Research Progress in Microporous Materials for Selective Adsorption and Separation of Methane from Low-Grade Gas
Abstract
:1. Introduction
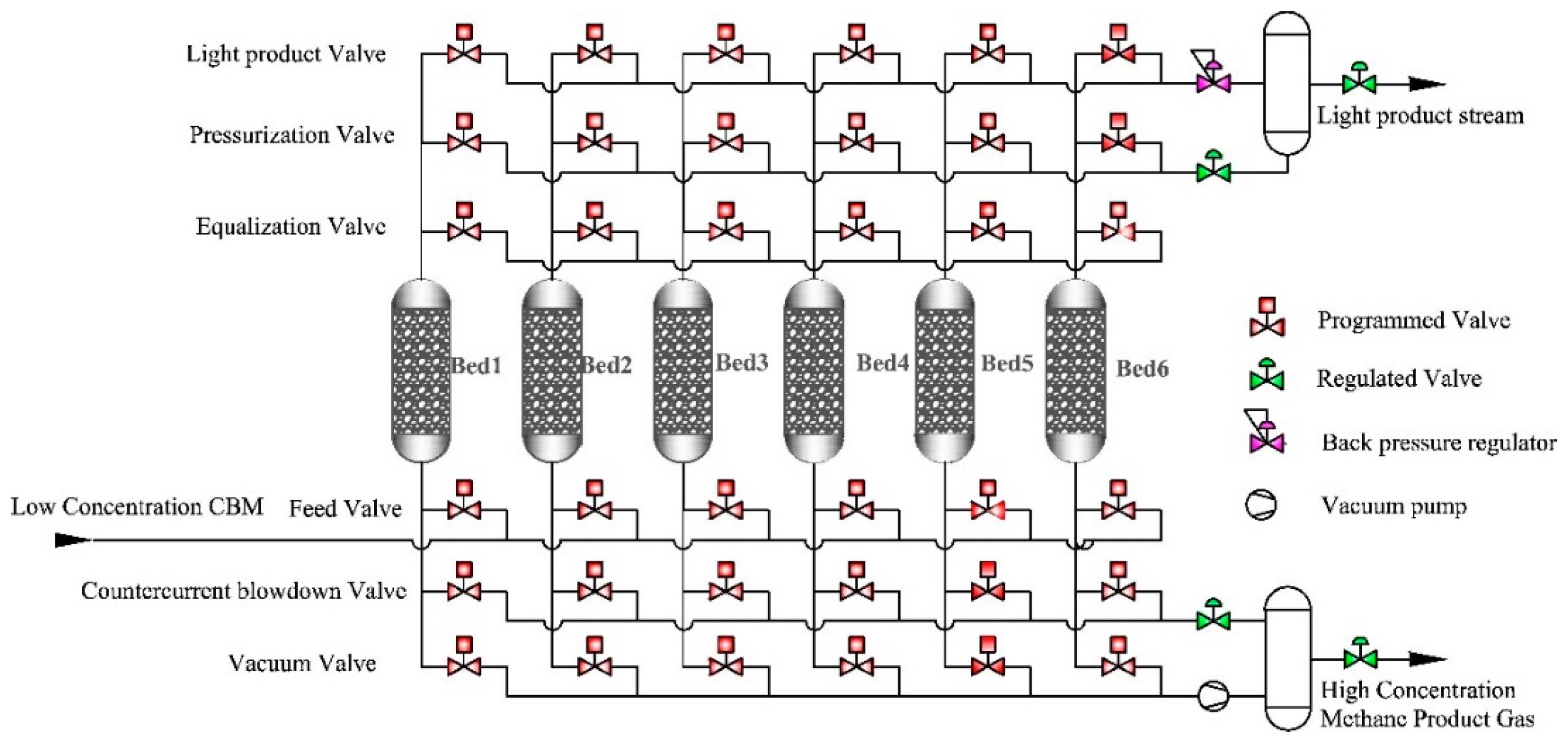
2. Adsorption Separation Technology and Processes
3. Porous Materials for CH4/N2 Adsorption Separation
3.1. Carbon-Based Adsorbent Materials
3.2. Zeolite Molecular Sieves
3.3. Metal/Organic Frameworks (MOFs)
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y. Promote Carbon Peaking and Carbon Neutrality with the Concept of Global Warming and Carbon Emission Reduction. BCP Bus. Manag. 2023, 39, 439–448. [Google Scholar] [CrossRef]
- Division, E.M. Unconventional Energy Resources: 2017 Review. Nat. Resour. Res. 2018, 28, 1661–1751. [Google Scholar]
- Disclaim Badr, O.; Probert, S.D.; O’Callaghan, P.W. Methane: A greenhouse gas in the Earth’s atmosphere. Appl. Energy 1992, 41, 95–113. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J.; Ai, B.; Song, M.; Hou, W. Determinants of global natural gas consumption and import–export flows. Energy Econ. 2019, 83, 588–602. [Google Scholar] [CrossRef]
- Weijermars, R.; Drijkoningen, G.; Heimovaara, T.J.; Rudolph, E.S.J.; Weltje, G.J.; Wolf, K.H.A.A. Unconventional gas research initiative for clean energy transition in Europe. J. Nat. Gas Sci. Eng. 2011, 3, 402–412. [Google Scholar] [CrossRef]
- Kuyper, J.; Schroeder, H.; Ola Linnér, B. Annual Review of Environment and Resources. Annu. Rev. Environ. Resour. 2018, 43, 343–368. [Google Scholar] [CrossRef]
- Whiting, G.J.; Chanton, J.P. Greenhouse carbon balance of wetlands: Methane emission versus carbon sequestration. Tellus B 2001, 53, 521–528. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Upgrade of Methane from Landfill Gas by Pressure Swing Adsorption. Energy Fuels 2005, 19, 2545–2555. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Pinnau, I.; He, Z.; Amo, K.D.; DaCosta, A.; Wijmans, J.G.; Baker, R.W. Membrane separation of nitrogen from natural gas: A case study from membrane synthesis to commercial deployment. J. Membr. Sci. 2010, 346, 270–279. [Google Scholar] [CrossRef]
- Saha, D.; Grappe, H.A.; Chakraborty, A.; Orkoulas, G. Postextraction Separation, On-Board Storage, and Catalytic Conversion of Methane in Natural Gas: A Review. Chem. Rev. 2016, 116, 11436–11499. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.; Graham, B.F.; Boxall, J.A.; Costa, J.D.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94, 123–154. [Google Scholar]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lee, K.; Isley, W.C.; Dzubak, A.L.; Verma, P.; Stoneburner, S.J.; Lin, L.; Howe, J.D.; Bloch, E.D.; Reed, D.A.; Hudson, M.R.; et al. Design of a metal-organic framework with enhanced back bonding for separation of N2 and CH4. J. Am. Chem. Soc. 2014, 136, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, M.; Farrusseng, D.; Valencia, S.; Aguado, S.; Ravon, U.; Rizzo, C.; Corma, A.; Mirodatos, C. Natural gas treating by selective adsorption: Material science and chemical engineering interplay. Chem. Eng. J. 2009, 155, 553–566. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, J. Experimental Study on Distillation Column Parameters for Liquefaction Device of Low Concentration Coalbed Methane. Processes 2021, 9, 606. [Google Scholar] [CrossRef]
- Janusz-Cygan, A.; Jaschik, J.; Tańczyk, M. Upgrading Biogas from Small Agricultural Sources into Biomethane by Membrane Separation. Membranes 2021, 11, 938. [Google Scholar] [CrossRef]
- Gong, H.; Chen, Z.; Yu, H.; Wu, W.; Weixing, W.; Pang, H.; Du, M. Methane recovery in a combined amine absorption and gas steam boiler as a self-provided system for biogas upgrading. Energy 2018, 157, 744–751. [Google Scholar] [CrossRef]
- Baek, S.; Ahn, Y.; Zhang, J.; Min, J.; Lee, H.; Lee, J.W. Enhanced methane hydrate formation with cyclopentane hydrate seeds. Appl. Energy 2017, 202, 32–41. [Google Scholar] [CrossRef]
