The Cytotoxic Activity and Metabolic Profiling of Hyptis rhomboidea Mart. et Gal
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic Activity Evaluation of H. rhomboidea Crude Extracts
2.2. Screening of the 95% Ethanol Extract for Cytotoxic Activity against Human Cancer Cell Lines
2.3. Metabolic Profiling of H. rhomboidea
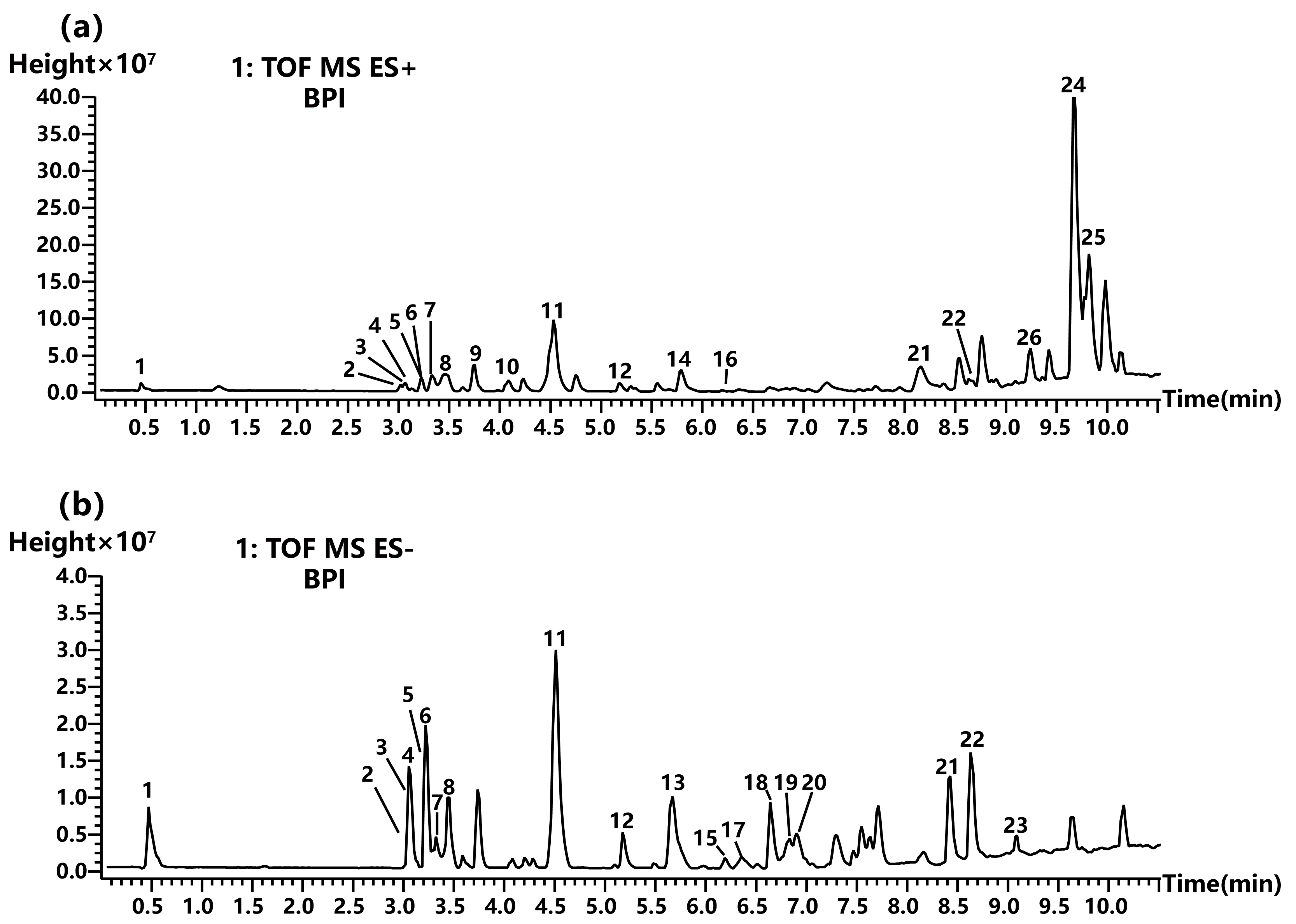
2.3.1. Non-Volatile Chemical Compound Analysis
| Peak No. | RT (min) | Molecular Weight (Da) | Putative Compound | Class | Molecular Formula | Fragment Ions m/z | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 0.488 | 378 | Geshoidin | Saccharides | C18H18O9 | 377.0846/313.0864/173.8846/161.8413/160.8414 | [28] |
| 2 | 3.071 | 610 | Rutin | Flavonoids | C27H30O16 | 519.0209/340.9951/303.0482/229.0467/153.0153 | [22] |
| 3 | 3.072 | 464 | Spiraeoside | Flavonoids | C21H20O12 | 465.1013/303.0482/229.0467/153.0151/137.0202 | [23] |
| 4 | 3.141 | 302 | Quercetin | Flavonoids | C15H10O7 | 303.0482/285.0369/257.0420/229.0467/153.0153 | [19] |
| 5 | 3.220 | 594 | Nicotiflorin | Flavonoids | C27H30O15 | 593.1539/285.0405/255.0302/227.0351 | [24] |
| 6 | 3.221 | 286 | Kaempferol | Flavonoids | C15H10O6 | 287.0539/213.0517/165.0154/153.0153/121.0255 | [20] |
| 7 | 3.289 | 448 | Astragalin | Flavonoids | C21H20O11 | 447.0931/284.0325/255.0296/227.0345 | [25] |
| 8 | 3.454 | 360 | Rosmarinic acid | Coumarins | C18H16O8 | 359.0773/197.0451/179.0346/161.0242/133.0290/134.0450 | [29] |
| 9 | 3.752 | 422 | Catechin 7-arabinofuranoside | Flavonoids | C20H22O10 | 423.1254/363.1036 | - |
| 10 | 4.083 | 382 | Resorcinolnaphthalein | Aromatic derivatives | C24H14O5 | 383.0852/347.1084/333.1290/287.0863/205.0828 | - |
| 11 | 4.547 | 428 | Theaflavic acid | Flavonoids | C21H16O10 | 453.0722/329.2333/160.8415 | [25] |
| 12 | 5.193 | 284 | Genkwanin | Flavonoids | C16H12O5 | 283.0607/268.0373/240.0415/211.0393/161.0239/151.0023 | [26] |
| 13 | 5.673 | 488 | Uncaric acid | Terpenoids | C30H48O5 | 487.3456/469.3322/425.3420 | - |
| 14 | 5.793 | 312 | 3′,4′,5′-Trimethoxyflavonol | Flavonoids | C18H16O6 | 329.1001/314.0764/296.0660/268.0708 | [21] |
| 15 | 6.204 | 488 | Asiatic acid | Terpenoids | C30H48O5 | 533.3480/487.3429 | [30] |
| 16 | 6.273 | 298 | 5-Hydroxy-4′,7-dimethoxyflavone | Flavonoids | C17H14O5 | 299.0893/284.0655/256.0708/184.0704 | [31] |
| 17 | 6.37 | 294 | 9-HOTrE | Aliphatic derivatives | C18H30O3 | 293.2122/275.9977/231.1745/171.9812 | [32] |
