Recent Advances on Two-Dimensional Nanomaterials Supported Single-Atom for Hydrogen Evolution Electrocatalysts
Abstract
1. Introduction
2. Synthetic Approaches and Characterization Methods
2.1. Synthetic Approaches of 2D Single-Atom Supports
2.1.1. “Top-Down” Method
2.1.2. “Bottom Up” Approach
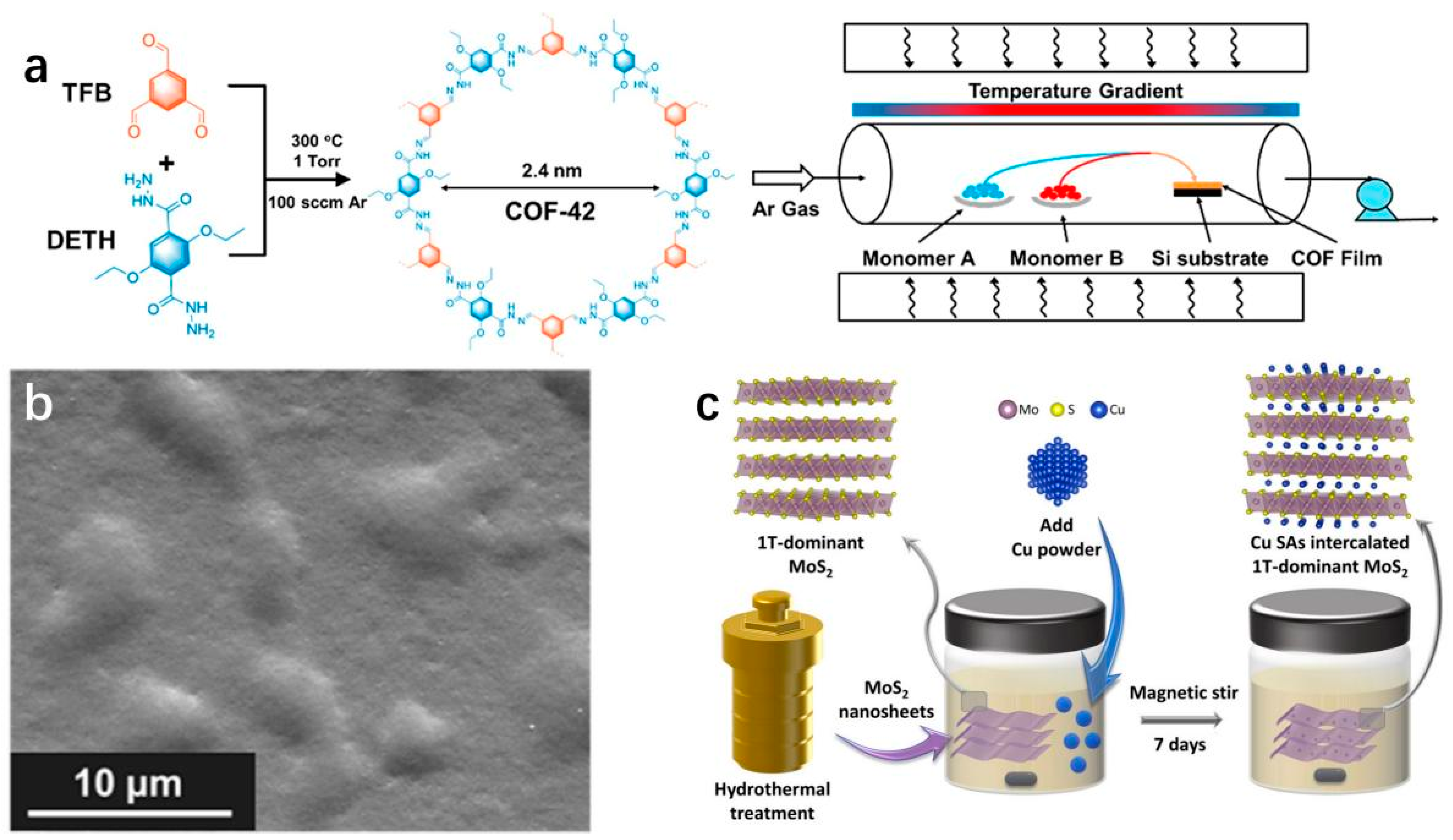
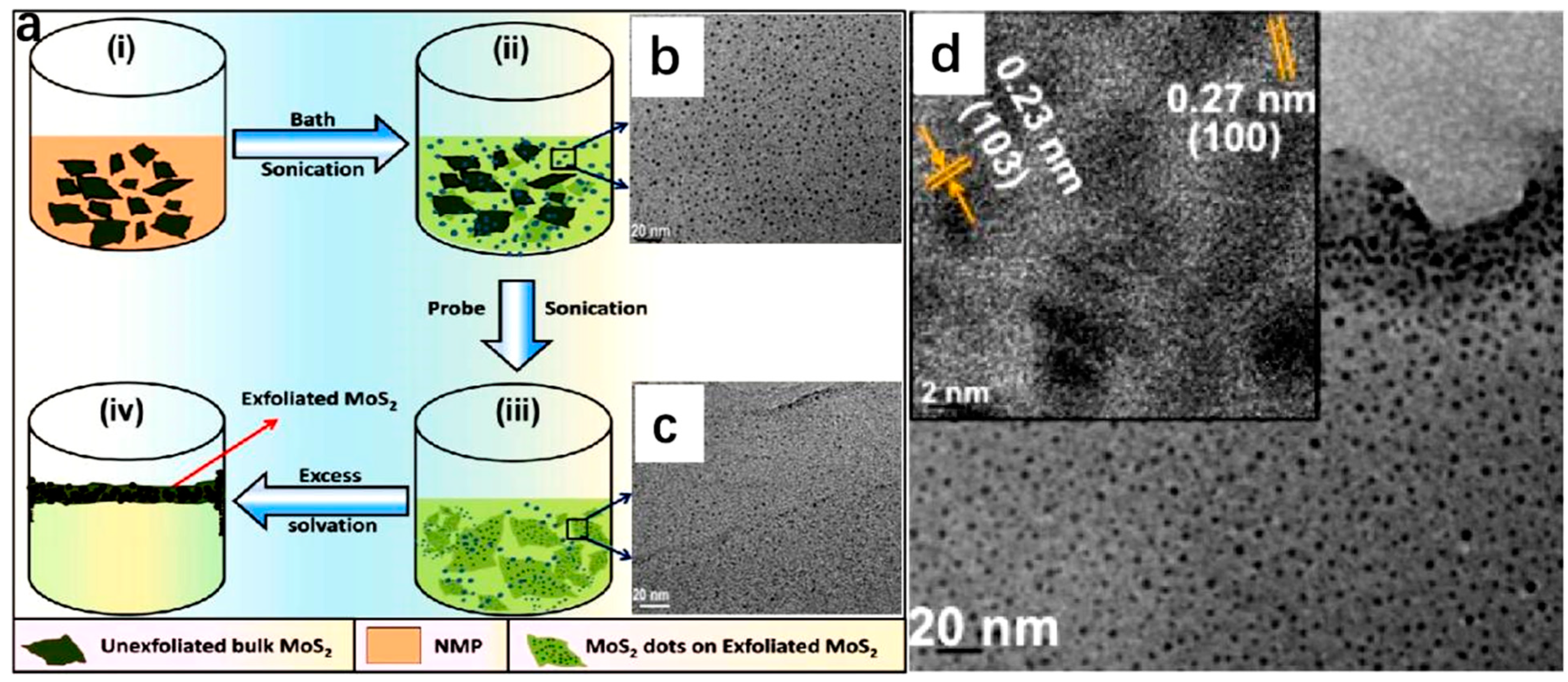
2.2. Synthetic Strategies of 2D-TMD-Supported SACs
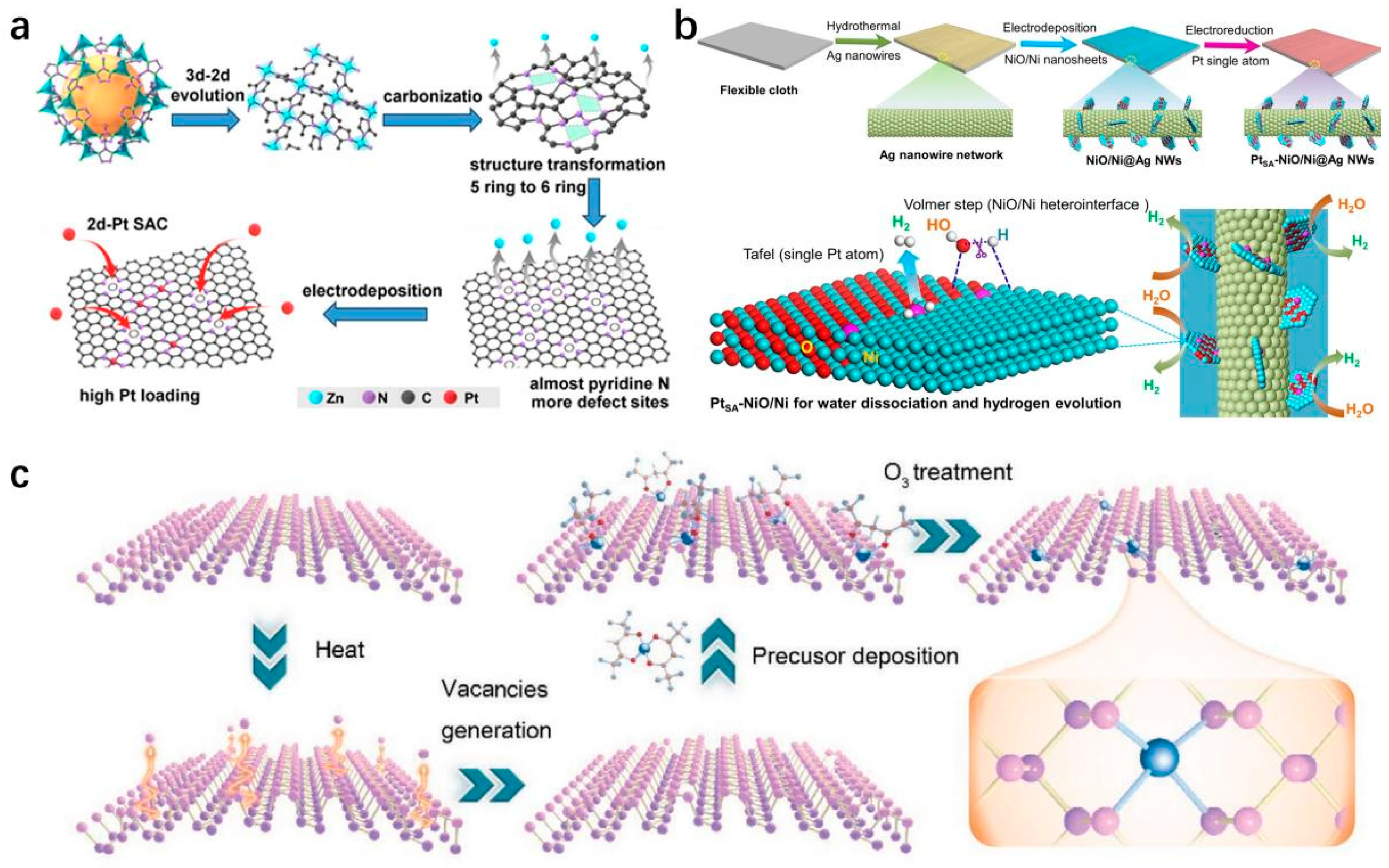
2.3. Characterization Methods
2.3.1. Imaging Characterization Techniques
2.3.2. Spectroscopic Characterization Techniques
2.3.3. Density Functional Theory (DFT)
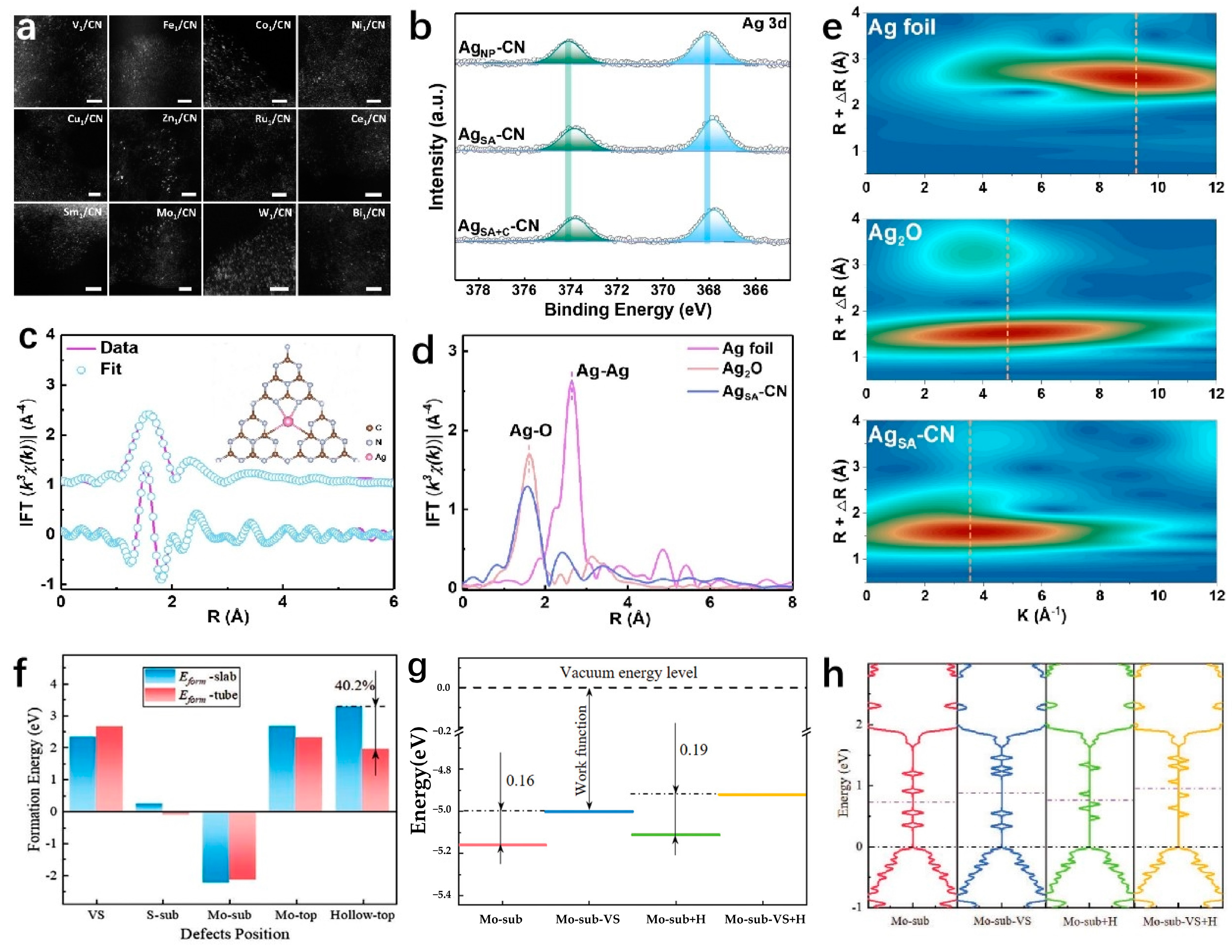
3. Different Types of Two-Dimensional Supported Single-Atoms
3.1. Two-Dimensional Carbon-Based Supports
3.2. Two-Dimensional Layered Double Hydroxides (LDHs) Supports
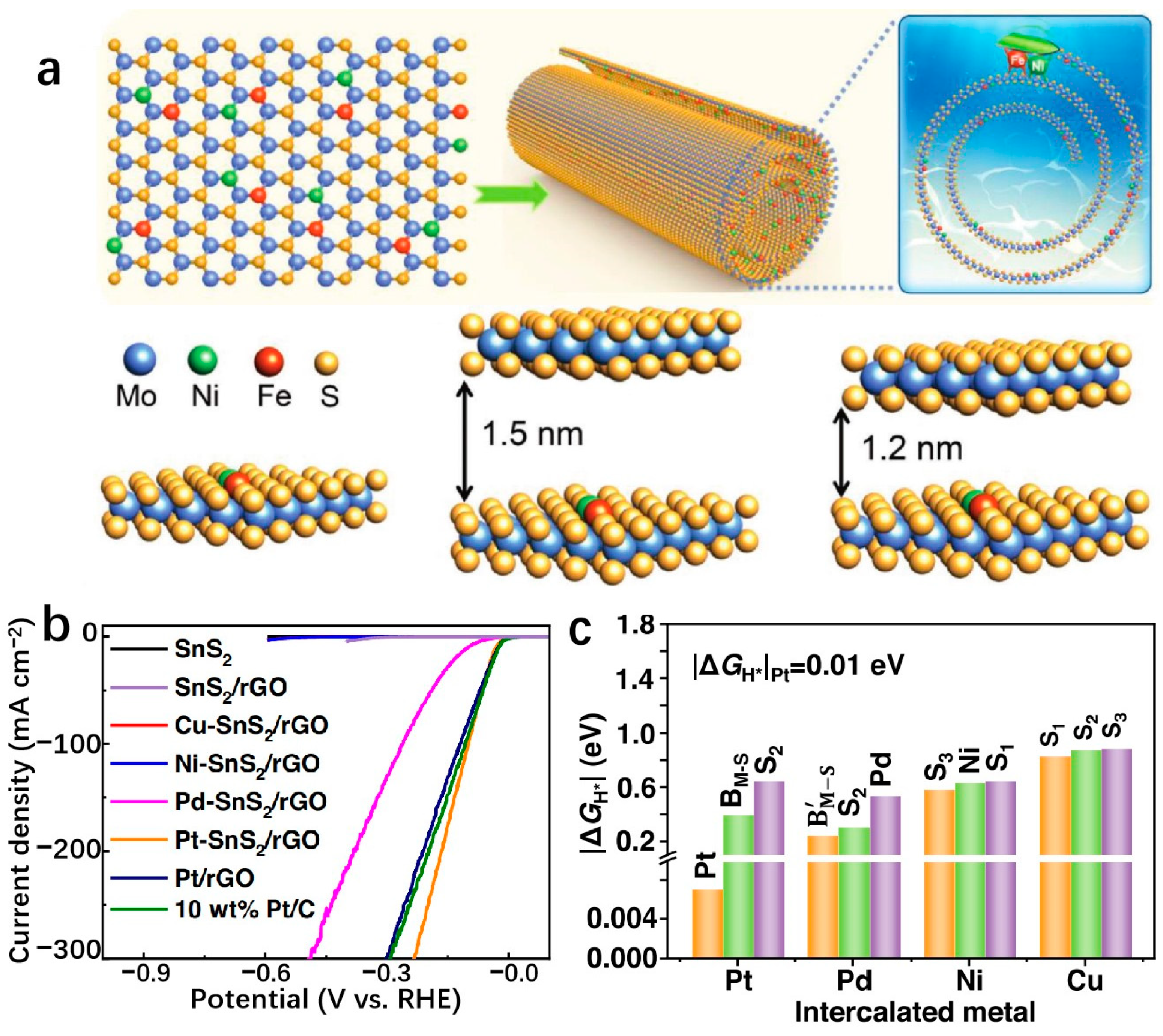
3.3. Two-Dimensional Transition-Metal Compounds Supports
3.4. Two-Dimensional MXenes and MBenes Supports
3.5. Other 2D Materials Single-Atom Supports
4. Manipulating the Microenvironment of SAs on Metal–2D Nanomaterials
4.1. Dopant Coordination Effect

4.2. Coordination Structure Effect
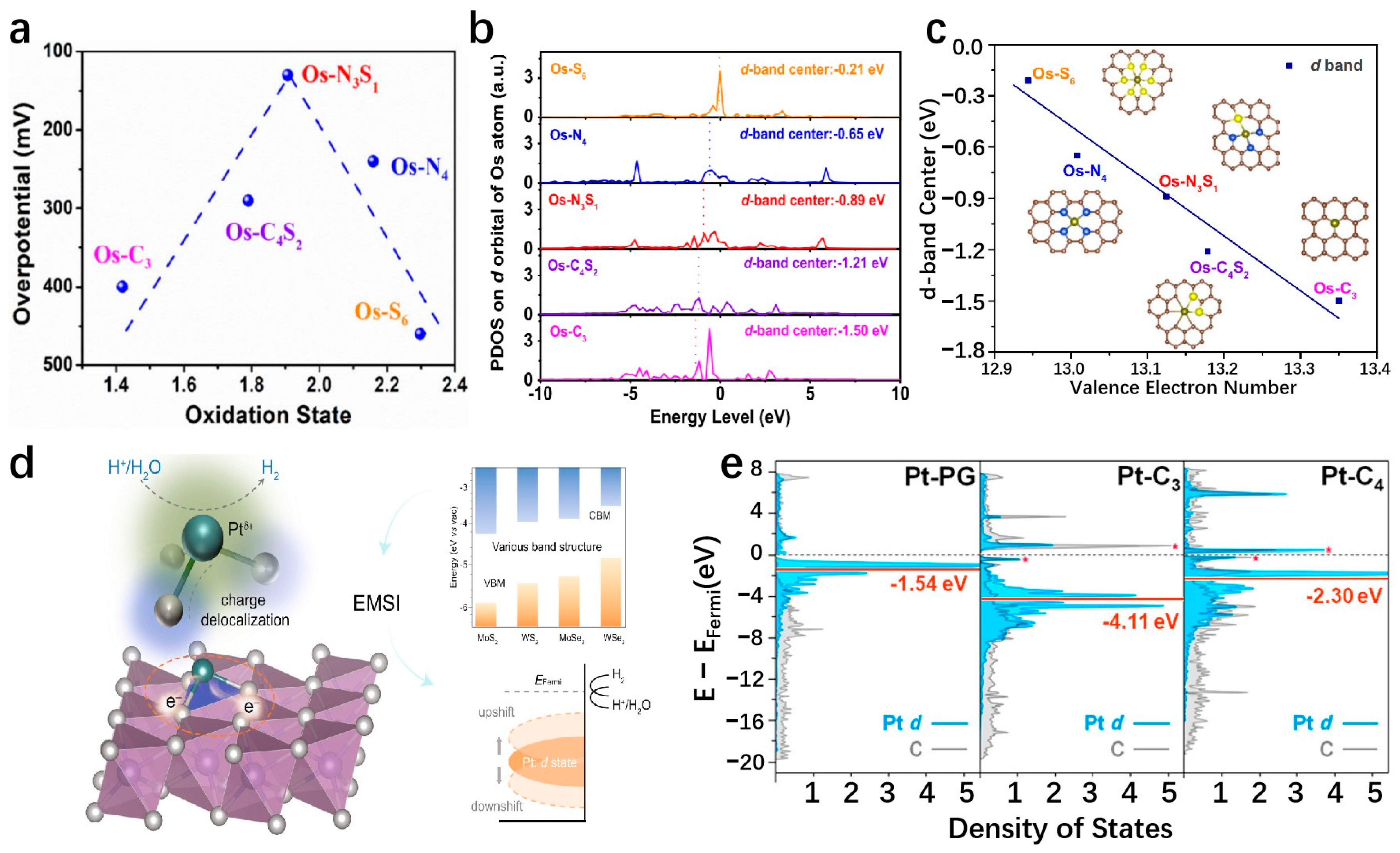
4.3. Defect Engineering
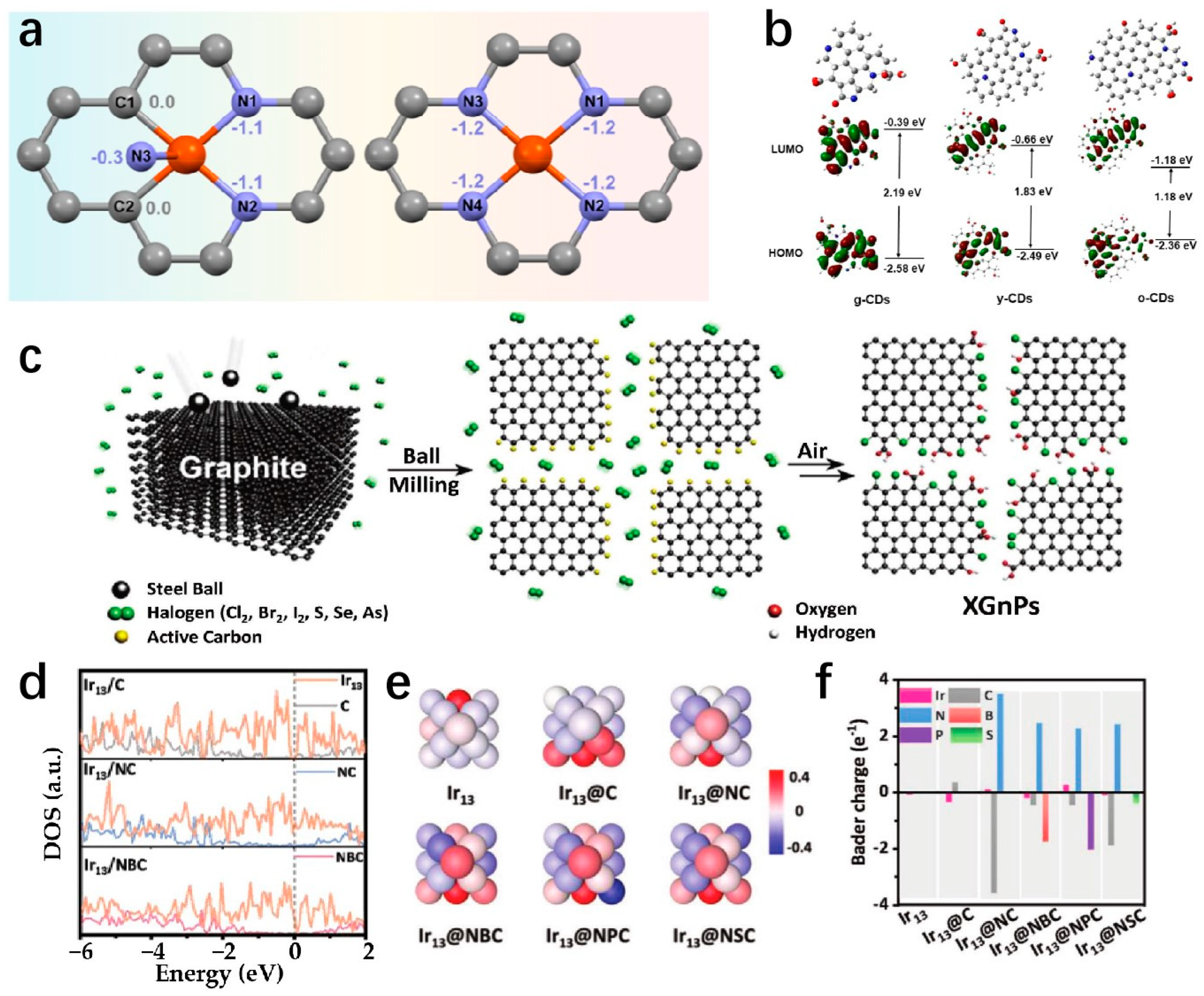
4.4. Charge Transfer
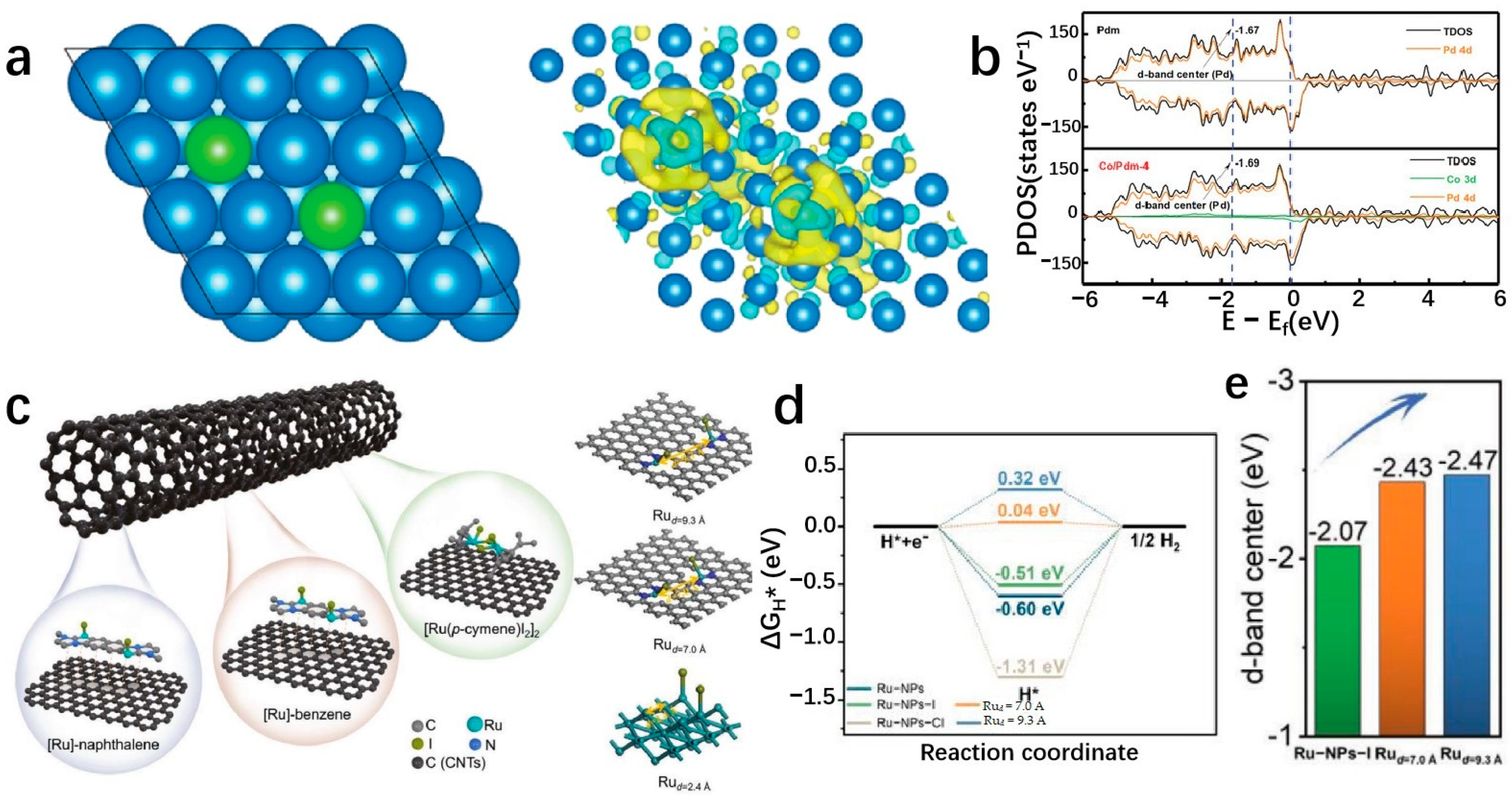
4.5. Confined Environment Structure Design
4.6. Other Methods for Manipulating the Microenvironment of Single Atoms
5. Conclusions and Outlook
5.1. Conclusions
5.2. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Grey, C.P.; Tarascon, J.M. Sustainability and in situ monitoring in battery development. Nat. Mater. 2016, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Rogelj, J.; den Elzen, M.; Hohne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 degrees C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef]
- Hanssen, S.V.; Daioglou, V.; Steinmann, Z.J.N.; Doelman, J.C.; Van Vuuren, D.P.; Huijbregts, M.A.J. The climate change mitigation potential of bioenergy with carbon capture and storage. Nat. Clim. Chang. 2020, 10, 1023–1029. [Google Scholar] [CrossRef]
- Heck, V.; Gerten, D.; Lucht, W.; Popp, A. Biomass-based negative emissions difficult to reconcile with planetary boundaries. Nat. Clim. Chang. 2018, 8, 151–155. [Google Scholar] [CrossRef]
- Zhou, D.; Ding, H.; Wang, Q.; Su, B. Literature review on renewable energy development and China’s roadmap. Front. Eng. Manag. 2020, 8, 212–222. [Google Scholar] [CrossRef]
- Xu, T.; Ding, X.; Cheng, H.; Han, G.; Qu, L. Moisture-Enabled Electricity from Hygroscopic Materials: A New Type of Clean Energy. Adv. Mater. 2024, 36, e2209661. [Google Scholar] [CrossRef]
- Klatzer, T.; Bachhiesl, U.; Wogrin, S.; Tomasgard, A. Ramping up the hydrogen sector: An energy system modeling framework. Appl. Energy 2024, 355, 122264. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of hydrogen energy: Biohydrogen production technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Jiao, Y.; Zheng, Y.; Luo, J.; Davey, K.; Qiao, S.-Z. Intermediate Modulation on Noble Metal Hybridized to 2D Metal-Organic Framework for Accelerated Water Electrocatalysis. Chem 2019, 5, 2429–2441. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Huang, W.; He, Q.; Hu, Y.; Li, Y. Molecular Heterostructures of Covalent Triazine Frameworks for Enhanced Photocatalytic Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2019, 58, 8676–8680. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Tang, Q.; Jiang, D.-e. Theoretical Advances in Understanding and Designing the Active Sites for Hydrogen Evolution Reaction. ACS Catal. 2022, 12, 8404–8433. [Google Scholar] [CrossRef]
- Niu, S.; Li, S.; Du, Y.; Han, X.; Xu, P. How to Reliably Report the Overpotential of an Electrocatalyst. ACS Energy Lett. 2020, 5, 1083–1087. [Google Scholar] [CrossRef]
- van der Heijden, O.; Park, S.; Eggebeen, J.J.J.; Koper, M.T.M. Non-Kinetic Effects Convolute Activity and Tafel Analysis for the Alkaline Oxygen Evolution Reaction on NiFeOOH Electrocatalysts. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216477. [Google Scholar] [CrossRef]
- Lu, X.; Tu, W.; Zhou, Y.; Zou, Z. Effects of Electrolyte Ionic Species on Electrocatalytic Reactions: Advances, Challenges, and Perspectives. Adv. Energy Mater. 2023, 13, 2300628. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-Atom Engineering to Ignite 2D Transition Metal Dichalcogenide Based Catalysis: Fundamentals, Progress, and Beyond. Chem. Rev. 2022, 122, 1273–1348. [Google Scholar] [CrossRef]
- Shan, J.; Li, M.; Allard, L.F.; Lee, S.; Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 2017, 551, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Somerville, S.V.; Gooding, J.J.; Tilley, R.D. Co-catalytic metal–support interactions in single-atom electro-catalysts. Nat. Rev. Mater. 2024, 9, 173–189. [Google Scholar] [CrossRef]
- Bhalothia, D.; Liu, H.-Y.; Chen, S.-H.; Tseng, Y.-T.; Li, W.; Dai, S.; Wang, K.-W.; Chen, T.-Y. The sub-nanometer In2O clusters on Ag nanoparticles with highly selective electrochemical CO2 reduction to formate. Chem. Eng. J. 2024, 481, 148295. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, Z.; Huang, Q.; Zhou, C.; Chen, W.; Wang, K.; Li, M.; Lin, F.; Luo, H.; Gu, Y.; et al. Single-Atom Cr-N4 Sites with High Oxophilicity Interfaced with Pt Atomic Clusters for Practical Alkaline Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 21432–21441. [Google Scholar] [CrossRef]
- Bhalothia, D.; Yan, C.; Hiraoka, N.; Ishii, H.; Liao, Y.F.; Dai, S.; Chen, P.C.; Chen, T.Y. Iridium Single Atoms to Nano-particles: Nurturing the Local Synergy with Cobalt-Oxide Supported Palladium Nanoparticles for Oxygen Reduction Reaction. Adv. Sci. 2024, 11, e2404076. [Google Scholar] [CrossRef] [PubMed]
- Bhalothia, D.; Yan, C.; Hiraoka, N.; Ishii, H.; Liao, Y.F.; Chen, P.C.; Wang, K.W.; Chou, J.P.; Dai, S.; Chen, T.Y. Pt-Mediated Interface Engineering Boosts the Oxygen Reduction Reaction Performance of Ni Hydroxide-Supported Pd Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 16177–16188. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, S.Y.; Guo, S. Recent progress on precious metal single atom materials for water splitting catalysis. SusMat 2021, 1, 194–210. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Zhang, J.; Qiao, J.; Huang, H.; Shi, J.; Wang, G. Advances of atomically dispersed catalysts from single-atom to clusters in energy storage and conversion applications. Prog. Mater. Sci. 2022, 128, 100964. [Google Scholar] [CrossRef]