- Fakhraei Ghazvini, M.; Vahedi, M.; Najafi Nobar, S.; Sabouri, F. Investigation of the MOF adsorbents and the gas adsorptive separation mechanisms. J. Environ. Chem. Eng. 2020, 9, 104790. [Google Scholar] [CrossRef]
- Fatehi, A.; Loughlin, K.F.; Hassan, M.M. Separation of methane—Nitrogen mixtures by pressure swing adsorption using a carbon molecular sieve. Gas Sep. Purif. 1995, 9, 199–204. [Google Scholar] [CrossRef]
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Águeda, V.I.; Gómez, P. Numerical simulation of a three-bed PSA cycle for the methane/nitrogen separation with silicalite. Sep. Purif. Technol. 2011, 77, 7–17. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Zhou, F.; Li, X.; Wei, K.; Song, J. Enrichment of Oxygen-Containing Low-Concentration Coalbed Methane with CMS-3KT as the Adsorbent. ACS Omega 2021, 6, 6914–6923. [Google Scholar] [CrossRef]
- Lu, B.; Shen, Y.; Tang, Z.; Zhang, D.; Chen, G. Vacuum pressure swing adsorption process for coalbed methane enrichment. Chin. J. Chem. Eng. 2020, 32, 264–280. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, G.; Chen, K.; Guo, J.; Webley, P.A.; Li, G.K. Capture of dilute methane with a novel dynamic-feed dual-reflux pressure swing adsorption process. AlChE J. 2021, 68, 17390. [Google Scholar] [CrossRef]
- Qian, Z.; Zhou, Y.; Yang, Y.; Li, P. Methane recovery from low-grade unconventional natural gas by the integrated mode of the conventional/improved vacuum pressure swing adsorption processes. Fuel 2023, 331, 125717. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Philippou, A.; Mackay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Corbin, D.R.; Shiflett, M.B. A Review of Porous Adsorbents for the Separation of Nitrogen from Natural Gas. Ind. Eng. Chem. Res. 2020, 59, 13355–13369. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yi, H.; Li, F.; Ning, P.; Tang, X.; Peng, J.; Li, Y.; Deng, H. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215–216, 635–642. [Google Scholar] [CrossRef]
- Rufford, T.E.; Watson, G.; Saleman, T.L.; Hofman, P.S.; Jensen, N.K.; May, E.F. Adsorption Equilibria and Kinetics of Methane + Nitrogen Mixtures on the Activated Carbon Norit RB3. Ind. Eng. Chem. Res. 2013, 52, 14270–14281. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, B.; Qi, Z.; Liu, Z.; Duan, S.; Du, X.; Xian, X. Effects of pore structure of granular activated carbons on CH4 enrichment from CH4/N2 by vacuum pressure swing adsorption. Sep. Purif. Technol. 2015, 146, 213–218. [Google Scholar] [CrossRef]
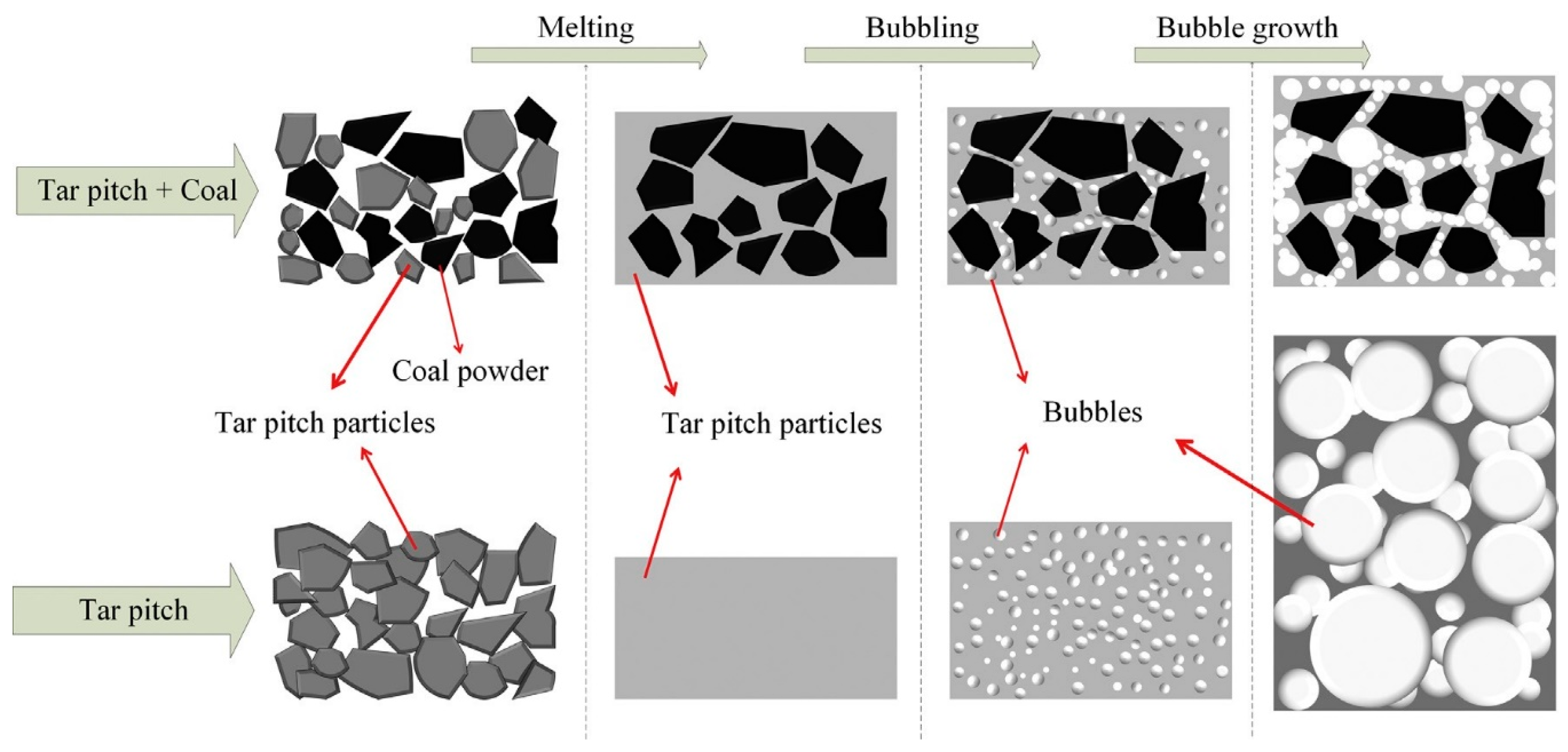
- Arami-Niya, A.; Rufford, T.E.; Zhu, Z. Activated carbon monoliths with hierarchical pore structure from tar pitch and coal powder for the adsorption of CO2, CH4 and N2. Carbon 2016, 103, 115–124. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, J.; Lin, Q.; Cao, J.X.; Liu, F.; Zheng, B. Preparation and Characterization of Activated Carbons from Bamboo Sawdust and Its Application for CH4 Selectivity Adsorption from a CH4/N2 System. Energy Fuels 2016, 30, 10730–10738. [Google Scholar] [CrossRef]
- Yao, K.X.; Chen, Y.; Lu, Y.; Zhao, Y.; Ding, Y. Ultramicroporous carbon with extremely narrow pore distribution and very high nitrogen doping for efficient methane mixture gases upgrading. Carbon 2017, 122, 258–265. [Google Scholar] [CrossRef]
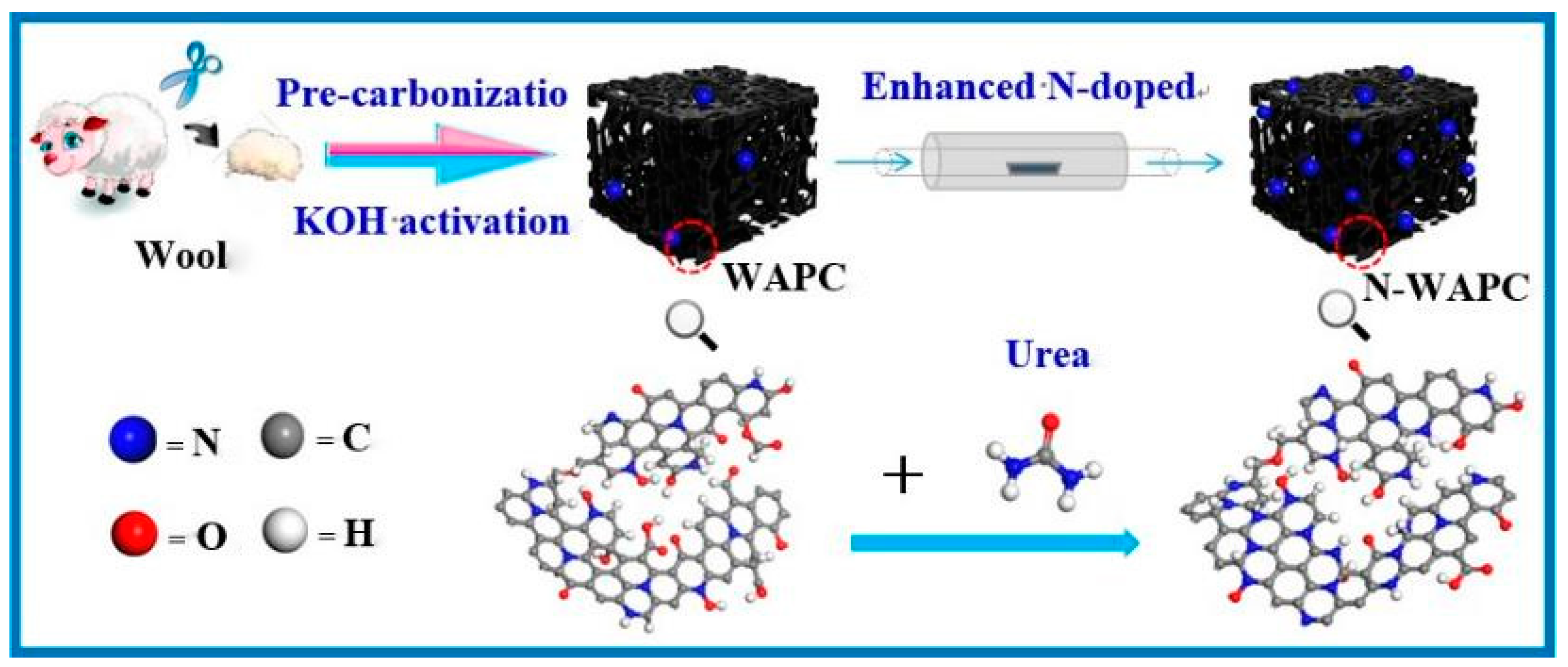
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped Porous Carbon Derived from KOH-Activated Waste Wool: A Promising Material for Selective Adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, P.; Wang, J.; Xu, M.; Deng, Q.; Zeng, Z.; Deng, S. Ultra-high surface area and nitrogen-rich porous carbons prepared by a low-temperature activation method with superior gas selective adsorption and outstanding supercapacitance performance. Chem. Eng. J. 2019, 355, 309–319. [Google Scholar] [CrossRef]
- Zhang, L.N.; Dong, Y.; Zhang, D.; Li, W.; Qin, H.; Luo, Z.; Shi, Y.; Lv, Y.; Zhang, C.; Pan, H.; et al. Facile preparation of nitrogen-doped microporous carbon from potassium citrate/urea for effective CH4 separation and uptake. Fuel 2023, 351, 128915. [Google Scholar]
