An Acid-Responsive Fluorescent Molecule for Erasable Anti-Counterfeiting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Elucidation of TPEPhDAT
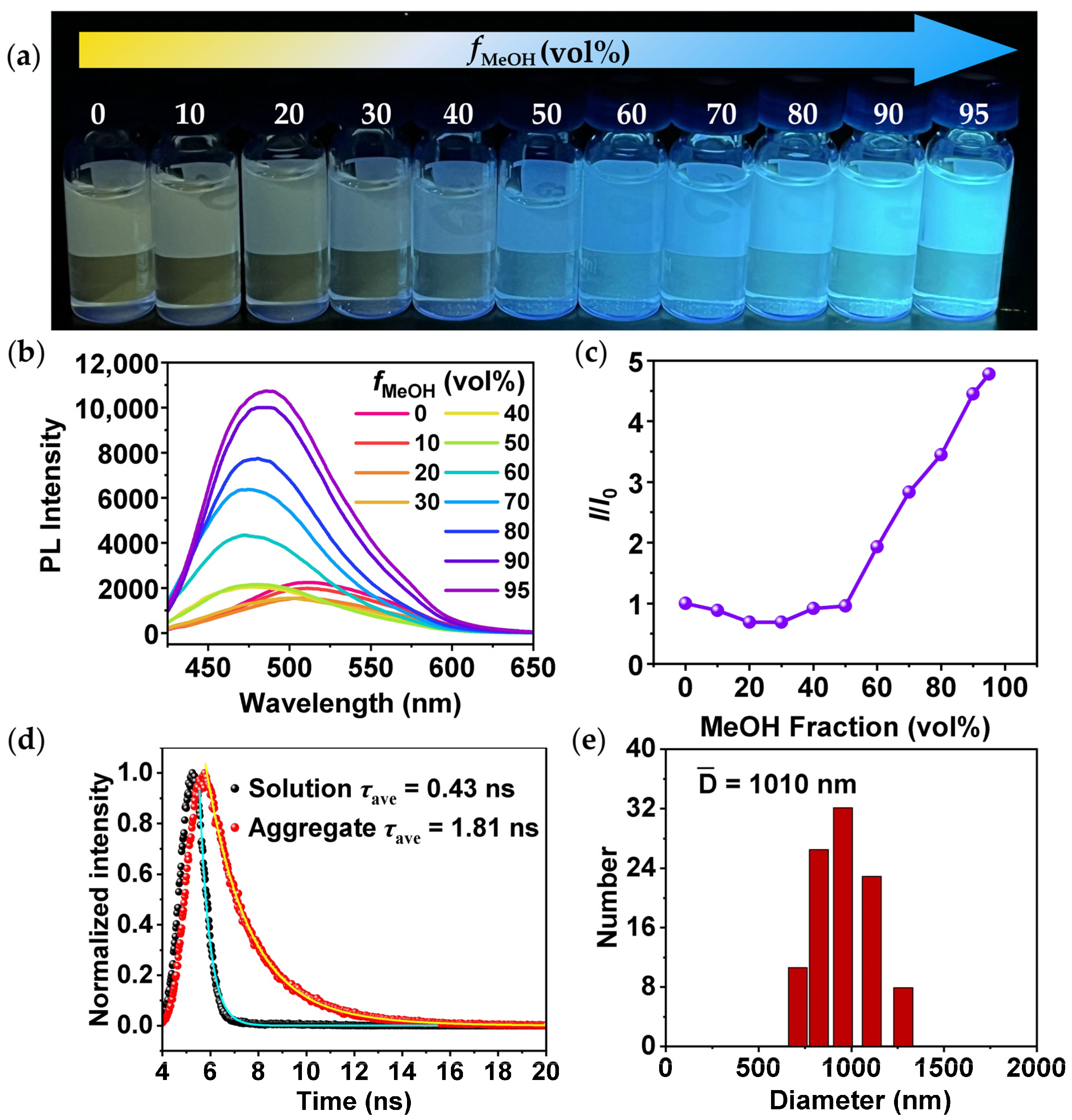
2.2. AIEE Characteristic of TPEPhDAT
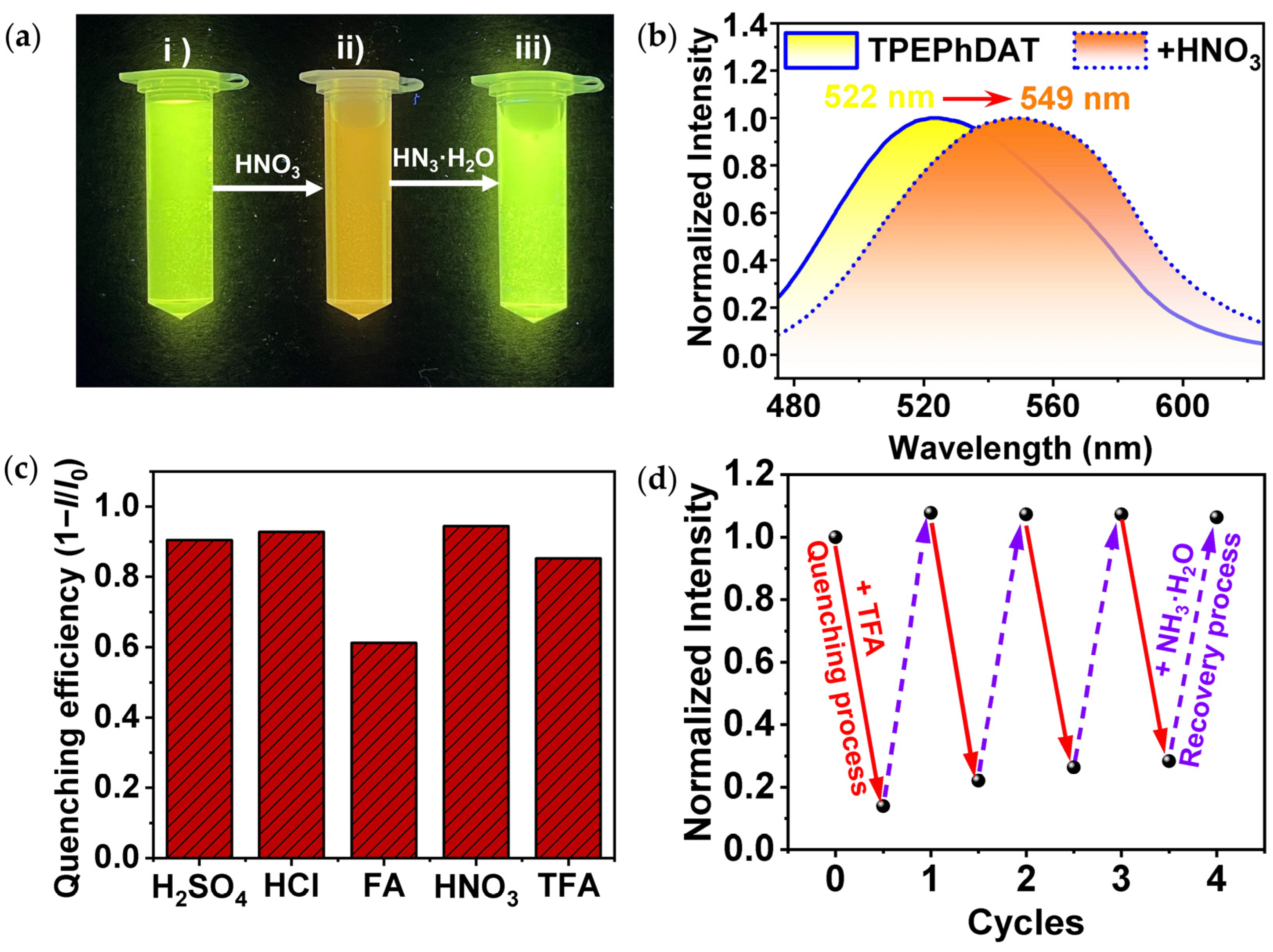
2.3. Acidochromism
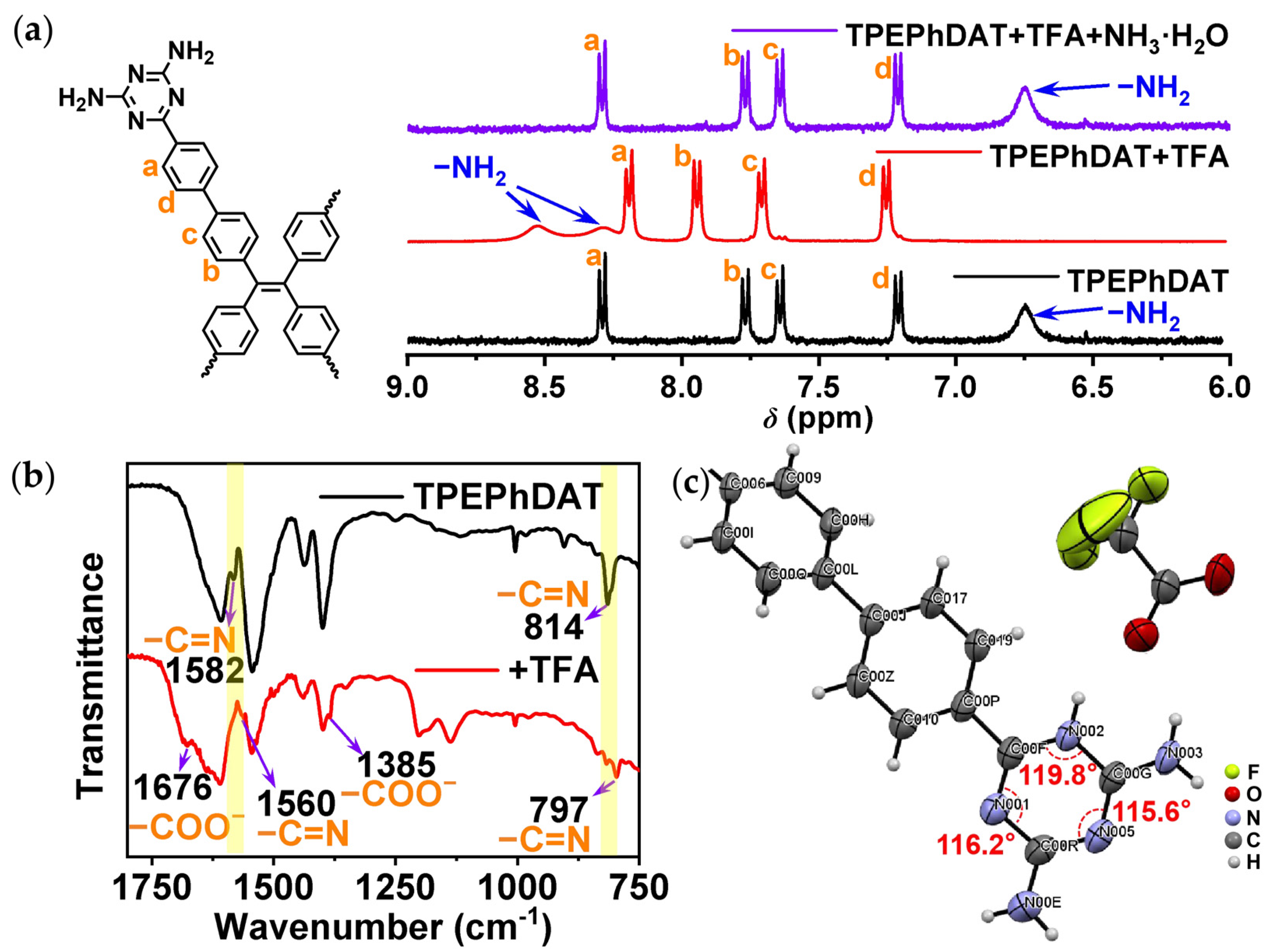
2.4. Mechanism of TPEPhDAT Binding to Acid
2.5. Possible Mechanism of Acid-Induced Fluorescence Quenching in TPEPhDAT
2.6. Erasable Anti-Counterfeiting Applications
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis of Tetrakis [4-(4′-Cyanophenyl)phenyl]ethene (TPEPhCN)
3.1.2. Synthesis of 6,6′,6″,6‴-(Ethene-1,1,2,2-tetrayltetrakis([1,1′-biphenyl]-4′,4-diyl))tetrakis(1,3,5-triazine-2,4-diamine) (TPEPhDAT)
3.1.3. Synthesis of TPEPhDAT-TFA Single Crystalline
3.1.4. Testing of Fluorescence Spectra of Acid-Induced Fluorescence Quenching of TPEPhDAT in Aqueous Solution
3.1.5. The Fabrication of Anti-Counterfeiting Inks
3.1.6. Density Functional Theory (DFT) Calculations
3.1.7. Introducing and Discussing the Double-Exponential Fitting Function
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.; Dai, J.; Sun, J.; Liu, Y.; Ma, X.; Liu, Z.; Ma, T.; Tan, J.; Yao, Z.; Wang, S.; et al. Fluorescence-based monitoring of the pressure-induced aggregation microenvironment evolution for an AIEgen under multiple excitation channels. Nat. Commun. 2022, 13, 5234. [Google Scholar] [CrossRef]
- Chen, C.X.; Yin, S.Y.; Wei, Z.W.; Qiu, Q.F.; Zhu, N.X.; Fan, Y.N.; Pan, M.; Su, C.Y. Pressure-Induced Multiphoton Excited Fluorochromic Metal-Organic Frameworks for Improving MPEF Properties. Angew. Chem. Int. Ed. 2019, 58, 14379–14385. [Google Scholar] [CrossRef]
- Nagasawa, H.; Kagawa, T.; Noborio, T.; Kanezashi, M.; Ogata, A.; Tsuru, T. Ultrafast Synthesis of Silica-Based Molecular Sieve Membranes in Dielectric Barrier Discharge at Low Temperature and Atmospheric Pressure. J. Am. Chem. Soc. 2020, 143, 35–40. [Google Scholar] [CrossRef]