- Xue, Y.; Huang, B.; Yi, Y.; Guo, Y.; Zuo, Z.; Li, Y.; Jia, Z.; Liu, H.; Li, Y. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat. Commun. 2018, 9, 1460. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Dai, Y.; Pu, Z.; Lao, Z.; Chen, Y.; Wang, M.; Zheng, X.; Zhu, J.; Zhang, W.; et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 2018, 13, 411–417. [Google Scholar] [CrossRef]
- Shao, X.-B.; Nian, Y.; Peng, S.-S.; Zhang, G.-S.; Gu, M.-X.; Han, Y.; Liu, X.-Q.; Sun, L.-B. Magnesium single-atom catalysts with superbasicity. Sci. China Chem. 2023, 66, 1737–1743. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A New Family of Promising Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2016, 7, 494–500. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Deng, D.; Bao, X. Two-dimensional materials confining single atoms for catalysis. Chin. J. Catal. 2017, 38, 1443–1453. [Google Scholar] [CrossRef]
- Aftab, S.; Hussain, S.; Al-Kahtani, A.A. Latest Innovations in 2D Flexible Nanoelectronics. Adv. Mater. 2023, 35, e2301280. [Google Scholar] [CrossRef]
- Guo, Y.T.; Yi, S.S. Recent Advances in the Preparation and Application of Two-Dimensional Nanomaterials. Materials 2023, 16, 5789. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Feng, Q.; Dong, W.; Kong, D.; Yang, Y.; Jia, L.; Liu, J.; Zhao, C.; Guo, D.; Tian, R.; et al. Controllable Growth of 2D V3S5 Single Crystal by Chemical Vapor Deposition. Adv. Funct. Mater. 2023, 34, 2308356. [Google Scholar] [CrossRef]
- Wang, Y.; Mei, L.; Li, Y.; Xia, X.; Cui, N.; Long, G.; Yu, W.; Chen, W.; Mu, H.; Lin, S. Integration of two-dimensional materials based photodetectors for on-chip applications. Phys. Rep. 2024, 1081, 1–46. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; Gu, C.; Choi, J.H.; Wang, S.; Jiang, J. Interlayer Charge Transfer Regulates Single-Atom Catalytic Activity on Electride/Graphene 2D Heterojunctions. J. Am. Chem. Soc. 2023, 145, 4774–4783. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Yang, V.N.; Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 2015, 137, 3076–3084. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Lee, S.; Zugic, B.; Huang, J.; Allard, L.F.; Flytzani-Stephanopoulos, M. A common single-site Pt(II)-O(OH)x- species stabilized by sodium on "active" and "inert" supports catalyzes the water-gas shift reaction. J. Am. Chem. Soc. 2015, 137, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Asakura, H.; Yan, N. Atomically Dispersed Rhodium on Self-Assembled Phosphotungstic Acid: Structural Features and Catalytic CO Oxidation Properties. Ind. Eng. Chem. Res. 2017, 56, 3578–3587. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Xie, Y. Surface Defects in Two-Dimensional Photocatalysts for Efficient Organic Synthesis. Matter 2020, 2, 842–861. [Google Scholar] [CrossRef]
- He, Z.; Zhao, R.; Chen, X.; Chen, H.; Zhu, Y.; Su, H.; Huang, S.; Xue, J.; Dai, J.; Cheng, S.; et al. Defect Engineering in Single-Layer MoS2 Using Heavy Ion Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 42524–42533. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, G.; Wang, W.; Xu, X.; Jiang, S.; Fortunato, E.; Martins, R. Energy-band engineering by 2D MXene doping for high-performance homojunction transistors and logic circuits. J. Mater. Sci. Technol. 2023, 159, 41–51. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, D.; Lagunas, F.; Atterberry, B.; Lei, M.; Hu, H.; Zhou, Z.; Filatov, A.S.; Jiang, D.E.; Rossini, A.J.; et al. Hybrid organic-inorganic two-dimensional metal carbide MXenes with amido- and imido-terminated surfaces. Nat. Chem. 2023, 15, 1722–1729. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, J.; Xia, Z.; Lyu, J.; Li, X.; Zheng, L.; Chen, C.; Chaemchuen, S.; Bu, T.; Verpoort, F.; et al. Correlating Single-Atomic Ruthenium Interdistance with Long-Range Interaction Boosts Hydrogen Evolution Reaction Kinetics. Adv. Mater. 2024, 36, e2310699. [Google Scholar] [CrossRef]
- Yin, X.P.; Wang, H.J.; Tang, S.F.; Lu, X.L.; Shu, M.; Si, R.; Lu, T.B. Engineering the Coordination Environment of Single-Atom Platinum Anchored on Graphdiyne for Optimizing Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 9382–9386. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, W.; Gao, J.; Pan, X.; Li, Y. Fundament and Application of Graphdiyne in Electrochemical Energy. Acc. Chem. Res. 2020, 53, 459–469. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Liu, X.; Xiao, W.; Luo, Z.; Wang, W.; Zhang, Y.; Liu, J.-C. Understanding the hydrogen evolution reaction activity of doped single-atom catalysts on two-dimensional GaPS4 by DFT and machine learning. J. Energy Chem. 2023, 81, 93–100. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Luo, J.; Zhang, G.; Lin, L.; Sun, F.; Li, M.; Dong, Z.; Li, X.-y. Modulating the coordination environment of Co single-atom catalysts through sulphur doping to efficiently enhance peroxymonosulfate activation for degradation of carbamazepine. Chem. Eng. J. 2023, 474, 145377. [Google Scholar] [CrossRef]
- Tsai, C.; Li, H.; Park, S.; Park, J.; Han, H.S.; Norskov, J.K.; Zheng, X.; Abild-Pedersen, F. Electrochemical generation of sulfur vacancies in the basal plane of MoS2 for hydrogen evolution. Nat. Commun. 2017, 8, 15113. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Lagos, M.J.; Manichev, V.; Fullon, R.; Song, X.; Voiry, D.; Chakraborty, S.; Zhang, W.; Batson, P.E.; et al. Single Atomic Vacancy Catalysis. ACS Nano 2019, 13, 9958–9964. [Google Scholar] [CrossRef]
- Su, J.; Zhang, S.; Liu, Q.; Hu, G.; Zhang, L. The janus in monodispersed catalysts: Synergetic interactions. J. Mater. Chem. A 2021, 9, 5276–5295. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, Y.; Li, X.; Ta, N.; Xu, J.; Nie, Y.; Cho, K.; Liu, J. Pocketlike Active Site of Rh1/MoS2 Single-Atom Catalyst for Selective Crotonaldehyde Hydrogenation. J. Am. Chem. Soc. 2019, 141, 19289–19295. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ouyang, Y.; Zhang, H.; Xiao, M.; Ge, J.; Jiang, Z.; Wang, J.; Tang, D.; Cao, X.; Liu, C.; et al. Chemically activating MoS2 via spontaneous atomic palladium interfacial doping towards efficient hydrogen evolution. Nat. Commun. 2018, 9, 2120. [Google Scholar] [CrossRef]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Xia, B.; Wang, T.; Jiang, X.; Zhang, T.; Li, J.; Xiao, W.; Xi, P.; Gao, D.; Xue, D.; Ding, J. Ar2+ Beam Irradiation-Induced Multivancancies in MoSe2 Nanosheet for Enhanced Electrochemical Hydrogen Evolution. ACS Energy Lett. 2018, 3, 2167–2172. [Google Scholar] [CrossRef]
