Radiolabeled Probes from Derivatives of Natural Compounds Used in Nuclear Medicine
Abstract
:1. Introduction
2. Curcumin Derivates
3. Stilbene Derivates
4. Other Natural Compound Derivates
4.1. Benzofuran Derivates
4.2. Chalcone Derivates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frey, K.A.; Royal, H.D.; Di Carli, M.F.; Dillehay, G.L.; Gordon, L.; Mankoff, D.A.; O’Malley, J.; Ramanna, L.; Rohren, E.; Segall, G.M.; et al. ABNM position statement: Nuclear medicine professional competency and scope of practice. J. Nucl. Med. 2011, 52, 994–997. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar]
- Sharma, R.; Aboagye, E. Development of radiotracers for oncology--the interface with pharmacology. Br. J. Pharmacol. 2011, 163, 1565–1585. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Bundschuh, R.A.; Bundschuh, L.; Fanti, S.; Javadi, M.S.; Higuchi, T.; Weich, A.; Pienta, K.J.; Buck, A.K.; Pomper, M.G.; et al. Novel Structured Reporting Systems for Theranostic Radiotracers. J. Nucl. Med. 2019, 60, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Witney, T.H.; Blower, P.J. The chemical tool-kit for molecular imaging with radionuclides in the age of targeted and immune therapy. Cancer Imaging 2021, 21, 18. [Google Scholar] [CrossRef]
- Fay, R.; Holland, J.P. The Impact of Emerging Bioconjugation Chemistries on Radiopharmaceuticals. J. Nucl. Med. 2019, 60, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar, Y.S.; Divgi, C. Current status of therapy of solid tumors. J. Nucl. Med. 2005, 46 (Suppl. S1), 141S–150S. [Google Scholar] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Schmidt, M.; Baum, R.P.; Simon, T.; Howman-Giles, R. Therapeutic nuclear medicine in pediatric malignancy. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 411–428. [Google Scholar]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B. 2014, 15, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Shetake, N.; Desai, S.; Kumar, A.; Samuel, G.; Pandey, B.N. Relevance of radiobiological concepts in radionuclide therapy of cancer. Int. J. Radiat. Biol. 2016, 92, 173–186. [Google Scholar] [CrossRef]
- Ferrier, M.G.; Radchenko, V. An Appendix of Radionuclides Used in Targeted Alpha Therapy. J. Med. Imaging Radiat. Sci. 2019, 50 (Suppl. S1), S58–S65. [Google Scholar] [CrossRef] [PubMed]
- Widel, M.; Przybyszewski, W.M.; Cieslar-Pobuda, A.; Saenko, Y.V.; Rzeszowska-Wolny, J. Bystander normal human fibroblasts reduce damage response in radiation targeted cancer cells through intercellular ROS level modulation. Mutat. Res. 2012, 731, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Gidaro, M.C.; Alcaro, S.; Secci, D.; Rivanera, D.; Mollica, A.; Agamennone, M.; Giampietro, L.; Carradori, S. Identification of new anti-Candida compounds by ligand-based pharmacophore virtual screening. J. Enzyme Inhib. Med. Chem. 2016, 31, 1703–1706. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. Compounds from Natural Sources as Protein Kinase Inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef]