- Li, Z.; Liu, Y.; Zhang, C.Z.; Yang, X.; Ren, J.; Jiang, L. Methane Recovery from Coal Bed Gas Using Modified Activated Carbons: A Combined Method for Assessing the Role of Functional Groups. Energy Fuels 2015, 29, 6858–6865. [Google Scholar] [CrossRef]
- Pan, H.; Yi, Y.; Lin, Q.; Xiang, G.; Zhang, Y.; Liu, F. Effect of Surface Chemistry and Textural Properties of Activated Carbons for CH4 Selective Adsorption through Low-Concentration Coal Bed Methane. J. Chem. Eng. Data 2016, 61, 2120–2127. [Google Scholar] [CrossRef]
- Du, S.; Wu, Y.; Wang, X.; Xia, Q.; Xiao, J.; Zhou, X.; Li, Z. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation. AlChE J. 2020, 66, 16231. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q. Microporous Carbon Adsorbents Prepared by Activating Reagent-Free Pyrolysis for Upgrading Low-Quality Natural Gas. ACS Sustain. Chem. Eng. 2020, 8, 977–985. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, S.; Li, L.; Wang, P.; Li, X.; Che, Y.; Li, X. Preparation of Carbon Molecular Sieves Used for CH4/N2 Separation. J. Chem. Eng. Data 2018, 63, 1737–1744. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Meng, Z.; Li, Y. Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve. Sep. Purif. Technol. 2019, 218, 130–137. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Zhang, M.; Liu, B.; Zheng, Z.; Hao, G.; Lu, A. Carbon Nanofibers with Gas Selective Layer Containing Rich and Accessible Ultramicropores for Methane/Nitrogen Separation. Chem. Eng. J. 2023, 462, 142118. [Google Scholar] [CrossRef]
- Yuan, D.; Zheng, Y.; Li, Q.; Lin, B.; Zhang, G.; Liu, J. Effects of pore structure of prepared coal-based activated carbons on CH4 enrichment from low concentration gas by IAST method. Powder Technol. 2018, 333, 377–384. [Google Scholar] [CrossRef]
- Qu, D.; Yang, Y.; Lu, K.; Yang, L.; Li, P.; Yu, J.; Ribeiro, A.M.; Rodrigues, A.E. Microstructure effect of carbon materials on the low-concentration methane adsorption separation from its mixture with nitrogen. Adsorption 2018, 24, 357–369. [Google Scholar] [CrossRef]
- Tang, R.; Dai, Q.; Liang, W.; Wu, Y.; Zhou, X.; Pan, H.; Li, Z. Synthesis of novel particle rice-based carbon materials and its excellent CH4/N2 adsorption selectivity for methane enrichment from Low-rank natural gas. Chem. Eng. J. 2020, 384, 123388. [Google Scholar] [CrossRef]
- Wang, S.; Wu, P.; Fu, J.; Yang, Q. Heteroatom-doped porous carbon microspheres with ultramicropores for efficient CH4/N2 separation with ultra-high CH4 uptake. Sep. Purif. Technol. 2021, 274, 119121. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, X.; Wang, J.A. Adsorption performance of activated carbon for methane with low concentration at atmospheric pressure. Energy Source Part A 2019, 43, 1337–1347. [Google Scholar] [CrossRef]
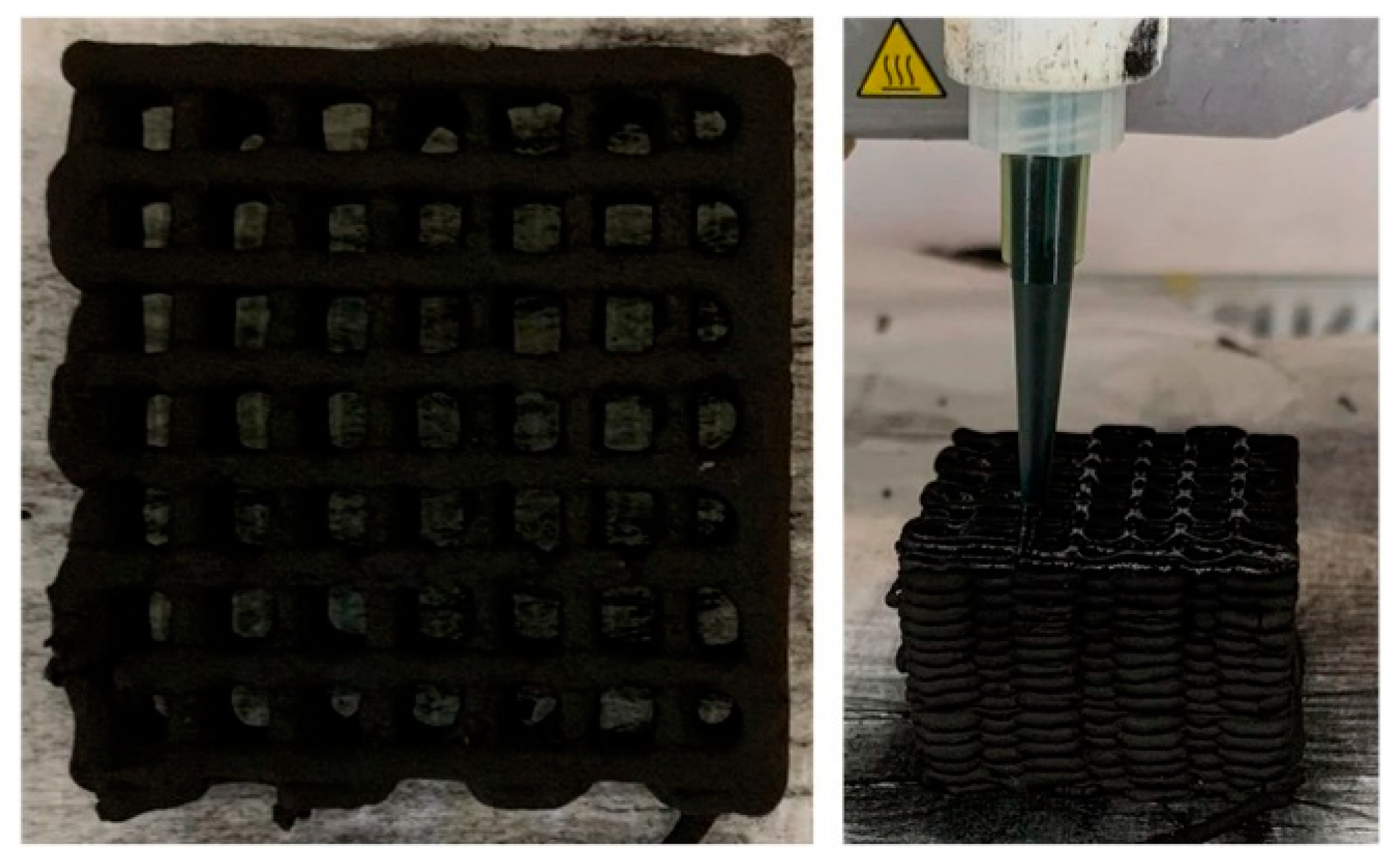
- Pereira, A.; Ferreira, A.F.; Rodrigues, A.E.; Ribeiro, A.M.; Regufe, M.J. Study of methane upgrading using an activated carbon 3D-printed adsorbent. J. Environ. Chem. Eng. 2023, 12, 111730. [Google Scholar] [CrossRef]
- Campo, M.C.; Magalhães, F.D.; Mendes, A.M. Comparative study between a CMS membrane and a CMS adsorbent: Part I—Morphology, adsorption equilibrium and kinetics. J. Membr. Sci. 2010, 346, 15–25. [Google Scholar] [CrossRef]
- Yang, Y.; Ribeiro, A.M.; Li, P.; Yu, J.; Rodrigues, A.E. Adsorption Equilibrium and Kinetics of Methane and Nitrogen on Carbon Molecular Sieve. Ind. Eng. Chem. Res. 2014, 53, 16840–16850. [Google Scholar] [CrossRef]
- Bae, Y.; Moon, J.; Ahn, H.W.; Lee, C. Effects of adsorbate properties on adsorption mechanism in a carbon molecular sieve. Korean J. Chem. Eng. 2004, 21, 712–720. [Google Scholar] [CrossRef]
- Gorska, O.; Cyganiuk, A.W.; Olejniczak, A.; Ilnicka, A.; Lukaszewicz, J.P. Salix viminaliswood as a new precursor for manufacturing of carbon molecular sieves for effective methane/nitrogen separation. Open Chem. 2015, 13, 748–755. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Zhang, C.Z.; Wang, H.; Zhang, E.; Xing, Y.; Xiao, P.; Yang, R.T.; Liu, Y.; Webley, P.A. Practical separation performance evaluation of coal mine methane upgrading with carbon molecular sieves. Chem. Eng. J. 2019, 367, 295–303. [Google Scholar] [CrossRef]
