Abstract
Hydrophilic and hydrophobic phenomena occur in aqueous solutions. Despite the complex nature of the molecular interactions, the propensity of molecules and ions to hydration is sometimes characterized by a single “hydration number”. Passynski’s method for determining the hydration numbers in dilute aqueous solutions belongs to the group of methods based on the analysis of the isentropic compressibility of a mixture. Isentropic compressibility is a thermodynamic material constant; thus, the paper deals with Passynski’s approach discussed in terms of thermodynamics. First, Passynski’s assumptions were applied to the volume of the mixture. Subsequent strict thermodynamic derivation led to a formula for the hydration number which resembled that of Onori rather than the original one. Passynski’s number turned out to be inconsistent with the thermodynamics and mechanics of fluids. This is a rather purely empirical measure of the slope of the dependence of isentropic compressibility on the solute mole fraction in a dilute aqueous solution. Being the quotient of the slope and the isentropic compressibility of pure water, Pasynski’s numbers are more convenient to analyze and discuss than the slopes themselves. Conclusions about molecular interactions based on these numbers must be treated with considerable caution.
Keywords:
solvation; hydration; aqueous solutions; compressibility; Pasynski; speed of sound; ideal mixture 1. Introduction
The lack of an unequivocal method for determining the molecular order in liquids causes many tentative concepts to exist even if they are not clearly defined. One such is that of the solvation number. Contrary to crystals, molecular aggregates in the liquid phase are often dynamic, labile, and of variable size. It seems rather difficult, if possible at all, to characterize complex solute-solvent interaction in liquids by just one number. The interactions in aqueous solutions involve hydrogen bonds that are manifested in hydrophilic and hydrophobic phenomena [1]. No wonder the hydration numbers depend not only on the system itself but also on the method of determination. A group of such methods is based on the analysis of the compressibility of aqueous solutions. One of these was suggested by Passynski (also spelled Pasynski) almost a century ago [2,3]. It is still applied (e.g., [3,4,5,6,7,8,9,10,11,12,13,14,15]), although its consistency with the thermodynamics and mechanics of fluids has never been verified. This paper is aimed at filling this gap.
Various solutes dissolved in water, both electrolytes and non-electrolytes, lower the compressibility of the liquid [3]. Passynski [2,3] assumed that the isentropic compressibility of binary aqueous solution results solely from the compressibility of bulk water, κS,1* > 0, while both the solute and water in the hydration sphere are incompressible: κS,2* = 0 and κS,1h = 0, respectively. The bulk water in the mixture is identical to pure water. Thus, the amount of compressible water decreases with the addition of the solute due to hydration, and each solute molecule binds several water molecules to form the hydrate. This led to the conclusion that the isentropic compressibility of such a mixture relates to that of pure water in the following manner:
where ni is the number of moles, xi is the mole fraction, i = 1 for water, i = 2 for the solute, and kP is the hydration number. The latter will be called “Passynski’s number” from here on to avoid confusion with other similar quantities reported in this work. In other words, Passynski arbitrarily assumed that the isentropic compressibility of the system is directly proportional to the quantity of bulk water. It seems questionable at the least. The following re-arranged Equation (1) gives the Passynski’s number:
where the molar ratio is equal to the mole fractions ratio: . Indeed, the kP depends on the concentration. For this reason, it is sometimes reported for infinitely diluted solutions [3], calculated as follows:
Isentropic compressibility can be calculated from the experimental speed of sound in the liquid, u, and its density, ρ, using Laplace’s formula as follows:
Precise and easy-to-operate speed of sound and density meters, e.g., [16], developed in the last decades, facilitated the routine measurements of the speed and density. That tempts researchers to calculate and report Passynski’s numbers identified as hydration numbers [3,4,5,6,7,8,9,10,11,12,13,14,15]. The author believes that this community will appreciate the present evaluation of Passynski’s method.
