Impact of Surface Pretreatment on the Corrosion Resistance and Adhesion of Thin Film Coating on SS316L Bipolar Plates for Proton-Exchange Membrane Fuel Cell Applications
Abstract
:1. Introduction
2. Material and Method
3. Results and Discussion
4. Conclusions
- (i)
- The pretreatment of SS316L substrate is necessary to achieve the desired surface conditions before coating, including surface roughness, hydrophobicity, and passive layer removal, which enable good adhesion of the subsequent coating.
- (ii)
- Etching SS316L with a V2A etchant solution of HNO3, HCl, and DI water in a 1:9:23 mole ratio for 7 min at room temperature (S1−7 min) revealed superior performance with optimal surface roughness, and a significantly increased hydrophobicity and the highest adhesion rating in comparison with others.
- (iii)
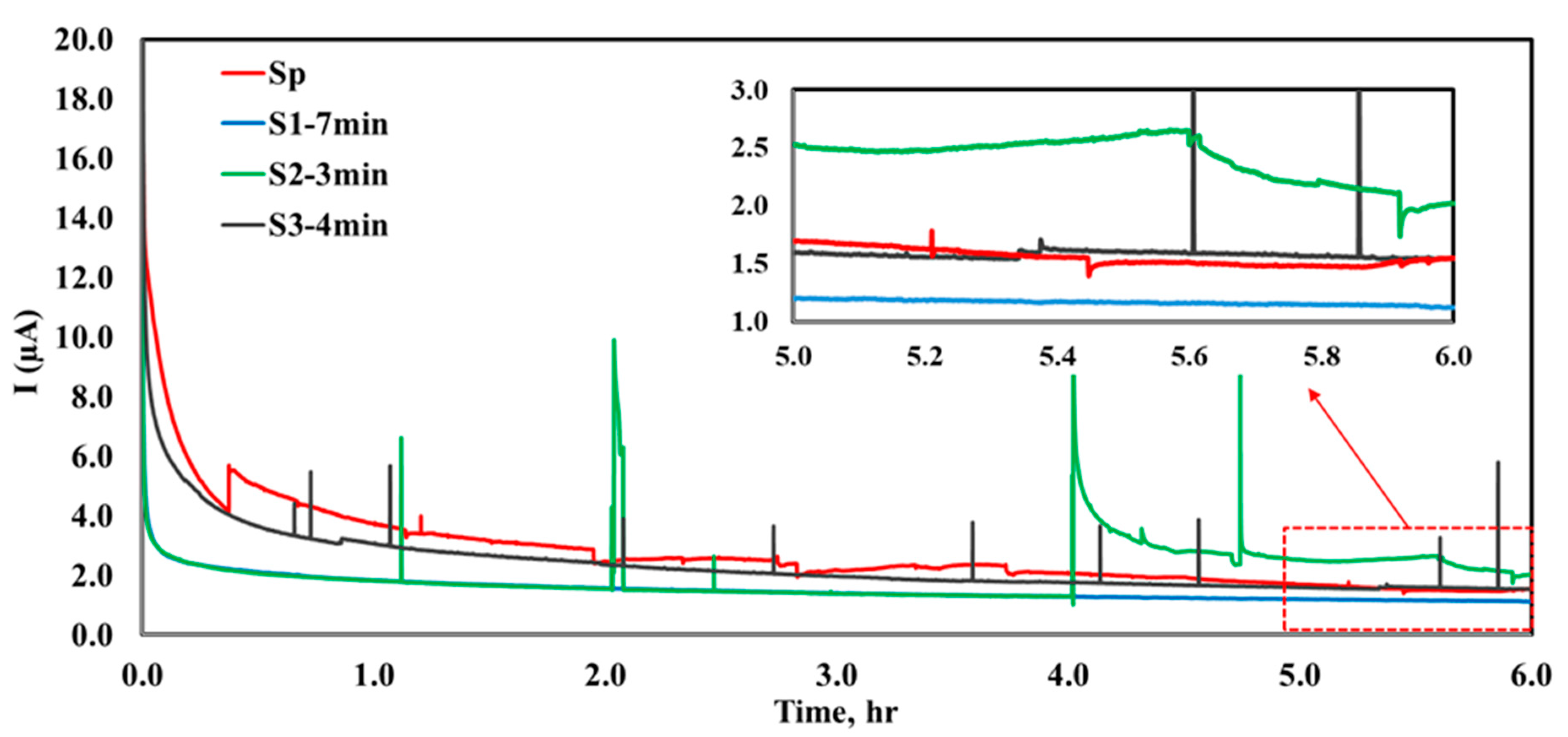
- Among all the Nb-coated samples, the S1−7 min pretreated sample exhibited the highest corrosion resistance, as indicated by the lowest corrosion current density Icorr (3.3 µA.cm−2) and the most positive corrosion potential Ecorr (−0.58 V vs. Hg2SO4 reference electrode). Additionally, it shows minimal fluctuation and the best stabilization after 6 h of potentiostatic polarization. The polished and uncoated SS316L sample showed high Ecorr (−0.63 V) and Icorr (10.6 µA.cm−2).
