments on both the 2 and 16 hydroxyl groups had a detrimental effect on anti-NSCLC activity (Figure 14) [84].
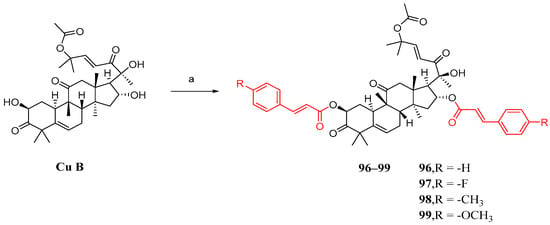
Figure 14.
Synthetic routes of compounds 96–99. Reagents and conditions: (a) 1,2-dichloroethane, 7e–7h, EDCI, DMAP, 37 °C, 16 h.
3.5. Study on Modification and Pharmacological Activity of Other Sites
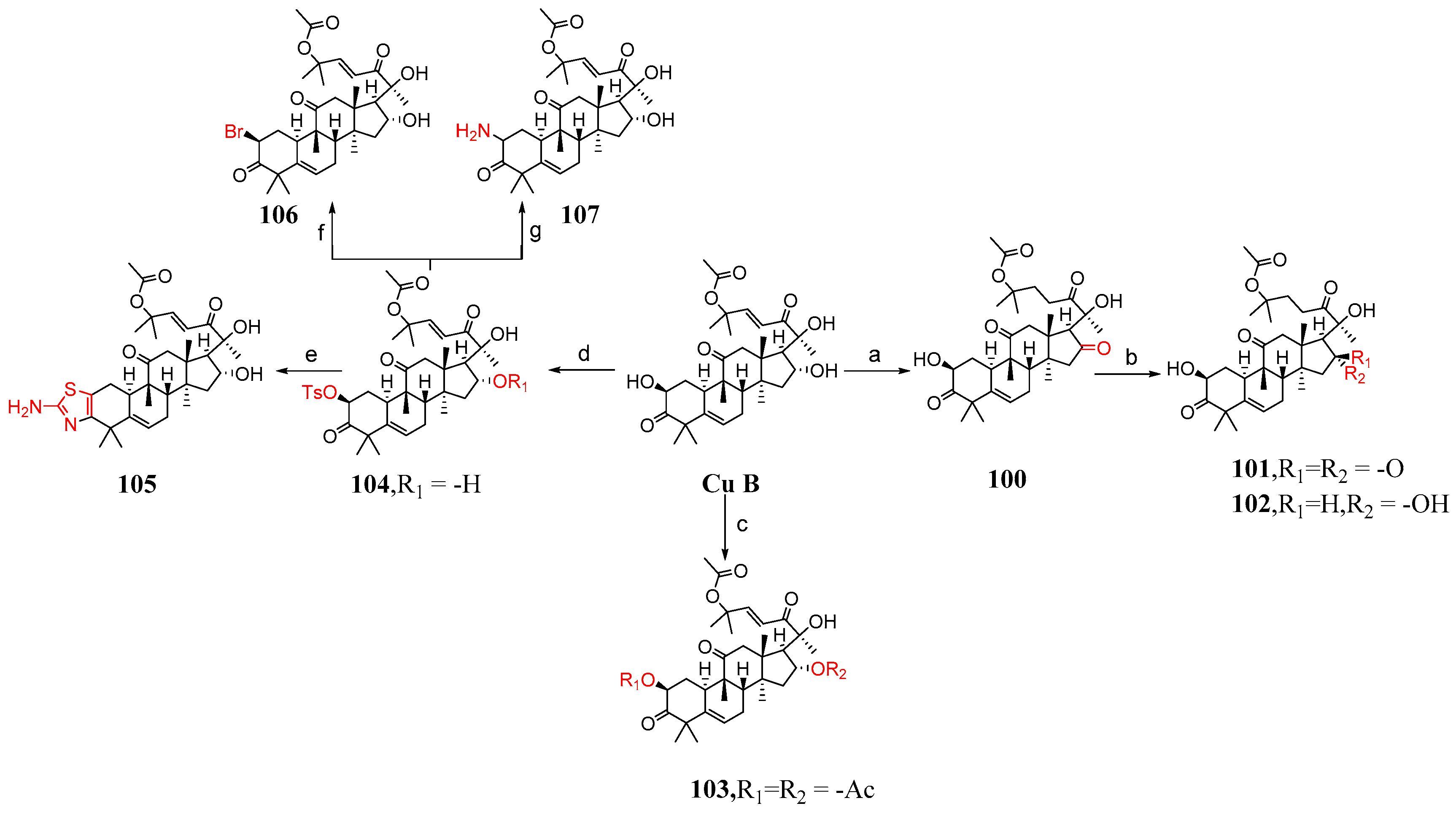
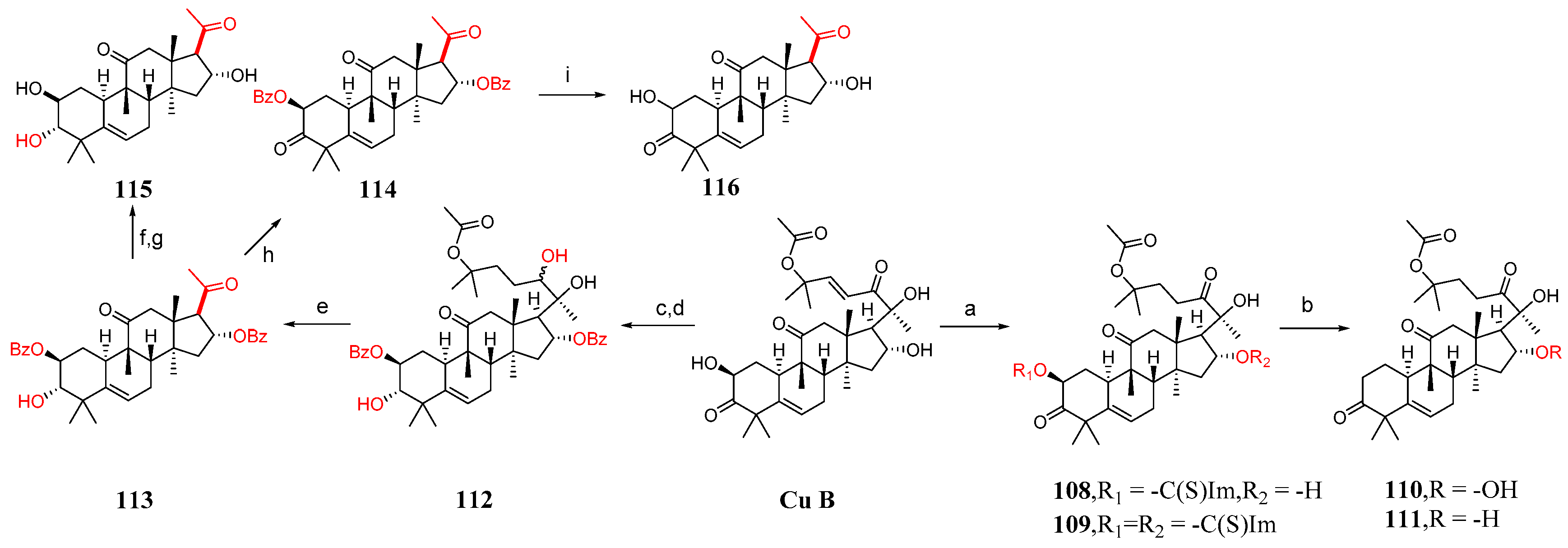
This section mainly describes C-2 and C-16 modification, A-ring modification, deoxygenation reactions, and side chain cleavage. Karen et al. [85] synthesized compounds 100–116 by modifying cucurbitacin B (Figure 15 and Figure 16). They added BaCO3 to cucurbitacin B and PCC and then synthesized compound 100. Compound 100 was treated with KOH in a mixed solvent of MeOH and DMF to form compounds 101 and 102. The hydroxyl group of cucurbitacin B was protected by reacting it with acetic anhydride and cucurbitacin B, resulting in the formation of 103. By adding 4-toluene sulfonyl chloride to DCM, DABCO reacted with cucurbitacin B at a low temperature to produce 104 or under microwave irradiation at 100 °C to yield 105. Compound 104 underwent a reaction with (Bu)4NBr in DMF, generating compound 106, or with sodium azide in DMF, producing compound 107. Cucurbitacin B reacted with 1,1′-thiocarbonyl diimidazole and dichloroethane at 60 °C to produce compounds 108 and 109. Compounds 110 and 111 were synthesized by treating compounds 108 and 109 with diphenylsilane and peroxide lauryl alcohol in refluxing toluene. Cucurbitacin B was reacted with benzoyl chloride and pyridine in DCM, followed by reduction using tert-butyl-lithium aluminum hydride to obtain compound 112. Compound 112 was further treated with periodic acid in MeOH to yield compound 113. Subsequently, compound 113 was subjected to a reaction with triethyl orthoformate in ethylene glycol, followed by p-toluene sulfonate addition, KOH hydrolysis, and subsequent HCl treatment to afford compound 115. Additionally, compound 113 underwent a reaction with PCC and BaCO3 in DCM, resulting in the formation of compound 114, which could be obtained through the synthesis step leading to compound 115.
