Modulating the Conductivity of Light-Responsive Ionic Liquid Crystals
Abstract
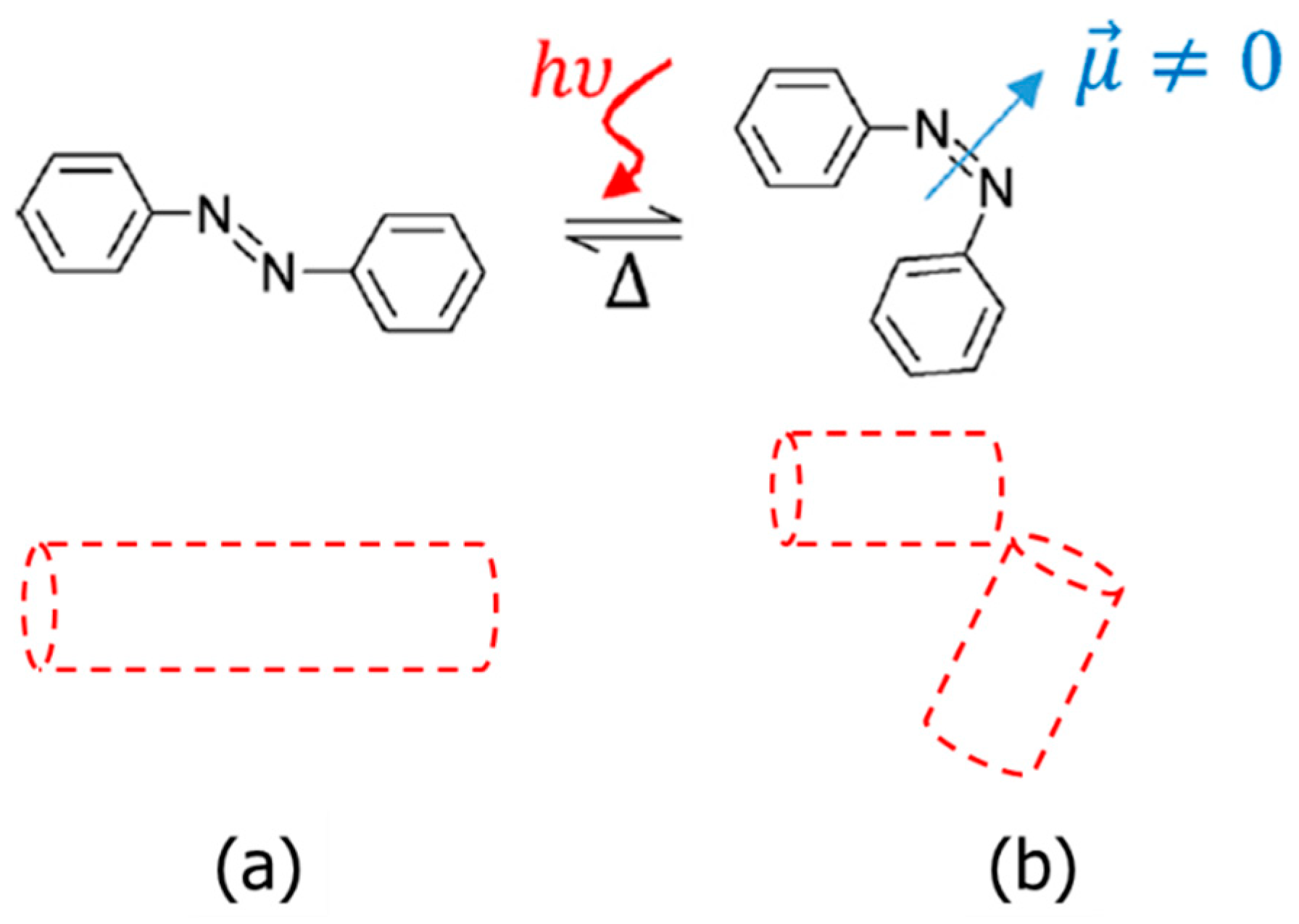
:1. Introduction
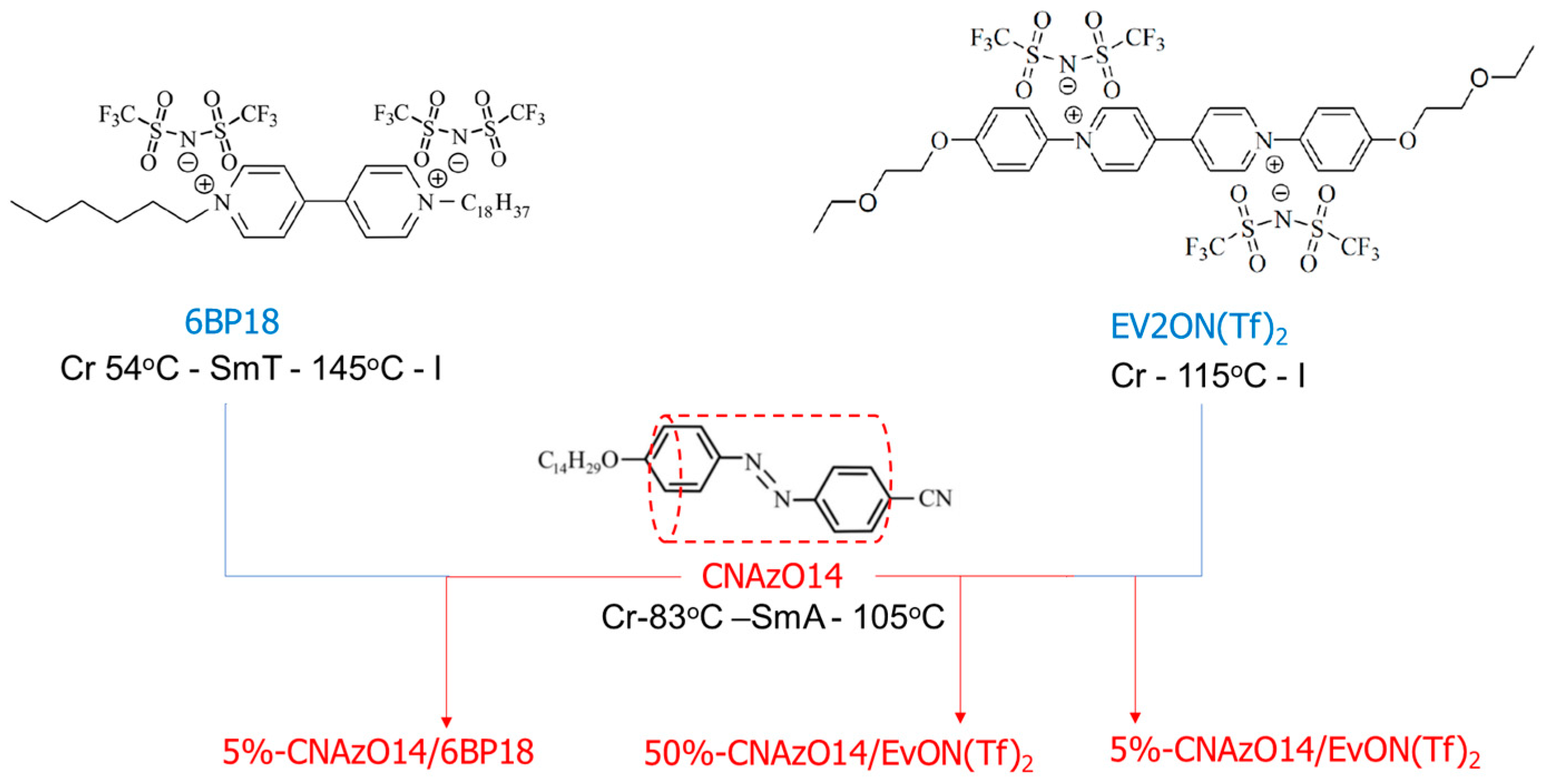
2. Experimental Procedure
2.1. Materials
2.2. Techniques and Methods
3. Results and Discussion
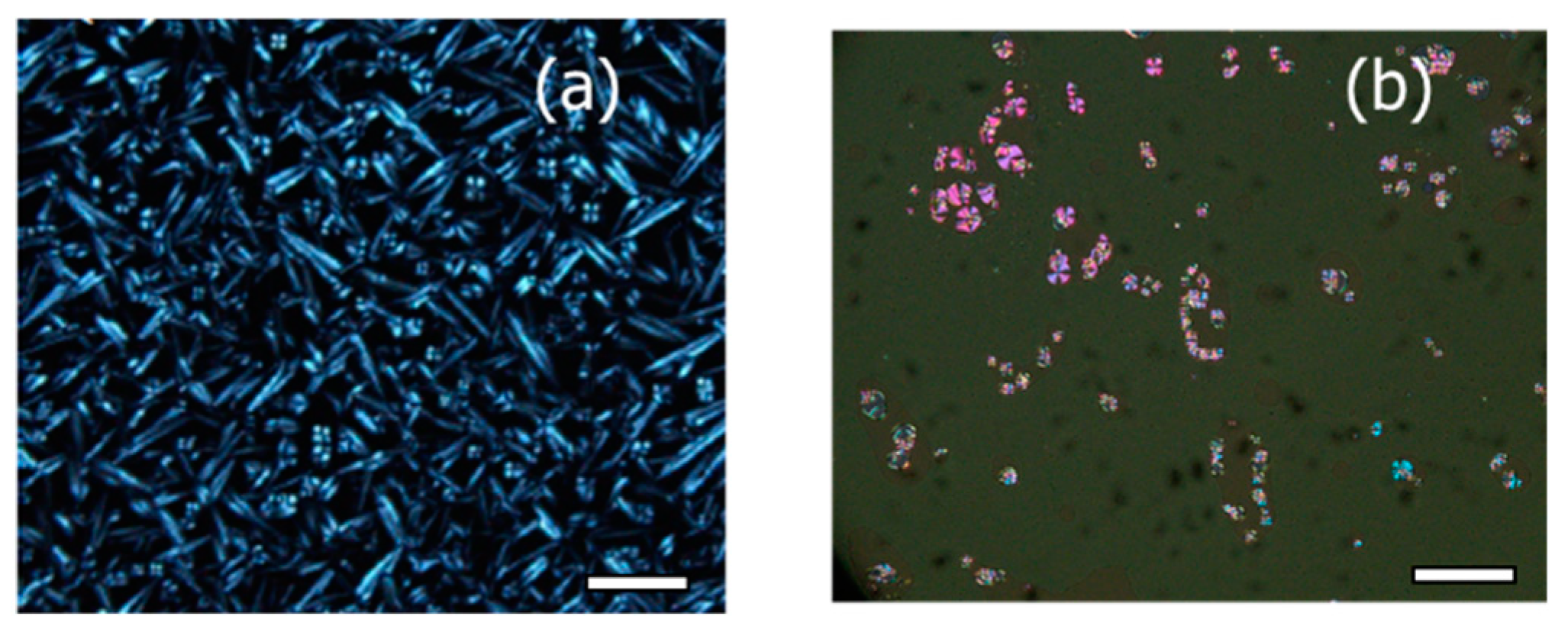
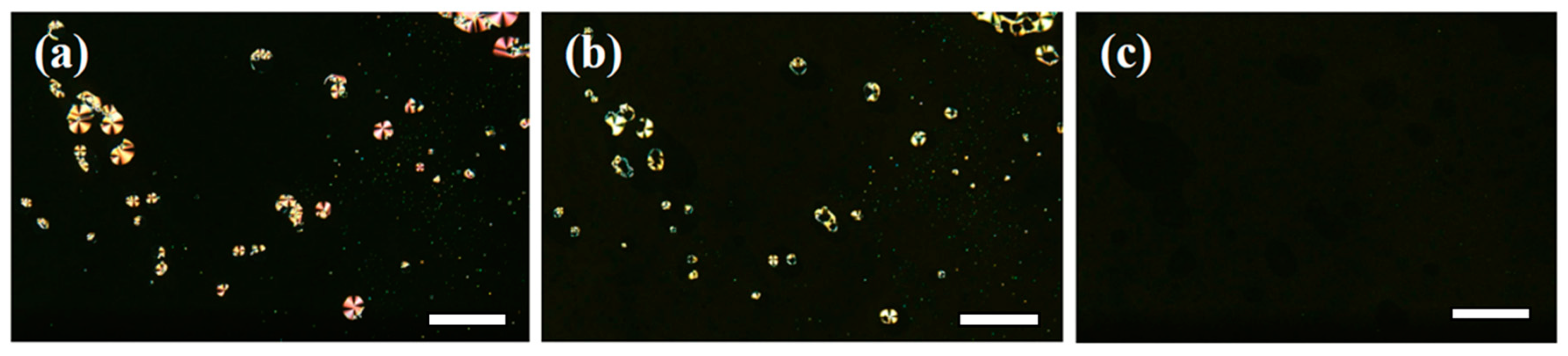
3.1. Phase Behaviour and Conductivity
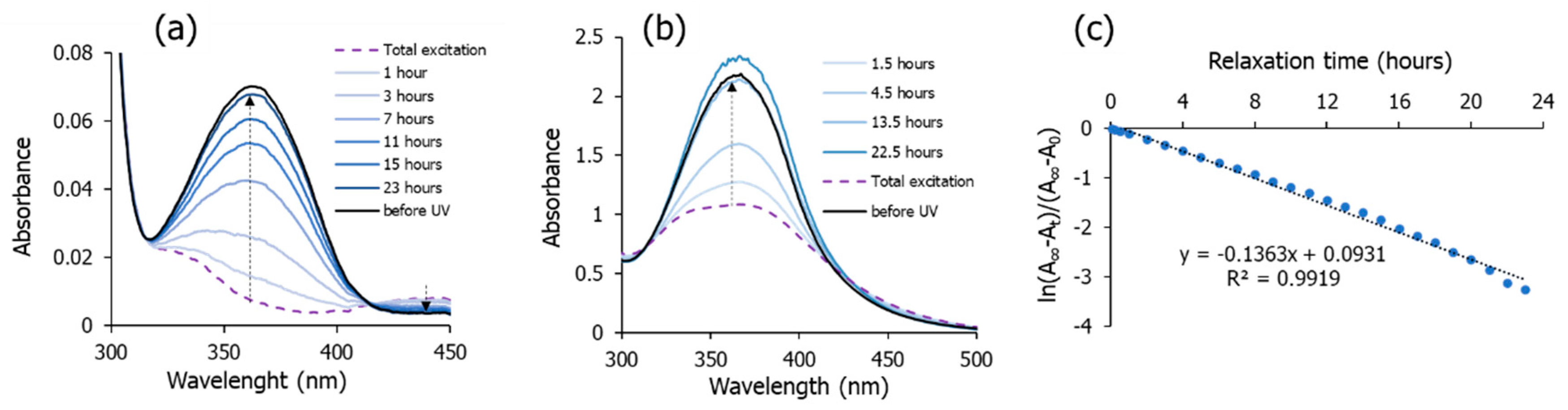
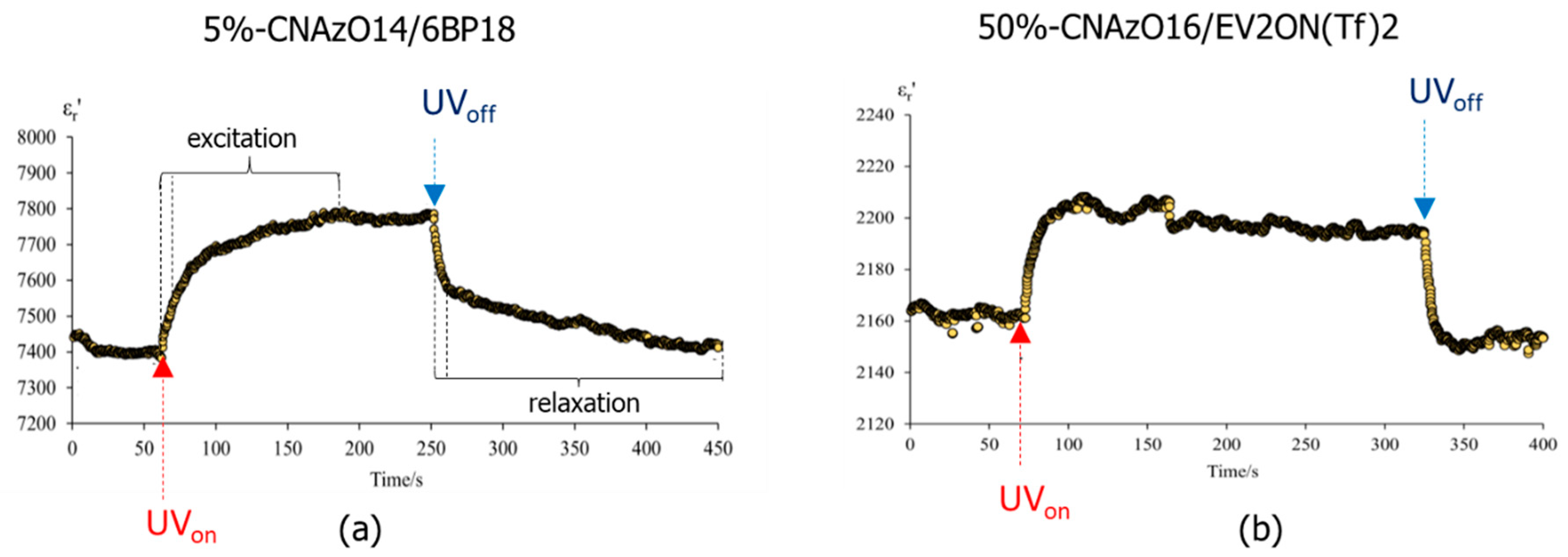
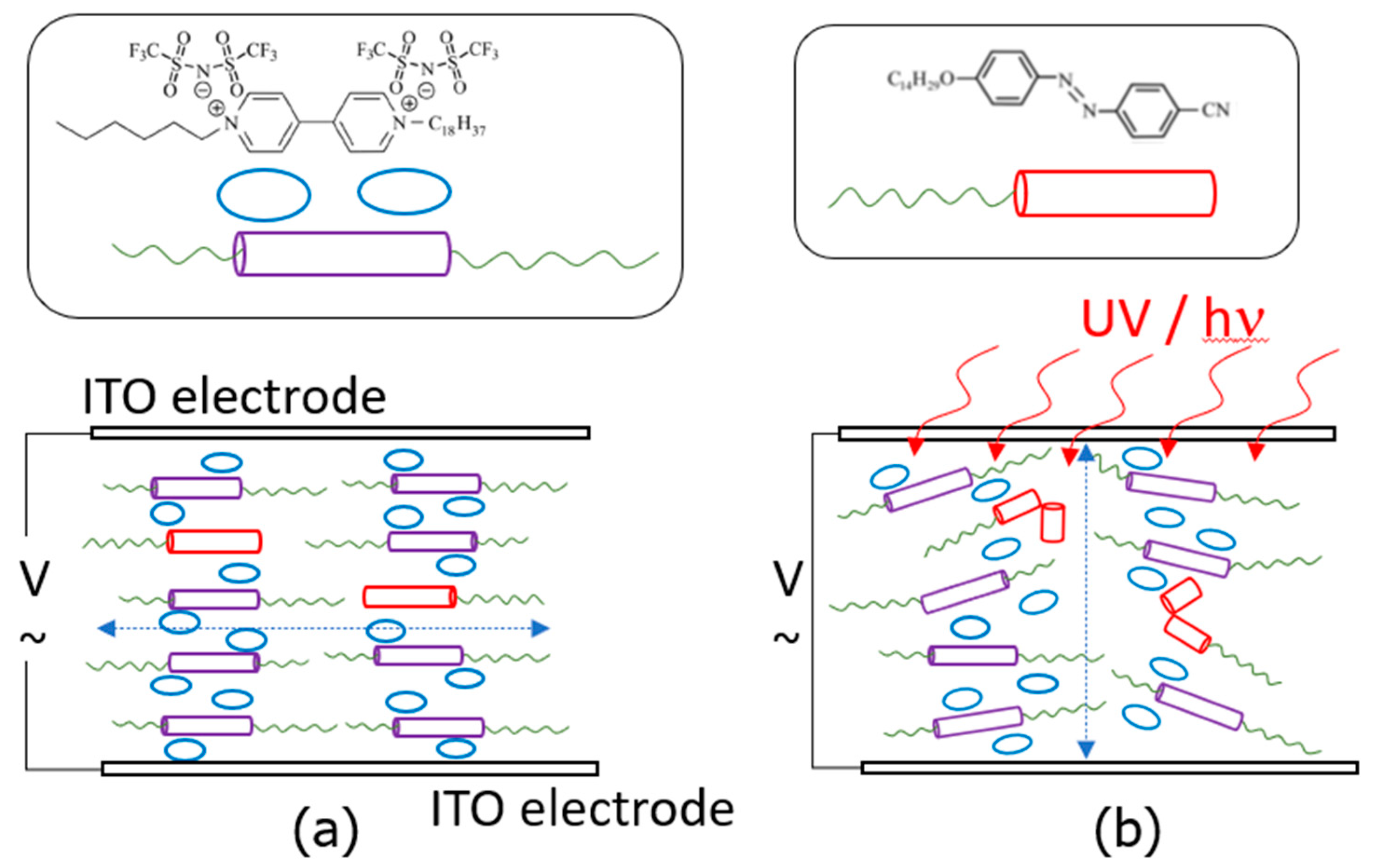
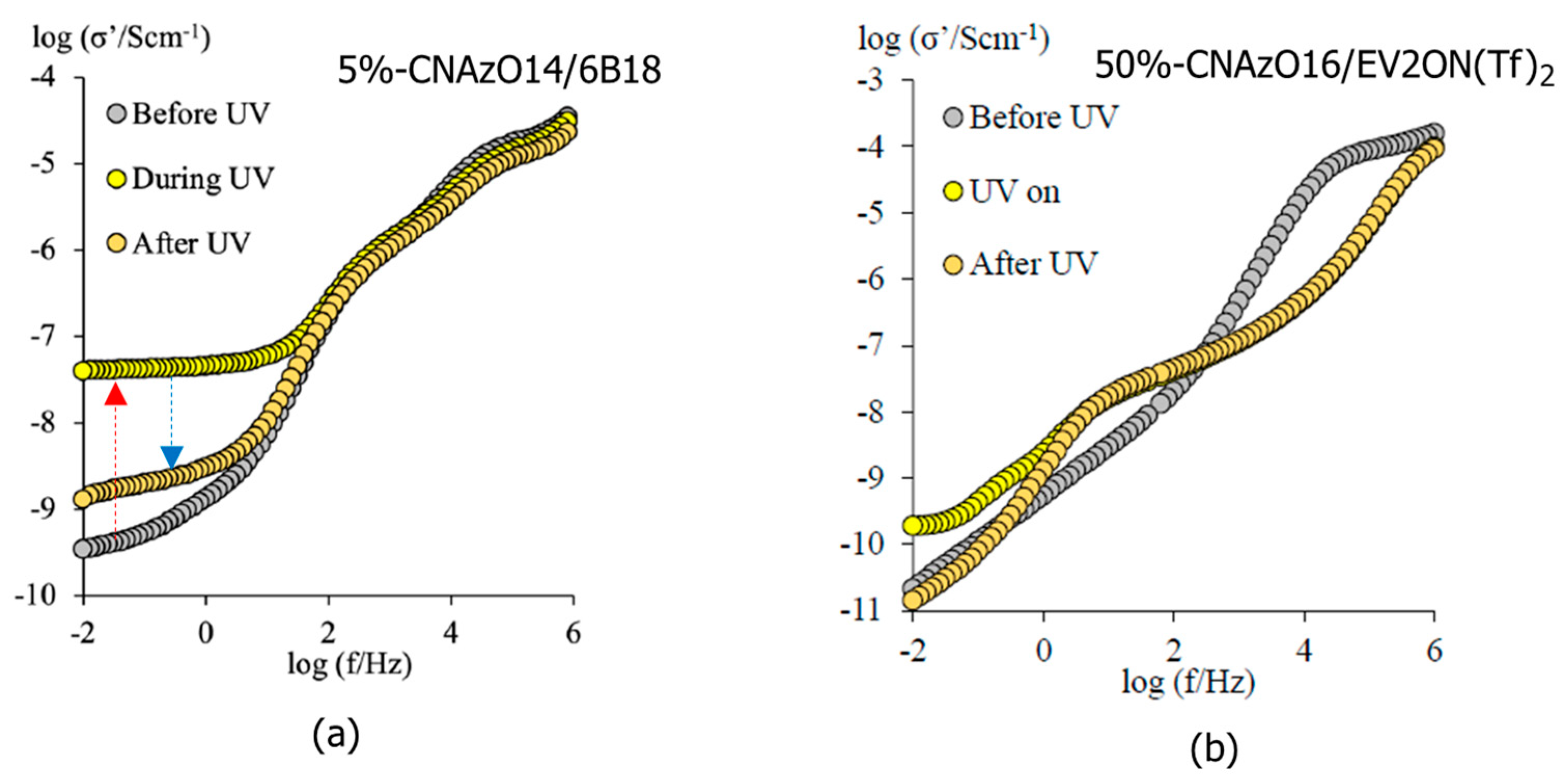
3.2. Light-Responsive Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Ruan, Q.; Yao, M.; Yuan, D.; Dong, H.; Liu, J.; Yuan, X.; Fang, W.; Zhao, G.; Zhang, H. Ionic liquid crystal electrolytes: Fundamental, applications and prospects. Nano Energy 2023, 106, 108087. [Google Scholar] [CrossRef]
- Álvarez Moisés, I.; Innocenti, A.; Somville, M.; Notredame, B.; Passerini, S.; Gohy, J.F. Liquid crystals as additives in solid polymer electrolytes for lithium metal batteries. Mrs Adv. 2023, 8, 797–802. [Google Scholar] [CrossRef]
- Salikolimi, K.; Sudhakar, A.; Ishida, Y. Functional Ionic Liquid Crystals. Langmuir 2020, 36, 11702–11731. [Google Scholar] [CrossRef] [PubMed]
- Kapernaum, N.; Lange, A.; Ebert, M.; Grunwald, M.A.; Haege, C.; Marino, S.; Zens, A.; Taubert, A.; Giesselmann, F.; Laschat, S. Current Topics in Ionic Liquid Crystals. Chempluschem 2022, 87, e202100397. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Cox, S.L.; Chen, S.L.; Smith, J.; King, D.; Han, H.; Velayutham, T.S.; Martinez-Felipe, A. Dicationic Ionic Salts as Electrolytes for the Next Generation of Batteries Including Solid State Batteries; IntechOpen: London, UK, 2024; pp. 1–19. [Google Scholar]
- Liu, Q.; Liu, H.; Zhang, W.; Ma, Q.; Xu, Q.; Hooshyari, K.; Su, H. Enhancing Polymer Electrolyte Membrane Fuel Cells with Ionic Liquids: A Review. Chem. A Eur. J. 2024, 30, e202303525. [Google Scholar] [CrossRef]
