Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents
Abstract
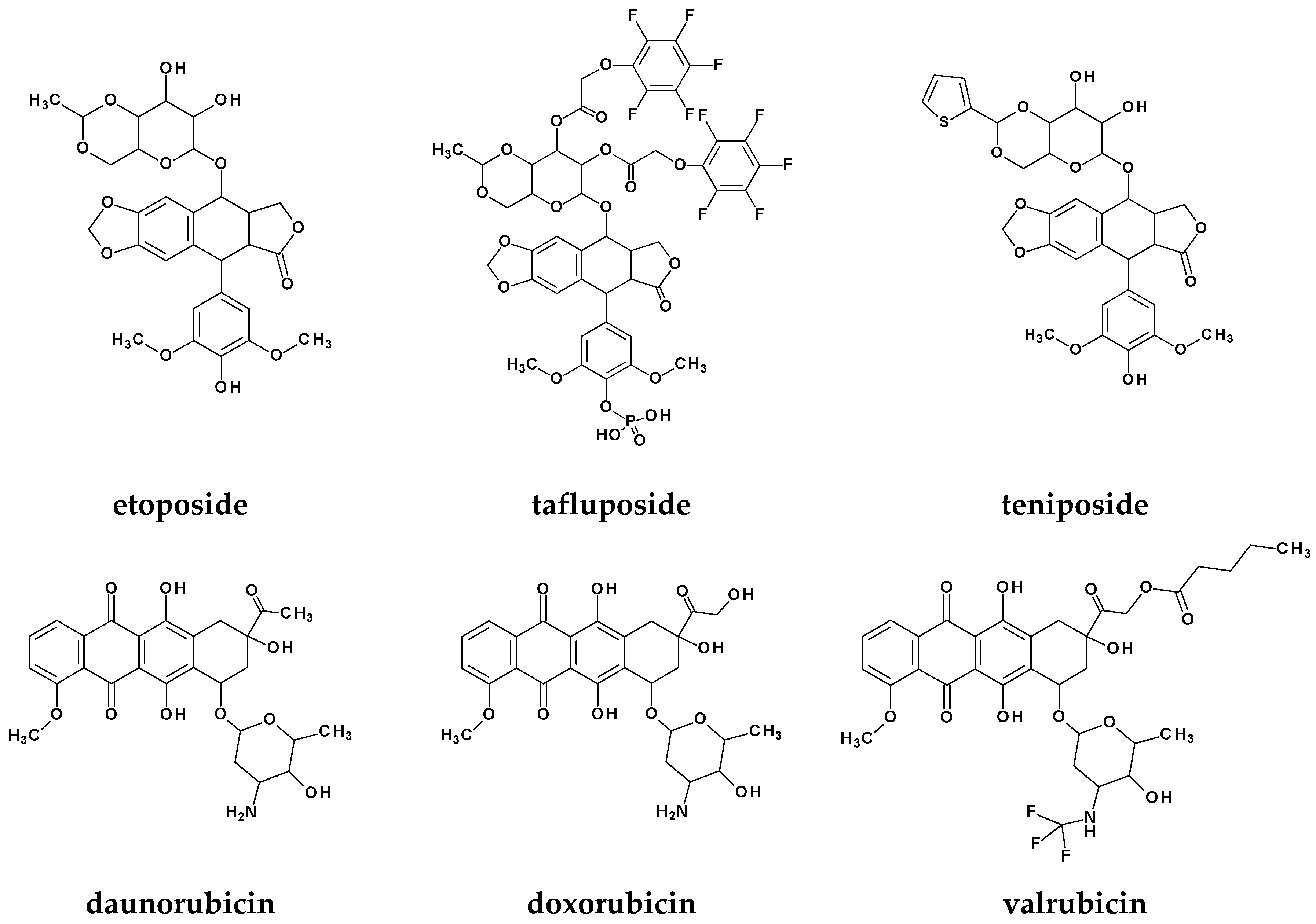
1. Introduction
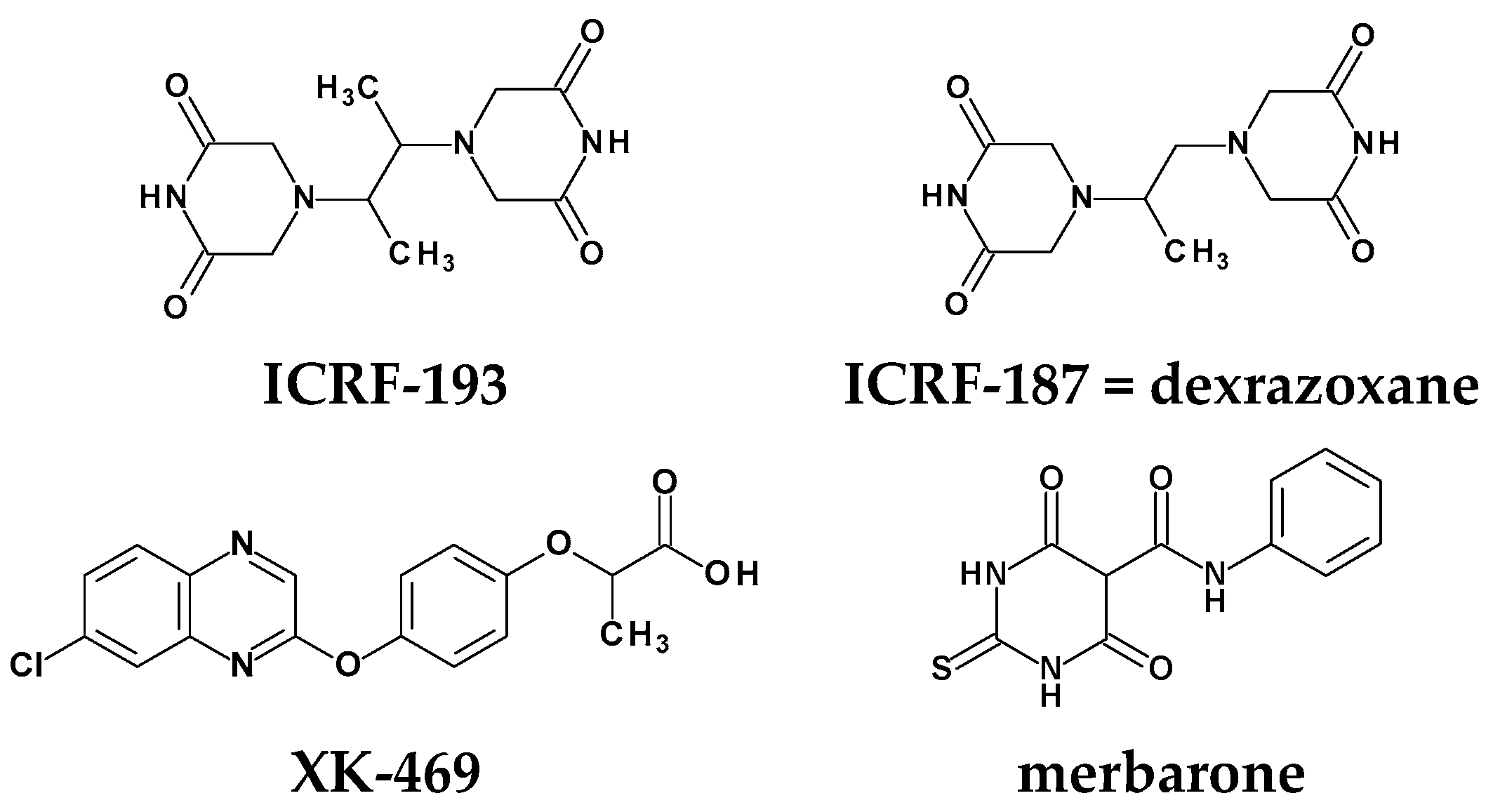
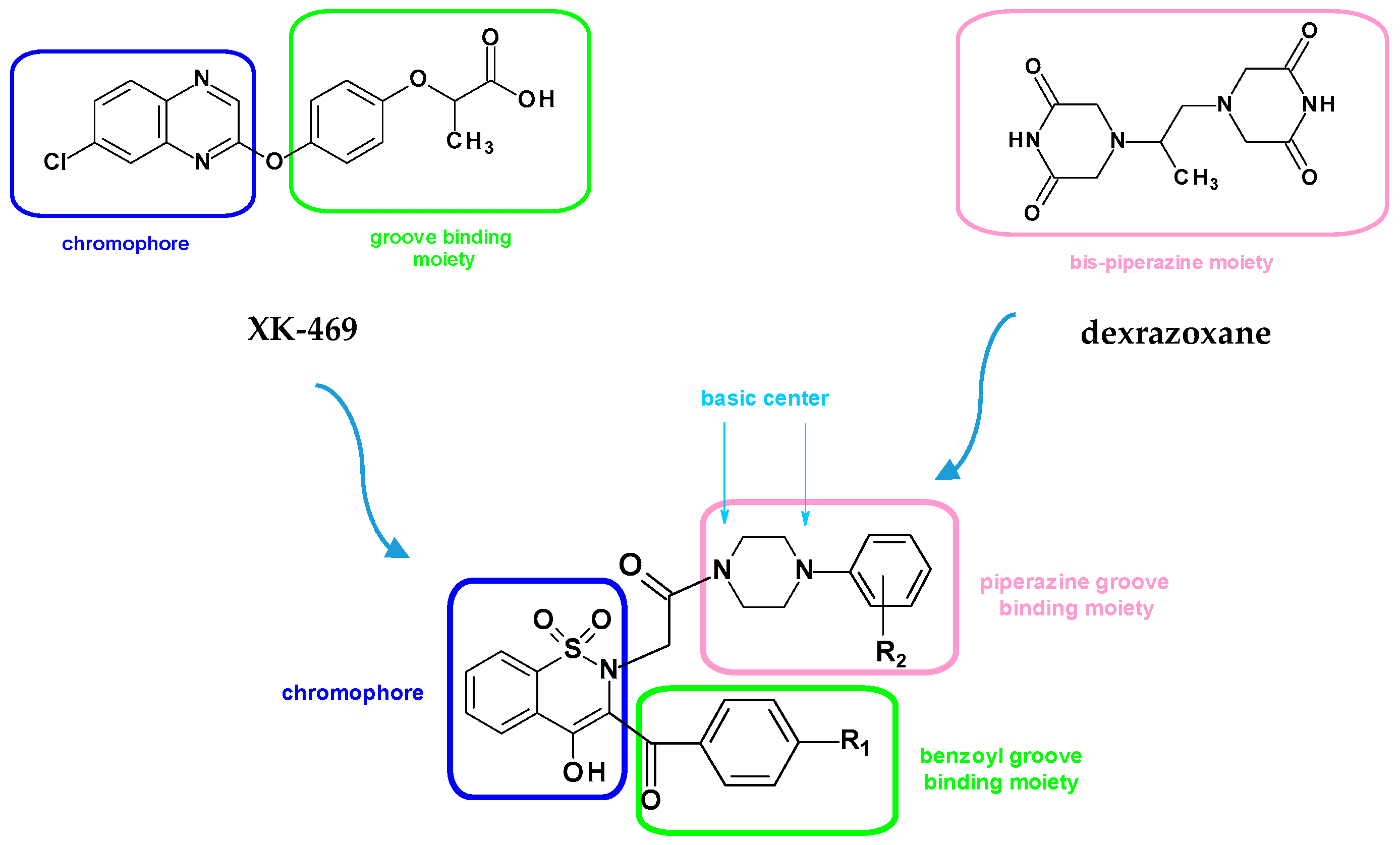
Rational Design of New Compounds
2. Results and Discussion
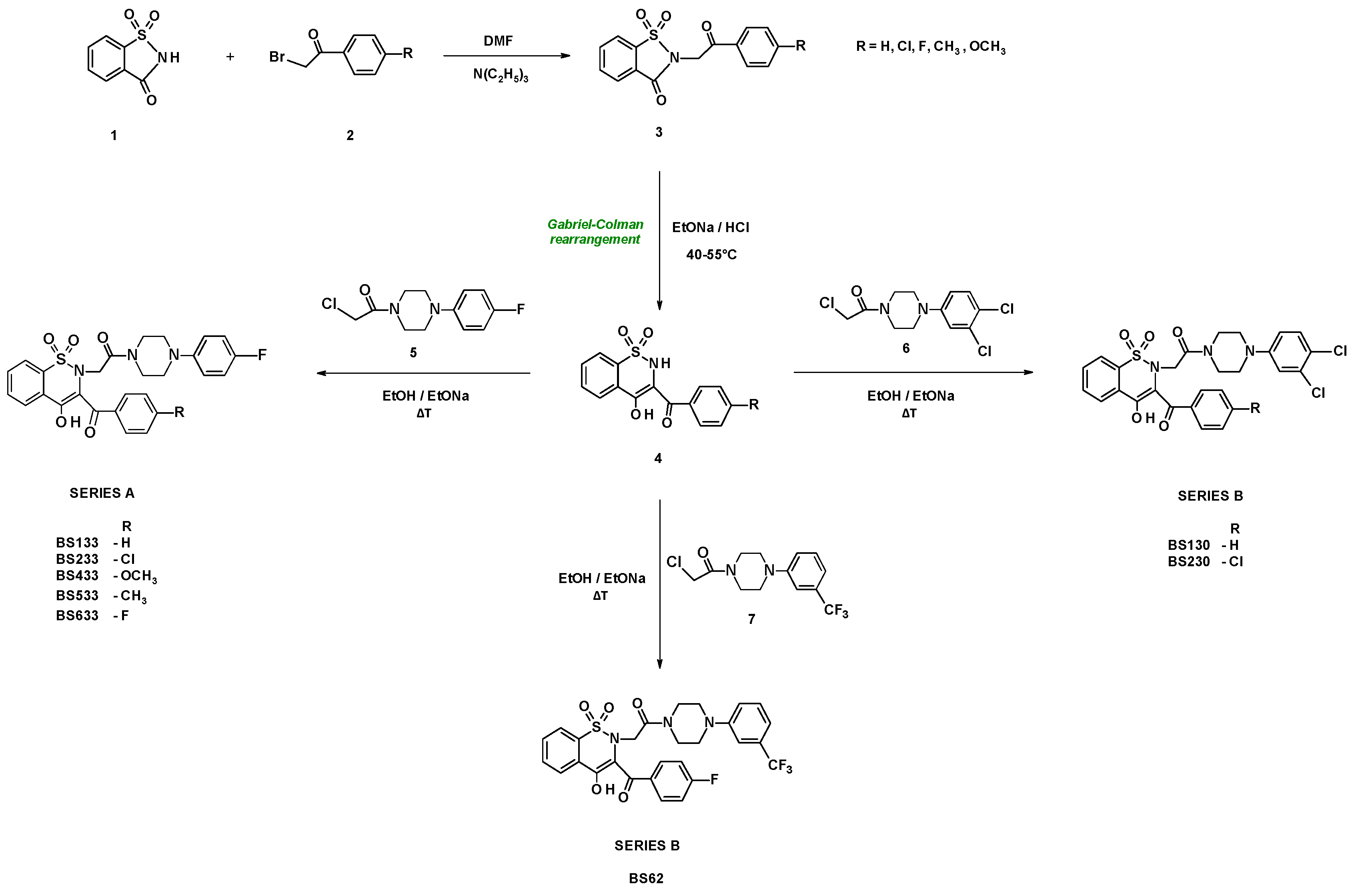
2.1. Chemistry
2.2. In Vitro Antiproliferative Activity