- Fu, X.; Tang, X.; Xu, Y.; Zhou, X.; Zhang, D. Microwave irradiation-induced alterations in physicochemical properties and methane adsorption capability of coals: An experimental study using carbon molecular sieve. Chin. J. Chem. Eng. 2024, 68, 165–180. [Google Scholar] [CrossRef]
- Ruthven, D.M. Molecular Sieve Separations. Chem. Ing. Tech. 2011, 83, 44–52. [Google Scholar] [CrossRef]
- Bol, R.; Harkness, D.D. The Use of Zeolite Molecular Sieves for Trapping Low Concentrations of CO2 from Environmental Atmospheres. Radiocarbon 1995, 37, 643–647. [Google Scholar] [CrossRef]
- Jensen, N.K.; Rufford, T.E.; Watson, G.; Zhang, D.; Chan, K.I.; May, E.F. Screening Zeolites for Gas Separation Applications Involving Methane, Nitrogen, and Carbon Dioxide. J. Chem. Eng. Data 2012, 57, 106–113. [Google Scholar] [CrossRef]
- Habgood, H.W. The Kinetics of Molecular Sieve Action. Sorption of Nitrogen-Methane Mixtures by Linde Molecular Sieve 4A. Can. J. Chem. 1958, 36, 1384–1397. [Google Scholar]
- Kennedy, D.; Mujcin, M.; Trudeau, E.; Tezel, F.H. Pure and Binary Adsorption Equilibria of Methane and Nitrogen on Activated Carbons, Desiccants, and Zeolites at Different Pressures. J. Chem. Eng. Data 2016, 61, 3163–3176. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Bajaj, H.C. Sorption of Methane and Nitrogen on Cesium Exchanged Zeolite-X: Structure, Cation Position and Adsorption Relationship. Ind. Eng. Chem. Res. 2014, 53, 6807–6814. [Google Scholar] [CrossRef]
- Mofarahi, M.; Bakhtyari, A. Experimental Investigation and Thermodynamic Modeling of CH4/N2 Adsorption on Zeolite 13X. J. Chem. Eng. Data 2015, 60, 683–696. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, X.; Sun, L.; Liu, X. Adsorption separation of carbon dioxide, methane and nitrogen on monoethanol amine modified β-zeolite. J. Nat. Gas Chem. 2009, 18, 167–172. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, W.; Li, L.; Li, J. Adsorption of CO2, CH4, and N2 on 8-, 10-, and 12-Membered Ring Hydrophobic Microporous High-Silica Zeolites: DDR, Silicalite-1, and Beta. Ind. Eng. Chem. Res. 2013, 52, 17856–17864. [Google Scholar] [CrossRef]
- Shang, H.; Li, Y.; Liu, J.; Tang, X.; Yang, J.; Li, J. CH4/N2 separation on methane molecules grade diameter channel molecular sieves with a CHA-type structure. Chin. J. Chem. Eng. 2019, 27, 1044–1049. [Google Scholar] [CrossRef]
- Yang, J.; Tang, X.; Liu, J.; Wang, J.; Shang, H.; Wu, L.; Li, J.; Deng, S. Down-sizing the crystal size of ZK-5 zeolite for its enhanced CH4 adsorption and CH4/N2 separation performances. Chem. Eng. J. 2021, 406, 126599. [Google Scholar] [CrossRef]
- Yaqi, W.; Danhua, Y.; Zeng, S.; Yang, L.; Dong, X.; Zhang, Q.; Xu, Y.P.; Liu, Z. Significant enhancement in CH4/N2 separation with amine-modified zeolite Y. Fuel 2021, 301, 121077. [Google Scholar]
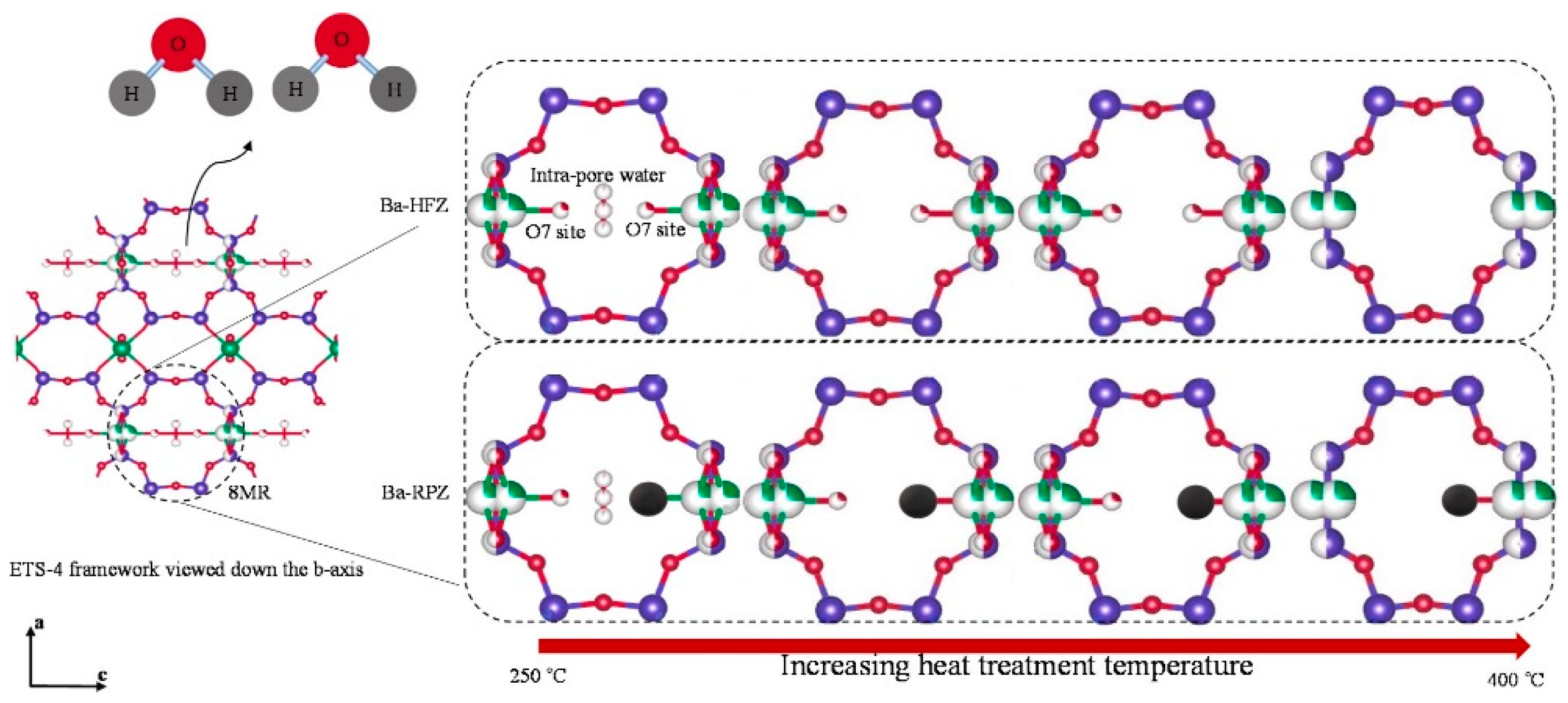
- Vosoughi, M.; Maghsoudi, H. Characterization of size-selective kinetic-based Ba-ETS-4 titanosilicate for nitrogen/methane separation: Chlorine-enhanced steric effects. Sep. Purif. Technol. 2021, 284, 120243. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Shang, H.; Wu, L.; Yang, J. Gas diffusion and adsorption capacity enhancement via ultrasonic pretreatment for hydrothermal synthesis of K-KFI zeolite with nano/micro-scale crystals. Microporous Mesoporous Mater. 2020, 297, 110036. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Liu, P.; Li, L.; Tang, X.; Shang, H.; Li, J.; Chen, B. K-Chabazite Zeolite Nanocrystal Aggregates for Highly Efficient Methane Separation. Angewandte Chem. 2021, 61, 16850. [Google Scholar]