- Gabryelczyk, A.; Swiderska-Mocek, A. Tailoring the Properties of Gel Polymer Electrolytes for Sodium-Ion Batteries Using Ionic Liquids: A Review. Chem. A Eur. J. 2024, 30, e202304207. [Google Scholar] [CrossRef]
- Pajak, M.; Hubkowska, K.; Monikowska, D.; Lota, G.; Czerwinski, A. Ionic liquids as electrolytes: New perspectives for protonic systems and Ni-MH batteries. A mini review. Energy Convers. Manag. X 2023, 20, 100500. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Koh, J.J.; King, D.; Han, H.; Heinrich, B.; Donnio, B.; Zaton, D.; Martinez-Felipe, A. Dicationic stilbazolium salts: Structural, thermal, optical, and ionic conduction properties. J. Mol. Liq. 2021, 341, 117311. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Noori, O.; Chen, S.L.; Han, H.; Fisch, M.R.; Robb, C.M.; Variyam, A.; Martinez-Felipe, A. Ionic liquid crystals: Synthesis and characterization via NMR, DSC, POM, X-ray diffraction and ionic conductivity of asymmetric viologen bistriflimide salts. J. Mol. Liq. 2021, 328, 115370. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Si, S.L.; Han, H.; Ishak, K.A.; Velayutham, T.S.; Bendaoud, U.; Martinez-Felipe, A. Dicationic Ionic Liquids based on Bis(4-oligoethyleneoxyphenyl) Viologen Bistriflimide Salts Exhibiting High Ionic Conductivities. J. Mol. Liq. 2022, 365, 120126. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Bielawski, C.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Suzawa, T.; Yokota, T.; Mukai, T.; Ohno, H.; Kato, T. Nano-segregated polymeric film exhibiting high ionic conductivities. J. Am. Chem. Soc. 2005, 127, 15618–15623. [Google Scholar] [CrossRef]
- Yoshio, M.; Kagata, T.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. One-dimensional ion-conductive polymer films: Alignment and fixation of ionic channels formed by self-organization of polymerizable columnar liquid crystals. J. Am. Chem. Soc. 2006, 128, 5570–5577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Moller, M.; Zhu, X.M.; Rueda, J.J.H.; Rosenthal, M.; Ivanov, D.A. From Channel-Forming Ionic Liquid Crystals Exhibiting Humidity-Induced Phase Transitions to Nanostructured Ion-Conducting Polymer Membranes. Adv. Mater. 2013, 25, 3543–3548. [Google Scholar] [CrossRef]
