Developments and Applications of Molecularly Imprinted Polymer-Based In-Tube Solid Phase Microextraction Technique for Efficient Sample Preparation
Abstract
1. Introduction
2. Overview of IT-SPME
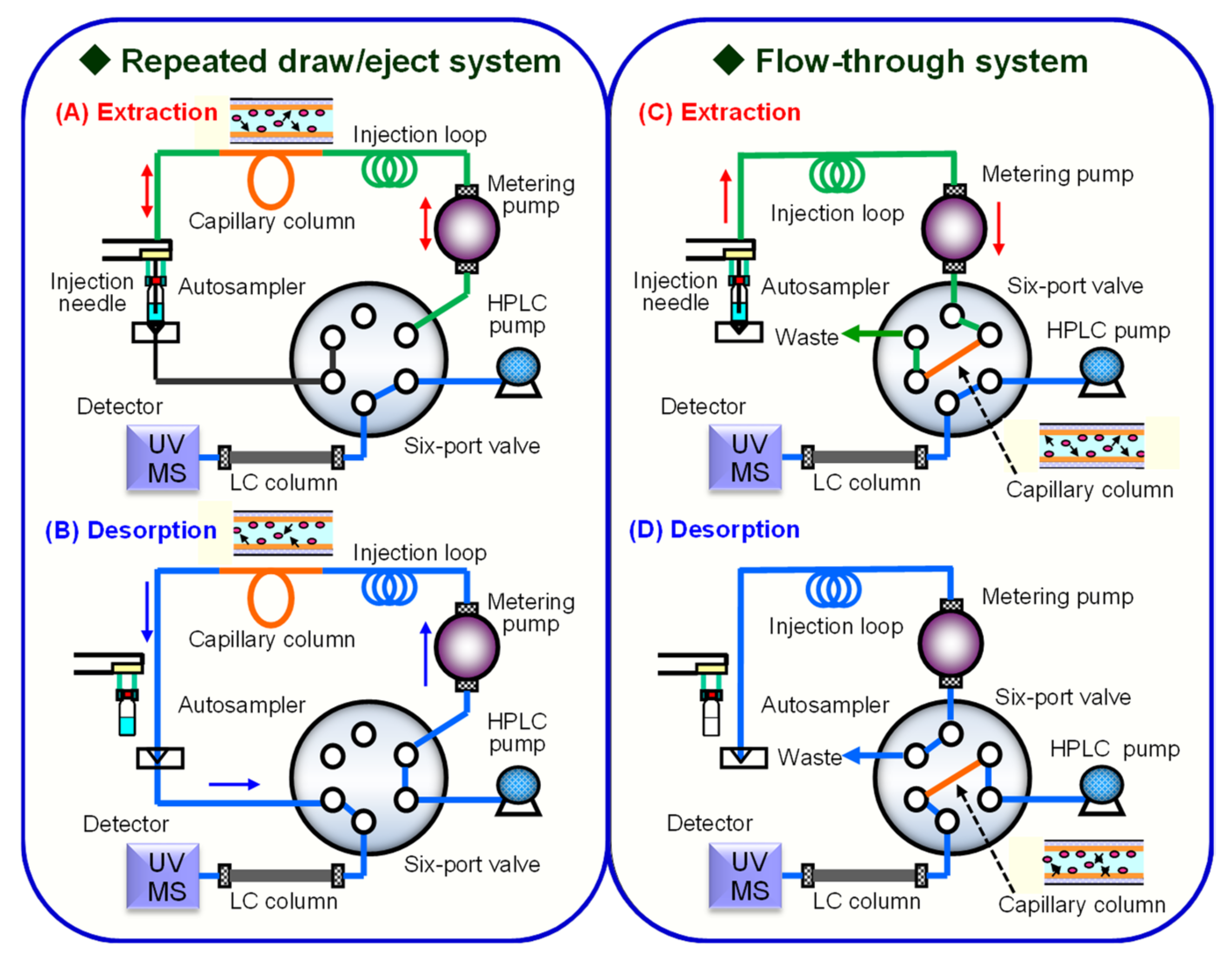
2.1. IT-SPME Operating System
2.2. Capillary Tube Configuration for IT-SPME
- Inner-surface-coated: includes wall-coated open tubular (WCOT) capillaries and porous layer open tubular (PLOT) capillaries, where the inner wall of the tube is coated with an adsorbent.
- Particle-packed: capillaries where adsorbent-coated particles are packed inside the tube.
- Fiber-packed: capillaries in which thin fibers are vertically packed inside the tube.
- Rod monolith: capillaries where a monolith is formed within the tube.
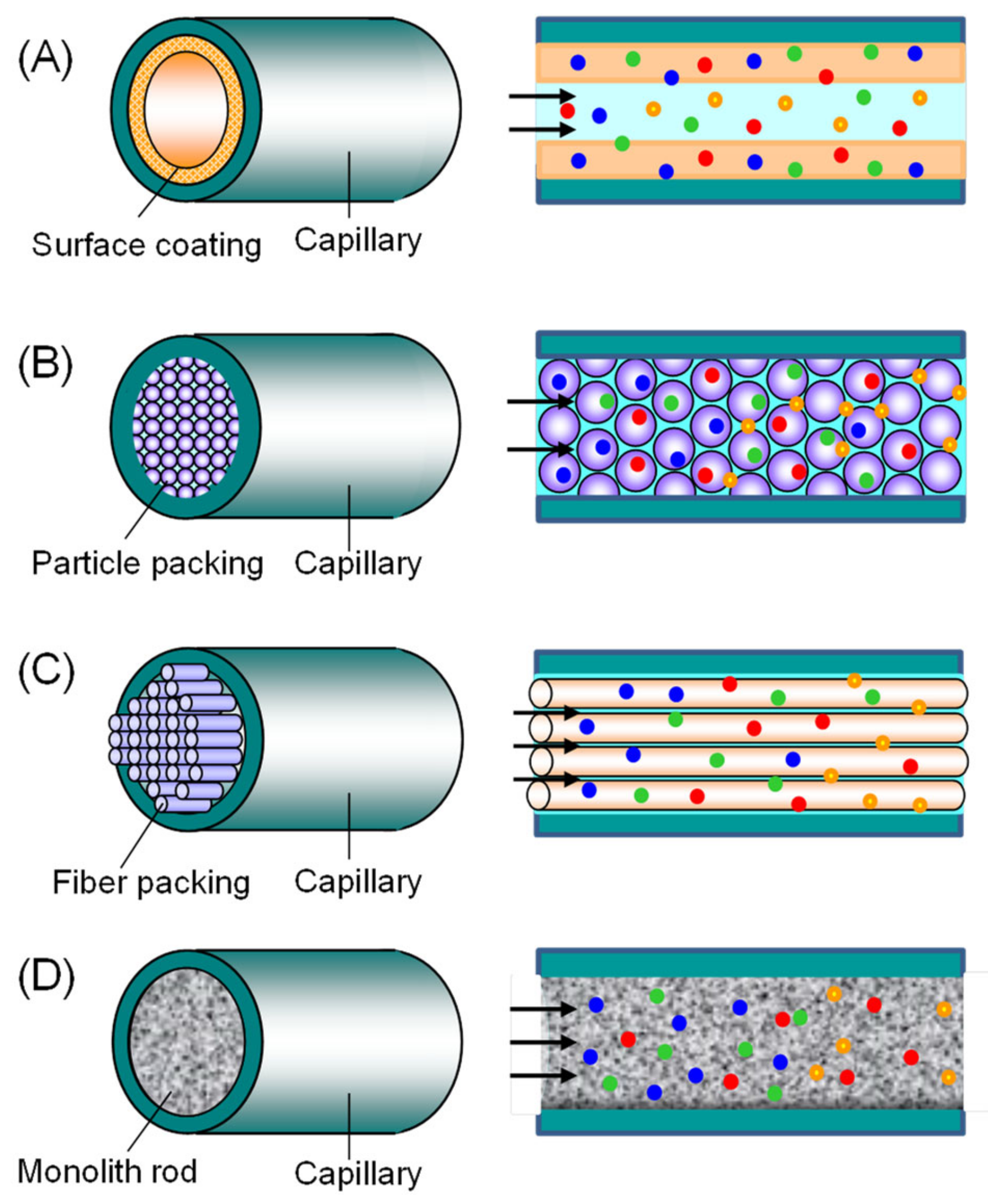
2.3. Extraction Phase of Capillary Tube
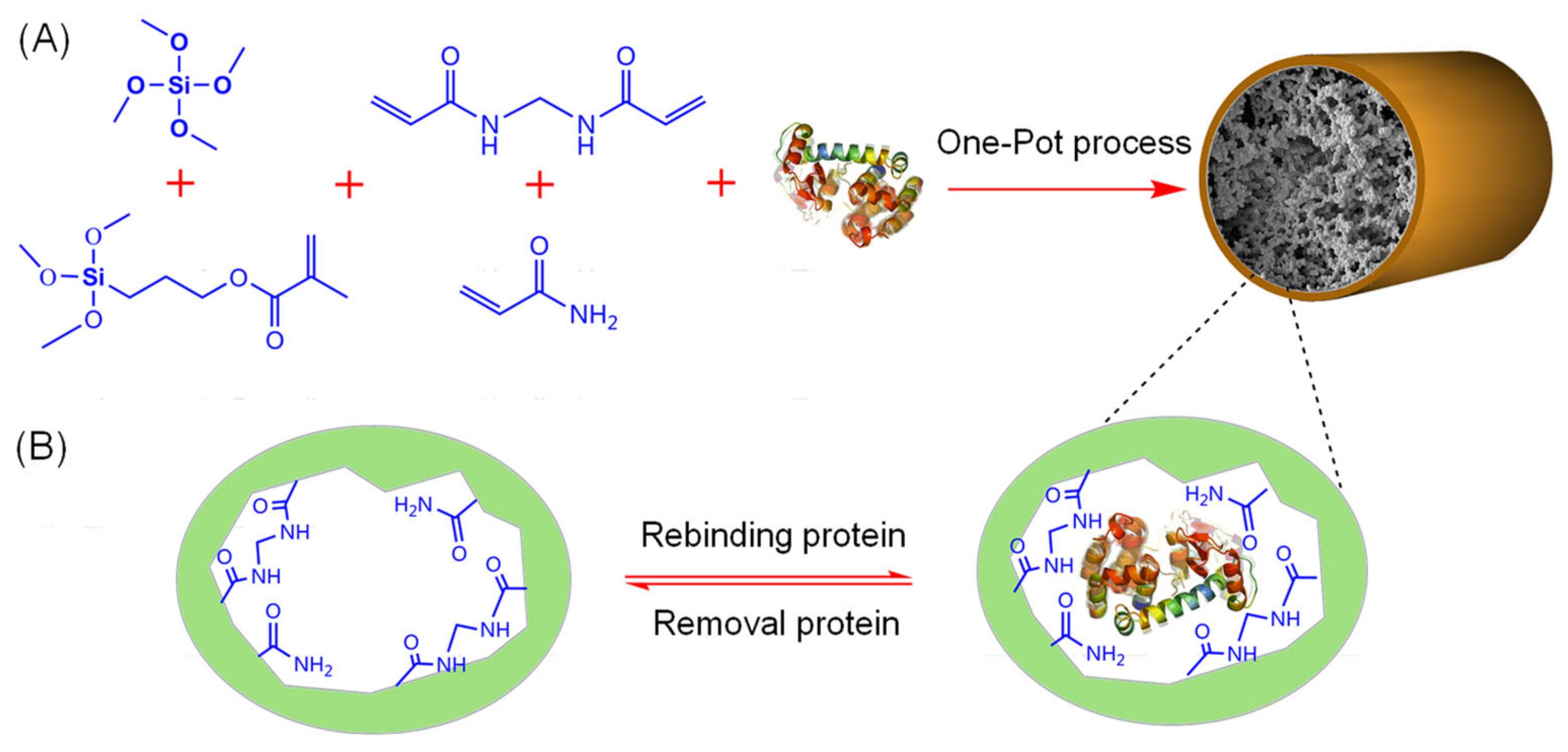
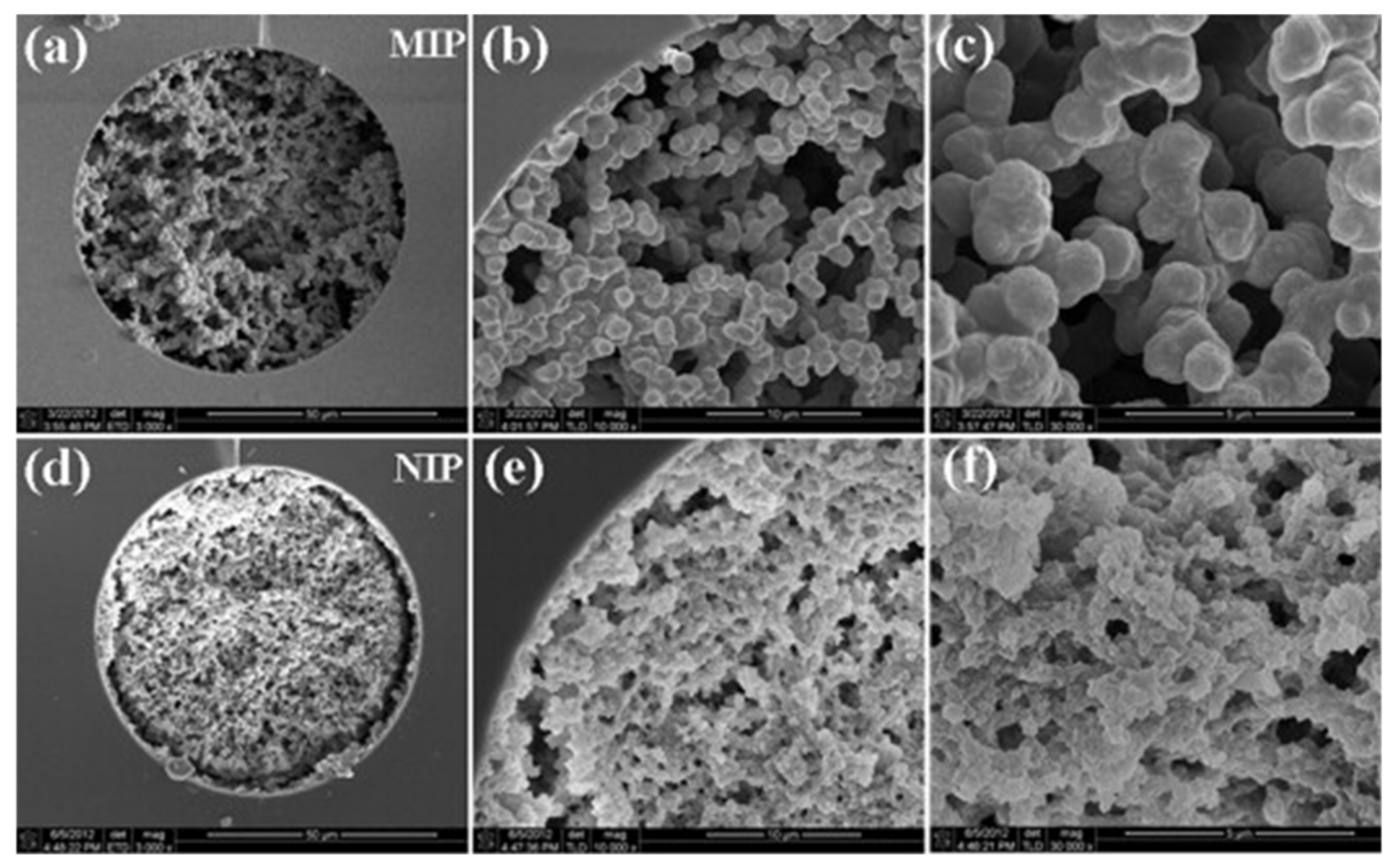
3. Fabrication of Molecularly Imprinted Polymers
3.1. Principles of Molecular Imprinting and Synthesis of MIPs
- Prearrangement: In the initial step, template molecules interact with functional monomers through non-covalent, covalent, or semi-covalent bonds, forming a host-guest complex. In non-covalent imprinting, the template and monomer form a monomer-template complex via non-covalent bonds (electrostatic interactions, hydrogen bonds, ion pair formation, van der Waals forces, π-π stacking, metal coordination, etc.). This method facilitates the removal of the template without chemical bond cleavage, preserving the polymer structure. However, the stability of these bonds can be sensitive to changes in the chemical environment, requiring careful optimization of reaction conditions. Excessive use of functional monomers may also introduce non-specific binding sites, reducing selectivity. In contrast, covalent imprinting involves reversible covalent bonding between the template and polymerizable groups. After polymerization, the template is cleaved, leaving functional groups correctly orientated for subsequent re-binding. Although this method requires suitable template-monomer complexes, it ensures accurate uptake of target analytes from aqueous solutions. However, the formation and cleavage of covalent bonds are unlikely to occur under mild conditions. Semi-covalent imprinting combines covalent and non-covalent interactions, offering a balance between template stability and re-binding efficiency.
