UiO-66 with Both Brønsted and Lewis Acid Sites for Catalytic Synthesis of Biodiesel
Abstract
1. Introduction
2. Results and Discussion
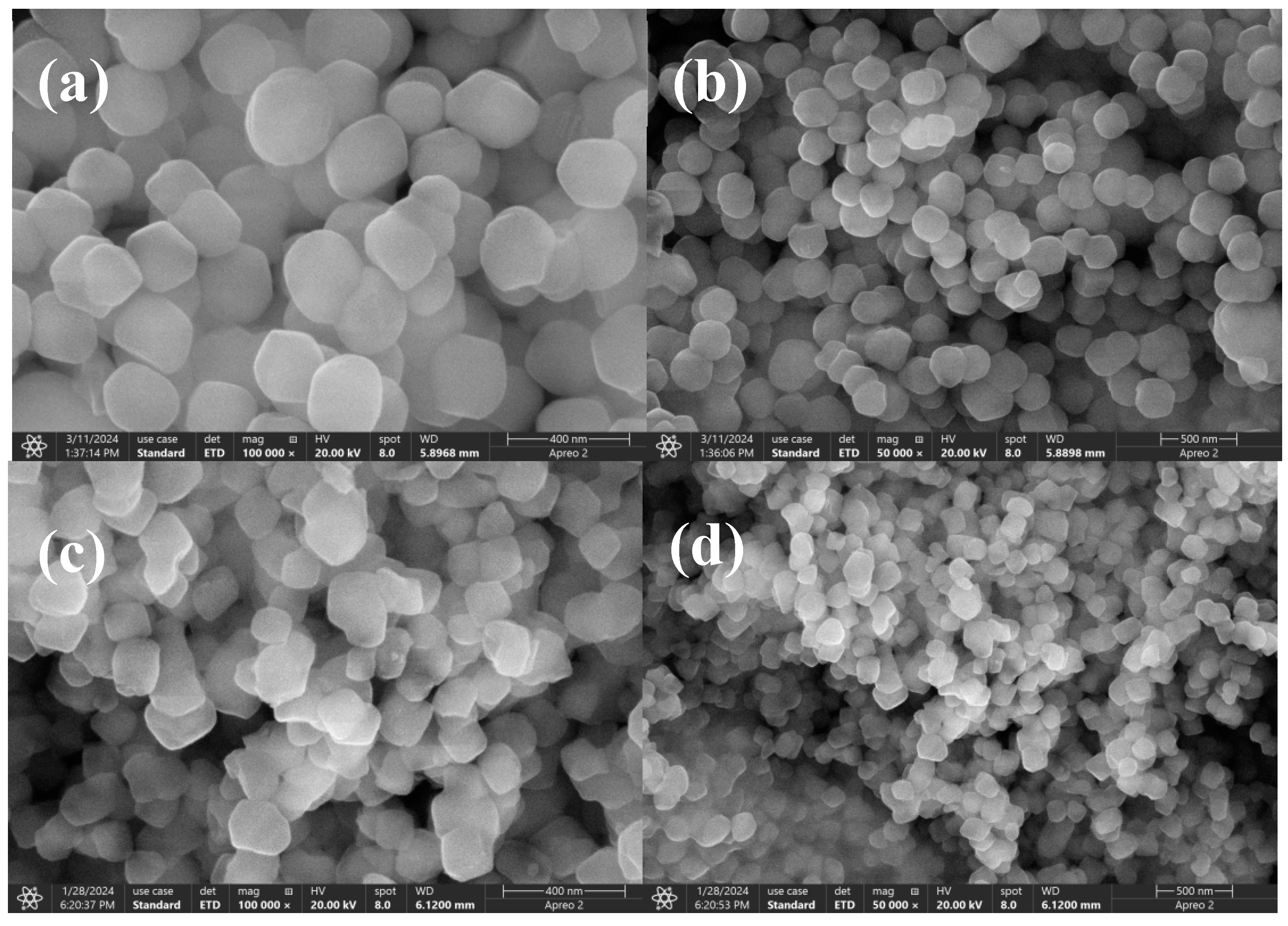
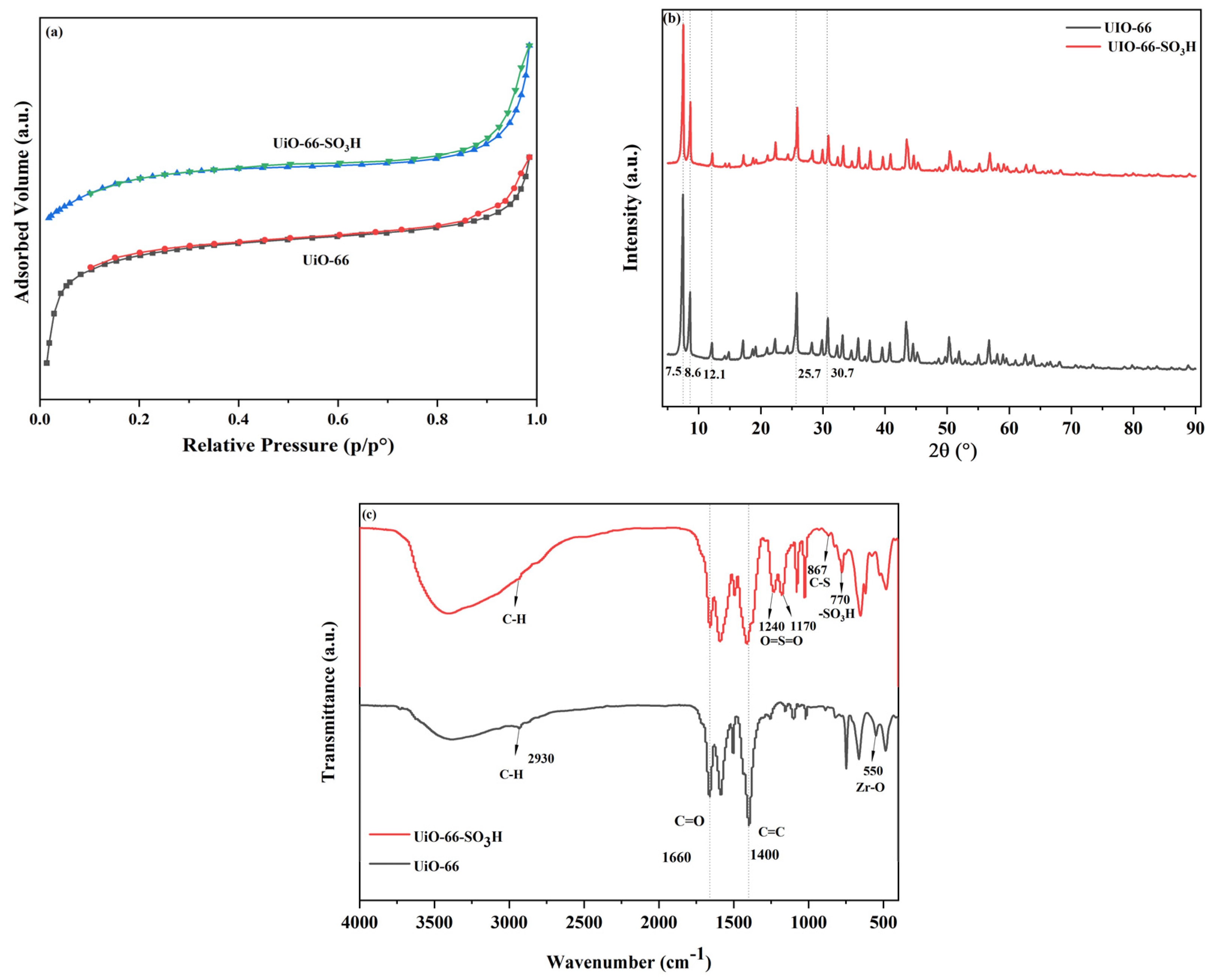
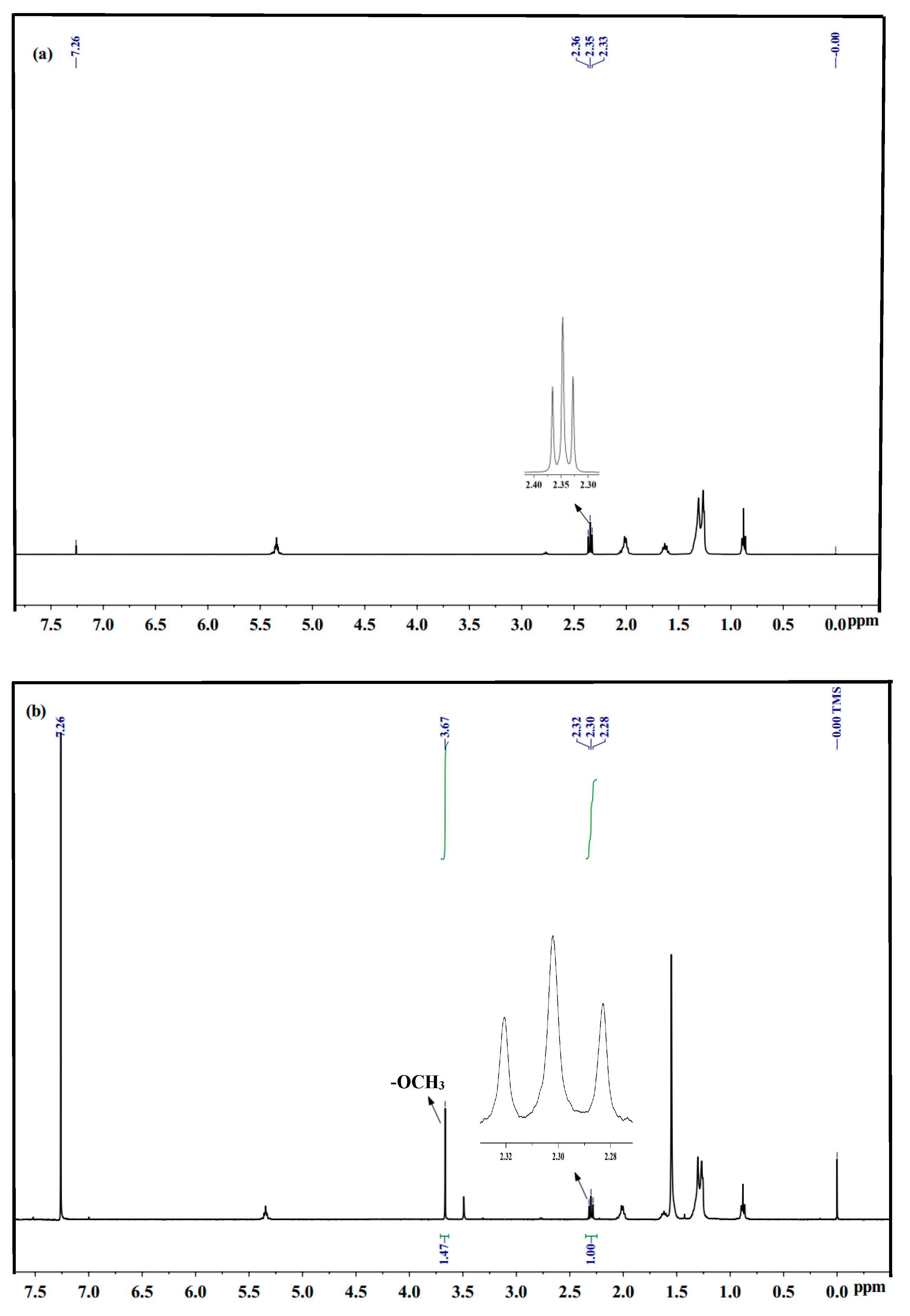
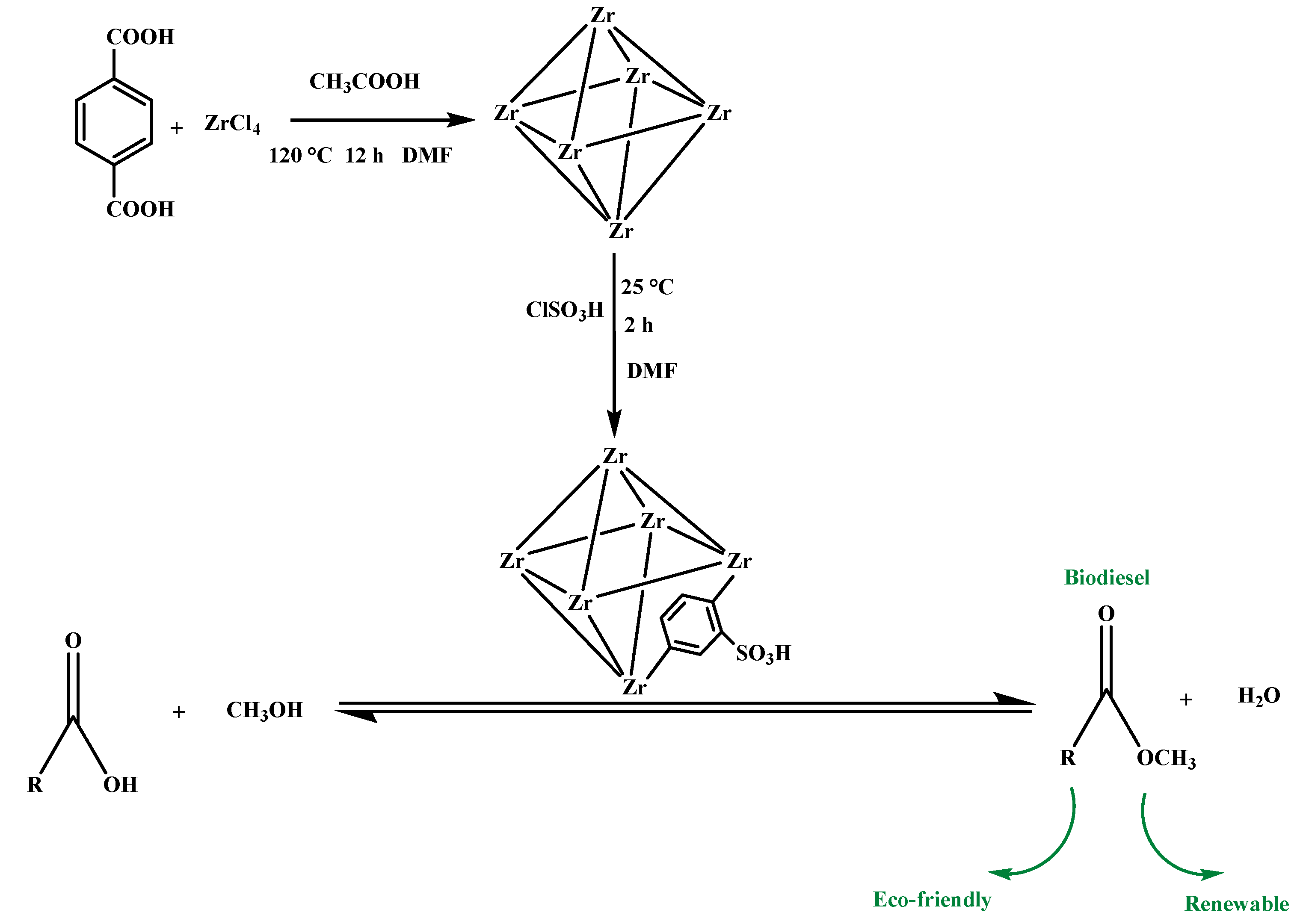
2.1. Catalysts Characterisation
2.2. Biodiesel Yield Analysis
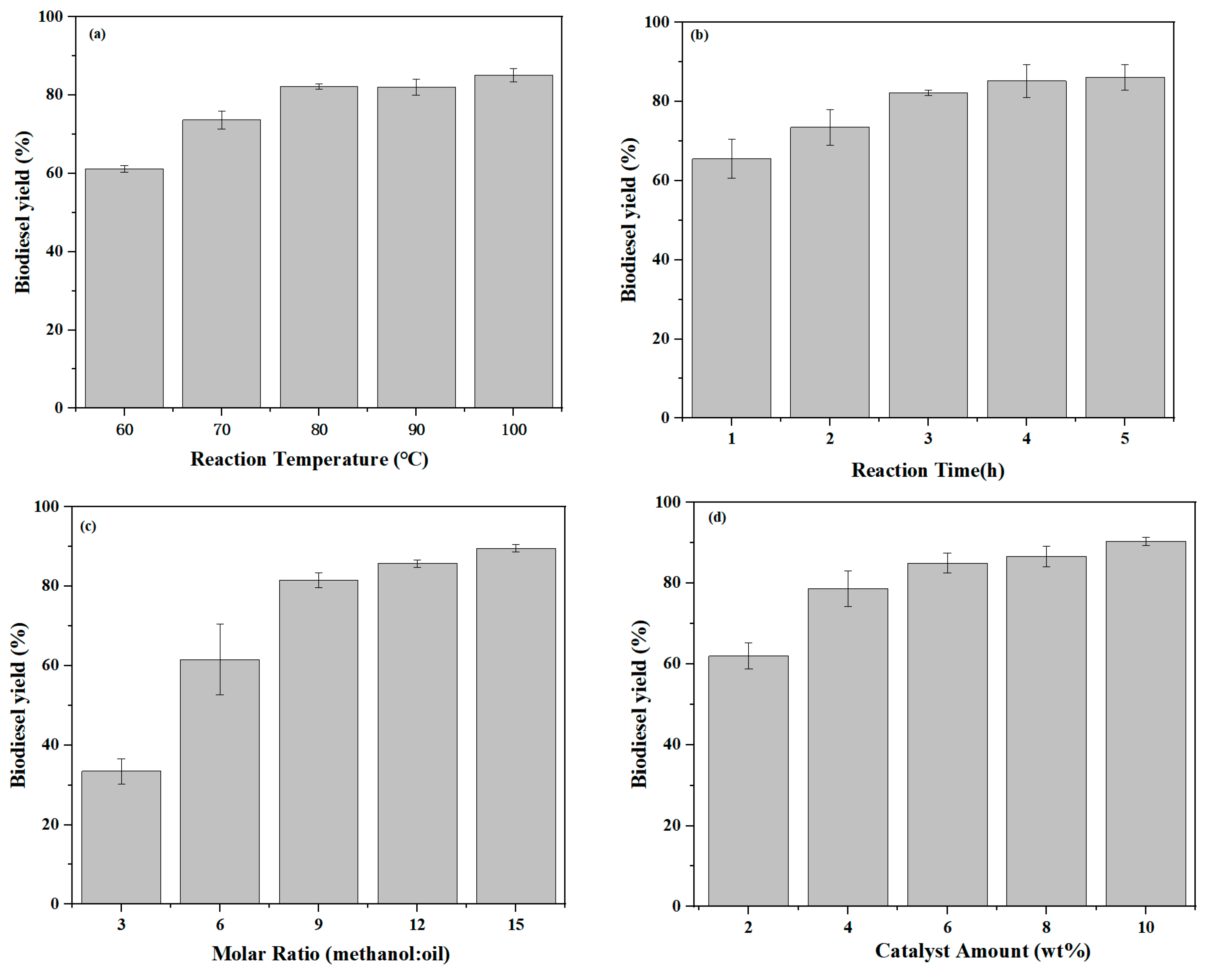
2.3. Optimisation of Reaction Conditions
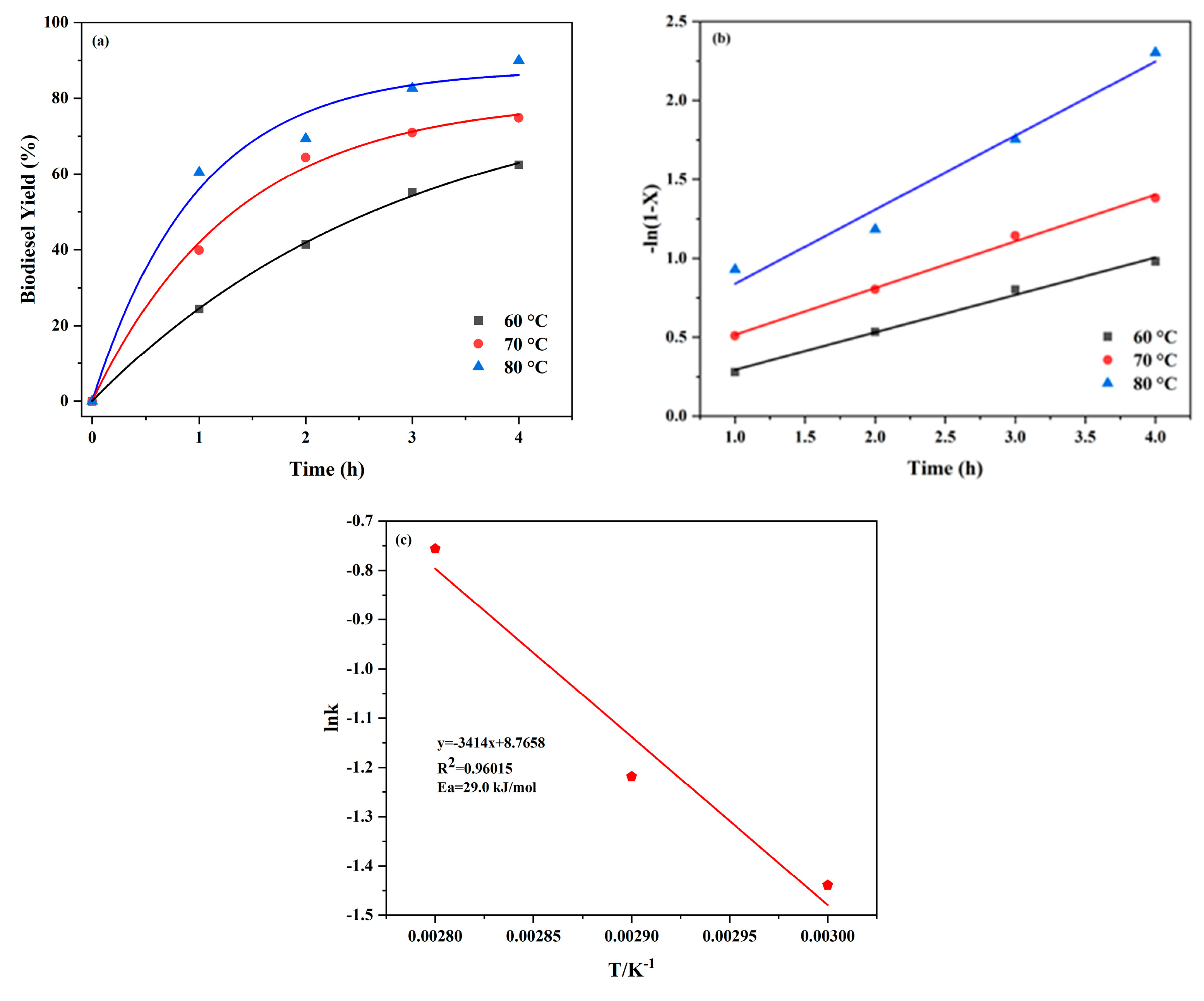
2.4. Kinetic Study
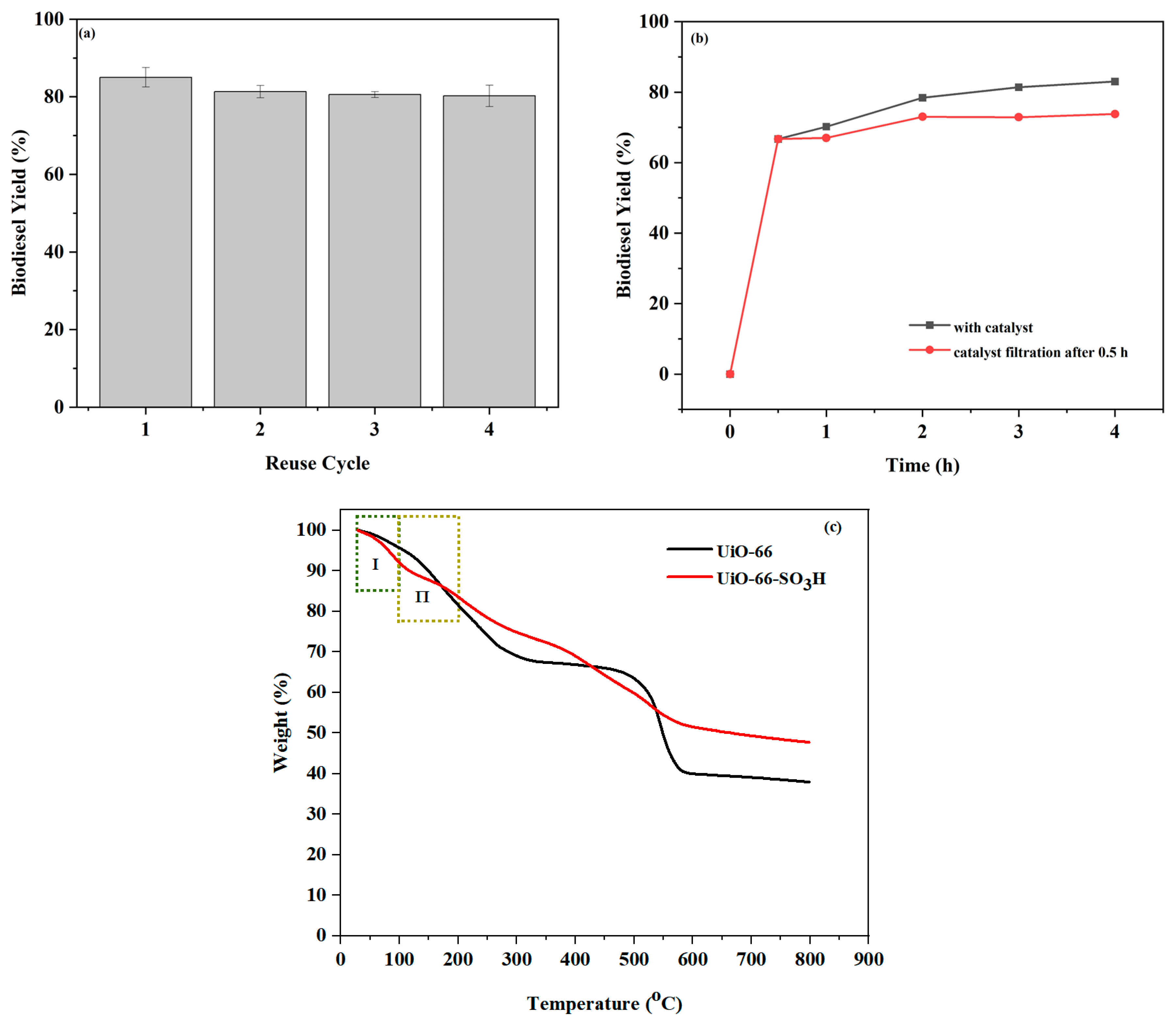
2.5. Reusability Studies of Catalysts
3. Materials and Methods
3.1. Materials
3.2. Preparation of Catalysts
3.3. Sulfonation of Catalysts
3.4. Characterisations
3.5. Acid Value Measurement
3.6. Determination of Acid Density
3.7. Preparation of Biodiesel by UiO-66-SO3H Catalysed Hydrolysis of Oleic Acid
3.8. Parameters Affecting Catalytic Activity
3.9. Reaction Kinetics Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezki, B.; Essamlali, Y.; Amadine, O.; Sair, S.; Aadil, M.; Len, C.; Zahouily, M. A comprehensive re-view on apatite-derived catalysts for sustainable biodiesel production: Classification, features and challenges. J. Environ. Chem. Eng. 2024, 12, 111913. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.F.; Shah, M.U.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for bio-diesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Asuquo, A.J.; Zhang, X.L.; Lin, L.T.; Li, J. Green synthesis of heterogeneous polymeric bio-based acid decorated with hydrophobic regulator for efficient catalytic production of biodiesel at low temperatures. Fuel 2022, 329, 125467. [Google Scholar] [CrossRef]
- Eswaramoorthi, Y.; Pandian, S.; Sahadevan, R. Sahadevan, Kinetic studies on the extraction of oil from a new feedstock (Chukrasia tabularis L. seed) for biodiesel production using a heterogeneous catalyst. Environ. Sci. Pollut. Res. 2023, 30, 14565–14579. [Google Scholar] [CrossRef]
