Synthesis and Anticancer Activity Assessment of Zelkovamycin Analogues
Abstract
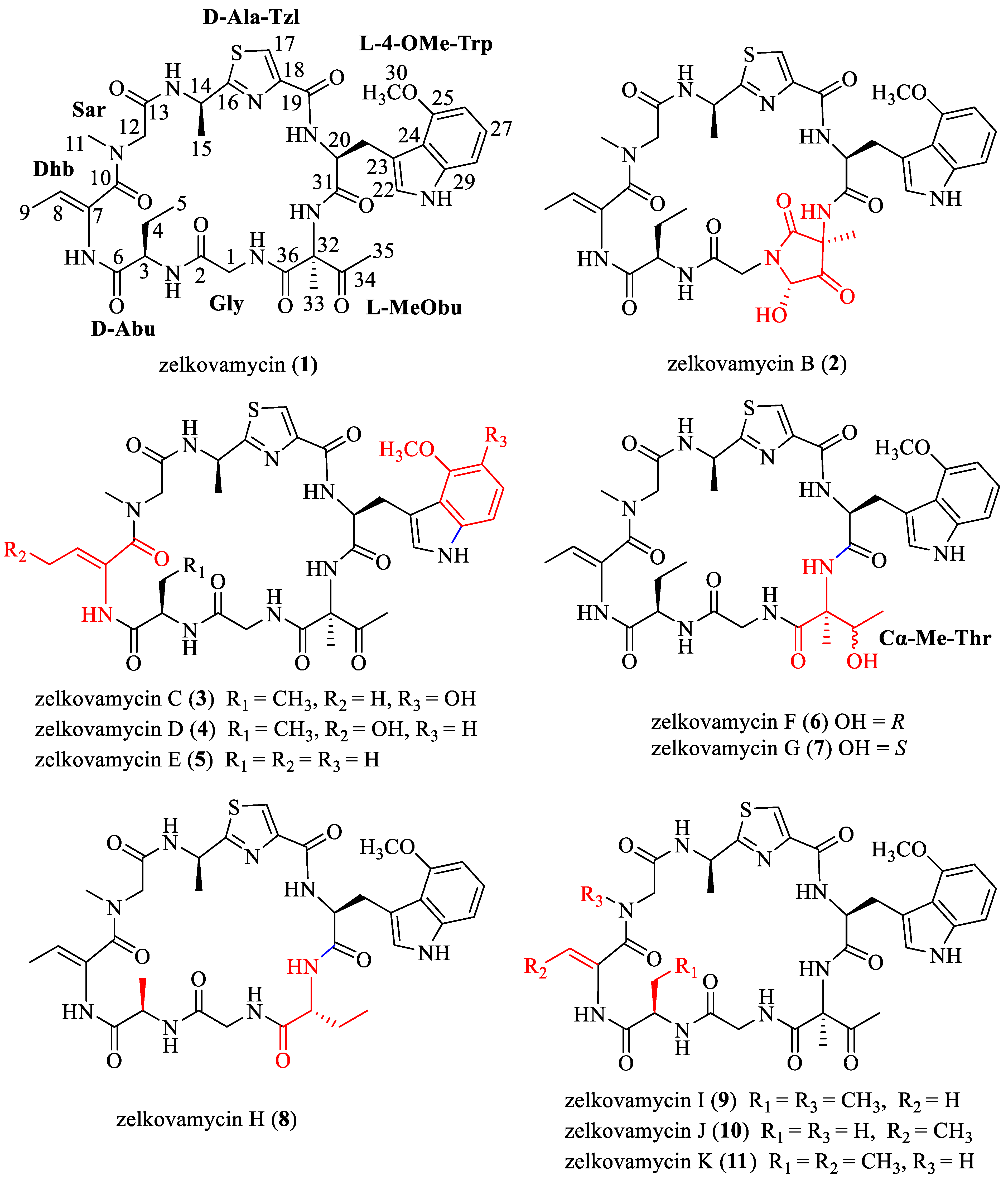
:1. Introduction
2. Results and Discussion
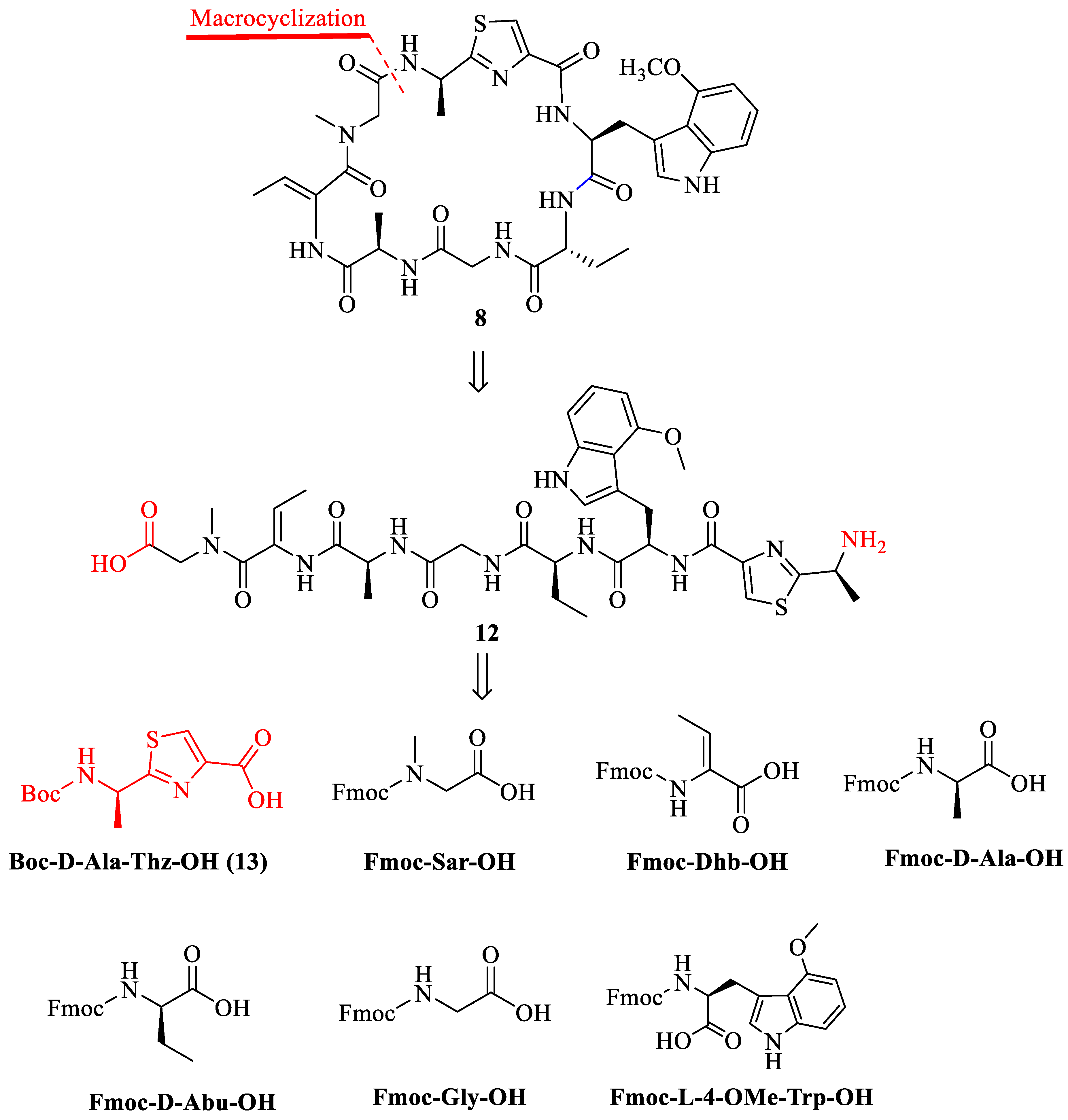
2.1. Synthesis of Zelkovamycin H
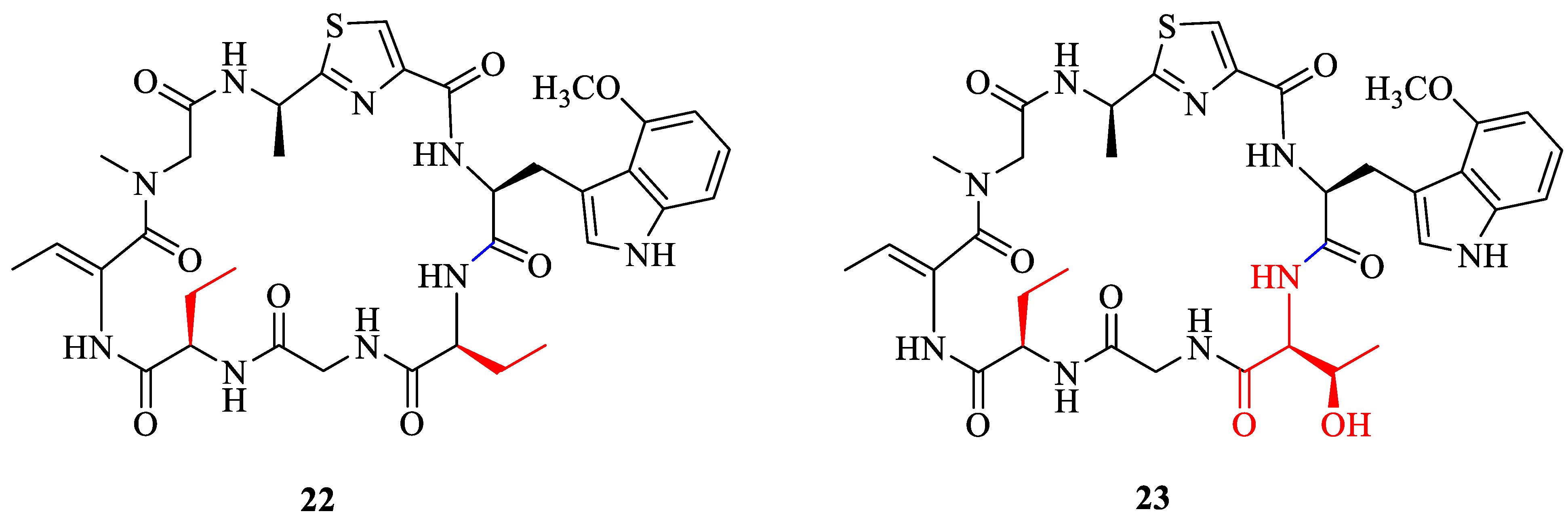
2.2. Synthesis of Analogue
2.3. Anticancer Evaluation
3. Materials and Methods
3.1. General Chemical Procedures
3.2. Synthesis of Boc-D-Ala-Thz-OH (13) [18,19,20,21]
3.3. Synthesis of Analogue 21 {Cyclo-[(Sar-Dhb-Ala-Gly-Abu-Trp(4-OMe)-Ala(Thz)]}
