Novel PDI-NH/PDI-COOH Supramolecular Junction for Enhanced Visible-Light Photocatalytic Phenol Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Structure Analysis
2.2. Photocatalytic Phenol Degradation
2.3. Active Species and Stability
2.4. Optical Absorption
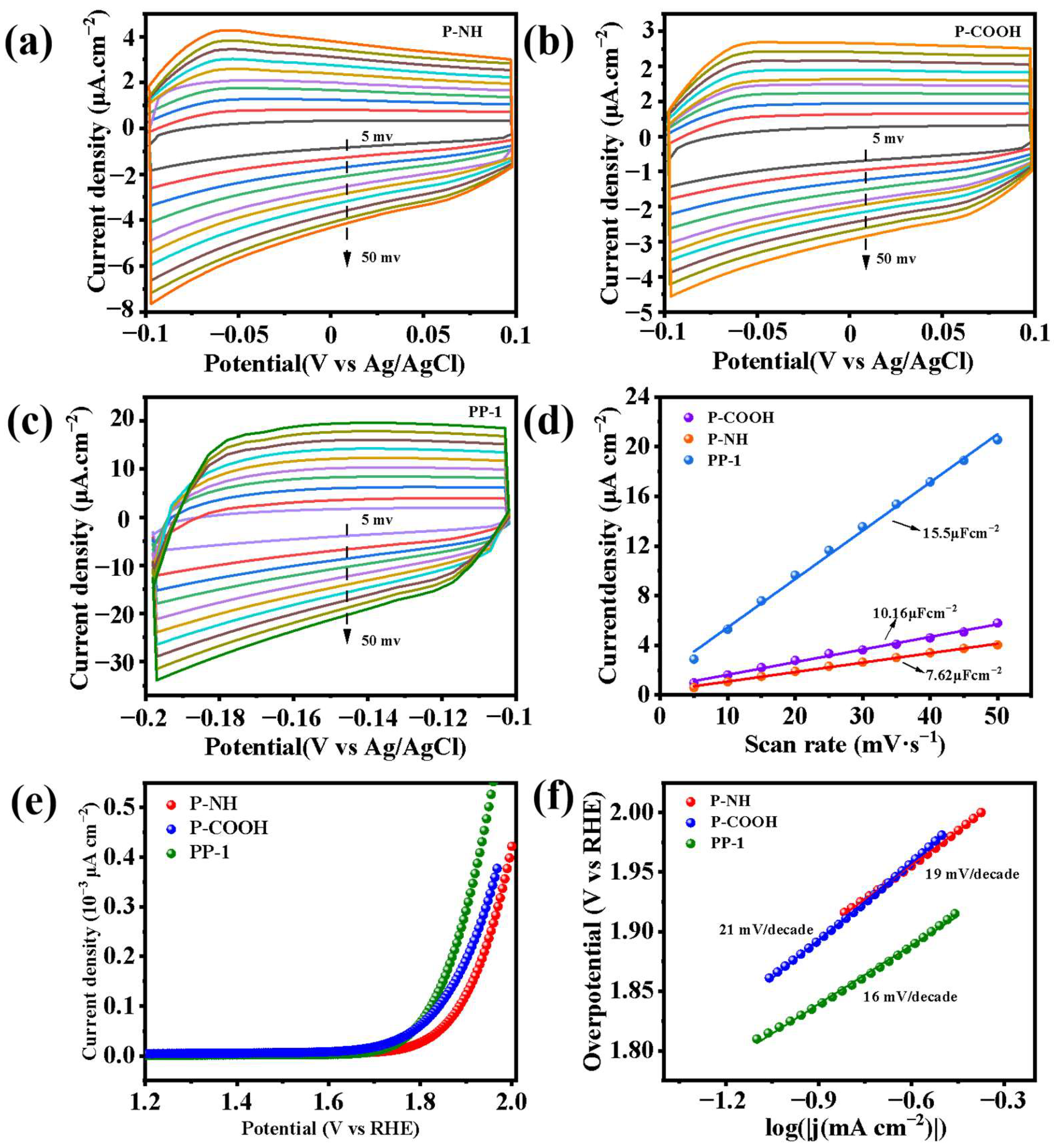
2.5. Charge Transfer Properties
2.6. Contact Angle
2.7. Built-in Electric Field and Mechanism
3. Experimental Section
3.1. Materials
3.2. Preparation of Polyxylene-3,4,9,10-Tetracarboxylic Dianhydride
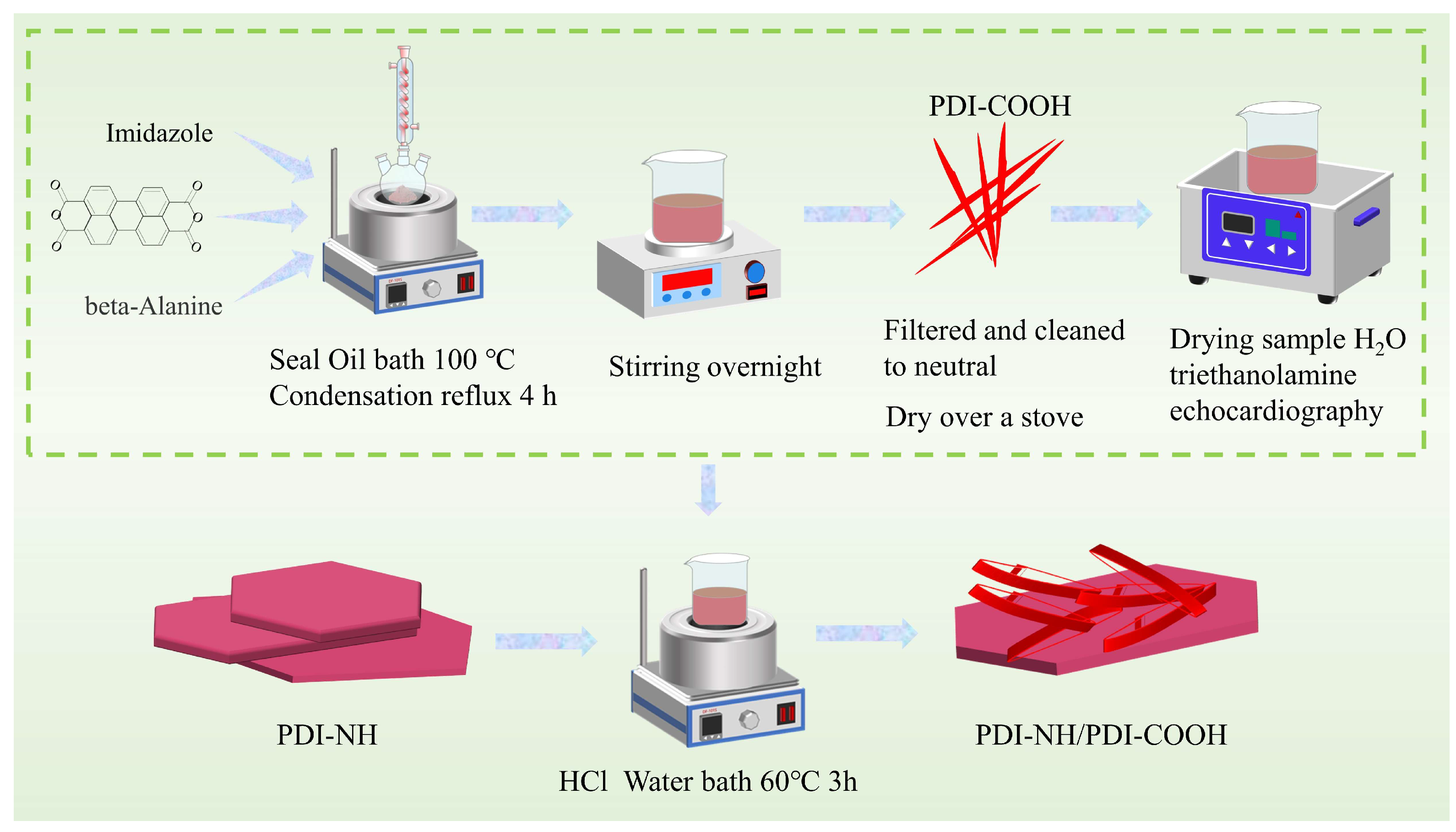
3.3. Synthesis of PDI-NH/PDI-COOH
3.4. Evaluation of Photocatalytic Performances
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.-H.; Park, J.-W.; Yip, A.C.K. Photocatalysts for Degradation of Dyes in Industrial Effluents: Opportunities and Challenges. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Chang, Y.S.; Ng, K.H.; Wu, T.Y.; Cheng, C.K. Photocatalytic Restoration of Liquid Effluent from Oil Palm Agroindustry in Malaysia Using Tungsten Oxides Catalyst. J. Clean. Prod. 2017, 162, 205–219. [Google Scholar] [CrossRef]
- De Andrade, P.M.; Dufrayer, C.R.; Ionashiro, E.Y.; De Brito, N.N. The Use of Metallurgical Waste for Heterogeneous Photo Fenton-Like Treatment of Cosmetic Effluent. J. Environ. Chem. Eng. 2020, 8, 104148. [Google Scholar] [CrossRef]
- Henrique, J.M.D.M.; Isidro, J.; Saez, C.; Dos Santos, E.V.; Rodrigo, M.A. Enhancing Electrokinetic Soil Flushing with Air Stripping for the Treatment of Soil Polluted with Phenol and O-Chlorophenol. Electrochim. Acta 2022, 432, 141189. [Google Scholar] [CrossRef]
- Yang, Z.-L.; Peng, D.-Y.; Zeng, H.-Y.; Xiong, J.; Li, S.; Ji, W.-Y.; Wu, B. Enhanced Photocatalytic Performance of Heterostructure BiOBr/PPy for Cr(VI) Reduction and Dye Degradation. Colloid. Surf. Physicochem. Eng. Asp. 2024, 680, 132647. [Google Scholar] [CrossRef]
- Alzahrani, F.M.A.; Anwar, M.; Farooq, A.; Alrowaili, Z.A.; Al-Buriahi, M.S.; Warsi, M.F. A New BiOCl–ZnFe2O4/CNTs Ternary Composite for Remarkable Photocatalytic Degradation Studies of a Herbicide and a Diazo Dye. Opt. Mater. 2024, 148, 114876. [Google Scholar] [CrossRef]
