Naturally Occurring Xanthones and Their Biological Implications
Abstract
1. Introduction
2. Methodology
3. History of Xanthones
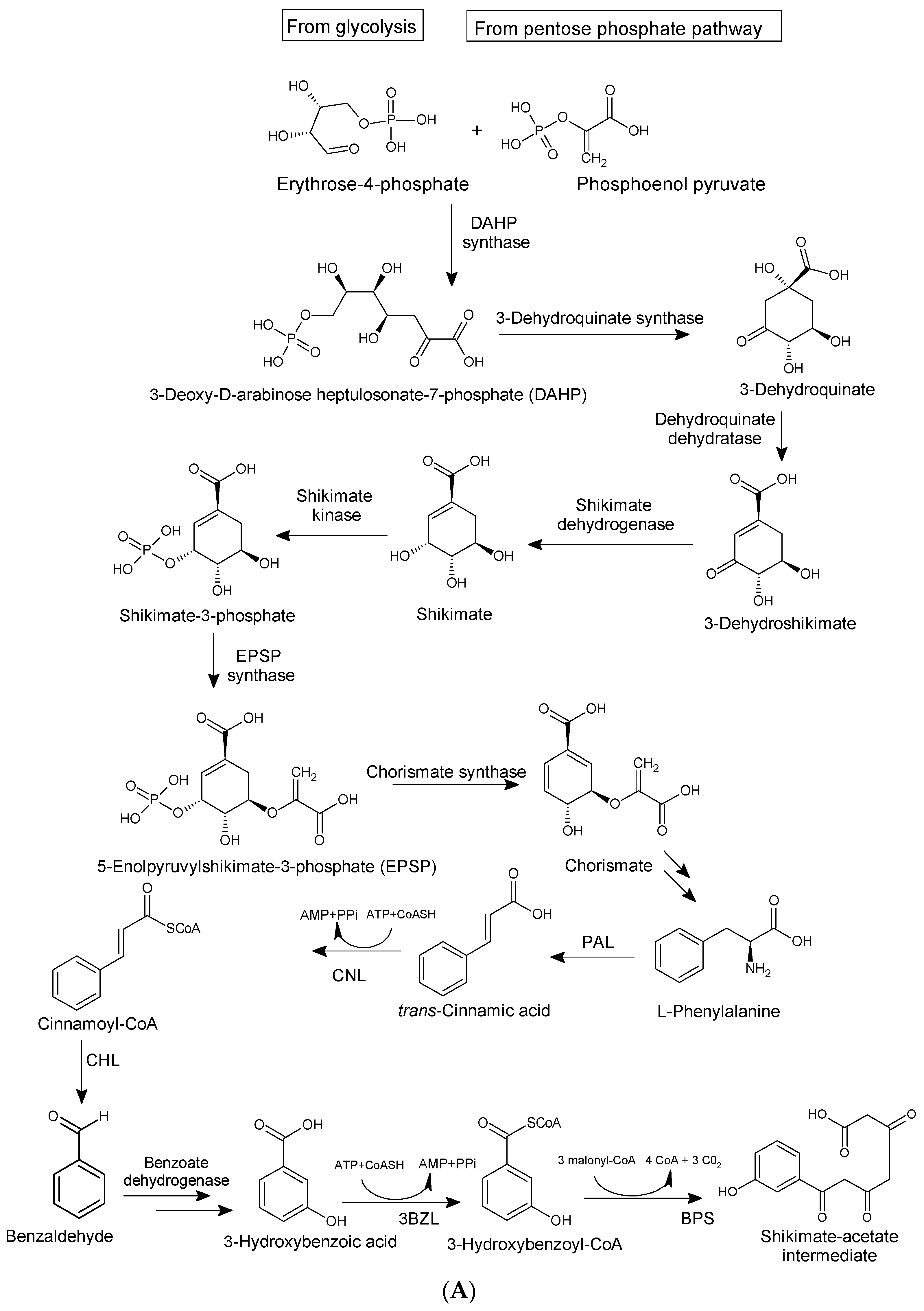
4. Natural Abundance, Classification, and Biosynthesis of Xanthones
5. Biological Activities of Natural Xanthones with Structure–Activity Relationship (SAR) Insight
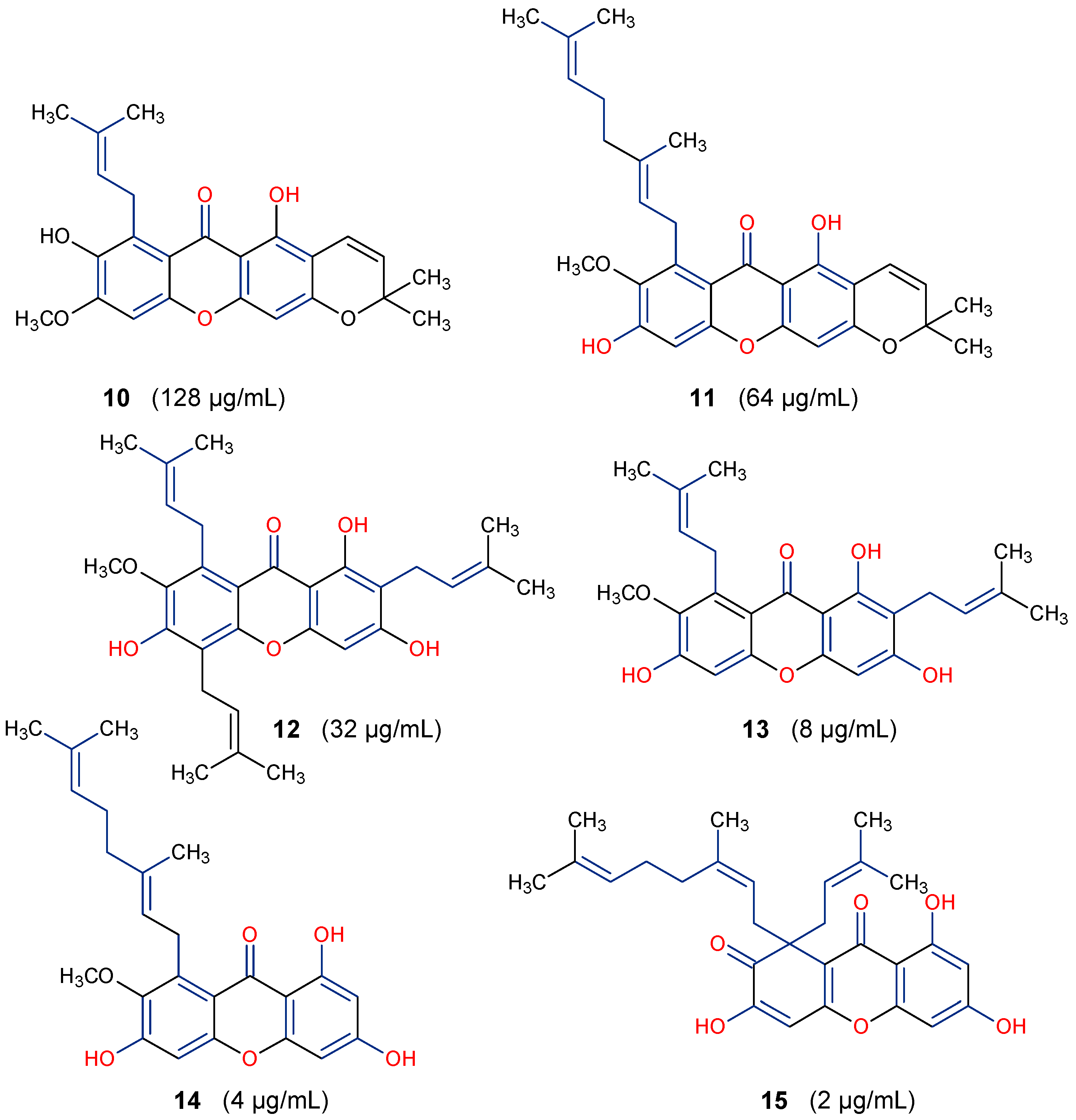
5.1. Antifungal Activity
5.2. Antibacterial Activity
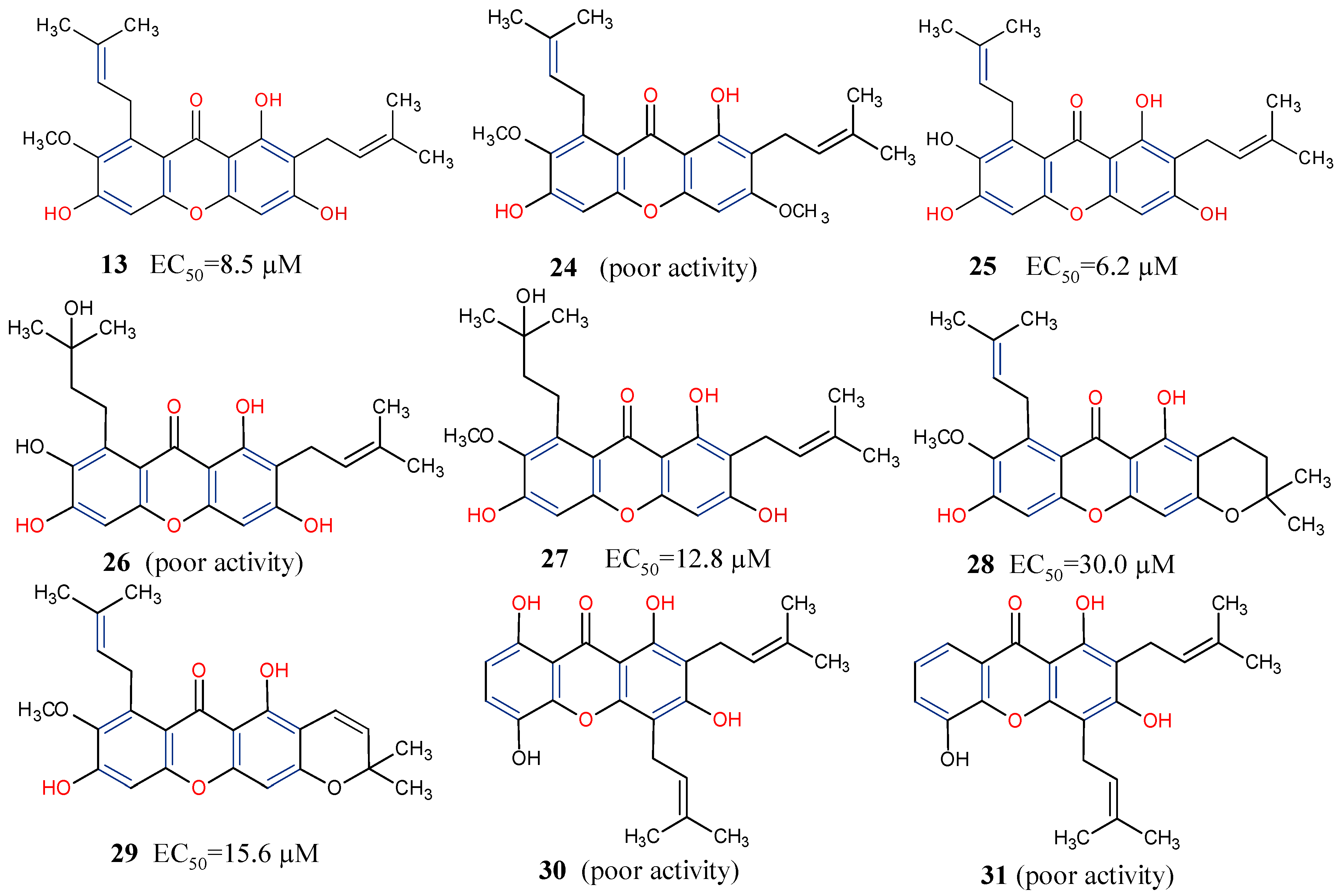
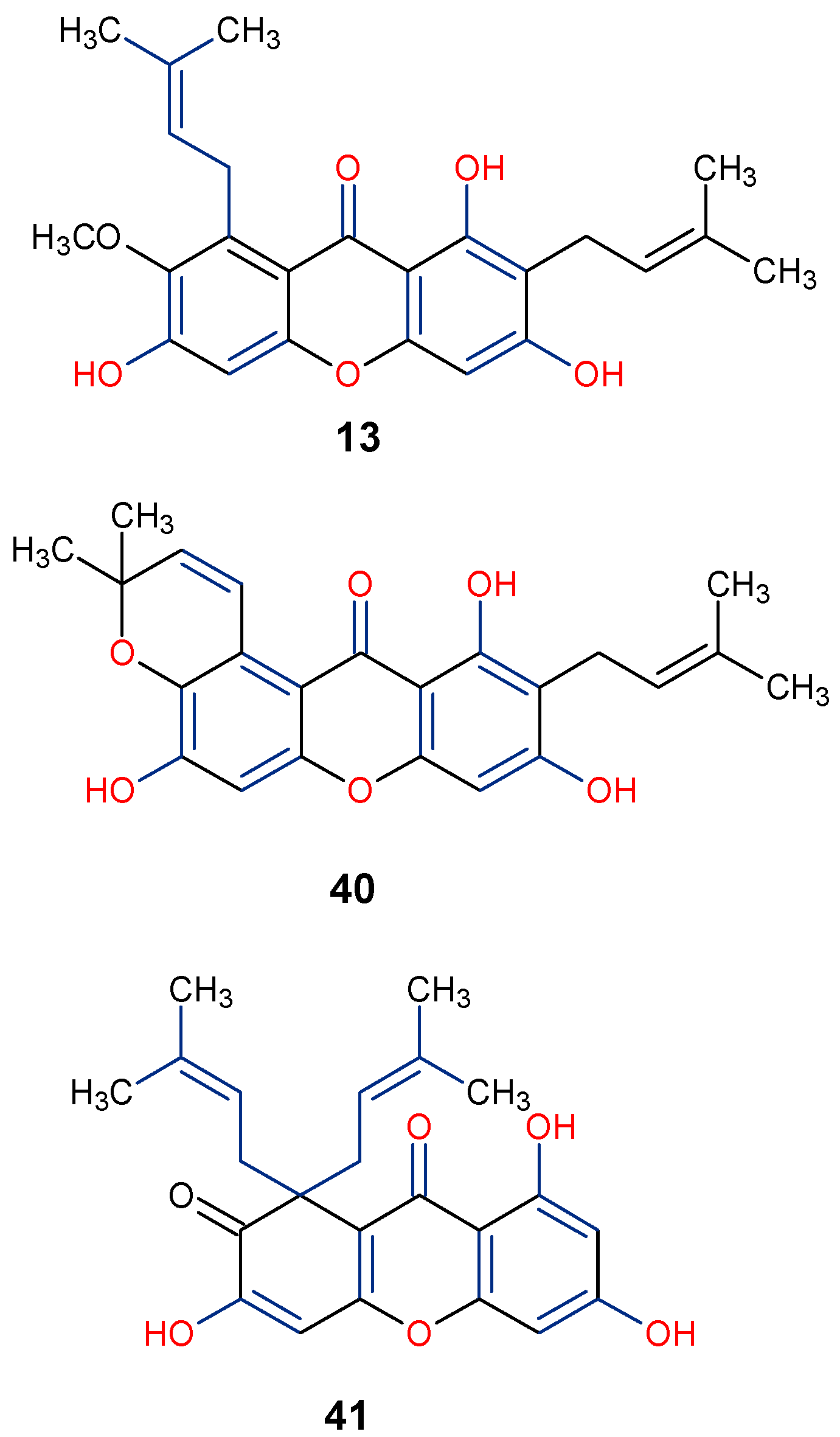
5.3. Anticancer Activity
5.4. Antioxidant Activity
5.5. Anti-Inflammatory Activity
5.6. Anti-HIV/AIDS Activity
5.7. Antidiabetic Activity
5.8. Insecticidal Activity
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Seedi, H.; El-Barbary, M.; El-Ghorab, D.; Bohlin, L.; Borg-Karlson, A.K.; Göransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.L.; Fernández, I.; Pedro, J.R.; Serrano, A. Xanthones from Hypericum reflexum. Phytochemistry 1990, 29, 3003–3006. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An update on the anticancer activity of xanthone derivatives: A review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef]
- Jindarat, S. Xanthones from mangosteen (Garcinia mangostana): Multi-targeting pharmacological properties. J. Med. Associat. Thailand 2014, 97, 196–201. [Google Scholar]
- Fernandes, C.; Carraro, M.; Ribeiro, J.; Araújo, J.; Tiritan, M.; Pinto, M. Synthetic chiral derivatives of xanthones: Biological activities and enantioselectivity studies. Molecules 2019, 24, 791. [Google Scholar] [CrossRef]
- Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar]
- Araújo, J.; Fernandes, C.; Pinto, M.; Tiritan, M. Chiral derivatives of xanthones with antimicrobial activity. Molecules 2019, 24, 314. [Google Scholar] [CrossRef]
- Cruz, M.I.; Cidade, H.; Pinto, M. Dual/multitargeted xanthone derivatives for Alzheimer’s disease: Where do we stand? Future Med. Chem. 2017, 9, 1611–1630. [Google Scholar] [CrossRef]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, a promising anti-inflammatory scaffold: Structure, activity, and drug like-ness analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef]
- Gunter, N.V.; Teh, S.S.; Lim, Y.M.; Mah, S.H. Natural xanthones and skin inflammatory diseases: Multitargeting mechanisms of action and potential application. Front. Pharmacol. 2020, 11, 594202. [Google Scholar] [CrossRef]
- Salman, Z.; Yu-Qing, J.; Bin, L.; Cai-Yun, P.; Iqbal, C.M.; Atta-ur, R.; Wei, W. Antioxidant nature adds further therapeutic value: An updated review on natural xanthones and their glycosides. Digit. Chin. Med. 2019, 2, 166–192. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Freitas, M.; Fernandes, E. A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem. 2018, 157, 1460–1479. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Wu, H.; Yuan, M.; Zheng, C.; Xu, H. Xan-thone glucosides: Isolation, bioactivity and synthesis. Molecules 2021, 26, 5575. [Google Scholar] [CrossRef]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone Biosynthetic pathway in plants: A review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef]
- Li, W.; Guofan, X.; Jianzhong, Z.; Yang, B.; Qian, Z.; Chen, Y.; Haiming, G.; Zhou, J.; Huang, L. 5,6-Dimethyl xanthone-4-acetic Acid Derivatives and Method of Preparing the Same. US Patents US20100099754A1, 8 January 2013. [Google Scholar]