2. Two-State Model of the Binary Aqueous Solution
Equations (1) and (2) seem to result from the formation of incompressible hydrates in the solution. However, different conclusions can be drawn if one considers the volume of the mixture first, and the compressibility only after that. Application of Passynski’s idea of hydration leads to the following formula for the extensive volume of the mixture:
where V1* is the molar volume of pure water, V2h is the molar volume of the hydrate, n1* is the quantity of bulk water (i.e., number of moles), and n2 is the quantity of the solute which is equal to the quantity of the hydrate expressed in moles. The molar volume of the hydrate, V2h, encompasses the molar volumes of the solvent and the hydration sphere. The n1* is related to the total number of water molecules, n1, and the hydration number, k, by the following Equation (6):
Consequently:
Equation (7) is the fundamental relationship for the volume of Passynski’s mixture. Thus, Passynski’s idea applied to the volume of the mixture led to the equation which is identical to that suggested by Onori [3,17].
The pressure derivative of V defines the compression of the system as follows:
where z is the temperature (T) or entropy (S) for the isothermal and isentropic processes, respectively. Like V, Kz is an extensive property. The compressibility is its intensive counterpart:
Isentropic compressibility has been used in the calculations of Passynski’s numbers. Now, we shall prove that Passynski’s Equation (2) cannot be obtained from the model Equation (7).
Substitution of Equation (7) to Equation (8) gives:
which for isothermal conditions reduces to the following:
Following Passynski, we assume that the hydrate is incompressible, , and the hydration number k does not depend on pressure, k = const. The latter is counterintuitive. One would expect that an increase in pressure should result in a disruption of the lattice-like structure of the bulk water and the growth of the incompressible hydration sphere. However taking this for granted, one obtains the following:
For 1 mole of the mixture, Equation (12) takes the form:
where Re-arranging Equation (13) gives the hydration number k:
or
where Vm is the molar volume of the mixture, κT is its isothermal compressibility, and V1* and κT,1* are the respective functions for pure water.
Now, a question arises whether Equations (14) and (15) are valid for ΚS and κS substituted for their isothermal counterparts. Equation (10) for the isentropic conditions and k = const. would take a form similar to the respective equation in Onori’s model [3,17], in which . Of crucial importance here is noticing the fundamental difference between isothermal and isentropic compressions, KT and KS. Only the first one is a Gibbsian property [18], which makes a substitution of KT for always correct. Similar substitution of KS for is not possible for the derivatives of volume on the right-hand side of Equation (10). For example:
rather than just
Indeed, the quantities given by Equations (16) and (17) differ one from another. Moreover, Equation (17) is ambiguous. Subscript S1* in Equation (16) denotes the constant partial entropy of the first component of the system, i.e., the bulk water here. The conditions in Equation (17) are less restrictive—subscript S indicates that just the total entropy of the system remained unchanged:
This does not imply S1* = const. for S = const. On the contrary, the changes in entropy of the mixture components, n1S1* and n2S1h, may cancel each other out resulting in the constant total entropy S. For the same reason the molar isothermal compression of the thermodynamic ideal mixture, KT,mid, is the mole-fraction weighted average of the isothermal compressions of its pure components, calculated as follows:
while for the isentropic compression:
A well-known thermodynamic relationship relates the two compressions one to another, e.g., [19]:
where is the isobaric molar thermal expansion and Cp,m is the isobaric heat capacity. Thus, the substitution of KT,m and KT,1* given by Equation (21) to Equation (14) relates the hydration number to the isentropic compression. An alternative is the following approximate relationship:
which results from the rather bold assumption:
Equations (14), (15) and (22) differ from Passynski’s Equation (2).
3. Discussion
We have shown that Passynski’s assumption applied to the volume leads to equations different from those suggested by himself. The two fundamental reservations about Passynski’s reasoning are (i) consideration of the isentropic rather than isothermal compressibility and (ii) the additivity rules for compressibility inconsistent with thermodynamics and mechanics of fluids. Indeed, the derived model of the mixture compressibility is not Passynski’s Equation (1) but that of Onori [17], simplified by the assumption about the incompressible hydration sphere. Passynski’s numbers (Equation (2)) are arbitrary rather than resulting from a thermodynamically justified model because the isentropic compressibility of the binary ideal mixture of the incompressible and compressible species is not the mole-fraction-weighted average of the two quantities [19].
