Alkylation of Complex Glycine Precursor (CGP) as a Prebiotic Route to 20 Proteinogenic Amino Acids Synthesis
Abstract
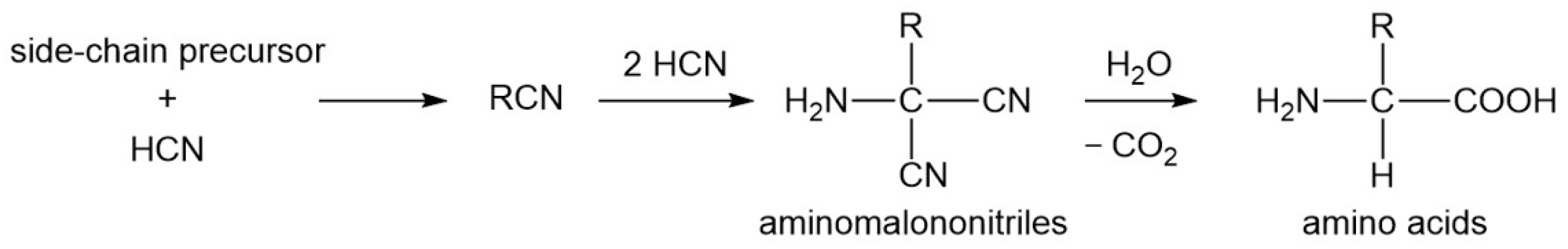
:1. Introduction
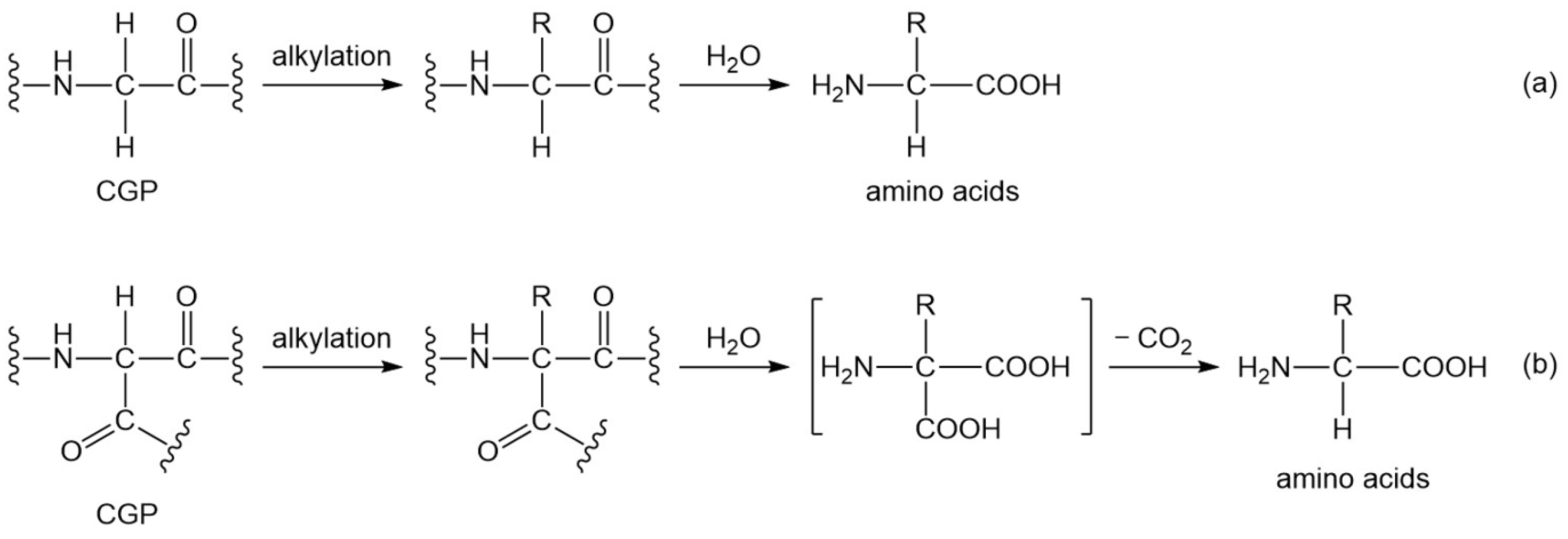
2. Basic Scheme of the New Polyglycine Scenario
3. Formation of the 20 Proteinogenic Amino Acids
3.1. Four Simple Amino Acids (Gly, Ala, Asn, and Asp)
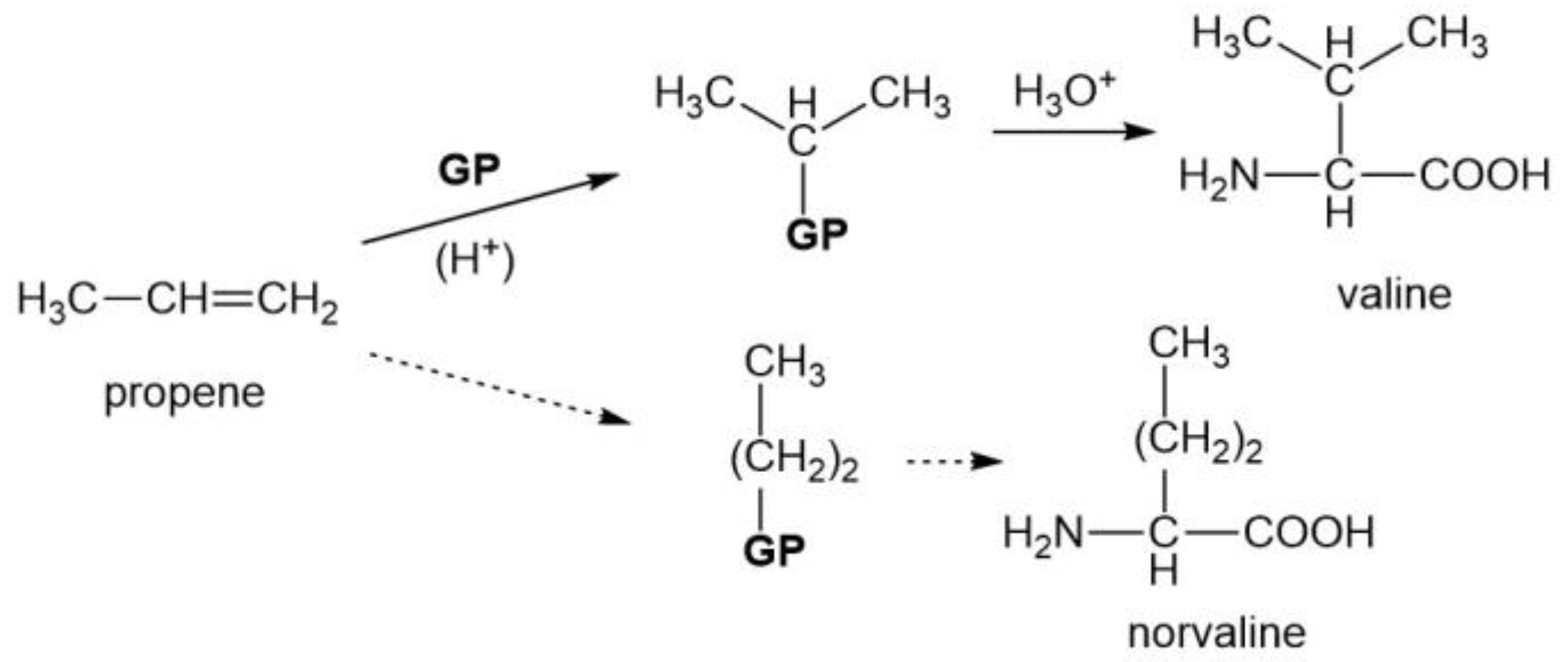
3.2. Three Aliphatic Amino Acids (Val, Ile, and Leu)
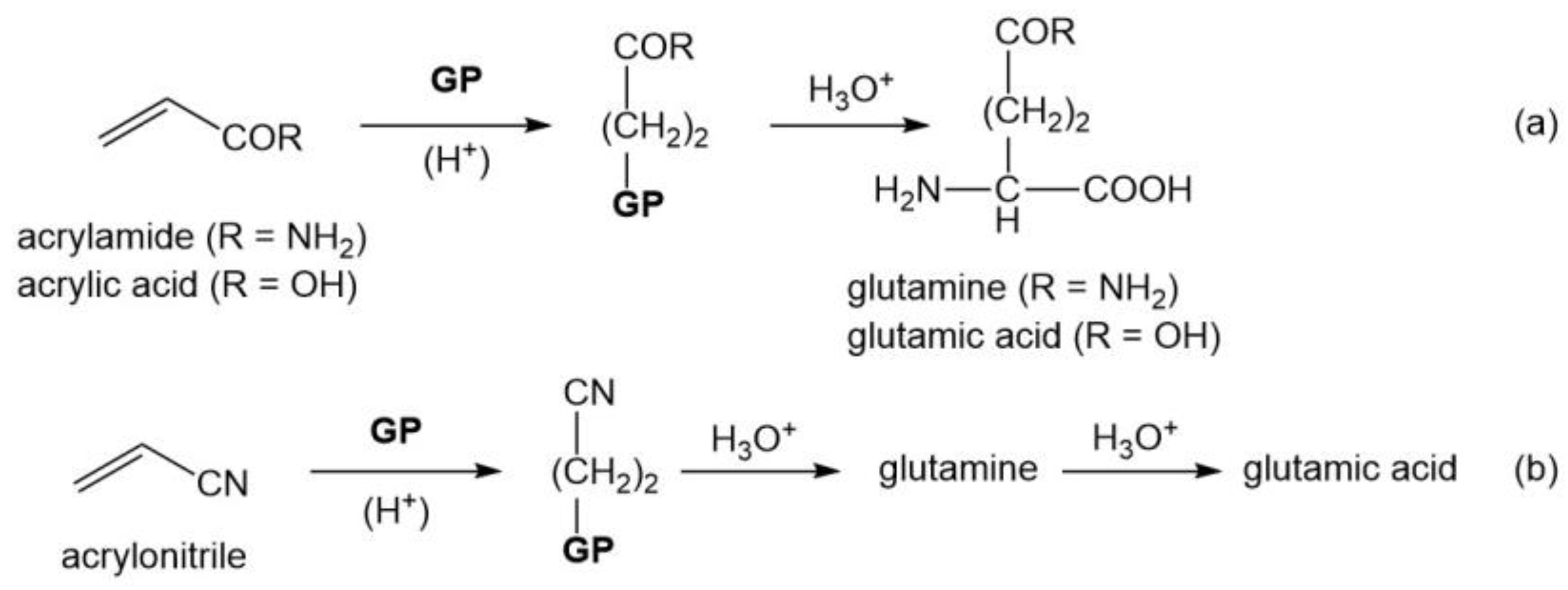
3.3. Four Oxygenated Amino Acids (Gln, Glu, Ser, and Thr)
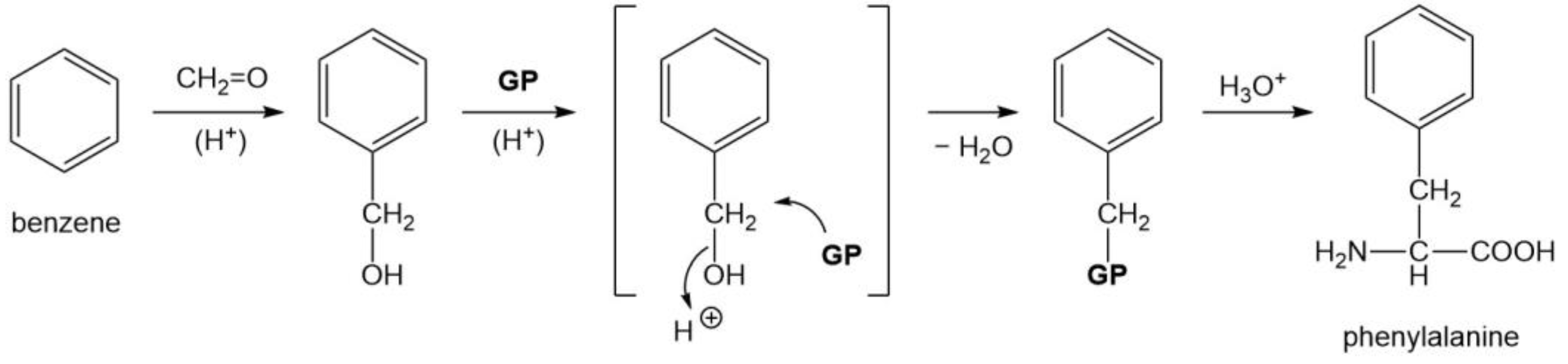
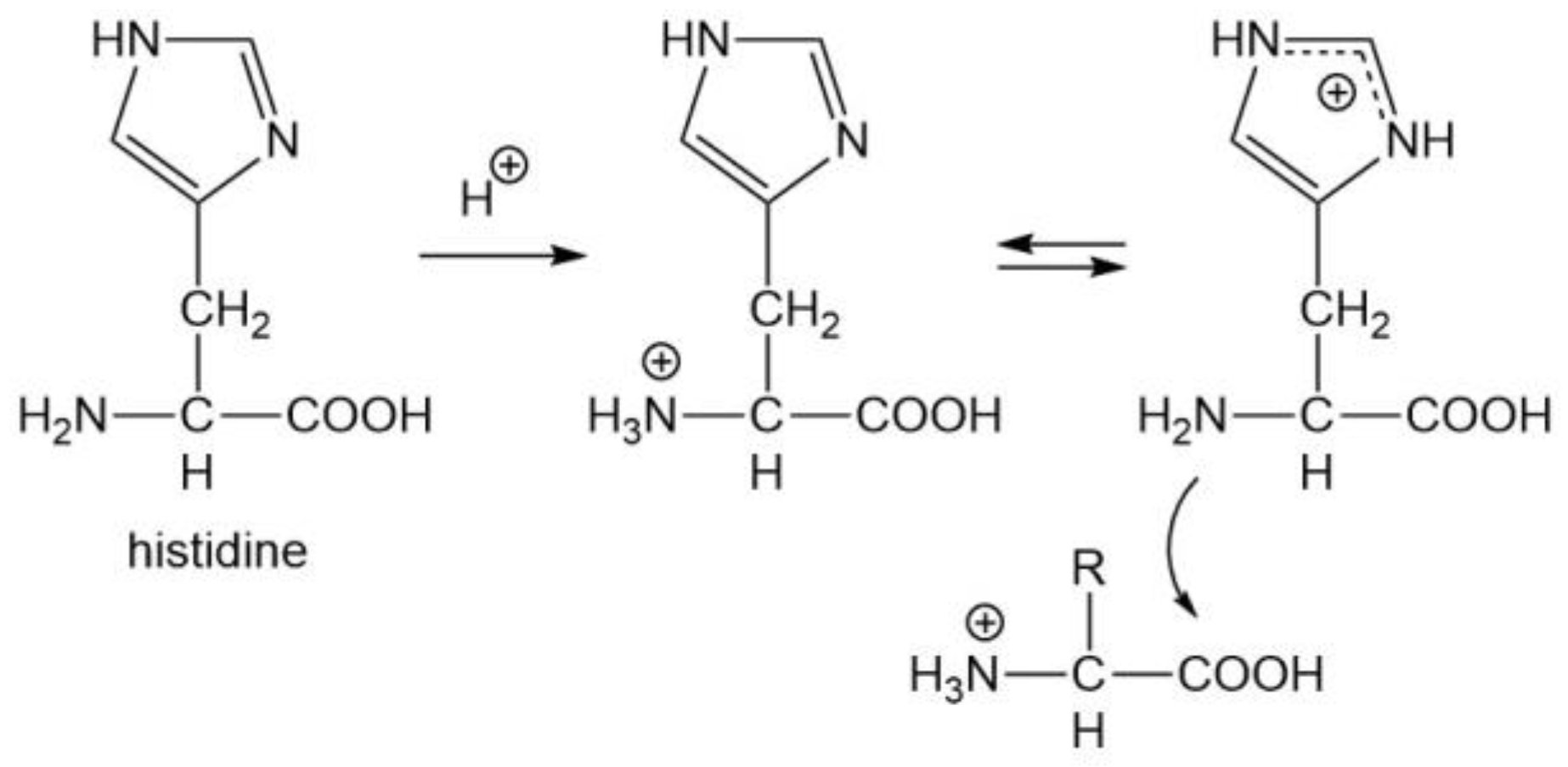
3.4. Four Aromatic Amino Acids (Phe, Tyr, His, and Trp)
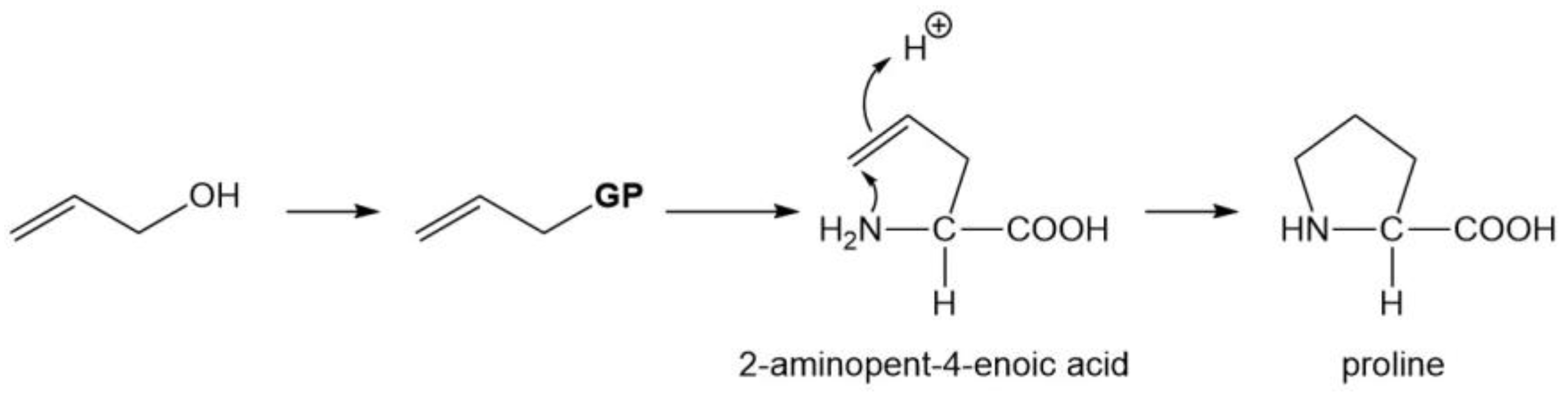
3.5. One Secondary Amino Acid (Pro)
3.6. Two More Basic Amino Acids (Lys and Arg)
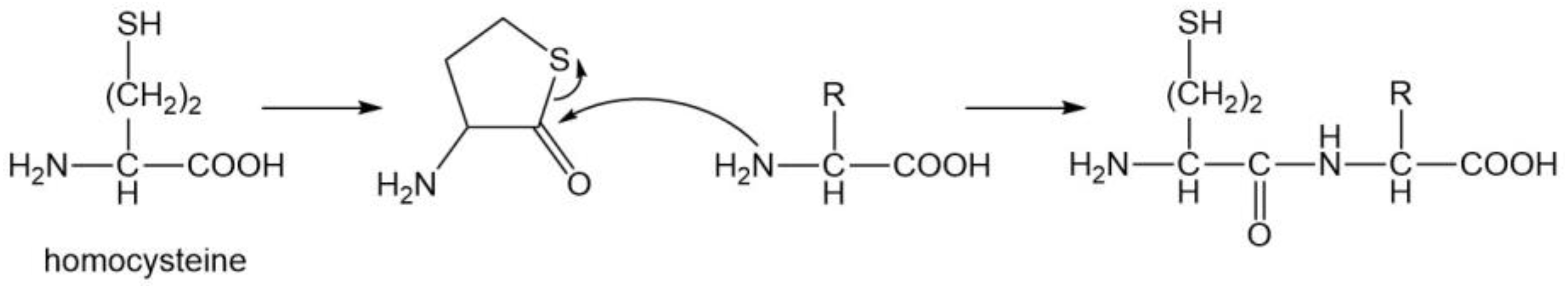
3.7. Two Sulfur-Containing Amino Acids (Cys and Met)
3.8. Why Are There No Other Precursor Compounds
4. Abiotic Peptide Formation
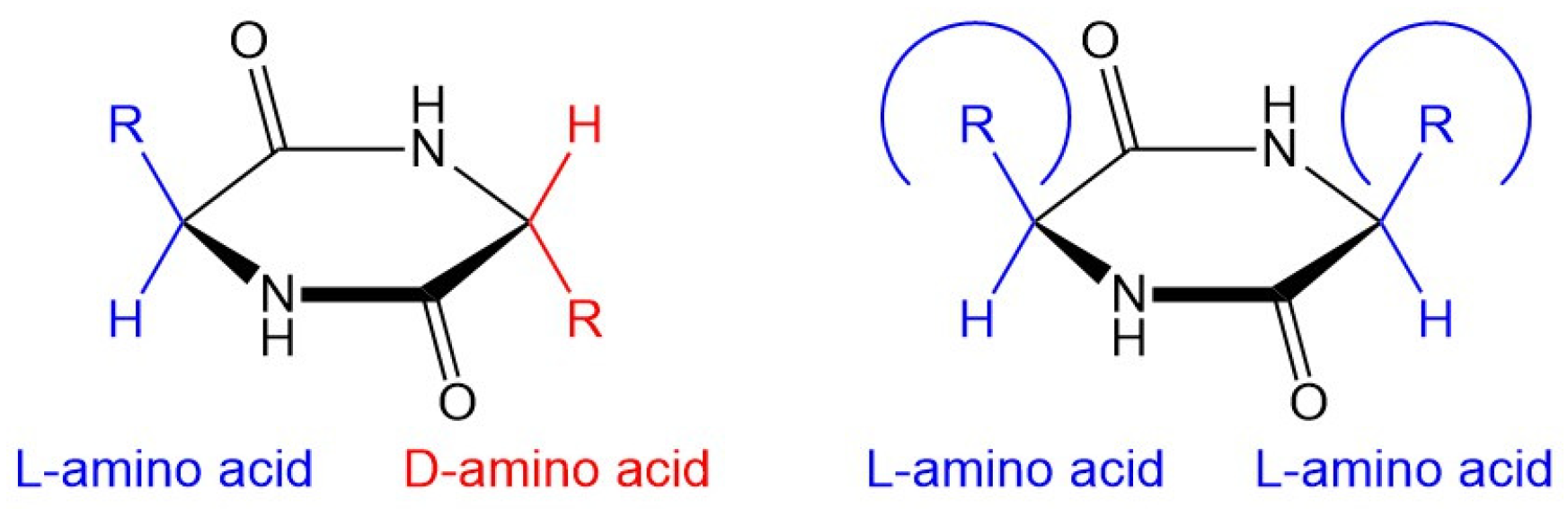
5. Asymmetry
6. Reaction Conditions and Environments