- (iv)
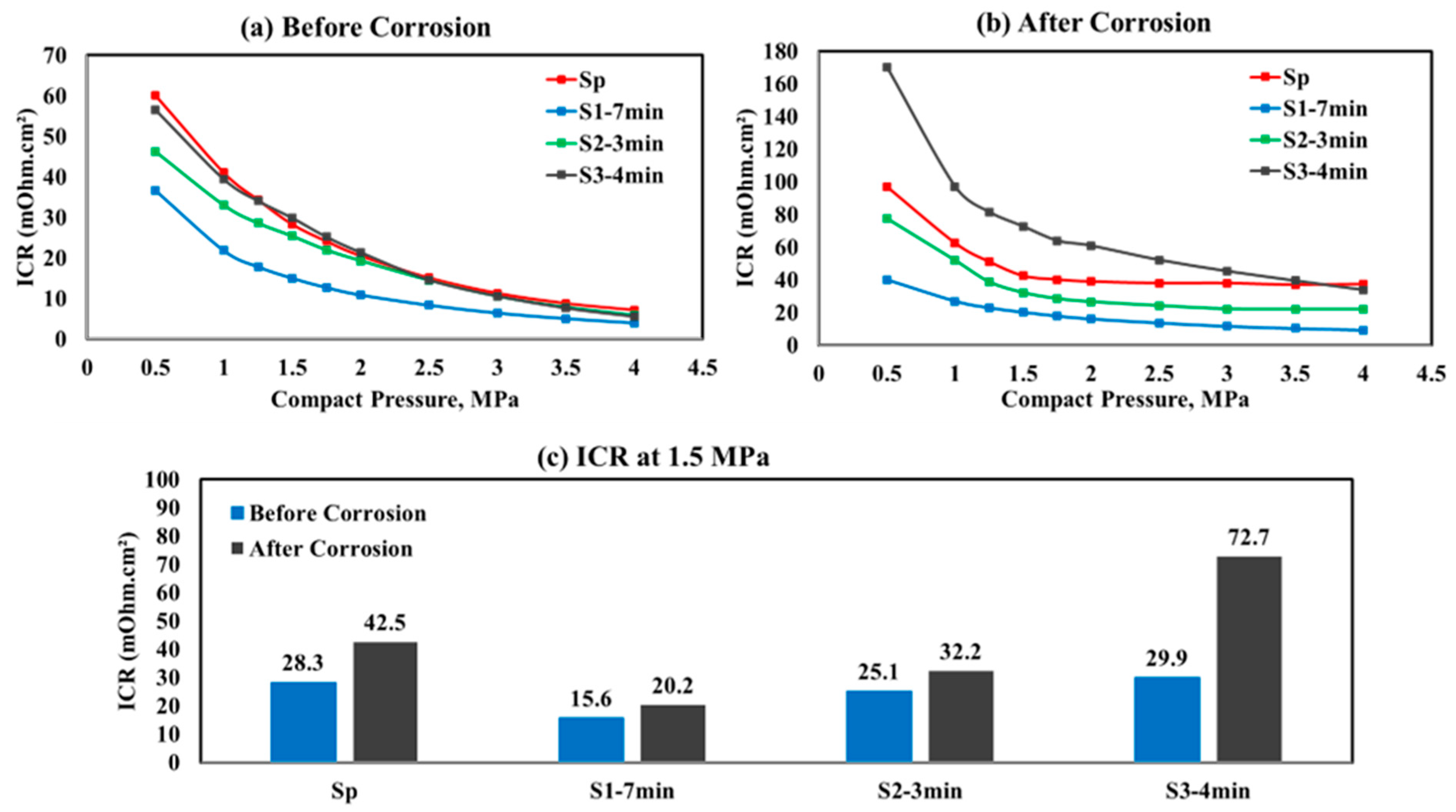
- The corrosion analysis findings align with the ICR values, where the S1−7 min pretreated sample exhibited the lowest measurements both before and after polarization (15.6 and 20.2 mΩ.cm2 at 1.5 MPa compression pressure, respectively), thereby enhancing electrical conductivity and improving electron transfer efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Liu, L.; Wang, Y.; Li, H.; Li, Z. Evaluation of vacuum heat-treated α-C films for surface protection of metal bipolar plates used in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 22983–22997. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Dong, G.; Zhang, M.; Jiao, K.; Leung, D.Y.C. Flexible fuel cells: A prospective review. Energy Rev. 2024, 3, 100099. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Ozden, A.; Li, X.; Hamdullahpur, F. A scaled-up proton exchange membrane fuel cell with enhanced performance and durability. Appl. Energy 2020, 268, 114956. [Google Scholar] [CrossRef]
- Tang, W.; Wang, S.; Li, S.; Yu, S.; Yuan, X.; Yang, C.; Li, W. Research progress on coating and coating technology of fuel cell metallic bipolar plate. Next Mater. 2024, 4, 100062. [Google Scholar] [CrossRef]
- Madhavan, P.V.; Shahgaldi, S.; Li, X. Modelling Anti-Corrosion Coating Performance of Metallic Bipolar Plates for PEM Fuel Cells: A Machine Learning Approach. Energy AI 2024, 17, 100391. [Google Scholar] [CrossRef]
- Alaefour, I.; Shahgaldi, S.; Ozden, A.; Li, X.; Hamdullahpur, F. The role of flow-field layout on the conditioning of a proton exchange membrane fuel cell. Fuel 2018, 230, 98–103. [Google Scholar] [CrossRef]
