PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3
Abstract
1. Introduction
2. Results and Discussion
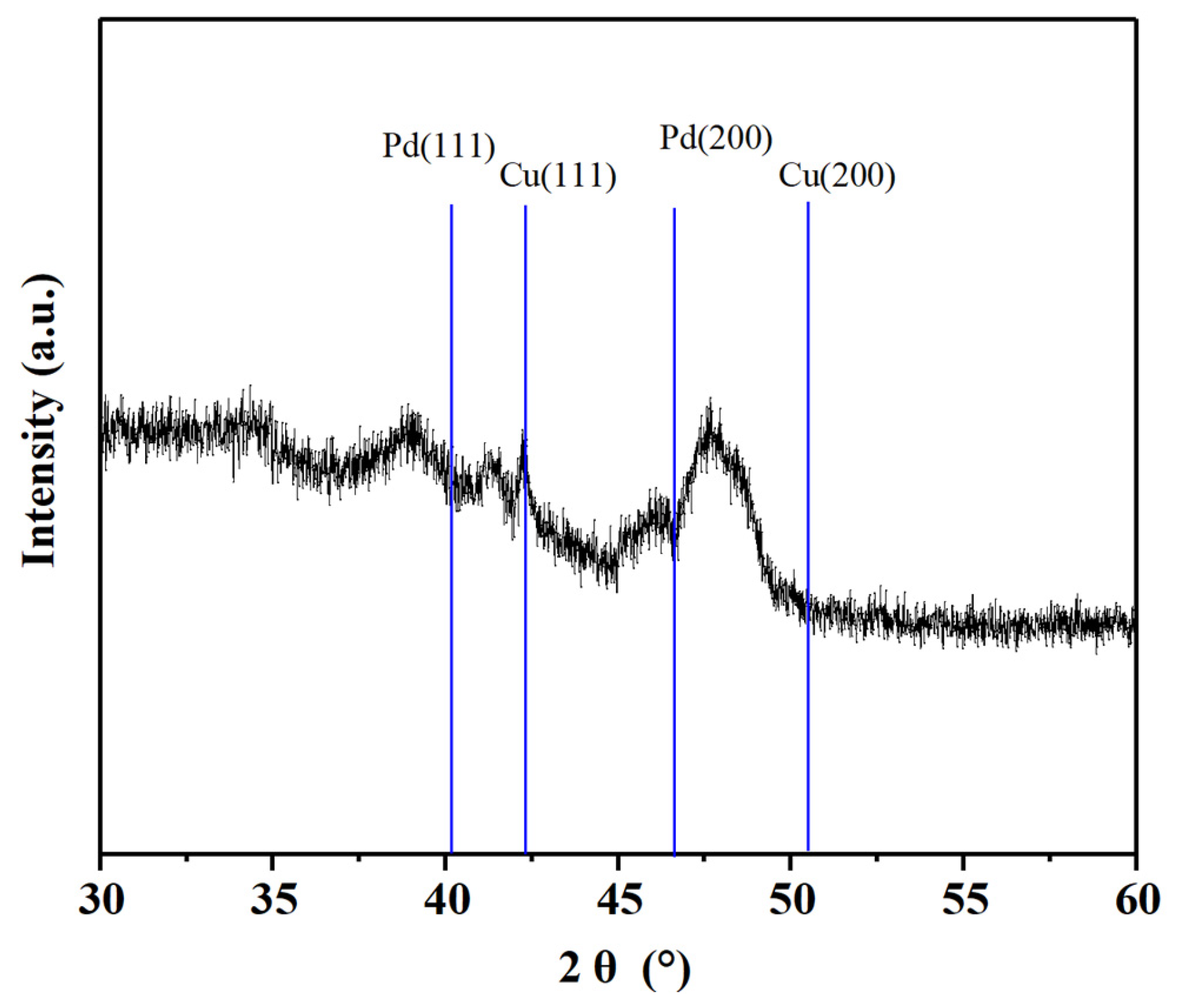
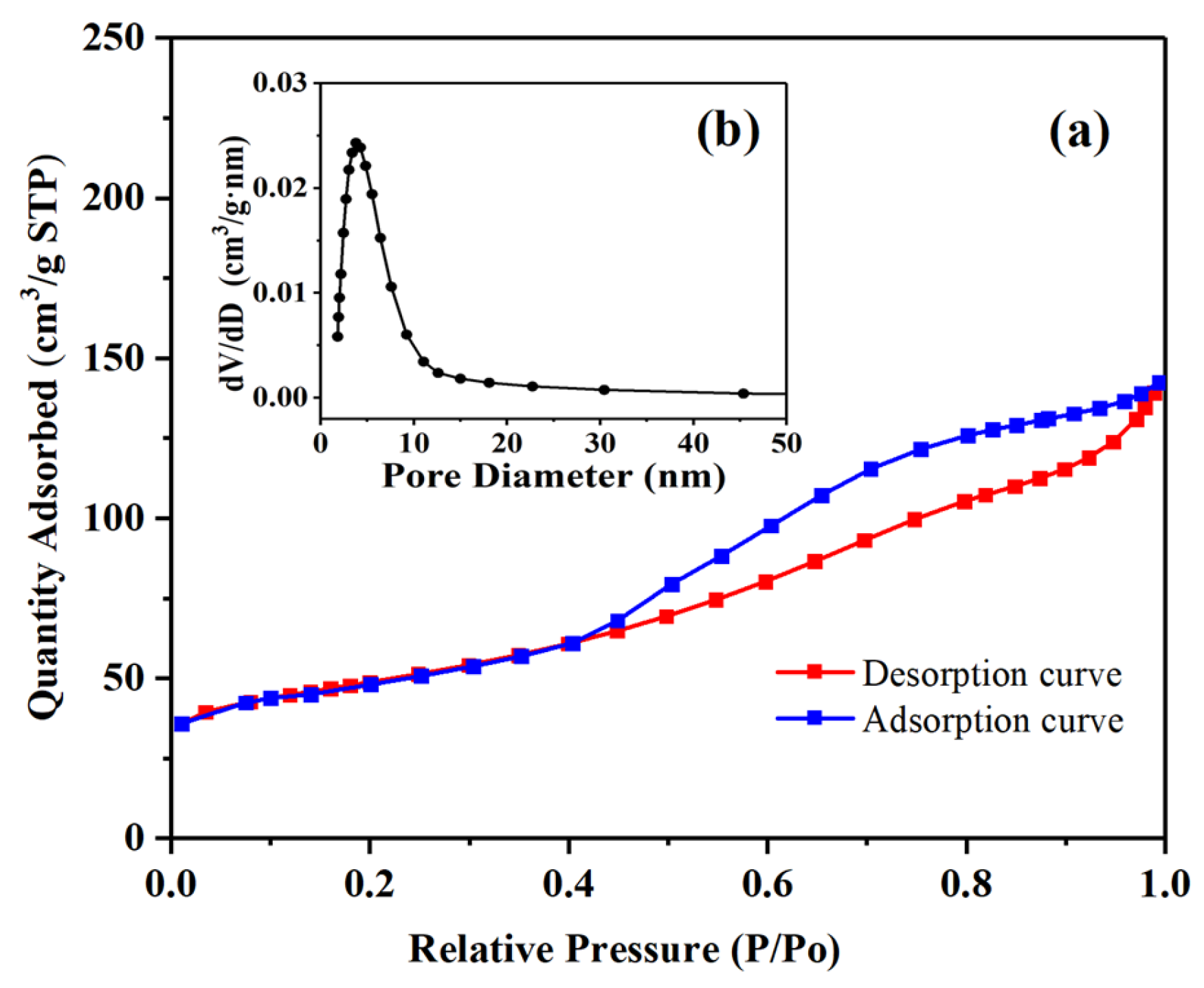
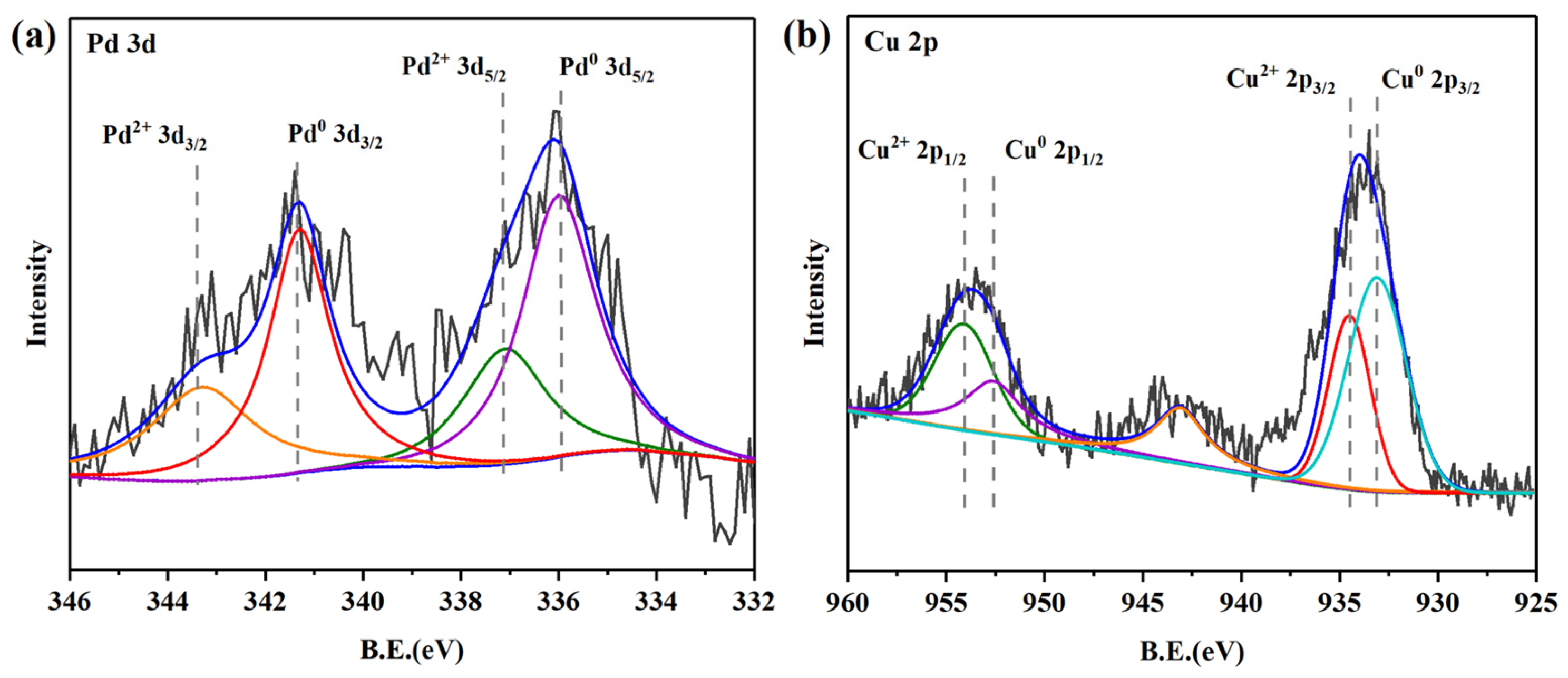
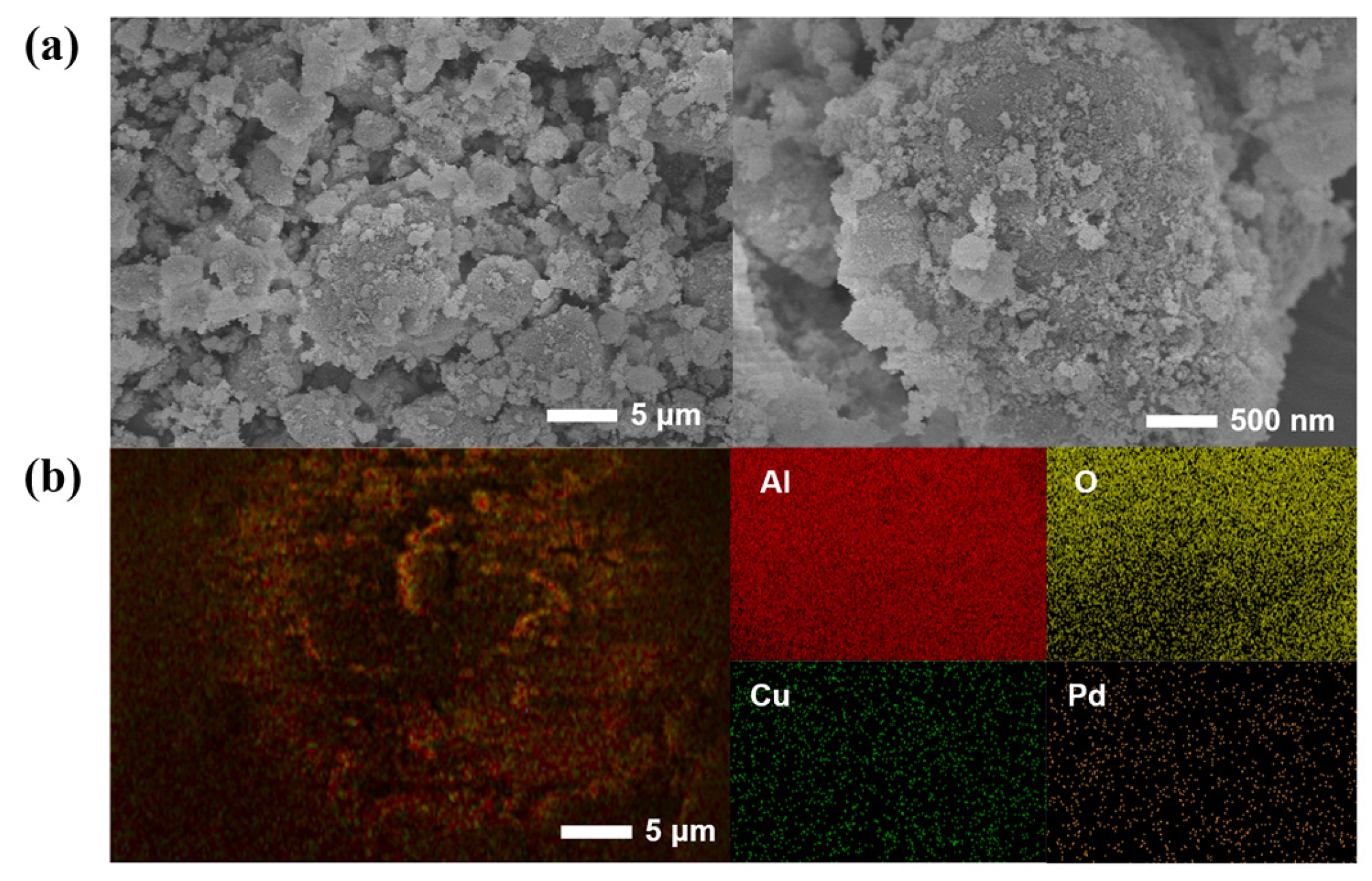
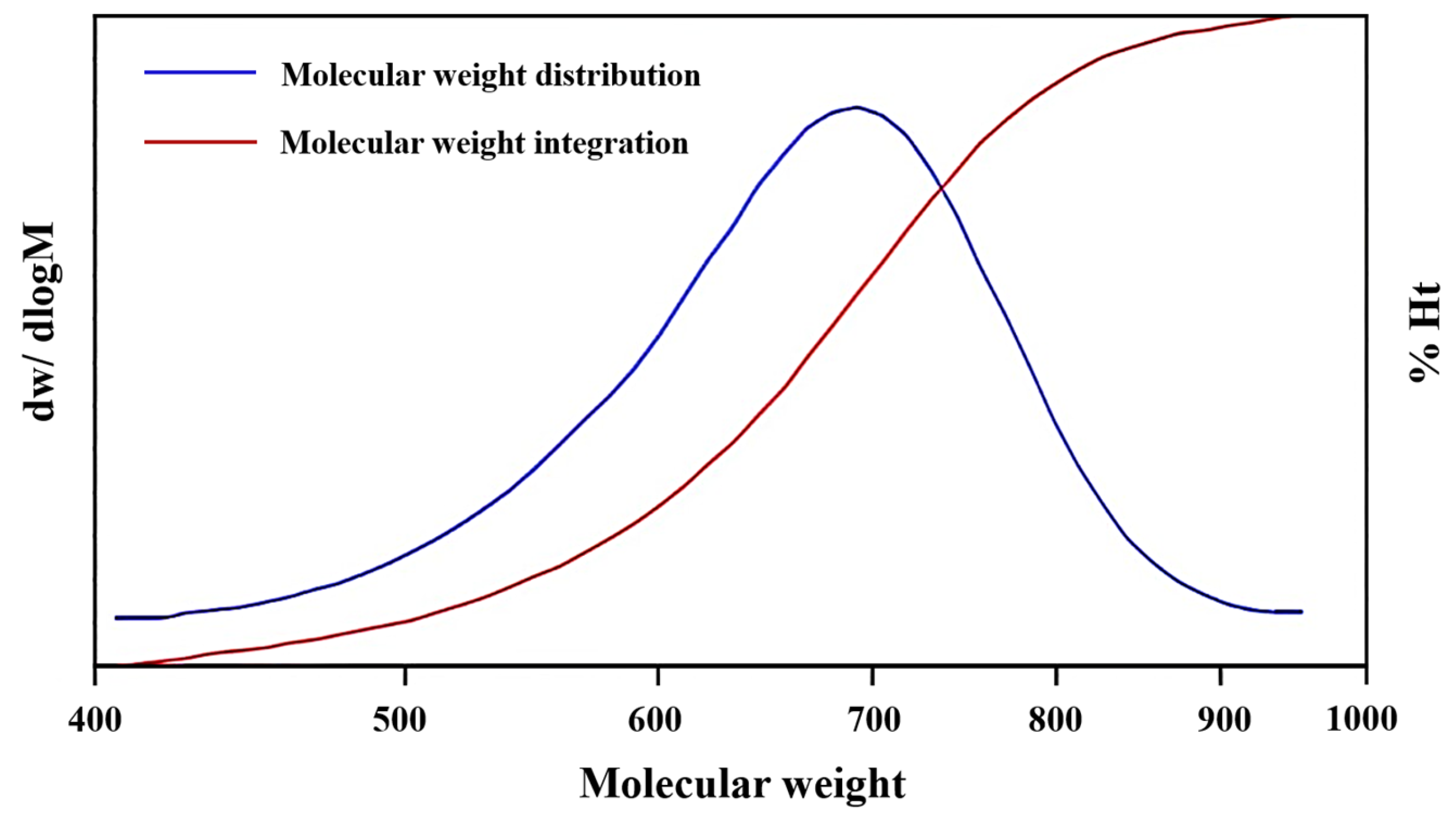
2.1. Characterization and Analysis of the Pd-Cu/γ-Al2O3 Catalysts
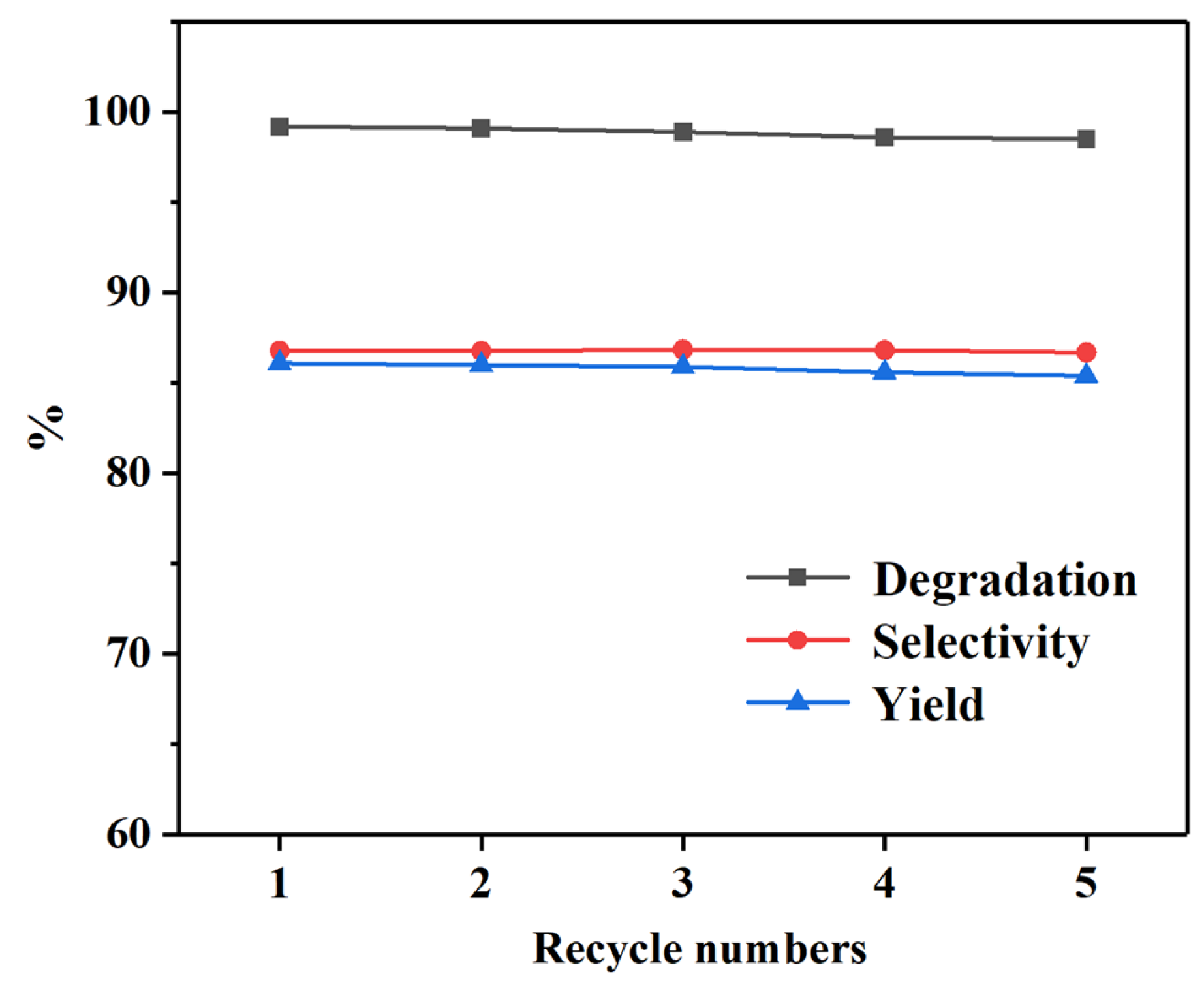
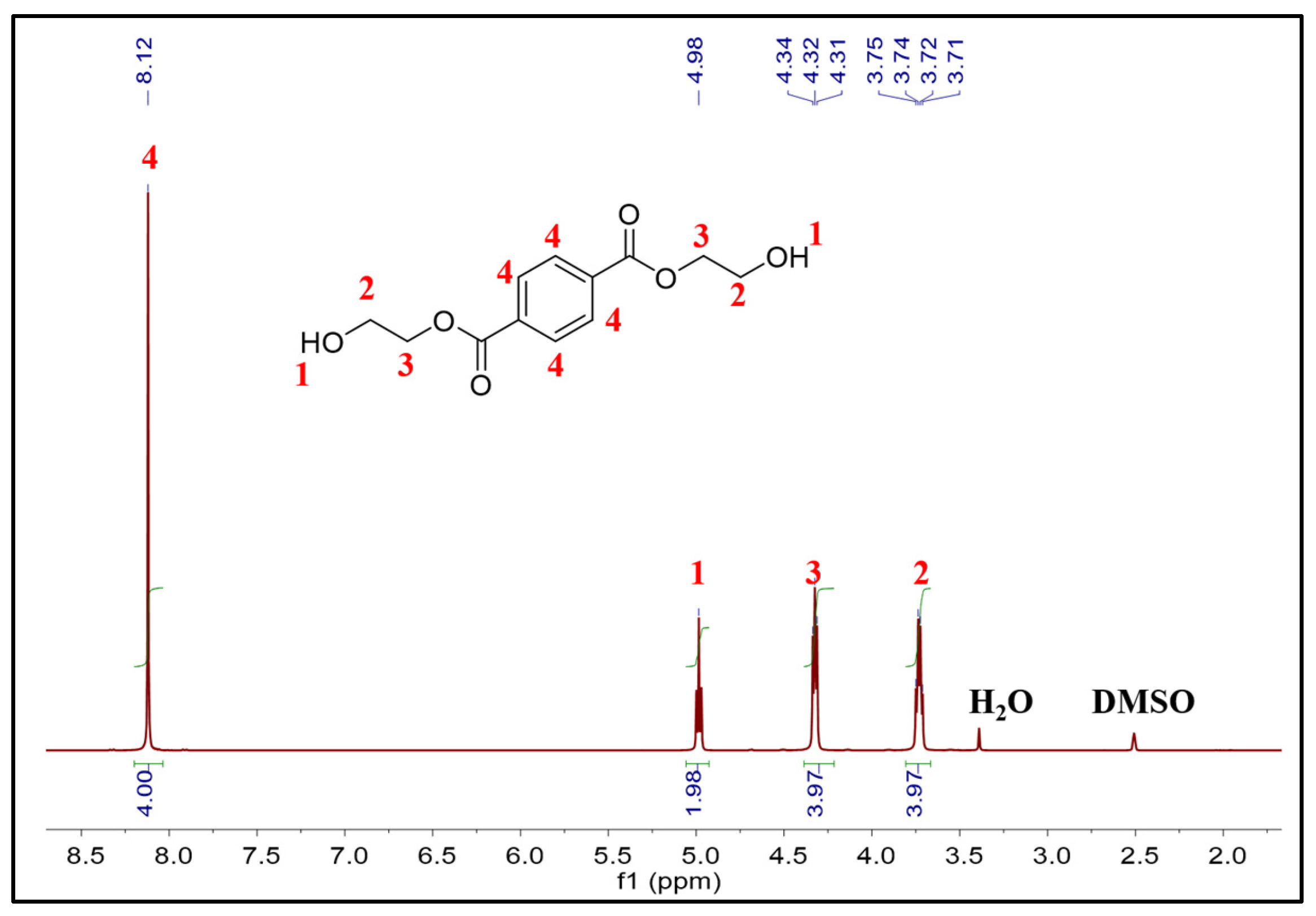
2.2. Catalytic Glycolysis of PET
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pd-Cu/γ-Al2O3 Catalysts
3.3. General Procedure for the PET Glycolysis
3.4. Characterization of Catalysts and Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abedsoltan, H. A focused review on recycling and hydrolysis techniques of polyethylene terephthalate. Polym. Eng. Sci. 2023, 63, 2651–2674. [Google Scholar] [CrossRef]