- Pham, V.P.; Yeom, G.Y. Recent Advances in Doping of Molybdenum Disulfide: Industrial Applications and Future Prospects. Adv. Mater. 2016, 28, 9024–9059. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 quantum dot-interspersed exfoliated MoS2 nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Backes, C.; Paton, K.R.; Harvey, A.; Gholamvand, Z.; McCauley, J.; Coleman, J.N. Large-Scale Production of Size-Controlled MoS2 Nanosheets by Shear Exfoliation. Chem. Mater. 2015, 27, 1129–1139. [Google Scholar] [CrossRef]
- Wang, S.; Xue, J.; Xu, D.; He, J.; Dai, Y.; Xia, T.; Huang, Y.; He, Q.; Duan, X.; Lin, Z. Electrochemical molecular intercalation and exfoliation of solution-processable two-dimensional crystals. Nat. Protoc. 2023, 18, 2814–2837. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Sun, B.; Sakai, N.; Ma, R.; Sasaki, T.; Wang, S.; Zhang, J.; Wang, G. 2D Superlattices for Efficient Energy Storage and Conversion. Adv. Mater. 2020, 32, e1902654. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, P.; Miao, M.; Fang, J.; Feng, X. TEMPO-Oxidized Nanofibrillated Cellulose Assisted Exfoliation of MoS2 /Graphene Composites for Flexible Paper-Anodes. Chem. Asian J. 2022, 17, e202200257. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, L.; Tang, Z.; Xu, Z.; Xie, C.; Guo, L.; Li, W. High-performance expanded graphite from flake graphite by microwave-assisted chemical intercalation process. J. Ind. Eng. Chem. 2023, 122, 562–572. [Google Scholar] [CrossRef]
- Cai, Z.X.; Wang, Z.L.; Xia, Y.J.; Lim, H.; Zhou, W.; Taniguchi, A.; Ohtani, M.; Kobiro, K.; Fujita, T.; Yamauchi, Y. Tailored Catalytic Nanoframes from Metal-Organic Frameworks by Anisotropic Surface Modification and Etching for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2021, 60, 4747–4755. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of two-dimensional titanium nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385–11391. [Google Scholar] [CrossRef]
- Daum, J.P.; Ajnsztajn, A.; Iyengar, S.A.; Lowenstein, J.; Roy, S.; Gao, G.H.; Tsai, E.H.R.; Ajayan, P.M.; Verduzco, R. Solutions Are the Problem: Ordered Two-Dimensional Covalent Organic Framework Films by Chemical Vapor Deposition. ACS Nano 2023, 17, 21411–21419. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, L.; Ge, Y.; Huang, Z.; Shi, Z.; Liu, J.; Zhai, W.; Liang, J.; Zhang, H. Wet-chemical synthesis of two-dimensional metal nanomaterials for electrocatalysis. Natl. Sci. Rev. 2022, 9, nwab142. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Selvarajan, P.; Wang, S.; Wan, T.; Xi, S.; Wang, X.; Xue, J.; Indirathankam, S.C.; Geng, X.; Qiao, L.; et al. Thermostable 1T-MoS2 Nanosheets Achieved by Spontaneous Intercalation of Cu Single Atoms at Room Temperature and Their Enhanced HER Performance. Small Struct. 2023, 4, 2300010. [Google Scholar] [CrossRef]
- Deng, Y.; Xi, X.; Xia, Y.; Cao, Y.; Xue, S.; Wan, S.; Dong, A.; Yang, D. 2D FeP Nanoframe Superlattices via Space-Confined Topochemical Transformation. Adv. Mater. 2022, 34, e2109145. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ge, L.; Liu, H.; Jiang, Z.; Jia, Y.; Li, Z.; Yang, D.; Hocking, R.K.; Li, M.; Zhang, L.; et al. A Surfactant-Free and Scalable General Strategy for Synthesizing Ultrathin Two-Dimensional Metal-Organic Framework Nanosheets for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2019, 58, 13565–13572. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, C.; Jia, S.; Meng, W.; Li, P.; Yan, S.; Cheng, Y.; Miao, J.; Zhang, L.; Gao, Y.; et al. Study of the growth mechanism of a self-assembled and ordered multi-dimensional heterojunction at atomic resolution. Front. Optoelectron. 2023, 16, 35. [Google Scholar] [CrossRef]
- Kan, X.; Ban, Y.; Wu, C.; Pan, Q.; Liu, H.; Song, J.; Zuo, Z.; Li, Z.; Zhao, Y. Interfacial Synthesis of Conjugated Two-Dimensional N-Graphdiyne. ACS Appl. Mater. Interfaces 2018, 10, 53–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, C.; Liu, C.; Zuo, M.; Qin, L.; Yan, X.; Xing, Y.; Li, H.; Si, R.; Zhou, S.; et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts. Nat. Commun. 2020, 11, 1215. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, F.; Huang, R.; Liu, X.; Zhang, Z.; Yao, T.; Zhang, Y.; Wu, Y. Symmetry Evolution Induced 2D Pt Single Atom Catalyst with High Density for Alkaline Hydrogen Oxidation. Adv. Mater. 2024, 36, e2404672. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Liu, Y.; Wang, C.; Jin, C.; Hou, J.; Yang, M.; Huo, D. Pt Single-Atom Catalyst on Co3O4 for the Electrocatalytic Detection of Glucose. ACS Appl. Nano Mater. 2024, 7, 13693–13700. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 3783. [Google Scholar] [CrossRef]
- Chen, C.; Ou, W.; Yam, K.M.; Xi, S.; Zhao, X.; Chen, S.; Li, J.; Lyu, P.; Ma, L.; Du, Y.; et al. Zero-Valent Palladium Single-Atoms Catalysts Confined in Black Phosphorus for Efficient Semi-Hydrogenation. Adv. Mater. 2021, 33, e2008471. [Google Scholar] [CrossRef]
- Yue, C.; Feng, C.; Sun, G.; Liu, N.; Hao, H.; Bao, W.; Zhang, X.; Sun, F.; Zhang, C.; Bi, J.; et al. Hierarchically stabilized Pt single-atom catalysts induced by an atomic substitution strategy for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2024, 17, 5227–5240. [Google Scholar] [CrossRef]
- Sun, Y.; Xue, Z.; Liu, Q.; Jia, Y.; Li, Y.; Liu, K.; Lin, Y.; Liu, M.; Li, G.; Su, C.Y. Modulating electronic structure of metal-organic frameworks by introducing atomically dispersed Ru for efficient hydrogen evolution. Nat. Commun. 2021, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, E.; Cui, S.; Yang, S.; Zou, X.; Gong, Y. Single-Atom Pt Anchored on Oxygen Vacancy of Monolayer Ti3C2Tx for Superior Hydrogen Evolution. Nano Lett. 2022, 22, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Cao, H.; Hao, Q.; Wang, R.; Liu, W.; Zuo, M.; Jia, C.; Zhang, Z.; Bao, J. Neighbouring Synergy in High-Density Single Ir Atoms on CoGaOOH for Efficient Alkaline Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. Engl. 2024, 63, e202404418. [Google Scholar] [CrossRef] [PubMed]
- Hai, X.; Xi, S.; Mitchell, S.; Harrath, K.; Xu, H.; Akl, D.F.; Kong, D.; Li, J.; Li, Z.; Sun, T.; et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat. Nanotechnol. 2022, 17, 174–181. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Evolution of Isolated Atoms and Clusters in Catalysis. Trends Chem. 2020, 2, 383–400. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, D.; Cao, D.; Zeng, X.C. Revisiting the universal principle for the rational design of single-atom electro-catalysts. Nat. Catal. 2024, 7, 207–218. [Google Scholar] [CrossRef]