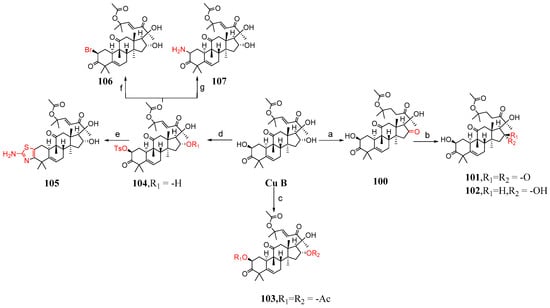
Figure 15.
Synthetic routes of compounds 100–107. Reagents and conditions: (a) PCC, BaCO3, CH2Cl2; (b) KOH, MeOH, DMF; (c) uccinic anhydride, Py, DMAP; (d) 4-toluenesulfonyl chloride, DABCO, CH2Cl2, 0 °C; (CH3CO)2O, Py, DMAP; (e) SC(NH2)2, EtOH, MW, 100 °C; (f) (Bu)4N−Br+, DMF; (g) NaN3, DMF, 70 °C.
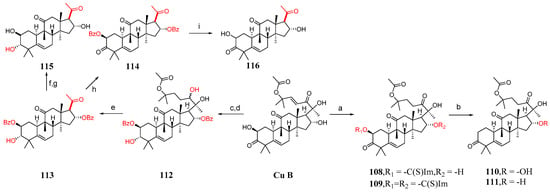
Figure 16.
Synthetic routes of compounds 108–116. Reagents and conditions: (a) 1,1′-thiocarbonyldiimidazol, Et2Cl2, 60 °C; (b) [CH3(CH2)10CO]2O2, Ph2SiH2, toluene, 115 °C; (c) C6H5COCl, Py, CH2Cl2; (d) C12H28AlO3Li, THF, 0 °C; (e) H5IO6, MeOH; (f) PCC, BaCO3, CH2Cl2; (g) ethylene glycol, HC(OEt)3, TsOH; (h) PCC, BaCO3, CH2Cl2; (i) ethylene glycol, HC(OEt)3, TsOH, KOH, MeOH, HCl, Et2O.
Compounds 104, 107, 108, and 110 demonstrated moderate activity against A549 tumor cells. Notably, compounds 107 and 108 were 100 times more potent than cucurbitacin B (Table 7). To some extent, substituting the 2-hydroxyl group of cucurbitacin B with different bioisosteres might potentially result in improved anti-tumor compounds.

Table 7.
Inhibitory effect of cucurbitacin B analogues on the proliferation of A549 cells.
4. Studies on Cucurbitacin B Preparations
Based on the unique activity of natural products, more and more natural products have been discovered and applied, and any drug must be prepared into a suitable application form before clinical use in order to solve the problems of achieving the best therapeutic effect of drugs, reducing adverse reactions, making different patients more convenient to use and accept, facilitating the carrying and transportation of drugs, etc. More and more new drug preparations appear in the public field of vision, such as microemulsions, solid liposomes, micelles, and so on [93,94,95].
Tian et al. [96] studied cucurbitacin B microemulsions (CuB-ME). An o/w CuB ME was prepared, and the CUP-ME formula was optimized, with azone:Tween 80:ethanol:water = 2.5:16.9:5.6:75.0 (w/w), where the nitrogen ketone can increase the solubility of CuB and promote the amount of skin permeability. While the water affects the particle size and permeability coefficient of CUP-ME, hydration can also promote the penetration of drugs. In addition, pharmacodynamic studies and irritant test results showed efficacy and safety. In conclusion, CuB-ME in this study can improve patient compliance and characterize the skin drug delivery process promoted by CUB-ME at the molecular level, which provides a reference value for the application of cucurbitacin B in the transdermal drug delivery system.
Wu et al. [97] prepared CuB-SD with a weight ratio of 1:7 by following the solvent method using PLX-407 as the carrier. After the dissolution test and pharmacokinetic study, it can be proved that solid dispersion technology can significantly improve the solubility and oral bioavailability of CuB, which can improve patient compliance.
Lv et al. [98], using carboxymethyl chitosan (CCS) as a bioadhesive polymer, glycerin as a plasticizer, and phospholipid–bile salt mixed micelles (PL-BS-MMs) as nanoscale carriers, developed an oral delivery system for muco-adhesive cucurbitacin B. After optimization, it has good physical properties and release characteristics in vitro. It can be proved by oral administration experiments in rabbits that a nano-scale chitosan film preparation can replace the oral dosage form because of its good pharmacokinetic characteristics. This oral mucosal adhesive membrane may provide an alternative route for the safe delivery of cucurbitacin B with better patient compliance and higher bioavailability.
Hu et al. [99] prepared solid lipid nanoparticles (Cu-BSLNs) loaded with cucurbitacin B by emulsification ultrasound. It was found that Cu-B SLNs can passively target tumors with the EPR effect and show higher accumulation in tumor interstitial space, which can improve the efficacy of cucurbitacin B and reduce the dose. This suggests that SLNs can increase the passive targeted delivery of drugs and reduce the dose and toxicity, which can improve the efficacy of insoluble drugs.
Chen et al. [100] conjured two cucurbitacin B molecules with diselenide bonds to form a dimeric prodrug, which was then assembled with FA-PEG-DSPE to finally produce an FA-Se-Se-NP nanomedicine. Diselenide bonds and CuB work synergistically to amplify oxidative stress and kill cancer triple-negative (TNBC) cells. FA-Se-Se-NPs have a longer blood circulation time and can also selectively accumulate in tumor tissue through passive targeting mediated by EPR effects. In an environment of high intracellular ROS and GSH levels, diselenide bonds can break and consume GSH, produce ROS, release CuB, and then produce ROS, amplify intracellular oxidative stress, and kill cancer cells. This new approach provides a promising strategy for cancer therapy, with significant effects on TNBC in vivo and in vitro.