- Kuznicki, S.M.; Bell, V.A.; Nair, S.; Hillhouse, H.W.; Jacubinas, R.M.; Braunbarth, C.M.; Toby, B.H.; Tsapatsis, M. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules. Nature 2001, 412, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, B.S.; Bhadra, S.J.; Marathe, R.; Farooq, S. Adsorption and Diffusion of Methane and Nitrogen in Barium Exchanged ETS-4. Ind. Eng. Chem. Res. 2011, 50, 3022–3035. [Google Scholar] [CrossRef]
- Jayaraman, A.; Hernández-Maldonado, A.J.; Yang, R.; Chinn, D.; Munson, C.L.; Mohr, D. Clinoptilolites for nitrogen/methane separation. Chem. Eng. Sci. 2004, 59, 2407–2417. [Google Scholar] [CrossRef]
- Sethia, G.; Somani, R.S.; Bajaj, H.C. Adsorption of carbon monoxide, methane and nitrogen on alkaline earth metal ion exchanged zeolite-X: Structure, cation position and adsorption relationship. RSC Adv. 2015, 5, 12773–12781. [Google Scholar] [CrossRef]
- Kennedy, D.; Tezel, F.H. Cation exchange modification of clinoptilolite-Screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous Mesoporous Mater. 2018, 262, 235–250. [Google Scholar] [CrossRef]
- Hao, X.; Hu, H.; Li, Z.; Wu, L.; Liu, X.; Zhang, Y. Adsorption Properties of Modified Clinoptilolite for Methane and Nitrogen. Materials 2018, 10, 2024. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Mujcin, M.; Alenko, T.; Tezel, F.H. Pure and mixture adsorption equilibria of methane and nitrogen onto clinoptilolite: Effects of Cs+ and Fe3+ exchanged cations on separation performance. Adsorption 2019, 25, 135–158. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Chen, K.; Yao, J.; Zavabeti, A.; Liu, J.; Li, G.K. Screening of Alkali Metal-Exchanged Zeolites for Nitrogen/Methane Separation. Langmuir 2023, 39, 1277–1287. [Google Scholar] [CrossRef]
- Hu, G.; Xiao, G.; Guo, Y.; Manning, M.; Chen, L.; Yu, L.; Li, K.G.; May, E.F. Separation of methane and nitrogen using ionic liquidic zeolites by pressure vacuum swing adsorption. AlChE J. 2022, 68, 17668. [Google Scholar] [CrossRef]
- Zhao, J.; Mousavi, S.H.; Xiao, G.; Mokarizadeh, A.; Moore, T.; Chen, K.; Gu, Q.; Singh, R.; Zavabeti, A.; Liu, J.; et al. Nitrogen Rejection from Methane via a “Trapdoor” K-ZSM-25 Zeolite. J. Am. Chem. Soc. 2021, 143, 15195–15204. [Google Scholar] [CrossRef]
- Ghasemi, F.; Alizadeh, M.; Azamat, J.; Erfan-Niya, H. Understanding the performance of RHO type zeolite membrane for CH4/N2 separation based on molecular dynamics and deep neural network methods. J. Mol. Graph. Model. 2023, 127, 108673. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.T.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 5, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.D.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Chemistry 2010, 16, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Munusamy, K.; Sethia, G.; Patil, D.; Somayajulu Rallapalli, P.; Somani, R.S.; Bajaj, H.C. Sorption of carbon dioxide, methane, nitrogen and carbon monoxide on MIL-101(Cr): Volumetric measurements and dynamic adsorption studies. Chem. Eng. J. 2012, 195, 359–368. [Google Scholar] [CrossRef]
- Möllmer, J.; Lange, M.; Möller, A.C.; Patzschke, C.; Stein, K.; Lässig, D.; Lincke, J.; Gläser, R.; Krautscheid, H.; Staudt, R. Pure and mixed gas adsorption of CH4 and N2 on the metal–organic framework Basolite® A100 and a novel copper-based 1,2,4-triazolyl isophthalate MOF. J. Mater. Chem. 2012, 22, 10274–10286. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Zhou, X.; Ng, S.W.; O’Keeffe, M.; Li, D. ROD-8, a rod MOF with a pyrene-cored tetracarboxylate linker: Framework disorder, derived nets and selective gas adsorption. CrystEngComm 2014, 16, 6291–6295. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Sun, T.; Guo, Y.; Wei, X.; Zhao, S.; Wang, S. Water Resistant and Flexible MOF Materials for Highly Efficient Separation of Methane from Nitrogen. Ind. Eng. Chem. Res. 2019, 58, 20392–20400. [Google Scholar] [CrossRef]