- Hu, D.; Xu, W.; Wang, G.; Liu, K.; Wang, Z.; Shi, Q.; Lin, S.; Liu, Z.; Fang, Y. A Mild-Stimuli-Responsive Fluorescent Molecular System Enables Multilevel Anti-Counterfeiting and Highly Adaptable Temperature Monitoring. Adv. Funct. Mater. 2022, 32, 2207895. [Google Scholar] [CrossRef]
- Shen, F.; Yang, W.; Cui, J.; Hou, Y.; Bai, G. Small-Molecule Fluorogenic Probe for the Detection of Mitochondrial Temperature In Vivo. Anal. Chem. 2021, 93, 13417–13420. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Han, Y.; Wang, Z.; Li, H.; Sun, S.; Xu, Y. A pH-sensitive ESIPT molecule with aggregation-induced emission and tunable solid-state fluorescence multicolor for anti-counterfeiting and food freshness detection. Chem. Eng. J. 2022, 428, 130986. [Google Scholar] [CrossRef]
- Hisaki, I.; Suzuki, Y.; Gomez, E.; Ji, Q.; Tohnai, N.; Nakamura, T.; Douhal, A. Acid Responsive Hydrogen-Bonded Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 2111–2121. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Wang, G.; Li, B.; Chen, H.; Li, H.; Zhao, Y. Photoresponsive supramolecular coordination polyelectrolyte as smart anticounterfeiting inks. Nat. Commun. 2021, 12, 1363. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Wang, H.-P.; Zhang, L.-Y.; Wei, S.-C.; Wei, Z.-W.; Pan, M.; Su, C.-Y. Coordinative-to-covalent transformation, isomerization dynamics, and logic gate application of dithienylethene based photochromic cages. Chem. Sci. 2020, 11, 8885–8894. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, J.; He, X.; Tao, W.; Shi, Y.; Xu, J.; Xu, L.; Tang, M.; Shen, D.; Bi, H.; et al. Tuned Intra- and Intermolecular Photoreactions of Tridentate Cyanostilbenes with Distinct Aggregated-State Photomechanical and Dispersed-State Photochromic Behaviors. Adv. Funct. Mater. 2023, 33, 2212886. [Google Scholar] [CrossRef]
- Hu, Z.B.; Li, L.H.; Han, Y.; Zhang, J.; Li, J.; Chen, Z.; Wu, S.; Zhang, Y.; Ye, H.Y.; Song, Y. A new insight into the unique magneto-optical effect of layered perovskite (C6H5C2H3FNH3)2MnCl4. Aggregate 2022, 4, e294. [Google Scholar] [CrossRef]
- Wei, W.; He, L.; Han, G.; Lu, Y.; Shi, S.; Yuan, Z.; Wang, X.; Li, Y.; Chen, B.; Zhang, Z.; et al. Stimulus-responsive hydrogen-bonded organic frameworks: Construction strategies, research progress and applications. Coord. Chem. Rev. 2024, 507, 215760. [Google Scholar] [CrossRef]