- Paoli, P. Enzymatic Inhibitors from Natural Sources: A Huge Collection of New Potential Drugs. Biomolecules 2021, 11, 133. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Dudoit, A.; Benbouguerra, N.; Richard, T.; Hornedo-Ortega, R.; Valls-Fonayet, J.; Coussot, G.; Saucier, C. α-Glucosidase Inhibitory Activity of Tannat Grape Phenolic Extracts in Relation to Their Ripening Stages. Biomolecules 2020, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules 2020, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Tisi, R.; Gaponenko, V.; Vanoni, M.; Sacco, E. Natural Products Attenuating Biosynthesis, Processing, and Activity of Ras Oncoproteins: State of the Art and Future Perspectives. Biomolecules 2020, 10, 1535. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Ahmed, N.; Giampietro, L. Editorial: Targeting tumor EMT-related signaling by natural products. Front. Pharmacol. 2023, 14, 1152328. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Taylor, E.; Kim, Y.; Zhang, K.; Chau, L.; Nguyen, B.C.; Rayalam, S.; Wang, X. Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules 2022, 27, 4396. [Google Scholar] [CrossRef]
- Wongso, H. Natural product-based radiopharmaceuticals: Focus on curcumin and its analogs, flavonoids, and marine peptides. J. Pharm. Anal. 2022, 12, 380–393. [Google Scholar] [CrossRef]
- Mari, M.; Carrozza, D.; Ferrari, E.; Asti, M. Applications of Radiolabelled Curcumin and Its Derivatives in Medicinal Chemistry. Int. J. Mol. Sci. 2021, 22, 7410. [Google Scholar] [CrossRef]
- Orteca, G.; Sinnes, J.P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954. [Google Scholar] [CrossRef]
- Rokka, J.; Snellman, A.; Zona, C.; La Ferla, B.; Nicotra, F.; Salmona, M.; Forloni, G.; Haaparanta-Solin, M.; Rinne, J.O.; Solin, O. Synthesis and evaluation of a (18)F-curcumin derivate for β-amyloid plaque imaging. Bioorg. Med. Chem. 2014, 22, 2753–2762. [Google Scholar] [CrossRef]
- Asti, M.; Ferrari, E.; Croci, S.; Atti, G.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Zerbini, A.; Saladini, M.; Versari, A. Synthesis and characterization of (68)Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and Alzheimer’s disease. Inorg. Chem. 2014, 53, 4922–4933. [Google Scholar] [CrossRef] [PubMed]