- Heidari, S.; Haghighi, M.; Shabani, M. Sunlight-Activated BiOCl/BiOBr–Bi24O31Br10 Photocatalyst for the Removal of Pharmaceutical Compounds. J. Clean. Prod. 2020, 259, 120679. [Google Scholar] [CrossRef]
- Han, A.; Zhang, H.; Lu, D.; Sun, J.; Chuah, G.K.; Jaenicke, S. Efficient Photodegradation of Chlorophenols by BiOBr/NaBiO3 Heterojunctioned Composites under Visible Light. J. Hazard. Mater. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Tang, Y.; Zhang, X.; Ji, S.; Luo, L.; Jiang, F. Photoinduced RhB-Sensitized Effect on a Novel AgI/BiOCl/Biochar Photocatalyst to Boost Its Photocatalytic Performance for 17α-Ethinyl Estradiol Degradation. Sep. Purif. Technol. 2024, 332, 125774. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.; Zhang, X.; Wang, S.; Duan, K.; Li, X.; Hu, Y.; Cheng, H. Direct Z-Scheme In2O3/AgI Heterojunction with Oxygen Vacancies for Efficient Molecular Oxygen Activation and Enhanced Photocatalytic Degradation of Tetracycline. Chem. Eng. J. 2023, 466, 143319. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Wang, J.; Li, A.; François-Xavier Corvini, P. BiOBr/Bi4O5Br2/PDI Constructed for Visible-Light Degradation of Endocrine Disrupting Chemicals: Synergistic Effects of Bi-Heterojunction and Oxygen Evolution. Chem. Eng. J. 2022, 433, 133622. [Google Scholar] [CrossRef]
- Ji, Q.; Cheng, X.; Kong, X.; Sun, D.; Wu, Y.; Xu, Z.; Liu, Y.; Duan, X.; He, H.; Li, S.; et al. Visible-Light Activation of Persulfate Ions by Z-Scheme Perylene Diimide/MIL-101(Cr) Heterojunction Photocatalyst towards Efficient Degradation of Iohexol. Chem. Eng. J. 2022, 435, 134947. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Alamgholiloo, H.; Noroozi Pesyan, N.; Zolfigol, M.A. A MOF-on-MOF Strategy to Construct Double Z-Scheme Heterojunction for High-Performance Photocatalytic Degradation. Appl. Catal. B Environ. 2023, 321, 122082. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Liu, Y.; Cai, T.; Dong, W.; Xia, X. A Novel Ti3C2 MXene/PDI Supramolecules Composite with Enhanced Photocatalytic Activities for Degradation of Tetracycline Hydrochloride under Visible-Light. J. Environ. Chem. Eng. 2022, 10, 107978. [Google Scholar] [CrossRef]