Tang et al. [101] used ion exchange resins to simply isolate collagen cationic CPs from bovine collagen peptide (CPs) to modify surface mixed nanomicelles (MM) and obtained CPS-modified CuB mixed nanomicelles (CUB-MS-CPS). In vitro, CPS-modified nanomicelles can lead to a significant increase in cell uptake and transport. Cub-ms-cps can enhance the oral absorption of CuB. Therefore, the use of CPs as a carrier may provide an effective method for oral drug delivery, which may help to address the problem of drug diffusion and penetration barriers in the intestine.
5. Research Progress on Cucurbitacin B in Agronomy
Nowadays, the existence of pests has made the economic situation of agriculture worse, and traditional synthetic chemical insecticides are still used to control pests, but these pesticides have caused damage to human health and the ecological environment, in addition to causing pest resistance [102,103,104]. CuB is the main secondary metabolite of the host plant calabash. It is easily extracted and isolated from plants such as calabash and is toxic to some arthropods [105,106,107,108]. A previous study showed that high concentrations of CuB can increase aphid mortality and decrease their fitness [109]. Therefore, we need to reduce the use of chemical pesticides and provide new ideas for the synthesis of novel biopesticides.
The survival and reproduction of animals need the help of taste. Grs is the first taste receptor found in the genome of fruit flies [110]. Suman et al. [111] conducted an experiment of the genetic screening method for electrophysiological examination, and the results show that flies have food resistance to Cucc-B, which is mediated by GR33a, and Cucc-B has an insecticidal effect. It can be proved that GR33a is the molecular sensor required for Cucc-B taste detection (CUCC-B). Because Cucc-B is widely found in cucurbitaceae plants and because of the specific GRs, the development of new highly effective insecticides based on cucurbitacin B is of paramount importance.
Sterols play an important role in cell membrane homeostasis and also affect steroid production in animals. Insects lack the ability to synthesize sterols, so they need sterol supplements for growth and development [112,113]. Insects can synthesize the steroid hormone ecdysone from dietary cholesterol in the steroid-producing organ, the prethymus (PG) [114]. Miwako et al. [115] investigated the effects of cucurbitacin B on the development of Drosophila melanogaster. The experimental results showed that CuB could prevent the physiological process of stunting their development. CuB destroyed any function related to sterols, and ecdysone biosynthesis in PG was inhibited. CuB can be used as an inhibitor of ecdysone biosynthesis and an antagonist of ecdysone receptors. In addition, a steroid endemic to arthropods is ecdysone, the biosynthetic pathway is an ideal target for IGRs, and CuB may serve as a candidate molecule for the development of a novel IGR that inhibits ecdysone biosynthesis.
The health of plants is closely linked to the subsurface microbial ecosystem [116]. Several studies have shown that plant-specific metabolites play an important role in the formation of the rhizosphere microbiota [117]. Zhong et al. [118] found that the transport of cucurbitacin B from melon roots to soil can selectively enrich two types of bacteria (Enterobacter and Bacillus) to regulate the rhizosphere microbiota, which in turn can produce strong resistance to the fusarium pathogen. The coordinated regulation of cucurbitacin biosynthesis and transport may be a potential mechanism to balance the pathogen resistance induced by cucurbitacin and reduce excessive energy depletion in plants.
Cotton aphids, which are distributed throughout the world, pose serious economic and ecological problems due to their rapid reproduction and high resistance to pesticides [103]. The development of insecticide resistance is rapid and widespread, threatening crop productivity. In contrast, biopesticides (e.g., fungi and plant extracts) thus become a better pest control option [119,120]. Zhao et al. [121] investigated the effects of cucurbitacin B on two host biotypes, cotton- and cucurbit-specialized aphids (CO and CU), and showed that cucurbitacin B significantly reduces fitness at the population level of cotton aphids and can alter important detoxification enzyme activity. Because of this property, CuB may have the opportunity to be developed as a pesticide for aphid control in agriculture, and it is also of great significance for the control and protection of cotton aphids and other insect pests.
Laurence et al. [122] experimentally demonstrated that cucurbitacin B prevents 20-hydroxyecdysone (20E) stimulation of the hormone reactivity reporter gene and also prevents the formation of the drosophila epidermal steroid receptor Ultraspiracle 20E complex with the Hsp27 epidermal steroid response element, demonstrating that cucurbitacin is the first well-defined insect steroid hormone antagonist.
20E is an important insect steroid hormone that can bind to a homologous nuclear receptor composed of an ECdysone receptor (EcR) and supercell (USP) to activate the expression of the 20E response gene, leading to subsequent metamorphosis [123]. Zou et al. [124] conducted experiments with Drosophila Kc and silkworm Bm12 cells to screen for 20E antagonists. The experimental results showed that cucurbitacin B could antagonize EcRE activity and gene expression in Kc and Bm12 cells induced by 20E. Cucurbitacin B caused hair loss defects and death in silkworms by antagonizing 20E, as well as skin loss defects and death in Voles melanogaster and cotton bollworms. Additionally, cucurbitacin B inhibited the growth and development of insects by antagonizing 20E. This study provided a reference value for the development of insect growth regulators.
Cowpea is sensitive to nematodes. Biocult and Nemafrici-BL phytofungicides can promote plant growth and inhibit nematode population density, and they can reduce the population density of Bacillus intestinalis in root and soil. Kgabo et al. [125] conducted experiments and found that the combined application of Biocult and Nemafrici-BL have antagonistic effects on the growth of cowpea and should be used separately in the production of cowpea.
Hafiz et al. [109] studied the effects of two concentrations of cucurbitacin B on adult worms (F0) and larvae and their offspring adults (F1), and the results showed that 25 ppm of cucurbitacin B can significantly reduce the adult lifespan and fertility of the F0 and F1 generations, and 100 ppm of cucurbitacin B can reduce the life span and fertility of the F0 generation and the life span of the F1 generation. These results indicate that host plants containing high concentrations of cucurbitacin B may protect themselves from the feeding activities of melon aphids by sublethal damage to their biological adaptability.
6. Conclusions and Prospects
After years of development, natural products are still a valuable source of drug discovery. Over the past decade, cucurbitacin B has attracted a great deal of interest as a natural product commonly used in traditional herbal medicines because of its antioxidant, anti-inflammatory, neuroprotective, and anticancer potential. Numerous studies have demonstrated that cucurbitacin B can regulate the cell cycle of various cancer cells through multifunctional pathways, induce autophagy and apoptosis, and exhibit significant anticancer effects in vitro and in vivo, thus attracting considerable attention. A variety of potential targets and signaling pathways related to cucurbitacin B and its derivatives have been identified, and a large number of cucurbitacin B derivatives with different modifications have appeared in front of the world, hoping to find more effective and safer drugs for cancer chemotherapy. It is exciting that in 2022, the research team found a direct action target IGF2BP1 in liver cancer cells, which will provide a feasible reference value for the development and application of cucurbitacin B. However, in order to advance cucurbitacin B and its derivatives into a viable treatment, there are still several questions and new directions for future development to be considered [15].
Firstly, this natural product has received considerable attention in the past, but the exact molecular mechanism of its involvement in the treatment of cancer and other diseases still needs to be further elucidated [126]. In the last decade or so, network pharmacology has been widely used in drug discovery to identify potential molecular mechanisms and therapeutic properties [127]. Some related studies have inspired investigators to identify new and unexplored targets for cucurbitacin B and its derivatives, such as one study demonstrating that cucurbitacin B can inhibit inflammation by inhibiting the IL-6/STAT3/HIF-1α signaling pathway through network pharmacology and in vivo and in vitro experiments [128]. Thus, it has potential protective and therapeutic effects on cholestatic liver injury (CLI), providing a reference value for future drug development and utilization.