- Bartik, L.; Whitfield, G.K.; Kaczmarska, M.; Lowmiller, C.L.; Moffet, E.W.; Furmick, J.K.; Hernandez, Z.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Curcumin: A novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer chemoprevention. J. Nutr. Biochem. 2010, 21, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Orteca, G.; Pisaneschi, F.; Rubagotti, S.; Liu, T.W.; Biagiotti, G.; Piwnica-Worms, D.; Iori, M.; Capponi, P.C.; Ferrari, E.; Asti, M. Development of a Potential Gallium-68-Labelled Radiotracer Based on DOTA-Curcumin for Colon-Rectal Carcinoma: From Synthesis to In Vivo Studies. Molecules 2019, 24, 644. [Google Scholar] [CrossRef]
- Ryu, E.K.; Choe, Y.S.; Lee, K.H.; Choi, Y.; Kim, B.T. Curcumin and dehydrozingerone derivatives: Synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Gama Sosa, M.A.; De Gasperi, R. Transgenic mouse models of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 69–81. [Google Scholar] [CrossRef]
- Shin, S.; Koo, H.-J.; Lee, I.; Choe, Y.S.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T. Synthesis and characterization of 18F-labeled hydrazinocurcumin derivatives for tumor imaging. RSC Adv. 2015, 5, 96733–96745. [Google Scholar] [CrossRef]
- Leporini, L.; Giampietro, L.; Amoroso, R.; Ammazzalorso, A.; Fantacuzzi, M.; Menghini, L.; Maccallini, C.; Ferrante, C.; Brunetti, L.; Orlando, G.; et al. In vitro protective effects of resveratrol and stilbene alkanoic derivatives on induced oxidative stress on C2C12 and MCF7 cells. J. Biol. Regul. Homeost. Agents 2017, 31, 589–601. [Google Scholar]
- Han, T.H.; Lee, J.; Harmalkar, D.S.; Kang, H.; Jin, G.; Park, M.K.; Kim, M.; Yang, H.A.; Kim, J.; Kwon, S.J.; et al. Stilbenoid derivatives as potent inhibitors of HIF-1α-centric cancer metabolism under hypoxia. Biomed. Pharmacother. 2024, 176, 116838. [Google Scholar] [CrossRef]
- Di Filippo, E.S.; Giampietro, L.; De Filippis, B.; Balaha, M.; Ferrone, V.; Locatelli, M.; Pietrangelo, T.; Tartaglia, A.; Amoroso, R.; Fulle, S. Synthesis and Biological Evaluation of Halogenated E-Stilbenols as Promising Antiaging Agents. Molecules 2020, 25, 5770. [Google Scholar] [CrossRef]
- Amoroso, R.; Leporini, L.; Cacciatore, I.; Marinelli, L.; Ammazzalorso, A.; Bruno, I.; De Filippis, B.; Fantacuzzi, M.; Maccallini, C.; Menghini, L.; et al. Synthesis, Characterization and Evaluation of Gemfibrozil-stilbene Hybrid as Antioxidant Agent. Lett. Drug. Des. Discov. 2018, 15, 1230–1238. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, C.; Sun, C.; Wang, L. Simultaneously regulating absorption capacities and antioxidant activities of four stilbene derivatives utilizing substitution effect: A theoretical and experimental study against UVB radiation. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2024, 304, 123325. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, L.; D’Angelo, A.; Giancristofaro, A.; Ammazzalorso, A.; De Filippis, B.; Di Matteo, M.; Fantacuzzi, M.; Linciano, P.; Maccallini, C.; Amoroso, R. Effect of stilbene and chalcone scaffolds incorporation in clofibric acid on PPARα agonistic activity. Med. Chem. 2014, 10, 59–65. [Google Scholar] [CrossRef]