- Avram, D.N.; Davidescu, C.M.; Dan, M.L.; Mirza-Rosca, J.C.; Hulka, I.; Stanciu, E.M.; Pascu, A. Corrosion resistance of NiCr(Ti) coatings for metallic bipolar plates. Mater. Today Proc. 2023, 72, 538–543. [Google Scholar] [CrossRef]
- Kwon, L.; Kang, J.G.; Baik, K.D.; Kim, K.; Ahn, C. Advancement and applications of PEMFC energy systems for large-class unmanned underwater vehicles: A review. Int. J. Hydrogen Energy 2024, 79, 277–294. [Google Scholar] [CrossRef]
- Mehdizadeh Chellehbari, Y.; Adavi, K.; Sayyad Amin, J.; Zendehboudi, S. A numerical simulation to effectively assess impacts of flow channels characteristics on solid oxide fuel cell performance. Energy Convers. Manag. 2021, 244, 114280. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Xu, R.; Zhen, Z.; Zeng, X.; Chen, X.; Cui, L. Research progress and prospect of the materials of bipolar plates for proton exchange membrane fuel cells (PEMFCs). Int. J. Hydrogen Energy 2024, 50, 711–743. [Google Scholar] [CrossRef]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 80, 100859. [Google Scholar] [CrossRef]
- Alaefour, I.; Shahgaldi, S.; Zhao, J.; Li, X. Synthesis and Ex-Situ characterizations of diamond-like carbon coatings for metallic bipolar plates in PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 11059–11070. [Google Scholar] [CrossRef]
- Varsan Madhavan, P.; Shahgaldi, S.; Li, X. Ex-situ Characterization of Nb-Ti Alloy/Pt Coated Stainless Steel Bipolar Plates for Proton Exchange Membrane Fuel Cells. Energy Convers. Manag. 2024, 311, 118536. [Google Scholar] [CrossRef]