Secondly, new drug delivery systems can be developed, which can improve the water solubility of many drugs. This strategy may also improve the water solubility of cucurbitacin B and its derivatives. A preparation of solid liposome nanoparticles of cucurbitacin B created by emulsifying ultrasonic hair increased its activity and decreased its toxicity and dose. Exploring novel drug delivery formulations containing nanosuspensions, nanogels, nanoparticles, micelles, and self-microemulsion systems will provide water-soluble, tumor-targeting, and potent cucurbitinoid Class B drugs for potential clinical applications [129,130,131,132,133].
Thirdly, compared with traditional pesticides, the impact of biopesticides is more moderate. Given the favorable conditions of cucurbitacin B in agriculture, there may be opportunities for it to be developed as a pesticide for agricultural pest control, which is also of great significance for the control and protection of related pests.
Finally, in a large amount of research in the literature, the design and synthesis of cucurbitacin B derivatives have mainly been tested against various cancer cells. In fact, many natural products are multifunctional agents that simultaneously offer opportunities for drug discovery in different therapeutic areas. For example, oleanane triterpene derivative CDDO-Me, which has strong antioxidant, anti-inflammatory, and cytoprotective effects in the low nanomolar range, has significant anti-proliferation and pro-apoptotic effects at relatively high concentrations [134,135]. Based on the multifunctional effects of CDDO-Me, several drugs have been tested in clinical trials, such as chronic kidney disease due to secondary diabetes (stage II), pulmonary hypertension (stage III), and advanced malignant melanoma (stage II). For the above studies, we should also pay attention to exploring other effects of cucurbitacin B, including anti-human immunodeficiency virus activity, antioxidant, blood pressure lowering, cardiac protection, and anti-cryptosporidium generated by cucurbitacin B and its derivatives [136,137,138,139].
As a naturally occurring compound, cucurbitacin B is widely distributed in nature and readily accessible. It serves as a crucial natural product platform for the development of drugs targeting cancer, inflammation, and other diseases. This paper aims to contribute to the research on cucurbitacin B by providing constructive suggestions and opinions for further investigation of its derivatives, thereby accelerating their development and application.
Author Contributions
Conceptualization and review methodologies—W.N. and Y.W. Original draft writing—W.N. and T.L. Editing—W.N., Y.W., X.T., J.L., Z.J., J.X., M.H., Q.S., H.G. and T.L. Figure creation—W.N., T.L. and H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (No. 82060628); Jilin Province Science and technology Department key research and development project (No. 20240305099YY); Jilin Provincial Scientific Technological Development Program (No. YDZJ202301ZYTS440); Scientific Research Project of the Department of Education of Liaoning Province (No. LJKMZ20221801); and Shenyang Medical College student research project (No. 20249089).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.; Wen, H.; Zhu, J.; Deng, H.; Jiang, X.; Ye, X.-Y.; Wang, L.; Xie, T.; Bai, R. Discovery of plant-derived anti-tumor natural products: Potential leads for anti-tumor drug discovery. Bioorganic Chem. 2024, 142, 106957. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.; Khaza’ai, H.; Aljaberi, M.A.; Shamsudin, M.N.; Idrus, Z. Safety and Neuroprotective Efficacy of Palm oil and Tocotrienol-Rich Fraction from Palm oil: A Systematic Review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.-R.; Jia, L.-Q.; Lei, M.; Gao, D.; Zhang, N.; Sha, L.; Liu, X.-H.; Liu, Y.-D. Natural products as pharmacological modulators of mitochondrial dysfunctions for the treatment of diabetes and its complications: An update since 2010. Pharmacol. Res. 2024, 200, 107054. [Google Scholar] [CrossRef]
- Huang, X.; Deng, H.; Shen, Q.-K.; Quan, Z.-S. Tanshinone IIA: Pharmacology, Total Synthesis, and Progress in Structure-modifications. Curr. Med. Chem. 2022, 29, 1959–1989. [Google Scholar] [CrossRef]
- Luo, Z.; Yin, F.; Wang, X.; Kong, L. Progress in approved drugs from natural product resources. Chin. J. Nat. Med. 2024, 22, 195–211. [Google Scholar] [CrossRef]
- Nabi, N.; Singh, S.; Saffeullah, P. An updated review on distribution, biosynthesis and pharmacological effects of artemisinin: A wonder drug. Phytochemistry 2023, 214, 113798. [Google Scholar] [CrossRef]
- Ghiulai, R.; Rosca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macasoi, I.; Olariu, T.; Dehelean, C.; Cretu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Fernandez-Aparicio, A.; Correa-Rodriguez, M.; Castellano, J.M.; Schmidt-RioValle, J.; Perona, J.S.; Gonzalez-Jimenez, E. Potential Molecular Targets of Oleanolic Acid in Insulin Resistance and Underlying Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1517. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Patra, J.K.; Singh, Y.D.; Panda, M.K.; Das, G.; Adetunji, C.O.; Michael, O.S.; Sytar, O.; Polito, L.; et al. Paclitaxel: Application in Modern Oncology and Nanomedicine-Based Cancer Therapy. Oxidative Med. Cell. Longev. 2021, 2021, 3687700. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Yao, Y.; Li, H.; Gao, C.; Sun, C.; Zhuang, J. Potential of cucurbitacin as an anticancer drug. Biomed. Pharmacother. 2023, 168, 115707. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, R.-L.; Xie, C.; Lin, Y.-L.; Zhang, L.; Qi, Y. Pharmacological basis for the medicinal use of muskmelon base (Pedicellus melo) for abdominal distention and constipation. J. Ethnopharmacol. 2012, 142, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Q.; Yang, H.; Zhang, X.-W.; Feng, N.; Wang, J.-K.; Liu, T.-T.; Zeng, K.-W.; Tu, P.-F. Allosteric Regulation of IGF2BP1 as a Novel Strategy for the Activation of Tumor Immune Microenvironment. Acs Cent. Sci. 2022, 8, 1102–1115. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, W.; Hao, W.; Ren, G.; Lu, J.; Chen, X. Cucurbitacin B Induces DNA Damage, G2/M Phase Arrest, and Apoptosis Mediated by Reactive Oxygen Species (ROS) in Leukemia K562 Cells. Anti-Cancer Agents Med. Chem. 2014, 14, 1146–1153. [Google Scholar] [CrossRef]