- Li, S.S.; Zheng, J.N.; Ma, X.W.; Shen, S.Y.; Liu, X.S.; Ma, Y.Y.; Li, Y.K.; Miao, D.; Wang, W.G.; Gong, D.P.; et al. Stilbene Derivatives from Bletilla striata and Their Antiviral Activity. Chem. Nat. Compd. 2023, 59, 621–624. [Google Scholar] [CrossRef]
- De Angelis, M.; De Filippis, B.; Balaha, M.; Giampietro, L.; Miteva, M.T.; De Chiara, G.; Palamara, A.T.; Nencioni, L.; Mollica, A. Nitrostilbenes: Synthesis and Biological Evaluation as Potential Anti-Influenza Virus Agents. Pharmaceuticals 2022, 15, 1061. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation-An Overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. Chem. Med. Chem. 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Giampietro, L.; Laghezza, A.; Cerchia, C.; Florio, R.; Recinella, L.; Capone, F.; Ammazzalorso, A.; Bruno, I.; De Filippis, B.; Fantacuzzi, M.; et al. Novel Phenyldiazenyl Fibrate Analogues as PPAR α/γ/δ Pan-Agonists for the Amelioration of Metabolic Syndrome. ACS Med. Chem Lett. 2019, 10, 545–551. [Google Scholar] [CrossRef]
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Attenuating Oxidative Stress and JNK/NF-κB Pathway in a SIRT1-Independent Mechanism. J. Cell Biochem. 2017, 118, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshiike, Y.; Takashima, A.; Hasegawa, K.; Naiki, H.; Yamada, M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: Implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003, 87, 172–181. [Google Scholar] [CrossRef]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35 Pt 5, 1156–1160. [Google Scholar] [CrossRef]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef]
- De Filippis, B.; De Lellis, L.; Florio, R.; Ammazzalorso, A.; Amoia, P.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R.; Veschi, S.; et al. Synthesis and cytotoxic effects on pancreatic cancer cells of resveratrol analogs. Med. Chem. Res. 2019, 28, 984–991. [Google Scholar] [CrossRef]
- de Medina, P.; Casper, R.; Savouret, J.F.; Poirot, M. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J. Med. Chem. 2005, 48, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, M.; Miller, K.D.; Sledge, G.W.; Hutchins, G.D.; Zheng, Q.H. Synthesis of radiolabeled stilbene derivatives as new potential PET probes for aryl hydrocarbon receptor in cancers. Bioorg. Med. Chem. Lett. 2006, 16, 5767–5772. [Google Scholar] [CrossRef]
- Zhang, W.; Oya, S.; Kung, M.P.; Hou, C.; Maier, D.L.; Kung, H.F. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl. Med. Biol. 2005, 32, 799–809. [Google Scholar] [CrossRef]
- Sabri, O.; Seibyl, J.; Rowe, C.; Barthel, H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015, 3, 13–26. [Google Scholar] [CrossRef]