- Chitra, V.; Narayanan, J. In vitro screening for anti-cholinesterase and antioxidant activity of extract of Garcinia hanburyi. Res. J. Pharm. Technol. 2018, 11, 2918–2921. [Google Scholar] [CrossRef]
- Marona, H. Synthesis and anticonvulsant effects of some aminoal-kanolic derivatives of xanthone. Pharmazie 1998, 53, 672–676. [Google Scholar]
- Keiser, J.; Vargas, M.; Winter, R. Anthelminthic properties of mangostin and mangostin diacetate. Parasitol. Int. 2012, 61, 369–371. [Google Scholar] [CrossRef]
- de Oliveira Caleare, A.; Lazarin-Bidóia, D.; Cortez, D.A.G.; Ueda-Nakamura, T.; Dias Filho, B.P.; de Oliveira Silva, S.; Nakamura, C.V. Trypanocidal activity of 1,3,7-trihydroxy-2-(3-methylbut-2-enyl)-xanthone isolated from Kielmeyera coriacea. Parasitol. Int. 2013, 62, 405–411. [Google Scholar] [CrossRef]
- Kurapati, K.R.; Atluri, V.S.; Samikkannu, T.; Garcia, G.; Nair, M.P. Natural products as anti-HIV agents and role in HIV-associated neurocognitive disorders (HAND): A brief overview. Front. Microbiol. 2016, 6, 1444. [Google Scholar] [CrossRef] [PubMed]
- Marona, H.; Librowski, T.; Cegła, M.; Erdođan, C.; Sahin, N.O. Antiarrhythmic and antihypertensive activity of some xanthone derivatives. Acta Poloniae Pharmaceut. Drug Res. 2008, 65, 383–390. [Google Scholar]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Hay, A.E.; Hélesbeux, J.J.; Duval, O.; Labaïed, M.; Grellier, P.; Richomme, P. Antimalarial xanthones from Calophyllum cale donicum and Garcinia vieillardii. Life Sci. 2004, 75, 3077–3085. [Google Scholar] [CrossRef]
- Yasunaka, K.; Abe, F.; Nagayama, A.; Okabe, H.; Lozada-Pérez, L.; López-Villafranco, E.; Muñiz, E.E.; Aguilar, A.; Reyes-Chilpa, R. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J. Ethnopharmacol. 2005, 97, 293–299. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, L.; Fu, W.W.; Liang, S.; Yang, Y.F.; Yuan, Q.H.; Yang, D.J.; Lu, A.P.; Xu, H.X. Prenylated benzoylphloroglucinols and xanthones from the leaves of Garcinia oblongifolia with anti-enteroviral activity. J. Nat. Prod. 2014, 77, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Al-Massarani, S.; El Gamal, A.; Al-Musayeib, N.; Mothana, R.; Basudan, O.; Al-Rehaily, A.; Farag, M.; Assaf, M.; El Tahir, K.; Maes, L. Phytochemical, antimicrobial and antiprotozoal evaluation of Garcinia mangostana pericarp and α-mangostin, its major xanthone derivative. Molecules 2013, 18, 10599–10608. [Google Scholar] [CrossRef]
- Lin, C.N.; Hsieh, H.K.; Liou, S.J.; Ko, H.H.; Lin, H.C.; Chung, M.I.; Ko, F.N.; Liu, H.W.; Teng, C.M. Synthesis and antithrombotic effect of xanthone derivatives. J. Pharm. Pharmacol. 2011, 48, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Durães, F.; Maia, M.; Sousa, E.; Pinto, M.M.M. Recent advances in the synthesis of xanthones and azaxanthones. Org. Chem. Front. 2020, 7, 3027–3066. [Google Scholar] [CrossRef]
- Meng, W.; Dong, Y.; Liu, J.; Wang, Z.; Zhong, X.; Chen, R.; Zhou, H.; Lin, M.; Jiang, L.; Gao, F.; et al. A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: A randomized, placebo-controlled, blinded, multicenter clinical trial. Trials 2009, 10, 30. [Google Scholar] [CrossRef]