- Dai, W.; Jiang, L.; Wang, J.; Pu, Y.; Zhu, Y.; Wang, Y.; Xiao, B. Efficient and Stable Photocatalytic Degradation of Tetracycline Wastewater by 3D Polyaniline/Perylene Diimide Organic Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2020, 397, 125476. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Jiang, W.; Yao, W.; Yang, H.; Zhu, Y. Self-Assembled Perylene Diimide Based Supramolecular Heterojunction with Bi2WO6 for Efficient Visible-Light-Driven Photocatalysis. Appl. Catal. B Environ. 2018, 232, 175–181. [Google Scholar] [CrossRef]
- Deng, Y.; Li, L.; Zeng, H.; Tang, R.; Zhou, Z.; Sun, Y.; Feng, C.; Gong, D.; Wang, J.; Huang, Y. Unveiling the Origin of High-Efficiency Charge Transport Effect of C3N5/C3N4 Homojunction for Activating Peroxymonosulfate to Degrade Atrazine under Visible Light. Chem. Eng. J. 2023, 457, 141261. [Google Scholar] [CrossRef]
- Ji, X.; Liu, X.; Guo, Y.; Zhang, J. Developing Visible Light Responsive Z-Scheme BN-PDI Photocatalysts with Good Degradation Performance for Antibiotics. Chem. Eng. J. 2021, 425, 131260. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Deng, Y.; Xiong, S.; Deng, J.; Li, L.; Zhou, Z.; Zheng, J.; Su, L.; Yang, L. π-π Stacked Step-Scheme PDI/g-C3N4/TiO2@Ti3C2 Photocatalyst with Enhanced Visible Photocatalytic Degradation towards Atrazine via Peroxymonosulfate Activation. Chem. Eng. J. 2022, 427, 131809. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Wang, Z.; Zhu, Y. Enhanced Visible Photocatalytic Oxidation Activity of Perylene Diimide/g-C3N4 n-n Heterojunction via π-π Interaction and Interfacial Charge Separation. Appl. Catal. B Environ. 2020, 271, 118933. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Guo, W.; Zhong, W.; Li, Q.; Tan, L.; Shang, L. Ratiometric Fluorescence Sensors for Heparin and Heparinase Based on Enhanced Excimer Emission of Perylene Probe Induced by Cationic Silver Nanoparticles. Sens. Actuators B Chem. 2020, 305, 127422. [Google Scholar] [CrossRef]
- Tao, B.; Ouyang, M.; Hua, Q.; Kong, C.; Zhang, J.; Li, W.; Bai, R.; Liu, J.; Lv, X.; Zhang, C. High Electrochromic Performance of Perylene Bisimide/ZnO Hybrid Films: An Efficient, Energy-Saving, and Green Route. ACS Appl. Mater. Interfaces 2023, 15, 13730–13739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, D.; Zhu, Y.; Zhou, S.; Guan, S. Supramolecular Packing Dominant Photocatalytic Oxidation and Anticancer Performance of PDI. Appl. Catal. B Environ. 2018, 231, 251–261. [Google Scholar] [CrossRef]