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, S.L. A production of amino acids under possible primitive Earth conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. Production of some organic compounds under possible primitive earth conditions. J. Am. Chem. Soc. 1955, 77, 2351–2361. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. II. Hydrogen cyanide, formaldehyde and ammonia. J. Mol. Evol. 1983, 19, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C.; Khare, B.N. Long-wavelength ultraviolet photoproduction of amino acids on the primitive Earth. Science 1971, 173, 417–420. [Google Scholar] [CrossRef]
- Harada, K.; Fox, S.W. Thermal synthesis of natural amino-acids from a postulated primitive terrestrial atmosphere. Nature 1964, 201, 335–336. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Lemmon, R.M.; Mariner, R.; Calvin, M. Formation of adenine by electron irradiation of methane, ammonia and water. Proc. Natl. Acad. Sci. USA 1963, 49, 737–740. [Google Scholar] [CrossRef]
- Bar-Nun, A.; Bar-Nun, N.; Bauer, S.H.; Sagan, C. Shock synthesis of amino acids in simulated primitive environments. Science 1970, 168, 470–473. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Furukawa, Y.; Kobayashi, T.; Sekine, T.; Terada, N.; Kakegawa, T. Impact-induced amino acid formation on Hadean Earth and Noachian Mars. Sci. Rep. 2020, 10, 9220. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kaneko, T.; Saito, T.; Oshima, T. Amino acid formation in gas mixtures by particle irradiation. Orig. Life Evol. Biosph. 1998, 28, 155–165. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ise, J.; Aoki, R.; Kinoshita, M.; Naito, K.; Udo, T.; Kunwar, B.; Takahashi, J.; Shibata, H.; Mita, H.; et al. Formation of amino acids and carboxylic acids in weakly reducing planetary atmospheres by solar energetic particles from the young sun. Life 2023, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, G.; Miller, S.L. Prebiotic synthesis in atmospheres containing CH4, CO, and CO2. I. Amino acids. J. Mol. Evol. 1983, 19, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison Meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Ertem, G.; Ferris, J.P.; Greenberg, J.M.; McCain, P.J.; Mendoza-Gomez, C.X.; Schutte, W. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig. Life Evol. Biosph. 1992, 22, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, T.; Kaneko, T.; Saito, T.; Kobayashi, K. Formation of organic compounds in simulated interstellar media with high energy particles. Bull. Chem. Soc. Jpn. 1997, 70, 1021–1026. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones Segovia, A.; Rosenbauer, H.; Thiemann, W.H.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef]
- Naraoka, H.; Takano, Y.; Dworkin, J.P.; Oba, Y.; Hamase, K.; Furusho, A.; Ogawa, N.O.; Hashiguchi, M.; Fukushima, K.; Aoki, D.; et al. Soluble organic molecules in samples of the carbonaceous asteroid (162173) Ryugu. Science 2023, 379, eabn9033. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Chan, Q.H.S.; Tachibana, S.; Kobayashi, K.; Zolensky, M.E. One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci. Adv. 2017, 3, e1602093. [Google Scholar] [CrossRef]
- Glavin, D.P.; Burton, A.S.; Elsila, J.E.; Aponte, J.C.; Dworkin, J.P. The search for chemical asymmetry as a potential biosignature in our solar system. Chem. Rev. 2020, 120, 4660–4689. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino acids of the Murchison meteorite: II. Five carbon acyclic primary β-, γ-, and δ-amino alkanoic acids. Geochim. Cosmochim. Acta 1985, 49, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Maruyama, S. Origins of building blocks of life: A review. Geoscience Frontiers 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Zaia, D.A.M.; Zaia, C.T.B.V.; De Santana, H. Which amino acids should be used in prebiotic chemistry studies? Orig. Life Evol. Biosph. 