- De Koning, C.B.; Giles, R.G.; Knight, L.S.; Niven, M.L.; Yorke, S.C. The ansamycins: Synthesis of the naphthoquinonoid nucleus of rifamycin W; crystal structure verification of a key naphthalenic intermediate. J. Chem. Soc. Perkin Trans. 1 1988, 8, 2477–2483. [Google Scholar] [CrossRef]
- Balan, J.; Fuska, J.; Kuhr, I.; Kuhrová, V. Bikaverin, an antibiotic from Gibberella fujikuroi, effective against Leishmania brasiliensis. Folia Microbiol. 1970, 15, 479–484. [Google Scholar] [CrossRef]
- Fuska, I.; Ivanitskaia, L.P.; Makukho, L.V.; Volkova, L.I. The effects of the antibiotics vermiculin PSX-1, bicaverin and duclauxin, isolated from fungi, on nucleic acid synthesis in several tumors. Antibiotiki 1974, 19, 890–893. [Google Scholar] [PubMed]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and Trafficking in Plants, Fungi and Lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, D.; Kjaer, A.; Pedersen, C.; Bu’Lock, J.D.; Smith, J.R. Bikaverin and norbikaverin, benzoxanthentrione pigments of Gibberella fujikuroi. J. Chem. Soc. C Org. 1971, 16, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Masters, K.S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef]
- Gul, S.; Aslam, K.; Pirzada, Q.; Rauf, A.; Khalil, A.A.; Semwal, P.; Bawazeer, S.; Al-Awthan, Y.S.; Bahattab, O.S.; Al Duais, M.A.; et al. Xanthones: A class of heterocyclic compounds with anticancer potential. Curr. Topics Med. Chem. 2022, 22, 1930–1949. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Chen, L.; Dong, Z.; Di, X.; Qiu, F. A new xanthone from Penicillium oxalicum. Chem. Nat. Compd. 2010, 46, 216. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Jia, C.; Xue, C.; Bai, J.; Li, Z.; Hua, H. Three new xanthones from the leaves of Garcinia lancilimba. J. Nat. Med. 2016, 70, 173–178. [Google Scholar] [CrossRef]
- Fujimoto, H.; Satoh, Y.; Yamaguchi, K.; Yamazaki, M. Manoamine oxidase inhibitory constituents from Anixiella micropertusa. Chem. Pharm. Bull. 1998, 46, 1506. [Google Scholar] [CrossRef]
- Rohr, M.; Kiefer, A.M.; Kauhl, U.; Groß, J.; Opatz, T.; Erkel, G. Anti-inflammatory dihydroxanthones from a Diaporthe species. Biol. Chem. 2021, 403, 89–101. [Google Scholar] [CrossRef]
- Zhang, W.; Krohn, K.; Ullah, Z.; Flörke, U.; Pescitelli, G.; Di Bari, L.; Antus, S.; Kurtan, T.; Rheinmeiner, J.; Draeger, S.; et al. New mono- and dimeric members of the secalonic acid family: Blennolides A–G isolated from the fungus Blennoria sp. Chem. Eur. J. 2008, 14, 4913. [Google Scholar] [CrossRef]
- Lu, S.; Tanaka, N.; Kawazoe, K.; Murakami, K.; Damdinjav, D.; Dorjbal, E.; Kashiwada, Y. Tetrahydroxanthones from Mongolian medicinal plant Gentianella amarella ssp. Acuta. J. Nat. Med. 2016, 70, 780–788. [Google Scholar] [CrossRef]
- Krick, A.; Kehraus, S.; Gerhauser, C.; Klimo, K.; Nieger, M.; Maier, A.; Fiebig, H.-H.; Atodiresei, I.; Raabe, G.; Fleischhauer, J.; et al. Potential cancer chemopreventive in vitro activities of monomeric xanthone derivatives from the marine algicolous fungus Monodictys putredinis. J. Nat. Prod. 2007, 70, 353–360. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Z.J.; Liu, J.K. Benzopyran-4-one derivatives from the fungus Ganoderma applanatum. Z. Naturforsch. 2007, 62b, 1329. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Aunchareon, P.; Komwijit, S.; Jones, E.B.G. Acremoxanthones A and B, novel antibiotic polyketides from the fungus Acremonium sp. BCC 31806. Tetrahedron Lett. 2009, 50, 284. [Google Scholar] [CrossRef]