- Polymerization: Polymerization is initiated by thermal or UV activation in the presence of cross-linkers and initiators, forming a highly cross-linked polymer network around the template molecules. This step creates the three-dimensional space necessary for molecular recognition.
- Template elution: In the final step, template molecules are removed from the polymer network through physicochemical methods such as hydrolysis or desorption, leaving MIPs with cavity sites complementary to the template molecules. However, in covalent imprinting, removal of the template by covalent bond cleavage under severe conditions may affect the functionality of the cavity.
3.2. Characteristics and Functionalization of MIPs
4. Developments and Applications of MIP IT-SPME
4.1. Selectivity and Extraction Efficiency of MIP IT-SPME
4.2. Fabrications of Various MIP Capillaries and Their Applications to IT-SPME
5. Conclusions and Perspective
- High selectivity and extraction efficiency: MIPs have specific binding sites that are complementary to the structure and functional groups of the target analyte, enabling highly selective adsorption. This results in the selective extraction of analytes from complex matrix samples, reducing matrix interference and improving the sensitivity and accuracy of analysis.
- High stability and reusability: MIPs offer superior chemical and physical stability compared to biorecognition materials such as antibodies and enzymes. They can withstand harsh conditions such as exposure to organic solvents and extreme pH environments. They can also be reused after washing, making them suitable for continuous analysis in IT-SPME.
- Ease of multifunctional modification: The outer surface of MIPs can be easily customized by incorporating various monomers to provide specific functional groups. This enables the enhancement of performance by creating hybrid materials, such as the integration of magnetic nanoparticles to for improved recycling or the inclusion of RAM to limit molecular permeability. Combining MIPs with other highly porous materials can also increase surface area and porosity, facilitating mass transfer of target analytes to binding sites.
- Cost reduction due to miniaturization: IT-SPME is cost-effective due to the small extraction phase of the capillary, which allows for rapid and efficient extraction and concentration. The miniaturization of MIP capillaries significantly reduces sample volume and solvent consumption. Additionally, MIPs are relatively simple and inexpensive to synthesize, facilitating mass production.
- Labor-saving through high throughput: IT-SPME can automate the extraction, desorption, and introduction of compounds into analytical instruments on-line using column-switching techniques. This automation enables high-throughput analysis of a large numbers of samples, saving labor and time.
- Environmental friendliness: The MIP-based IT-SPME method uses minimal organic solvents, reducing health hazards to analysts, minimizing waste, and promoting environmentally friendly sample preparation. However, MIP preparation still requires the use of hazardous organic solvents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kataoka, H.; Saito, K.; Yokoyama, A. Sampling and Sample Preparation for Clinical and Pharmaceutical Analysis. In Handbook of Sample Preparation; Pawliszyn, J., Lord, H.L., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2011; pp. 285–311. ISBN 978-0470099346. [Google Scholar] [CrossRef]
- Kataoka, H. Sample Preparation for Liquid Chromatography. In Handbooks in Separation Science: Liquid Chromatography, 3rd ed.; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 1; Volume 2, pp. 1–49. ISBN 978-0323999694. [Google Scholar] [CrossRef]
- Kataoka, H. In-tube solid-phase microextraction: Current trends and future perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Z.S.; Duan, R.; Wang, X.; Yang, Y.S.; Dong, L.Y.; Wang, X.H. Advances and applications of in-tube solid-phase microextraction for analysis of proteins. J. Chromatogr. A 2021, 1640, 461962. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid Phase Microextraction: A Two-Decade Overview. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, Y.; Sun, D.; Yan, S.; Wen, Y.; Wang, Y.; Li, G.; Liu, H.; Li, J.; Song, Z. Recent Advances in Molecularly Imprinted Polymers for Antibiotic Analysis. Molecules 2023, 28, 335. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Razavi, N. Application of molecularly-imprinted polymers in solid-phase microextraction techniques. TrAC Trends Anal. Chem. 2015, 73, 81–90. [Google Scholar] [CrossRef]
- Ansari, S.; Karim, M. Recent progress, challenges and trends in trace determination of drug analysis using molecularly imprinted solid-phase microextraction technology. Talanta 2017, 164, 612–625. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Mansour, F.R. Homogeneous liquid-liquid extraction as an alternative sample preparation technique for biomedical analysis. J. Sep. Sci. 2022, 45, 185–209. [Google Scholar] [CrossRef]
- Poole, C.F. Core concepts and milestones in the development of solid-phase extraction. In Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 1; pp. 1–36. ISBN 978-0-12-816906-3. [Google Scholar] [CrossRef]
- Hamidi, S. Recent Advances in Solid-Phase Extraction as a Platform for Sample Preparation in Biomarker Assay. Crit. Rev. Anal. Chem. 2023, 53, 199–210. [Google Scholar] [CrossRef]