| 18 | 6.684 | 472 | Maslinic acid | Terpenoids | C30H48O4 | 472.3516/471.3482/405.3159 | [33] |
| 19 | 6.788 | 296 | α-Dimorphecolic acid | Aliphatic derivatives | C18H32O3 | 295.2278/277.2173/171.3940 | [34] |
| 20 | 6.902 | 472 | Corosolic acid | Terpenoids | C30H48O4 | 472.3513/471.3484 | [35] |
| 21 | 8.159 | 456 | Ursolic acid | Terpenoids | C30H48O3 | 455.3534/407.3311 | [36] |
| 22 | 8.628 | 334 | 4-Ethenyloctahydro-2-hydroxy-4,5′,8′a-trimethyl-1′-oxospiro[cyclopentane-1,2′(1′H)-naphthalene]-5′-carboxylic acid | Terpenoids | C20H30O4 | 333.2288/235.8418/137.8908 | - |
| 23 | 9.091 | 356 | Rutamarin | Coumarins | C21H24O5 | 355.1585/119.9463 | [37] |
| 24 | 9.681 | 598 | 12b-O-[deca-2Z,4E-dienoyl]-13a-isobutyl-5-ene-7-oxo-4b-phorbol | Aromatic derivatives | C34H46O9 | 621.3096/533.2560/434.2414 | - |
| 25 | 9.772 | 390 | 2-acetoxy-4-pentadecylbenzoic acid | Aliphatic derivatives | C24H38O4 | 413.2644/301.1388 | - |
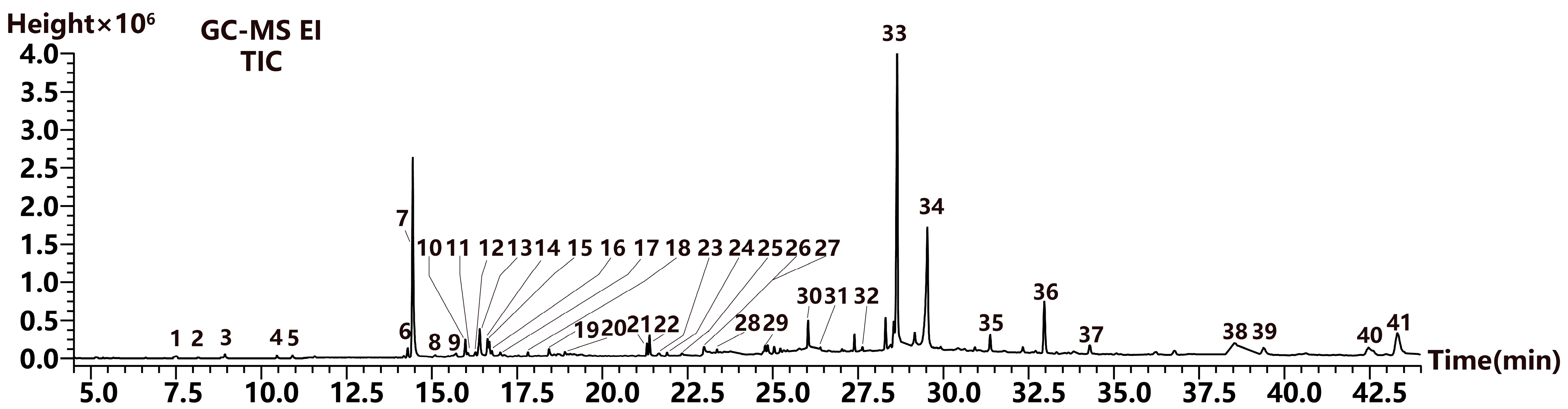
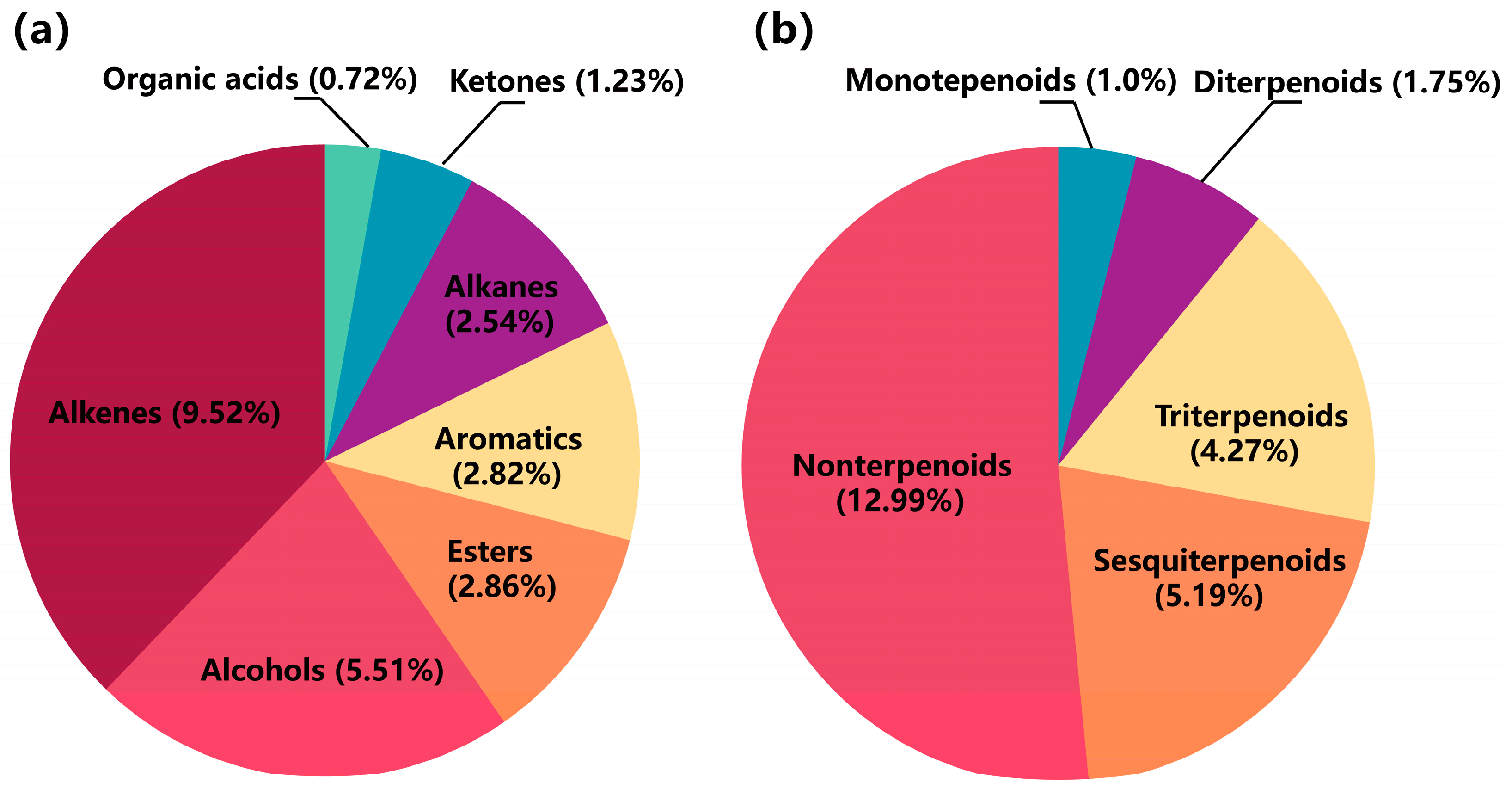
2.3.2. Volatile Compound Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Perparation of Crude Extract
4.3. Cytotoxic Activity Assay
4.4. UPLC-QTOF-MS Analysis of Non-Volatile Compounds
4.5. GC-MS Analysis of Volatile Compounds
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2022. J Natl. Cancer Cent. 2024, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual report to the nation on the status of cancer, Part 1: National cancer statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, antioxidant, and anti-tumor activities of sargassum linearifolium and cystoseira crinita from egyptian mediterranean coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef]
- He, S.M.; Xu, J.; Liu, X.J.; Zhen, Y.S. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 2021, 11, 3379–3392. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.-S. Phytochemical profiling and biological activities of Quercus sp. galls (oak galls): A systematic review of studies published in the last 5 years. Plants 2023, 12, 3873. [Google Scholar] [CrossRef]
- Haif, S.K.; Al Kury, L.T.; Talib, W.H. Combination of thymoquinone and intermittent fasting as a treatment for breast cancer implanted in mice. Plants 2024, 13, 35. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ozleyen, A.; Tumer, T.B.; Adetunji, C.O.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, M.; Cao, H.; Du, X.; Zhang, X.; Wang, J.; Bi, X. Research progress on the synergistic anti-tumor effect of natural anti-tumor components of Chinese herbal medicine combined with chemotherapy drugs. Pharmaceuticals 2023, 16, 1734. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Tang, L.; Zheng, N.N.; An, S.; Wang, Y.X.; Zhang, W.X.; Hu, Y.N. Determination of antioxidant activity in vitro from Hyptis rhomboidea Mart. et Gal. ethanol extracts. Appl. Mech. Mater. 2014, 472, 824–828. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Peter, R.; Hong, D.Y.; Aedo, C.; Cai, J.; Aiken, S.; Chang, C.S.; Akiyama, S.; Alexander, C.; Zhang, M.Z.; et al. Flora of China; Science Press: Beijing, China, 1994; Volume 17, p. 267. [Google Scholar]
- Cheng, F.-R.; Cui, H.-X.; Tang, L.; Yuan, K. Antitumor effects of ethanol extracts from Hyptis rhomboidea in H22 tumor-bearing mice. Pharmacogn. Mag. 2017, 13, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.F.; Lee, S.S. Neolignans as xanthine oxidase onhibitors from Hyptis rhomboidea. Phytochemistry 2014, 101, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and caryophyllene oxide: A variety of chemical transformations and biological activities. Chem. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- Kadasah, S.F.; Radwan, M.O. Overview of ursolic acid potential for the treatment of metabolic disorders, autoimmune diseases, and cancers via nuclear receptor pathways. Biomedicines 2023, 11, 2845. [Google Scholar] [CrossRef]
- Quan, F.; Luan, X.; Zhang, J.; Gao, W.; Yan, J.; Li, P. Cytotoxic isopentenyl phloroglucinol compounds from Garcinia xanthochymus using LC-MS-based metabolomics. Metabolites 2023, 13, 258. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H.; Wang, M.; Li, X.; Wang, L.; Xue, M. Elucidation of Quercetin by Electron Spray Ionization Mass Spectrometry. J. Chin. Mass. Spectrom. Soc. 2009, 30, 374–378. [Google Scholar]
- Jan, R.; Asaf, S.; Paudel, S.; Lubna; Lee, S.; Kim, K.M. Discovery and validation of a novel step catalyzed by OsF3H in the flavonoid biosynthesis pathway. Biology 2021, 10, 32. [Google Scholar] [CrossRef]
- Du, Z.Y.; Ng, H.F.; Zhang, K.; Zeng, H.Q.; Wang, J. Ionic liquid mediated cu-catalyzed cascade michael-oxidation: Efficient synthesis of flavones under mild reaction conditions. Org. Biomol. Chem. 2011, 9, 6930–6933. [Google Scholar] [CrossRef]
- He, J.; Feng, Y.; Ouyang, H.-z.; Yu, B.; Chang, Y.-x.; Pan, G.-x.; Dong, G.-y.; Wang, T.; Gao, X.-m. A sensitive LC–MS/MS method for simultaneous determination of six flavonoids in rat plasma: Application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013, 84, 189–195. [Google Scholar] [CrossRef]