- Barnard, E.; Rubio Arias, J.J.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Islam, M.S.; Gupta, I.; Xia, L.; Pitchai, A.; Shannahan, J.; Mitra, S. Generation of Eroded Nanoplastics from Domestic Wastes and Their Impact on Macrophage Cell Viability and Gene Expression. Molecules 2024, 29, 2033. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Likozar, B.; Saha, B. Chemical Recycling Processes of Waste Polyethylene Terephthalate Using Solid Catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef]
- Guo, Z.; Adolfsson, E.; Tam, P.L. Nanostructured micro particles as a low-cost and sustainable catalyst in the recycling of PET fiber waste by the glycolysis method. Waste Manage. 2021, 126, 559–566. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, X.; Ju, Z.; Liu, B.; Yao, H.; Xu, J.; Zhou, Q.; Hu, Y.; Zhang, S. Alcoholysis of polyethylene terephthalate to produce dioctyl terephthalate using choline chloride-based deep eutectic solvents as efficient catalysts. Green Chem. 2019, 21, 897–906. [Google Scholar] [CrossRef]
- Ügdüler, S.; Van Geem, K.M.; Denolf, R.; Roosen, M.; Mys, N.; Ragaert, K.; De Meester, S. Towards closed-loop recycling of multilayer and coloured PET plastic waste by alkaline hydrolysis. Green Chem. 2020, 22, 5376–5394. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Du, R.; Zhou, X.; Liu, X.; Xu, S.; Wang, Y.-Z. Cosolvent-promoted selective non-aqueous hydrolysis of PET wastes and facile product separation. Green Chem. 2022, 24, 3284–3292. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Y.; Li, Z.; Xu, M.; Wang, Y.; Ge, R.; Kong, X.; Zheng, L.; Duan, H. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 2021, 12, 4679. [Google Scholar] [CrossRef]
- Syeda, Z.; Chen, H.-Y.; Leung, M.-K. Amino alcohols as recycling-catalysts for degradation of PET to oligoethylene terephthalates. Polym. Degrad. Stab. 2023, 214, 110387. [Google Scholar] [CrossRef]
- George, N.; Kurian, T. Sodium Carbonate Catalyzed Aminolytic Degradation of PET. Prog. Rubber Plast. Recycl. Technol. 2016, 32, 153–165. [Google Scholar] [CrossRef]