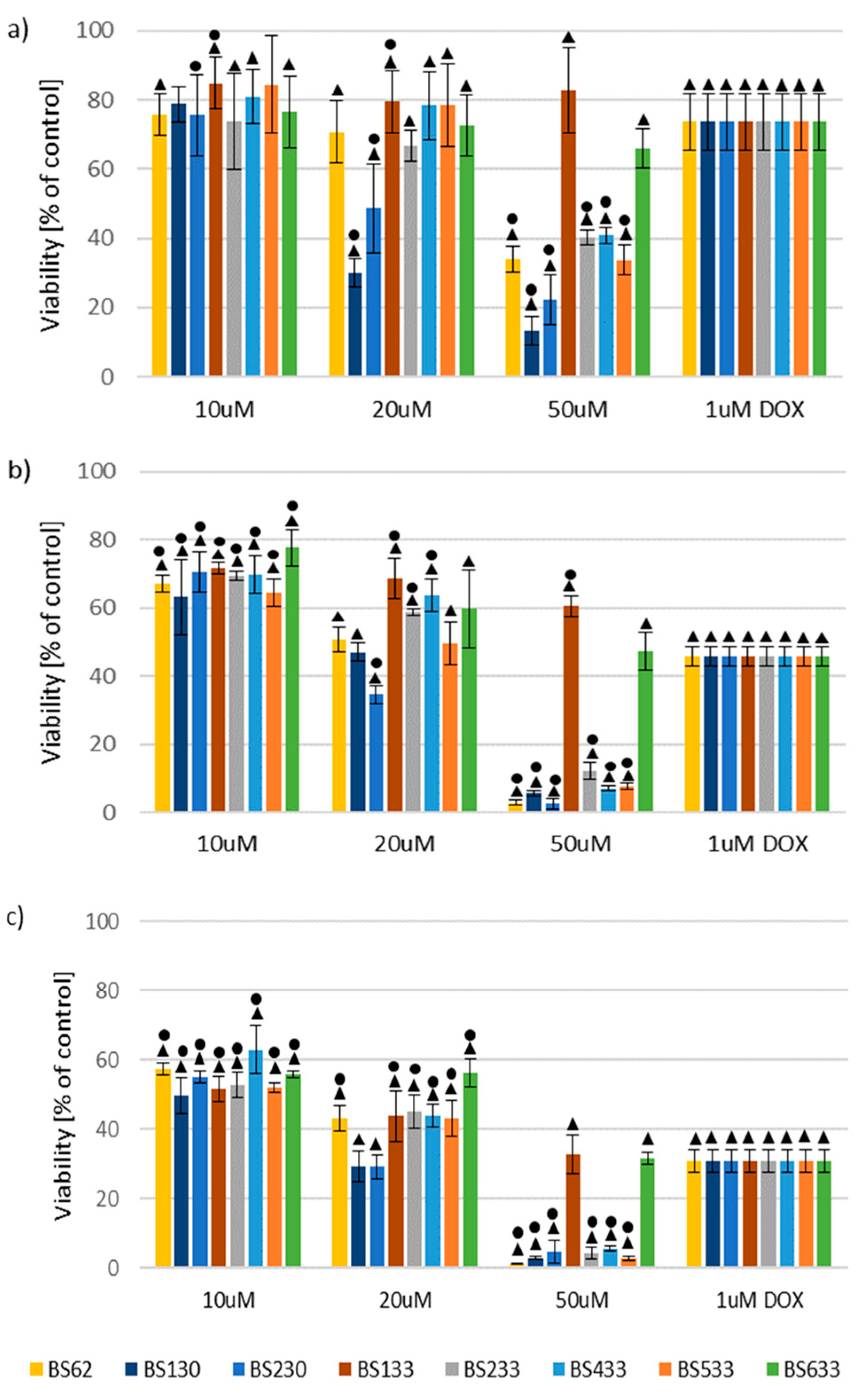
2.2.1. Effect of New Compounds on MCF10A and MCF7 Cell Viability
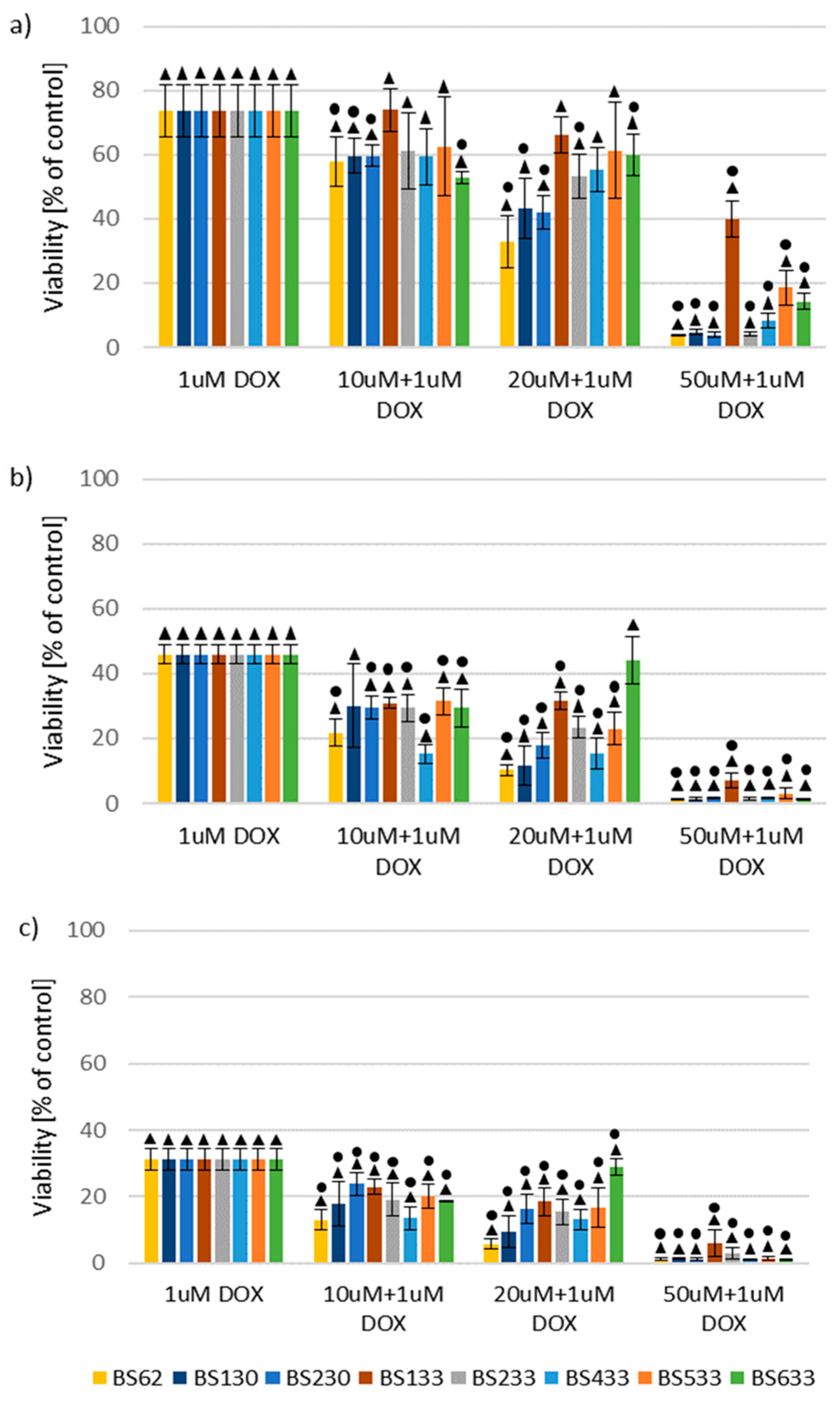
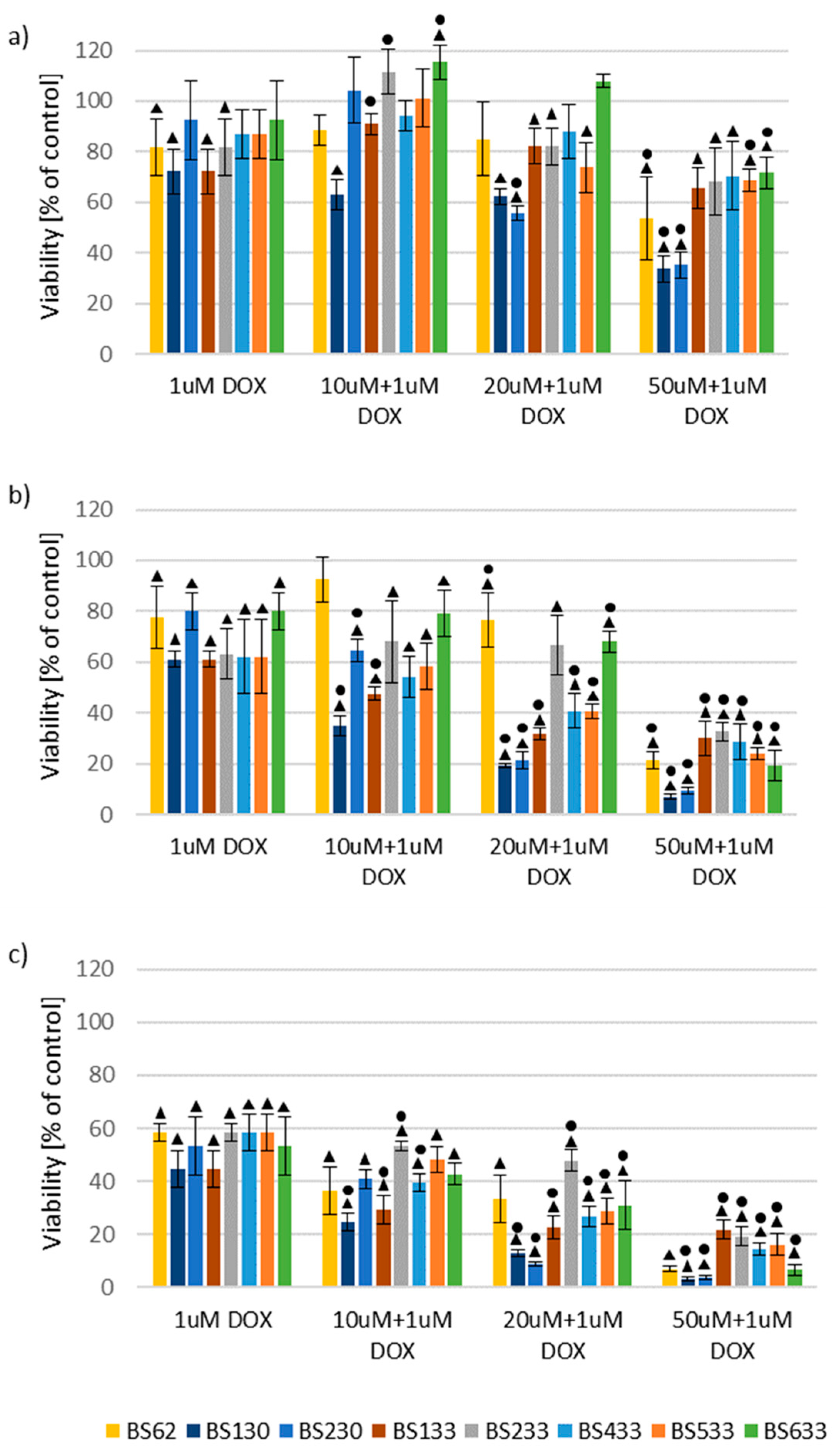
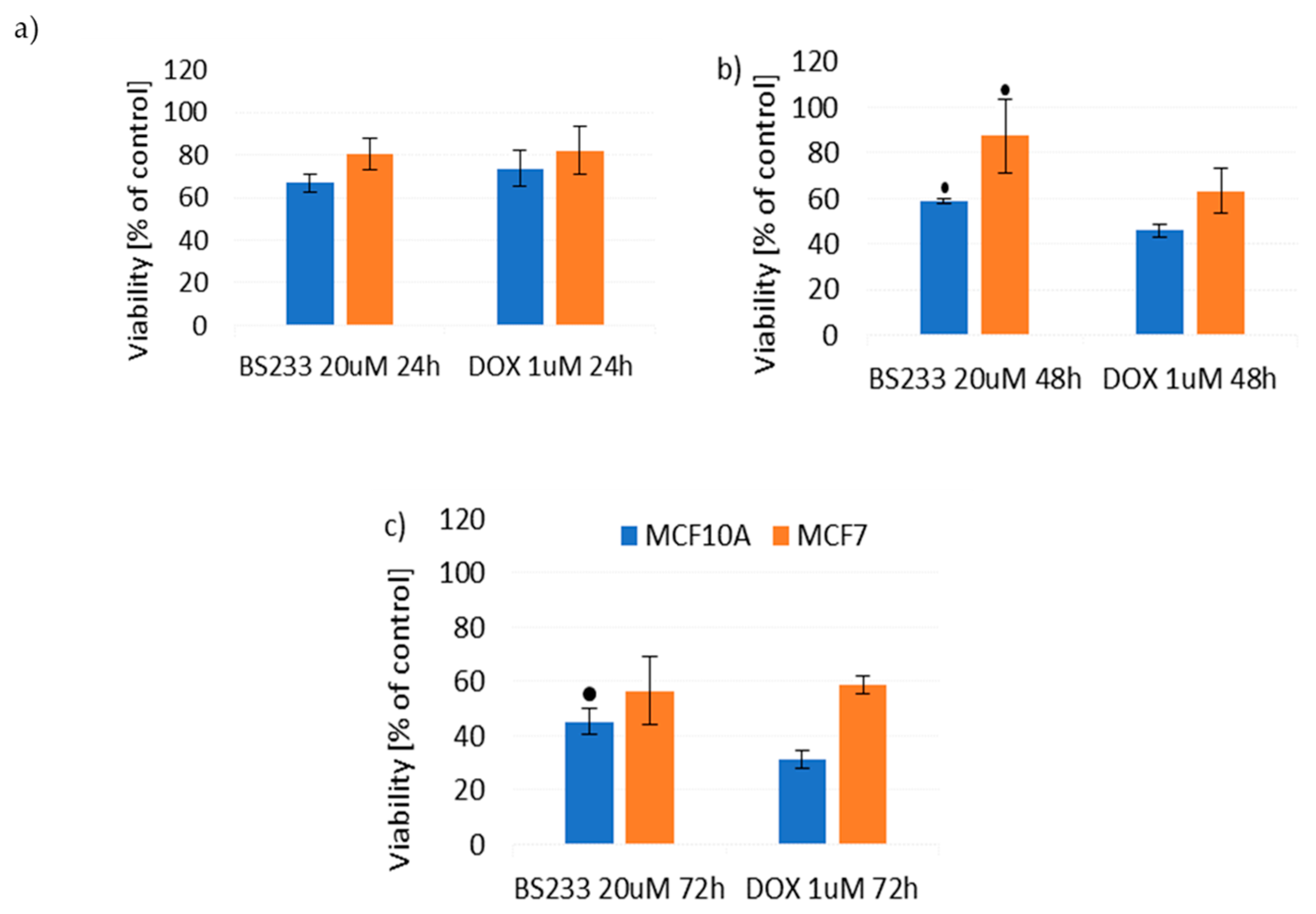
2.2.2. Effect of New Compounds in Combination with Doxorubicin on MCF10A and MCF7 Cell Viability
Effect of New Compounds in Combination with Doxorubicin on MCF10A Cell Viability