- Cai, X.; Dai, X.; Li, Z.; Chen, J.; Wang, X.; Zhang, M. An UPLC-MS/MS method for quantification of spiraeoside in mouse blood and its application to a pharmacokinetic and bioavailability study. Acta Chromatogr. 2022, 35, 272–277. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.J.; Kim, J.B. Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Chen, C.; Zhong, S.; Li, M.; Xu, K.; Zhu, Y.; Li, P.; You, S.; Jin, S. Chemical and quality analysis of beauty tea processed from fresh leaves of tieguanyin variety with different puncturing degrees. Foods 2023, 12, 1737. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Liang, C.; Diao, X.; Cheng, X.; Liao, M.; Zhang, L. Metabolism studies on hydroxygenkwanin and genkwanin in human liver microsomes by UHPLC-Q-TOF-MS. Xenobiotica 2018, 48, 332–341. [Google Scholar] [CrossRef]
- McOtshana, Z.K.S.; McGaw, L.J.; Kemboi, D.; Fouche, G.; Famuyide, I.M.; Krause, R.W.M.; Siwe-Noundou, X.; Tembu, V.J. Cytotoxicity and antimicrobial activity of isolated compounds from Monsonia angustifolia and Dodonaea angustifolia. J. Ethnopharmacol. 2023, 301, 115170. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Munyao Mutie, F.; Xu, Y.-B.; Saleri, F.D.; Hu, G.-W.; Guo, M.-Q. Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals 2020, 13, 55. [Google Scholar] [CrossRef]
- Wang, X.; Qian, Y.; Li, X.; Jia, X.; Yan, Z.; Han, M.; Qiao, M.; Ma, X.; Chu, Y.; Zhou, S.; et al. Rapid determination of rosmarinic acid and its two bioactive metabolites in the plasma of rats by LC-MS/MS and application to a pharmacokinetics study. Biomed. Chromatogr. 2021, 35, e4984. [Google Scholar] [CrossRef]
- Paemanee, A.; Rattanabunyong, S.; Ketngamkum, Y.; Siriwaseree, J.; Pongpamorn, P.; Romyanon, K.; Tangphatsornruang, S.; Kuaprasert, B.; Choowongkomon, K. Mass spectrometry and synchrotron-ftir microspectroscopy reveal the anti-inflammatory activity of bua bok extracts. Phytochem. Anal. 2022, 33, 1086–1098. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, K.-S.; Lee, C.; Chang, Y.-H. Synthesis of a complete series of o-methyl analogues of naringenin and apigenin. Bull. Korean Chem. Soc. 2007, 28, 2527–2530. [Google Scholar]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A. A new HPLC-MS method for measuring maslinic acid and oleanolic acid in HT29 and HepG2 human cancer cells. Int. J. Mol. Sci. 2015, 16, 21681–21694. [Google Scholar] [CrossRef] [PubMed]