A good illustration could be the compressibility of the thermodynamic ideal binary mixture of a monoatomic gas with a diatomic homonuclear one. Both pure gases and their mixture fulfill the equation of state:
where R is the universal gas constant. The isothermal compressibility of the two gases, as well as their mixtures, is κT = p−1. The pure gases differ only in the isobaric heat capacities, equal to 5/2R and 7/2R, respectively. The isentropic compressibility can be calculated from the modified Equation (21), written as follows:
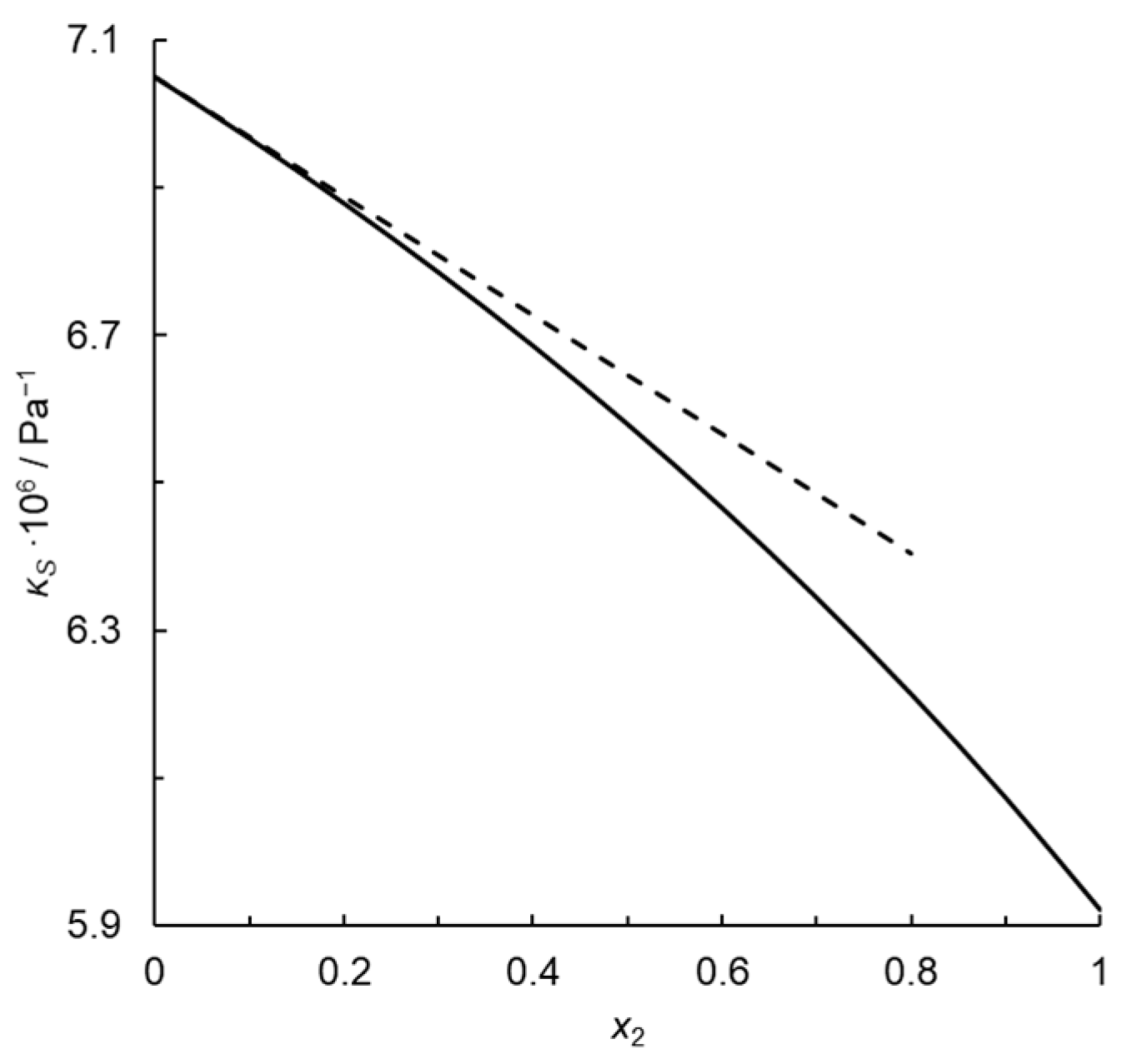
where , Vm = V1* = V2*, and Cp,m follow the ideal mixing rule:
Passynski’s number calculated for the monoatomic gas infinitely diluted in the diatomic one is 0.114 rather than 0, which would be expected for a fully random distribution of molecules. Moreover, Passynski’s number depends on concentration because isentropic compressibility does not depend rectilinearly on the mole fraction, as illustrated in Figure 1. The equation analogous to Passynski’s Equation (2) but with isothermal compressibility instead of an isentropic one is as follows:
where kP′ = 0, because κT = const. in the whole concentration range.
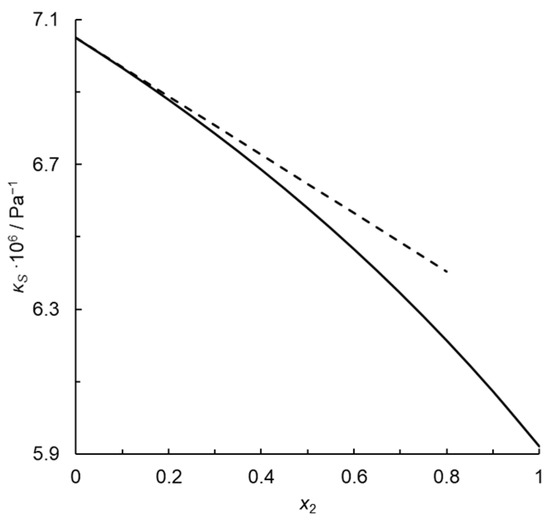
Figure 1.