- Zahid, A.; Mukhtar, Z.; Shahid, S.; Almasoud, N.; Shariq, M.; Almutib, E.; Alasmari, A.; Alshehri, K.; Al-Gethami, W.; Hassan, K.F.; et al. Efficient Production of Biodiesel by the Transesterification of Neem Oil Using Ni-Doped ZnO Nanoparticles as Heterogeneous Catalysts. Arab. J. Sci. Eng. 2024, 49, 1–10. [Google Scholar] [CrossRef]
- Ningaraju, C.; Yatish, K.V.; Prakash, M.R.; Sakar, M.; Balakrishna, G.R. Simultaneous refining of biodiesel-derived crude glycerol and synthesis of value-added powdered catalysts for biodiesel production: A green chemistry approach for sustainable biodiesel industries. J. Clean. Prod. 2022, 363, 132448. [Google Scholar] [CrossRef]
- Gadore, V.; Mishra, S.R.; Ahmaruzzaman, M. Ahmaruzzaman, Advances in photocatalytic biodiesel production: Preparation methods, modifications and mechanisms. Fuel 2024, 362, 130749. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.P.; Yang, S. Functional Nanomaterials-Catalyzed Production of Biodiesel. Curr. Nanosci. 2020, 16, 376–391. [Google Scholar] [CrossRef]
- Zhang, M.J.; Jiao, X.P.; Ren, D.Z.; Huang, C.X.; Li, Y.; Chen, D.F.; Huo, Z.B. Design of anti-saponification Ca-based catalyst with porous-shell structure from crustacean waste for biodiesel production. Fuel 2024, 363, 131006. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.Y.; Fan, J.H.; Han, Z.W. Synthesis, catalysts and enhancement technologies of biodiesel from oil feedstock—A review. Sci. Total Environ. 2023, 904, 166982. [Google Scholar] [CrossRef]
- Sun, X.K.; Xu, L.Y.; Yuan, H.L.; Wang, G.L.; Maleki, B. Boosted conversion of restaurant waste oil into biodiesel using Fe3O4@UiO-66-NH2 magnetic heterogeneous nanocatalyst and its application on the diesel engine: Optimization via RSM. Renew. Eengry 2024, 223, 120007. [Google Scholar] [CrossRef]
- Wang, A.P.; Quan, W.X.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for ef-ficient biodiesel production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Bekele, D.T.; Shibeshi, N.T.; Reshad, A.S. Catalytic Performance Investigation of Alkali and Bifunctional Catalysts Derived from Lignocellulosic Biomasses for Biodiesel Synthesis from Waste Fry-ing Oil. ACS Omega 2024, 9, 2815–2829. [Google Scholar] [CrossRef]