- Xia, C.; Qiu, Y.; Xia, Y.; Zhu, P.; King, G.; Zhang, X.; Wu, Z.; Kim, J.Y.T.; Cullen, D.A.; Zheng, D.; et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 2021, 13, 887–894. [Google Scholar] [CrossRef]
- Yan, B.; Song, H.; Yang, G. A facile and green large-scale fabrication of single atom catalysts for high photocatalytic H2 evolution activity. Chem. Eng. J. 2022, 427, 131795. [Google Scholar] [CrossRef]
- Ding, X.; Liu, D.; Zhao, P.; Chen, X.; Wang, H.; Oropeza, F.E.; Gorni, G.; Barawi, M.; Garcia-Tecedor, M.; de la Pena O’Shea, V.A.; et al. Dynamic restructuring of nickel sulfides for electrocatalytic hydrogen evolution reaction. Nat. Commun. 2024, 15, 5336. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhu, X.; Nga, T.T.T.; Liu, Q.; Wu, Y.; Yang, P.; Zhou, Y.; Xiao, Z.; Dong, C.L.; Fu, X.; et al. Ultra-Low-Potential Methanol Oxidation on Single-Ir-Atom Catalyst. Angew. Chem. Int. Ed. Engl. 2024, 63, e202404713. [Google Scholar] [CrossRef]
- Hu, C.; Hu, J.; Zhu, Z.; Lu, Y.; Chu, S.; Ma, T.; Zhang, Y.; Huang, H. Orthogonal Charge Transfer by Precise Positioning of Silver Single Atoms and Clusters on Carbon Nitride for Efficient Piezocatalytic Pure Water Splitting. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212397. [Google Scholar] [CrossRef]
- Wang, B.; Chu, S.; Zheng, L.; Li, X.; Zhang, J.; Zhang, F. Application of X-Ray Absorption Spectroscopy in Electrocatalytic Water Splitting and CO2 Reduction. Small Sci. 2021, 1, 2100023. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, R.; Wray, L.A.; Chen, J.; Zhuo, Z.; Chen, Y.; Yan, S.; Pan, F.; Hussain, Z.; Yang, W. Quantitative probe of the transition metal redox in battery electrodes through soft x-ray absorption spectroscopy. J. Phys. D Appl. Phys. 2016, 49, 413003. [Google Scholar] [CrossRef]
- Tata, C. Hind Lever Chem merger approved. Focus Surfactants 2004, 2004, 1–8. [Google Scholar]
- van Bokhoven, J. Recent developments in X-ray absorption spectroscopy. Phys. Chem. Chem. Phys. 2010, 12, 5502. [Google Scholar] [PubMed]
- Zhang, Q.; Ma, Y.; Lu, Y.; Zhou, X.; Lin, L.; Li, L.; Yan, Z.; Zhao, Q.; Zhang, K.; Chen, J. Designing Anion-Type Water-Free Zn2+ Solvation Structure for Robust Zn Metal Anode. Angew. Chem. Int. Ed. Engl. 2021, 60, 23357–23364. [Google Scholar] [CrossRef]
- Sellies, L.; Spachtholz, R.; Bleher, S.; Eckrich, J.; Scheuerer, P.; Repp, J. Single-molecule electron spin resonance by means of atomic force microscopy. Nature 2023, 624, 64–68. [Google Scholar] [CrossRef]
- Keeble, D.J.; Wiktor, J.; Pathak, S.K.; Phillips, L.J.; Dickmann, M.; Durose, K.; Snaith, H.J.; Egger, W. Identification of lead vacancy defects in lead halide perovskites. Nat. Commun. 2021, 12, 5566. [Google Scholar] [CrossRef]
- Yeh, K.-Y.; Janik, M.J. Density Functional Theory Methods for Electrocatalysis. In Computational Catalysis; RSC Publishing: Cambridge, UK, 2013; pp. 116–156. [Google Scholar]
- Jiao, S.; Kong, M.; Hu, Z.; Zhou, S.; Xu, X.; Liu, L. Pt Atom on the Wall of Atomic Layer Deposition (ALD)-Made MoS2 Nanotubes for Efficient Hydrogen Evolution. Small 2022, 18, e2105129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shou, H.; Qiao, S.; Cao, Y.; Zhang, P.; Wei, S.; Chen, S.; Wu, X.; Song, L. Analyzing the Active Site and Predicting the Overall Activity of Alloy Catalysts. J. Am. Chem. Soc. 2024, 146, 15167–15175. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, L.; Favre-Réguillon, A.; De Bellefon, C. Comments on “Nanoporous aluminosilicate mediated transacetalization reactions: Application in glycerol valorization” by A.E. Graham et al. Catal. Sci. Technol. 2012, 2, 2258. Catal. Sci. Technol. 2013, 3, 1644–1645. [Google Scholar]
- Liu, C.; Pan, G.; Liang, N.; Hong, S.; Ma, J.; Liu, Y. Ir Single Atom Catalyst Loaded on Amorphous Carbon Materials with High HER Activity. Adv. Sci. 2022, 9, e2105392. [Google Scholar] [CrossRef]
- Liu, X.; Deng, Y.; Zheng, L.; Kesama, M.R.; Tang, C.; Zhu, Y. Engineering Low-Coordination Single-Atom Cobalt on Graphitic Carbon Nitride Catalyst for Hydrogen Evolution. ACS Catal. 2022, 12, 5517–5526. [Google Scholar] [CrossRef]
- Lv, X.; Wei, W.; Wang, H.; Huang, B.; Dai, Y. Holey graphitic carbon nitride (g-CN) supported bifunctional single atom electrocatalysts for highly efficient overall water splitting. Appl. Catal. B Environ. 2020, 264, 118521. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Yuan, P.; Jia, Y.; Zhuang, L.; Zhang, H.; Yan, X.; Liu, G.; Zhao, Y.; Liu, J.; et al. Single Carbon Vacancy Traps Atomic Platinum for Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2022, 144, 2171–2178. [Google Scholar] [CrossRef]
- Narendra Kumar, A.V.; Muthu Prabhu, S.; Shin, W.S.; Yadav, K.K.; Ahn, Y.; Abdellattif, M.H.; Jeon, B.-H. Prospects of non-noble metal single atoms embedded in two-dimensional (2D) carbon and non-carbon-based structures in electrocatalytic applications. Coord. Chem. Rev. 2022, 467, 214613. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, Y.; Li, T.; Chen, Z.; Jiang, Q.; Zhao, Z.; Liang, X.; Hu, C. Unraveling the High-Activity Origin of Single-Atom Iron Catalysts for Organic Pollutant Oxidation via Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 8318–8328. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, S.; An, L.; Zhu, J.; Xiao, J.; Zhao, X.; Wang, D. Strain-Engineered Ru-NiCr LDH Nanosheets Boosting Alkaline Hydrogen Evolution Reaction. ACS Catal. 2024, 14, 3466–3474. [Google Scholar] [CrossRef]
- Dang, Y.; Li, X.; Chen, Z.; Zhao, X.; Ma, B.; Chen, Y. Hierarchical MoN@NiFe-LDH Heterostructure Nanowire Array for Highly Efficient Electrocatalytic Hydrogen Evolution. Small 2023, 19, e2303932. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Q.; Dong, L.; Wu, H.B.; Yu, X.-Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- Wang, S.; Wu, J.; Xu, Y.; Liang, D.; Li, D.; Chen, D.; Liu, G.; Feng, Y. Boosting Efficient Alkaline Hydrogen Evolution Reaction of CoFe-Layered Double Hydroxides Nanosheets via Co-Coordination Mechanism of W-Doping and Oxygen Defect Engineering. Small 2024, 20, 2311221. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, T.; Jiang, X.; Liu, X.; Xin, L.; Yang, H.; Kuang, Y.; Sun, X. Catalytic applications of single-atom metal-anchored hydroxides: Recent advances and perspective. Mater. Rep. Energy 2022, 2, 100146. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, C.; Wang, Z.; Han, C.B.; Zhang, Q.; Ke, X.; Liu, J.; Wang, H. Seamlessly conductive Co(OH)2 tailored atomically dispersed Pt electrocatalyst with a hierarchical nanostructure for an efficient hydrogen evolution reaction. Energy Environ. Sci. 2020, 13, 3082–3092. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Huo, L.; Tang, J.; Li, S.; Jiang, K.; He, Q.; Dong, H.; Gong, Y.; Hu, Z. Precise Atomic Structure Regulation of Single-Atom Platinum Catalysts toward Highly Efficient Hydrogen Evolution Reaction. Small 2024, 20, e2309509. [Google Scholar] [CrossRef]