- Kim, M.; Park, S.Y.; Jin, M.L.; Park, G.; Son, H.-J. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Park, G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci. Lett. 2015, 609, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh, T.A.; Mishra, A.K.; Sharma, S.; Mehdi, M.M. Aging, oxidative stress and degenerative diseases: Mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609–662. [Google Scholar] [CrossRef]
- Sallam, A.M.; Esmat, A.; Abdel-Naim, A.B. Cucurbitacin-B attenuates CCl4-induced hepatic fibrosis in mice through inhibition of STAT-3. Chem. Biol. Drug Des. 2018, 91, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xu, H.; Zhong, Y.; Zhang, X.; Zeng, T.; Li, L.; Xu, G.; Li, M.; Liu, J.; Yang, T. Identification and characterization of the Cucurbitacins, a novel class of small-molecule inhibitors of Tropomyosin receptor kinase a. BMC Complement. Altern. Med. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Cheng, C.; Yan, W.; Guo, R.; Wang, Y.; Zhang, H.; Chai, J.; Cheng, Y.; Zhang, F. Cucurbitacin B induces ferroptosis in oral leukoplakia via the SLC7A11/mitochondrial oxidative stress pathway. Phytomedicine 2024, 129, 155548. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Grossman, S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem. Biophys. Res. Commun. 2007, 364, 181–186. [Google Scholar] [CrossRef]
- Low, A.; Mak, E.; Rowe, J.B.; Markus, H.S.; O’Brien, J.T. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res. Rev. 2019, 53, 100916. [Google Scholar] [CrossRef]
- Andrusiow, S.; Pawlak, Z.; Stanczykiewicz, B.; Bogunia-Kubik, K.; Koszewicz, M. Chronic inflammatory demyelinating polyradiculoneuropathy in patients with diabetes mellitus-treatment with intravenous immunoglobulins: A systematic review. Biomed. Pharmacother. 2023, 164, 114974. [Google Scholar] [CrossRef]
- Chen, B.; Collen, L.V.; Mowat, C.; Isaacs, K.L.; Singh, S.; Kane, S.V.; Farraye, F.A.; Snapper, S.; Jneid, H.; Lavie, C.J.; et al. Inflammatory Bowel Disease and Cardiovascular Diseases. Am. J. Med. 2022, 135, 1453–1460. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Rava, M.; Real, F.X.; Hartmann, A.; Allory, Y.; Malats, N. Inflammatory Biomarkers and Bladder Cancer Prognosis: A Systematic Review. Eur. Urol. 2014, 66, 1078–1091. [Google Scholar] [CrossRef]
- Temmerman, J.; Engelborghs, S.; Bjerke, M.; D’Haeseleer, M. Cerebrospinal fluid inflammatory biomarkers for disease progression in Alzheimer’s disease and multiple sclerosis: A systematic review. Front. Immunol. 2023, 14, 1162340. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, L.; Zhou, Y.; Fang, Q. Cucurbitacin B alleviates cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome-mediated inflammation and reducing oxidative stress. Biosci. Biotechnol. Biochem. 2022, 86, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, R.; Fang, P.; Ye, Z.-Q.; Zhao, Y.; Zhou, Y.; Zhang, K.-Q.; Li, L. NLRP3 inflammasome inhibitor cucurbitacin B suppresses gout arthritis in mice. J. Mol. Endocrinol. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, Y.; Deng, X.; Liu, M.; Luo, W. Cucurbitacin B supplementation reduces inflammatory responses and alveolar bone loss via regulating MPO, COX-2 and RANK/RANKL/OPG signals in a rodent model of ligature -induced periodontitis. J. King Saud Univ. Sci. 2020, 32, 1889–1895. [Google Scholar] [CrossRef]
- Liu, P.; Tan, X.-Y.; Zhang, H.-Q.; Su, K.-L.; Shang, E.-X.; Xiao, Q.-L.; Guo, S.; Duan, J.-A. Optimal compatibility proportional screening of Trichosanthis Pericarpium- Trichosanthis Radix and its anti- Inflammatory components effect on experimental zebrafish and coughing mice. J. Ethnopharmacol. 2024, 319, 117096. [Google Scholar] [CrossRef]
- Aljohani, O.S. Phytochemical evaluation of Cucumis prophetarum: Protective effects against carrageenan-induced prostatitis in rats. Drug Chem. Toxicol. 2022, 45, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.-B.; Jiang, B.; Shi, T.-S.; Li, W.-Y.; Chen, W.-J.; Zhu, B.-L.; Qin, Z.-H. Cucurbitacin B Exerts Significant Antidepressant-Like Effects in a Chronic Unpredictable Mild Stress Model of Depression: Involvement of the Hippocampal BDNF-TrkB System. Int. J. Neuropsychopharmacol. 2023, 26, 680–691. [Google Scholar] [CrossRef]
- Li, J.; Sun, K.; Muroi, M.; Gao, L.; Chang, Y.-T.; Osada, H.; Xiang, L.; Qi, J. Cucurbitacin B induces neurogenesis in PC12 cells and protects memory in APP/PS1 mice. J. Cell. Mol. Med. 2019, 23, 6283–6294. [Google Scholar] [CrossRef]
- Liu, Z.; Kumar, M.; Kabra, A. Cucurbitacin B exerts neuroprotection in a murine Alzheimer’s disease model by modulating oxidative stress, inflammation, and neurotransmitter levels. Front. Biosci. -Landmark 2022, 27, 71. [Google Scholar] [CrossRef]
- Arques, S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann. De Cardiol. Et D’angeiologie 2020, 69, 192–200. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Z.; Wu, Q.-Q.; Jiang, X.-H.; Yuan, Y.; Chang, W.; Bian, Z.Y.; Zhu, J.X.; Tang, Q.-Z. Cucurbitacin B Protects Against Pressure Overload Induced Cardiac Hypertrophy. J. Cell. Biochem. 2017, 118, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Y.; Xu, M.; Chen, L.; Sun, Z.; Gong, J.; Li, Y.; Jiang, T. Cucurbitacin B protects against myocardial ischemia-reperfusion injury through activating JAK2/STAT3 signaling pathway. Cell. Mol. Biol. 2023, 69, 155–161. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cao, M.; Tang, Y.; Luo, Y.; Gu, F.; Zhu, Y.; Liu, X.; Yan, C.; Hu, W.; Wang, S.; Chao, X.; et al. Natural compounds modulating mitophagy: Implications for cancer therapy. Cancer Lett. 2024, 582, 216590. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Sflakidou, E.; Leonidis, G.; Foroglou, E.; Siokatas, C.; Sarli, V. Recent Advances in Natural Product-Based Hybrids as Anti-Cancer Agents. Molecules 2022, 27, 6632. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, X.-D.; Tang, P.Y.-Z.; Li, H.-M.; Liu, X.; Zhong, J.-J.; Tang, Y.-J. Advances of antitumor drug discovery in traditional Chinese medicine and natural active products by using multi-active components combination. Med. Res. Rev. 2023, 43, 1778–1808. [Google Scholar] [CrossRef]