- Mundt, S.; Kreitlow, S.; Jansen, R. Fatty acids with antibacterial activity from the cyanobacterium oscillatoria redekei HUB 051. J. Appl. Phycol. 2003, 15, 263–267. [Google Scholar] [CrossRef]
- Li, B.; Wu, J.; Li, X. Quantitative determination of corosolic acid in rat plasma by LC-MS/MS-ESI: Application to a pharmacokinetic study. Pak. J. Pharm. Sci. 2014, 27, 511–516. [Google Scholar]
- Tan, Z.-R.; Chen, Y.; Zhou, G.; Cao, S.; Peng, X.-D.; Wang, Y.-C.; Peng, X.-J.; Zhang, W.; Zhou, H.-H. LC–MS–MS quantitative determination of ursolic acid in human plasma and its application to pharmacokinetic studies. Chromatographia 2010, 72, 1107–1113. [Google Scholar] [CrossRef]
- Karahisar, E.; Tugay, O.; Orhan, I.E.; Sezer Senol Deniz, F.; Vlad Luca, S.; Skalicka-Wozniak, K.; Sahin, M. Metabolite profiling by hyphenated liquid chromatographic mass spectrometric technique (HPLC-DAD-ESI-Q-TOF-MS/MS) and neurobiological potential of Haplophyllum sahinii and H. vulcanicum Extracts. Chem. Biodivers. 2019, 16, e1900333. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, F.X.; Yang, S.X.; Qiu, Y.; Yuan, K. Chemical constituents of Hyptis rhomboidea and their antifungal activity. China J. Chin. Mater. Med. 2014, 39, 2284–2288. [Google Scholar]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids regulate tumor-associated macrophages—From structure-activity relationship to clinical potential (review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Hill, C.U.F.K.; Saad, S.E.A.; Britton, R.G.; Gescher, A.J.; Sale, S.; Brown, K.; Howells, L.M. Inhibition of prostate cancer cell growth by 3′,4′,5′-trimethoxyflavonol (TMFol). Cancer Chemother. Pharm. 2015, 76, 179–185. [Google Scholar] [CrossRef]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, B.; Yin, S.; Xie, S.; Huang, K.; Wang, J.; Yang, W.; Liu, H.; Zhang, G.; Liu, X.; et al. Quercetin induces autophagy-associated death in HL-60 cells through CaMKKβ/AMPK/mTOR signal pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2022, 54, 1244–1256. [Google Scholar] [PubMed]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Everton Freitas de Morais, E.F.; de Oliveira, L.Q.R.; Farias Morais, H.G.d.; Souto Medeiros, M.R.d.; Freitas, R.d.A.; Rodini, C.O.; Coletta, R.D. The Anticancer Potential of Kaempferol: A Systematic Review Based on In Vitro Studies. Cancers 2024, 16, 585. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Pandey, P.; Ramniwas, S.; Verma, M.; Rautela, I.; Khan, F.; Shah, M.A. A comprehensive review uncovering the anticancerous potential of genkwanin (plant-derived compound) in several human carcinomas. Open Chem. 2024, 22, 20240003. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Antitumor activity of rosmarinic acid-loaded silk fibroin nanoparticles on HeLa and MCF-7 cells. Polymers 2021, 13, 3169. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Ma, L.; Wang, J.; Yu, Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021, 12, 889. [Google Scholar] [CrossRef]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Nile, A.; Nile, S.H.; Cespedes-Acuña, C.L.; Oh, J.-W. Spiraeoside extracted from red onion skin ameliorates apoptosis and exerts potent antitumor, antioxidant and enzyme inhibitory effects. Food Chem. Toxicol. 2021, 154, 112327. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, S.A.; Hong, S.L.; Abdul Malek, S.N. Rutamarin, an Active Constituent from Ruta angustifolia Pers., Induced Apoptotic Cell Death in the HT29 Colon Adenocarcinoma Cell Line. Pharmacogn. Mag. 2017, 13, S179–S188. [Google Scholar] [PubMed]
- Spanova, M.; Daum, G. Squalene–biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar]
- Choi, J.-M.; Lee, E.-O.; Lee, H.-J.; Kim, K.H.; Ahn, K.s.; Shim, B.; Kim, N.-I.; Song, M.C.; Baek, N.-I.; Kim, S.-H. Identification of campesterol from Chrysanthemum coronarium L. and its antiangiogenic activities. Phytother. Res. 2007, 21, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Nan, N.; Cao, K.X.; Ma, C.; Yang, G.W.; Yu, M.W.; Yang, L.; Li, J.P.; Wang, X.M.; et al. Elemene inhibits the migration and invasion of 4T1 murine breast cancer cells via heparanase. Mol. Med. Rep. 2017, 16, 794–800. [Google Scholar] [CrossRef]
- La Torre, C.; Loizzo, M.R.; Frattaruolo, L.; Plastina, P.; Grisolia, A.; Armentano, B.; Cappello, M.S.; Cappello, A.R.; Tundis, R. Chemical profile and bioactivity of Rubus idaeus L. fruits grown in conventional and aeroponic systems. Plants 2024, 13, 1115. [Google Scholar] [CrossRef]
- O’Toole, S.A.; Sheppard, B.L.; McGuinness, E.P.J.; Gleeson, N.C.; Yoneda, M.; Bonnar, J. The MTS assay as an indicator of chemosensitivity/resistance in malignant gynaecological tumours. Cancer Detect. Prev. 2003, 27, 47–54. [Google Scholar] [CrossRef]
- Li, P.; Bai, G.; He, J.; Liu, B.; Long, J.; Morcol, T.; Peng, W.; Quan, F.; Luan, X.; Wang, Z.; et al. Chromosome-level genome assembly of Amomum tsao-ko provides isights into the biosynthesis of flavor compounds. Hortic. Res. 2022, 9, uhac211. [Google Scholar] [CrossRef]

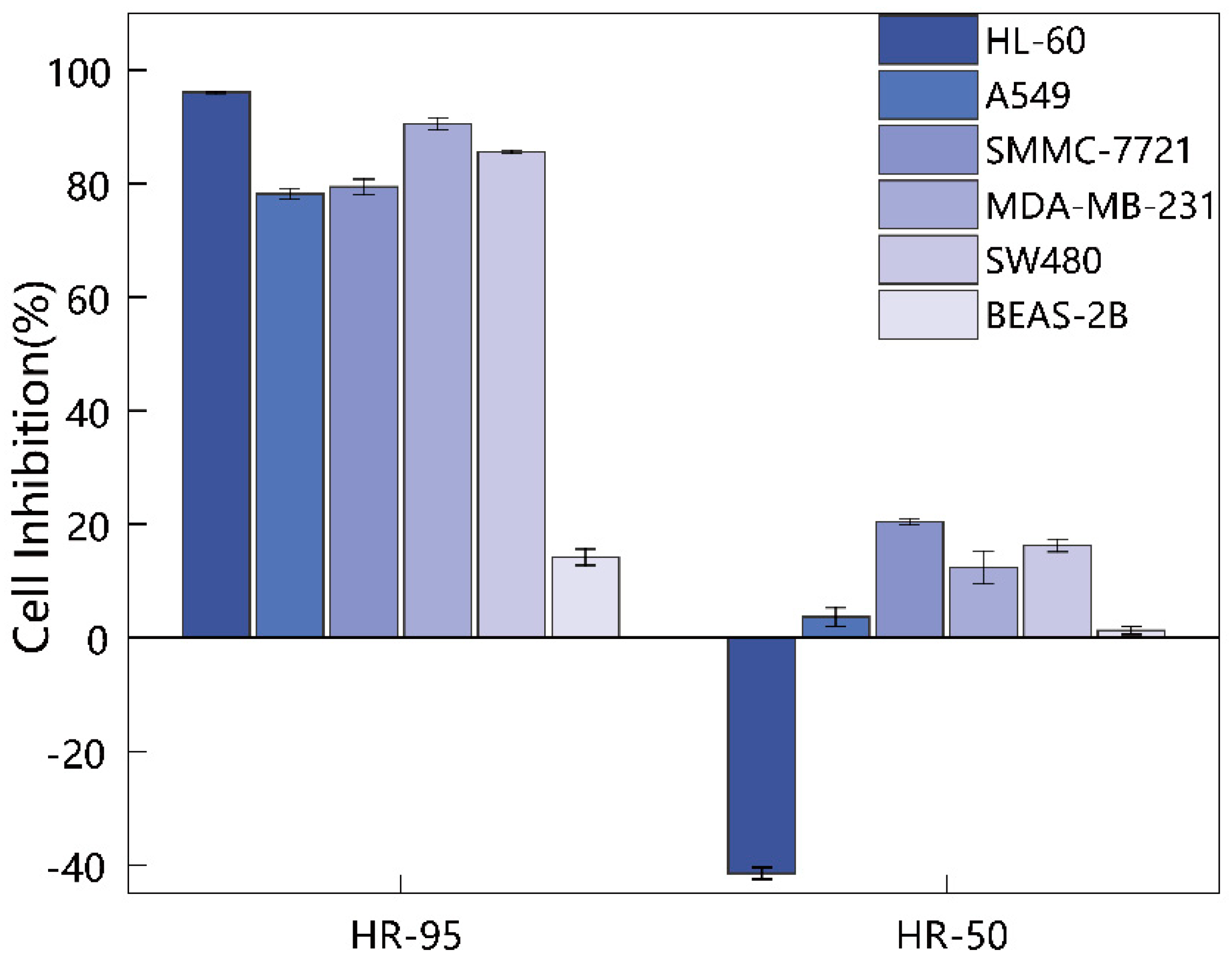