- Da, Y.; Jiang, R.; Tian, Z.; Han, X.; Chen, W.; Hu, W. The applications of single-atom alloys in electrocatalysis: Progress and challenges. SmartMat 2022, 4, e1136. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, T.; Song, Z.; Wu, Z.; Bai, S.; Liu, G.; Sun, X.; Wang, Y.; Hu, S.; Zheng, L.; et al. Atomic Modulation of Single Dispersed Ir Species on Self-Supported NiFe Layered Double Hydroxides for Efficient Electrocatalytic Overall Water Splitting. ACS Catal. 2023, 13, 11195–11203. [Google Scholar] [CrossRef]
- Raphael Ezeigwe, E.; Dong, L.; Wang, J.; Wang, L.; Yan, W.; Zhang, J. MOF-deviated zinc-nickel-cobalt ZIF-67 electrode material for high-performance symmetrical coin-shaped supercapacitors. J. Colloid Interface Sci. 2020, 574, 140–151. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, Y.; Duan, X.; Chen, P.; Wang, S.; Qiu, X.; Ye, L.; Tu, X. Heterostructured MoO3 Anchored Defect-Rich NiFe-LDH/NF as a Robust Self-Supporting Electrocatalyst for Overall Water Splitting. Small 2024, 20, e2307797. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yuan, Y.; Chen, G.; Ye, Z.; Guo, Z.; Zhao, Y. Interface Engineering Induced Multi-Scale Self-Assembly NiFe-LDH Heterostructures for High-Performance Water Electrolysis. ChemSusChem 2024, 17, e202400812. [Google Scholar] [CrossRef]
- Yu, J.; Yu, F.; Yuen, M.-F.; Wang, C. Two-dimensional layered double hydroxides as a platform for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 9389–9430. [Google Scholar] [CrossRef]
- Fu, H.; Chen, Z.; Chen, X.; Jing, F.; Yu, H.; Chen, D.; Yu, B.; Hu, Y.H.; Jin, Y. Modification Strategies for Development of 2D Material-Based Electrocatalysts for Alcohol Oxidation Reaction. Adv. Sci. 2023, e2306132. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Fu, Q.; Han, J.; Wang, X.; Xu, P.; Yao, T.; Zhong, J.; Zhong, W.; Liu, S.; Gao, T.; Zhang, Z.; et al. 2D Transition Metal Dichalcogenides: Design, Modulation, and Challenges in Electrocatalysis. Adv. Mater. 2021, 33, e1907818. [Google Scholar] [CrossRef]
- He, Y.; Wang, T.L.; Zhang, M.; Wang, T.W.; Wu, L.F.; Zeng, L.; Wang, X.; Boubeche, M.; Wang, S.; Yan, K.; et al. Discovery and Facile Synthesis of a New Silicon Based Family as Efficient Hydrogen Evolution Reaction Catalysts: A Computational and Experimental Investigation of Metal Monosilicides. Small 2021, 17, e2006153. [Google Scholar] [CrossRef]
- Xu, J.; Shao, G.; Tang, X.; Lv, F.; Xiang, H.; Jing, C.; Liu, S.; Dai, S.; Li, Y.; Luo, J.; et al. Frenkel-defected monolayer MoS2 catalysts for efficient hydrogen evolution. Nat. Commun. 2022, 13, 2193. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Lin, X.; Liu, G.; Ling, C.; Xi, S.; Chen, B.; Ge, Y.; Tan, C.; Lai, Z.; et al. Phase-dependent growth of Pt on MoS2 for highly efficient H2 evolution. Nature 2023, 621, 300–305. [Google Scholar] [CrossRef]
- Liu, W.; Kong, Y.; Wang, B.; Li, X.; Liu, P.; Santiago, A.R.P.; He, T. Computational Study of the Curvature-Promoted Anchoring of Transition Metals for Water Splitting. Nanomaterials 2021, 11, 3173. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Tian, W.; Zhang, H.; Li, J.; Wang, S.; Wang, Y. Non-Metal Single-Phosphorus-Atom Catalysis of Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2020, 59, 23791–23799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kitchaev, D.A.; Lebens-Higgins, Z.; Vinckeviciute, J.; Zuba, M.; Reeves, P.J.; Grey, C.P.; Whittingham, M.S.; Piper, L.F.J.; Van der Ven, A.; et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 2022, 7, 522–540. [Google Scholar] [CrossRef]
- Duan, H.; Wang, C.; Li, G.; Tan, H.; Hu, W.; Cai, L.; Liu, W.; Li, N.; Ji, Q.; Wang, Y.; et al. Single-Atom-Layer Catalysis in a MoS2 Monolayer Activated by Long-Range Ferro-magnetism for the Hydrogen Evolution Reaction: Beyond Single-Atom Catalysis. Angew. Chem. Int. Ed. Engl. 2021, 60, 7251–7258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liang, Y.; Zhang, H.; Geng, Z.; Zeng, J. Doping regulation in transition metal compounds for electrocatalysis. Chem. Soc. Rev. 2021, 50, 9817–9844. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J. Applications of MXene-Based Single-Atom Catalysts. Small Struct. 2023, 4, 2200354. [Google Scholar] [CrossRef]
- Sun, Z.; Li, R.; Xi, Q.; Xie, F.; Jian, X.; Gao, X.; Li, H.; Yu, Z.; Liu, J.; Zhang, X.; et al. Single atom supported on MXenes for the alkaline hydrogen evolution reaction: Species, coordination environment, and action mechanism. Phys. Chem. Chem. Phys. 2023, 25, 13728–13740. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Z.; Liu, Q.; Sun, P.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Single-atom Ru anchored in nitrogen-doped MXene (Ti3C2Tx) as an efficient catalyst for the hydrogen evolution reaction at all pH values. J. Mater. Chem. A 2020, 8, 24710–24717. [Google Scholar] [CrossRef]
- Zou, Y.; Kazemi, S.A.; Shi, G.; Liu, J.; Yang, Y.; Bedford, N.M.; Fan, K.; Xu, Y.; Fu, H.; Dong, M.; et al. Ruthenium single-atom modulated Ti3C2Tx MXene for efficient alkaline electrocatalytic hydrogen production. EcoMat 2022, 5, e12274. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varadhan, P.; Fu, H.C.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, e1903841. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Han, Z.-C.; Zhao, S.; Yang, T.; Yan, D.-Z.; Yu, H.-J. Theoretical insights into efficient oxygen evolution reaction using non-noble metal single-atom catalysts on W2CO2 MXene. Rare Met. 2024, 43, 1–13. [Google Scholar] [CrossRef]
- Wei, J.; Xiao, K.; Chen, Y.; Guo, X.-P.; Huang, B.; Liu, Z.-Q. In situprecise anchoring of Pt single atoms in spinel Mn3O4 for a highly efficient hydrogen evolution reaction. Energy Environ. Sci. 2022, 15, 4592–4600. [Google Scholar] [CrossRef]
- Chellasamy, G.; Arumugasamy, S.K.; Kuppusamy, S.; Ekambaram, V.; Rajagopalan, K.; Venkateswarlu, S.; Deivasigamani, P.; Choi, M.J.; Govindaraju, S.; Yun, K. MXene–MOF architectural hybrid-supported nickel single-atom catalysts for hydrogen evolution reactions. J. Mater. Chem. A 2024, 12, 1115–1127. [Google Scholar] [CrossRef]
- Saha, S.; Das, G.M.; Chhetri, S.; Vadivel, G. A detailed investigation of the effect of graphene/Ag nanoparticles on the ion exchange properties of PVDF membrane for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 66, 185–194. [Google Scholar] [CrossRef]