- Luo, W.-W.; Zhao, W.-W.; Lu, J.-J.; Wang, Y.-T.; Chen, X.-P. Cucurbitacin B suppresses metastasis mediated by reactive oxygen species (ROS) via focal adhesion kinase (FAK) in breast cancer MDA-MB-231 cells. Chin. J. Nat. Med. 2018, 16, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.-L.; Yuan, J.-W.; Zhang, H.-R.; Liu, D.; Hao, J.; Ji, W.; Wu, X.-Z.; Chen, D. Cucurbitacin B inhibits the migration and invasion of breast cancer cells by altering the biomechanical properties of cells. Phytother. Res. 2019, 33, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wakimoto, N.; Xing, H.; Lu, D.; Huynh, T.; Wang, X.; Black, K.L.; Koeffler, H.P. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int. J. Cancer 2008, 123, 1364–1375. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, Y.; Liu, W.; Ma, F.; Zhou, Y.; Chen, M.; Chang, J.; Wang, Y.; Yang, G.; He, G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014, 10, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-L.; Tao, W.-H.; Yang, T.-X.; Qiao, J.-G. Anticancer effect of cucurbitacin B on MKN-45 cells via inhibition of the JAK2/STAT3 signaling pathway. Exp. Ther. Med. 2016, 12, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Yang, R.; Zhou, T.; Ke, W.; Si, Y.; Yang, S.; Zhang, T.; Liu, X.; Zhang, L.; et al. Cucurbitacin B inhibits gastric cancer progression by suppressing STAT3 activity. Arch. Biochem. Biophys. 2020, 684, 108314. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Gao, M.X.; Yang, K. Cucurbitacin B inhibits cell proliferation and induces apoptosis in human osteosarcoma cells via modulation of the JAK2/STAT3 and MAPK pathways. Exp. Ther. Med. 2017, 14, 805–812. [Google Scholar] [CrossRef]
- Wu, H.; Ma, T.; He, M.; Xie, W.; Wang, X.; Lu, L.; Wang, H.; Cui, Y. Cucurbitacin B modulates M2 macrophage differentiation and attenuates osteosarcoma progression via PI3K/AKT pathway. Phytother. Res. 2024, 38, 2215–2233. [Google Scholar] [CrossRef]
- Yu, Z.; Liang, S.; Ji, L.; Cheng, Y.; Yan, W.; Gao, R.; Zhang, F. Network pharmacological analysis and experimental study of cucurbitacin B in oral squamous cell carcinoma. Mol. Divers. 2023. [Google Scholar] [CrossRef]
- Tao, B.; Wang, D.; Yang, S.; Liu, Y.; Wu, H.; Li, Z.; Chang, L.; Yang, Z.; Liu, W. Cucurbitacin B Inhibits Cell Proliferation by Regulating X-Inactive Specific Transcript Expression in Tongue Cancer. Front. Oncol. 2021, 11, 651648. [Google Scholar] [CrossRef]
- Alafnan, A.; Khalifa, N.E.; Hussain, T.; Osman, M.E. Cucurbitacin-B instigates intrinsic apoptosis and modulates Notch signaling in androgen-dependent prostate cancer LNCaP cells (vol 14, 1206981, 2023). Front. Pharmacol. 2023, 14, 1206981. [Google Scholar]
- Alafnan, A.; Alamri, A.; Hussain, T.; Rizvi, S.M.D. Cucurbitacin-B Exerts Anticancer Effects through Instigation of Apoptosis and Cell Cycle Arrest within Human Prostate Cancer PC3 Cells via Downregulating JAK/STAT Signaling Cascade. Pharmaceuticals 2022, 15, 1229. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, M.; Chen, Y.; Xu, S.; Guo, Y.; Zhao, L. Cucurbitacin B suppresses proliferation of pancreatic cancer cells by ceRNA: Effect of miR-146b-5p and lncRNA-AFAP1-AS1. J. Cell. Physiol. 2019, 234, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Z.; Chen, Y.-Y.; Liu, Q.-P.; Feng, Z.-H.; Zhang, L.; Zhang, H. Cucurbitacin B suppresses hepatocellular carcinoma progression through inducing DNA damage-dependent cell cycle arrest. Phytomedicine 2024, 126, 155177. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Y.; Yan, X.; Li, J.; Lin, B.; Dai, L.; Xu, C.; Li, H.; Li, D.; Yang, T.; et al. Cucurbitacin B exhibits antitumor effects on CD133+HepG2 liver cancer stem cells by inhibiting JAK2/STAT3 signaling pathway. Anti-Cancer Drugs 2021, 32, 548–557. [Google Scholar] [CrossRef]
- Liu, J.-H.; Li, C.; Cao, L.; Zhang, C.-H.; Zhang, Z.-H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Pharm. Biol. 2022, 60, 154–162. [Google Scholar] [CrossRef]
- Yuan, R.; Fan, Q.; Liang, X.; Han, S.; He, J.; Qin-Qin, W.; Gao, H.; Feng, Y.; Yang, S. Cucurbitacin B inhibits TGF-β1-induced epithelial-mesenchymal transition (EMT) in NSCLC through regulating ROS and PI3K/Akt/mTOR pathways. Chin. Med. 2022, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, L.; Tang, H.; Wang, W.; Lin, Y. Cucurbitacin B enhances apoptosis in gefitinib resistant non-small cell lung cancer by modulating the miR-17-5p/STAT3 axis. Mol. Med. Rep. 2021, 24, 710. [Google Scholar] [CrossRef]
- Yu, X.; Chen, W.; Zhang, J.; Gao, X.; Cui, Q.; Song, Z.; Du, J.; Lv, W. Antitumor activity and mechanism of cucurbitacin B in A549/DDP cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 396, 1095–1103. [Google Scholar] [CrossRef]
- Mao, D.; Liu, A.H.; Wang, Z.P.; Zhang, X.W.; Lu, H. Cucurbitacin B inhibits cell proliferation and induces cell apoptosis in colorectal cancer by modulating methylation status of BTG3. Neoplasma 2019, 66, 593–602. [Google Scholar] [CrossRef]
- Huang, J.-L.; Liang, L.; Xie, P.-E.; Sun, W.-L.; Wang, L.; Cai, Z.-W. Cucurbitacin B induces apoptosis in colorectal cells through reactive oxygen species generation and endoplasmic reticulum stress pathways. Exp. Ther. Med. 2023, 26, 484. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, B.; Wei, H.; Zeng, H.; Sheng, D.; Zhang, Y. Cucurbitacin B controls M2 macrophage polarization to suppresses metastasis via targeting JAK-2/STAT3 signalling pathway in colorectal cancer. J. Ethnopharmacol. 2022, 287, 114915. [Google Scholar] [CrossRef]
- Dandawate, P.; Subramaniam, D.; Panovich, P.; Standing, D.; Krishnamachary, B.; Kaushik, G.; Thomas, S.M.; Dhar, A.; Weir, S.J.; Jensen, R.A.; et al. Cucurbitacin B and I inhibits colon cancer growth by targeting the Notch signaling pathway. Sci. Rep. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Kaewmeesri, P.; Kukongviriyapan, V.; Prawan, A.; Kongpetch, S.; Senggunprai, L. Cucurbitacin B Diminishes Metastatic Behavior of Cholangiocarcinoma Cells by Suppressing Focal Adhesion Kinase. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kurman, Y.; Kiliccioglu, I.; Dikmen, A.U.; Esendagli, G.; Bilen, C.Y.; Sozen, S.; Konac, E. Cucurbitacin B and cisplatin induce the cell death pathways in MB49 mouse bladder cancer model. Exp. Biol. Med. 2020, 245, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Kariya, R.; Sittithumcharee, G.; Okada, S. Cucurbitacin B induces apoptosis of primary effusion lymphoma via disruption of cytoskeletal organization. Phytomedicine 2021, 85, 153545. [Google Scholar] [CrossRef]
- Ding, Y.; Xue, X. Medicinal Chemistry Strategies for the Modification of Bioactive Natural Products. Molecules 2024, 29, 689. [Google Scholar] [CrossRef]