- Zhong, J.; Hou, B.; Zhang, W.; Zhang, S.; Zhao, Y.; Zhao, C. Corrosion Resistance of Plasma-Sprayed FeCrMoSi Amorphous. Molecules 2023, 28, 6718. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Sun, Y.; Shen, X.; Zhu, Y. The Microstructure and Properties of Nitrided Chromium Coating on 316 Stainless Steels Fabricated by Electrodeposition and Electrolytic Nitriding for Pemfc Bipolar Plate. Int. J. Electrochem. Sci. 2021, 16, 210742. [Google Scholar] [CrossRef]
- Choi, J.H.; Eun Kang, H.; Kim, D.J.; Soo Yoon, Y. A Comprehensive Review of Stainless-Steel Bipolar Plate Coatings and Their Role in Mitigating Corrosion in Aggressive Proton-Exchange Membrane Fuel Cells Environments. Chem. Eng. J. 2024, 493, 152662. [Google Scholar] [CrossRef]
- Kang, H.E.; Kim, S.H.; Choi, J.H.; Kim, D.J.; Yoon, Y.S. Selective etching-induced surface modifications of FeCrAl alloy bipolar plates: Mechanisms for enhanced corrosion resistance and hydrophobicity. Chem. Eng. J. 2024, 493, 152409. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Facile fabrication of superhydrophobic surfaces from austenitic stainless steel (AISI 304) by chemical etching. Appl. Surf. Sci. 2018, 439, 598–604. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Chauhan, P.; Yadav, K.; Kumar, A. Effect on the corrosion resistance property of aluminum substrate by altering the wetting behavior. J. Coat. Technol. Res. 2023, 20, 1639–1648. [Google Scholar] [CrossRef]