- Zhang, T.L.; Zhao, C.; Liu, Z.J.; Xu, T.Y.; Lin, H.Y.; Zhou, S.W.; Li, Y.H.; Tong, F. Reversible Acid-Base Responsive Fluorescence Changes of Solutions and Crystals Based on Anthracenyl Pyridyl Derivatives. ChemPhotoChem 2023, 7, e202300194. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Deng, W.J.; Zhao, K.Q.; Redshaw, C.; Donnio, B. Phenanthrothiophene-Triazine Star-Shaped Discotic Liquid Crystals: Synthesis, Self-Assembly, and Stimuli-Responsive Fluorescence Properties. Chem.-Eur. J. 2024, 30, e202400296. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Zhu, W.; Ding, Y.; Liu, S.; Wang, Q.; Chen, Y. A versatile color and fluorescence pH sensor based on AIE and open-loop synergy effect: Crystal structure and its application in cell imaging. Dyes Pigment. 2021, 190, 109310. [Google Scholar] [CrossRef]
- Das, S.; Das, M.; Das, U.K.; Chandra Samanta, B.; Bag, A.; Patra, A.; Bhattacharya, N.; Maity, T. Spirolactam ring locking and unlocking tuned solvent regulated unique Hg(II) sensing by a novel AIE active Rhodamine −1, 2 diamino propane-based Schiff chemosensor and its pH sensor performance. Dyes Pigment. 2024, 222, 111884. [Google Scholar] [CrossRef]
- Kalita, A.; Malik, A.H.; Sarma, N.S. Stimuli-Responsive Naphthalene Diimide as Invisible Ink: A Rewritable Fluorescent Platform for Anti-Counterfeiting. Chem.-Asian J. 2020, 15, 1074–1080. [Google Scholar] [CrossRef]
- Yu, C.-M.; Wang, P.-H.; Liu, Q.; Cai, L.-Z.; Guo, G.-C. Modulating Fading Time of Photochromic Compounds by Molecular Design for Erasable Inkless Printing and Anti-counterfeiting. Cryst. Growth Des. 2021, 21, 1323–1328. [Google Scholar] [CrossRef]
- Wang, S.F.; Lin, J.R.; Ishiwari, F.; Fukushima, T.; Masuhara, H.; Sugiyama, T. Spatiotemporal Dynamics of Aggregation-Induced Emission Enhancement Controlled by Optical Manipulation. Angew. Chem. Int. Ed. 2020, 59, 7063–7068. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Wang, B.; Yang, X.; Zou, B.; Yang, B.; Lu, S. Pressure-triggered aggregation-induced emission enhancement in red emissive amorphous carbon dots. Nanoscale Horiz. 2019, 4, 1227–1231. [Google Scholar] [CrossRef]