- Jin, J.; Schwingenschlögl, U. Exploration of two-dimensional molybdenum-borides and potential applications. NPJ 2D Mater. Appl. 2022, 6, 49. [Google Scholar] [CrossRef]
- Yu, E.; Pan, Y. Exploring the hydrogen evolution catalytic activity of the orthorhombic and hexagonal borophene as the hydrogen storage material. Electrochim. Acta 2022, 435, 141391. [Google Scholar] [CrossRef]
- Lee, E.; Fokwa, B.P.T. Nonprecious Metal Borides: Emerging Electrocatalysts for Hydrogen Production. Acc. Chem. Res. 2022, 55, 56–64. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Peng, Q.; Zhou, J.; Sun, Z. Mo2B2 MBene-supported single-atom catalysts as bifunctional HER/OER and OER/ORR electrocatalysts. J. Mater. Chem. A 2021, 9, 433–441. [Google Scholar] [CrossRef]
- Park, S.J.; Nguyen, T.H.; Tran, D.T.; Dinh, V.A.; Lee, J.H.; Kim, N.H. Delaminated MBene sheets beyond usual 2D transition metal materials for securing Pt single atoms to boost hydrogen evolution. Energy Environ. Sci. 2023, 16, 4093–4104. [Google Scholar] [CrossRef]
- Wang, X.; Tai, G.; Wu, Z.; Hu, T.; Wang, R. Ultrathin molybdenum boride films for highly efficient catalysis of the hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 23471–23475. [Google Scholar] [CrossRef]
- Wang, A.; Shen, L.; Zhao, M.; Wang, J.; Zhou, W.; Li, W.; Feng, Y.; Liu, H. Tungsten boride: A 2D multiple Dirac semi-metal for the hydrogen evolution reaction. J. Mater. Chem. C 2019, 7, 8868–8873. [Google Scholar] [CrossRef]
- Saha, S. 2D stacking of graphene and boron nitride for efficient metal free overall water splitting. Int. J. Hydrogen Energy 2024, 51, 503–514. [Google Scholar] [CrossRef]
- Bai, X.; Guan, J. MXenes for electrocatalysis applications: Modification and hybridization. Chin. J. Catal. 2022, 43, 2057–2090. [Google Scholar] [CrossRef]
- Anand, R.; Nissimagoudar, A.S.; Umer, M.; Ha, M.; Zafari, M.; Umer, S.; Lee, G.; Kim, K.S. Late Transition Metal Doped MXenes Showing Superb Bifunctional Electrocatalytic Activities for Water Splitting via Distinctive Mechanistic Pathways. Adv. Energy Mater. 2021, 11, 2102388. [Google Scholar] [CrossRef]
- Rao, Y.; Kim, E.; Dai, Z.; He, J.; Li, Y.; Lu, N. Size-dependent shape characteristics of 2D crystal blisters. J. Mech. Phys. Solids 2023, 175, 105286. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, J.; Liu, H.; Zhang, Y.W.; Gao, J.; Zhao, J. Robust Sandwiched B/TM/B Structures by Metal Intercalating into Bilayer Borophene Leading to Excellent Hydrogen Evolution Reaction. Adv. Energy Mater. 2023, 13, 2301331. [Google Scholar] [CrossRef]
- Li, C.; Xue, J.-Y.; Zhang, W.; Li, F.-L.; Gu, H.; Braunstein, P.; Lang, J.-P. Accelerating water dissociation at carbon supported nanoscale Ni/NiO heterojunction electrocatalysts for high-efficiency alkaline hydrogen evolution. Nano Res. 2022, 16, 4742–4750. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Chen, C.; Wang, R.; Xie, M.; Wan, S.; Zhang, R.; Cong, L.; Lu, H.; Han, Y.; et al. Single-atom platinum with asymmetric coordination environment on fully conjugated covalent organic framework for efficient electrocatalysis. Nat. Commun. 2024, 15, 2556. [Google Scholar] [CrossRef]
- Yang, P.; Cao, Z.; Long, Y.; Liu, D.; Huang, W.; Zhan, S.; Li, M. Regulating the Local Electronic Structure of Copper Single Atoms with Unsaturated B,O-Coordination for Selective 1O2 Generation. ACS Catal. 2023, 13, 12414–12424. [Google Scholar] [CrossRef]
- Di Liberto, G.; Morales-Garcia, A.; Bromley, S.T. An unconstrained approach to systematic structural and energetic screening of materials interfaces. Nat. Commun. 2022, 13, 6236. [Google Scholar] [CrossRef]
- Mosallanezhad, A.; Wei, C.; Ahmadian Koudakan, P.; Fang, Y.; Niu, S.; Bian, Z.; Liu, B.; Huang, T.; Pan, H.; Wang, G. Interfacial synergies between single-atomic Pt and CoS for enhancing hydrogen evolution reaction catalysis. Appl. Catal. B Environ. 2022, 315, 121534. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Wang, J. Oxidative-Atmosphere-Induced Strong Metal-Support Interaction and Its Catalytic Application. Acc. Chem. Res. 2023, 56, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lu, R.; Yu, R.; Zhao, H.; Wu, D.; Yao, Y.; Yu, K.; Zhu, J.; Ji, P.; Pu, Z.; et al. Tuning Active Metal Atomic Spacing by Filling of Light Atoms and Resulting Reversed Hydrogen Adsorption-Distance Relation-ship for Efficient Catalysis. Nanomicro. Lett. 2023, 15, 168. [Google Scholar]
- Wang, J.-H.; Yang, S.-W.; Ma, F.-B.; Zhao, Y.-K.; Zhao, S.-N.; Xiong, Z.-Y.; Cai, D.; Shen, H.-D.; Zhu, K.; Zhang, Q.-Y.; et al. RuCo alloy nanoparticles embedded within N-doped porous two-dimensional carbon nanosheets: A high-performance hydrogen evolution reaction catalyst. Tungsten 2023, 6, 114–123. [Google Scholar] [CrossRef]
- Tang, H.; Gu, H.; Li, Z.; Chai, J.; Qin, F.; Lu, C.; Yu, J.; Zhai, H.; Zhang, L.; Li, X.; et al. Engineering the Coordination Interface of Isolated Co Atomic Sites Anchored on N-Doped Carbon for Effective Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 46401–46409. [Google Scholar] [CrossRef]
- Liu, G.; Wang, W.; Zeng, P.; Yuan, C.; Wang, L.; Li, H.; Zhang, H.; Sun, X.; Dai, K.; Mao, J.; et al. Strengthened d-p Orbital Hybridization through Asymmetric Coordination Engineering of Single-Atom Catalysts for Durable Lithium-Sulfur Batteries. Nano Lett. 2022, 22, 6366–6374. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zheng, X.; Lin, L.; Xu, H.; Xu, G. Reaction Time-Controlled Synthesis of Multicolor Carbon Dots for White Light-Emitting Diodes. ACS Appl. Nano Mater. 2023, 6, 2478–2490. [Google Scholar] [CrossRef]
- Shu, L.; Mukherjee, M.; Xu, X.; Wang, K.; Wu, X. A Survey on Gas Leakage Source Detection and Boundary Tracking with Wireless Sensor Networks. IEEE Access 2016, 4, 1700–1715. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of Carbon Materials for Metal-Free Electrocatalysis. Adv. Mater. 2019, 31, e1804672. [Google Scholar] [CrossRef]
- Xue, D.; Cheng, J.; Yuan, P.; Lu, B.A.; Xia, H.; Yang, C.C.; Dong, C.L.; Zhang, H.; Shi, F.; Mu, S.C.; et al. Boron-Tethering and Regulative Electronic States Around Iridium Species for Hydrogen Evolution. Adv. Funct. Mater. 2022, 32, 2113191. [Google Scholar] [CrossRef]