- Li, K.; Xie, W.L. Enhanced biodiesel production from low-value acidic oils using ordered hierarchical macro-mesoporous MoAl@H-SiO2 catalyst. Fuel 2024, 364, 131105. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.B.; Zhou, K.C.; Yang, S. Chemo-catalytic Esterification and Transesterification over Organic Polymer-Based Catalysts for Biodiesel Synthesis. Curr. Org. Chem. 2019, 23, 2190–2203. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, G.; Shi, Y.; Pang, H. Synthesis and catalytic application of defective MOF materials. Cell Rep. Phys. Sci. 2023, 4, 101301. [Google Scholar] [CrossRef]
- Ediati, R.; Zulfa, L.L.; Putrilia, R.D.; Hidayat, A.; Sulistiono, D.O.; Rosyidah, A.; Martak, F.; Har-tanto, D. Synthesis of UiO-66 with addition of HKUST-1 for enhanced adsorption of RBBR dye. Arab. J. Chem. 2023, 16, 104637. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Ma, H.; Wang, Z.H.; Zhao, N.; Wang, S. Fabrication of Fe3O4@UiO-66-SO3H core-shell functional adsorbents for highly selective and efficient removal of organic dyes. New J. Chem. 2019, 43, 7770–7777. [Google Scholar] [CrossRef]
- Hu, D.W.; Miao, S.S.; Zhang, P.F.; Wu, S.Y.; He, Y.P.; Meng, Q.W. Modulated synthesis of cesium phosphomolybdate encapsulated in hierarchical porous UiO-66 for catalysing alkene epoxidation. RSC Adv. 2023, 13, 33533–33540. [Google Scholar] [CrossRef]
- Le, S.K.; Jin, Q.J.; Han, J.A.; Zhou, H.C.; Liu, Q.S.; Yang, F.; Miao, J.; Liu, P.P.; Zhu, C.Z.; Xu, H.T. Rare earth element-modified MOF materials: Synthesis and photocatalytic applications in environmental remediation. Rare Met. 2024, 1–17, 1390–1406. [Google Scholar] [CrossRef]
- Fonseca, J.; Meng, L.X.; Imaz, I.; Maspoch, D. Self-assembly of colloidal metal-organic framework (MOF) particles. Chem. Soc. Rev. 2023, 52, 2528–2543. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.A.; Wang, Y.; Ren, Z.Y.; Shen, H.X.; Sheng, J.; Zhang, K.; Wang, J.W.; Wang, X.M. Preparation of ILe@cu@MOF catalyst and its application in biodiesel catalysis. Coatings 2023, 13, 49. [Google Scholar] [CrossRef]