- Zhang, F.; Shang, H.; Zhai, B.; Li, X.; Zhang, Y.; Wang, X.; Li, J.; Yang, J. Thermodynamic-Kinetic Synergistic Separation of CH4/N2 on A Robust Aluminum-Based Metal-Organic Framework. IChE J. 2022, 69, e18079. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, P.; Liu, J.; Shen, F.; Zhang, Y.; Chai, K.; Ying, Y.; Kang, C.; Zhang, Z.; Ji, H. Enhancing CH4/N2 separation performance within aluminum-based Metal-Organic Frameworks: Influence of the pore structure and linker polarity. Sep. Purif. Technol. 2022, 286, 120446. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Wei, Y.; Yan, T.; Wang, J.; Liu, D.; Chen, J. Discovery of a Scalable Metal–Organic Framework with a Switchable Structure for Efficient CH4/N2 Separation. Chem. Mater. 2023, 35, 4286–4296. [Google Scholar] [CrossRef]
- He, Y.; Xiang, S.; Zhang, Z.; Xiong, S.; Fronczek, F.; Krishna, R.; O’Keeffe, M.; Chen, B. A microporou lanthanide-tricarboxylate framework with the potential for purification of natural gas. Chem. Commun. 2012, 48, 10856–10858. [Google Scholar] [CrossRef]
- Ma, H.; Ren, H.; Zou, X.; Meng, S.; Sun, F.; Zhu, G. Post-metalation of porous aromatic frameworks for highly efficient carbon capture from CO2 + N2 and CH4 + N2 mixtures. Polym. Chem. 2014, 5, 144–152. [Google Scholar] [CrossRef]
- Hu, J.; Sun, T.; Liu, X.; Zhao, S.; Wang, S. Rationally tuning the separation performances of [M3(HCOO)6] frameworks for CH4/N2 mixtures via metal substitution. Microporous Mesoporous Mater. 2016, 225, 456–464. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, J.; Shang, H.; Bai, H.; Zhao, Y.Q.; Yang, J.; Dong, J.; Li, J. Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage. Sep. Purif. Technol. 2020, 230, 115850. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.Y.; Yoon, T.U.; Kim, M.B.; Park, W.; Han, H.H.; Kong, C.; Park, C.; Kim, J.; Bae, Y. Improved methane/nitrogen separation properties of zirconium-based metal–organic framework by incorporating highly polarizable bromine atoms. Chem. Eng. J. 2020, 399, 125717. [Google Scholar] [CrossRef]
- Wang, S.; Shivanna, M.; Yang, Q. Nickel-Based Metal-Organic Frameworks for Coal-Bed Methane Purification with Record CH4/N2 Selectivity. Angew. Chem. 2022, 61, e202201017. [Google Scholar] [CrossRef] [PubMed]
- Qadir, S.; Gu, Y.; Ali, S.; Li, D.; Zhao, S.; Wang, S.; Xu, H.; Wang, S. A thermally stable isoquinoline based ultra-microporous metal-organic framework for CH4 separation from coal mine methane. Chem. Eng. J. 2022, 428, 131136. [Google Scholar] [CrossRef]
- Chang, M.; Wang, F.; Wei, Y.; Yang, Q.; Wang, J.; Liu, D.; Chen, J. Separation of CH4/N2 by an Ultra-Stable Metal-Organic Framework with the Highest Brea. AlChE J. 2022, 68, 17794. [Google Scholar] [CrossRef]
- Guo, P.; Chen, Y.; Chang, M.; Li, Y.; Yang, Q.; Liu, D. A Stable Cu(I)-Based Ultramicroporous NKMOF-8-Br with High CH4 Uptake for Efficient Separation of CH4/N2 Mixtures. J. Chem. Eng. Data 2022, 67, 1654–1662. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Zhou, F.; Sheng, B.; Sun, H.; Zheng, F.; Yang, Q.; Zhang, Z.; Ren, Q.; Bao, Z. Zr-Based Metal–Organic Framework with Wall-Shared Dual Ultramicroporous Channels for Effective CH4/N2 Separation. Ind. Eng. Chem. Res. 2023, 62, 13144–13152. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Yang, Q.; Liu, D. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation. Chem. Eng. J. 2020, 408, 127294. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Li, J.; Chen, Y.; Li, J. Adsorption and molecular simulation of CO2 and CH4 in two-dimensional metal–organic frameworks with the same layered substrate. CrystEngComm 2013, 15, 6782–6789. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Wang, J.; Zhang, Z.; Yang, Q.; Yang, Y.; Ren, Q.; Bao, Z. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework. AlChE J. 2018, 64, 3681–3689. [Google Scholar] [CrossRef]
- Niu, Z.; Cui, X.; Pham, T.; Lan, P.C.; Xing, H.; Forrest, K.A.; Wojtas, L.; Space, B.; Ma, S. A Metal-Organic Framework Based Methane Nano-trap for the Capture of Coal-Mine Methane. Angew. Chem. 2019, 58, 1–5. [Google Scholar]