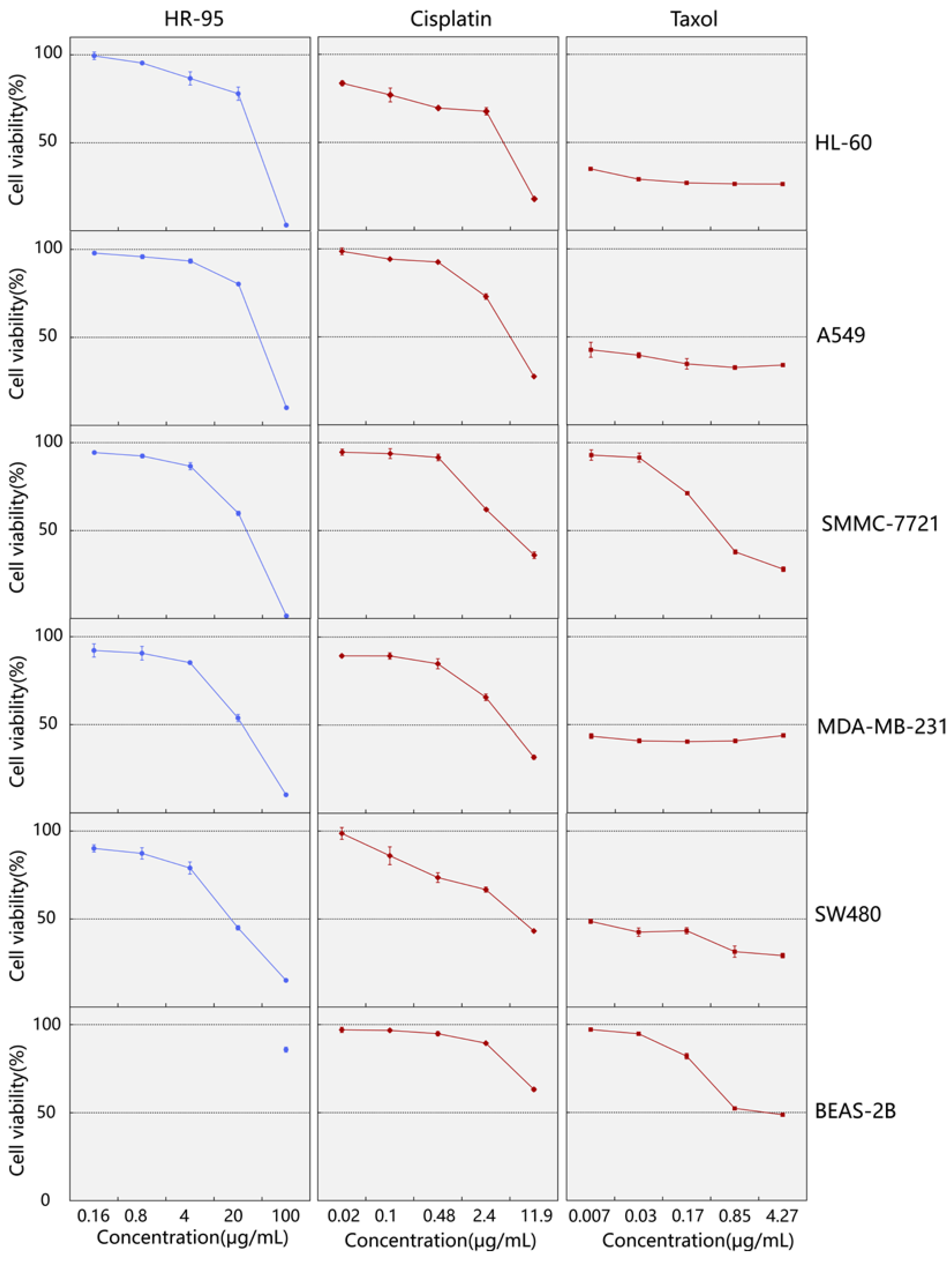
| Groups | HL-60 | A549 | SMMC-7721 | MDA-MB-231 | SW480 | BEAS-2B |
|---|---|---|---|---|---|---|
| HR-95 | 96.10 ± 0.13 | 78.24 ± 0.90 | 79.43 ± 1.35 | 90.54 ± 1.01 | 85.64 ± 0.26 | 14.20 ± 1.43 |
| HR-50 | −41.52 ± 1.03 | 3.64 ± 1.64 | 20.40 ± 0.57 | 12.35 ± 2.92 | 16.24 ± 1.11 | 1.27 ± 0.68 |
| Groups | HL-60 | A549 | SMMC-7721 | MDA-MB-231 | SW480 | BEAS-2B |
|---|---|---|---|---|---|---|
| HR-95 | 36.44 ± 1.82 | 40.02 ± 0.58 | 26.20 ± 0.77 | 22.95 ± 1.59 | 15.81 ± 0.63 | >100 |
| Cisplatin | 4.25 ± 0.22 | 5.40 ± 0.12 | 5.05 ± 0.20 | 5.07 ± 0.39 | 7.52 ± 0.38 | 23.44 ± 1.14 |
| Taxol | <0.007 | <0.007 | 0.466 ± 0.25 | <0.007 | <0.007 | 2.44 ± 0.42 |
| Peak No. | RT (min) | Compound | Molecular Formula | Library No. | Match | Retention Index | Relative Content (%) | |
|---|---|---|---|---|---|---|---|---|
| Average RI | Lib.RI | |||||||
| 1 | 7.495 | m-Cymene | C10H14 | 535-77-3 | 801 | 1026 | 1023 | 0.16% |
| 2 | 8.155 | γ-Terpinene | C10H16 | 99-85-4 | 838 | 1060 | 1060 | 0.13% |
| 3 | 8.940 | Linalool | C10H18O | 78-70-6 | 848 | 1100 | 1099 | 0.28% |
| 4 | 10.455 | endo-Borneol | C10H18O | 507-70-0 | 902 | 1178 | 1167 | 0.21% |
| 5 | 10.915 | Estragole | C10H12O | 140-67-0 | 890 | 1201 | 1196 | 0.22% |
| 6 | 14.175 | α-Ylangene | C15H24 | 14912-44-8 | 880 | 1378 | 1372 | 0.14% |
| 7 | 14.290 | (-)-alpha-Copaene | C15H24 | 3856-25-5 | 895 | 1384 | 1376 | 0.62% |
| 8 | 15.090 | Caryophyllene | C15H24 | 87-44-5 | 845 | 1404 | 1419 | 0.18% |
| 9 | 15.710 | Humulene | C15H24 | 6753-98-6 | 839 | 1467 | 1454 | 0.21% |
| 10 | 15.985 | γ-Muurolene | C15H24 | 30021-74-0 | 927 | 1483 | 1477 | 0.98% |
| 11 | 16.060 | α-Muurolene | C15H24 | 31983-22-9 | 919 | 1488 | 1499 | 0.14% |
| 12 | 16.275 | β-Eudesmene | C15H24 | 17066-67-0 | 836 | 1500 | 1486 | 0.24% |
| 13 | 16.405 | 2,4-Di-tert-butylphenol | C14H22O | 96-76-4 | 870 | 1509 | 1519 | 1.56% |
| 14 | 16.630 | γ-Cadinene | C15H24 | 39029-41-9 | 939 | 1523 | 1513 | 0.79% |
| 15 | 16.690 | δ-Cadinene | C15H24 | 483-76-1 | 855 | 1526 | 1524 | 0.49% |
| 16 | 16.760 | Calamenene | C15H22 | 483-77-2 | 870 | 1531 | 1523 | 0.38% |
| 17 | 17.010 | α-Cadinene | C15H24 | 24406-05-1 | 870 | 1547 | 1538 | 0.20% |
| 18 | 17.820 | Caryophyllene oxide | C15H24O | 1139-30-6 | 800 | 1597 | 1581 | 0.32% |
| 19 | 18.435 | Muurola-4,10(14)-dien-1β-ol | C15H24O | 257293-90-6 | 844 | 1638 | 1635 | 0.44% |
| 20 | 18.685 | 10-epi-α-Cadinol | C15H26O | 1474790 | 870 | 1654 | 1580 | 0.06% |