2008, 38, 469–488. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kaneko, T.; Takahashi, J.; Takano, Y.; Yoshida, S. High-molecular-weight complex organics in interstellar space and their relevance to origin of life. In Astrobiology: Emergence, Search and Detection of Life; Basiuk, V.A., Ed.; American Scientific Publishers: Valencia, CA, USA, 2009; Chapter 7. [Google Scholar]
- Cleaves II, H.J. The origin of the biologically coded amino acids. J. Theor. Biol. 2010, 263, 490–498. [Google Scholar] [CrossRef]
- Makarov, M.; Rocha, A.C.S.; Krystufek, R.; Cherepashuk, I.; Dzmitruk, V.; Charnavets, T.; Faustino, A.M.; Labl, M.; Fujishima, K.; Fried, S.D.; et al. Early selection of the amino acid alphabet was adaptively shaped by biophysical constraints of foldability. J. Am. Chem. Soc. 2023, 145, 5320–5329. [Google Scholar] [CrossRef]
- Akahori, S. Asymmetric synthesis of amino acids and formation of primitive proteins [translated from Japanese]. Kagaku 1955, 25, 54–58. [Google Scholar]
- Kuroda, C. A plausible entry to 20 proteinogenic amino acids via aminomalononitrile derivatives. Nat. Prod. Commun. 2023, 18, 1–3. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sarker, P.K.; Ono, K.; Kawamoto, Y.; Obayashi, Y.; Kaneko, T.; Yoshida, S.; Mita, H.; Yabuta, H.; Yamagishi, A. Formation, alteration and delivery of exogenous high molecular weight of organic compounds: Objectives of the Tanpopo Mission from the point of view of chemical evolution. Trans. JSASS Aerospace Tech. Jpn. 2012, 10, Tp_7–Tp_11. [Google Scholar] [CrossRef]
- Lee, H.M.; Choe, J.C. Formation of glycine from HCN and H2O: A computational mechanistic study. Chem. Phys. Lett. 2017, 675, 6–10. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Asano, S.; Tani, A.; Yoda, I.; Kobayashi, K. Gamma-ray-induced amino acid formation in aqueous small bodies in the early solar system. ACS Cent. Sci. 2022, 8, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.J.; Jamieson, C.S.; Osamura, Y.; Kaiser, R.I. A combined experimental and computational investigation on the synthesis of acetaldehyde in interstellar ices. Astrophys. J. 2005, 624, 1097–1115. [Google Scholar] [CrossRef]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B.H. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef]