Effect of New Compounds in Combination with Doxorubicin on MCF7 Cell Viability
2.2.3. Structure–Activity Relationship (SAR) Analysis
SAR Analysis in the Study of the Effect of New Compounds on MCF10A and MCF7 Cell Viability
SAR Analysis in the Study of the Effect of New Compounds in Combination with Doxorubicin on MCF10A and MCF7 Cells Viability
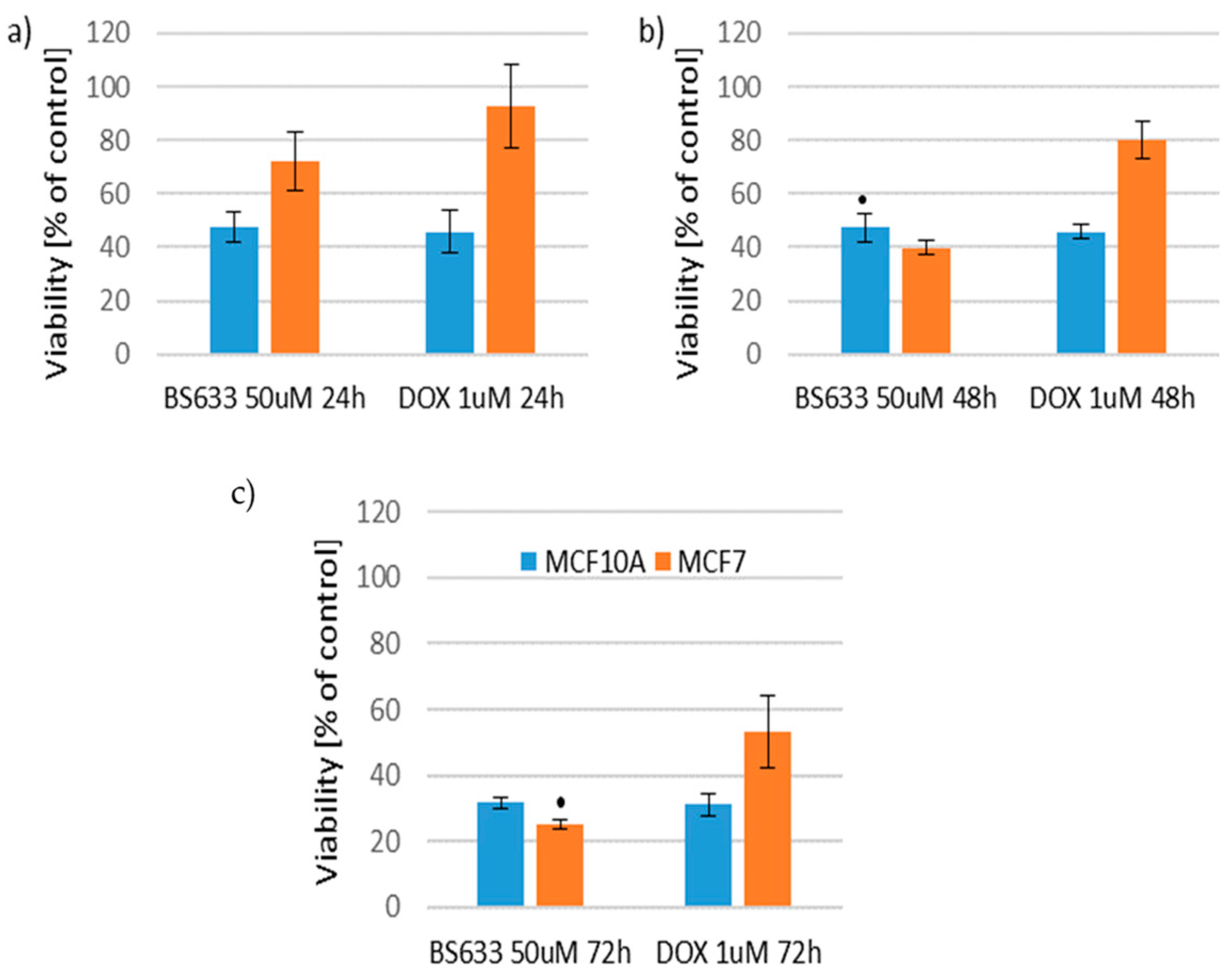
2.2.4. Therapeutic Potential of the Tested Compounds
- Decreased viability of MCF7 cells while maintaining toxicity similar to DOX towards MCF10A cells;
- Decreased toxicity towards MCF10A cells while maintaining toxicity similar to DOX towards MCF7 cells;
- Decreased viability of MCF7 cells while simultaneously reducing toxicity towards MCF10A cells.