- Cho, B.K. Nanostructured organic electrolytes. RSC Adv. 2014, 4, 395–405. [Google Scholar] [CrossRef]
- Martinez-Felipe, A. Liquid crystal polymers and ionomers for membrane applications. Liq. Cryst. 2011, 38, 1607–1626. [Google Scholar] [CrossRef]
- Ueda, S.; Kagimoto, J.; Ichikawa, T.; Kato, T.; Ohno, H. Anisotropic Proton-Conductive Materials Formed by the Self-Organization of Phosphonium-Type Zwitterions. Adv. Mater. 2011, 23, 3071–3074. [Google Scholar] [CrossRef]
- Concellon, A.; Hernandez-Ainsa, S.; Barbera, J.; Romero, P.; Serrano, J.L.; Marcos, M. Proton conductive ionic liquid crystalline poly(ethyleneimine) polymers functionalized with oxadiazole. RSC Adv. 2018, 8, 37700–37706. [Google Scholar] [CrossRef]
- Concellon, A.; Liang, T.; Schenning, A.; Serrano, J.L.; Romero, P.; Marcos, M. Proton-conductive materials formed by coumarin photocrosslinked ionic liquid crystal dendrimers. J. Mater. Chem. C 2018, 6, 1000–1007. [Google Scholar] [CrossRef]
- Alauddin, S.M.; Ibrahim, A.R.; Aripin, N.F.K.; Velayutham, T.S.; Abou-Zied, O.K.; Martinez-Felipe, A. New side-chain liquid crystalline terpolymers with anhydrous conductivity: Effect of azobenzene substitution on light response and charge transfer. Eur. Polym. J. 2021, 146, 110246. [Google Scholar] [CrossRef]
- Alauddin, S.M.; Aripin, N.F.K.; Velayutham, T.S.; Martinez-Felipe, A. Liquid Crystalline Copolymers Containing Sulfonic and Light-Responsive Groups: From Molecular Design to Conductivity. Molecules 2020, 25, 2579. [Google Scholar] [CrossRef] [PubMed]