- Maiese, W.M.; Lechevalier, M.P.; Lechevalier, H.A.; Korshalla, J.; Goodman, J.; Wildey, M.J.; Kuck, N.; Greenstein, M. LL-E19085 alpha, a novel antibiotic from Micromonospora citrea: Taxonomy, fermentation and biological activity. J. Antibiot. 1989, 42, 846. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nishino, C.; Ohya, J.; Sato, S.; Mikawa, T.; Shiobara, Y.; Kodama, M. Actinoplanones C, D, E, F, and G, new cytotoxic polycyclic xanthones from Actinoplanes sp. J. Antibiot. 1988, 41, 502. [Google Scholar] [CrossRef]
- Finnegan, R.A.; Bachman, P.L. Natural occurrence of 2-hydroxyxanthone. J. Pharm. Sci. 1965, 54, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Haerani, S.N.; Raksat, A.; Pudhom, K. Two new xanthones from the root of Thai Calophyllum inophyllum and their toxicity against colon and liver cancer cells. J. Nat. Med. 2021, 75, 670–674. [Google Scholar] [CrossRef]
- Teh, S.; Ee, G.; Mah, S. Evaluation of nitric oxide inhibition effect in LPS-stimulated RAW 264.7 macrophages by phytochemical constituents from Mesua beccariana, Mesua congestiflora, and Mesua ferrea. Med. Chem. Res. 2017, 26, 3240–3246. [Google Scholar] [CrossRef]
- Liang, B.; Li, H.R.; Xu, L.Z.; Yang, S.L. Xanthones from the roots of Cudrania fruticosa Wight. J. Asian Nat. Prod. Res. 2007, 9, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Huang, S.T. Xanthones from Swertia nervosa and their inhibitory effects on nitric oxide production. Chem. Nat. Compd. 2020, 56, 733–735. [Google Scholar] [CrossRef]
- Ghosal, S.; Biswas, K.; Chaudhuri, R.K. Chemical constituents of Gentianaceae XXIV: Anti-Mycobacterium tuberculosis activity of naturally occurring xanthones and synthetic analogs. J. Pharm. Sci. 1978, 67, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Valentão, P.; Andrade, P.B.; Silva, E.; Vicente, A.; Santos, H.; Bastos, M.L.; Seabra, R.M. Methoxylated xanthones in the quality control of small centaury (Centaurium erythraea) flowering tops. J. Agric. Food Chem. 2002, 50, 460–463. [Google Scholar] [CrossRef]
- Zhang, W.D.; Fu, P.; Liu, R.H.; Li, T.-Z.; Li, H.L.; Zhang, W.; Chen, H.S. A new bisxanthone from Hypericum japonicum. Fitoterapia 2007, 78, 74–75. [Google Scholar] [CrossRef]
- Cortez, D.A.G.; Young, M.C.M.; Marston, A.; Wolfender, J.L.; Hostettmann, K. Xanthones, triterpenes and a biphenyl from Kielmeyera coriacea. Phytochemistry 1998, 47, 1367–1374. [Google Scholar] [CrossRef]
- Kumla, D.; Dethoup, T.; Gales, L.; Pereira, J.A.; Freitas-Silva, J.; Costa, P.M.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. Erubescensoic Acid, a new polyketide and a xanthonopyrone SPF-3059-26 from the culture of the marine sponge-associated fungus Penicillium erubescens KUFA 0220 and antibacterial activity evaluation of some of its constituents. Molecules 2019, 24, 208. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Li, W.; Li, Q.; Ai, H.L.; Li, Z.H.; Huang, R.; Feng, T.; Liu, J.K. Piperidine alkaloids and xanthone from the roots of Caulophyllum robustum Maxim. Fitoterapia 2018, 132, 22–25. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; El-labbad, E.M.; Al-Azzawi, M.A.; Ashmawy, N.S. A New Xanthone Glycoside from Mangifera indica L.: Physicochemical Properties and In Vitro Anti-Skin Aging Activities. Molecules 2022, 27, 2609. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Lewis, J.R. Biogenesis of xanthones in Gentiana lutea. J. Chem. Soc. C 1971, 1971, 629–631. [Google Scholar] [CrossRef]