- Shi, H.; Peng, J.; Deng, F.; Li, X.; Zhou, J.; Zhang, Y.; Luo, X. Preferential degradation of ofloxacin on all-organic molecularly imprinted PDI/g-C3N4 photocatalyst via specific molecular recognition. Sep. Purif. Technol. 2025, 353, 128499. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Bai, X.; Wang, X.; Sun, B.; Li, D.; Zhao, L.; Zong, R.; Hao, D. In-Plane Polarization Induced by the Hydrogen Bonding and π–π Stacking of Functionalized PDI Supramolecules for the Efficient Photocatalytic Degradation of Organic Pollutants. Mater. Chem. Front. 2020, 4, 2673–2687. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Zhang, Q.; Huang, B.; Wang, P.; Qin, X.; Zhang, X.; Dai, Y. Self-Assembled Supramolecular System PDINH on TiO2 Surface Enhances Hydrogen Production. J. Colloid Interface Sci. 2018, 525, 136–142. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, B.; Liu, G.; Xu, C.; Ji, Q.; Xiang, W.; Sun, D.; Zhong, Q.; He, H.; Yazi, L.; et al. Perylene Diimide Supermolecule (PDI) as a Novel and Highly Efficient Cocatalyst for Photocatalytic Degradation of Tetracycline in Water: A Case Study of PDI Decorated Graphitic Carbon Nitride/Bismuth Tungstate Composite. J. Colloid Interface Sci. 2022, 615, 849–864. [Google Scholar] [CrossRef]
- Akple, M.S.; Low, J.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.; Zhang, J. Enhanced Visible Light Photocatalytic H2-Production of g-C3N4/WS2 Composite Heterostructures. Appl. Surf. Sci. 2015, 358, 196–203. [Google Scholar] [CrossRef]
- Angelella, M.; Wang, C.; Tauber, M.J. Resonance Raman Spectra of a Perylene Bis(Dicarboximide) Chromophore in Ground and Lowest Triplet States. J. Phys. Chem. A 2013, 117, 9196–9204. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Bai, X.; Zong, R.; Zhu, Y. Self-Assembled PDINH Supramolecular System for Photocatalysis under Visible Light. Adv. Mater. 2016, 28, 7284–7290. [Google Scholar] [CrossRef]