- Vanti, L.; Mohd Alauddin, S.; Zaton, D.; Aripin, N.F.K.; Giacinti-Baschetti, M.; Imrie, C.T.; Ribes-Greus, A.; Martinez-Felipe, A. Ionically conducting and photoresponsive liquid crystalline terpolymers: Towards multifunctional polymer electrolytes. Eur. Polym. J. 2018, 109, 124–132. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Bhowmik, P.; Han, H.; Cebe, J.; Burchett, R.; Acharya, B.; Kumar, S. Ambient temperature thermotropic liquid crystalline viologen bis(triflimide) salts. Liq. Cryst. 2003, 30, 1433–1440. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Al-Karawi, M.K.M.; Killarney, S.T.; Dizon, E.J.; Chang, A.; Kim, J.; Chen, S.L.; Principe, R.C.G.; Ho, A.; Han, H.; et al. Thermotropic Liquid-Crystalline and Light-Emitting Properties of Bis(4-aalkoxyphenyl) Viologen Bis(triflimide) Salts. Molecules 2020, 25, 2435. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Han, H.S.; Nedeltchev, I.K.; Cebe, J.J. Room-temperature thermotropic ionic liquid crystals: Viologenbis(triflimide) salts. Mol. Cryst. Liq. Cryst. 2004, 419, 27–46. [Google Scholar] [CrossRef]
- Bhowmik, P.K.; Killarney, S.T.; Li, J.R.A.; Koh, J.J.; Han, H.; Sharpnack, L.; Agra-Kooijman, D.M.; Fisch, M.R.; Kumar, S. Thermotropic liquid-crystalline properties of extended viologen bis(triflimide) salts. Liq. Cryst. 2018, 45, 872–885. [Google Scholar] [CrossRef]
- Causin, V.; Saielli, G. Effect of asymmetric substitution on the mesomorphic behaviour of low-1melting viologen salts of bis(trifluoromethanesulfonyl)amide. J. Mater. Chem. 2009, 19, 9153–9162. [Google Scholar] [CrossRef]
- Kumar, G.S.; Neckers, D.C. Photochemistry of Azobenzene-Containing Polymers. Chem. Rev. 1989, 89, 1915–1925. [Google Scholar] [CrossRef]
- Chaganava, I.; Kilosanidze, B.; Kakauridze, G.; Oriol, L.; Pinol, M.; Martinez-Felipe, A. Induction of the vector polyphotochromism in side-chain azopolymers. J. Photochem. Photobiol. A Chem. 2018, 354, 70–77. [Google Scholar] [CrossRef]
- Sumitani, R.; Mochida, T. Switchable ionic conductivity and viscoelasticity of ionogels containing photo- and thermo-responsive organometallic ionic liquids. J. Mol. Liq. 2021, 342, 117510. [Google Scholar] [CrossRef]