- Merkel, D.R.; Kuang, W.; Malhotra, D.; Petrossian, G.; Zhong, L.; Simmons, K.L.; Zhang, J.; Cosimbescu, L. Waste PET Chemical Processing to Terephthalic Amides and Their Effect on Asphalt Performance. ACS Sustain. Chem. Eng. 2020, 8, 5615–5625. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis Acid–Base Synergistic Catalysis for Polyethylene Terephthalate Degradation by 1,3-Dimethylurea/Zn(OAc)2 Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2018, 7, 3292–3300. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, Y.; Lu, X.; Zhang, S. First-Row Transition Metal-Containing Ionic Liquids as Highly Active Catalysts for the Glycolysis of Poly(ethylene terephthalate) (PET). ACS Sustain. Chem. Eng. 2015, 3, 340–348. [Google Scholar] [CrossRef]
- Yue, Q.; Xiao, L.; Zhang, M.; Bai, X. The Glycolysis of Poly(ethylene terephthalate) Waste: Lewis Acidic Ionic Liquids as High Efficient Catalysts. Polymers 2013, 5, 1258–1271. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Liao, Z.; Duan, Y.; Guo, L.; Zheng, R.; Wang, L.; Chen, Y.; Zhang, L.; Qian, X. Preparation of a heteropoly acid ionic liquid and its application in the catalytic degradation of bottle-grade PET. New J. Chem. 2023, 47, 4337–4345. [Google Scholar] [CrossRef]
- Martín, A.J.; Mondelli, C.; Jaydev, S.D.; Pérez-Ramírez, J. Catalytic processing of plastic waste on the rise. Chem 2021, 7, 1487–1533. [Google Scholar] [CrossRef]
- Muszyński, M.; Nowicki, J.; Zygadło, M.; Dudek, G. Comparsion of Catalyst Effectiveness in Different Chemical Depolymerization Methods of Poly(ethylene terephthalate). Molecules 2023, 28, 6385. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate)(PET). Green Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Lima, G.R.; Monteiro, W.F.; Ligabue, R.; Santana, R.M.C. Titanate Nanotubes as New Nanostrutured Catalyst for Depolymerization of PET by Glycolysis Reaction. Mater. Res. 2017, 20, 588–595. [Google Scholar] [CrossRef]