- Ben, H.; Liu, Y.; Liu, X.; Liu, X.; Ling, C.; Liang, C.; Zhang, L. Diffusion-Controlled Z-Scheme-Steered Charge Separation across PDI/BiOI Heterointerface for Ultraviolet, Visible, and Infrared Light-Driven Photocatalysis. Adv. Funct. Mater. 2021, 31, 2102315. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Huang, S.; Xu, J.; Liu, L.; Li, J.; Jing, J.; Zhu, Y. Electron-Enriched Supramolecular PDI-SiO2 Promoting PDS Activation for Enhanced Photocatalytic Advanced Oxidation. Appl. Catal. B Environ. 2024, 340, 123262. [Google Scholar] [CrossRef]
- Yoo, P.S.; Amaranatha Reddy, D.; Jia, Y.; Bae, S.E.; Huh, S.; Liu, C. Magnetic Core-Shell ZnFe2O4/ZnS Nanocomposites for Photocatalytic Application under Visible Light. J. Colloid Interface Sci. 2017, 486, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.; Zhang, Y. Preparation of Organic-Inorganic PDI/BiO2-x Photocatalyst with Boosted Photocatalytic Performance. J. Taiwan Inst. Chem. Eng. 2022, 132, 104111. [Google Scholar] [CrossRef]
- Lan, Z.; Yu, Y.; Yao, J.; Cao, Y. The Band Structure and Photocatalytic Mechanism of MoS2-Modified C3N4 Photocatalysts with Improved Visible Photocatalytic Activity. Mater. Res. Bull. 2018, 102, 433–439. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Li, J.; Wang, Y.; Jin, Z. Unique Synergistic Effects of ZIF-9(Co)-Derived Cobalt Phosphide and CeVO4 Heterojunction for Efficient Hydrogen Evolution. Chin. J. Catal. 2020, 41, 82–94. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Zeng, Q.; Zheng, Y.; Sun, W.; Meng, X. Ball Milling Synthesis of Porous g-C3N4 Ultrathin Nanosheets Functionalized with Alkynyl Groups for Strengthened Photocatalytic Activity. Sep. Purif. Technol. 2022, 282, 120097. [Google Scholar] [CrossRef]
- Yang, J.; Miao, H.; Li, W.; Li, H.; Zhu, Y. Designed Synthesis of a P-Ag2S/n-PDI Self-Assembled Supramolecular Heterojunction for Enhanced Full-Spectrum Photocatalytic Activity. J. Mater. Chem. A 2019, 7, 6482–6490. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Wang, J.; Ouyang, Z.; Li, J.; Wei, G.; Su, Z. Interactive Oxidation–Reduction Reaction for the in Situ Synthesis of Graphene–Phenol Formaldehyde Composites with Enhanced Properties. ACS Appl. Mater. Interfaces 2014, 6, 4254–4263. [Google Scholar] [CrossRef]