- Rocha, L.; Marston, A.; Auxiliadora, M.; Kaplan, C.; Stoeckli-Evans, H.; Thull, U.; Hostettmann, K. An antifungal γ-pyrone and xanthones with monoamine oxidase inhibitory activity from Hypericum brasiliense. Phytochemistry 1994, 36, 1381–1385. [Google Scholar] [CrossRef]
- Rath, G.; Potterat, O.; Mavi, S.; Hostettmann, K. Xanthones from Hypericum roeperanum. Phytochemistry 1996, 43, 513–520. [Google Scholar] [CrossRef]
- Ogundele, S.B.; Oriola, A.O.; Oyedeji, A.O.; Olorunmola, F.O.; Agbedahunsi, J.M. Flavonoids from stem bark of Artocarpus altilis (Parkinson ex FA Zorn) Fosberg. Chem. Afr. 2022, 5, 1921–1935. [Google Scholar] [CrossRef]
- Sriyatep, T.; Siridechakorn, I.; Maneerat, W.; Pansanit, A.; Ritthiwigrom, T.; Andersen, R.J.; Laphookhieo, S. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod. 2015, 78, 265–271. [Google Scholar] [CrossRef]
- Araya-Cloutier, C.; Vincken, J.P.; van de Schans, M.G.M.; Hageman, J.; Schaftenaar, G.; den Besten, H.M.W.; Gruppen, H. QSAR-based molecular signatures of prenylated (iso)flavonoids underlying antimicrobial potency against and membrane-disruption in Gram-positive and Gram-negative bacteria. Sci. Rep. 2018, 8, 9267. [Google Scholar] [CrossRef]
- Lee, Y.M.; Li, H.; Hong, J.; Cho, H.Y.; Bae, K.S.; Kim, M.A.; Kim, D.-K.; Jung, J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010, 33, 231–235. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, R.; Guo, Z.; She, Z.; Lin, Y. A new xanthone derivative from mangrove endophytic fungus No. ZSU-H16. Chem. Nat. Compd. 2010, 46, 348–351. [Google Scholar] [CrossRef]
- Yang, R.; Li, P.; Li, N.; Zhang, Q.; Bai, X.; Wang, L.; Xiao, Y.; Sun, L.; Yang, Q.; Yan, J. Xanthones from the pericarp of Garcinia mangostana. Molecules 2017, 22, 683. [Google Scholar] [CrossRef]
- Zamakshshari, N.H.; Ee, G.C.L.; Ismail, I.S.; Ibrahim, Z.; Mah, S.H. Cytotoxic xanthones isolated from Calophyllum depressinervosum and Calophyllum buxifolium with antioxidant and cytotoxic activities. Food Chem. Toxicol. 2019, 133, 110800. [Google Scholar] [CrossRef]
- Oanh, V.T.K.; Thoa, H.T.; Hang, N.T.M.; Phuong, D.T.L.; Lien, N.T.P.; Popova, M.; Trusheva, B.; Bankova, V.; Le, T.N. New dihydrochromene and xanthone derivatives from Lisotrigona furva propolis. Fitoterapia 2021, 149, 104821. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. The purple mangosteen (Garcinia mangostana): Defining the anticancer potential of selected xanthones. Pharmacol. Res. 2022, 175, 106032. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Shan, T.; Ma, Q.; Guo, K.; Liu, J.; Li, W.; Wang, F.; Wu, E. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr. Mol. Med. 2011, 11, 666–677. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 54, 301–305. [Google Scholar] [CrossRef]