- Mahdavijalal, M.; Petio, C.; Staffilano, G.; Mandrioli, R.; Protti, M. Innovative Solid-Phase Extraction Strategies for Improving the Advanced Chromatographic Determination of Drugs in Challenging Biological Samples. Molecules 2024, 29, 2278. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, S.; Taghvimi, A.; Mazouchi, N. Micro Solid Phase Extraction Using Novel Adsorbents. Crit. Rev. Anal. Chem. 2021, 51, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Bedair, A.; Abdelhameed, R.M.; Hammad, S.F.; Abdallah, I.A.; Mansour, F.R. Applications of metal organic frameworks in dispersive micro solid phase extraction (D-μ-SPE). J. Chromatogr. A 2024, 1732, 465192. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, É.A.; Jiang, R.; Rodríguez-Lafuente, A.; Gionfriddo, E.; Pawliszyn, J. A critical review of the state of the art of solid-phase microextraction of complex matrices I. Environmental analysis. TrAC Trends Anal. Chem. 2015, 71, 224–235. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Li, G. Recent advance on microextraction sampling technologies for bioanalysis. J. Chromatogr. A 2024, 1720, 464775. [Google Scholar] [CrossRef]
- Boyacı, E.; Rodríguez-Lafuente, Á.; Gorynski, K.; Mirnaghi, F.; Souza-Silva, É.A.; Hein, D.; Pawliszyn, J. Sample preparation with solid phase microextraction and exhaustive extraction approaches: Comparison for challenging cases. Anal. Chim. Acta 2015, 873, 14–30. [Google Scholar] [CrossRef]
- Owczarzy, A.; Kulig, K.; Piordas, K.; Piśla, P.; Sarkowicz, P.; Rogóż, W.; Maciążek-Jurczyk, M. Solid-phase microextraction—A future technique in pharmacology and coating trends. Anal Methods 2024, 16, 3164–3178. [Google Scholar] [CrossRef]
- Jin, H.-F.; Shi, Y.; Cao, J. Recent advances and applications of novel advanced materials in solid-phase microextraction for natural products. TrAC Trends Anal. Chem. 2024, 178, 117858. [Google Scholar] [CrossRef]
- Camino-Sánchez, F.J.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Santos-Fandila, A.; Vílchez, J.L. Stir bar sorptive extraction: Recent applications, limitations and future trends. Talanta 2014, 130, 388–399. [Google Scholar] [CrossRef]
- Hasan, C.K.; Ghiasvand, A.; Lewis, T.W.; Nesterenko, P.N.; Paull, B. Recent advances in stir-bar sorptive extraction: Coatings, technical improvements, and applications. Anal. Chim. Acta 2020, 1139, 222–240. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Zhang, Q.; Zang, L.; Chen, B.; Hu, B. Stir bar sorptive extraction and its application. J. Chromatogr. A 2021, 1637, 461810. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Said, R.; Abdel-Rehim, M. Sorbent, device, matrix and application in microextraction by packed sorbent (MEPS): A review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, J.; Han, S.; Ji, X.; Li, C.; Feng, J.; Sun, M. Recent advances in micro- and nanomaterial-based adsorbents for pipette-tip solid-phase extraction. Mikrochim. Acta 2021, 188, 189. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; Yang, F.Q. Applications of magnetic solid-phase extraction in the sample preparation of natural product analysis (2020–2023). J. Sep. Sci. 2024, 47, e2400082. [Google Scholar] [CrossRef] [PubMed]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Green aspects, developments and perspectives of liquid phase microextraction techniques. Talanta 2014, 119, 34–45. [Google Scholar] [CrossRef]
- Hansen, F.; Øiestad, E.L.; Pedersen-Bjergaard, S. Bioanalysis of pharmaceuticals using liquid-phase microextraction combined with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113446. [Google Scholar] [CrossRef]
- Bayatloo, M.R.; Tabani, H.; Nojavan, S.; Alexovič, M.; Ozkan, S.A. Liquid-Phase Microextraction Approaches for Preconcentration and Analysis of Chiral Compounds: A Review on Current Advances. Crit. Rev. Anal. Chem. 2023, 53, 1623–1637. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent advances of in-tube solid-phase microextraction. TrAC Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Fernández-Amado, M.; Prieto-Blanco, M.C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Strengths and weaknesses of in-tube solid-phase microextraction: A scoping review. Anal. Chim. Acta 2016, 906, 41–57. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Ballester-Caudet, A.; Verdú-Andrés, J.; Herráez-Hernández, R.; Molins-Legua, C.; Campíns-Falcó, P. In-tube solid-phase microextraction. In Solid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 387–427. ISBN 978-0-12-816906-3. [Google Scholar] [CrossRef]
- Queiroz, M.E.; Melo, L.P. Selective capillary coating materials for in-tube solid-phase microextraction coupled to liquid chromatography to determine drugs and biomarkers in biological samples: A review. Anal. Chim. Acta 2014, 826, 1–11. [Google Scholar] [CrossRef]
- Ponce-Rodríguez, H.D.; Verdú-Andrés, J.; Herráez-Hernández, R.; Campíns-Falcó, P. Innovations in Extractive Phases for In-Tube Solid-Phase Microextraction Coupled to Miniaturized Liquid Chromatography: A Critical Review. Molecules 2020, 25, 2460. [Google Scholar] [CrossRef] [PubMed]
- Grecco, C.F.; de Souza, I.D.; Queiroz, M.E.C. Novel materials as capillary coatings for in-tube solid-phase microextraction for bioanalysis. J. Sep. Sci. 2021, 44, 1662–1693. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Oliveira, I.G.C.; Queiroz, M.E.C. Innovative extraction materials for fiber-in-tube solid phase microextraction: A review. Anal. Chim. Acta 2021, 1165, 238110. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Queiroz, M.E.C. Organic-silica hybrid monolithic sorbents for sample preparation techniques: A review on advances in synthesis, characterization, and applications. J. Chromatogr. A 2024, 1713, 464518. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic liquids: Solvents and sorbents in sample preparation. J. Sep. Sci. 2018, 41, 209–235. [Google Scholar] [CrossRef]
- de Faria, H.D.; Abrão, L.C.; Santos, M.G.; Barbosa, A.F.; Figueiredo, E.C. New advances in restricted access materials for sample preparation: A review. Anal. Chim. Acta 2017, 959, 43–65. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, J.; Zhang, K.; Lian, H.; Li, G. Novel applications of molecularly-imprinted polymers in sample preparation. TrAC Trends Anal. Chem. 2013, 43, 37–52. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, M. Molecularly imprinted polymers for on-line extraction techniques. Bioanalysis 2015, 7, 2145–2153. [Google Scholar] [CrossRef]