- Guo, M.; Jin, J.; Zhao, D.; Rong, Z.; Cao, L.-Q.; Li, A.-H.; Sun, X.-Y.; Jia, L.-Y.; Wang, Y.-D.; Huang, L.; et al. Research Advances on Anti-Cancer Natural Products. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef]
- Jung, M.E.; Lui, R.M. Studies toward the Total Syntheses of Cucurbitacins B and D. J. Org. Chem. 2010, 75, 7146–7158. [Google Scholar] [CrossRef]
- Suebsakwong, P.; Wang, J.; Ithetkam, P.; Weerapreeyaku, N.; Wu, J.; Du, Y.; Yao, Z.J.; Li, J.-X.; Suksamrarn, A. A Bioreductive Prodrug of Cucurbitacin B Significantly Inhibits Tumor Growth in the 4T1 Xenograft Mice Model. Acs Med. Chem. Lett. 2019, 10, 1400–1406. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Xing, Y.; Sun, Y.-Q.; Liu, C.; Xu, Q.; Shang, F.F.; Zhang, R.H.; Jin, X.-J.; Chen, F.; Lee, J.J.; et al. Ginsengenin derivatives synthesized from 20(R)-panaxotriol: Synthesis, characterization, and antitumor activity targeting HIF-1 pathway. J. Ginseng Res. 2022, 46, 738–749. [Google Scholar] [CrossRef]
- Liu, H.-J.; Huang, X.; Shen, Q.-K.; Deng, H.; Li, Z.; Quan, Z.-S. Design, Synthesis, and Anticancer Activity Evaluation of Hybrids of Azoles and Barbituric Acids. Iran. J. Pharm. Res. 2021, 20, 144–155. [Google Scholar]
- Ma, Q.; Bian, M.; Gong, G.; Bai, C.; Liu, C.; Wei, C.; Quan, Z.-S.; Du, H.-H. Synthesis and Evaluation of Bakuchiol Derivatives as Potent Anti-inflammatory Agents in Vitro and in Vivo. J. Nat. Prod. 2022, 85, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, C.-Y.; Gong, G.-H.; Quan, Z.-S. Synthesis, in vitro and in vivo biological evaluation of novel lappaconitine derivatives as potential anti-inflammatory agents. Acta Pharm. Sin. B 2020, 10, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.-F.; Lu, Q.; Lin, T.; Pu, M.; Xiao, R.; Liu, W.; Deng, H.; Guo, H.; Quan, Z.-S.; Ding, C.; et al. Discovery of Triazolyl Derivatives of Cucurbitacin B Targeting IGF2BP1 against Non-Small Cell Lung Cancer. J. Med. Chem. 2023, 66, 12931–12949. [Google Scholar] [CrossRef]
- Lang, K.L.; Silva, I.T.; Zimmermann, L.A.; Machado, V.R.; Teixeira, M.R.; Ivana Lapuh, M.; Alejandra Galetti, M.; Alejandro Palermo, J.; Myriam Cabrera, G.; Campos Bernardes, L.S.; et al. Synthesis and cytotoxic activity evaluation of dihydrocucurbitacin B and cucurbitacin B derivatives. Bioorganic Med. Chem. 2012, 20, 3016–3030. [Google Scholar] [CrossRef]
- Ge, W.; Chen, X.; Han, F.; Liu, Z.; Wang, T.; Wang, M.; Chen, Y.; Ding, Y.; Zhang, Q. Synthesis of Cucurbitacin B Derivatives as Potential Anti-Hepatocellular Carcinoma Agents. Molecules 2018, 23, 3345. [Google Scholar] [CrossRef]
- Ai, Y.; Kang, F.; Huang, Z.; Xue, X.; Lai, Y.; Peng, S.; Tian, J.; Zhang, Y. Synthesis of CDDO-Amino Acid-Nitric Oxide Donor Trihybrids as Potential Antitumor Agents against Both Drug-Sensitive and Drug-Resistant Colon Cancer. J. Med. Chem. 2015, 58, 2452–2464. [Google Scholar] [CrossRef]
- Duan, W.; Li, J.; Inks, E.S.; Chou, C.J.; Jia, Y.; Chu, X.; Li, X.; Xu, W.; Zhang, Y. Design, Synthesis, and Antitumor Evaluation of Novel Histone Deacetylase Inhibitors Equipped with a Phenylsulfonylfuroxan Module as a Nitric Oxide Donor. J. Med. Chem. 2015, 58, 4325–4338. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Liu, M.-M.; Chen, X.-Y.; Huang, Y.-Q.; Feng, P.; Guo, Y.-L.; Yang, G.; Chen, Y. Hybrids of Phenylsulfonylfuroxan and Coumarin as Potent Antitumor Agents. J. Med. Chem. 2014, 57, 9343–9356. [Google Scholar]
- Tang, W.; Xie, J.; Xu, S.; Lv, H.; Lin, M.; Yuan, S.; Bai, J.; Hou, Q.; Yu, S. Novel Nitric Oxide-Releasing Derivatives of Brusatol as Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Nitric Oxide Release Studies. J. Med. Chem. 2014, 57, 7600–7612. [Google Scholar] [CrossRef]
- Zhuo, N.; Ma, J.; Cao, L.; Chen, L.; Nan, F. Protecting-Group-Free One-Step Palladium-Catalyzed Coupling on C25 of Cucurbitacin B Expands Chemical Diversity with Improved Cytotoxicity against A549 Cells. Chin. J. Chem. 2022, 40, 1662–1666. [Google Scholar] [CrossRef]
- Ahmadi, M.; Siavashy, S.; Ayyoubzadeh, S.M.; Kecili, R.; Ghorbani-Bidkorbeh, F. Controllable Synthesis of Polymeric Micelles by Microfluidic Platforms for Biomedical Applications: A Systematic Review. Iran. J. Pharm. Res. 2021, 20, e124514. [Google Scholar]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnuerch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, J.; Zhang, Q.; Fang, L.; Liu, C.; Xu, H. Development and Evaluation of Cucurbitacin B Microemulsion: The Effect of Oil Phase and Aqueous Phase on Drug Percutaneous Absorption Based on ATR-FTIR Spectroscopy and Molecular Modeling. Aaps Pharmscitech 2020, 21, 258. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, Z.; Xiao, Y.; Chang, J.; Liu, P.; Liu, C.; Liu, X. Preparation, characterization and pharmacokinetics of Cucurbitacin B solid dispersion. OpenNano 2022, 8, 100088. [Google Scholar] [CrossRef]
- Lv, Q.; Shen, C.; Li, X.; Shen, B.; Yu, C.; Xu, P.; Xu, H.; Han, J.; Yuan, H. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv. 2015, 22, 351–358. [Google Scholar] [CrossRef]
- Hu, H.; Liu, D.; Zhao, X.; Qiao, M.; Chen, D. Preparation, characterization, cellular uptake and evaluation in vivo of solid lipid nanoparticles loaded with cucurbitacin B. Drug Dev. Ind. Pharm. 2013, 39, 770–779. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Lu, X.; Li, Y.; Lu, C. Diselenium-linked dimeric prodrug nanomedicine breaking the intracellular redox balance for triple-negative breast cancer targeted therapy. Eur. J. Pharm. Biopharm. 2023, 193, 16–27. [Google Scholar] [CrossRef]
- Tang, L.; Fu, L.; Zhu, Z.; Yang, Y.; Sun, B.; Shan, W.; Zhang, Z. Modified mixed nanomicelles with collagen peptides enhanced oral absorption of Cucurbitacin B: Preparation and evaluation. Drug Deliv. 2018, 25, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Allen-Perkins, A.; Estrada, E. Mathematical modelling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2019, 475, 20190136. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef] [PubMed]
- Zust, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.R.; Hoffman, C.A. Chemical Feeding Deterrent Mobilized in Response to Insect Herbivory and Counteradaptation by Epilachna tredecimnotata. Science 1980, 209, 414–416. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins—An insight into medicinal leads from nature. Pharmacogn. Rev. 2015, 9, 12–18. [Google Scholar]
- Garg, S.; Kaul, S.C.; Wadhwa, R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review). Int. J. Oncol. 2018, 52, 19–37. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metcalf, R.A.; Rhodes, A.M. Cucurbitacins as kairomones for diabroticite beetles. Proc. Natl. Acad. Sci. USA 1980, 77, 3769–3772. [Google Scholar] [CrossRef]