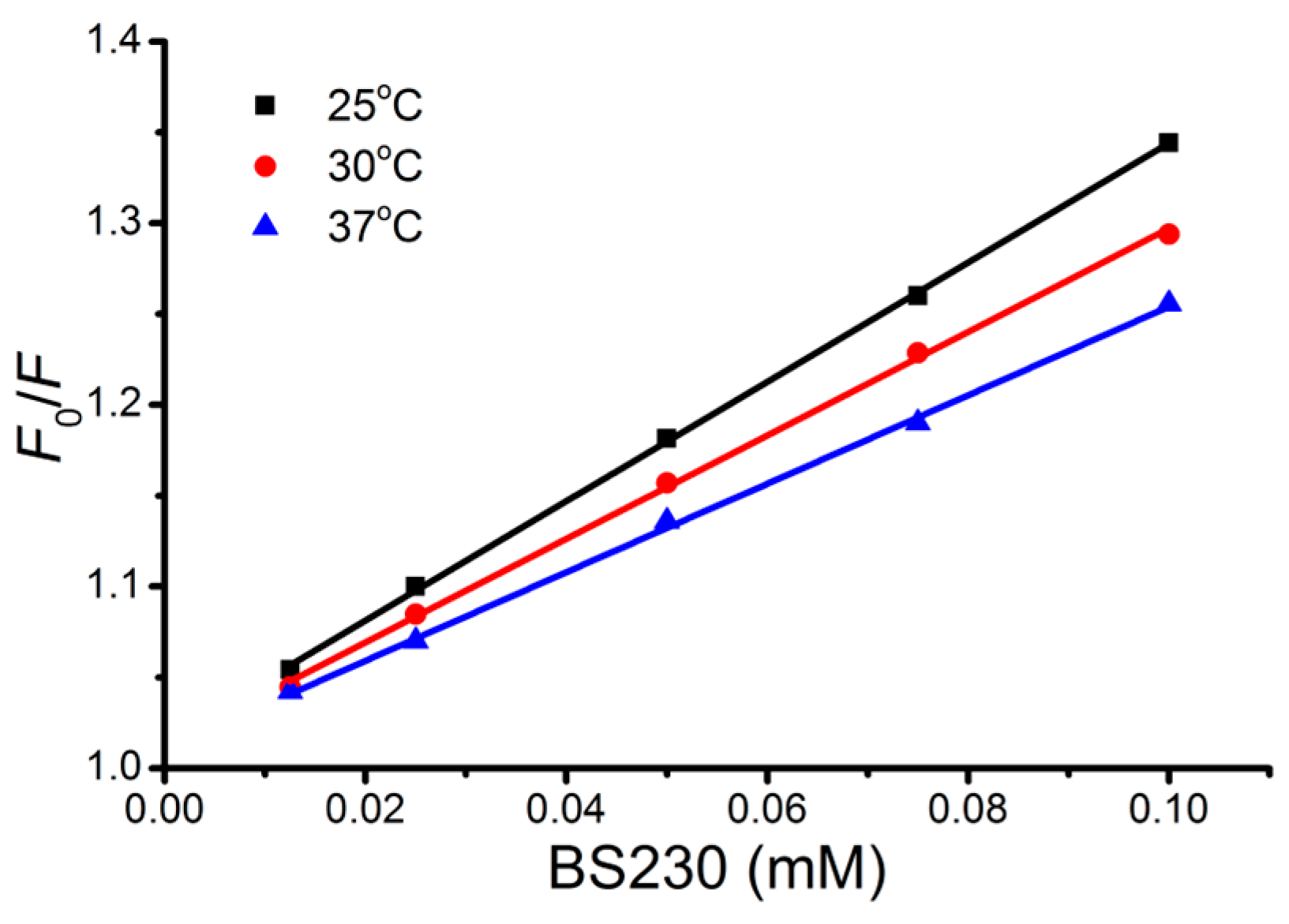
2.3. Fluorescence Spectroscopic Study of Binding to DNA
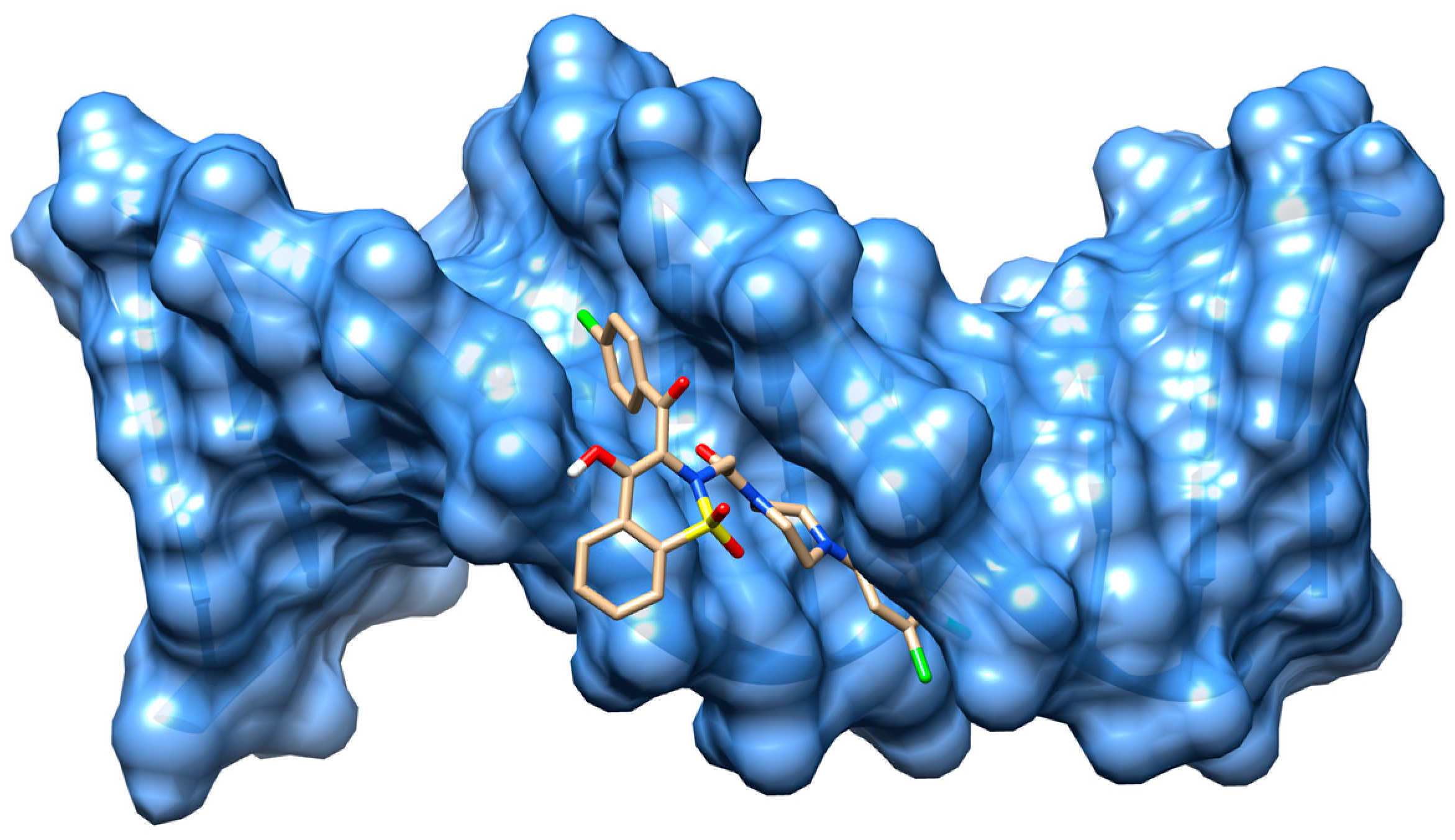
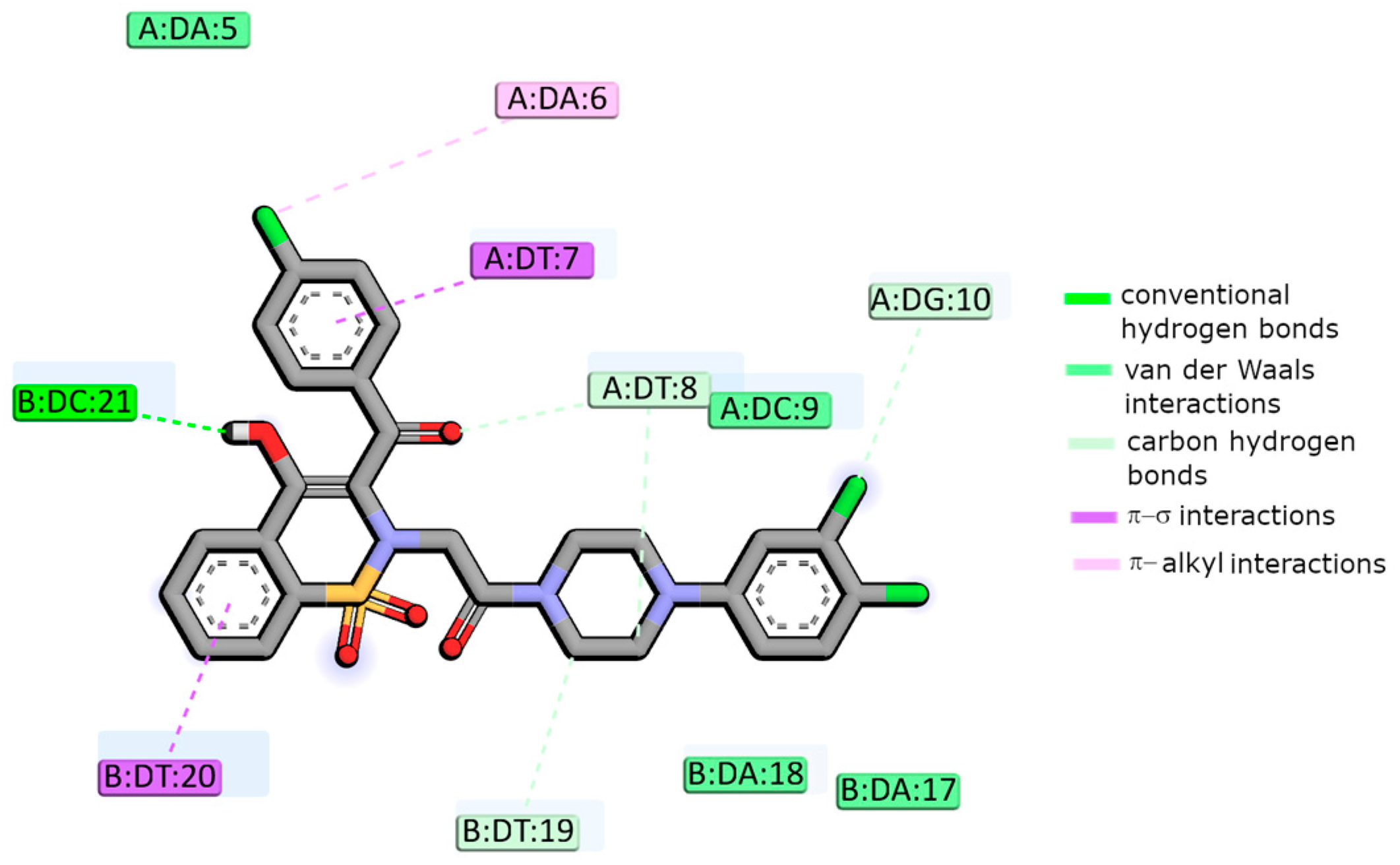
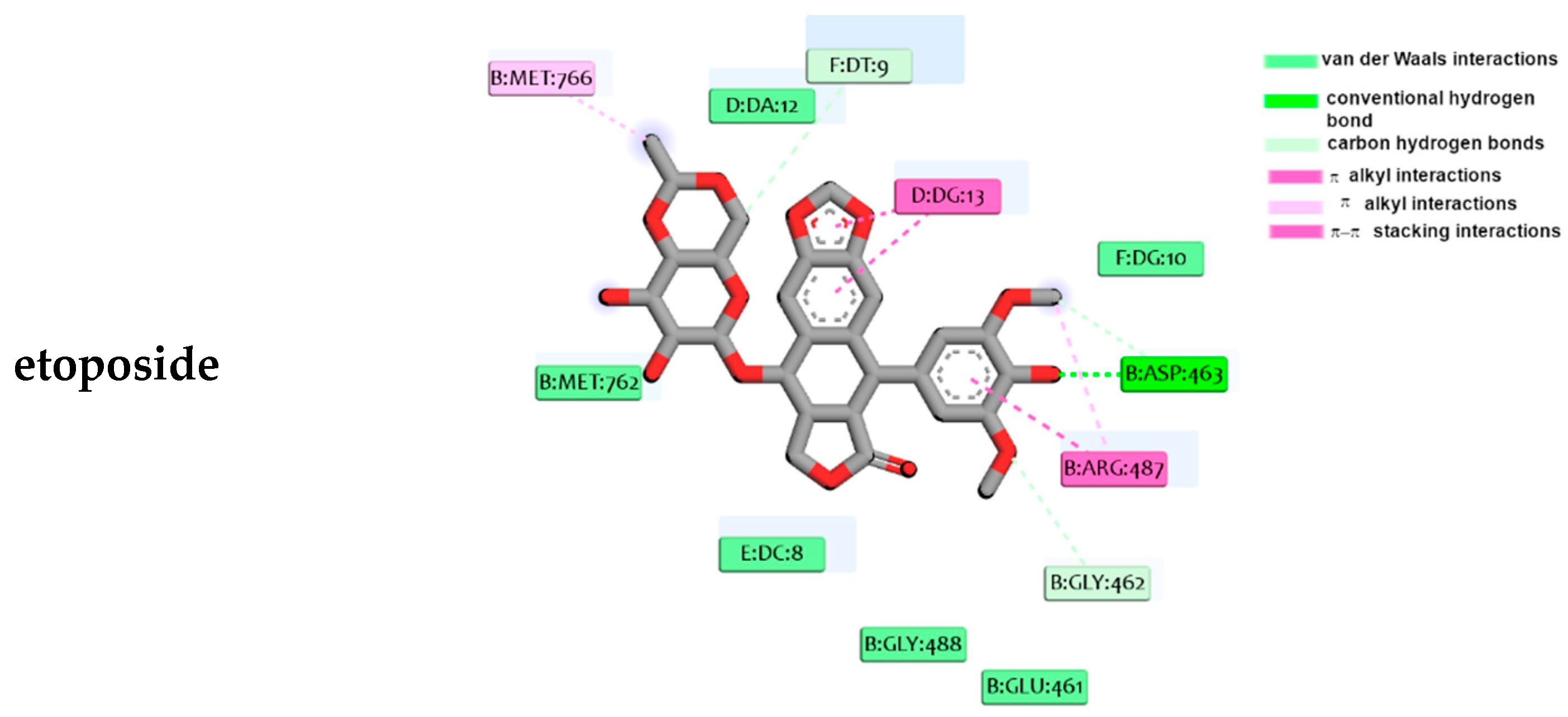
2.4. Molecular Docking Studies
3. Summary
4. Materials and Methods
4.1. Chemistry
- General procedure for the preparation of series A and series B compounds
- Synthesis and properties of series A compounds
- Synthesis and properties of series B compounds
4.2. Biological Experiments
4.2.1. Cell Lines
4.2.2. Cell Culture
4.2.3. Preparation of Compounds for In Vitro Testing
4.2.4. Cell Viability Testing
4.2.5. Statistical Analysis
4.3. Fluorescence Studies
4.4. Molecular Docking Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 8 February 2024).
- Svoboda, M.; Poprach, A.; Dobes, S.; Kiss, I.; Vyzula, R. Cardiac Toxicity of Targeted Therapies Used in the Treatment for Solid Tumours: A Review. Cardiovasc. Toxicol. 2012, 12, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Robles-Flores, M. Fighting Cancer Resistance: An Overview. Methods Mol. Biol. 2021, 2174, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Delgado, J.L.; Hsieh, C.M.; Chan, N.L.; Hiasa, H. Topoisomerases as Anticancer Targets. Biochem. J. 2018, 475, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L.; Soans, E.; Rogojina, A.; Seth, A.; Mishina, M. Topoisomerase Assays. Curr. Protoc. Pharmacol. 2012, 57, 3.3.1–3.3.27. [Google Scholar] [CrossRef] [PubMed]
- You, F.; Gao, C. Topoisomerase Inhibitors and Targeted Delivery in Cancer Therapy. Curr. Top. Med. Chem. 2019, 19, 713–729. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Penumallu, N.R.; Shafi, S.; Kamal, A. Prospects of Topoisomerase Inhibitors as Promising Anti-Cancer Agents. Pharmaceuticals 2023, 16, 1456. [Google Scholar] [CrossRef]
- Hande, K.R. Topoisomerase II Inhibitors. Update Cancer Ther. 2008, 3, 13–26. [Google Scholar] [CrossRef]
- Jensen, L.H.; Nitiss, K.C.; Rose, A.; Dong, J.; Zhou, J.; Hu, T.; Osheroff, N.; Jensen, P.B.; Sehested, M.; Nitiss, J.L. A Novel Mechanism of Cell Killing by Anti-Topoisomerase II Bisdioxopiperazines. J. Biol. Chem. 2000, 275, 2137–2146. [Google Scholar] [CrossRef]
- Montero, V.; Montana, M.; Khoumeri, O.; Correard, F.; Estève, M.A.; Vanelle, P. Synthesis, In Vitro Antiproliferative Activity, and In Silico Evaluation of Novel Oxiranyl-Quinoxaline Derivatives. Pharmaceuticals 2022, 15, 781. [Google Scholar] [CrossRef]
- Alousi, A.M.; Boinpally, R.; Wiegand, R.; Parchment, R.; Gadgeel, S.; Heilbrun, L.K.; Wozniak, A.J.; DeLuca, P.; LoRusso, P.M. A Phase 1 Trial of XK469: Toxicity Profile of a Selective Topoisomerase IIbeta Inhibitor. Investig. New Drugs 2007, 25, 147–154. [Google Scholar] [CrossRef]