- Lin, W.; Lu, Y.-R.; Peng, W.; Luo, M.; Chan, T.-S.; Tan, Y. Atomic bridging modulation of Ir–N, S co-doped MXene for accelerating hydrogen evolution. J. Mater. Chem. A 2022, 10, 9878–9885. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, S.; Chai, Y.M.; Dong, B. Ligand Modulation of Active Sites to Promote Cobalt-Doped 1T-MoS2 Electrocatalytic Hydrogen Evolution in Alkaline Media. Angew. Chem. Int. Ed. Engl. 2023, 62, e202313845. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hybertsen, M.S.; Wu, Q. A Physical Model for Understanding the Activation of MoS2 Basal-Plane Sulfur Atoms for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2020, 59, 14835–14841. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Du, H.; Dong, W.; Ye, S.; Liu, H.; Sun, H.; Huang, K.; Li, H.; Tang, Y.; et al. Oxophilic Tm-Sites in MoS2 Trigger Thermodynamic Spontaneous Water Dissociation for Enhanced Hydrogen Evolution. Adv. Energy Mater. 2024, 14, 2401716. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, J.; Zhang, J.; Xi, P.; Tao, K.; Gao, D.; Xue, D. P Dopants Triggered New Basal Plane Active Sites and Enlarged Interlayer Spacing in MoS2 Nanosheets toward Electrocatalytic Hydrogen Evolution. ACS Energy Lett. 2017, 2, 745–752. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, P.; Zhang, J.; Song, W.; Feng, Y.P.; Gao, D.; Ding, J. Dual-Functional N Dopants in Edges and Basal Plane of MoS2 Nanosheets Toward Efficient and Durable Hydrogen Evolution. Adv. Energy Mater. 2016, 7, 1602086. [Google Scholar] [CrossRef]
- Yan, P.; Yang, T.; Lin, M.; Guo, Y.; Qi, Z.; Luo, Q.; Yu, X.Y. “One Stone Five Birds” Plasma Activation Strategy Synergistic with Ru Single Atoms Doping Boosting the Hydrogen Evolution Performance of Metal Hydroxide. Adv. Funct. Mater. 2023, 33, 2301343. [Google Scholar] [CrossRef]
- Li, X.; Rong, H.; Zhang, J.; Wang, D.; Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, X.; Liu, J.N.; Wang, J.; Wan, X.; Wang, C.; Li, X.Y.; Shui, J.; Song, L.; Peng, H.J.; et al. Molecular Recognition Regulates Coordination Structure of Single-Atom Sites. Angew. Chem. Int. Ed. Engl. 2023, 62, e202313028. [Google Scholar] [CrossRef]
- Tan, W.; Xie, S.; Le, D.; Diao, W.; Wang, M.; Low, K.B.; Austin, D.; Hong, S.; Gao, F.; Dong, L.; et al. Fine-tuned local coordination environment of Pt single atoms on ceria controls catalytic reactivity. Nat. Commun. 2022, 13, 7070. [Google Scholar] [CrossRef]
- He, C.; Lee, C.H.; Meng, L.; Chen, H.T.; Li, Z. Selective Orbital Coupling: An Adsorption Mechanism in Single-Atom Catalysis. J. Am. Chem. Soc. 2024, 146, 12395–12400. [Google Scholar] [CrossRef]
- Cao, D.; Xu, H.; Li, H.; Feng, C.; Zeng, J.; Cheng, D. Volcano-type relationship between oxidation states and catalytic activity of single-atom catalysts towards hydrogen evolution. Nat. Commun. 2022, 13, 5843. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, Z.R.; Xiao, Y.Y.; Yin, Y.C.; Huang, W.M.; Huang, Z.C.; Zheng, Y.Z.; Mu, F.Y.; Huang, R.; Shi, G.Y.; et al. Electronic metal-support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Yi, J.; Xiang, D.Y. Defects in Carbon-Based Materials for Electrocatalysis: Synthesis, Recognition, and Advances. Acc. Chem. Res. 2023, 56, 948–958. [Google Scholar]
- Su, H.; Pan, X.; Li, S.; Zhang, H.; Zou, R. Defect-engineered two-dimensional transition metal dichalcogenides towards electrocatalytic hydrogen evolution reaction. Carbon Energy 2023, 5, e296. [Google Scholar] [CrossRef]
- Liu, J.; Tang, C.; Ke, Z.; Chen, R.; Wang, H.; Li, W.; Jiang, C.; He, D.; Wang, G.; Xiao, X. Optimizing Hydrogen Adsorption by d–d Orbital Modulation for Efficient Hydrogen Evolution Catalysis. Adv. Energy Mater. 2022, 12, 2103301. [Google Scholar] [CrossRef]
- Wang, L.; Kong, Y.; Cai, H.; Sun, J.; Jiang, X.; Li, X.; Hu, Q.; Yang, H.; He, C. Modulation of d-band electron enables efficient CO2 electroreduction towards CO on Ni nanoparticles. J. Mater. Chem. A 2024, 12, 16403–16409. [Google Scholar] [CrossRef]
- Xiao, M.; Zhu, J.; Li, S.; Li, G.; Liu, W.; Deng, Y.-P.; Bai, Z.; Ma, L.; Feng, M.; Wu, T.; et al. 3d-Orbital Occupancy Regulated Ir-Co Atomic Pair Toward Superior Bifunctional Oxygen Electrocatalysis. ACS Catal. 2021, 11, 8837–8846. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Zhao, J.; Zhang, S.; Wang, J.; Zhang, H.; Zhang, J.; Dong, Q.; Zhang, W.; Hu, W.; et al. Stabilizing Pt Single Atoms through Pt-Se Electron Bridges on Vacancy-enriched Nickel Selenide for Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2023, 62, e202308686. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Li, N.; Chen, G.; Hou, L. Applications of vacancy defect engineering in persulfate activation: Performance and internal mechanism. J. Hazard Mater. 2023, 449, 130971. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Zhang, Y.; Sun, Y.; Ma, K.; Xie, Y.; Zheng, W.; Tian, Z.; Kang, Z.; Zhang, Y. Vacancy Defects in 2D Transition Metal Dichalcogenide Electrocatalysts: From Aggregated to Atomic Configuration. Adv. Mater. 2023, 35, e2206576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Si, H.; Zhang, Q.; Wu, J.; Gao, L.; Wei, X.; Sun, Y.; Liao, Q.; Zhang, Z.; et al. Single-Atom Vacancy Defect to Trigger High-Efficiency Hydrogen Evolution of MoS2. J. Am. Chem. Soc. 2020, 142, 4298–4308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.C.; Ma, X.; Li, J.; Yuan, Q.; Zhang, P.; Wang, M.; Li, J.; Li, M.; Wang, S.; et al. Vacancy Defects Inductive Effect of Asymmetrically Coordinated Single-Atom Fe horizontal line N3 S1 Active Sites for Robust Electrocatalytic Oxygen Reduction with High Turnover Frequency and Mass Activity. Adv. Mater. 2024, 36, e2308243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Yang, J.; Tu, T.X.; Li, W.Q.; Zhang, P.F.; Zhou, Y.; Li, J.F.; Li, J.T.; Sun, S.G. Evolution of Cationic Vacancy Defects: A Motif for Surface Restructuration of OER Precatalyst. Angew. Chem. Int. Ed. Engl. 2021, 60, 26829–26836. [Google Scholar] [CrossRef]
- Lei, Y.J.; Lu, X.; Yoshikawa, H.; Matsumura, D.; Fan, Y.; Zhao, L.; Li, J.; Wang, S.; Gu, Q.; Liu, H.K.; et al. Understanding the charge transfer effects of single atoms for boosting the performance of Na-S batteries. Nat. Commun. 2024, 15, 3325. [Google Scholar] [CrossRef]
- Yang, S.; Si, Z.; Li, G.; Zhan, P.; Liu, C.; Lu, L.; Han, B.; Xie, H.; Qin, P. Single Cobalt Atoms Immobilized on Palladium-Based Nanosheets as 2D Single-Atom Alloy for Efficient Hydrogen Evolution Reaction. Small 2023, 19, e2207651. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, B.; Chen, S. Carbon-Supported Single Atom Catalysts for Electrochemical Energy Conversion and Storage. Adv. Mater. 2018, 30, e1801995. [Google Scholar] [CrossRef]
- Qin, R.; Liu, P.; Fu, G.; Zheng, N. Strategies for Stabilizing Atomically Dispersed Metal Catalysts. Small Methods 2017, 2, 1700286. [Google Scholar] [CrossRef]