- Wi, R.; Imran, M.; Lee, K.G.; Yoon, S.H.; Cho, B.G.; Kim, D.H. Effect of support size on the catalytic activity of metal-oxide-doped silica particles in the glycolysis of polyethylene terephthalate. J. Nanosci. Nanotechnol. 2011, 11, 6544–6549. [Google Scholar] [CrossRef]
- Park, G.; Bartolome, L.; Lee, K.G.; Lee, S.J.; Kim, D.H.; Park, T.J. One-step sonochemical synthesis of a graphene oxide-manganese oxide nanocomposite for catalytic glycolysis of poly(ethylene terephthalate). Nanoscale 2012, 4, 3879–3885. [Google Scholar] [CrossRef]
- Stéphanie, L.; Benoît, H.; Alain, B.; André, R.; Jean-Paul, P. Determination of surface composition of alloy nanoparticles and relationships with catalytic activity in Pd–Cu/SiO2 cogelled xerogel catalysts. Appl. Catal. A 2004, 270, 201–208. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Wan, H.; Zheng, J.; Zheng, S. Aqueous bromate reduction by catalytic hydrogenation over Pd/Al2O3 catalysts. Appl. Catal. B 2010, 96, 307–313. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, H. In Situ Immobilizing Ni Nanoparticles to FDU-12 via Trehalose with Fine Size and Location Control for CO2 Methanation. ACS Sustain. Chem. Eng. 2020, 8, 2093–2105. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Evans, D.G.; Duan, X.; Li, D. Catalytic hydrogenation of ethylanthraquinone over highly dispersed eggshell Pd/SiO2–Al2O3 spherical catalysts. Appl. Catal. A 2010, 382, 240–245. [Google Scholar] [CrossRef]
- Yang, Y.; Rioux, R.M. Highly stereoselective anti-Markovnikov hydrothiolation of alkynes and electron-deficient alkenes by a supported Cu-NHC complex. Green Chem. 2014, 16, 3916–3925. [Google Scholar] [CrossRef]
- Somayeh, M.; Bouldo, M.G.; Mojtaba, E. Controlled Glycolysis of Poly(ethylene terephthalate) to Oligomers under Microwave Irradiation Using Antimony(III) Oxide. ACS Appl. Polym. Mater. 2023, 5, 6574–6584. [Google Scholar] [CrossRef]

| Entry | Cat 1 | Cat 2 | Cat 3 | Cat 4 | Cat 5 |
|---|---|---|---|---|---|
| Theoretical ratio | 1:1 | 1:1.5 | 1:2 | 1:2.5 | 1:3 |
| Actual value (%) | 1.85:1.98 | 1.81:2.96 | 1.88:3.95 | 1.88:4.96 | 1.87:5.94 |
| PET degradation rate | 36% | 49% | 57% | 62% | 63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Qin, E.; Huang, H.; Wang, Y.; Li, M. PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3. Molecules 2024, 29, 4305. https://doi.org/10.3390/molecules29184305
Zhou L, Qin E, Huang H, Wang Y, Li M. PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3. Molecules. 2024; 29(18):4305. https://doi.org/10.3390/molecules29184305
Chicago/Turabian StyleZhou, Lei, Enbo Qin, Hao Huang, Yuanyou Wang, and Mingxin Li. 2024. "PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3" Molecules 29, no. 18: 4305. https://doi.org/10.3390/molecules29184305
APA StyleZhou, L., Qin, E., Huang, H., Wang, Y., & Li, M. (2024). PET Glycolysis to BHET Efficiently Catalyzed by Stable and Recyclable Pd-Cu/γ-Al2O3. Molecules, 29(18), 4305. https://doi.org/10.3390/molecules29184305