- El-Adl, K.; El-Helby, A.G.A.; Sakr, H.; Elwan, A. Design, Synthesis, Molecular Docking and Anti-Proliferative Evaluations of [1,2,4]Triazolo[4,3-a]Quinoxaline Derivatives as DNA Intercalators and Topoisomerase II Inhibitors. Bioorg. Chem. 2020, 105, 104399. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.E.; Velea, L.M. Intercalative Binding of Small Molecules to Nucleic Acids. Curr. Org. Chem. 2005, 4, 915–929. [Google Scholar] [CrossRef]
- Avendaño, C.; Menéndez, J.C. Medicinal Chemistry of Anticancer Drugs, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–740. [Google Scholar] [CrossRef]
- Aparicio Gallego, G.; Díaz Prado, S.; Jiménez Fonseca, P.; García Campelo, R.; Cassinello Espinosa, J.; Antón Aparicio, L.M. Cyclooxygenase-2 (COX-2): A Molecular Target in Prostate Cancer. Clin. Transl. Oncol. 2007, 9, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Szczȩśniak-Sięga, B.; Gȩbczak, K.; Gȩbarowski, T.; Maniewska, J. Synthesis, COX-1/2 Inhibition and Antioxidant Activities of New Oxicam Analogues Designed as Potential Chemopreventive Agents. Acta Biochim. Pol. 2018, 65, 199–207. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Wesołowska, O.; Szczęśniak-Siega, B.; Puła, B.; Dziegiel, P.; Maniewska, J.; Malinka, W.; Palko-Łabuz, A.; Michalak, K. Effect of New Oxicam Derivatives on Efflux Pumps Overexpressed in Resistant a Human Colorectal Adenocarcinoma Cell Line. Anticancer Res. 2015, 35, 2835–2840. [Google Scholar] [PubMed]
- Środa-Pomianek, K.; Michalak, K.; Palko-Łabuz, A.; Uryga, A.; Szczęśniak-Sięga, B.; Wesołowska, O. Simvastatin Strongly Augments Proapoptotic, Anti-Inflammatory and Cytotoxic Activity of Oxicam Derivatives in Doxorubicin-Resistant Colon Cancer Cells. Anticancer Res. 2019, 39, 727–734. [Google Scholar] [CrossRef]
- Shan, Y.Y.; Zhang, C.M.; Tang, L.Q.; Liu, Z.P.; Bearss, N.R.; Sarver, J.G.; Luniwal, A.; Erhardt, P.W. Syntheses of 2,3-Diarylated 2H-Benzo[e][1,2]Thiazine 1,1-Dioxides and Their 3,4-Dihydro Derivatives, and Assessment of Their Inhibitory Activity against MCF-7 Breast Cancer Cells. Med. Chem. 2011, 7, 561–571. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Szczęśniak-Sięga, B.; Szczuka, I.; Fortuna, P.; Zawadzki, M.; Kubiak, A.; Mierzchała-Pasierb, M.; Fleszar, M.G.; Lewandowski, Ł.; Serek, P.; et al. L-Arginine/Nitric Oxide Pathway Is Altered in Colorectal Cancer and Can Be Modulated by Novel Derivatives from Oxicam Class of Non-Steroidal Anti-Inflammatory Drugs. Cancers 2020, 12, 2594. [Google Scholar] [CrossRef]
- Szczuka, I.; Wierzbicki, J.; Serek, P.; Szczęśniak-Sięga, B.M.; Krzystek-Korpacka, M. Heat Shock Proteins Hspa1 and Hsp90aa1 Are Upregulated in Colorectal Polyps and Can Be Targeted in Cancer Cells by Anti-Inflammatory Oxicams with Arylpiperazine Pharmacophore and Benzoyl Moiety Substitutions at Thiazine Ring. Biomolecules 2021, 11, 1588. [Google Scholar] [CrossRef]
- Lewandowska, P.; Szczuka, I.; Bednarz-Misa, I.; Szczęśniak-Sięga, B.M.; Krzystek-Korpacka, M. Analogues against Macrophage-Associated Chemokines in Colorectal Cancer. Molecules 2021, 26, 7375. [Google Scholar] [CrossRef]