Isentropic compressibility of the thermodynamic ideal binary mixture of a monoatomic gas with a diatomic homonuclear one at T = 273.15 K and p = 101,325 Pa (solid line), and tangent to this curve at x2 = 0 (dashed line); x2—mole fraction of the monoatomic gas.
The above considerations suggest that Passynski’s number is not a sensical characteristic of hydration. On the other hand, Passynski’s approach gave intuitively acceptable results for homologous series of alcohols, polyols, carbohydrates, polymers, and amino acids [3]. Burakowski and Gliński [3] thoroughly discussed this and five other methods of calculating the “hydration numbers” from the isentropic compressibility of dilute aqueous solutions or directly from the speed of sound in such systems. They noted that the isentropic compressibility of dilute solutions of nonelectrolytes (x2 < 0.01) can be satisfactorily interpolated by an empirical equation:
where κS,1* is a constant parameter, the coefficient a can be obtained by the least squares regression, and . The κS expressed by Equation (28) substituted to Equation (2) gives the Passynski’s number, calculated as follows:
for x2 < 0.01, and
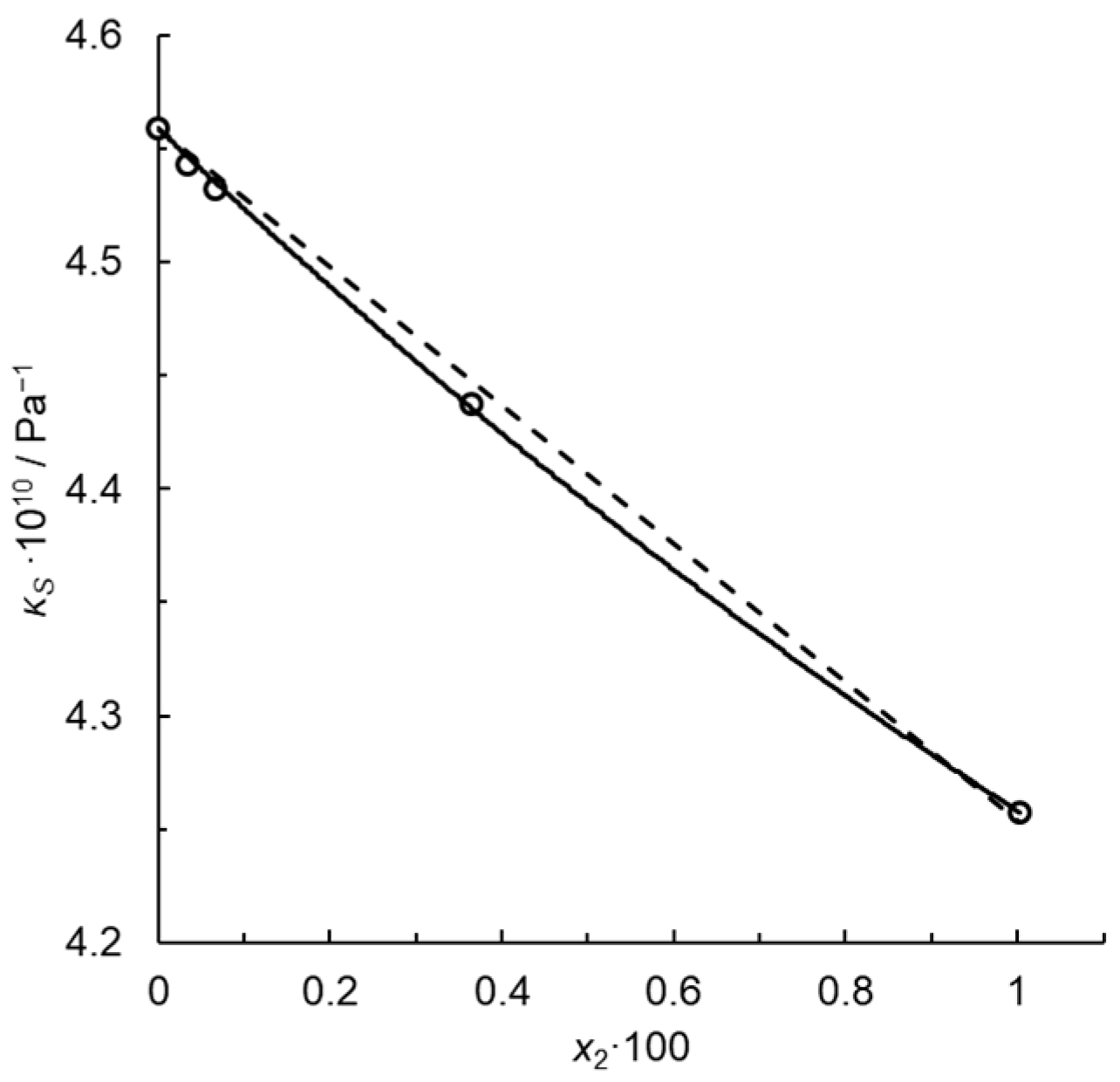
for the infinitely dilute solution. Note that kP for x2 = 0.01, i.e., for the maximum solute concentration, is just 1% lower than , because in this approximation. Thus, kP is constant in this concentration range within the uncertainty of the fit limits. Figure 1 illustrates the fit for dilute solutions of N-methylpiperidine in water at a temperature T = 293.15 K. Passynski’s number calculated using the linear Equation (28) is 6.69 ± 0.54 at the confidence level of 0.95 for infinite dilution, and determination coefficient R2 = 0.9961. Despite a high R2, the dependence of κS on x2 is not rectilinear but rather a convex downward function, approximated by a parabola in Figure 2.
with constant parameter κS,1* and fitted coefficients a and b. Here, no systematic deviations from the regression line occur and the determination coefficient is much higher, R2 = 0.9995. For infinite dilution, we write the following:
and = 7.88 ± 1.39 at the confidence level of 0.95. Such convex functions are typical of the isentropic compressibility isotherms for dilute aqueous solutions, e.g., [15]. Thus, Passynski’s numbers depend on the function applied to interpolate the empirical dependency of κS on x2. Their values must be calculated using carefully selected interpolation functions which provide random distribution of the residual deviations.