- Nguyen, P.; Scheuermann, A.; Nikolaev, A.; Chabinyc, M.; Bates, C.; de Alaniz, J. Reversible Modulation of Conductivity in Azobenzene Polyelectrolytes Using Light. ACS Appl. Polym. Mater. 2023, 5, 4698–4703. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, Y.; Chang, C.; Lee, M.; Chen, Y.; Lee, L.; Chang, M.; Chen, J. Boosting Ion Conductivities: Light-Modulated Azobenzene-Based Ionic Liquids in Vertical Nanochannels. ACS Appl. Mater. Interfaces 2023, 15, 45418–45425. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sano, K.; Araoka, F. Anisotropic fluid with phototunable dielectric permittivity. Nat. Commun. 2022, 13, 1142. [Google Scholar] [CrossRef]
- Yamakado, R.; Hara, M.; Nagano, S.; Seki, T.; Maeda, H. Photo-Responsive Soft Ionic Crystals: Ion-Pairing Assemblies of Azobenzene Carboxylates. Chem. A Eur. J. 2017, 23, 9244–9248. [Google Scholar] [CrossRef]
- Filippi, N.; Mezalira, D.; Ovalle, S.; Westphal, E. Study of the mesomorphic behaviour through the structure modification of azo and acetylene pyridinium and imidazolium-based ionic liquid crystals. Liq. Cryst. 2016, 43, 2163–2190. [Google Scholar] [CrossRef]
- Babamale, H.; Ng, S.; Tang, W.; Yam, W. Azobenzene-Imidazolium ionic liquid crystals: Phase properties and photoisomerization in solution state. J. Mol. Struct. 2024, 1303, 137494. [Google Scholar] [CrossRef]
- Wuckert, E.; Harjung, M.; Kapernaum, N.; Mueller, C.; Frey, W.; Baro, A.; Giesselmann, F.; Laschat, S. Photoresponsive ionic liquid crystals based on azobenzene guanidinium salts. Phys. Chem. Chem. Phys. 2015, 17, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Zaton, D.; Karamoula, A.; Strachan, G.J.; Storey, J.M.D.; Imrie, C.T.; Martinez-Felipe, A. Photo-driven effects in twist-bend nematic phases: Dynamic and memory response of liquid crystalline dimers. J. Mol. Liq. 2021, 344, 117680. [Google Scholar] [CrossRef]
- Choudhury, T.; Shen, Y.; Rao, N.; Clark, N. Dinuclear ortho-metallated palladium(II) azobenzene complexes with acetato and chloro bridges: Influence of polar substituents on the mesomorphic properties. J. Organomet. Chem. 2012, 712, 20–28. [Google Scholar] [CrossRef]