- Krzyzak, E.; Szczȩśniak-Siȩga, B.; Malinka, W. Synthesis and Thermal Behaviour of New 1,2-benzothiazine Long-Chain Aryl-Piperazine Derivatives. J. Therm. Anal. Calorim. 2014, 115, 793–802. [Google Scholar] [CrossRef][Green Version]
- Ling, X.; Zhong, W.; Huang, Q.; Ni, K. Spectroscopic Studies on the Interaction of Pazufloxacin with Calf Thymus DNA. J. Photochem. Photobiol. B Biol. 2008, 93, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Tripathi, P.; Parashar, P.; Maurya, V.; Malik, Z.; Singh, R.; Yadav, P.; Tandon, V. Biological Evaluation of Novel 1H-Benzo[d]imidazole Derivatives as Potential Anticancer Agents Targeting Human Topoisomerase I. ACS Omega 2022, 7, 2861–2880. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.L.; Thatikonda, S.; Sigalapalli, D.K.; Sagar, A.; Kiranmai, G.; Kalle, A.M.; Alvala, M.; Godugu, C.; Nagesh, N.; Babu, B.N. Design and synthesis of β-carboline linked aryl sulfonyl piperazine derivatives: DNA topoisomerase II inhibition with DNA binding and apoptosis inducing ability. Bioorg. Chem. 2020, 101, 103983. [Google Scholar] [CrossRef]
- Yoshimura, A.; Koski, S.R.; Fuchs, J.; Saito, A.; Nemykin, V.N.; Zhdankin, V.Z. Saccharin-Based μ-Oxo Imidoiodane: A Readily Available and Highly Reactive Reagent for Electrophilic Amination. Chem.-A Eur. J. 2015, 21, 5328–5331. [Google Scholar] [CrossRef]
- Szczeęśniak-Sięga, B.; Maniewska, J.; Poła, A.; Środa-Pomianek, K.; Malinka, W.; Michalak, K. Synthesis of New Piroxicam Derivatives and Their Influence on Lipid Bilayers. Acta Pol. Pharm.—Drug Res. 2014, 71, 1045–1050. [Google Scholar] [PubMed]
- Muszalska, I.; Ciemniejewski, M.P.; Lesniewska, M.A.; Szkatuła, D.; Malinka, W. Forced Degradation and Photodegradation Studies of Pyrrolo[3,4-c]Pyridine-1,3-Dione Derivatives as Analgesic Active Compounds Using HPLC, UV and IR Spectrometry, and HPLC/MS Methods. J. AOAC Int. 2015, 98, 1248–1259. [Google Scholar] [CrossRef]
- Colabufo, N.A.; Berardi, F.; Perrone, R.; Rapposelli, S.; Digiacomo, M.; Vanni, M.; Balsamo, A. 2-[(3-Methoxyphenylethyl)Phenoxy]-Based ABCB1 Inhibitors: Effect of Different Basic Side-Chains on Their Biological Properties. J. Med. Chem. 2008, 51, 7602–7613. [Google Scholar] [CrossRef]
- Janek, T.; Czyżnikowska, Ż.; Łukaszewicz, M.; Gałęzowska, J. The Effect of Pseudomonas Fluorescens Biosurfactant Pseudofactin II on the Conformational Changes of Bovine Serum Albumin: Pharmaceutical and Biomedical Applications. J. Mol. Liq. 2019, 288, 111001. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–954. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01, GaussView 5.0; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chen, S.F.; Wu, C.C.; Liao, Y.W.; Lin, T.S.; Liu, K.T.; Chen, Y.S.; Li, T.K.; Chien, T.C.; Chan, N.L. Producing Irreversible Topoisomerase II-Mediated DNA Breaks by Site-Specific Pt(II)-Methionine Coordination Chemistry. Nucleic Acids Res. 2017, 45, 10861–10871. [Google Scholar] [CrossRef] [PubMed]