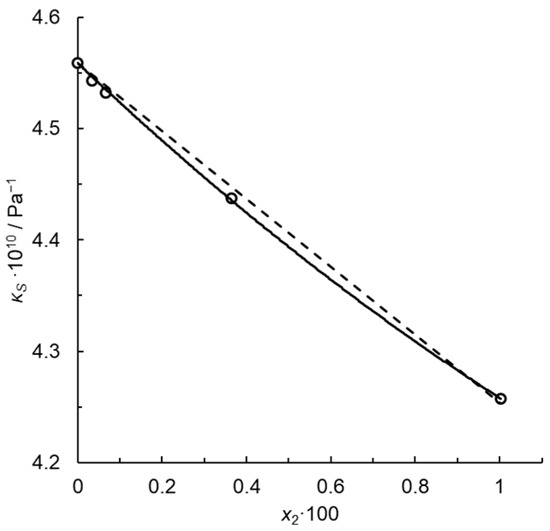
Figure 2.
Isentropic compressibility of the system water (1) + N-methylpiperidine at T = 293.15 K. Points—calculated using Equation (4) from the speeds of ultrasound and densities reported in [20]; solid line—Equation (31); dashed line—Equation (28).
Equations (29) and (30) illustrate the fact that Passynski’s number is just an empirical characteristic of the slope of the κS(x2) function. However, Passynski’s number seems to be related to the molecular structure of the solute. For example, it increases by 1.0 per methylene group in normal alcohol molecules, while by 0.75 in those of α,ω-diols [3]. Noteworthy is that other “hydration numbers” defined by Shiio [21,22,23], Yasunaga [24,25], Isemura and Goto [26,27], Millero [28,29], and Onori [17], and discussed by Burakowski and Gliński [3], do not fulfill the condition of thermodynamic consistency also.
4. Conclusions
Passynski’s numbers, often called the “hydration numbers” are inconsistent with thermodynamics and the mechanics of fluids. Equation (2) for Passynski’s number does not result from the equation for the volume of the binary mixture based on his model assumptions. The latter resembles rather the equation suggested by Onori [17]. A simple proof for this inconsistency is that Passynski’s number for the solute in the ideal binary mixture of perfect gases differs from zero for the gases with different heat capacities.
Passynski’s number seems to be a purely empirical measure of the slope of the dependence of isentropic compressibility on the solute mole fraction in dilute aqueous solution (Equations (29) and (30)). The quotient of the slope and the isentropic compressibility of pure water are indeed more convenient to analyze and discuss than the slope itself because it is commonly a small number between one and ten. It is seldom smaller than one, e.g., 0.9 for hydrogen peroxide [3]. Doubtless, hydration affects compressibility and is related to the slope of the κS(x2) function in this way. However, the effects of structure-making and structure-breaking effects of solute molecules in water cannot be explained by such a simple model. Muller’s observation made almost 40 years ago is still worth remembering: “Whether or not a particular solute is perceived as a structure maker thus depends on what data are chosen as a basis for judgment and on personal preferences as to how the data should be construed.” [30].
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are included within the article.