- Okazawa, Y.; Kondo, K.; Akita, M.; Yoshizawa, M. Polyaromatic Nanocapsules Displaying Aggregation-Induced Enhanced Emissions in Water. J. Am. Chem. Soc. 2014, 137, 98–101. [Google Scholar] [CrossRef]
- Huang, J.; Yao, W.; Cui, X.; Si, L.; Yang, D.; Liu, X.; Liu, W. Robust, self-healing AIE fluorescent supramolecular elastomers for smart anti-counterfeiting. Chem. Eng. Sci. 2024, 293, 120030. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, Y.; Guo, Y.; Wang, Y.; Ma, L. AIE activity, mechanochromism, acidchromism, and high-level anti-counterfeiting based on multifunctional tetraphenylvinyl imidazolopyridine. J. Lumin. 2024, 273, 120678. [Google Scholar] [CrossRef]
- Li, P.; He, Y.; Arman, H.D.; Krishna, R.; Wang, H.; Weng, L.; Chen, B. A microporous six-fold interpenetrated hydrogen-bonded organic framework for highly selective separation of C2H4/C2H6. Chem. Commun. 2014, 50, 13081–13084. [Google Scholar] [CrossRef]
- Wang, H.; Li, B.; Wu, H.; Hu, T.-L.; Yao, Z.; Zhou, W.; Xiang, S.; Chen, B. A Flexible Microporous Hydrogen-Bonded Organic Framework for Gas Sorption and Separation. J. Am. Chem. Soc. 2015, 137, 9963–9970. [Google Scholar] [CrossRef]
- Song, C.-L.; Li, Z.; Wu, J.-R.; Lu, T.; Yang, Y.-W. Intramolecular Through-Space Interactions Induced Emission of Pillar[4]arene[1]dicyanobenzene. Chem. Mater. 2022, 34, 10181–10189. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, H.; Zhou, Z.; Liu, J.; Sung, H.H.Y.; Williams, I.D.; Halpert, J.E.; Zhao, Z.; Tang, B.Z. How do molecular interactions affect fluorescence behavior of AIEgens in solution and aggregate states? Sci. China Chem. 2021, 65, 135–144. [Google Scholar] [CrossRef]