- Syed, Y.Y.; Deeks, E. [(18)F]Florbetaben: A review in β-amyloid PET imaging in cognitive impairment. CNS Drugs 2015, 29, 605–613. [Google Scholar] [CrossRef]
- Baratto, L.; Park, S.Y.; Hatami, N.; Gulaka, P.; Vasanawala, S.; Yohannan, T.K.; Herfkens, R.; Witteles, R.; Iagaru, A. 18F-florbetaben whole-body PET/MRI for evaluation of systemic amyloid deposition. EJNMMI Res. 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Auvity, S.; Tonietto, M.; Caillé, F.; Bodini, B.; Bottlaender, M.; Tournier, N.; Kuhnast, B.; Stankoff, B. Repurposing radiotracers for myelin imaging: A study comparing 18F-florbetaben, 18F-florbetapir, 18F-flutemetamol,11C-MeDAS, and 11C-PiB. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 490–501. [Google Scholar] [CrossRef]
- Cassano Cassano, R.; Genovesi, D.; Vergaro, G.; Giorgetti, A.; Aimo, A.; Del Giudice, M.L.; Galimberti, S.; Emdin, M.; Buda, G. [18F]-florbetaben PET/CT is sensitive for cardiac AL amyloidosis. Eur. J. Clin. Investig 2024, e14270, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.S.; Mathis, C.A.; Klunk, W.E. Positron emission tomography radioligands for in vivo imaging of Aβ plaques. J. Labelled Comp. Radiopharm. 2013, 56, 89–95. [Google Scholar] [CrossRef]
- Filippi, L.; Chiaravalloti, A.; Bagni, O.; Schillaci, O. 18F-labeled radiopharmaceuticals for the molecular neuroimaging of amyloid plaques in Alzheimer’s disease. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 268–281. [Google Scholar]
- Mittal, P.; Singh, N.; Chaturvedi, S.; Jyoti, A.; Mishra, A.K.; Hazari, P.P. Comprehensive review on design perspective of PET ligands based on β-amyloids, tau and neuroinflammation for diagnostic intervention of Alzheimer’s disease. Clin. Transl. Imaging 2021, 9, 153–175. [Google Scholar] [CrossRef]
- Datar, Y.; Clerc, O.F.; Cuddy, S.A.M.; Kim, S.; Taylor, A.; Neri, J.C.; Benz, D.C.; Bianchi, G.; Yee, A.J.; Sanchorawala, V.; et al. Quantification of right ventricular amyloid burden with 18F-florbetapir positron emission tomography/computed tomography and its association with right ventricular dysfunction and outcomes in light-chain amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Tago, T.; Toyohara, J.; Fujimaki, R.; Tatsuta, M.; Song, R.; Hirano, K.; Iwai, K.; Tanaka, H. Effects of 18F-fluorinated neopentyl glycol side-chain on the biological characteristics of stilbene amyloid-β PET ligands. Nucl. Med. Biol. 2021, 94–95, 38–45. [Google Scholar] [CrossRef]
- Lee, I.; Choe, Y.S.; Choi, J.Y.; Lee, K.H.; Kim, B.T. Synthesis and evaluation of ¹⁸F-labeled styryltriazole and resveratrol derivatives for β-amyloid plaque imaging. J. Med. Chem. 2012, 55, 883–892. [Google Scholar] [CrossRef]