- Manabe, K.; Iimura, S.; Sun, X.-M.; Kobayashi, S. Dehydration reactions in water. Brønsted acid-surfactant-combined catalyst for ester, ether, thioether, and dithioacetal formation in water. J. Am. Chem. Soc. 2002, 124, 11971–11978. [Google Scholar] [CrossRef]




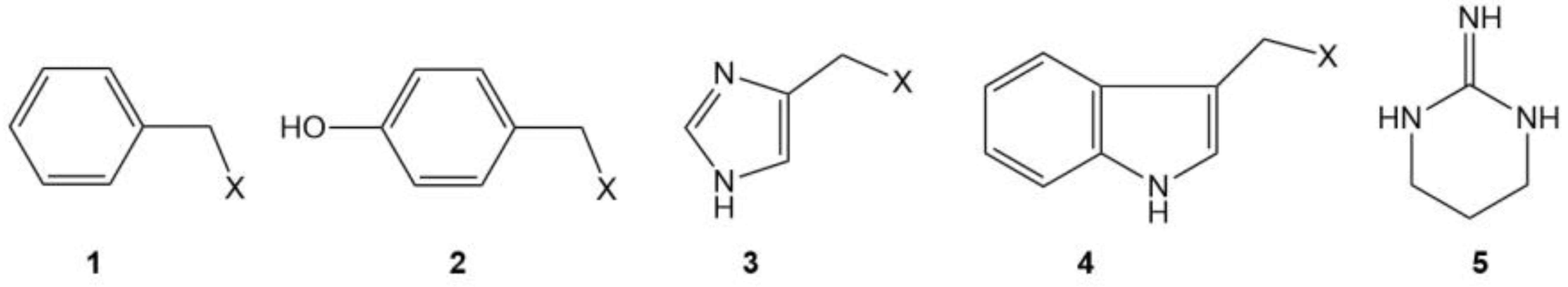
| Amino Acid | Side-Chain Precursor |
|---|---|
| Asn | H2NCH2CONH2 |
| Asp | H2NCH2COOH (Gly) |
| Val | CH2=CHMe |
| Ile | CHMe=CHMe, CH2=CHEt |
| Leu | CH2=CMe2 |
| Gln | CH2=CHCONH2, CH2=CHCN |
| Glu | CH2=CHCOOH, CH2=CHCN |
| Ser | HCHO |
| Thr | MeCHO |
| Phe | 1 2 |
| Tyr | 2 2 |
| His | 3 2 |
| Trp | 4 2 |
| Pro | CH2=CHCH2X 2 |
| Lys | pyrrolidine |
| Arg | 5 |
| Cys | CH2=S |
 | |
| No. | Features |
|---|---|
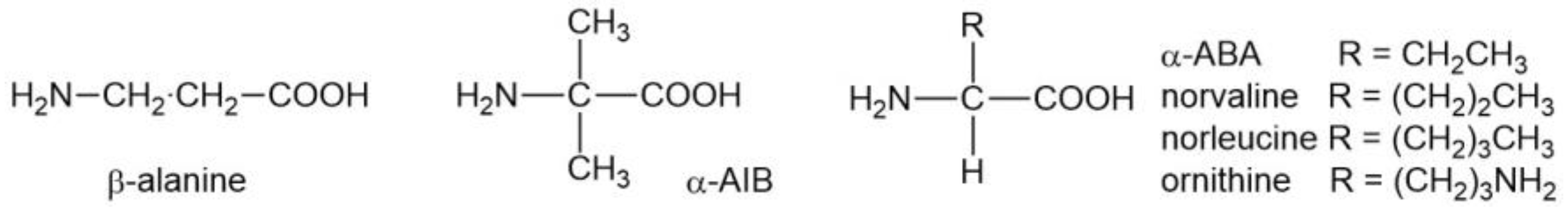
| i | All are α-amino acids. β-Alanine, for example, is not a member. |
| ii | One hydrogen atom is attached to the α-carbon. αAIB, for example, is not a member. |
| iii | All, except for Gly, have l-configuration with respect to the α-carbon. |
| iv | As aliphatic side chains, methyl (Ala), propyl (Val), and butyl (Ile/Leu) are present, but ethyl (αABA) is absent. |
| v | Only branched alkyl groups are present for the propyl and butyl side chains. Norvaline and norleucine are not members. |
| vi | All aromatic amino acids have one methylene between the aromatic ring and the α-carbon. |