- Lee, M.M.S.; Yu, E.Y.; Yan, D.; Chau, J.H.C.; Wu, Q.; Lam, J.W.Y.; Ding, D.; Kwok, R.T.K.; Wang, D.; Tang, B.Z. The Role of Structural Hydrophobicity on Cationic Amphiphilic Aggregation-Induced Emission Photosensitizer-Bacterial Interaction and Photodynamic Efficiency. ACS Nano 2023, 17, 17004–17020. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, Y.; Xiao, D.; Liu, J.; Zhao, Z.; Liu, Z.; Lam, J.W.Y.; Tang, B.Z. An Intelligent AIEgen with Nonmonotonic Multiresponses to Multistimuli. Adv. Sci. 2020, 7, 2001845. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Y.; Shen, H.; Lam, J.W.Y.; Sun, J.; Zhang, H.; Tang, B.Z. Regulating the proximity effect of heterocycle-containing AIEgens. Nat. Commun. 2023, 14, 3772. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Bian, G.; Chen, Y.; Ouyang, M.; Hu, B.; Zhang, C. Cyanostilben-based derivatives: Mechanical stimuli-responsive luminophors with aggregation-induced emission enhancement. Photochem. Photobiol. Sci. 2012, 11, 1414–1421. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef]
- Tang, B.Z.; Geng, Y.; Lam, J.W.Y.; Li, B.; Jing, X.; Wang, X.; Wang, F.; Pakhomov, A.B.; Zhang, X.X. Processible Nanostructured Materials with Electrical Conductivity and Magnetic Susceptibility: Preparation and Properties of Maghemite/Polyaniline Nanocomposite Films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Gao, M.; Hong, Y.; Chen, B.; Wang, Y.; Zhou, W.; Wong, W.W.H.; Zhou, J.; Smith, T.A.; Zhao, Z. AIE conjugated polyelectrolytes based on tetraphenylethene for efficient fluorescence imaging and lifetime imaging of living cells. Polym. Chem. 2017, 8, 3862–3866. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular Rotor Measures Viscosity of Live Cells via Fluorescence Lifetime Imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, T.; Chen, X.; Chen, Q.; Hao, J.; Song, W.; Zeng, Y.; Xue, P. Guest-Regulated Luminescence and Force-Stimuli Response of a Hydrogen-Bonded Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 32270–32277. [Google Scholar] [CrossRef]
- Zadehnazari, A.; Khosropour, A.; Altaf, A.A.; Rosen, A.S.; Abbaspourrad, A. Tetrazine-Linked Covalent Organic Frameworks With Acid Sensing and Photocatalytic Activity. Adv. Mater. 2024, 36, 2311042. [Google Scholar] [CrossRef]