- Cook, A.G.; Inkster, R.T.; Martinez-Felipe, A.; Ribes-Greus, A.; Hamley, I.W.; Imrie, C.T. Synthesis and phase behaviour of a homologous series of polymethacrylate-based side-chain liquid crystal polymers. Eur. Polym. J. 2012, 48, 821–829. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Santonja-Blasco, L.; Badia, J.D.; Imrie, C.T.; Ribes-Greus, A. Characterization of Functionalized Side-Chain Liquid Crystal Methacrylates Containing Nonmesogenic Units by Dielectric Spectroscopy. Ind. Eng. Chem. Res. 2013, 52, 8722–8731. [Google Scholar] [CrossRef]
- Zentel, R.; Strobl, G.R.; Ringsdorf, H. Dielectric-Relaxation of Liquid-Crystalline Polyacrylates and Polymethacrylates. Macromolecules 1985, 18, 960–965. [Google Scholar] [CrossRef]
- Colomer, F.R.; Duenas, J.M.M.; Ribelles, J.L.G.; Barralesrienda, J.M.; Deojeda, J.M.B. Side-Chain Liquid-Crystalline Poly(n-Maleimides). 5. Dielectric-Relaxation Behavior of Liquid-Crystalline Side-Chain and Amorphous Poly(n-Maleimides)—A Comparative Structural Study. Macromolecules 1993, 26, 155–166. [Google Scholar] [CrossRef]
- Liebsch, J.; Strachan, R.; Suthaharan, S.; Dominguez-Candela, I.; Auria-Soro, C.; San-Millan, A.; Walker, R.; Chilukuri, B.; Ros, M.; Martinez-Felipe, A. Tailoring the dielectric and ferroelectric response of mixtures containing bent-core liquid crystals through light-irradiation and composition. J. Mol. Liq. 2024, 399, 124371. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Zulkhairi, I.; Pintre, I.; Aripin, N.F.K.; Lora-Garcia, J.; Fombuena, V.; Ros, M.B.; Martinez-Felipe, A. Light-responsive bent-core liquid crystals as candidates for energy conversion and storage. J. Mater. Chem. C 2022, 10, 18200–18212. [Google Scholar] [CrossRef]
- Prasad, S.K. Photo-Stimulated and Photo-Suppressed Phase Transitions. Mol. Cryst. Liq. Cryst. 2009, 509, 1059–1069. [Google Scholar] [CrossRef]