- Yousaf, H.K.; Shan, T.; Chen, X.; Ma, K.; Shi, X.; Desneux, N.; Biondi, A.; Gao, X. Impact of the secondary plant metabolite Cucurbitacin B on the demographical traits of the melon aphid, Aphis gossypii. Sci. Rep. 2018, 8, 16473. [Google Scholar] [CrossRef]
- Clyne, P.J.; Warr, C.G.; Carlson, J.R. Candidate taste receptors in Drosophila. Science 2000, 287, 1830–1834. [Google Scholar] [CrossRef]
- Rimal, S.; Sang, J.; Dhakal, S.; Lee, Y. Cucurbitacin B Activates Bitter-Sensing Gustatory Receptor Neurons via Gustatory Receptor 33a in Drosophila melanogaster. Mol. Cells 2020, 43, 530–538. [Google Scholar] [PubMed]
- Cooke, J.; Sang, J.H. Utilization of sterols by larvae of Drosophila melanogaster. J. Insect Physiol. 1970, 16, 801–812. [Google Scholar] [CrossRef]
- Niwa, R.; Niwa, Y.S. The Fruit Fly Drosophila melanogaster as a Model System to Study Cholesterol Metabolism and Homeostasis. Cholesterol 2011, 2011, 176802. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Fujinaga, D.; Inaba, K.; Funahashi, T.; Fujikawa, Y.; Inoue, H.; Kataoka, H.; Niwa, R.; Ono, H. The plant-derived triterpenoid, cucurbitacin B, but not cucurbitacin E, inhibits the developmental transition associated with ecdysone biosynthesis in Drosophila melanogaster. J. Insect Physiol. 2021, 134, 104294. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.-X.; Bai, Y.-C.; Reed, J.; Qu, B.; Goossens, A.; Nutzmann, H.-W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef]
- Koprivova, A.; Schuck, S.; Jacoby, R.P.; Klinkhammer, I.; Welter, B.; Leson, L.; Martyn, A.; Nauen, J.; Grabenhorst, N.; Mandelkow, J.F.; et al. Root-specific camalexin biosynthesis controls the plant growth-promoting effects of multiple bacterial strains. Proc. Natl. Acad. Sci. USA 2019, 116, 15735–15744. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xun, W.; Wang, X.; Tian, S.; Zhang, Y.; Li, D.; Zhou, Y.; Qin, Y.; Zhang, B.; Zhao, G.; et al. Root-secreted bitter triterpene modulates the rhizosphere microbiota to improve plant fitness. Nat. Plants 2022, 8, 887–896. [Google Scholar] [CrossRef]
- Cheke, R.A. New pests for old as GMOs bring on substitute pests. Proc. Natl. Acad. Sci. USA 2018, 115, 8239–8240. [Google Scholar] [CrossRef]
- Constantine, K.L.; Kansiime, M.K.; Mugambi, I.; Nunda, W.; Chacha, D.; Rware, H.; Makale, F.; Mulema, J.; Lamontagne-Godwin, J.; Williams, F.; et al. Why don’t smallholder farmers in Kenya use more biopesticides? Pest Manag. Sci. 2020, 76, 3615–3625. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Luo, J.; Niu, L.; Hua, H.; Zhang, S.; Cui, J. Potential of Cucurbitacin B and Epigallocatechin Gallate as Biopesticides against Aphis gossypii. Insects 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Whiting, P.; Girault, J.P.; Lafont, R.; Dhadialla, T.S.; Cress, D.E.; Mugat, B.; Antoniewski, C.; Lepesant, J.A. Cucurbitacins are insect steroid hormone antagonists acting at the ecdysteroid receptor. Biochem. J. 1997, 327 Pt 3, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M.; Cherbas, P.; Truman, J.W. Ecdysone receptors and their biological actions. Vitam. Horm. 2000, 60, 1–73. [Google Scholar]
- Zou, C.; Liu, G.; Liu, S.; Liu, S.; Song, Q.; Wang, J.; Feng, Q.; Su, Y.; Li, S. Cucurbitacin B acts a potential insect growth regulator by antagonizing 20-hydroxyecdysone activity. Pest Manag. Sci. 2018, 74, 1394–1403. [Google Scholar] [CrossRef]
- Pofu, K.M.; Mashela, P.W. Interactive Effects of Filamentous Fungi and Cucurbitacin Phytonematicide on Growth of Cowpea and Suppression of Meloidogyne enterolobii. Front. Microbiol. 2022, 13, 765051. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Boezio, B.; Audouze, K.; Ducrot, P.; Taboureau, O. Network-based Approaches in Pharmacology. Mol. Inform. 2017, 36, 1700048. [Google Scholar] [CrossRef]
- Dai, S.; Wu, R.; Fu, K.; Li, Y.; Yao, C.; Liu, Y.; Zhang, F.; Zhang, S.; Guo, Y.; Yao, Y.; et al. Exploring the effect and mechanism of cucurbitacin B on cholestatic liver injury based on network pharmacology and experimental verification. J. Ethnopharmacol. 2024, 322, 117584. [Google Scholar] [CrossRef]
- Aizawa, H.; Kimura, S.; Abe, S.; Sano, M. Gel network amplifies Nano-Scale adsorption at Solid/Liquid interface to Sub-Millimeter-Scale. J. Colloid Interface Sci. 2022, 626, 276–282. [Google Scholar] [CrossRef]
- Dokania, S.; Joshi, A.K. Self-microemulsifying drug delivery system (SMEDDS)—challenges and road ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Eilers, A.; Witt, S.; Walter, J. Aptamer-Modified Nanoparticles in Medical Applications. Aptamers Biotechnol. 2020, 174, 161–193. [Google Scholar]
- Kovalchuk, N.M.; Johnson, D.; Sobolev, V.; Hilal, N.; Starov, V. Interactions between nanoparticles in nanosuspension. Adv. Colloid Interface Sci. 2019, 272, 102020. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Jiang, Y.; McClements, D.J.; Liu, F.; Liu, X. Self-assembled nano-micelles of lactoferrin peptides: Structure, physicochemical properties, and application for encapsulating and delivering curcumin. Food Chem. 2022, 387, 132790. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Sporn, M.B. Synthetic Oleanane Triterpenoids: Multifunctional Drugs with a Broad Range of Applications for Prevention and Treatment of Chronic Disease. Pharmacol. Rev. 2012, 64, 972–1003. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef]
- Dai, S.; Wang, C.; Zhao, X.; Ma, C.; Fu, K.; Liu, Y.; Peng, C.; Li, Y. Cucurbitacin B: A review of its pharmacology, toxicity, and pharmacokinetics. Pharmacol. Res. 2023, 187, 106587. [Google Scholar] [CrossRef]
- Guedes Silvestre, G.F.; de Lucena, R.P.; Alves, H.d.S. Cucurbitacins and the Immune System: Update in Research on Anti-inflammatory, Antioxidant, and Immunomodulatory Mechanisms. Curr. Med. Chem. 2022, 29, 3774–3789. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Ras, R.; Widmer, G. Effect of Ginsenoside-Rh2 and Curcurbitacin-B on Cryptosporidium parvum in vitro. Exp. Parasitol. 2020, 212, 107873. [Google Scholar] [CrossRef]
- Chen, J.-C.; Zhang, G.-H.; Zhang, Z.-Q.; Qiu, M.-H.; Zheng, Y.-T.; Yang, L.-M.; Yu, K.-B. Octanorcucurbitane and cucurbitane triterpenoids from the tubers of Hemsleya endecaphylla with HIV-1 inhibitory activity. J. Nat. Prod. 2008, 71, 153–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).