- Gama, M.R.; Bottoli, C.B. Molecularly imprinted polymers for bioanalytical sample preparation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 107–121. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Huang, S.; Xu, J.; Zheng, J.; Zhu, F.; Xie, L.; Ouyang, G. Synthesis and application of magnetic molecularly imprinted polymers in sample preparation. Anal. Bioanal. Chem. 2018, 410, 3991–4014. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation. Molecules 2019, 24, 2889. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Bottaro, C.S. A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples. J. Chromatogr. A 2020, 1614, 460603. [Google Scholar] [CrossRef] [PubMed]
- Martín-Esteban, A. Green molecularly imprinted polymers for sustainable sample preparation. J. Sep. Sci. 2022, 45, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Suzaei, F.M.; Daryanavard, S.M.; Abdel-Rehim, A.; Bassyouni, F.; Abdel-Rehim, M. Recent molecularly imprinted polymers applications in bioanalysis. Chem. Zvesti. 2023, 77, 619–655. [Google Scholar] [CrossRef]
- de Toffoli, A.L.; Maciel, E.V.S.; Fumes, B.H.; Lanças, F.M. The role of graphene-based sorbents in modern sample preparation techniques. J. Sep. Sci. 2018, 41, 288–302. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; Mejía-Carmona, K.; Jordan-Sinisterra, M.; da Silva, L.F.; Vargas Medina, D.A.; Lanças, F.M. The Current Role of Graphene-Based Nanomaterials in the Sample Preparation Arena. Front. Chem. 2020, 8, 664. [Google Scholar] [CrossRef]
- Borsatto, J.V.B.; Lanças, F.M. Recent Trends in Graphene-Based Sorbents for LC Analysis of Food and Environmental Water Samples. Molecules 2023, 28, 5134. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M.; Otárola-Jiménez, J. Magnetic solid-phase extraction using carbon nanotubes as sorbents: A review. Anal. Chim. Acta 2015, 892, 10–26. [Google Scholar] [CrossRef]
- Ng, N.T.; Kamaruddin, A.F.; Wan Ibrahim, W.A.; Sanagi, M.M.; Abdul Keyon, A.S. Advances in organic-inorganic hybrid sorbents for the extraction of organic and inorganic pollutants in different types of food and environmental samples. J. Sep. Sci. 2018, 41, 195–208. [Google Scholar] [CrossRef]
- Öztürk, E.E.; Dalgıç Bozyiğit, G.; Büyükpınar, Ç.; Bakırdere, S. Magnetic Nanoparticles Based Solid Phase Extraction Methods for the Determination of Trace Elements. Crit. Rev. Anal. Chem. 2022, 52, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cejuela, H.M.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Recent Advances in Affinity MOF-Based Sorbents with Sample Preparation Purposes. Molecules 2020, 25, 4216. [Google Scholar] [CrossRef] [PubMed]
- Vardali, S.C.; Manousi, N.; Barczak, M.; Giannakoudakis, D.A. Novel Approaches Utilizing Metal-Organic Framework Composites for the Extraction of Organic Compounds and Metal Traces from Fish and Seafood. Molecules 2020, 25, 513. [Google Scholar] [CrossRef] [PubMed]
- González-Sálamo, J.; Jiménez-Skrzypek, G.; Ortega-Zamora, C.; González-Curbelo, M.Á.; Hernández-Borges, J. Covalent Organic Frameworks in Sample Preparation. Molecules 2020, 25, 3288. [Google Scholar] [CrossRef] [PubMed]
- Torabi, E.; Mirzaei, M.; Bazargan, M.; Amiri, A. A critical review of covalent organic frameworks-based sorbents in extraction methods. Anal. Chim. Acta 2022, 1224, 340207. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Silva, M.S.; Tavares, A.P.M.; de Faria, H.D.; Sales, M.G.F.; Figueiredo, E.C. Molecularly Imprinted Solid Phase Extraction Aiding the Analysis of Disease Biomarkers. Crit. Rev. Anal. Chem. 2022, 52, 933–948. [Google Scholar] [CrossRef]
- Sobiech, M.; Luliński, P. Molecularly imprinted solid phase extraction—Recent strategies, future prospects and forthcoming challenges in complex sample pretreatment process. TrAC Trends Anal. Chem. 2024, 174, 117695. [Google Scholar] [CrossRef]
- Zhang, M.; Zeng, J.; Wang, Y.; Chen, X. Developments and trends of molecularly imprinted solid-phase microextraction. J. Chromatogr. Sci. 2013, 51, 577–586. [Google Scholar] [CrossRef]
- Shahhoseini, F.; Azizi, A.; Bottaro, C.S. A critical evaluation of molecularly imprinted polymer (MIP) coatings in solid phase microextraction devices. TrAC Trends Anal. Chem. 2022, 156, 116695. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Recent advances and future trends in molecularly imprinted polymers-based sample preparation. J. Sep. Sci. 2023, 46, 2300157. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.O.; Batista Junior, A.C.; Brito, C.C.S.M.; Rocha, Y.A.; Chaves, A.R. Molecularly imprinted polymers in solid-phase microextraction: Enhancing the analysis of pharmaceutical compounds across diverse sample matrices. J. Pharm. Biomed. Anal. Open 2024, 4, 100037. [Google Scholar] [CrossRef]
- Eisert, R.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Pawliszyn, J.; Arthur, C.L. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Kataoka, H. Automated sample preparation using in-tube solid-phase microextraction and its application—A review. Anal. Bioanal. Chem. 2002, 373, 31–45. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Saito, K. Online column-switching sample preparation for liquid chromatography. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Comprehensive Sampling and Sample Preparation, 2nd ed.; Soylak, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; CH 00061 Chapter 14547; ISBN 978-0-12-409547-2. [Google Scholar] [CrossRef]
- Moliner-Martínez, Y.; Prima-Garcia, H.; Ribera, A.; Coronado, E.; Campíns-Falcó, P. Magnetic in-tube solid phase microextraction. Anal. Chem. 2012, 84, 7233–7240. [Google Scholar] [CrossRef]