- Hu, F.; Li, X.; Ding, Z.; Wang, L.; Ge, C.; Xu, L.; Li, S.-S. Divergent synthesis of [3,4]-fused 3-alkenyl-oxindoles via propargyl alcohol-triggered C(sp3)–H functionalization. ACS Catal. 2022, 12, 943–952. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, F.; Wu, H.; Guo, F.-W.; Wang, L.; Du, F.-Y.; Li, S.-S. Controllable synthesis of N-heterocycles via hydride transfer strategy-enabled formal [5 + 1] and [5 + 2] cyclizations. Org. Lett. 2024, 26, 332–337. [Google Scholar] [CrossRef]
- Vemu, B.; Nauman, M.C.; Veenstra, J.P.; Johnson, J.J. Structure-activity relationship of xanthones for inhibition of cyclin-dependent kinase 4 from mangosteen (Garcinia mangostana L.). Int. J. Nutr. 2019, 4, 38–45. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Chen, B.; Luo, X.; Chen, D. Structure-activity relationship and prediction of the electron-transfer potential of the xanthones series. Chem. Open 2018, 7, 730–736. [Google Scholar] [CrossRef]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Yamakuni, T.; Aoki, K.; Nakatani, K.; Kondo, N.; Oku, H.; Ishiguro, K.; Ohizumi, Y. Garcinone B reduces prostaglandin E2 release and NF-κB-mediated transcription in C6 rat glioma cells. Neurosci. Lett. 2006, 394, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Chen, C.H.; Chu, S.J.; Chen, J.H.; Lai, J.H.; Sytwu, H.K.; Chang, D.M. Interleukin (IL)-23 p19 expression induced by IL-1 β in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-κ B and AP-1 dependent pathway. Rheumatology 2007, 46, 1266–1273. [Google Scholar] [CrossRef]
- Zuo, J.; Yin, Q.; Wang, Y.W.; Li, Y.; Lu, L.M.; Xiao, Z.G.; Wang, G.D.; Luan, J.J. Inhibition of NF-κB pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int. Immunopharmacol. 2018, 56, 78–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.M.; Zhang, M.M.; Wang, R.; Liang, C.H.; Zhao, Y.M.; Deng, Y.Y.; Liu, Y.P.; Fu, Y.H. Xanthones with Potential Anti-Inflammatory and Anti-HIV Effects from the Stems and Leaves of Cratoxylum cochinchinense. Molecules 2023, 28, 6050. [Google Scholar] [CrossRef]
- Wang, J.N.; Hou, C.Y.; Liu, Y.L.; Lin, L.Z.; Gil, R.R.; Cordell, G.A. Swertifrancheside, an HIV-reverse transcriptase inhibitor and the first flavone-xanthone dimer, from Swertia franchetiana. J. Nat. Prod. 1994, 57, 211–217. [Google Scholar] [CrossRef]
- Groweiss, A.; Cardellina, J.H.; Boyd, M.R. HIV-Inhibitory Prenylated Xanthones and Flavones from Maclura tinctoria. J. Nat. Prod. 2000, 63, 1537–1539. [Google Scholar] [CrossRef]
- Saikia, R.; Pathak, K.; Pramanik, P.; Islam, M.d.A.; Karmakar, S.; Gogoi, S.; Pathak, M.P.; Das, D.; Sahariah, J.J.; Ahmad, M.Z.; et al. Exploring the therapeutic potential of xanthones in diabetes management: Current insights and future directions. Eur. J. Med. Chem. Rep. 2024, 12, 100189. [Google Scholar] [CrossRef]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Son, J.; Accili, D. Reversing pancreatic β-cell dedifferentiation in the treatment of type 2 diabetes. Exp. Mol. Med. 2023, 2023, 1–7. [Google Scholar] [CrossRef]
- Kumar, V.; Prakash, C.B.K.G.; Miohd Rashid, F.A.A.; Khan, J.A.J.; Firoz, A.A.V. α-Mangostin mediated pharmacological modulation of hepatic carbohydrate metabolism in diabetes-induced wistar rat. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 255–276. [Google Scholar] [CrossRef]