- Dai, Q.Q.; Yang, Z.F.; Li, J.; Cao, Y.; Tang, H.B.; Wei, X.C. Zirconium-based MOFs-loaded ionic liquid-catalyzed preparation of biodiesel from Jatropha oil. Renew. Energy 2021, 163, 1588–1594. [Google Scholar] [CrossRef]
- Yang, J.; Cong, W.J.; Zhu, Z.Y.; Miao, Z.D.; Wang, Y.T.; Nelles, M.; Fang, Z. Microwave-assisted one-step production of biodiesel from waste cooking oil by magnetic bifunctional SrO-ZnO/MOF catalyst. J. Clean. Prod. 2023, 395, 136182. [Google Scholar] [CrossRef]
- Saidi, M.; Amirnia, R. Recent advances on application of Metal-Organic framework based catalysts in biodiesel production Process: A review of catalyst types and Activity, challenges and opportunities. Fuel 2024, 363, 130905. [Google Scholar] [CrossRef]
- Asuquo, A.J.; Zhang, X.L.; Lin, L.T.; Li, J. Green heterogeneous catalysts derived from fermented kola nut pod husk for sustainable biodiesel production. Int. J. Green Energy 2024, 21, 2218–2227. [Google Scholar] [CrossRef]
- Ruan, Z.H.; Wang, X.; Yuan, X. Improved catalytic performance and stability of defected UiO-66-SO3H in the esterification reaction of cyclohexene with cyclohexanecarboxylic acid. J. Porous Mater. 2022, 29, 1957–1968. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Li, Y.C.; Hu, Y.L.; Li, H.; Xu, C.C.; Yang, S. Functionalized organic-inorganic hybrid porous coordination polymer-based catalysts for biodiesel production via trans/esterification. Green Chem. 2022, 24, 7763–7786. [Google Scholar] [CrossRef]
- Tatay, S.; Martinez-Giménez, S.; Rubio-Gaspar, A.; Gómez-Oliveira, E.; Castells-Gil, J.; Dong, Z.Y.; Mayoral, A.; Almora-Barrios, N.; Padial, N.M.; Marti-Gastaldo, C. Synthetic control of correlated disorder in UiO-66 frameworks. Nat. Commun. 2023, 14, 6962. [Google Scholar] [CrossRef]
- Zhong, L.H.; Liao, X.Q.; Cui, H.S.; Luo, H.; Lv, Y.; Liu, P.L. Hydrogenation of α,β-unsaturated aldehydes over defective UiO-66 with Frustrated Lewis Pairs: Modulation of densities of defect sites via tailoring ligand-vacancies. Appl. Catal. B-Environ. 2024, 342, 123421. [Google Scholar] [CrossRef]