- Hodée, M.; Lenne, A.; Rodríguez-López, J.; Robin-le Guen, F.; Katan, C.; Achelle, S.; Fihey, A. Influence of (de)protonation on the photophysical properties of phenol-substituted diazine chromophores: Experimental and theoretical studies. New J. Chem. 2021, 45, 19132–19144. [Google Scholar] [CrossRef]
- Xu, J.; Wu, G.; Wang, Z.; Zhang, X. Generation of 2D organic microsheets from protonated melamine derivatives: Suppression of the self assembly of a particular dimension by introduction of alkyl chains. Chem. Sci. 2012, 3, 3227–3230. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Cui, D.-L.; Jing, H.-P. Melaminium sulfate. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 2008, 64, o351. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Wang, C.; Gao, X.; Zhou, Y.; Chen, B.; Li, P. Self-Healing Hydrogen-Bonded Organic Frameworks for Low-Concentration Ammonia Capture. J. Am. Chem. Soc. 2023, 146, 627–634. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, J.; Xiong, Z.; Yang, X.; Li, Y.; Zhang, H.; Xiang, S.; Chen, B.; Zhang, Z. Smart-Responsive HOF Heterostructures with Multiple Spatial-Resolved Emission Modes toward Photonic Security Platform. Adv. Mater. 2023, 36, 2309130. [Google Scholar] [CrossRef]
- Zheng, P.; Abdurahman, A.; Liu, G.; Liu, H.; Zhang, Y.; Zhang, M. An instantaneously-responded, ultrasensitive, reutilizable fluorescent probe to sarin substitute both in solution and in gas phase. Sens. Actuators B 2020, 322, 128611. [Google Scholar] [CrossRef]
- Zheng, P.; Abdurahman, A.; Zhang, Z.; Feng, Y.; Zhang, Y.; Ai, X.; Li, F.; Zhang, M. A simple organic multi-analyte fluorescent prober: One molecule realizes the detection to DNT, TATP and Sarin substitute gas. J. Hazard. Mater. 2021, 409, 124500. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, B. Configuration-regulated highly luminescent hydrogen-organic frameworks for detection of phenelzine and propofol. Inorg. Chem. Front. 2024, 11, 1099–1107. [Google Scholar] [CrossRef]
- Gu, K.; Meng, Z.; Liu, X.; Wu, Y.; Qi, X.; Ren, Y.; Yu, Z.-Q.; Tang, B.Z. A gated strategy stabilizes room-temperature phosphorescence. Aggregate 2023, 4, e337. [Google Scholar] [CrossRef]