Acknowledgments
The author is indebted to Małgorzata Jóźwiak from the Faculty of Chemistry, University of Lodz, Poland, for inspiring discussions.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Franks, F. Water: 2nd Edition—A Matrix of Life. The Royal Society of Chemistry: Cambridge, UK, 2000. [Google Scholar]
- Passynski, A. Compressibility and solvation of solutions of electrolytes. Acta Physicochim. URSS 1938, 8, 385–418. [Google Scholar]
- Burakowski, A.; Gliński, J. Hydration Numbers of Nonelectrolytes from Acoustic Methods. Chem. Rev. 2012, 112, 2059–2081. [Google Scholar] [CrossRef] [PubMed]
- Burakowski, A.; Gliński, J. Hydration of the Zwitterionic Forms of Amino Acids from the Acoustic Passynski Method. Acta Phys. Pol. A 2008, 114, A39–A44. [Google Scholar] [CrossRef]
- Burakowski, A.; Gliński, J. Hydration of urea and its derivatives from acoustic and volumetric methods. Chem. Phys. Lett. 2015, 641, 40–43. [Google Scholar] [CrossRef]
- Chakraborty, N.; Juglan, K.C.; Kumar, H. Temperature-dependent thermodynamic and physicochemical studies of glycols in aqueous biotin solutions. J. Mol. Liq. 2021, 337, 116605. [Google Scholar] [CrossRef]
- Clavijo-Penagos, J.A.; Gracia-Fadrique, J.; Romero, C.M. Effect of temperature on the volumetric and thermoacoustic properties of polyoxyethylene (10) cetyl ether: C16E10 and polyoxyethylene (20) cetyl ether: C16E20 in aqueous solution. J. Mol. Liq. 2024, 398, 124225. [Google Scholar] [CrossRef]
- Cruz, Y.P.; Esteso, M.A.; Romero, C.M. Effect of temperature on the partial molar volumes and the partial molar compressibilities of amino alcohols in aqueous solution. J. Chem. Thermodyn. 2021, 160, 106521. [Google Scholar] [CrossRef]
- Dagade, D.H.; Shinde, S.P.; Madkar, K.R.; Barge, S.S. Density and sound speed study of hydration of 1-butyl-3-methylimidazolium based amino acid ionic liquids in aqueous solutions. J. Chem. Thermodyn. 2014, 79, 192–204. [Google Scholar] [CrossRef]
- Gliński, J.; Burakowski, A. Is the hydration number of a non-electrolyte additive with length and constituents of the solute molecule? Eur. Phys. J. Spec. Top. 2008, 154, 275–279. [Google Scholar] [CrossRef]
- Jóźwiak, M.; Burakowski, A.; Tyczyńska, M.; Komudzińska, M. Compression of selected glymes in N,N-dimethylformamide plus water. The hydration numbers and hydrophobic hydration process of glymes. J. Mol. Liq. 2022, 362, 119722. [Google Scholar] [CrossRef]
- Krakowiak, J.; Wawer, J. Hydration of urea and its derivatives—Volumetric and compressibility studies. J. Chem. Thermodyn. 2014, 79, 109–117. [Google Scholar] [CrossRef]
- Musale, S.P.; Patil, K.R.; Gavhane, R.J.; Dagade, D.H. Density and Speed-of-Sound Measurements for Dilute Binary Mixtures of Diethylammonium-Based Protic Ionic Liquids with Water. J. Chem. Eng. Data 2018, 63, 1859–1876. [Google Scholar] [CrossRef]
- Warmińska, D.; Kloskowski, A. Influence of temperature and anion type on thermophysical properties of aqueous solutions of morpholine based amino acid ionic liquids. J. Chem. Thermodyn. 2023, 187, 107148. [Google Scholar] [CrossRef]
- Kiełek, K.; Marczak, W. Hydration of Non-electrolytes in H2O and D2O Investigated by Passynski’s Method. Int. J. Thermophys. 2010, 31, 77–84. [Google Scholar] [CrossRef]
- Density and Sound Velocity Meter: DSA 5000 M, Anton Paar. Available online: https://www.anton-paar.com/uk-en/products/details/density-and-sound-velocity-meter-dsa-5000-m/ (accessed on 26 July 2024).