- Gouda, S.P.; Shi, D.; Basumatary, S.; Li, H.; Piloto-Rodríguez, R.; Rokhum, S.L. Sulfamic acid modi-fied UiO-66 metal-organic framework for biodiesel production: Process optimization using response sur-face methodology, kinetics, and thermodynamic study. Chem. Eng. J. 2024, 480, 148154. [Google Scholar] [CrossRef]
- Chen, F.; Kim, S.; Barpaga, D.; Fulton, J.L.; Motkuri, R.K.; Gutiérrez, O.Y.; Camaioni, D.M.; Lercher, J.A. Activity of bronsted acid sites in UiO-66 for cyclohexanol dehydration. Top. Catal. 2023, 66, 1196–1201. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Azarifar, D.; Dalirana, S.; Oveisi, A.R. The UiO-66-SO3H metal-organic framework as a green catalyst for the facile synthesis of dihydro-2-oxypyrrole derivatives. RSC Adv. 2016, 6, 29182–29189. [Google Scholar] [CrossRef]
- Sung, Y.S.; Lin, L.Y.; Lin, H.Y. Study of pH value effect on synthesizing UIO-66 and carbonized UIO-66 as active material for solid-state supercapacitors. J. Taiwan Inst. Chem. Eng. 2020, 116, 197–204. [Google Scholar] [CrossRef]
- Gouda, S.P.; Shi, D.; Basumatary, S.; Li, H.; Piloto-Rodríguez, R.; Rokhum, S.L. Synthesis of acidic modified UiO-66 by concentrated sulphation form and its application in the production of fatty acids ethyl esters. Can. J. Chem. Eng. 2024, 102, 399–412. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Liu, T.Y.; Ho, C.K.; Li, C.; Chan, K.Y. Imparting UiO-66 with fast cation exchange property via sulfonating organic linkers for selective adsorption. Sep. Purif. Technol. 2021, 260, 118219. [Google Scholar] [CrossRef]
- Ruan, H.; Pan, N.; Wang, C.; Yu, L.; Liao, J.; Shen, J. Functional UiO-66 series membranes with high perm selectivity of monovalent and bivalent anions for electrodialysis applications. Ind. Eng. Chem. Res. 2021, 60, 4086–4096. [Google Scholar] [CrossRef]
- Yangcheng, R.; Li, J.W.; He, J.D.; Zheng, Y.X.; Yu, H.J.; Chen, C.L.; Wang, J.J. Carboxyl-Decorated UiO-66 Supporting Pd Nanoparticles for Efficient Room-Temperature Hydrodeoxygenation of Lignin Derivatives. Small 2024, 20, 2309821. [Google Scholar] [CrossRef]