- Noor, A.; Hayne, D.J.; Lim, S.; Van Zuylekom, J.K.; Cullinane, C.; Roselt, P.D.; McLean, C.A.; White, J.M.; Donnelly, P.S. Copper Bis(thiosemicarbazonato)-stilbenyl Complexes That Bind to Amyloid-β Plaques. Inorg. Chem. 2020, 59, 11658–11669. [Google Scholar] [CrossRef]
- Baumann, B.; Bohnenstengel, F.; Siegmund, D.; Wajant, H.; Weber, C.; Herr, I.; Debatin, K.M.; Proksch, P.; Wirth, T. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J. Biol. Chem. 2002, 277, 44791–44800. [Google Scholar] [CrossRef] [PubMed]
- Proksch, P.; Giaisi, M.; Treiber, M.K.; Palfi, K.; Merling, A.; Spring, H.; Krammer, P.H.; Li-Weber, M. Rocaglamide derivatives are immunosuppressive phytochemicals that target NF-AT activity in T cells. J. Immunol. 2005, 174, 7075–7084. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.C.; Montagnoli, T.L.; Da Silva, J.S.; de Alencar, A.K.N.; Reina Gamba, L.E.; Alves, B.E.O.; da Silva, M.M.C.; Trachez, M.M.; do Nascimento, J.H.M.; Pimentel-Coelho, P.M.; et al. New Benzofuran N-Acylhydrazone Reduces Cardiovascular Dysfunction in Obese Rats by Blocking TNF-Alpha Synthesis. Drug. Des. Devel. Ther. 2020, 14, 3337–3350. [Google Scholar] [CrossRef] [PubMed]
- Radadiya, A.; Shah, A. Bioactive benzofuran derivatives: An insight on lead developments, radioligands and advances of the last decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef]
- Cheng, Y.; Ono, M.; Kimura, H.; Ueda, M.; Saji, H. Technetium-99m labeled pyridyl benzofuran derivatives as single photon emission computed tomography imaging probes for β-amyloid plaques in Alzheimer’s brains. J. Med. Chem. 2012, 55, 2279–2286. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Capponi, P.C.; Mari, M.; Ferrari, E.; Asti, M. Radiolabeled Chalcone Derivatives as Potential Radiotracers for β-Amyloid Plaques Imaging. Molecules 2023, 28, 3233. [Google Scholar] [CrossRef]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone Derivatives: Anti-inflammatory Potential and Molecular Targets Perspectives. Curr. Top. Med. Chem. 2017, 17, 3146–3169. [Google Scholar] [CrossRef]
- Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A Systematic Review on Anti-diabetic Properties of Chalcones. Curr. Med. Chem. 2020, 27, 2257–2321. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Chen, W.; Hui, J.; Ji, J.; Hu, S.; Zhou, J.; Wang, Y.; Liang, G. Chemopreventive effect of chalcone derivative, L2H17, in colon cancer development. BMC Cancer 2015, 15, 870. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Lu, Q.; Xie, J.; Wu, J.; Chen, C. A novel chalcone derivative exerts anti-inflammatory and anti-oxidant effects after acute lung injury. Aging 2019, 11, 7805–7816. [Google Scholar] [CrossRef]
- Henry, E.J.; Bird, S.J.; Gowland, P.; Collins, M.; Cassella, J.P. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J. Antibiot. 2020, 73, 299–308. [Google Scholar] [CrossRef]