- Vlcak, P.; Fojt, J.; Drahokoupil, J.; Brezina, V.; Sepitka, J.; Horazdovsky, T.; Miksovsky, J.; Cerny, F.; Lebeda, M.; Haubner, M. Influence of surface pre-treatment with mechanical polishing, chemical, electrochemical and ion sputter etching on the surface properties, corrosion resistance and MG-63 cell colonization of commercially pure titanium. Mater. Sci. Eng. C 2020, 115, 111065. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.T.; Oh, K.; Singh, P.M.; Breedveld, V.; Hess, D.W. Wettability control of stainless steel surfaces via evolution of intrinsic grain structures. J. Mater. Sci. 2016, 51, 5196–5206. [Google Scholar] [CrossRef]
- Pillis, M.F.; de Oliveira, M.C.L.; Antunes, R.A. Surface chemistry and the corrosion behavior of magnetron sputtered niobium oxide films in sulfuric acid solution. Appl. Surf. Sci. 2018, 462, 344–352. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Z.Q.; Lu, H.B.; Ke, H.B.; Yuan, Y.; Dong, Y.M.; Meng, X.K. Corrosion protection of NiNb metallic glass coatings for 316SS by magnetron sputtering. J. Mater. Sci. Technol. 2021, 79, 88–98. [Google Scholar] [CrossRef]
- Cho, M.G.; Kang, U.; Lim, S.H.; Han, S. a-Phase Tantalum Film Deposition Using Bipolar High-Power Impulse Magnetron Sputtering Technique. Thin Solid. Film. 2023, 767, 139668. [Google Scholar] [CrossRef]
- Xu, X.; Gou, Y.; Zhan, X.; Xie, F.; Zhang, K.; Sun, S.; Shao, Z. Investigation of the effect of the TaC/α-C coating process on the properties of stainless steel bipolar plates. Int. J. Hydrogen Energy 2024, 55, 98–109. [Google Scholar] [CrossRef]
- Li, L.; Ye, D.; Xiang, Y.; Guo, W. Effect of deposition temperature on columnar structure of α-C nano-coatings of PEMFC metal bipolar plates. Int. J. Electrochem. Sci. 2023, 18, 100188. [Google Scholar] [CrossRef]