- Ichiki, H.; Miura, T.; Kubo, M.; Ishihara, E.; Komatsu, Y.; Tanigawa, K.; Okada, M. New antidiabetic compounds, mangiferin and its glucoside. Biol. Pharm. Bull. 1998, 21, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Chaubey, P.; Suvarna, V. Role of natural products in alleviation of rheumatoid arthritis—A review. J. Food Biochem. 2021, 45, e13673. [Google Scholar] [CrossRef]
- Ondeyka, J.G.; Dombrowski, A.W.; Polishook, J.P.; Felcetto, T.; Shoop, W.L.; Guan, Z.; Singh, S.B. Isolation and insecticidal/anthelmintic activity of xanthonol, a novel bis-xanthone, from a non-sporulating Fungal species. J. Antibiot. 2006, 59, 288–292. [Google Scholar] [CrossRef]
- Maiese, W.M.; Korshalla, J.; Goodman, J.; Torrey, M.J.; Kantor, S.; Labeda, D.P.; Greenstein, M. Simaomicin (LL-D42067), a novel antibiotic from Actinomadura madurae. I. Taxonomy, fermentation and biological activity. J. Antibiot. 1990, 43, 1059–1063. [Google Scholar] [CrossRef]
- Yu, H.-Q.; Li, G.; Lou, H.-X. Isolation, biosynthesis, and biological activity of polycyclic xanthones from Actinomycetes. Front. Microbiol. 2022, 13, 922089. [Google Scholar] [CrossRef]
- Annang, F.; Pérez-Victoria, I.; Pérez-Moreno, G.; Domingo, E.; González, I.; Tormo, J.R.; Martín, J.; Ruiz-Pérez, L.M.; González-Pacanowska, O.G.D.; Vicente, F.; et al. MDN-0185, an antiplasmodial polycyclic xanthone isolated from Micromonospora sp. CA-256353. J. Nat. Prod. 2018, 81, 1687–1691. [Google Scholar] [CrossRef]
- Ke, H.; Morrisey, J.M.; Qu, S.; Chantarasriwong, O.; Mather, M.W.; Theodorakis, E.A.; Vaidya, A.B. Caged Garcinia xanthones, a novel chemical scaffold with potent antimalarial activity. Antimicrob. Agents Chemother. 2017, 61, e01220-16. [Google Scholar] [CrossRef]
- Li, J.; Nie, X.; Rangsinth, P.; Wu, X.; Zheng, C.; Cheng, Y.; Shiu, P.H.; Li, R.; Lee, S.M.; Fu, C.; et al. Structure and activity relationship analysis of xanthones from mangosteen: Identifying garcinone E as a potent dual EGFR and VEGFR2 inhibitor. Phytomedicine 2024, 122, 155140. [Google Scholar] [CrossRef]
- Cardozo-Muñoz, J.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Lopez-Vallejo, F.; Patiño-Ladino, O.J. Multitarget action of xanthones from Garcinia mangostana against α-amylase, α-glucosidase and pancreatic lipase. Molecules 2022, 27, 3283. [Google Scholar] [CrossRef]







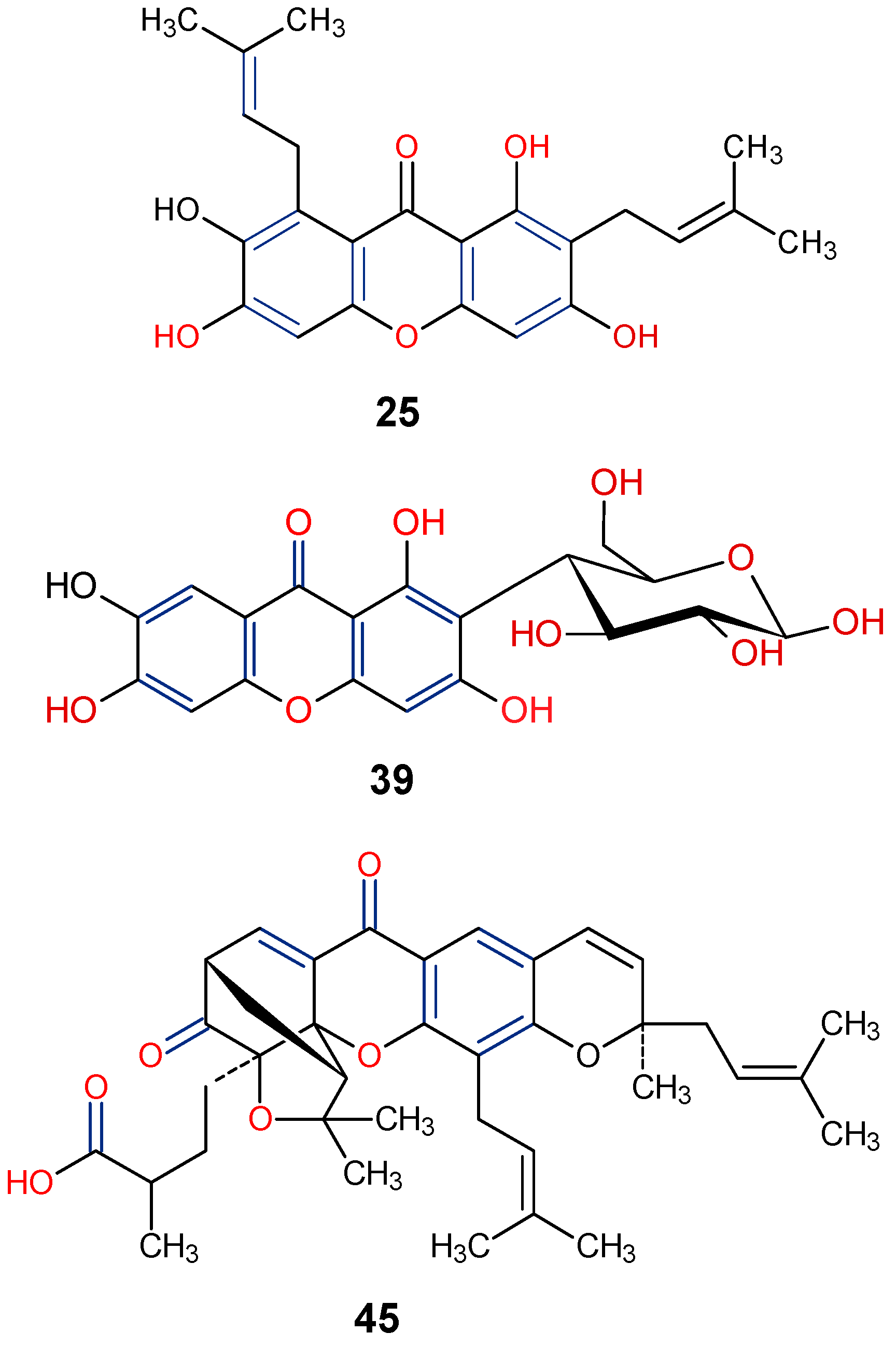
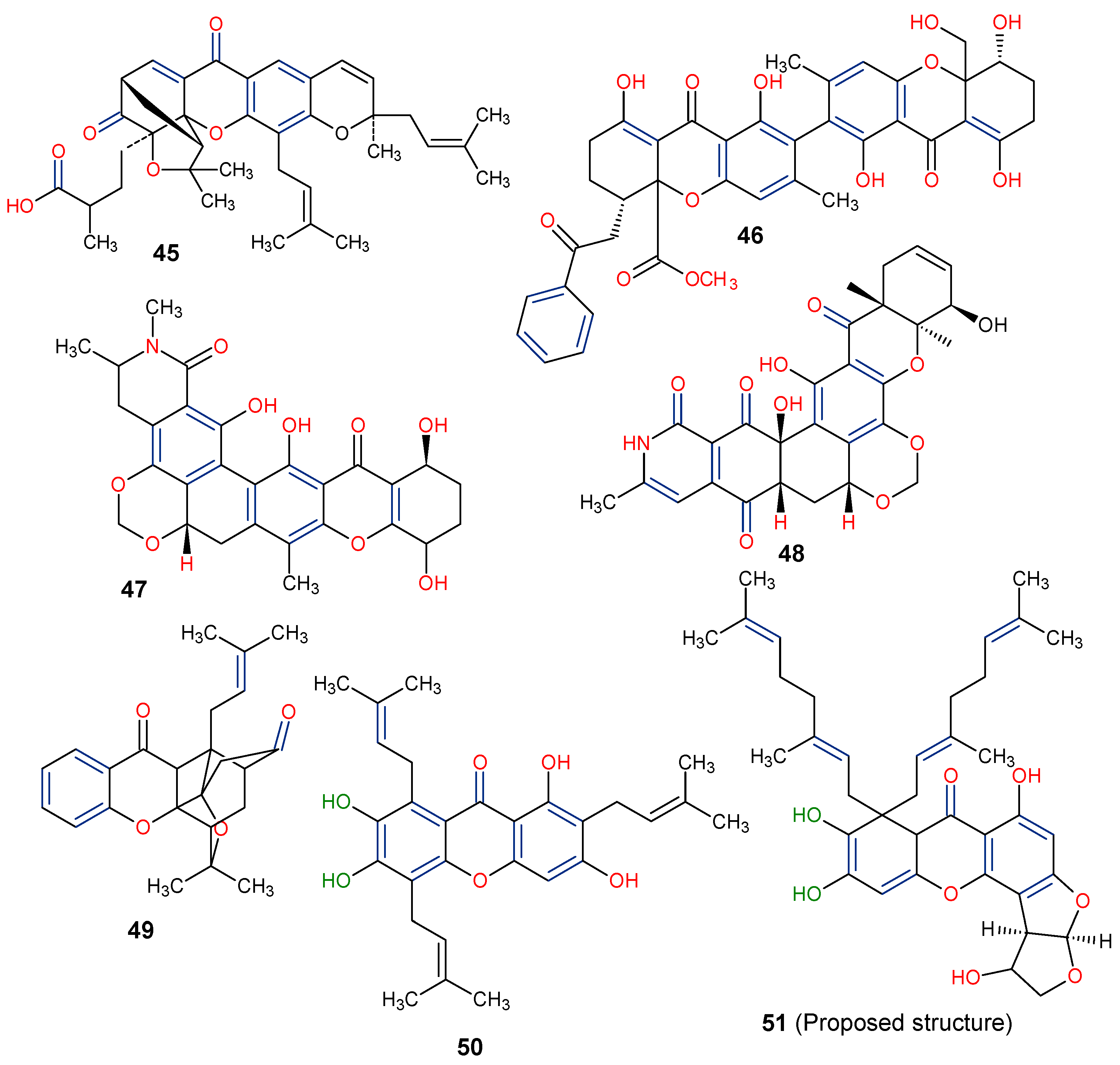
| Parent Class | Structural Example | Natural Source (Family) | Reference |
|---|---|---|---|
| Based on the level of oxidation of the C-ring | |||
| Monomers 1 (Xanthones) | 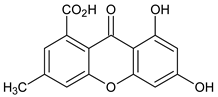 6,8-Dihydroxy-3-methylxanthone-1-carboxylic acid | Penicillium oxalicum (Trichocomaceae) | [37] |
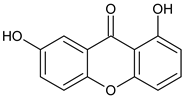 1,7-Dihydroxyxanthone 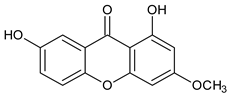 Gentisin | Garcinia lancilimba (Guttiferae) | [38] | |
| Monomer 2 (Dihydroxanthones) | 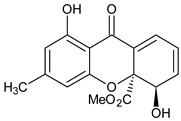 Nidulalin A | Anixiella micropertusa (Sordariaceae) | [39] |
 Dihydroxanthone AGI-B4 | Diaporthe species (Diaporthaceae) | [40] | |
| Monomer 3 (Tetrahydroxanthones) | 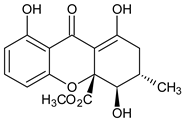 Blennolide B (Hemi-secalonic acid E) | Blennoria species (Coleosporiaceae) | [41] |