- de Mello, M.V.P.; Abrahim-Vieira, B.A.; Domingos, T.F.S.; de Jesus, J.B.; de Sousa, A.C.C.; Rodrigues, C.R.; Souza, A.M.T. A comprehensive review of chalcone derivatives as antileishmanial agents. Eur. J. Med. Chem. 2018, 150, 920–929. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, L.; Huang, X.; Wang, X.; Gong, M. Chalcone hybrids and their antimalarial activity. Arch. Pharm. 2020, 353, e1900350. [Google Scholar] [CrossRef]
- Giampietro, L.; D’Angelo, A.; Giancristofaro, A.; Ammazzalorso, A.; De Filippis, B.; Fantacuzzi, M.; Linciano, P.; Maccallini, C.; Amoroso, R. Synthesis and structure-activity relationships of fibrate-based analogues inside PPARs. Bioorg. Med. Chem. Lett. 2012, 22, 7662–7666. [Google Scholar] [CrossRef]
- Mazumder, R.; Ichudaule Ghosh, A.; Deb, S.; Ghosh, R. Significance of Chalcone Scaffolds in Medicinal Chemistry. Top. Curr. Chem. 2024, 382, 22. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem. 2021, 108, 104681. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ikeoka, R.; Watanabe, H.; Kimura, H.; Fuchigami, T.; Haratake, M.; Saji, H.; Nakayama, M. Synthesis and evaluation of novel chalcone derivatives with (99m)Tc/Re complexes as potential probes for detection of β-amyloid plaques. ACS Chem. Neurosci. 2010, 1, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, M.; Dai, J.; Wang, X.; Yu, P.; Yang, Y.; Jia, J.; Fu, H.; Ono, M.; Jia, H.; et al. Novel cyclopentadienyl tricarbonyl complexes of (99m)Tc mimicking chalcone as potential single-photon emission computed tomography imaging probes for β-amyloid plaques in brain. J. Med. Chem. 2013, 56, 471–482. [Google Scholar] [CrossRef]
- Gu, J.; Gui, Y.; Chen, L.; Yuan, G.; Lu, H.Z.; Xu, X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS ONE 2013, 8, e62839. [Google Scholar] [CrossRef] [PubMed]
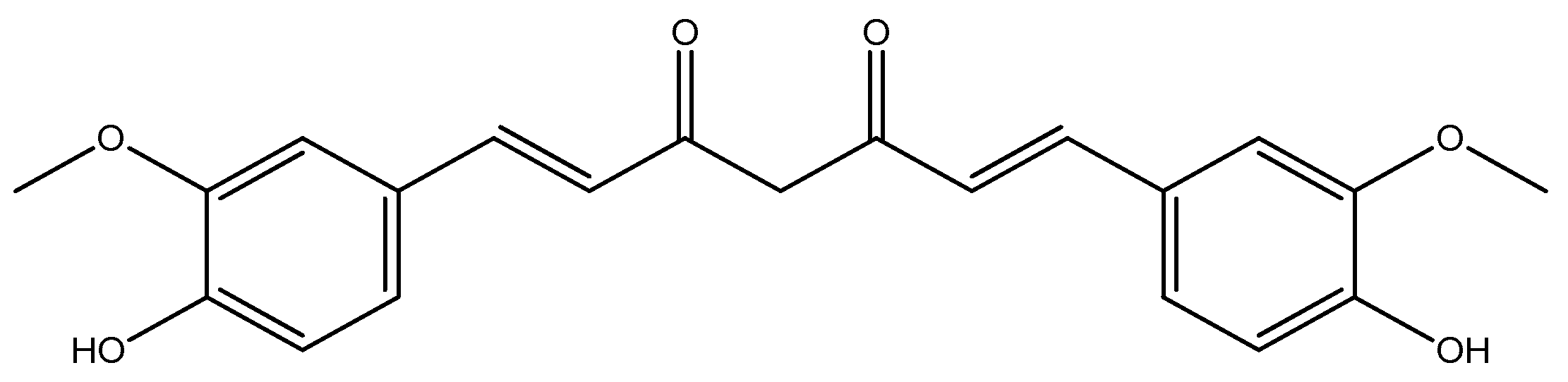
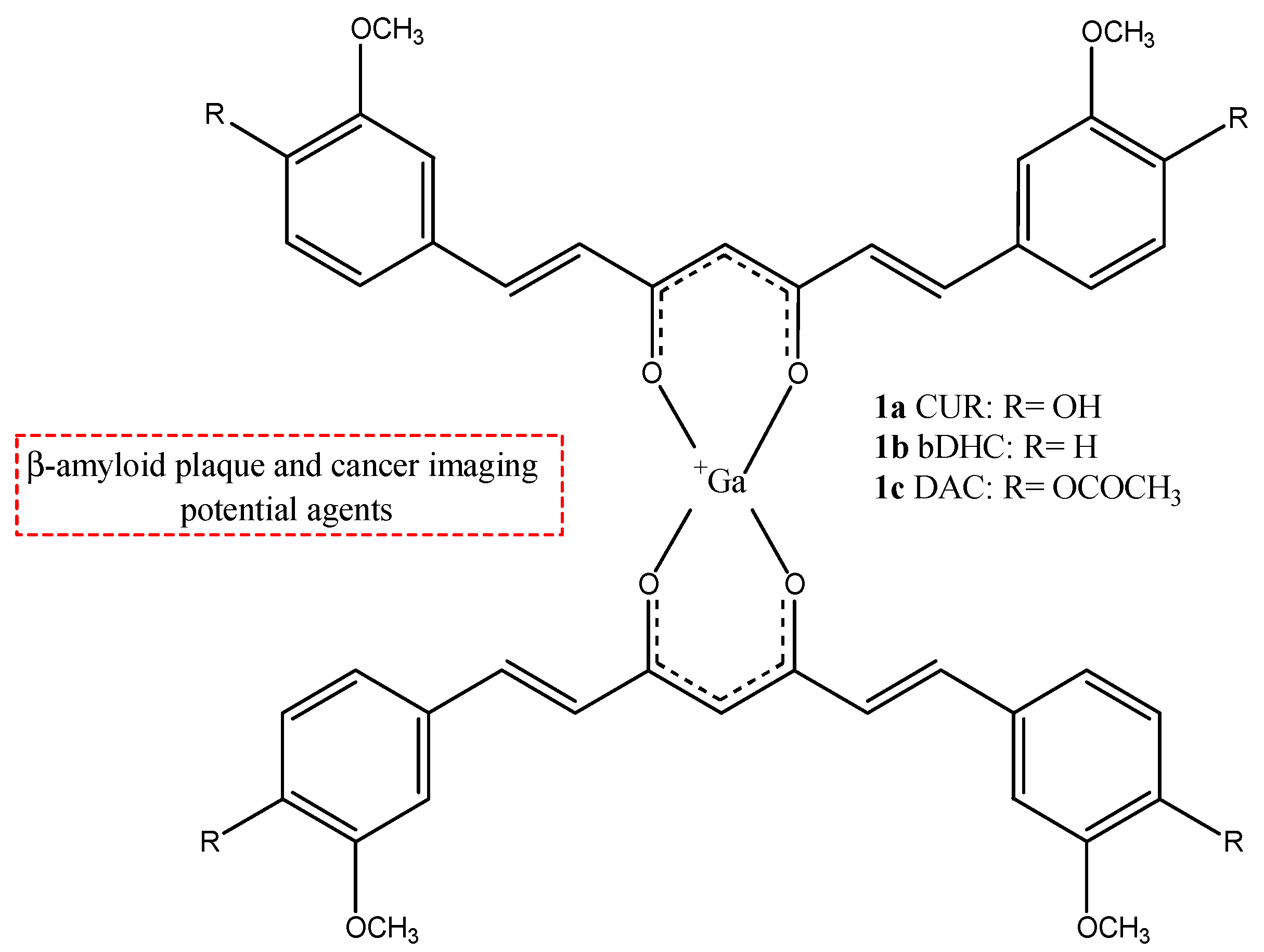

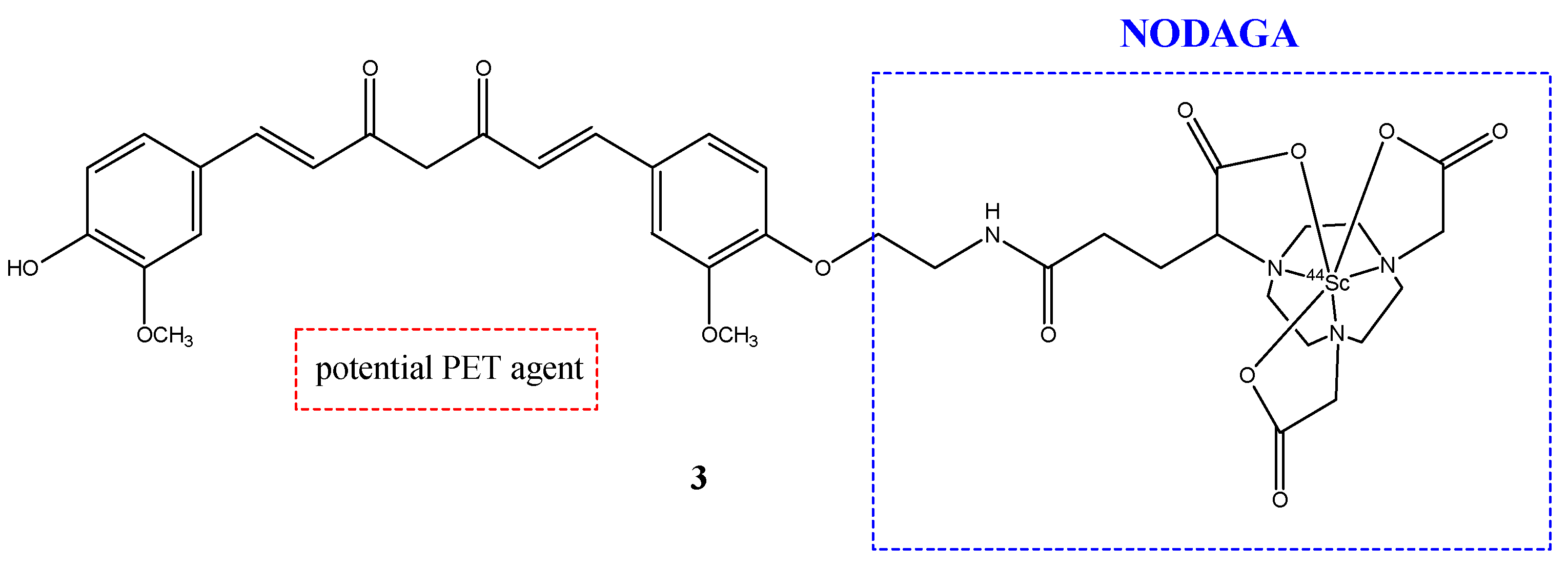
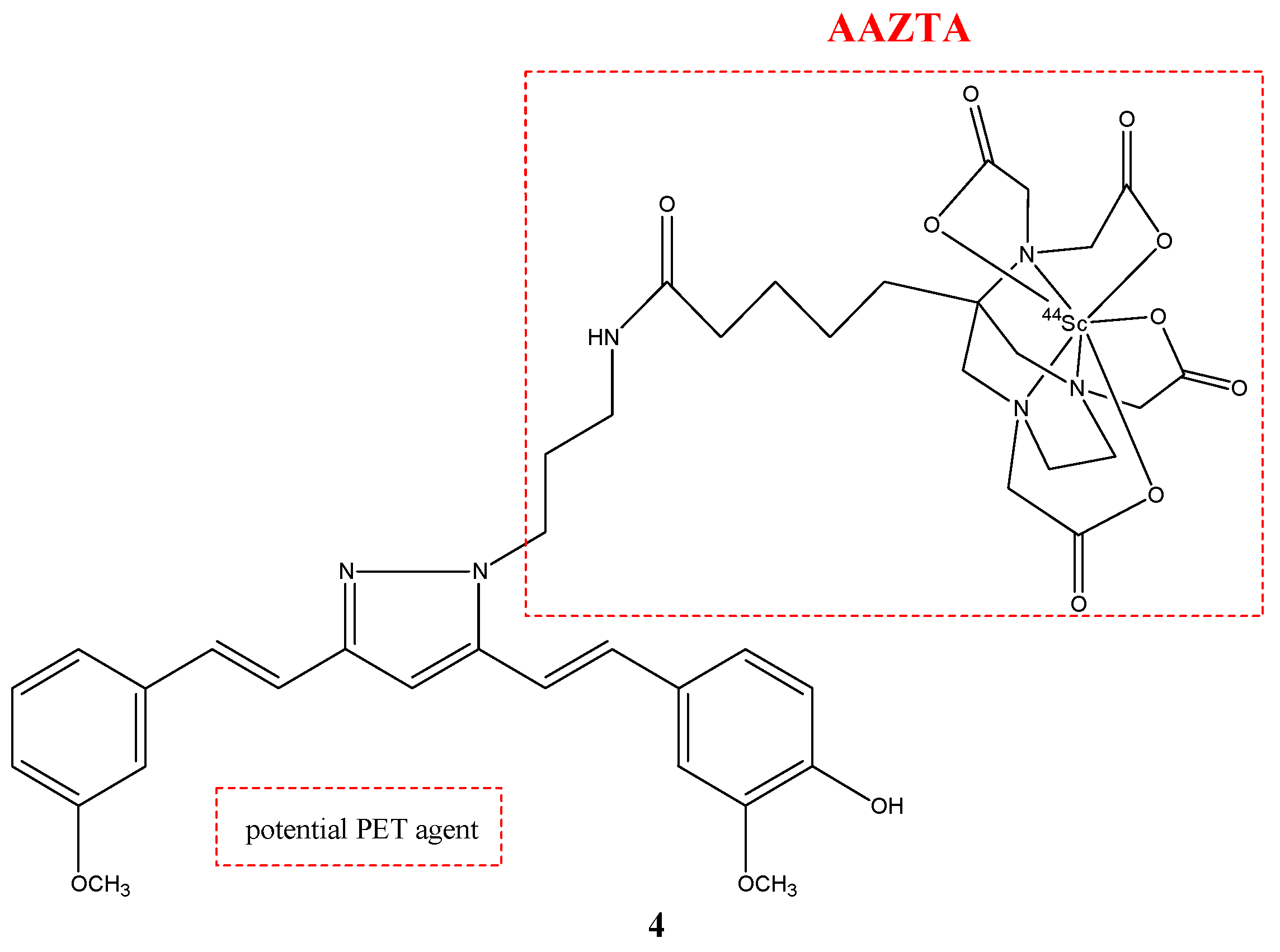

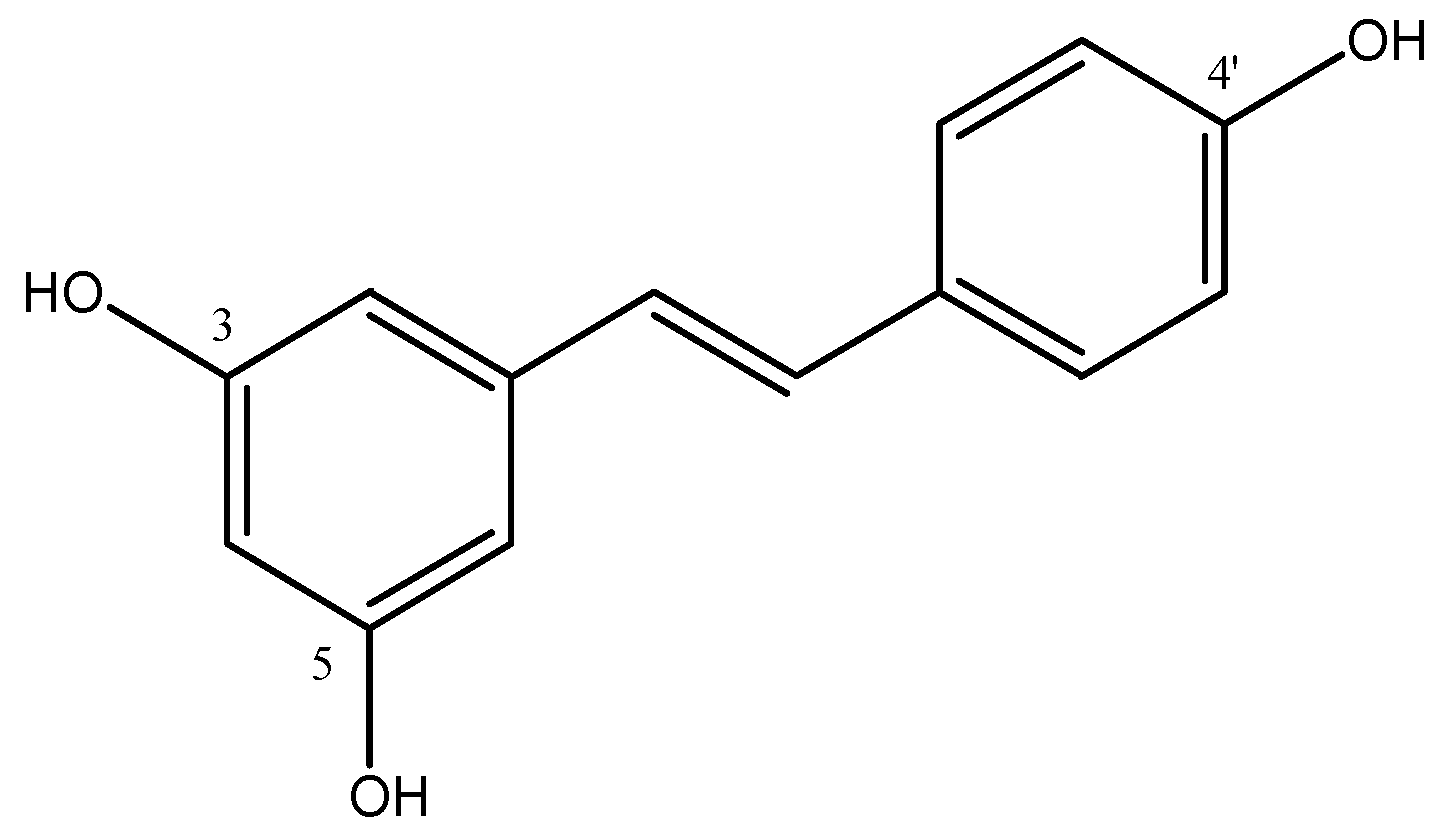

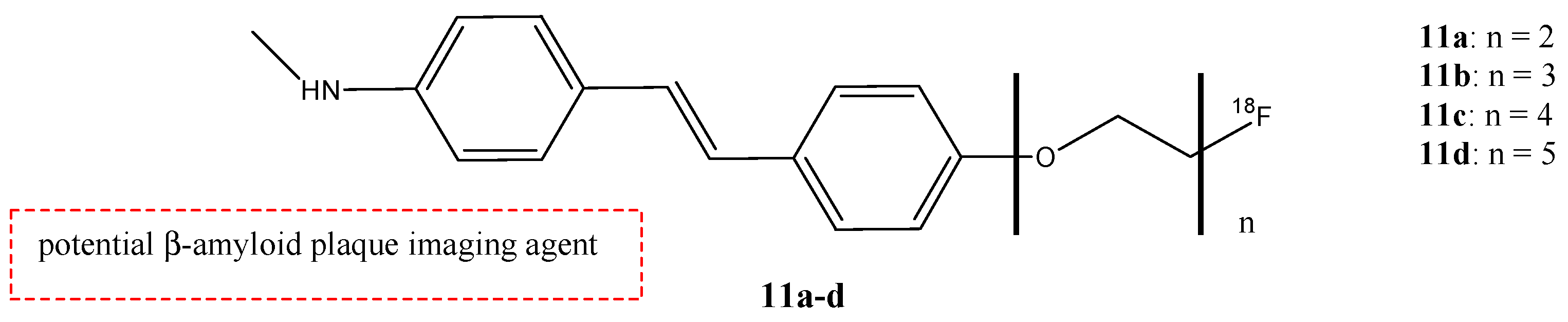
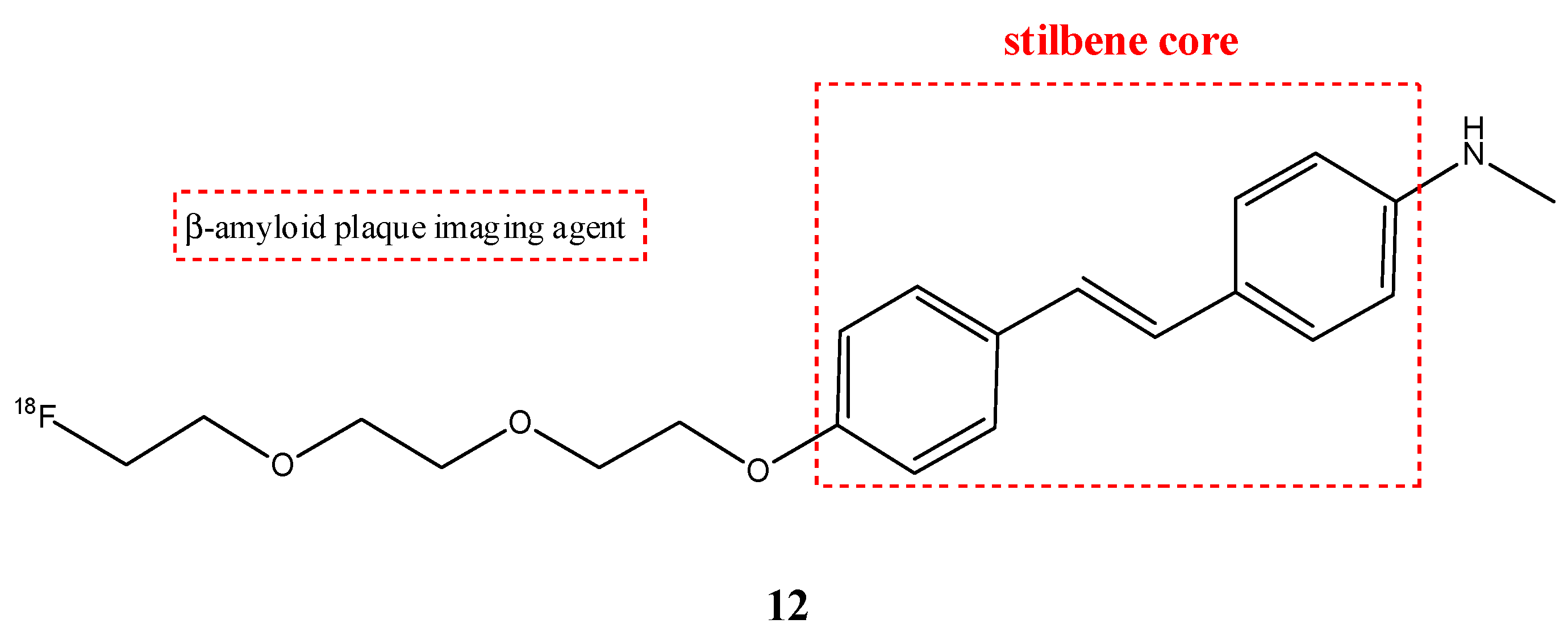

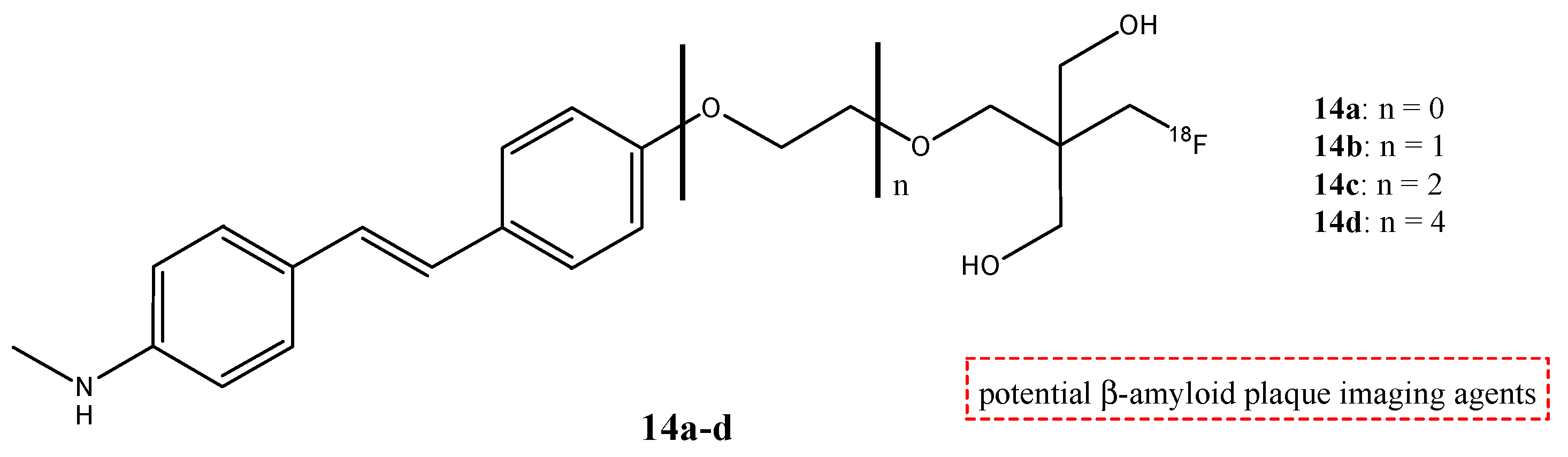






| Compounds | Structures | Radionuclides | Pharmacological Effects | References |
|---|---|---|---|---|
| 1a–c | 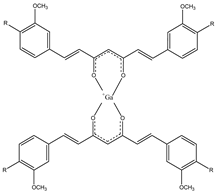 | 68Ga | Potential use for imaging of β-amyloid plaque and cancer. | [32] |
| 2 |  | 68Ga | Potential use of colon rectal cancer. | [34] |
| 3 | 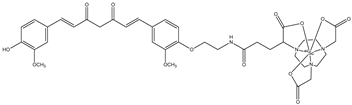 | 44Sc | Potential PET agent. | [30] |
| 4 | 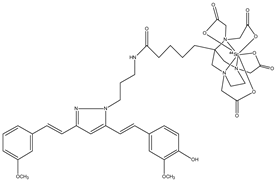 | 44Sc | Potential PET agent. | [30] |
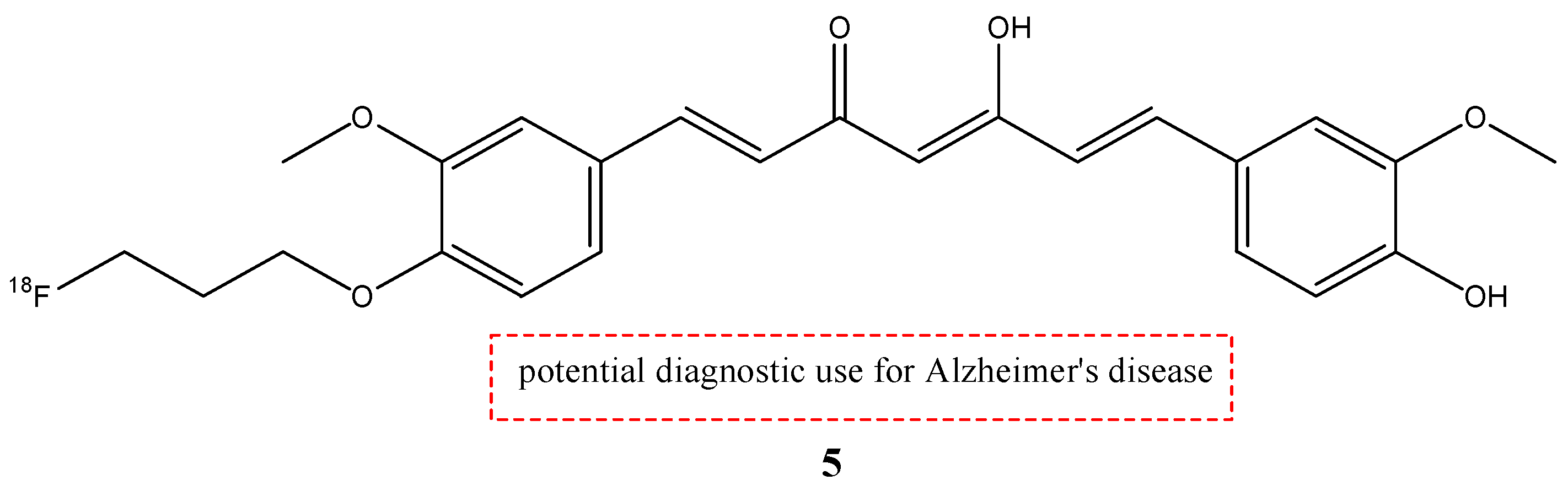
| 5 |  | 18F | Potential diagnostic use for Alzheimer’s disease. | [35] |