- Shen, H.; Li, Y.; Li, Y. Self-assembly and tunable optical properties of intramolecular charge transfer molecules. Aggregate 2020, 1, 57–68. [Google Scholar] [CrossRef]
- Jiang, G.; Ma, Y.; Ding, J.; Liu, J.; Liu, R.; Zhou, P. N-Protonation as a Switch of the Twisted Excited States with ππ* or nπ* Character and Correlation with the π-Electrons Characteristic of Rotatable Bonds. Chem.-Eur. J. 2023, 29, e202300625. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, Z.; Shen, S.; Liang, K.; Shao, Q.; Cong, Z.; Wang, H. Reversible Specific Vapoluminescence Behavior in Pure Organic Crystals through Hydrogen-Bonding Docking Strategy. Adv. Opt. Mater. 2019, 7, 1801549. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, M.; Yang, Z.; Qiu, H.; Kou, S.; Padhiar, M.A.; Bhatti, A.S.; Gaponenko, N.V. Pressure-Driven Transformation of CsPbBrI2 Nanoparticles into Stable Nanosheets in Solution through Self-Assembly. J. Phys. Chem. Lett. 2020, 11, 9862–9868. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Meng, H.; Chai, Y.; Chen, X.; Xu, J.; Liu, X.; Liu, W.; Guldi, D.M.; Zhu, Y. Enhancing Built-in Electric Fields for Efficient Photocatalytic Hydrogen Evolution by Encapsulating C60 Fullerene into Zirconium-Based Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2023, 135, e202217897. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Gao, X.; Niu, Q.; Jin, M.; Wang, Y.; Alshahrani, T.; Sun, H.-L.; Chen, B.; Li, Z.; Li, P. An Acid-Responsive Fluorescent Molecule for Erasable Anti-Counterfeiting. Molecules 2024, 29, 4335. https://doi.org/10.3390/molecules29184335
Liu J, Gao X, Niu Q, Jin M, Wang Y, Alshahrani T, Sun H-L, Chen B, Li Z, Li P. An Acid-Responsive Fluorescent Molecule for Erasable Anti-Counterfeiting. Molecules. 2024; 29(18):4335. https://doi.org/10.3390/molecules29184335
Chicago/Turabian StyleLiu, Jiabao, Xiangyu Gao, Qingyu Niu, Mingyuan Jin, Yijin Wang, Thamraa Alshahrani, He-Lue Sun, Banglin Chen, Zhiqiang Li, and Peng Li. 2024. "An Acid-Responsive Fluorescent Molecule for Erasable Anti-Counterfeiting" Molecules 29, no. 18: 4335. https://doi.org/10.3390/molecules29184335