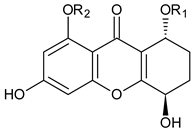 Amarellin A: R1 = Glc, R2 = H Amarellin B: R1 = Xyl, R2 = H Amarellin C: R1 = H, R2 = Glc | Gentianella amarella ssp. Acuta (Gentianaceae) | [42] | |
| Monomer 4 (Hexahydroxanthones) | 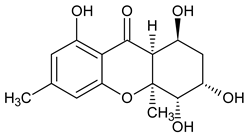 Monodictysin A | Monodictys putredinis (Thrombiaceae) | [43] |
 Applanatin A | Ganoderma applanatum (Ganodermataceae) | [44] | |
| Dimers and Heterodimers 1 (Xanthones) |  Acremoxanthone A | Acremonium sp. (Hypocreaceae) | [45] |
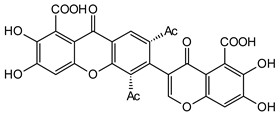 Vinaxanthone | Penicillium glabrum (Trichocomaceae) | [35] | |
| Dimers and Heterodimers 1 (Dihydroxanthones) | 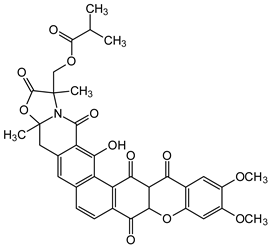 Citreamicin β | Micromonospora citrea (Micromonosporaceae) | [46] |
| Dimers and Heterodimers 3 (Tetrahydroxanthone) | 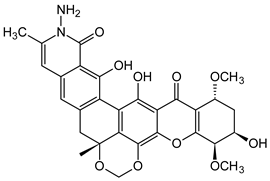 Actinoplanone C | Actinoplanes species (Micromonosporaceae) | [47] |
| Based on the level of oxygenation/type of ring residue | |||
| Simple oxygenated xanthones (mono-oxygenated) | 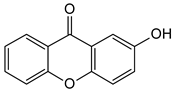 2-Hydroxyxanthone | Mammea americana (Calophyllaceae) | [48,49] |
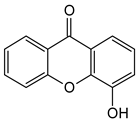 4-Hydroxyxanthone | Calophyllum inophyllum (Calophyllaceae) | ||
| Di-oxygenated | 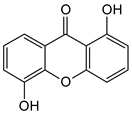 1,5-Dihydroxyxanthone | Mesua ferrea (Calophyllaceae) | [50] |
| Tri-oxygenated | 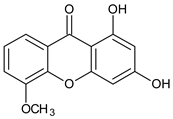 1,3-Dihydroxy-5-methoxyxanthone | Cudrania fruticose (Moraceae) | [51] |
| Tetra-oxygenated | 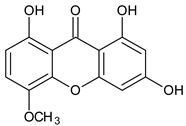 1,3-Dihydroxy-5methoxyxanthone | Swertia nervosa (Gentianaceae) | [52] |
| Penta-oxygenated |  Anomalin B | Swertia purpurascens (Gentianaceae) | [53] |
| Hexa-oxygenated |  1,8-Dihydroxy-2,3,4,6-tetramethoxyxanthone | Centaurium erythraea (Gentianaceae) | [54] |
| Prenylated and related xanthones | 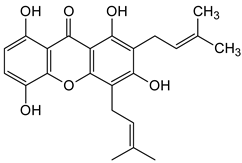 Gartanin  9-Hydroxycalabaxanthone | Garcinia mangostana (Guttiferae) | [3] |
| Bisxanthones |  Jacarelhyperols D | Hypericum japonicum (Guttiferae) | [55] |
| Xanthonolignoids | 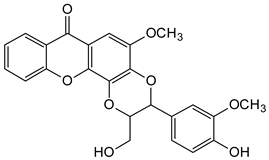 Kielcorin | Kielmeyera coriacea (Guttiferae) | [56] |
| Other miscellaneous xanthones | 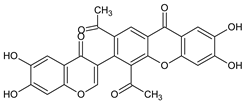 Xanthonopyrone, SPF-3059-26 | Penicillium erubescens KUFA 0220 (Aspergillaceae) isolated from the marine sponge Neopetrosia sp. | [57] |
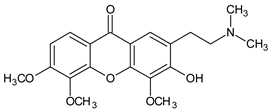 2-(2-(Dimethylamino)ethyl)-3-hydroxy-4,5,6-trimethoxy-9H-xanthen-9-one | Caulophyllum robustum (Berberidaceae) | [58] | |
 Isomangiferin | Mangifera indica (Anacardiaceae) | [59] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriola, A.O.; Kar, P. Naturally Occurring Xanthones and Their Biological Implications. Molecules 2024, 29, 4241. https://doi.org/10.3390/molecules29174241
Oriola AO, Kar P. Naturally Occurring Xanthones and Their Biological Implications. Molecules. 2024; 29(17):4241. https://doi.org/10.3390/molecules29174241
Chicago/Turabian StyleOriola, Ayodeji O., and Pallab Kar. 2024. "Naturally Occurring Xanthones and Their Biological Implications" Molecules 29, no. 17: 4241. https://doi.org/10.3390/molecules29174241
APA StyleOriola, A. O., & Kar, P. (2024). Naturally Occurring Xanthones and Their Biological Implications. Molecules, 29(17), 4241. https://doi.org/10.3390/molecules29174241