- Lv, D.; Wu, Y.; Chen, J.; Tu, Y.; Yuan, Y.; Wu, H.; Chen, Y.; Liu, B.; Xi, H.; Li, Z.; et al. Improving CH4/N2 selectivity within isomeric Al-based MOFs for the highly selective capture of coal-mine methane. AlChE J. 2020, 66, e16287. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Chen, R.; Zhang, Z.; Yang, Q.; Yang, Y.; Su, B.; Ren, Q.; Bao, Z. A robust two–dimensional layered metal–organic framework for efficient separation of methane from nitrogen. Sep. Purif. Technol. 2022, 281, 119911. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, C.J.; Xu, S.; Shi, J.; Hu, Y.; Liu, G.; Wu, L. An effective approach for CH4/N2 separation using aluminum-based metal-organic frameworks (MOFs) prepared from coal fly ash. Fuel 2024, 360, 130562. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Yan, J.; Lv, D.; Chen, X.; Xu, F.; Zhou, X.; Li, Z. High-pressure separation performance of Ni(TMBDC)(DABCO)0.5 featured low-polarity channel for CH4/N2 mixture. Sep. Purif. Technol. 2023, 335, 126019. [Google Scholar] [CrossRef]
- Liu, P.; Fan, L.; Li, J.; Wu, Z.; Wang, Y.; Chen, Y.; Gao, J.; Yang, J.; Li, J.; Chen, B.; et al. A Microporous Titanium Metal–Organic Framework with Double Nanotraps for Record CH4/N2 Separation. Chem. Mater. 2024, 36, 2925–2932. [Google Scholar] [CrossRef]
- Feng, W.; Wu, H.; Jin, J.; Liu, D.; Meng, H.; Yun, J.; Mi, J. Transformation of Al-CDC from 3D crystals to 2D nanosheets in macroporous polyacrylates with enhanced CH4/N2 separation efficiency and stability. Chem. Eng. J. 2022, 429, 132285. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, J.; Liu, X.; Sun, T.; Zhao, S.; Wang, S. Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2. Chem. Eng. J. 2017, 327, 564–572. [Google Scholar] [CrossRef]
- Knaebel, K.S.; Hill, F.B. Pressure swing adsorption: Development of an equilibrium theory for gas separations. Chem. Eng. Sci. 1985, 40, 2351–2360. [Google Scholar] [CrossRef]
- Serbezov, A.; Sotirchos, S.V. Particle-bed model for multicomponent adsorption-based separations: Application to pressure swing adsorption. Chem. Eng. Sci. 1999, 54, 5647–5666. [Google Scholar] [CrossRef]
- Golubyatnikov, O.O.; Akulinin, E.I.; Dvoretsky, S.I.; Dvoretsky, D.S. To the problem of forming the equation system for pressure swing adsorption mathematical model. Chem. Prod. Process Model. 2021, 17, 681–699. [Google Scholar] [CrossRef]


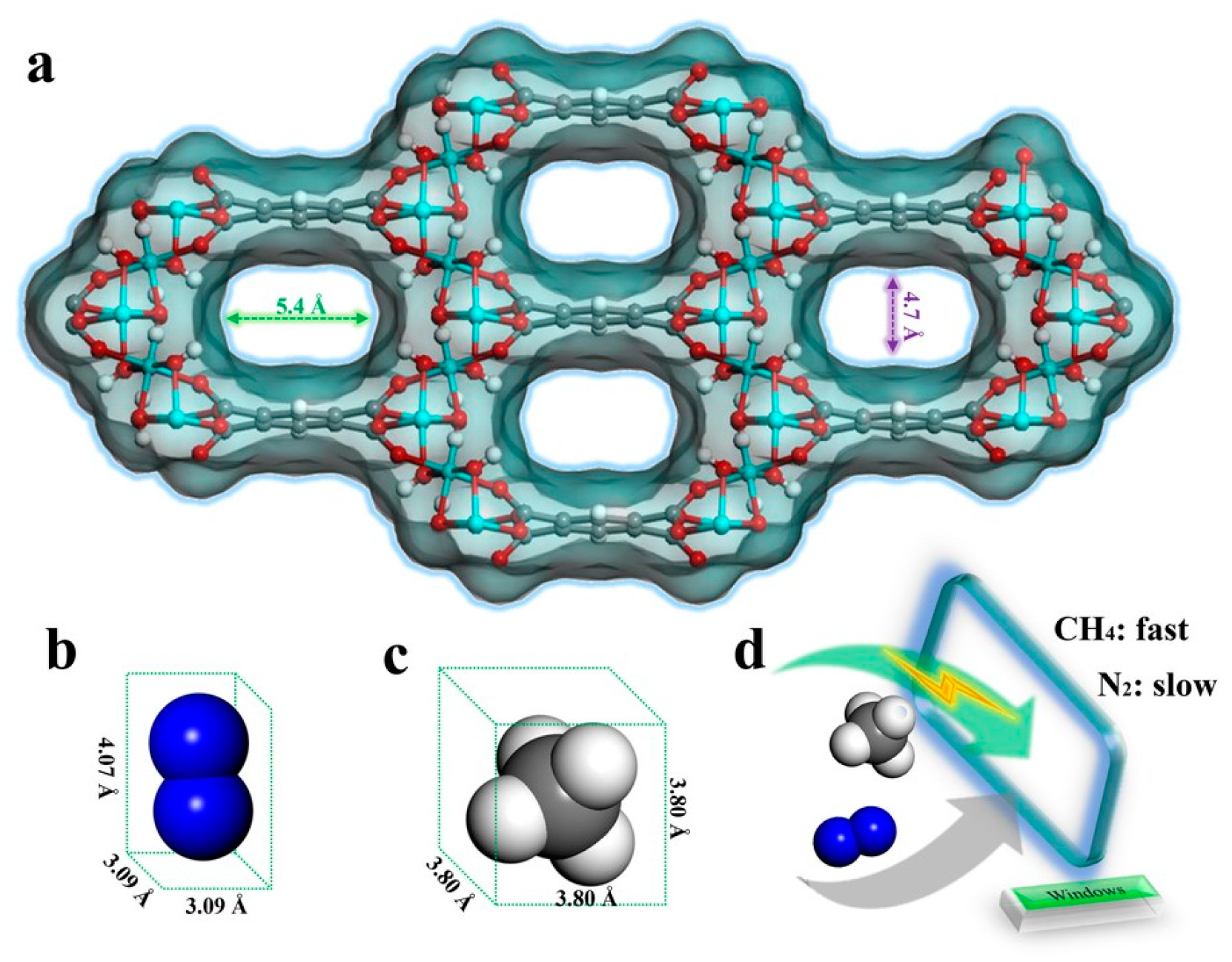
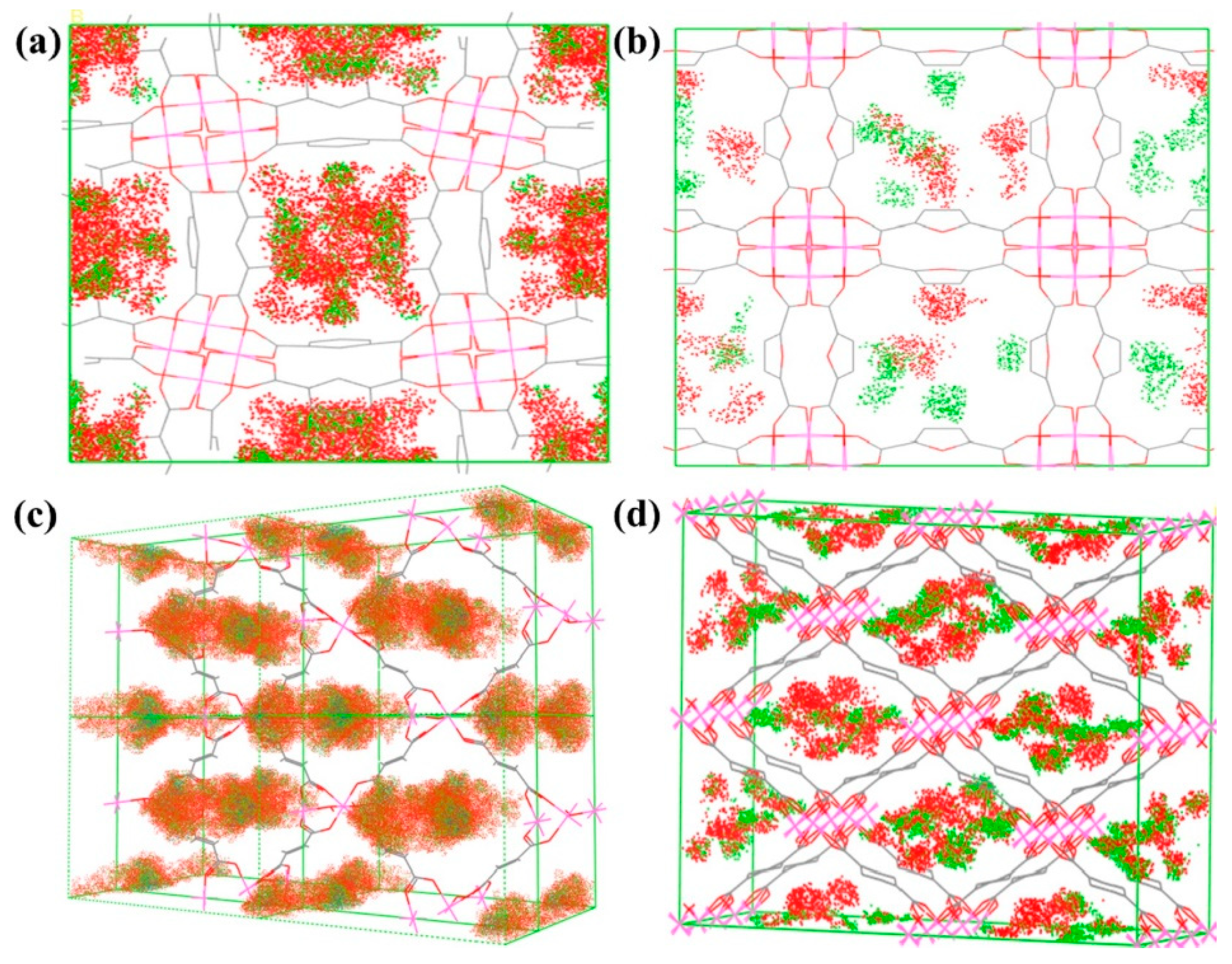

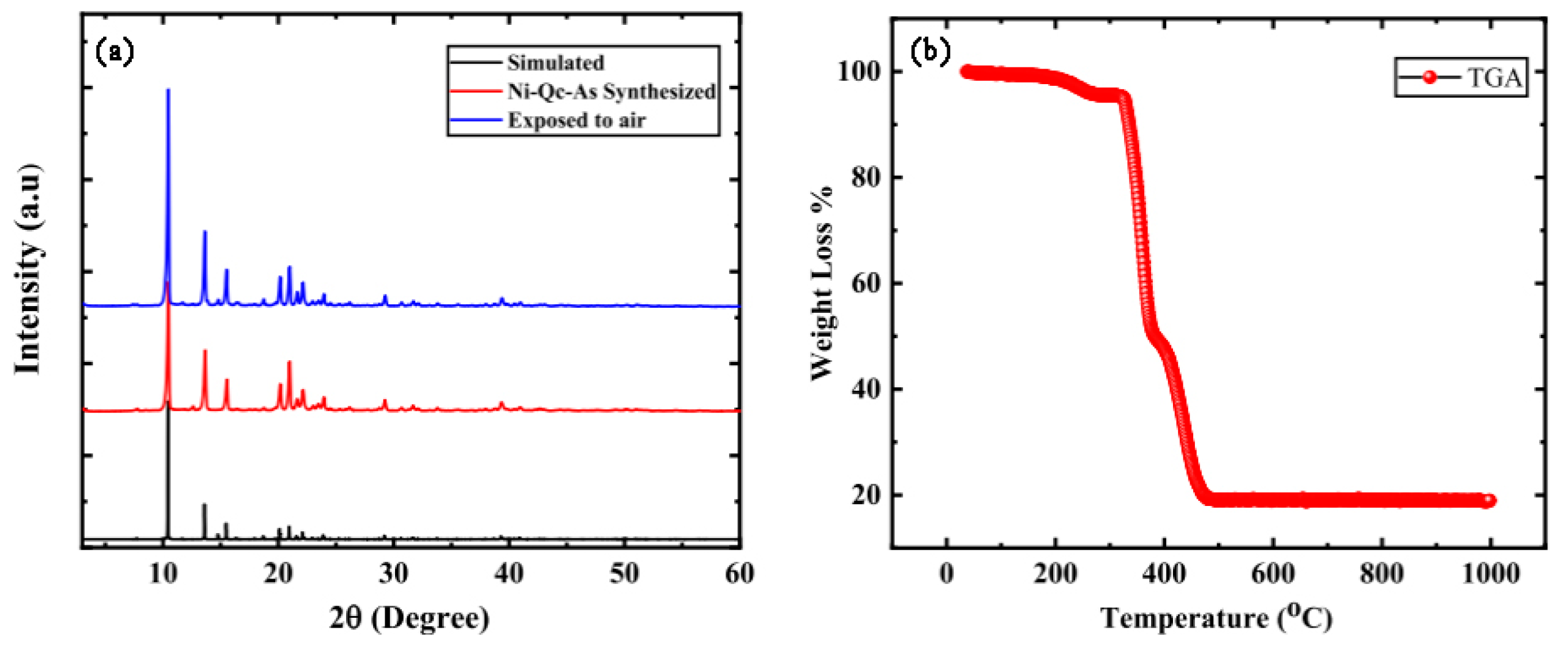
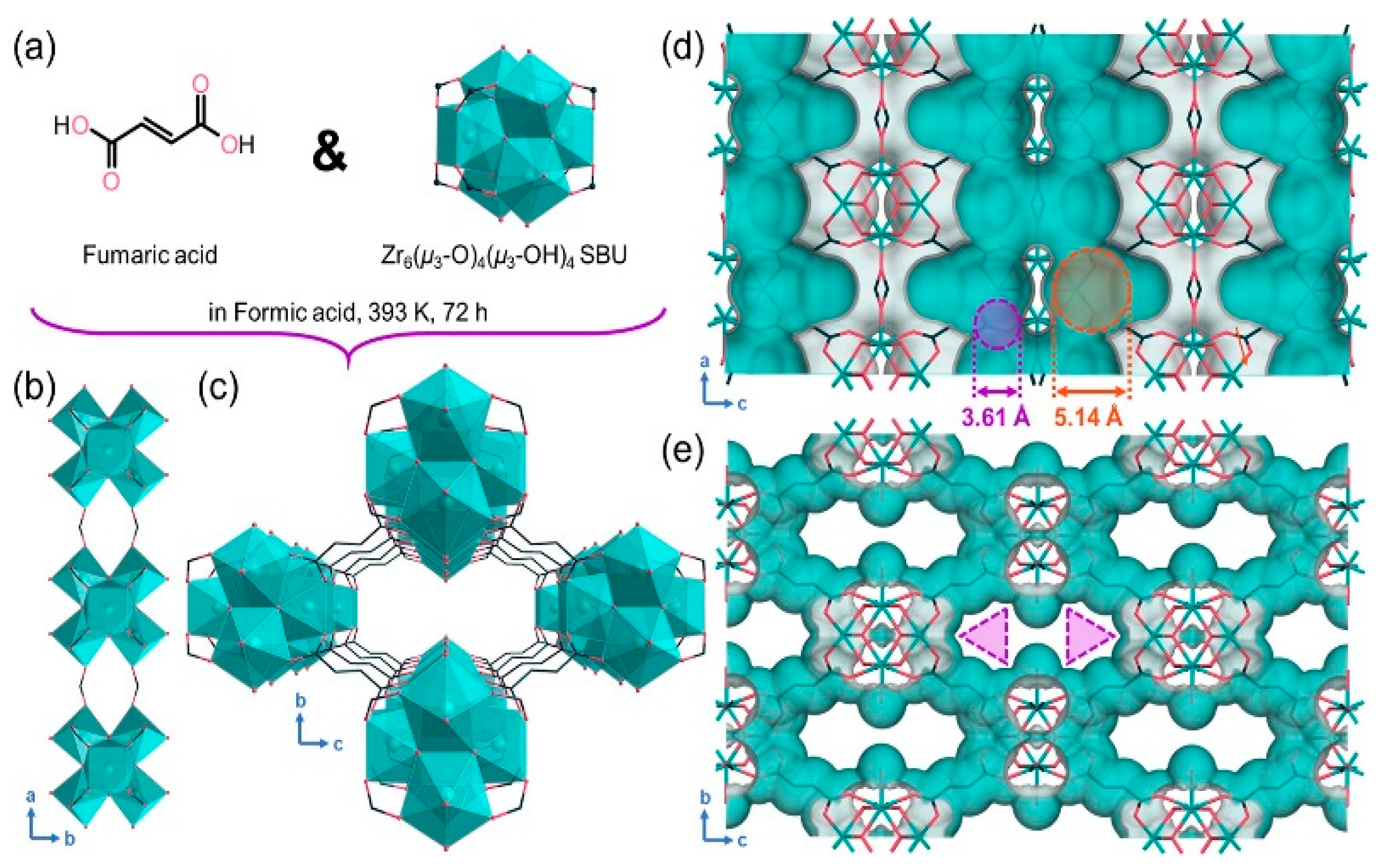
| Molecular | /nm | /nm3 | m) | m2) | Tc/K |
|---|---|---|---|---|---|
| CH4 | 0.380 | 2.448 × 10−3 | 0 | 0.067 × 10−40 | 190 |
| N2 | 0.364 | 1.710 × 10−3 | 0 | 5.134 × 10−40 | 126 |
| Purification Technology | Principle | Strengths and Weaknesses |
|---|---|---|
| Cryogenic distillation technology [16] | Utilizes the difference in boiling points between CH4 and N2 to separate the mixed gases after liquefaction. | The product gas has a high purity and recovery rate of CH4, but the operation conditions are demanding, the equipment investment is substantial, and the risk factor is high. |
| Membrane separation technology [17] | Utilizes the difference in the permeation rates of CH4 and N2 through the membrane, driven by pressure, through steps of dissolution, diffusion, and desorption to achieve purification. | High separation efficiency, low energy consumption, simple operation, and sustainable operation, but low membrane selectivity, high cost, and poor mechanical properties. |
| Chemical absorption technology [18] | Utilizes specific chemical absorbents to react with impurities in the CH4 gas, thereby achieving CH4 purification. | Good purification effect, low operating pressure, minimal CH4 loss, but high energy consumption for processing, complex regeneration process, and high investment cost. |
| Hydrate technology [19] | Utilizes the preferential encapsulation of CH4 over N2 in hydrate cavities when gas and water form hydrates under low temperature and high-pressure conditions, thereby achieving the separation of mixed gases. | Safe technology, highly efficient and energy-saving, with low pressure loss and short industrial trial process, but with low separation efficiency and relatively low product purity. |