- Xia, S.; Tao, J.; Zhao, Y.; Men, Y.; Chen, C.; Hu, Y.; Zhu, G.; Chu, Y.; Yan, B.; Chen, G. Application of waste derived magnetic acid-base bifunctional CoFe/biochar/CaO as an efficient catalyst for biodiesel production from waste cooking oil. Chemosphere 2024, 350, 141104. [Google Scholar] [CrossRef]
- Luo, Q.Z.; Li, J.J.; Yun, P.F.; Qian, L.B.; Zhang, J.S.; He, C.Q.; Hu, X.J.; Jiang, H.F. Thin-film composite membrane for desalination containing a sulfonated UiO-66 material. J. Mater. Sci. 2023, 58, 3134–3146. [Google Scholar] [CrossRef]
- Elnasr, T.; Al-Enezi, A.T.; Hussein, M.F.; Bielal, H.; Alhumaimess, M.S.; El-Ossaily, Y.A.; Hassan, H.; Alnahwa, L.; Aldawsari, A.M.; Alsohaimi, I.H. Sustainable biodiesel production from waste olive oil: Utilizing olive pulp-derived catalysts for environmental and economic benefits. Sustain. Chem. Pharm. 2024, 37, 101426. [Google Scholar] [CrossRef]
- Zheng, B.H.; Chen, L.; He, L.J.; Wang, H.; Li, H.; Zhang, H.; Yang, S. Facile synthesis of chitosan-derived sulfonated solid acid catalysts for realizing highly effective production of biodiesel. Ind. Crops Prod. 2024, 210, 118058. [Google Scholar] [CrossRef]
- Wang, A.P.; Zhang, H.; Li, H.; Yang, S. Efficient Production of Methyl Oleate Using a Biomass-Based Solid Polymeric Catalyst with High Acid Density. Adv. Polym. Technol. 2019, 2019, 4041631. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.N.; Li, H.; Wang, Y.G.; Shen, Z.F.; Wang, Y.Q.; Li, L.F.; Li, X.; Xu, H.Y.; Zhou, Z.M.; et al. Direct production of biodiesel from crude Euphorbia lathyris L. Oil catalyzed by multifunctional mesoporous composite materials. Fuel 2022, 309, 122172. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.L.; Tan, X.; Li, H.; Yang, S. Catalytic high-yield biodiesel production from fatty acids and non-food oils over a magnetically separable acid nanosphere. Ind. Crops Prod. 2021, 173, 114126. [Google Scholar] [CrossRef]