- Rusen, E.; Mocanu, A.; Nistor, L.C.; Hudhomme, P.; Diacon, A. Anionic Polymerization by an Electron Transfer Process from a CdSe Quantum Dot–Perylenediimide (PDI) System. RSC Adv. 2015, 5, 28228–28232. [Google Scholar] [CrossRef]
- Liscio, A.; Palermo, V.; Gentilini, D.; Nolde, F.; Müllen, K.; Samorì, P. Quantitative Measurement of the Local Surface Potential of π-Conjugated Nanostructures: A Kelvin Probe Force Microscopy Study. Adv. Funct. Mater. 2006, 16, 1407–1416. [Google Scholar] [CrossRef]
- Yang, J.; Jing, J.; Li, W.; Zhu, Y. Electron Donor–Acceptor Interface of TPPS/PDI Boosting Charge Transfer for Efficient Photocatalytic Hydrogen Evolution. Adv. Sci. 2022, 9, 2201134. [Google Scholar] [CrossRef] [PubMed]










| Semiconductors | ECB (vs. NHE) | EVB (vs. NHE) | Eg (eV) |
|---|---|---|---|
| P-COOH | −0.41 | +1.83 | 2.24 |
| P-NH | −0.28 | +2.01 | 2.29 |
| PP-1 | −0.26 | +1.99 | 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Luo, X.; Wang, F.; Xiang, W.; Zhou, C.; Huang, W.; Lu, K.; Li, S.; Zhou, M.; Yang, K. Novel PDI-NH/PDI-COOH Supramolecular Junction for Enhanced Visible-Light Photocatalytic Phenol Degradation. Molecules 2024, 29, 4196. https://doi.org/10.3390/molecules29174196
Xu Y, Luo X, Wang F, Xiang W, Zhou C, Huang W, Lu K, Li S, Zhou M, Yang K. Novel PDI-NH/PDI-COOH Supramolecular Junction for Enhanced Visible-Light Photocatalytic Phenol Degradation. Molecules. 2024; 29(17):4196. https://doi.org/10.3390/molecules29174196
Chicago/Turabian StyleXu, Yongzhang, Xingrui Luo, Fulin Wang, Wentao Xiang, Chensheng Zhou, Weiya Huang, Kangqiang Lu, Shaoyu Li, Man Zhou, and Kai Yang. 2024. "Novel PDI-NH/PDI-COOH Supramolecular Junction for Enhanced Visible-Light Photocatalytic Phenol Degradation" Molecules 29, no. 17: 4196. https://doi.org/10.3390/molecules29174196
APA StyleXu, Y., Luo, X., Wang, F., Xiang, W., Zhou, C., Huang, W., Lu, K., Li, S., Zhou, M., & Yang, K. (2024). Novel PDI-NH/PDI-COOH Supramolecular Junction for Enhanced Visible-Light Photocatalytic Phenol Degradation. Molecules, 29(17), 4196. https://doi.org/10.3390/molecules29174196