- Safari, M.; Yamini, Y. Application of magnetic nanomaterials in magnetic in-tube solid-phase microextraction. Talanta 2021, 221, 121648. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Manbohi, A.; Heydar, K.T. Electrochemically controlled in-tube solid phase microextraction. Anal. Chim. Acta 2015, 853, 335–341. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M. Development of electrochemically controlled packed-in-tube solid phase microextraction method for sensitive analysis of acidic drugs in biological samples. Talanta 2018, 185, 80–88. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Electrochemically controlled fiber-in-tube solid-phase microextraction method for the determination of trace amounts of antipsychotic drugs in biological samples. J. Sep. Sci. 2018, 41, 3598–3606. [Google Scholar] [CrossRef]
- Yu, Q.-W.; Ma, Q.; Feng, Y.-Q. Temperature-response polymer coating for in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Talanta 2011, 84, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rodriguez-Lafuente, A.; Pawliszyn, J. Thermoelectric-based temperature-controlling system for in-tube solid-phase microextraction. J. Sep. Sci. 2014, 37, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.W.; Zou, C.J.; Luo, N.; Weng, Q.F.; Cai, L.S.; Wu, Y.C.; Xing, J. Determination of urinary 8-hydroxy-2′-deoxyguanosine by capillary electrophoresis with molecularly imprinted monolith in-tube solid phase microextraction. Chin. Chem. Lett. 2010, 21, 85–88. [Google Scholar] [CrossRef]
- Shuo, Z.H.A.O.; Hao-Tian, W.A.N.G.; Ke, L.I.; Jing, Z.; Xia-Yan, W.A.N.G.; Guang-Sheng, G.U.O. Fast Determination of Residual Sulfonamides in Milk by In-Tube Solid-Phase Microextraction Coupled with Capillary Electrophoresis-Laser Induced Fluorescence. Chin. J. Anal. Chem. 2018, 46, e1810–e1816. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, W.; Lin, Z.; Bushman, L.R.; Anderson, P.L.; Ouyang, Z. In-capillary microextraction for direct mass spectrometry analysis of biological samples. Talanta 2018, 189, 451–457. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Shamsayei, M.; Safari, M.; Rezaei, F. On-line electrochemically controlled in-tube solid phase microextraction of inorganic selenium followed by hydride generation atomic absorption spectrometry. Anal. Chim. Acta 2016, 922, 37–47. [Google Scholar] [CrossRef]
- Maciel, E.V.S.; de Toffoli, A.L.; Lanças, F.M. Current status and future trends on automated multidimensional separation techniques employing sorbent-based extraction columns. J. Sep. Sci. 2019, 42, 258–272. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An Overview of the Most Common Lab-Made Coating Materials in Solid Phase Microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Shamsipur, M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Mullett, W.M.; Martin, P.; Pawliszyn, J. In-tube moleculary imprinted polymer solid-phase microextraction for the selective determination of propranolol. Anal. Chem. 2001, 73, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Zarejousheghani, M.; Möder, M.; Borsdorf, H. A new strategy for synthesis of an in-tube molecularly imprinted polymer-solid phase microextraction device: Selective off-line extraction of 4-nitrophenol as an example of priority pollutants from environmental water samples. Anal. Chim. Acta 2013, 798, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.D.; Melo, L.P.; Jardim, I.C.S.F.; Monteiro, J.C.S.; Nakano, A.M.S.; Queiroz, M.E.C. Selective molecularly imprinted polymer combined with restricted access material for in-tube SPME/UHPLC-MS/MS of parabens in breast milk samples. Anal. Chim. Acta 2016, 932, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, H.; Yamini, Y.; Seidi, S.; Ghahramanifard, F. Preparation and evaluation of a novel molecularly imprinted polymer coating for selective extraction of indomethacin from biological samples by electrochemically controlled in-tube solid phase microextraction. Anal. Chim. Acta 2016, 913, 76–85. [Google Scholar] [CrossRef]
- Kefayati, H.; Yamini, Y.; Shamsayei, M.; Abdi, S. Molecularly imprinted polypyrrole@CuO nanocomposite as an in-tube solid-phase microextraction coating for selective extraction of carbamazepine from biological samples. J. Pharm. Biomed. Anal. 2021, 204, 114256. [Google Scholar] [CrossRef]
- Song, X.; Li, X.; Wang, J.; Huang, X. Adoption of new strategy for molecularly imprinted polymer based in-tube solid phase microextraction to improve specific recognition performance and extraction efficiency. Microchem. J. 2023, 194, 109224. [Google Scholar] [CrossRef]
- Chaves, A.R.; Queiroz, M.E.C. In-tube solid-phase microextraction with molecularly imprinted polymer to determine interferon alpha 2a in plasma sample by high performance liquid chromatography. J. Chromatogr. A 2013, 1318, 43–48. [Google Scholar] [CrossRef]
- Hu, Y.; Song, C.; Li, G. Fiber-in-tube solid-phase microextraction with molecularly imprinted coating for sensitive analysis of antibiotic drugs by high performance liquid chromatography. J. Chromatogr. A 2012, 1263, 21–27. [Google Scholar] [CrossRef]
- Zhang, S.-W.; Xing, J.; Cai, L.-S.; Wu, C.-Y. Molecularly imprinted monolith in-tube solid-phase microextraction coupled with HPLC/UV detection for determination of 8-hydroxy-2′-deoxyguanosine in urine. Anal. Bioanal. Chem. 2009, 395, 479–487. [Google Scholar] [CrossRef]
- Lei, X.; Huang, T.; Wu, X.; Mangelings, D.; Eeckhaut, A.V.; Bongaerts, J.; Terryn, H.; Heyden, Y.V. Fabrication of a molecularly imprinted monolithic column via the epitope approach for the selective capillary microextraction of neuropeptides in human plasma. Talanta 2022, 243, 123397. [Google Scholar] [CrossRef] [PubMed]