- Gouda, S.P.; Ngaosuwan, K.; Assabumrungrat, S.; Selvaraj, M.; Halder, G.; Rokhum, S.L. Microwave assisted biodiesel production using sulfonic acid-functionalized metal-organic frameworks UiO-66 as a heterogeneous catalyst. Renew. Energy 2022, 197, 161–169. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.P.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
- Choksi, H.; Pandian, S.; Gandhi, Y.H.; Deepalakshmi, S. Studies on production of biodiesel from Madhuca indica oil using a catalyst derived from cotton stalk. Energy Source Part A 2021, 43, 3424–3433. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Feng, L.; Zhang, X. Hierarchically ordered porous solid acid: Preparation and application as a biodiesel catalyst. ChemistrySelect 2022, 7, e202200514. [Google Scholar] [CrossRef]
- Kushwaha, T.; Ao, S.; Ngaosuwan, K.; Assabumrungrat, S.; Gurunathan, B.; Rokhum, S.L. Esterification of oleic acid to biodiesel using biowaste-based solid acid catalyst under microwave irradiation. Environ. Prog. Sustain. 2023, 42, e14170. [Google Scholar] [CrossRef]
- Amouhadi, E.; Fazaeli, R.; Aliyan, H. Biodiesel production via esterification of oleic acid catalyzed by MnO2@Mn(btc) as a novel and heterogeneous catalyst. J. Chin. Chem. Soc. 2019, 66, 608–613. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Li, H.X.; Xu, Z.L.; Li, P.; Zhan, Z.M.; Li, P.P.; Xu, S.J. Ceramic hollow fiber NF membrane incorporating UiO-66 for the chlorinated hydrocarbons removal. Chem. Eng. J. 2022, 435, 134789. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, H.; Li, H.; Yang, S. Electrovalent bifunctional acid enables heterogeneously catalytic production of biodiesel by (trans)esterification of non-edible oils. Fuel 2022, 310, 122273. [Google Scholar] [CrossRef]
- Palhares, H.G.; Andrade, P.; Moissette, A.; Volkringer, C.; Loiseau, T.; Houmard, M.; Nunes, E. Simultaneous adsorption of anionic and cationic species on UiO-66 and MIL-125 MOFs: Mechanisms & selectivity. Microporous Mesoporous Mater. 2024, 368, 113010. [Google Scholar] [CrossRef]
- Manaf, I.; Embong, N.H.; Khazaai, S.; Rahim, M.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