- Prasad, S.K.; Madhuri, P.L.; Satapathy, P.; Yelamaggad, C.V. A soft-bent dimer composite exhibiting twist-bend nematic phase: Photo-driven effects and an optical memory device. Appl. Phys. Lett. 2018, 112, 253701. [Google Scholar] [CrossRef]
- Prasad, S.K.; Nair, G.G.; Hegde, G.; Sandhya, K.L.; Rao, D.S.S.; Lobo, C.V.; Yelamaggad, C.V. Photoinduced effects in nematic liquid crystals. Phase Transit. 2005, 78, 443–455. [Google Scholar] [CrossRef]
- Dunmur, D.; Luckhurst, G.; de la Fuente, M.; Diez, S.; Jubindo, M. Dielectric relaxation in liquid crystalline dimers. J. Chem. Phys. 2001, 115, 8681–8691. [Google Scholar] [CrossRef]
- Velayutham, T.S.; Azmina, N.M.S.; Manickam-Achari, V.; Roche, A.; Ramesh, R.; Martinez-Felipe, A. A new light-responsive resistive random-access memory device containing hydrogen-bonded complexes. J. Photochem. Photobiol. A Chem. 2021, 404, 112914. [Google Scholar] [CrossRef]
- Ruslim, C.; Komitov, L.; Matsuzawa, Y.; Ichimura, K. Effect of conformations of trans- and cis-azobenzenes on photoinduced anchoring transitions in a nematic liquid crystal. Jpn. J. Appl. Phys. Part 2 Lett. 2000, 39, L104–L106. [Google Scholar] [CrossRef]
- Tan, Y.; Fu, Z.; Zeng, Y.; Chen, H.; Liao, S.; Zhang, J.; Dai, J. Highly stable photochromic crystalline material based on a close-packed layered metal-viologen coordination polymer. J. Mater. Chem. 2012, 22, 17452–17455. [Google Scholar] [CrossRef]
- Li, Z.; Xue, L.; Mu, Y.; Zhao, B. Viologen-derived material showing photochromic, visually oxygen responsive, and photomodulated luminescence behaviors. Crystengcomm 2021, 23, 1019–1024. [Google Scholar] [CrossRef]
- Liang, T.; van Kuringen, H.P.C.; Mulder, D.J.; Tan, S.; Wu, Y.; Borneman, Z.; Nijmeijer, K.; Schenning, A. Anisotropic Dye Adsorption and Anhydrous Proton Conductivity in Smectic Liquid Crystal Networks: The Role of Cross-Link Density, Order, and Orientation. ACS Appl. Mater. Interfaces 2017, 9, 35218–35225. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Lu, Z.; Henderson, P.A.; Picken, S.J.; Norder, B.; Imrie, C.T.; Ribes-Greus, A. Synthesis and characterisation of side chain liquid crystal copolymers containing sulfonic acid groups. Polymer 2012, 53, 2604–2612. [Google Scholar] [CrossRef]