- Chen, M.; Ding, J.C.; Kwon, S.H.; Wang, Q.; Zhang, S. Corrosion resistance and conductivity of NbN-coated 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Corros. Sci. 2022, 196, 110042. [Google Scholar] [CrossRef]
- Jeżowski, P.; Nowicki, M.; Grzeszkowiak, M.; Czajka, R.; Béguin, F. Chemical etching of stainless steel 301 for improving performance of electrochemical capacitors in aqueous electrolyte. J. Power Sources 2015, 279, 555–562. [Google Scholar] [CrossRef]
- Li, K.; Zeng, X.; Li, H.; Lai, X. A study on the fabrication of superhydrophobic iron surfaces by chemical etching and galvanic replacement methods and their anti-icing properties. Appl. Surf. Sci. 2015, 346, 458–463. [Google Scholar] [CrossRef]
- Gümpel, P.; Hörtnagl, A. Influence of the surface condition on corrosion behavior of stainless steel. Mater. Corros. 2016, 67, 607–620. [Google Scholar] [CrossRef]
- Vander Voort, G.F.; Lucas, G.M.; Manilova, E.P. Metallography and Microstructures of Stainless Steels and Maraging Steels. Metallogr. Microstruct. 2018, 9, 670–700. [Google Scholar] [CrossRef]
- Shi, K.; Li, X.; Zhao, Y.; Li, W.-W.; Wang, S.-B.; Xie, X.-F.; Yao, L.; Jensen, J.O.; Li, Q.-F. Corrosion behavior and conductivity of TiNb and TiNbN Coated Steel For Metallic Bipolar Plates. Appl. Sci. 2019, 9, 2568. [Google Scholar] [CrossRef]
- Zipperian, D.C. Metallographic Handbook; PACE Technologies: Tucson, AZ, USA, 2011. [Google Scholar]
- Kim, Y.S.; Lee, I.S.; Choi, J.Y.; Jun, S.; Kim, D.; Cha, B.C.; Kim, D.W. Corrosion behavior of niobium-coated 316L stainless steels as metal bipolar plates for polymer electrolyte membrane fuel cells. Materials 2021, 14, 4972. [Google Scholar] [CrossRef]