- Courtois, J.; Fischer, G.; Sellergren, B.; Irgum, K. Molecularly imprinted polymers grafted to flow through poly(trimethylolpropane trimethacrylate) monoliths for capillary-based solid-phase extraction. J. Chromatogr. A 2006, 1109, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, Y.; Sun, X.; Yang, H.; Zhang, L.; Chen, G. One-pot preparation of a molecularly imprinted hybrid monolithic capillary column for selective recognition and capture of lysozyme. J. Chromatogr. A 2013, 1284, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, J.; Tan, X.; Sun, L.; Yu, R.; Yang, H.; Chen, G. Preparation of boronate-functionalized molecularly imprinted monolithic column with polydopamine coating for glycoprotein recognition and enrichment. J. Chromatogr. A 2013, 1319, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Szumski, M.; Grzywiński, D.; Prus, W.; Buszewski, B. Monolithic molecularly imprinted polymeric capillary columns for isolation of aflatoxins. J. Chromatogr. A 2014, 1364, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bouvarel, T.; Delaunay, N.; Pichon, V. Selective extraction of cocaine from biological samples with a miniaturized monolithic molecularly imprinted polymer and on-line analysis in nano-liquid chromatography. Anal. Chim. Acta 2020, 1096, 89–99. [Google Scholar] [CrossRef]
- Marchioni, C.; Vieira, T.M.; Crotti, A.E.M.; Crippa, J.A.; Queiroz, M.E.C. In-tube solid-phase microextraction with a dummy molecularly imprinted monolithic capillary coupled to ultra-performance liquid chromatography-tandem mass spectrometry to determine cannabinoids in plasma samples. Anal. Chim. Acta 2020, 1099, 145–154. [Google Scholar] [CrossRef]


| Surface imprinting | MIPs are typically fabricated in layers on hard particles, forming high affinity recognition sites on the substrate surface. The size of the imprinting cavities on the polymer surface can be effectively controlled, and uniformly distributed sites not only increase the adsorption capacity of the MIP and improve the rebinding rates of the recognition sites to the imprinted molecules, but also enhance the adsorption and separation efficiency of the imprinted material. |
| Nanoimprinting | Nanoimprinting technology is used to prepare nanostructured MIPs offering advantages such as high resolution, fast processing speed, high throughput, material compatibility, and low cost. These benefits improve the adsorption capacity, recombination rate, and site accessibility of the MIPs. |
| Living/controlled radical polymerization technology | Common methods include nitroxide-mediated free radical polymerization, atom transfer radical polymerization, and reversible addition cleavage chain transfer polymerization. Advantages include: (1) a wide range of polymerizable monomers, controllable polymer molecular weight, and narrow molecular weight distribution; (2) mild reaction conditions, low polymerization reaction temperatures, and compatibility with various solvents; (3) structural functional control, with the use of “reactive” features and functionalized end groups allowing for the preparation of polymers with complex compositions and structures; (4) a linear increase in polymer molecular weight with the conversion rate. |
| Multi-template imprinting | This technique uses multiple target molecules as templates to form various recognition sites in a single polymer material. Multiple template MIPs can simultaneously recognize multiple target molecules, allowing for the concurrent extraction, separation, analysis, and detection of different species, thus greatly expanding the practical applications of MIPs. |
| Multi-functional monomer imprinting | Multifunctional monomer imprinting techniques utilize non-covalent bonds between two or more functional monomers and a template molecule to create different forces with selective adsorption capacity. This improves the selectivity of the MIP for the template molecule, thereby enhancing its enrichment capacity. |
| Dummy template imprinting | Structural analogues of the target compound are used as template molecules when the target compound is either unsuitable for use as a template molecule or susceptible to degradation. |
| Analyte | Template | Polymerization Composition 1 and Conditions (Monomer/Crosslinker/Initiator/Porogen) 1 | Capillary Tube Configuration | IT-SPME Operation | Enrichment, Sensitivity 2 | IF 3 | Matrix | Detection 4 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Estrogen-related compounds | β-Estradiol | VP/EGDMA/AIBN/CH2Cl2, 50 °C for 4.5 h, template/VP/EGDMA (1:6:30). In-situ synthesis of MIP in a fused silica capillary surface by insertion of fluorocarbon yarn (65 cm × 0.20 mm). | Inner-surface-coated fused silica capillary (60 cm × 0.32 mm ID). | Draw/ejection on-line | EF: 1.9–16.4 | 1.8–3.6 | Water | HPLC-UV | - |
| 4-Nitrophenol | 4-Nitrophenol | MAA/EGDMA/AIBN/acetonitrile, 60 °C for 4 h. In-situ synthesis of MIP in a glass capillary surface by insertion of a metal rod. | Inner-surface-coated glass-capillary (100 μL). | Syringe pump off-line | LOD: 0.33 ng mL−1 | - | Environ-mental water | HPLC-DAD | [88] |
| Parabens | Benzyl-paraben | MIP: VP/EGDMA/AIBN/acetonitrile in capillary at 50 °C for 4 h, template/monomer (1:4), RAM: hydrophilic monomer GDMA/PPDS at 70 °C for 20 h. | Inner-surface-coated fused silica capillary (50 mm × 0.53 mm ID). | Syringe pump off-line | LLOQ: 3–10 ng mL−1 | - | Breast milk | UHPLC-MS/MS | [89] |
| Indomethacin | Indomethacin | PY/EGDMA/AIBN/MeOH:H2O (2:1, v/v), cyclic voltammetry in the potential range between −1.0 and ~1.0 V during 30 cycles (scan rate: 50 mV/s). | Inner-surface-coated stainless-steel tube (10 cm × 0.75 mm ID). | Electrochemi-cal control flow-through on-line | LOD: 0.6–2.0 ng mL−1 | - | Urine, plasma, blood | HPLC-UV | [90] |
| Carbamazepine | Carbam-azepine | Molecularly imprinted polypyrrole coated on CuO by electrodeposition, cyclic voltammetry in the potential range (0~+3 V during 30 cycles (scan rate: 70 mV/s). | Inner-surface-coated copper tube (10 cm × 0.78 mm ID). | Flow-through on-line | LOQ: 0.1 ng mL−1 | - | Urine, plasma | HPLC-UV | [91] |