- Brown, A.W.; Martinez-Felipe, A. Ionic conductivity mediated by hydrogen bonding in liquid crystalline 4-n-alkoxybenzoic acids. J. Mol. Struct. 2019, 1197, 487–496. [Google Scholar] [CrossRef]
- Nagao, Y. Progress on highly proton-conductive polymer thin films with organized structure and molecularly oriented structure. Sci. Technol. Adv. Mater. 2020, 21, 79–91. [Google Scholar] [CrossRef]
- Yang, X.H.; Tan, S.; Liang, T.; Wei, B.Z.; Wu, Y. A unidomain membrane prepared from liquid-crystalline poly(pyridinium 4-styrene sulfonate) for anhydrous proton conduction. J. Membr. Sci. 2017, 523, 355–360. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendaoud, U.; Bhowmik, P.K.; Chen, S.L.; Han, H.; Cox, S.L.; Liebsch, J.; Ros, M.B.; Selvi Velayutham, T.; Aripin, N.F.K.; Martinez-Felipe, A. Modulating the Conductivity of Light-Responsive Ionic Liquid Crystals. Molecules 2024, 29, 4459. https://doi.org/10.3390/molecules29184459
Bendaoud U, Bhowmik PK, Chen SL, Han H, Cox SL, Liebsch J, Ros MB, Selvi Velayutham T, Aripin NFK, Martinez-Felipe A. Modulating the Conductivity of Light-Responsive Ionic Liquid Crystals. Molecules. 2024; 29(18):4459. https://doi.org/10.3390/molecules29184459
Chicago/Turabian StyleBendaoud, Umama, Pradip K. Bhowmik, Si L. Chen, Haesook Han, Seonghyeok L. Cox, Jasmin Liebsch, M. Blanca Ros, Thamil Selvi Velayutham, Nurul Fadhilah Kamalul Aripin, and Alfonso Martinez-Felipe. 2024. "Modulating the Conductivity of Light-Responsive Ionic Liquid Crystals" Molecules 29, no. 18: 4459. https://doi.org/10.3390/molecules29184459
APA StyleBendaoud, U., Bhowmik, P. K., Chen, S. L., Han, H., Cox, S. L., Liebsch, J., Ros, M. B., Selvi Velayutham, T., Aripin, N. F. K., & Martinez-Felipe, A. (2024). Modulating the Conductivity of Light-Responsive Ionic Liquid Crystals. Molecules, 29(18), 4459. https://doi.org/10.3390/molecules29184459