| 21 | 21.305 | Neophytadiene | C20H38 | 504-96-1 | 920 | 1836 | 1837 | 0.64% |
| 22 | 21.385 | Hexahydrofarnesyl acetone | C18H36O | 502-69-2 | 904 | 1842 | 1844 | 1.23% |
| 23 | 21.675 | Diisobutyl phthalate | C16H22O4 | 84-69-5 | 877 | 1863 | 1870 | 0.15% |
| 24 | 21.895 | Neophytadiene | C20H38 | 504-96-1 | 910 | 1879 | 1774 | 0.26% |
| 25 | 22.350 | Farnesyl palmitate | C31H56O2 | 157501-12-7 | 810 | 1913 | 3225 | 0.18% |
| 26 | 22.940 | Dibutyl phthalate | C16H22O4 | 84-74-2 | 825 | 1959 | 1965 | 0.09% |
| 27 | 22.980 | n-Hexadecanoic acid | C16H32O2 | 57-10-3 | 851 | 1962 | 1968 | 0.72% |
| 28 | 23.360 | Hexadecanoic acid, ethyl ester | C18H36O2 | 628-97-7 | 903 | 1991 | 1993 | 0.21% |
| 29 | 24.850 | Phytol | C20H40O | 150-86-7 | 892 | 2111 | 2114 | 0.67% |
| 30 | 26.035 | 2-Hexadecen-1-ol, 3,7,11,15- Tetramethyl-, acetate | C22H42O2 | 76337-16-1 | 911 | 2212 | 2232 | 1.50% |
| 31 | 26.395 | Tributyl acetylcitrate | C20H34O8 | 77-90-7 | 801 | 2243 | 2250 | 0.14% |
| 32 | 27.625 | 4,8,12,16-Tetramethylheptadecan -4-olide | C21H40O2 | 96168-15-9 | 804 | 2354 | 2364 | 0.28% |
| 33 | 28.645 | Unknown | - | - | - | - | - | 19.86% |
| 34 | 29.530 | Unknown | - | - | - | - | - | 14.39% |
| 35 | 31.370 | Heptacosane | C27H56 | 593-49-7 | 857 | 2699 | 2700 | 1.14% |
| 36 | 32.960 | Squalene | C30H50 | 111-02-4 | 925 | 2816 | 2832 | 4.27% |
| 37 | 34.290 | Nonacosane | C29H60 | 630-03-5 | 830 | 2907 | 2900 | 0.94% |
| 38 | 38.550 | Dotriacontane | C32H66 | 544-85-4 | 820 | 3197 | 3200 | 0.47% |
| 39 | 39.385 | Vitamin E | C29H50O2 | 59-02-9 | 940 | 3254 | 3149 | 0.73% |
| 40 | 42.480 | Campesterol | C28H48O | 474-62-4 | 800 | 3465 | 3131 | 1.00% |
| 41 | 43.320 | Stigmasterol | C29H48O | 83-48-7 | 840 | 3522 | 3170 | 2.85% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Gao, W.; Jahan, I.; Zhai, R.; Yao, K.; Yan, J.; Li, P. The Cytotoxic Activity and Metabolic Profiling of Hyptis rhomboidea Mart. et Gal. Molecules 2024, 29, 4216. https://doi.org/10.3390/molecules29174216
Zhang J, Gao W, Jahan I, Zhai R, Yao K, Yan J, Li P. The Cytotoxic Activity and Metabolic Profiling of Hyptis rhomboidea Mart. et Gal. Molecules. 2024; 29(17):4216. https://doi.org/10.3390/molecules29174216
Chicago/Turabian StyleZhang, Jian, Wenjie Gao, Israt Jahan, Run Zhai, Kaiwei Yao, Jian Yan, and Ping Li. 2024. "The Cytotoxic Activity and Metabolic Profiling of Hyptis rhomboidea Mart. et Gal" Molecules 29, no. 17: 4216. https://doi.org/10.3390/molecules29174216