| 2,4-Dinitroaniline (2,4-DNA) | 2,4-DNA | VI/EDMA/AIBN/1-propanol:1,4-butanediol (1:1)/Fe3O4 nanoparticles pre-modified with γ-MAPS at 70 °C for 12 h, template/VI/EDMA (4:1:4). | Inner-surface-coated fused silica capillary (2 cm × 0.53 mm ID). | Magnetic field control flow-through on-line | LOD: 60 pg mL−1 | 3.1 | Environ-mental water | HPLC-DAD | [92] |
| Propranolol | Racemic propranolol | MAA/EGDMA/AIBN/toluene at 60 °C for 18 h, template/monomer (1:2) | Particle-packed PEEK tube (80 mm × 0.76 mm ID). | Draw/ejection on-line | LOD: 0.32 μg mL−1 | - | Serum | HPLC-UV | [87] |
| Interferon alpha 2a | Interferon alpha 2a | Two-step sol–gel procedure: APS/TEOS/deionized water:0.1 M HCl: absolute (EtOH) (1:1.4:1.7) + silanes, kept at room temperature for 24 h and dried at 50 °C for 48 h. | Particle-packed PEEK tube (50 mm × 0.02 inch ID). | Draw/ejection on-line | - | - | Plasma | HPLC-FD | [93] |
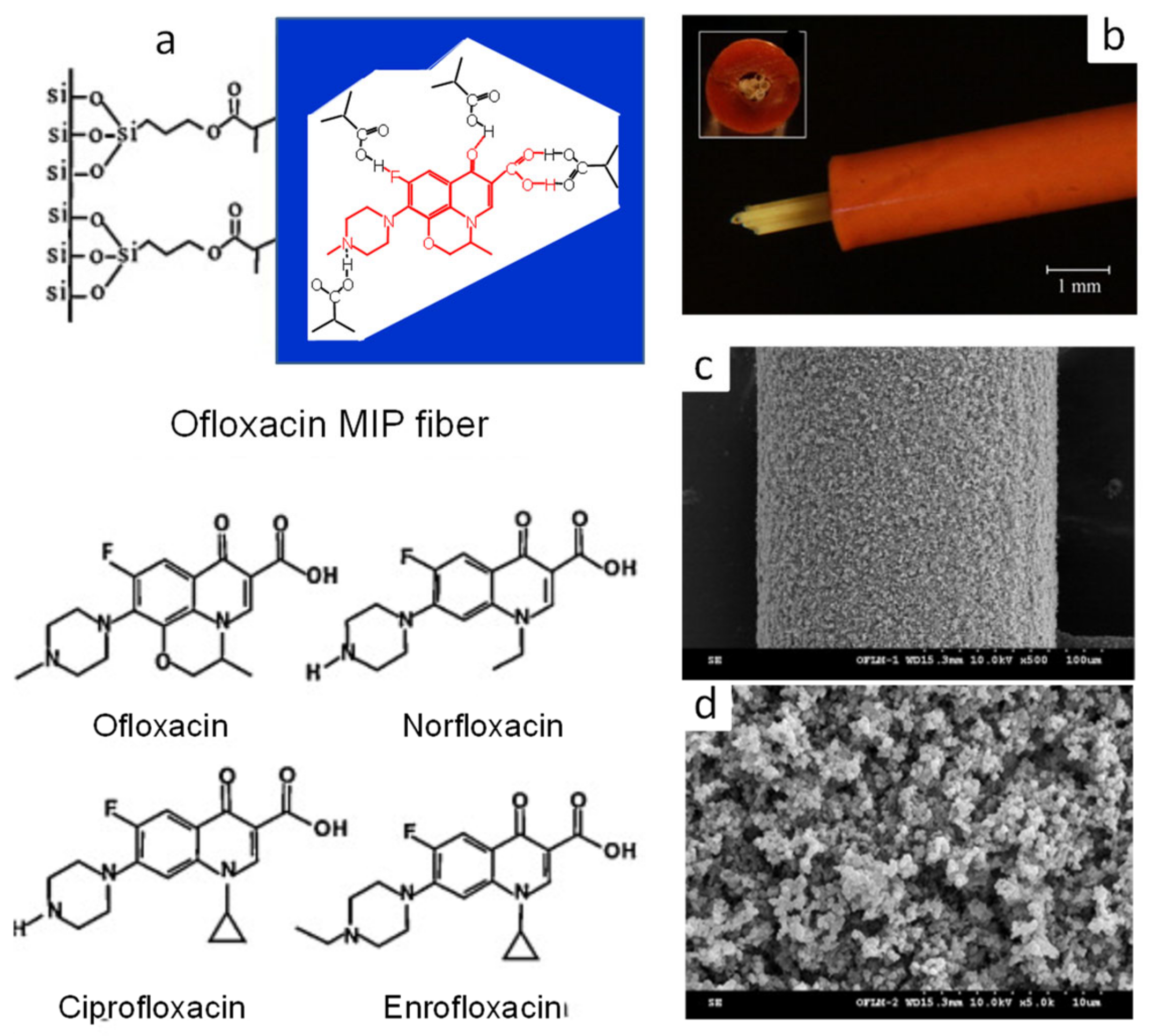
| Four fluoro-quinolone antibiotics | Ofloxacin, sulfadiazine | MAA/TRIM/AIBN/CH3CN:H2O (6:1, v/v), silica fiber (10 cm × 0.125 mm) coating in glass capillary (10 cm × 1.0 mm ID) at 60 °C for 3 h. | Fiber-packed (6 cm × 6 fibers) PEEK tube (0.5 mm ID). | Flow-through on-line | EF: 69–136, LOD: 16–110 pg mL−1 | - | Pork liver | HPLC-UV | [94] |
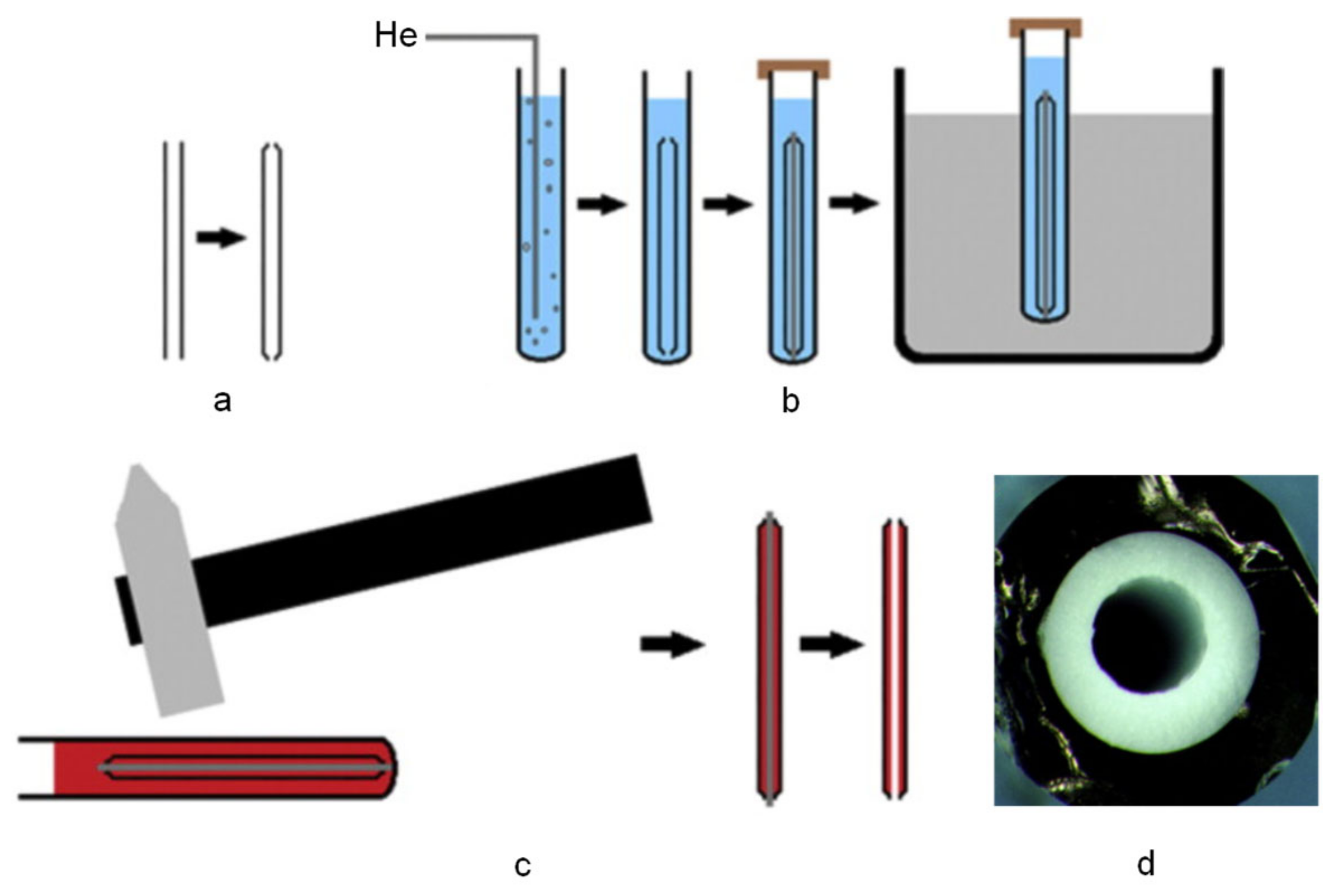
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | Guanosine | Monolith: TEPM/methanol, 40 °C for 12 h; MIP monolith: VP/MBA/AIBN/dodecanol, in capillary at 60 °C for 18 h. | Rod monolith in fused silica capillary (50 mm × 0.53 mm ID). | Syringe pump off-line | EF: 76, LOD: 957 pg mL−1 | - | Urine | HPLC-UV | [95] |
| Neurotensin, neuromedin N | Pro-Tyr-Ile-Leu | MIP monolith: MAA/EGDMA/AIBN/MeOH/acetonitrile/isooctane, 60 °C for 16 h template/MAA (1:3). | Inner-surface-coated fused silica capillary (2 cm × 0.53 mm ID). | Syringe pump off-line | LOD: 0.9–1.0 ng mL−1 | 5.7–13.4 | Plasma | HPLC-UV | [96] |
| Anaesthetics (bupivacaine, mepivacaine, S-ropivacaine) | Bupivacaine, mepivacaine, ropivacaine | Monolith: TRIM/EDMA/BME/2,2,4-trimethylpentane/toluene (80:20, w/w), UV; MIP monolith: MAA/EDMA/AIBN/toluene, Template/MAA/EDMA (0.33:4:20), UV, 1 h | Rod monolith in UV transparent capillaries (70 mm × 0.1 mm ID). | Flow-through on-line | - | 12–72 | Water | HPLC-UV | [97] |
| Lysozyme | Lysozyme | Co-precursors: PEG/TMOS/γ-MAPS MIP hybrid monolith: co-precursors + AAm/MBA/AIBN/MeOH:H2O (5:3, v/v), in capillary at 40 and 60 °C for 12 h. | Rod monolith in fused silica capillary (25 cm × 75 μm ID). | Flow-through on-line | - | 1.91 | Serum, egg white | pCEC-UV (capLC) | [98] |
| Glycoprotein | Horseradish peroxidase (HRP) | Monolith: VPBA/PETA/AIBN/ethylene glycol: cyclohexanol, in capillary at 75 °C for 12 h; MIP monolith: immobilization of HRP on VPBA-based monolith and poly-dopamine (pDA) coating with DA and APS. | Rod monolith in fused silica capillary (25 cm × 75 μm ID). | Flow-through on-line | - | 2.76 | Serum | pCEC-UV | [99] |
| Aflatoxins | 5,7-Dimethoxy-coumarin | Monolith: γ-MAPS/TRIM/BME or AIBN/2,2,4-trimethylpentane/toluene (80:20, w/w), UV for 1 h and 60 °C for 24 h MIP monolith: MAA/EGDMA/AIBN/toluene, template/MAA/EGDMA (0.3:4:20), UV, 1 h | Rod monolith in UV transparent fused capillary (70 mm × 0.1 mm ID). | Flow-through on-line | - | - | Water | MicroLC-LIF | [100] |
| Cocaine and its metabolite | Cocaine | MIP monolith: MAA/EGDMA/AIBN/acetonitrile-isooctane (9:1), 60 °C for 24 h template/MAA/EGDMA (1:4:20). | Inner-surface-coated fused capillary (50 mm × 0.1 mm ID). | Flow-through on-line | LOD: 14.5–6.1 ng mL−1 | 2.2–3.2 | Plasma, saliva | NanoLC-UV | [101] |
| Cannabinoids | Hydrogenated cannabidiol | MIP monolith: MAA/EGDMA/AIBN/CH2Cl2, 60 °C for 24 h, template/MAA (1:3). | Inner-surface-coated fused capillary (10 cm × 0.53 mm ID). | Flow-through on-line | LLOQ: 10 ng mL−1 | - | Plasma | UHPLC-MS/MS | [102] |
| Compound | IF 1 | EF 2 | Compound | IF | EF | Compound | IF | EF |
|---|---|---|---|---|---|---|---|---|
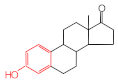 Estrone | 2.78 | 16.4 | 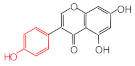 Genistein | 3.56 | 12.2 |  Nonylphenol | 1.39 | 4.27 |
 β-Estradiol | 2.35 | 3.60 |  Bisphenol A | 2.64 | 5.26 |  Di-n-butyl phthalate | 1.21 | 2.54 |
 Estriol | 2.43 | 1.93 |  Progesterone | 1.50 | 2.63 |  Di-2-ethylhexyl phthalate | 0.86 | 1.45 |
 Ethinylestradiol | 2.29 | 5.60 |  Testosterone | 0.96 | 1.03 |  Polychlorinated biphenyl (PCBs) | 0.92 | 13.7 |
 Diethylstilbestrol (DES) | 1.79 | 2.63 |  Corticosterone | 0.77 | 0.77 |  Dichlorodiphenyl- trichloroethane (DDT) | 1.16 | 19.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, H.; Ishizaki, A.; Saito, K.; Ehara, K. Developments and Applications of Molecularly Imprinted Polymer-Based In-Tube Solid Phase Microextraction Technique for Efficient Sample Preparation. Molecules 2024, 29, 4472. https://doi.org/10.3390/molecules29184472
Kataoka H, Ishizaki A, Saito K, Ehara K. Developments and Applications of Molecularly Imprinted Polymer-Based In-Tube Solid Phase Microextraction Technique for Efficient Sample Preparation. Molecules. 2024; 29(18):4472. https://doi.org/10.3390/molecules29184472
Chicago/Turabian StyleKataoka, Hiroyuki, Atsushi Ishizaki, Keita Saito, and Kentaro Ehara. 2024. "Developments and Applications of Molecularly Imprinted Polymer-Based In-Tube Solid Phase Microextraction Technique for Efficient Sample Preparation" Molecules 29, no. 18: 4472. https://doi.org/10.3390/molecules29184472
APA StyleKataoka, H., Ishizaki, A., Saito, K., & Ehara, K. (2024). Developments and Applications of Molecularly Imprinted Polymer-Based In-Tube Solid Phase Microextraction Technique for Efficient Sample Preparation. Molecules, 29(18), 4472. https://doi.org/10.3390/molecules29184472