- Su, J.; Ge, R.; Dong, Y.; Hao, F.; Chen, L. Recent progress in single-atom electrocatalysts: Concept, synthesis, and applications in clean energy conversion. J. Mater. Chem. A 2018, 6, 14025–14042. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S. Oxygen electroreduction catalyzed by gold nanoclusters: Strong core size effects. Angew. Chem. Int. Ed. Engl. 2009, 48, 4386–4389. [Google Scholar] [CrossRef]
- Perez-Alonso, F.J.; McCarthy, D.N.; Nierhoff, A.; Hernandez-Fernandez, P.; Strebel, C.; Stephens, I.E.; Nielsen, J.H.; Chorkendorff, I. The effect of size on the oxygen electroreduction activity of mass-selected platinum nanoparticles. Angew. Chem. Int. Ed. Engl. 2012, 51, 4641–4643. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Peng, Y.; Yue, Y.; Hu, Y.; Liang, X.; Yin, P.; Guo, L. Synthesis of concave gold nanocuboids with high-index facets and their enhanced catalytic activity. Chem Commun. 2015, 51, 11591–11594. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.Y.; Sun, S.G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Sheng, T.; Xu, Y.F.; Jiang, Y.X.; Huang, L.; Tian, N.; Zhou, Z.Y.; Broadwell, I.; Sun, S.G. Structure Design and Performance Tuning of Nanomaterials for Electrochemical Energy Conversion and Storage. Acc. Chem. Res. 2016, 49, 2569–2577. [Google Scholar] [CrossRef]
- Tan, X.; Tahini, H.A.; Smith, S.C. Defect Engineering in Graphene-Confined Single-Atom Iron Catalysts for Room-Temperature Methane Conversion. J. Phys. Chem. C 2021, 125, 12628–12635. [Google Scholar] [CrossRef]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef]
- Perivoliotis, D.K.; Tagmatarchis, N. Recent advancements in metal-based hybrid electrocatalysts supported on graphene and related 2D materials for the oxygen reduction reaction. Carbon 2017, 118, 493–510. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.L.; Guo, S. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, W.; Hu, C.; Luo, X.; Zeng, W.; Gong, X.; Yang, Y.; Yu, T.; Lei, W.; Yuan, C. Interlayer-Confined NiFe Dual Atoms within MoS2 Electrocatalyst for Ultra-Efficient Acidic Overall Water Splitting. Adv. Mater. 2023, 35, e2300505. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, W.; Xu, M.; Wang, E.; Wei, Y.; Liu, W.; Gu, X.; Liu, L.; Chen, Q.; Zhai, P.; et al. Single atom catalysts in Van der Waals gaps. Nat. Commun. 2022, 13, 6863. [Google Scholar] [CrossRef]
- Luo, T.; Huang, J.; Hu, Y.; Yuan, C.; Chen, J.; Cao, L.; Kajiyoshi, K.; Liu, Y.; Zhao, Y.; Li, Z.; et al. Fullerene Lattice-Confined Ru Nanoparticles and Single Atoms Synergistically Boost Electrocatalytic Hydrogen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2213058. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Zhang, C.; Chang, H.H.; Zhang, Y.; Li, L.; Chen, H.Y.; Peng, S. Manipulating the Microenvironment of Single Atoms by Switching Support Crystallinity for Industrial Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317220. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Zeng, S.; Jia, Z.; Ma, F.X.; Sun, L.; Cheng, L.; Pan, J.; Bao, Y.; Mao, Z.; Bu, Y.; et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution. Nat. Commun. 2022, 13, 6249. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Jadhav, A.R.; Yang, T.; Hwang, Y.; Wang, H.; Wang, L.; Luo, Y.; Kumar, A.; Lee, J.; et al. Unraveling the Function of Metal-Amorphous Support Interactions in Single-Atom Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. Engl. 2022, 61, e202114160. [Google Scholar] [CrossRef] [PubMed]
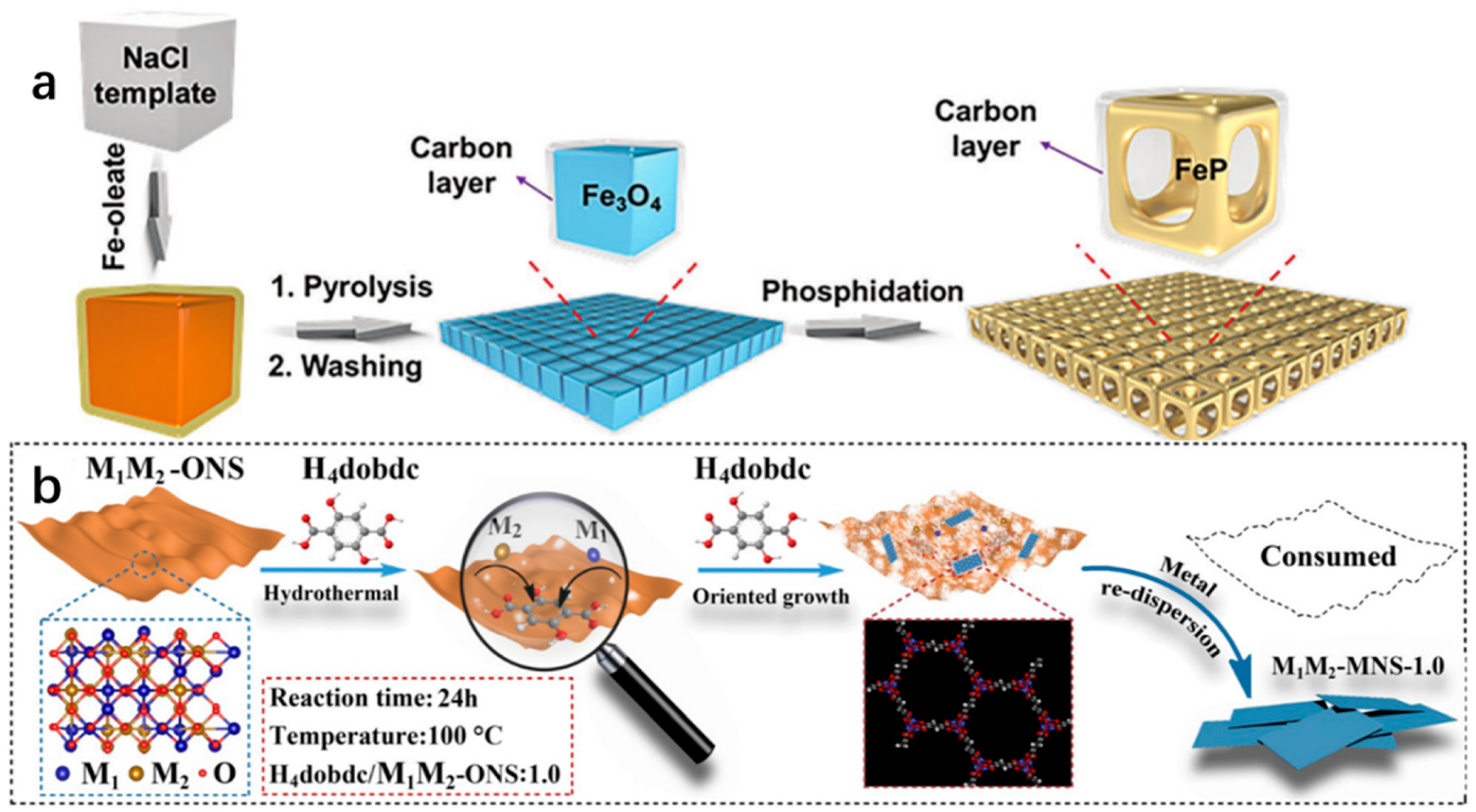
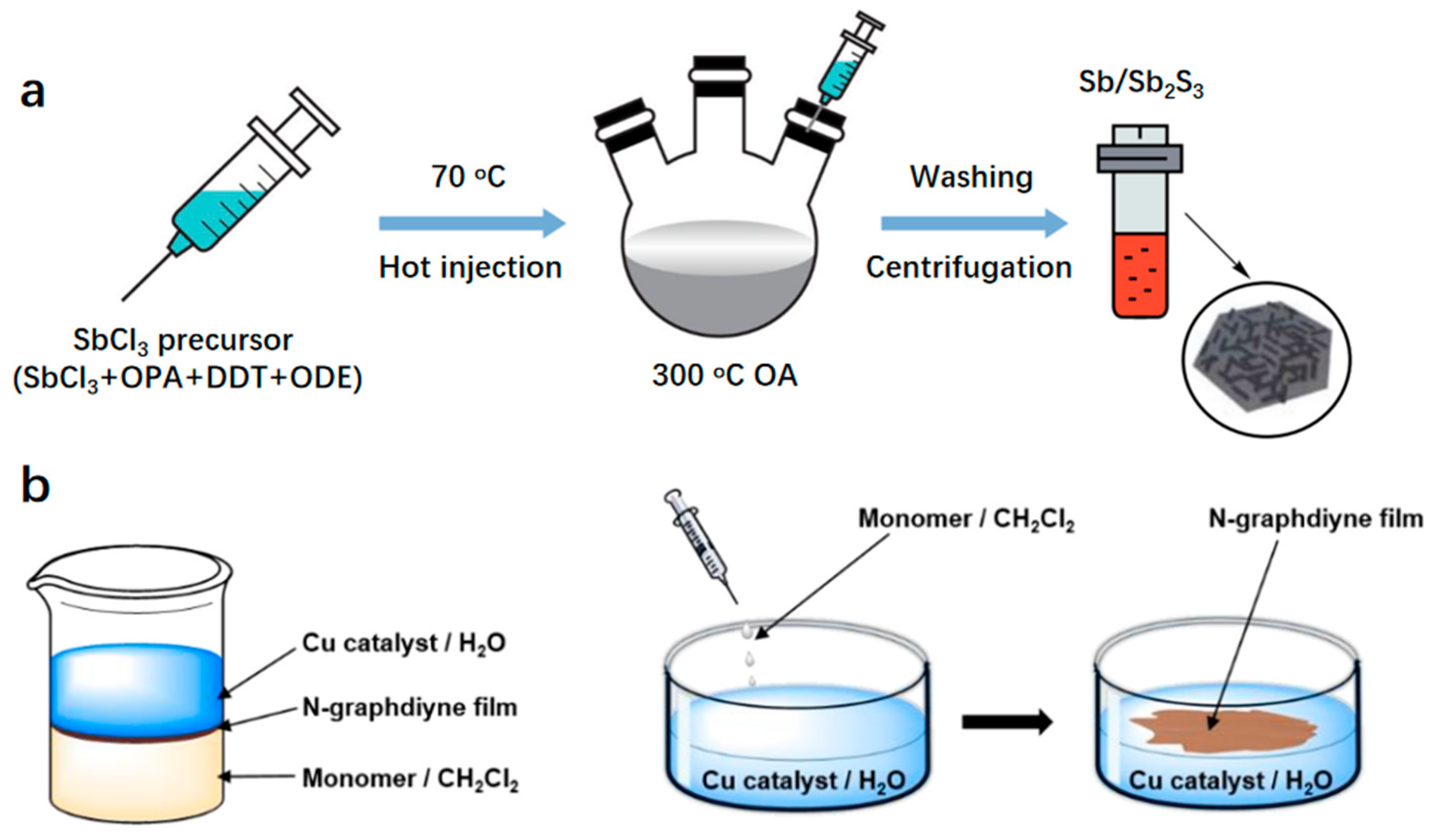
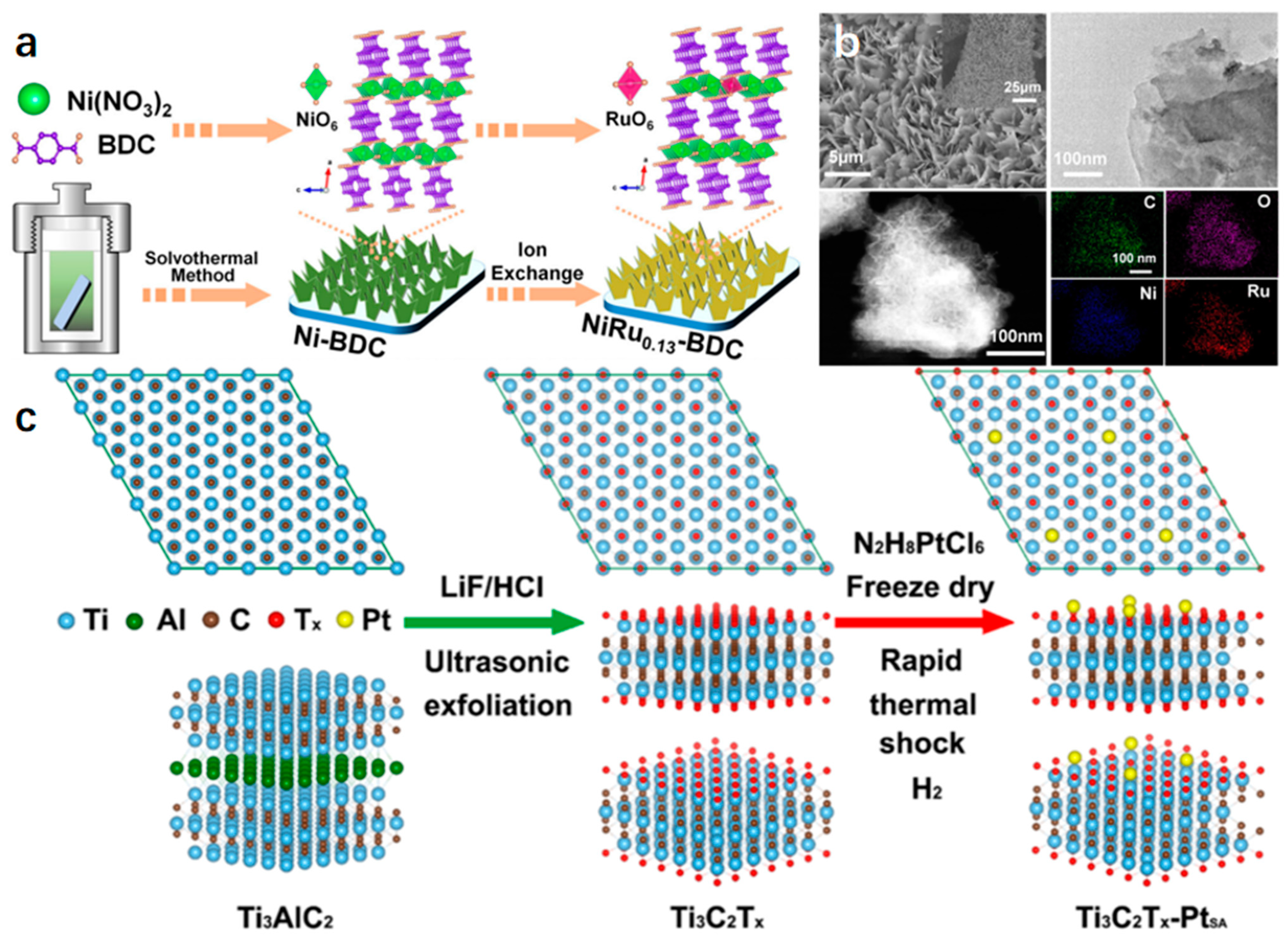
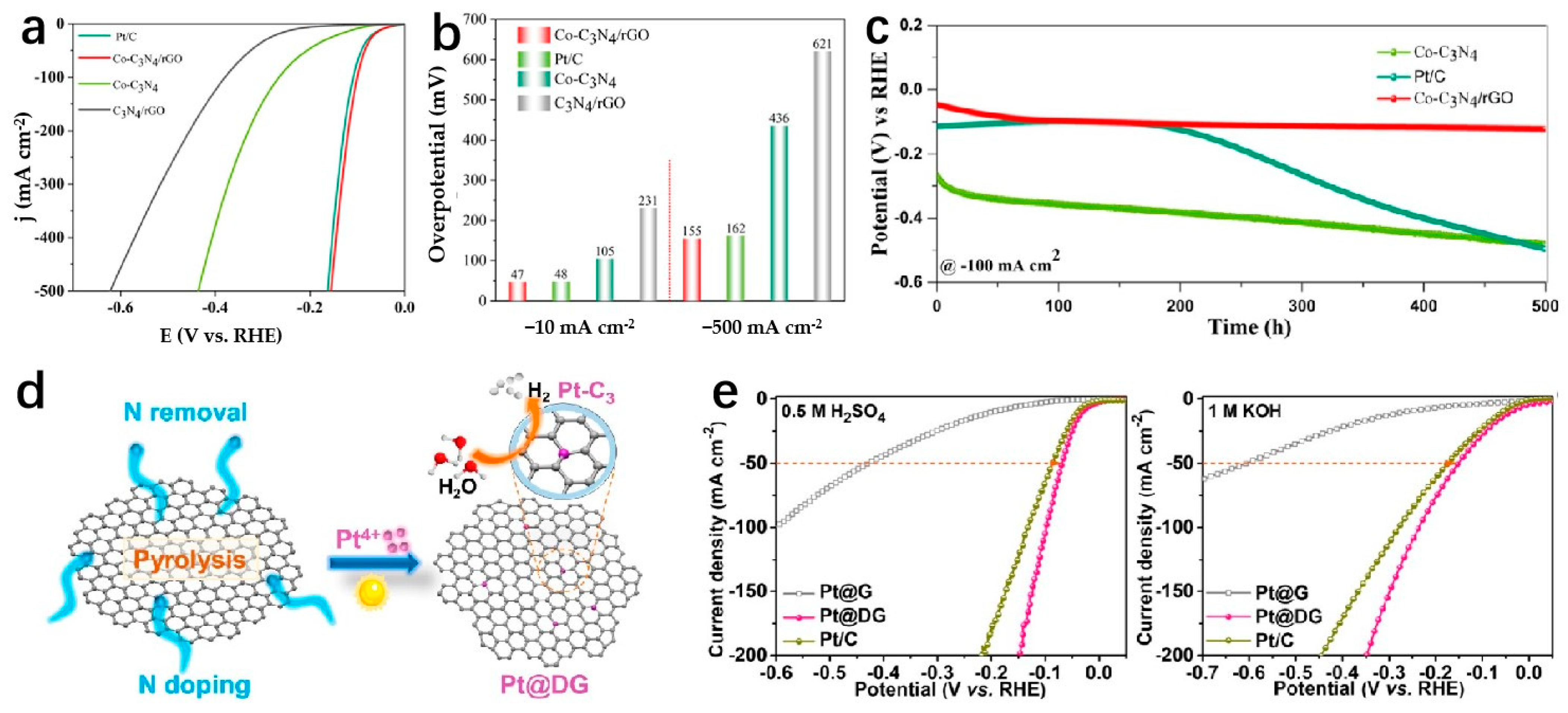


| Catalyst | Electrolyte | Overpotential (mV) | Current Density (mA cm−2) | Tafel Slope (mV dec−1) | Strategy | Ref. |
|---|---|---|---|---|---|---|
| Co-CNG | 1 M KOH | 47 | 10 | 44 | Annealing process | [106] |
| Pt@DG | 0.5 M H2SO4 | 30 | 10 | 53 | Annealing process | [107] |
| 1 M KOH | 37 | 10 | - | |||
| PtSACo(OH)2@Ag | 1 M KOH | 29 | 10 | 35.72 | Electrochemical phase transformation | [116] |
| Ru1/D-NiFe LDH | 1 M KOH | 18 | 10 | 29 | Electrochemical deposition | [117] |
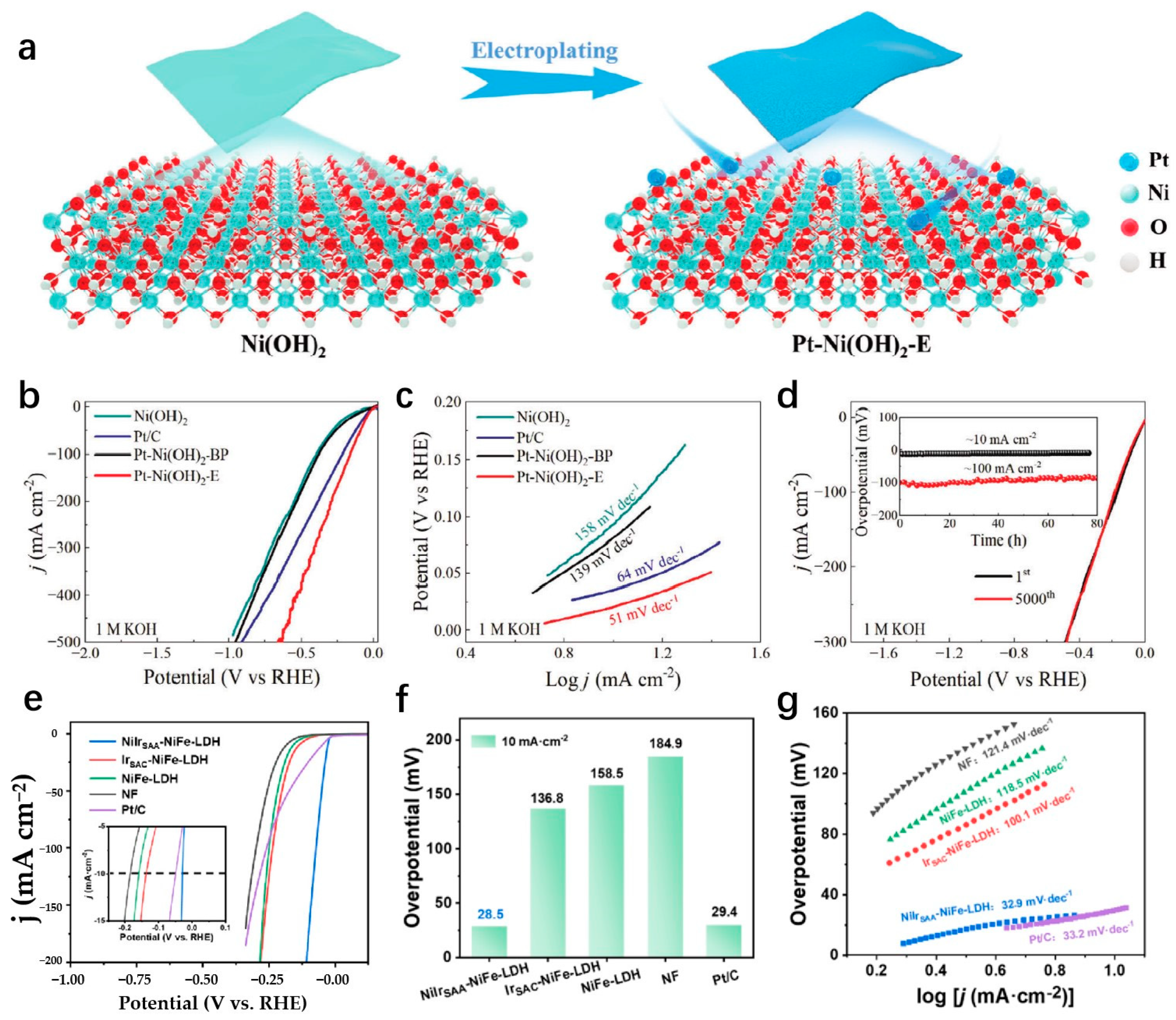
| Pt-Ni(OH)2-E | 1 M KOH | 21 | 10 | 51 | Electrochemical deposition | [118] |
| 1 M PBS | 34 | 10 | - | |||
| NiIrSAA-NiFe-LDH | 1 M KOH | 28.5 | 10 | 32.9 | Hydrothermal method | [120] |
| s-Pt/1T′-MoS2 | 0.5 M H2SO4 | 50 | 10 | 10 | Electrochemical intercalation | [130] |
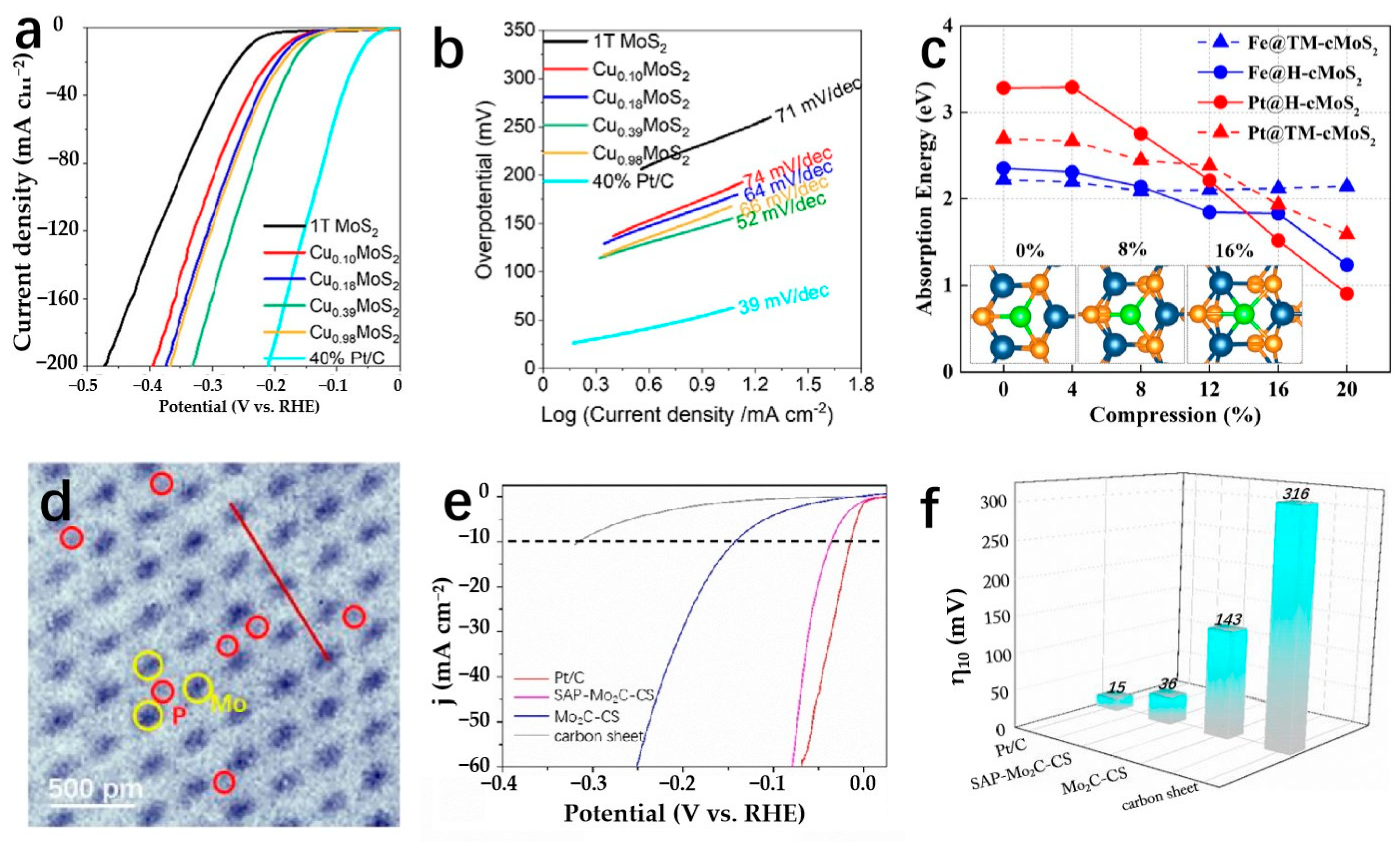
| Cu0.39MoS2 | 0.5 M H2SO4 | 250 | 10 | 52 | Hydrothermal synthesis | [72] |
| SAP-Mo2C-CS | 0.5 M H2SO4 | 36 | 10 | 38.1 | Pressurized gas-assisted process | [132] |
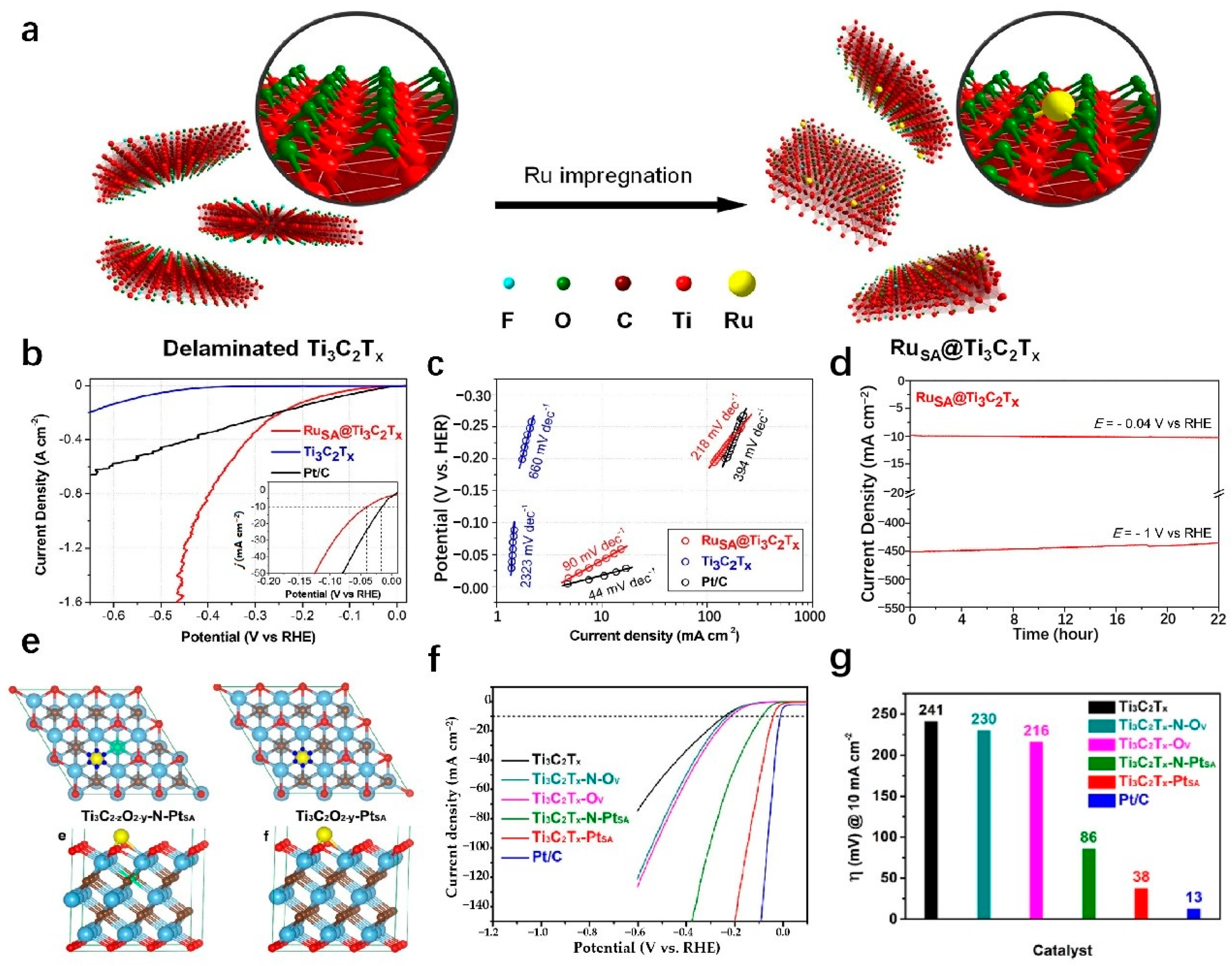
| RuSA-N-Ti3C2Tx | 1 M KOH | 27 | 10 | 29 | Acoustic wave assisted liquid phase stripping | [138] |
| 0.5 M H2SO4 | 23 | 10 | 42 | |||
| 1 M PBS | 81 | 10 | - | |||
| RuSA@Ti3C2Tx | 1 M KOH | 425.7 | 1000 | - | Wet chemistry impregnation | [139] |
| RuSA-N-S-Ti3C2Tx | 0.5 M H2SO4 | 76 | 10 | 90 | Annealing process | [140] |
| Ti3C2Tx-PtSA | 0.5 M H2SO4 | 38 | 10 | 45 | Acoustic wave assisted liquid phase stripping | [84] |
| MXene-Fe-MOF | 0.5 M H2SO4 | 52 | 10 | ~30 | Selective etching-assisted liquid phase exfoliation. | [143] |
| h-BN | 1 M KOH | 28 | 10 | 25 | Annealing method | [152] |
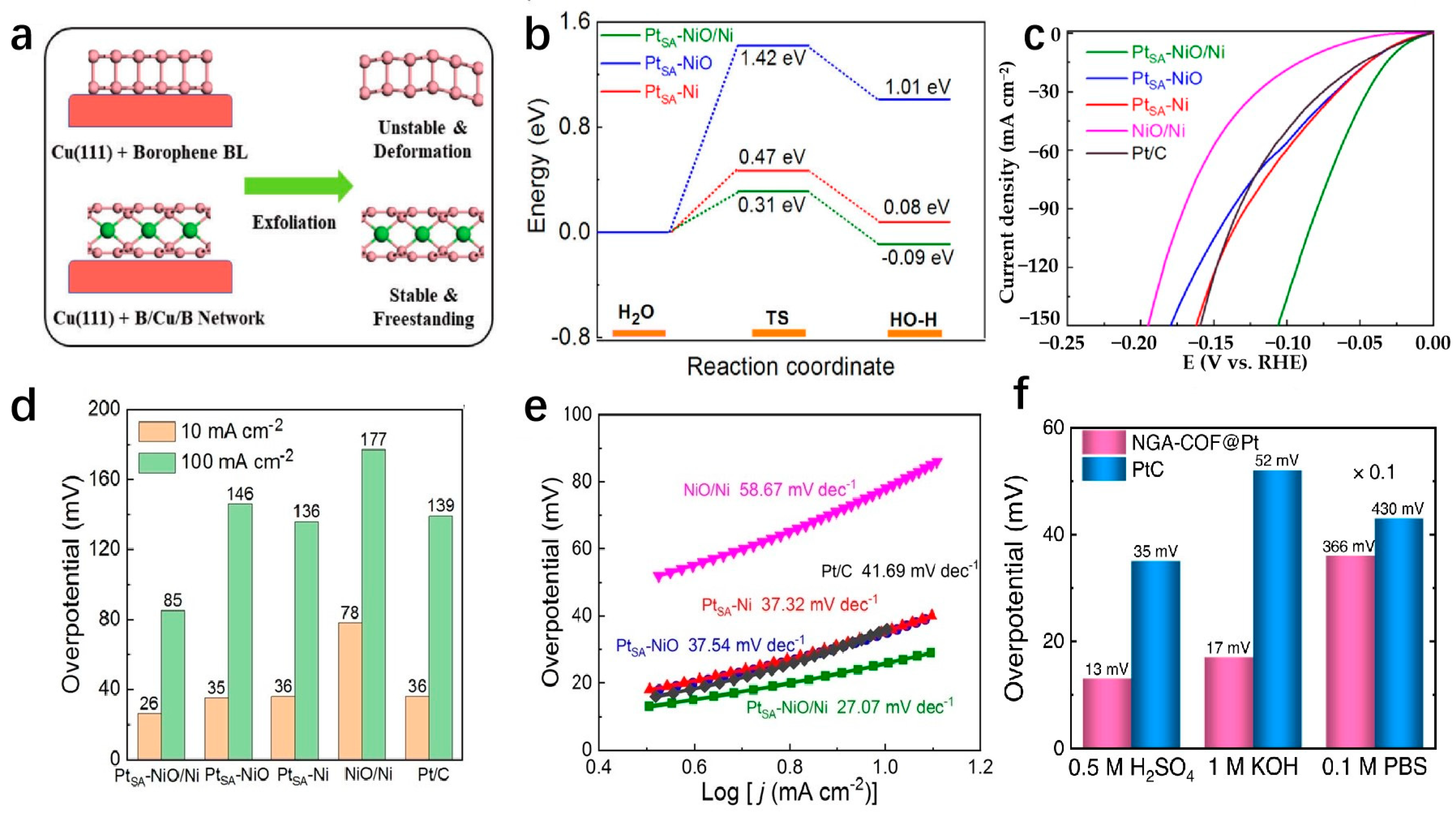
| NGA-COF@Pt | NGA-COF@Pt | 13 | 10 | 21.88 | Electrochemical modification strategy | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Yuan, D.; Yu, T.; Lei, C.; Kou, Z.; Huang, B.; Lyu, S.; Zhang, F.; Wan, T. Recent Advances on Two-Dimensional Nanomaterials Supported Single-Atom for Hydrogen Evolution Electrocatalysts. Molecules 2024, 29, 4304. https://doi.org/10.3390/molecules29184304
Fu K, Yuan D, Yu T, Lei C, Kou Z, Huang B, Lyu S, Zhang F, Wan T. Recent Advances on Two-Dimensional Nanomaterials Supported Single-Atom for Hydrogen Evolution Electrocatalysts. Molecules. 2024; 29(18):4304. https://doi.org/10.3390/molecules29184304
Chicago/Turabian StyleFu, Kangkai, Douke Yuan, Ting Yu, Chaojun Lei, Zhenhui Kou, Bingfeng Huang, Siliu Lyu, Feng Zhang, and Tongtao Wan. 2024. "Recent Advances on Two-Dimensional Nanomaterials Supported Single-Atom for Hydrogen Evolution Electrocatalysts" Molecules 29, no. 18: 4304. https://doi.org/10.3390/molecules29184304
APA StyleFu, K., Yuan, D., Yu, T., Lei, C., Kou, Z., Huang, B., Lyu, S., Zhang, F., & Wan, T. (2024). Recent Advances on Two-Dimensional Nanomaterials Supported Single-Atom for Hydrogen Evolution Electrocatalysts. Molecules, 29(18), 4304. https://doi.org/10.3390/molecules29184304