- ASTM D3359-23; Standard Test Methods for Measuring Adhesion by Tape Test 1. ASTM International: West Conshohocken, PA, USA, 2023. [CrossRef]
- Okolo, B.; Lamparter, P.; Welzel, U.; Mittemeijer, E.J. Stress, texture, and microstructure in niobium thin films sputter deposited onto amorphous substrates. J. Appl. Phys. 2004, 95, 466–476. [Google Scholar] [CrossRef]
- Jin, J.; He, Z.; Zhao, X. Effect of Al content on the corrosion resistance and conductivity of metal nitride coating in the cathode environment of PEMFCs. Mater. Chem. Phys. 2020, 245, 122739. [Google Scholar] [CrossRef]
- Leng, Y.; Yang, D.; Ming, P.; Zhang, C. A comparative study of corrosion resistance evaluation of bipolar plate materials for proton exchange membrane fuel cell. eTransportation 2021, 10, 100139. [Google Scholar] [CrossRef]
- Ijaodola, O.; Ogungbemi, E.; Khatib, F.N.; Wilberforce, T.; Ramadan, M.; El Hassan, Z.; Thompson, J.; Olabi, A.G. Evaluating the effect of metal bipolar plate coating on the performance of proton exchange membrane fuel cells. Energies 2018, 11, 3203. [Google Scholar] [CrossRef]
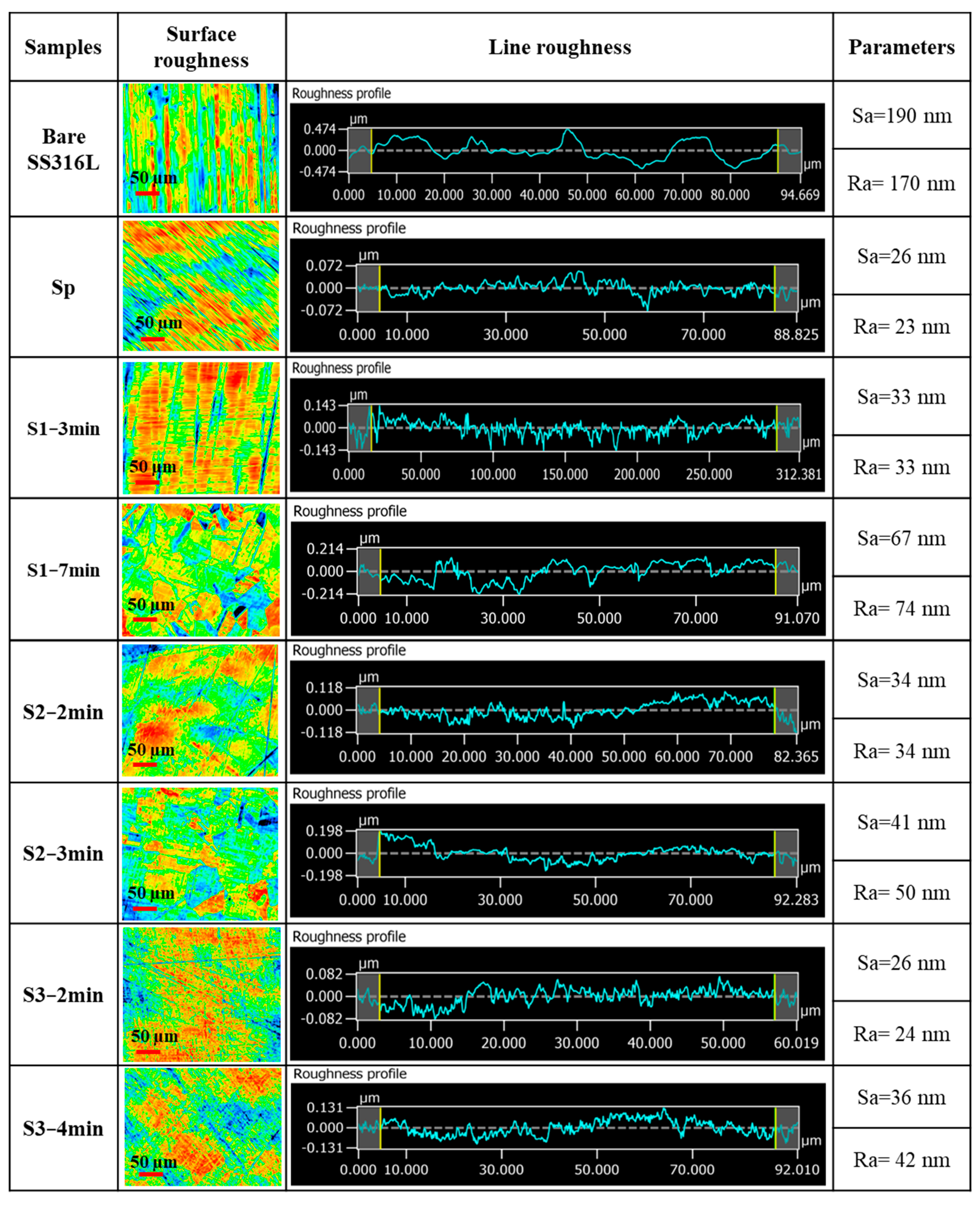
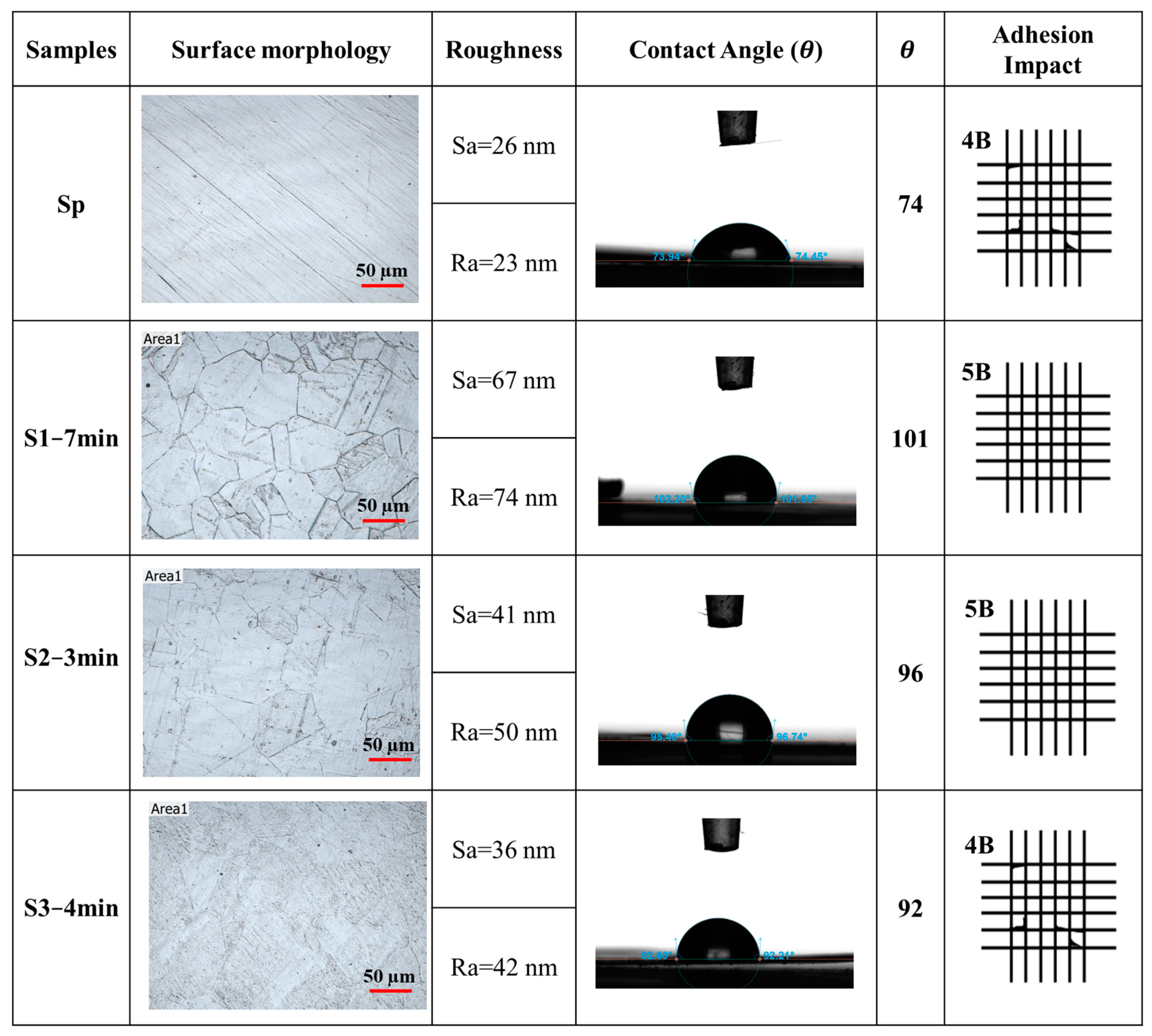
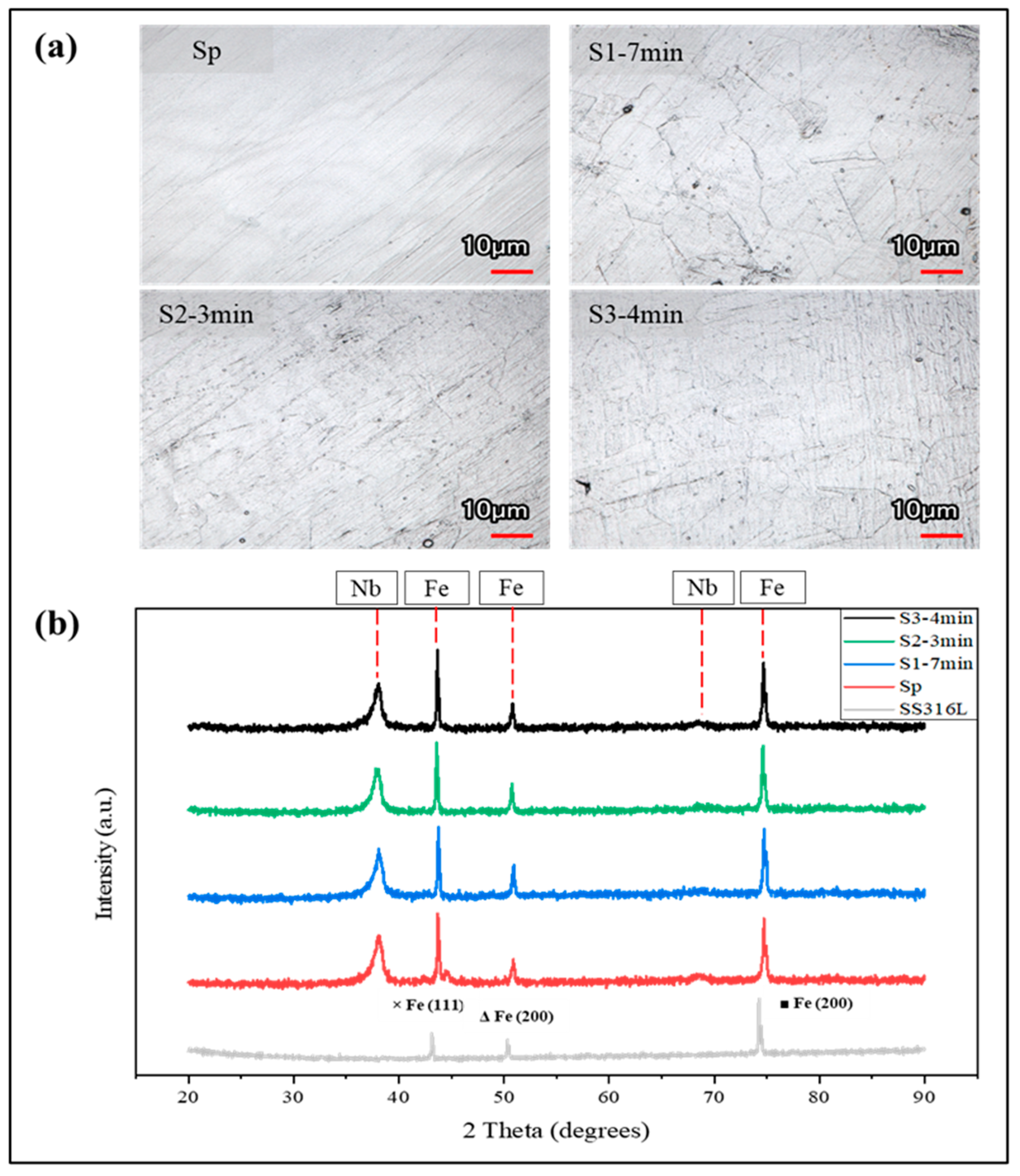
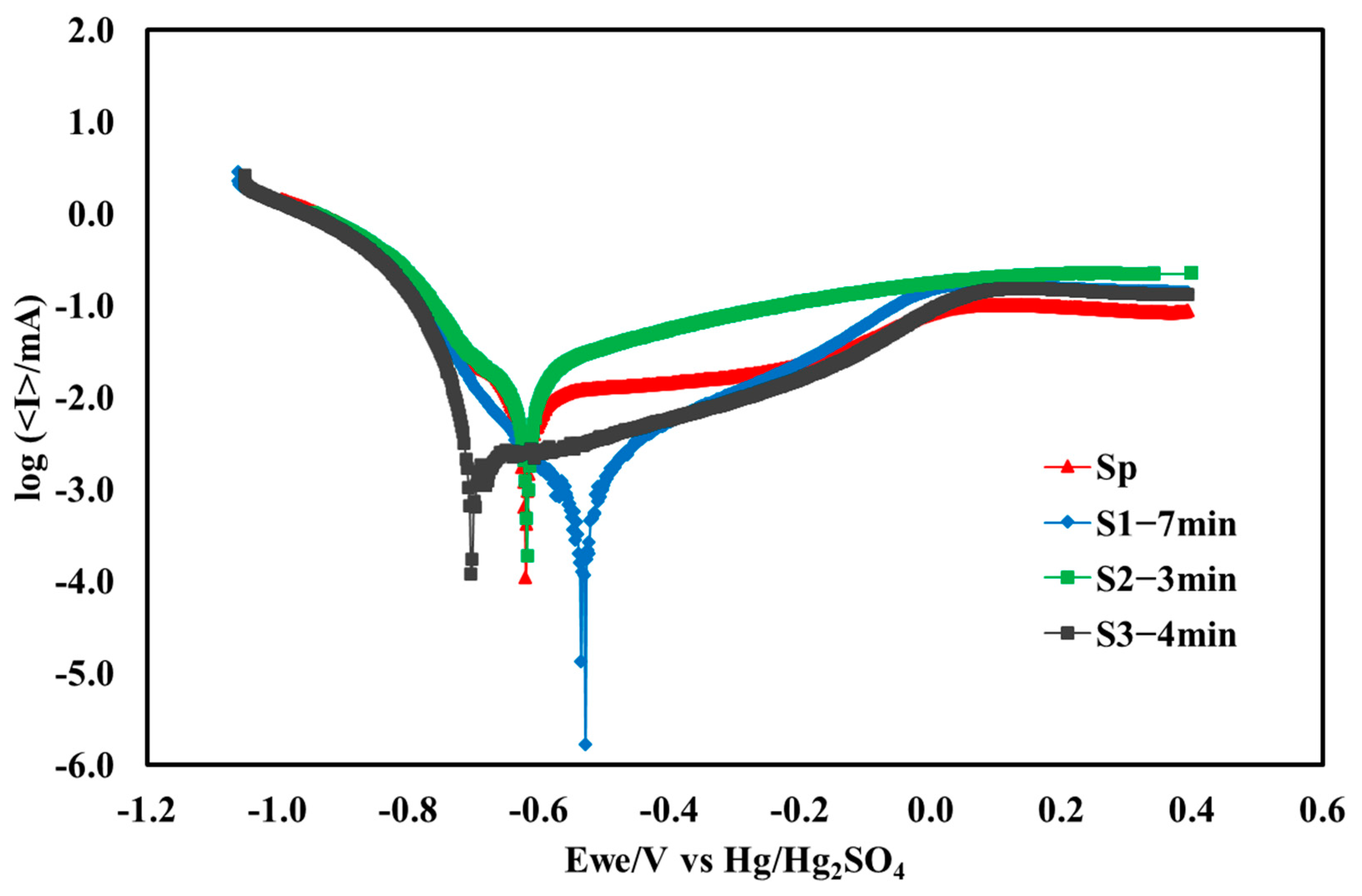
| Samples | Procedure | Immersion Time | Name |
|---|---|---|---|
| Polished SS316L (Sp) | SiC abrasive paper with grit sizes ranging from 400 to 4000 | - | Sp |
| Etchant 1 (S1) [32,34] | HCl 119 mL, HNO3 12 mL, Di Water 119 mL (1:9:23) mole ratio | 3 min | S1−3 min |
| 7 min | S1−7 min | ||