| Sample | BET Surface Area (m2/g) | Pore Volume (cm³/g) | Average Particle Size (nm) |
|---|---|---|---|
| UiO-66-SO3H | 913.8 | 0.09520 | 245.7 |
| UiO-66 | 1226 | 0.1029 | 194.7 |
| Temperature (°C) | Reaction Rate Constant (k) | Coefficient of Determination (R2) |
|---|---|---|
| 60 | 0.2372 | 0.9928 |
| 70 | 0.2958 | 0.9958 |
| 80 | 0.4693 | 0.9759 |
| Catalysts | Activation Energy (KJ/mol) | Reference |
|---|---|---|
| UiO-66-SO3H | 35.3 | [46] |
| UiO-66/SA | 41.5 | [31] |
| Fe3O4@SiO2-SO3H | 47.9 | [45] |
| UiO-66-SO3H | 29.0 | This work |
| NO. | Catalyst | Feedstock | Reaction Conditions | Biodiesel Yield (Conversion) (%) | Ref. |
|---|---|---|---|---|---|
| 1 | cotton stalk | Madhuca indica oil | T = 60 °C, CA = 5 wt%, M/O = 18:1, t = 5 h | 89.2 | [48] |
| 2 | HOP CLPS-SO3H-7 | oleic acid | T = 60 °C, CA = 6 wt%, M/O = 10:1, t = 6 h | 93.7 | [49] |
| 3 | WP-SO3H-6 | oleic acid | T = 100 °C, CA = 8 wt%, M/O = 20:1, t = 20 h | 94.4 | [50] |
| 4 | MnO2@Mn(btc) | oleic acid | T = 100 °C, CA = 3 wt%, M/O = 12:1, t = 12 h | 98 | [51] |
| 5 | UiO-66-SO3H | oleic acid | T = 80 °C, CA = 6 wt%, M/O = 9:1, t = 3 h | 90.0 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, Z.; Wu, X.; Quan, W.; Chen, Q.; Wang, A. UiO-66 with Both Brønsted and Lewis Acid Sites for Catalytic Synthesis of Biodiesel. Molecules 2024, 29, 4195. https://doi.org/10.3390/molecules29174195
Wang Y, Yang Z, Wu X, Quan W, Chen Q, Wang A. UiO-66 with Both Brønsted and Lewis Acid Sites for Catalytic Synthesis of Biodiesel. Molecules. 2024; 29(17):4195. https://doi.org/10.3390/molecules29174195
Chicago/Turabian StyleWang, Yu, Zhimin Yang, Xichang Wu, Wenxuan Quan, Qi Chen, and Anping Wang. 2024. "UiO-66 with Both Brønsted and Lewis Acid Sites for Catalytic Synthesis of Biodiesel" Molecules 29, no. 17: 4195. https://doi.org/10.3390/molecules29174195
APA StyleWang, Y., Yang, Z., Wu, X., Quan, W., Chen, Q., & Wang, A. (2024). UiO-66 with Both Brønsted and Lewis Acid Sites for Catalytic Synthesis of Biodiesel. Molecules, 29(17), 4195. https://doi.org/10.3390/molecules29174195