- Mallena, S.; Lee, M.P.H.; Bailly, C.; Neidle, S.; Kumar, A.; Boykin, D.W.; Wilson, W.D. Thiophene-Based Diamidine Forms a “Super” AT Binding Minor Groove Agent. Am. Chem. Soc. 2004, 126, 13659–13669. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A Web Server for Automatic Structure Matching and RMSD Calculations among Identical and Similar Compounds in Protein-Ligand Docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef] [PubMed]
- Tylińska, B.; Wiatrak, B.; Czyżnikowska, Ż.; Cieśla-Niechwiadowicz, A.; Gębarowska, E.; Janicka-Kłos, A. Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study. Int. J. Mol. Sci. 2021, 22, 3825. [Google Scholar] [CrossRef]
- Chen, D.; Menche, G.; Power, T.D.; Sower, L.; Peterson, J.W.; Schein, C.H. Accounting for Ligand-Bound Metal Ions in Docking Small Molecules on Adenylyl Cyclase Toxins. Proteins 2007, 67, 593–605. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio. Discovery Studio Visualizer; BIOVIA Discovery Studio: San Diego, CA, USA, 2019. [Google Scholar]







| Compound | Ksv (1 × 103) (M−1) | kq (1 × 1011) (M−1 s−1) | K (1 × 103) (M−1) | n | R2 |
|---|---|---|---|---|---|
| BS62 | 2.16 | 2.16 | 2.34 | 0.92 | 0.995 |
| BS130 | 3.32 | 3.32 | 3.21 | 0.99 | 0.997 |
| BS133 | 1.35 | 1.35 | 0.61 | 1.02 | 0.994 |
| BS230 | 2.41 | 2.41 | 2.86 | 1.01 | 0.996 |
| BS233 | 1.98 | 1.98 | 2.13 | 0.97 | 0.997 |
| BS433 | 3.23 | 3.23 | 2.64 | 0.99 | 0.995 |
| BS533 | 3.06 | 3.06 | 3.96 | 0.99 | 0.995 |
| BS633 | 3.41 | 3.41 | 3.12 | 1.01 | 0.998 |
| Compound | ∆Gbinding to DNA [kJ/mol] | ∆Gbinding to Topo IIα [kJ/mol] |
|---|---|---|
| BS62 | −61.1 | −63.2 |
| BS130 | −61.3 | −63.2 |
| BS133 | −57.1 | −63.1 |
| BS230 | −63.2 | −66.6 |
| BS233 | −60.2 | −64.2 |
| BS433 | −62.2 | −65.6 |
| BS533 | −58.7 | −64.1 |
| BS633 | −58.4 | −63.0 |
| etoposide | −48.3 | −44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczęśniak-Sięga, B.M.; Zaręba, N.; Czyżnikowska, Ż.; Janek, T.; Kepinska, M. Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents. Molecules 2024, 29, 4282. https://doi.org/10.3390/molecules29184282
Szczęśniak-Sięga BM, Zaręba N, Czyżnikowska Ż, Janek T, Kepinska M. Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents. Molecules. 2024; 29(18):4282. https://doi.org/10.3390/molecules29184282
Chicago/Turabian StyleSzczęśniak-Sięga, Berenika M., Natalia Zaręba, Żaneta Czyżnikowska, Tomasz Janek, and Marta Kepinska. 2024. "Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents" Molecules 29, no. 18: 4282. https://doi.org/10.3390/molecules29184282
APA StyleSzczęśniak-Sięga, B. M., Zaręba, N., Czyżnikowska, Ż., Janek, T., & Kepinska, M. (2024). Rational Design, Synthesis, Molecular Docking, and Biological Evaluations of New Phenylpiperazine Derivatives of 1,2-Benzothiazine as Potential Anticancer Agents. Molecules, 29(18), 4282. https://doi.org/10.3390/molecules29184282