| Etchant 2 (S2) [32,34] | Ferric Chloride 45 gr, Copper Ammonium Chloride 9 gr, HCl 150 mL, Di Water 75 mL. | 2 min | S2−2 min |
| 3 min | S2−3 min | ||
| Etchant 3 (S3) [32,34] | Ferric Chloride 8.5 gr, Cupric Chloride 2.4 gr, Alcohol 122 mL, HCl 122 mL, HNO3 6 mL. | 2 min | S3−2 min |
| 4 min | S3−4 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdizadeh Chellehbari, Y.; Gupta, A.; Li, X.; Shahgaldi, S. Impact of Surface Pretreatment on the Corrosion Resistance and Adhesion of Thin Film Coating on SS316L Bipolar Plates for Proton-Exchange Membrane Fuel Cell Applications. Molecules 2024, 29, 4319. https://doi.org/10.3390/molecules29184319
Mehdizadeh Chellehbari Y, Gupta A, Li X, Shahgaldi S. Impact of Surface Pretreatment on the Corrosion Resistance and Adhesion of Thin Film Coating on SS316L Bipolar Plates for Proton-Exchange Membrane Fuel Cell Applications. Molecules. 2024; 29(18):4319. https://doi.org/10.3390/molecules29184319
Chicago/Turabian StyleMehdizadeh Chellehbari, Yasin, Abhay Gupta, Xianguo Li, and Samaneh Shahgaldi. 2024. "Impact of Surface Pretreatment on the Corrosion Resistance and Adhesion of Thin Film Coating on SS316L Bipolar Plates for Proton-Exchange Membrane Fuel Cell Applications" Molecules 29, no. 18: 4319. https://doi.org/10.3390/molecules29184319
APA StyleMehdizadeh Chellehbari, Y., Gupta, A., Li, X., & Shahgaldi, S. (2024). Impact of Surface Pretreatment on the Corrosion Resistance and Adhesion of Thin Film Coating on SS316L Bipolar Plates for Proton-Exchange Membrane Fuel Cell Applications. Molecules, 29(18), 4319. https://doi.org/10.3390/molecules29184319