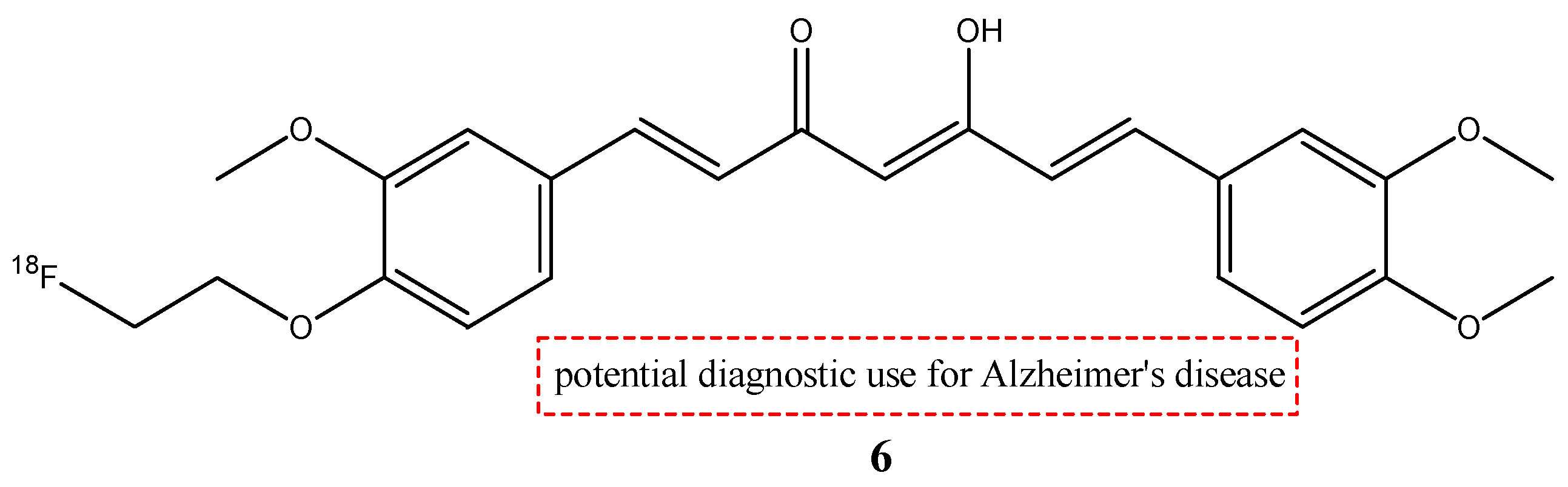
| 6 |  | 18F | Potential diagnostic use for Alzheimer’s disease. | [29] |
| 7 | 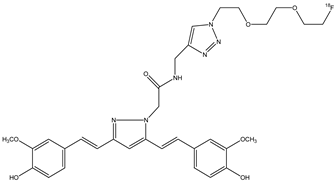 | 18F | Potential diagnostic use for Alzheimer’s disease. | [33] |
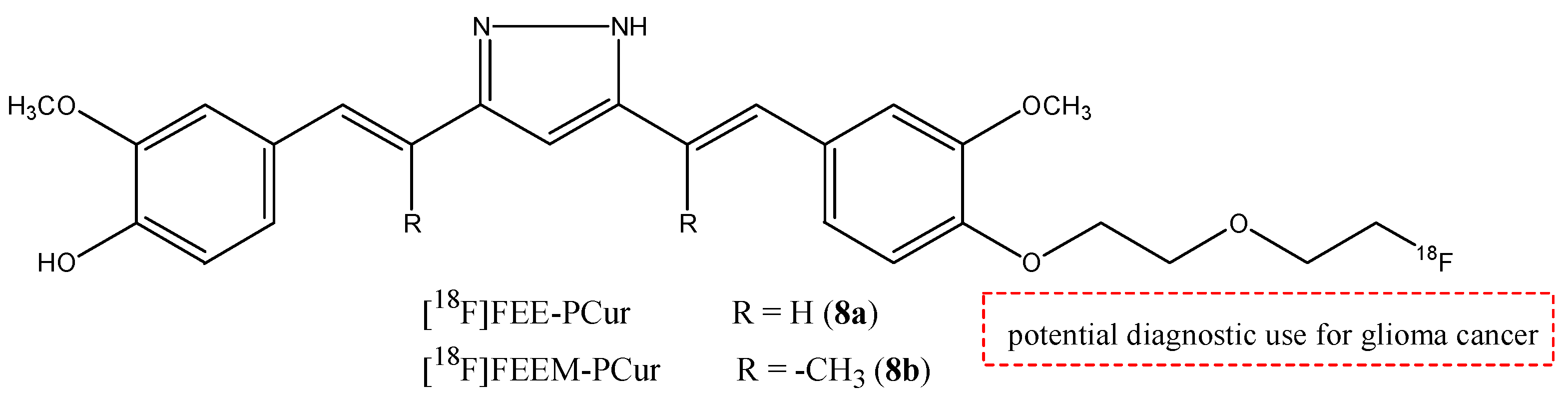
| 8a,b | 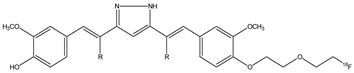 | 18F | Potential diagnostic use for glioma cancer. | [38] |
| 9a–c |  | 18F | Potential PET cancer imaging agents. | [57] |
| 10a–c |  | 18F | Potential PET cancer imaging agents. | [57] |
| 11a–d | 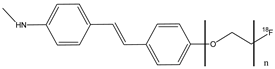 | 18F | Potential β-amyloid plaque imaging agent. | [58] |
| 12 | 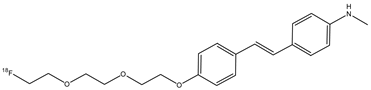 | 18F | β-amyloid plaque imaging agent. | [59] |
| 13 | 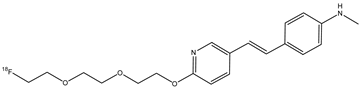 | 18F | β-amyloid plaque imaging agent | [65] |
| 14a–d | 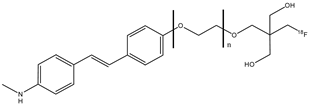 | 18F | Potential β-amyloid plaque imaging agents. | [68] |
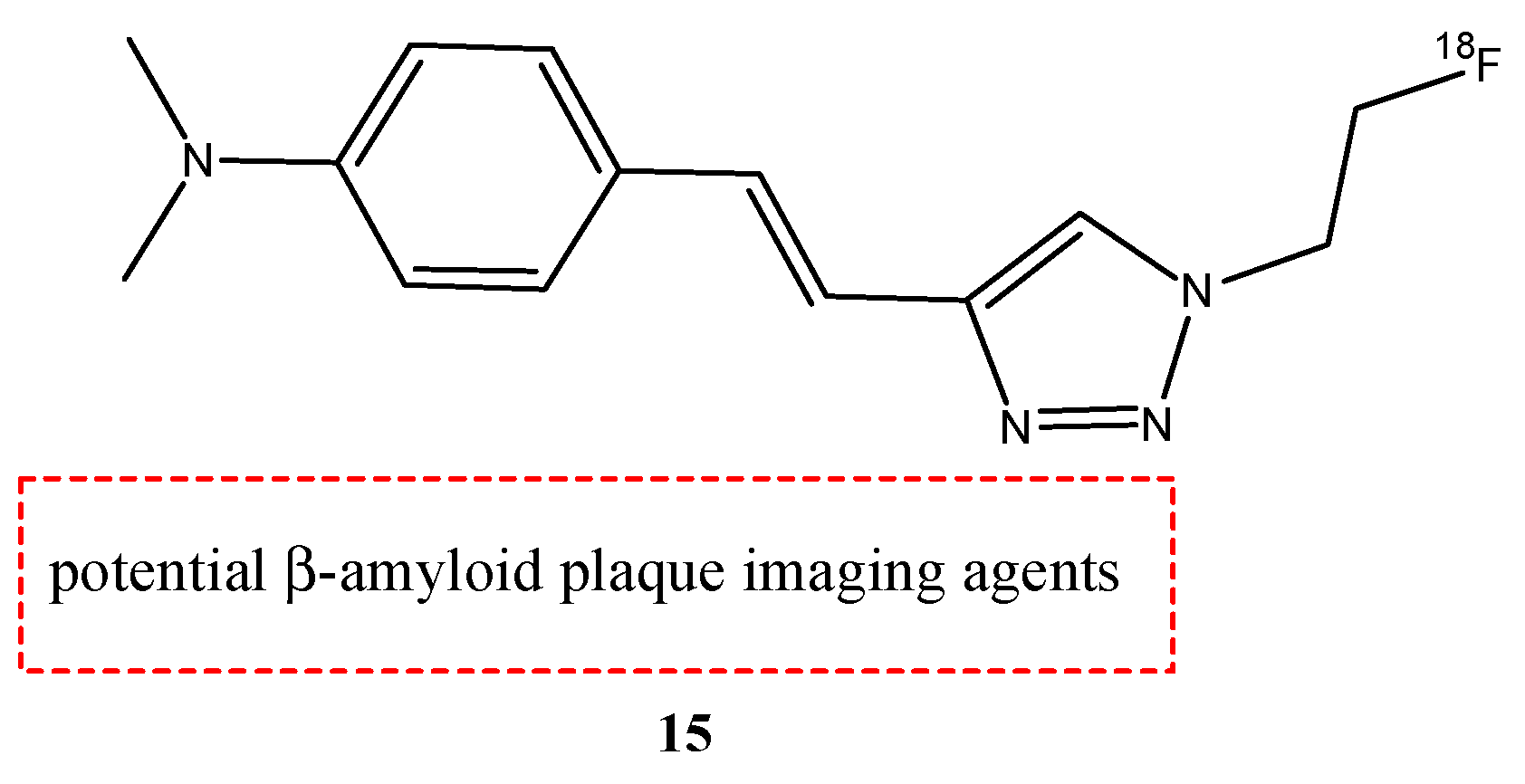
| 15 | 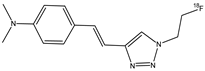 | 18F | Potential β-amyloid plaque imaging agents. | [69] |
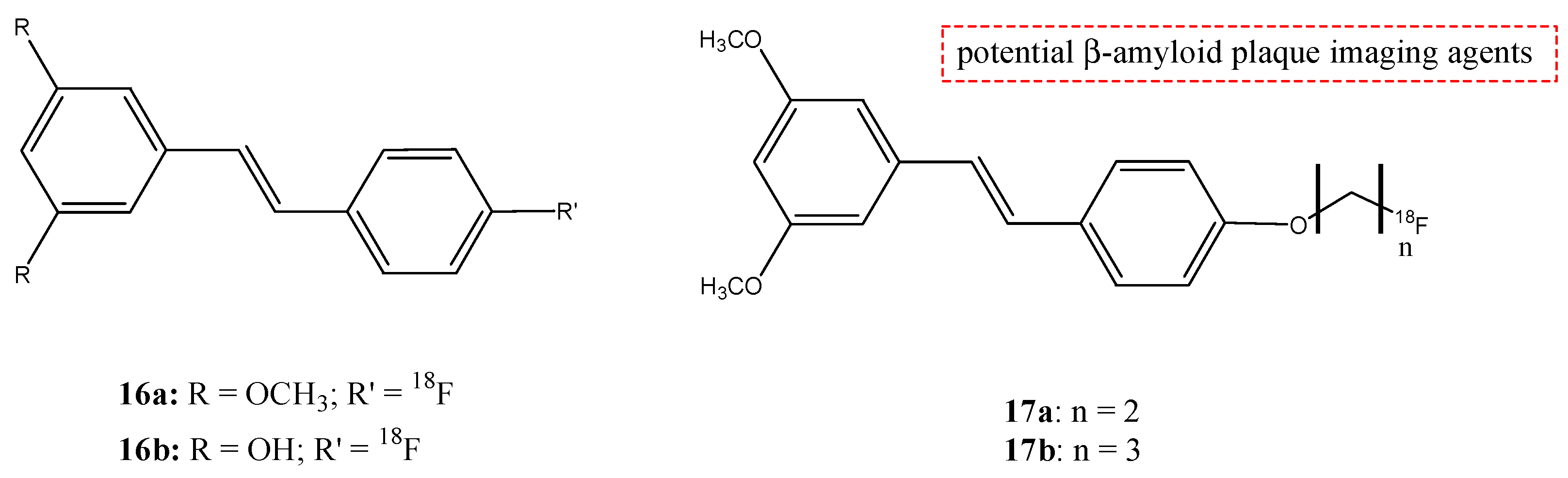
| 16a,b | 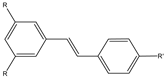 | 18F | Potential β-amyloid plaque imaging agents. | [69] |
| 17a,b | 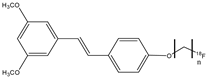 | 18F | Potential β-amyloid plaque imaging agents. | [69] |
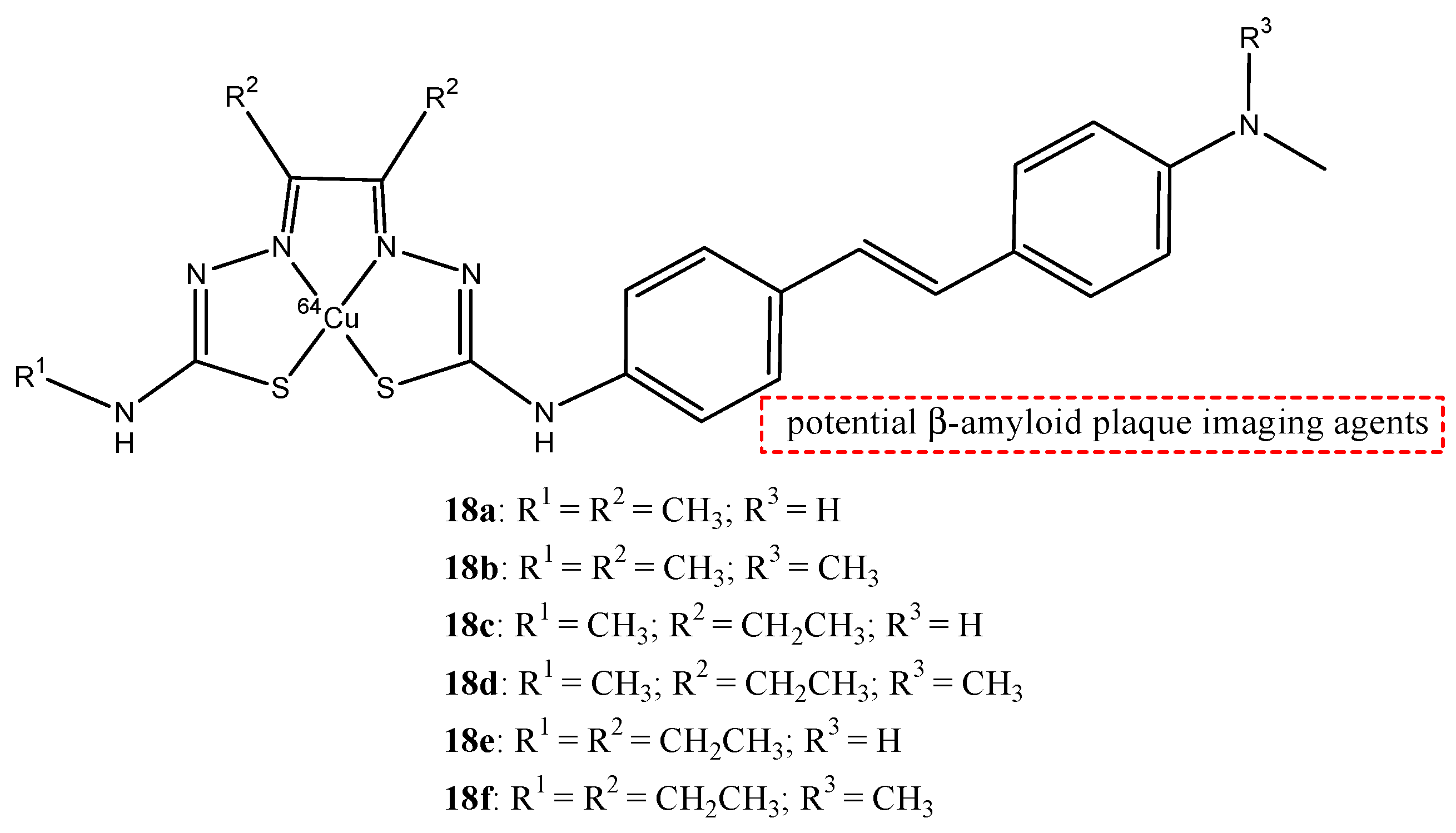
| 18a–f |  | 64Cu | Potential β-amyloid plaque imaging agents. | [70] |
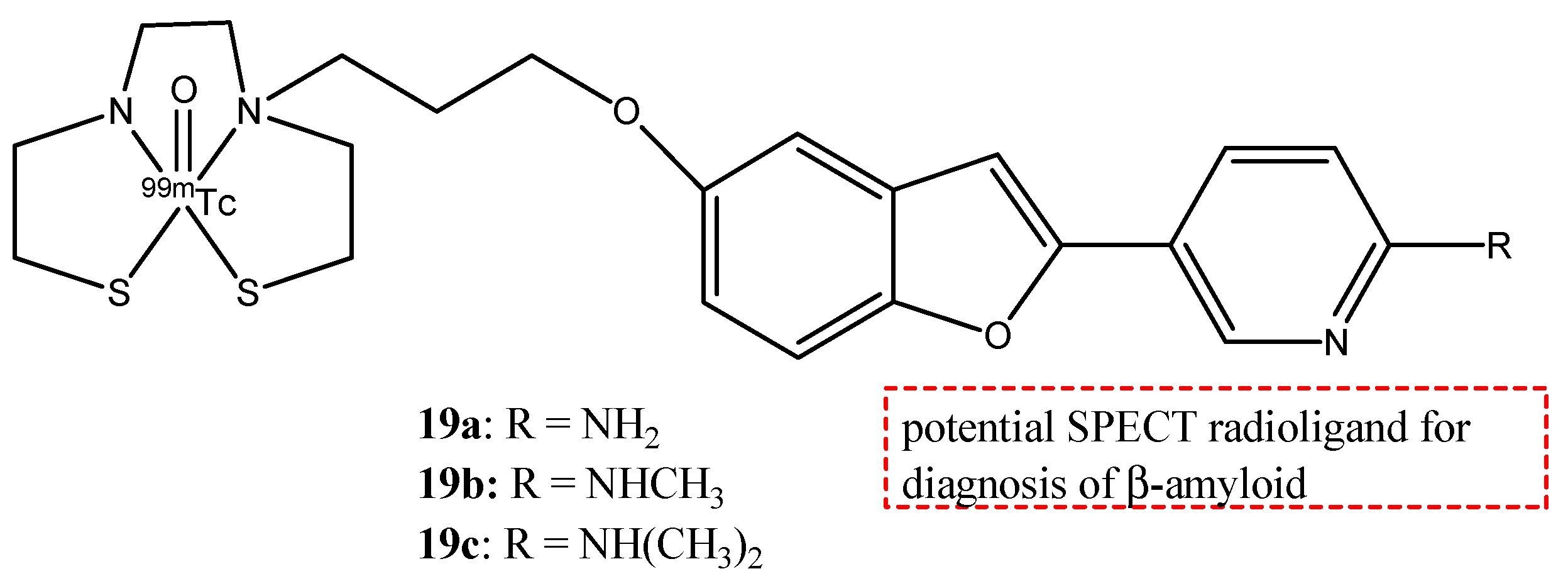
| 19a–c |  | 99mTc | Potential SPECT radioligand for diagnosis of β-amyloid. | [75] |
| 20a–e | 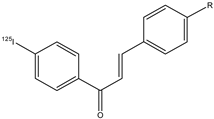 | 125I | Potential β-amyloid plaque imaging agents. | [77] |
| 21 | 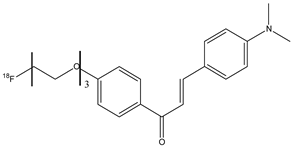 | 18F | Potential β-amyloid plaque imaging agents. | [77] |
| 22a–d | 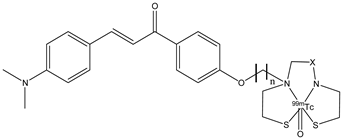 | 99mTc | Potential SPECT radioligand for diagnosis of β-amyloid. | [77] |
| 23a–c | 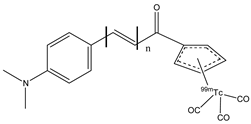 | 99mTc | Potential SPECT radioligand for diagnosis of β-amyloid. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesse, G.; Tolomeo, A.; De Filippis, B.; Giampietro, L. Radiolabeled Probes from Derivatives of Natural Compounds Used in Nuclear Medicine. Molecules 2024, 29, 4260. https://doi.org/10.3390/molecules29174260
Tesse G, Tolomeo A, De Filippis B, Giampietro L. Radiolabeled Probes from Derivatives of Natural Compounds Used in Nuclear Medicine. Molecules. 2024; 29(17):4260. https://doi.org/10.3390/molecules29174260
Chicago/Turabian StyleTesse, Giuseppe, Anna Tolomeo, Barbara De Filippis, and Letizia Giampietro. 2024. "Radiolabeled Probes from Derivatives of Natural Compounds Used in Nuclear Medicine" Molecules 29, no. 17: 4260. https://doi.org/10.3390/molecules29174260