| vii | Lys has four methylenes. Its shorter analogues, such as ornithine, are not members. |
| viii | Arg has three methylenes. Its shorter analogues are not members. |
| ix | Gln and Glu have two methylenes. |
| x | Tyr has an OH group on the para-position. Its meta- and ortho-isomers are not members. |
| xi | Trp has an indole group substituted at C-3. |
| xii | One amino acid (Pro) has a secondary amino group. |
| xiii | Structurally similar amino acids are present (Asp/Gln, Asp/Glu, and Val/Ile/Leu). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, C.; Kobayashi, K. Alkylation of Complex Glycine Precursor (CGP) as a Prebiotic Route to 20 Proteinogenic Amino Acids Synthesis. Molecules 2024, 29, 4403. https://doi.org/10.3390/molecules29184403
Kuroda C, Kobayashi K. Alkylation of Complex Glycine Precursor (CGP) as a Prebiotic Route to 20 Proteinogenic Amino Acids Synthesis. Molecules. 2024; 29(18):4403. https://doi.org/10.3390/molecules29184403
Chicago/Turabian StyleKuroda, Chiaki, and Kensei Kobayashi. 2024. "Alkylation of Complex Glycine Precursor (CGP) as a Prebiotic Route to 20 Proteinogenic Amino Acids Synthesis" Molecules 29, no. 18: 4403. https://doi.org/10.3390/molecules29184403