- Onori, G. Ionic hydration in sodium chloride solutions. J. Chem. Phys. 1988, 89, 510–516. [Google Scholar] [CrossRef]
- Reis, J.C.R.; Blandamer, M.J.; Davis, M.I.; Douhéret, G. The concepts of non-Gibbsian and non-Lewisian properties in chemical thermodynamics. Phys. Chem. Chem. Phys. 2001, 3, 1465–1470. [Google Scholar] [CrossRef]
- Douhéret, G.; Davis, M.I.; Reis, J.C.R.; Blandamer, M.J. Isentropic Compressibilities—Experimental Origin and the Quest for their Rigorous Estimation in Thermodynamically Ideal Liquid Mixtures. ChemPhysChem 2001, 2, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Marczak, W.; Łężniak, M.; Zorębski, M.; Lodowski, P.; Przybyła, A.; Truszkowska, D.; Almásy, L. Water-induced aggregation and hydrophobic hydration in aqueous solutions of N-methylpiperidine. RSC Adv. 2013, 3, 22053–22064. [Google Scholar] [CrossRef]
- Shiio, H.; Ogawa, T.; Yoshihashi, H. Measurement of the Amount of Bound Water by Ultrasonic Interferometer. J. Am. Chem. Soc. 1955, 77, 4980–4982. [Google Scholar] [CrossRef]
- Shiio, H.; Yoshihashi, H. Measurement of the Amount of Bound Water by Ultrasonic Interferometer. II. Polyvinyl Alcohol and its Partially Substituted Acetates. J. Phys. Chem. 1956, 60, 1049–1061. [Google Scholar] [CrossRef]
- Shiio, H. Ultrasonic Interferometer Measurements of the Amount of Bound Water. Saccharides. J. Am. Chem. Soc. 1958, 80, 70–73. [Google Scholar] [CrossRef]
- Yasunaga, T.; Hirata, Y.; Kawano, Y.; Miura, M. Ultrasonic Studies of the Hydration of Various Compounds in an Ethanol-Water Mixed Solvent. I. Hydration of Inorganic Compounds. Bull. Chem. Soc. Jpn. 1964, 37, 867–871. [Google Scholar] [CrossRef]
- Yasunaga, T.; Usui, I.; Iwata, K.; Miura, M. Ultrasonic Studies of the Hydration of Various Compounds in an Ethanol- Water Mixed Solvent. II. The Hydration of Organic Compounds. Bull. Chem. Soc. Jpn. 1964, 37, 1658–1660. [Google Scholar] [CrossRef]
- Isemura, T.; Goto, S. Studies of the Hydration and the Structure of Water and Their Roles in Protein Structure. II. The Hydration of Electrolytes by Ultrasonic Interferometry and Its Temperature Dependence. Bull. Chem. Soc. Jpn. 1964, 37, 1690–1693. [Google Scholar] [CrossRef]
- Goto, S.; Isemura, T. Studies of the Hydration and the Structure of Water and Their Roles in Protein Structure. IV. The Hydration of Amino Acids and Oligopeptides. Bull. Chem. Soc. Jpn. 1964, 37, 1697–1701. [Google Scholar] [CrossRef]
- Millero, F.J.; Ward, G.K.; Lepple, F.K.; Hoff, E.V. Isothermal Compressibility of Aqueous Sodium Chloride, Magnesium Chloride, Sodium Sulfate, and Magnesium Sulfate Solutions from 0 to 45° at 1 Atm. J. Phys. Chem. 1974, 78, 1636–1643. [Google Scholar] [CrossRef]
- Millero, F.J.; Lo Surdo, A.; Shin, C.J. The Apparent Molal Volumes and Adiabatic Compressibilities of Aqueous Amino Acids at 25 °C. J. Phys. Chem. 1978, 82, 784–792. [Google Scholar] [CrossRef]
- Muller, N. Is There a Region of Highly Structured Water Around a Nonpolar Solute Molecule? J. Solut. Chem. 1988, 17, 661–672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).