| Adsorption separation technology [20,21] | Utilizes the high selectivity of solid adsorbents for CH4, leveraging the significant differences in gas adsorption capacities to achieve efficient CH4 purification. | Mature technology, low energy consumption, simple process, flexible operation, but high performance requirements for adsorbents. |
| AC | CH4/N2 Selectivity | CH4 Adsorption Capacity (mmol/g) | Reference |
|---|---|---|---|
| GAC(C-12) | 3.17 d | 2.3 d | [32] |
| C-12 | 4.8 e | 13.0 e | [33] |
| AC-1-400 | 3.38 f | 0.87 f | [34] |
| ClCTF-1-650 | 8.1 b | 1.47 b | [35] |
| N-WAPC | 7.62 b | 1.01 b | [36] |
| OTSS-2-450 | 4.9 b | 0.85 b | [37] |
| ACK2N1 | 7.11 a | 3.0 a | [38] |
| AC NH3·H2O-10% | 4.62 b | 1.1 b | [39] |
| KCl/AC | 5.33 b | 2.849 b | [40] |
| SCs | 5.7 b | 1.86 b | [41] |
| C-PVDC 700 | 14.7 b | 1.57 b | [42] |
| CMS-G | 4.74 c | 1.41 c | [43] |
| CMS-P-N | 3.32 b | 0.95 b | [44] |
| PCF-Co-0.5 | 0.97 b | 6.8 b | [45] |
| Zeolites | CH4/N2 Selectivity | CH4 Adsorption Capacity (mmol/g) | Reference |
|---|---|---|---|
| 5A | 2.5 b | 0.71 a | [66] |
| 13X | 1.23 b | 0.5 a | [66] |
| silicalite-1 | 3.92 b | 0.652 a | [66] |
| Beta | 2.58 b | 0.558 a | [66] |
| Chabazite-K | 5.5 a | 0.7 a | [67] |
| SAPO-34 | 3.1 a | 0.73 a | [67] |
| SSZ-13 | 2.7 a | 1.38 a | [67] |
| Nano-ZK-5 | 4.4 a | 1.3 a | [68] |
| TMAY | 6.32 a | 0.52 a | [69] |
| ChY | 6.5 a | 0.41 a | [69] |
| Na-ETS-4 | 2.65 c | 0.44 | [70] |
| HT-K-KFI | 4.6 a | 0.83 a | [71] |
| K-Chabazite | 5.5 a | 0.70 a | [72] |
| MOF | CH4/N2 Adsorptive Selectivity | CH4 Adsorption Capacity (mmol/g)−1 | Reference |
|---|---|---|---|
| MOF-5 | 1.13 d | 0.13 b | [87] |
| MOF-177 | 4.00 d | 0.59 b | [87] |
| [Cu(Me-4py-trz-ia)] | 4.5 b | 0.71 b | [88] |
| Basolite A100 | 5.0 b | 1.12 b | [88] |
| ROD-8 | 9.0 b | 0.77 b | [89] |
| Co-MA-BPY | 7.2 b | 0.92 b | [90] |
| AL-CDC@PA | 13.75 d | 1.32 b | [90] |
| MIL-120Al | 6.0 b | 1.3 b | [91] |
| AL-Fum | 17.2 a | 1.14 c | [92] |
| MIL-53(AL) | 6.8 a | 0.57 a | [92] |
| CAU-10-H | 7.0 a | 0.74 a | [92] |
| MIL-160 | 8.8 a | 0.47 c | [92] |
| SBMOF-1 | 11.5 b | 0.92 b | [93] |
| UTSA-30 | 5.0 b | 0.60 b | [94] |
| PAF-26-COOH | 4.2 b | 0.54 b | [95] |
| Ni3(HCOO)6 | 6.5 b | 0.81 b | [96] |
| ZIF-94 | 7.0 b | 1.51 b | [97] |
| UiO-66-Br2 | 5.06 b | 0.72 b | [98] |
| Ni(ina)2 | 15.8 b | 1.67 b | [99] |
| Ni-Qc-5 | 7.0 b | 1.30 b | [100] |
| NKMOF-8-Me | 9.0 d | 1.76 b | [101] |
| NKMOF-8-Br | 8.9 d | 1.84 b | [102] |
| MIP-203-F | 8.9 b | 1.16 b | [103] |
| Cu-MOF-SCH3 | 15.0 b | 0.67 b | [104] |
| [Cu(1,3-BDC)(H2O)]·2H2O | 2.1 d | 0.34 b | [105] |
| Cu(1,3-BDC)(PY)2 | 20.1 d | 0.72 b | [105] |
| [Co3(C4O4)2(OH)2] | 12.5 b | 0.88 b | [106] |
| ATC-Cu | 9.7 b | 2.90 b | [107] |
| CAU-21-BPDC | 11.9 b | 0.99 b | [108] |
| CAU-8-BPDC | 4.9 b | 0.85 b | [108] |
| Ni(4-DPDS)2CrO4 | 7.3 a | 0.95 a | [109] |
| CFAs-FumMOF-1 | 4.56 b | 0.844–0.895 b | [110] |
| Ni(TMBDC)(DABCO)0.5 | 5.1 e | 4.23 e | [111] |
| ZSTU-1 | 12–21.6 b | 1.37 b | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, D.; Chen, P.; Li, C.; Yan, Y.; Zhao, R.; Yue, Q.; Qiao, Y. Research Progress in Microporous Materials for Selective Adsorption and Separation of Methane from Low-Grade Gas. Molecules 2024, 29, 4404. https://doi.org/10.3390/molecules29184404
Su D, Chen P, Li C, Yan Y, Zhao R, Yue Q, Qiao Y. Research Progress in Microporous Materials for Selective Adsorption and Separation of Methane from Low-Grade Gas. Molecules. 2024; 29(18):4404. https://doi.org/10.3390/molecules29184404
Chicago/Turabian StyleSu, Dongrui, Panpan Chen, Cunlei Li, Yongfei Yan, Ranlei Zhao, Qingyou Yue, and Yupeng Qiao. 2024. "Research Progress in Microporous Materials for Selective Adsorption and Separation of Methane from Low-Grade Gas" Molecules 29, no. 18: 4404. https://doi.org/10.3390/molecules29184404