3.3.1. Loading First Amino Acid Fmoc-Sar-OH to 2-CTC Resin for the Synthesis of 17
3.3.2. Synthesis of Chain Peptide on the Resin (18)
3.3.3. Cleavage of Peptide from Resin to Synthesize 19
3.3.4. Macrolactamization to Synthesize 21
3.4. Synthesis of the Analogues
3.5. Anticancer Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Song, X.; Ku, C.F.; Si, T.X.; Jaiswal, Y.S.; Williams, L.L.; Lu, D.Y.; Huang, J.J.; He, Z.D.; Wang, M.Z. Synthesis and Biological Activities Assessment of 4-, 6-, and 9-Phenylphenalenone Derivatives. ChemistrySelect 2022, 7, e202203793. [Google Scholar] [CrossRef]
- Zhang, H.; Tomoda, H.; Tabata, N.; Oohori, M.; Shinose, M.; Takahashi, Y.; Omura, S. Zelkovamycin, a New Cyclic Peptide Antibiotic from Streptomyces sp. K96-0670. I. Production, Isolation and Biological Properties. J. Antibiot. 1999, 52, 29–33. [Google Scholar] [CrossRef]
- Tabata, N.; Tomoda, H.; Zhang, H.; Uchida, R.; Omura, S. Zelkovamycin, a New Cyclic Peptide Antibiotic from Streptomyces sp. K96-0670 II. Structure Elucidation. J. Antibiot. 1999, 52, 34–39. [Google Scholar] [CrossRef]
- Tarantini, F.S.; Brunati, M.; Taravella, A.; Carrano, L.; Parenti, F.; Hong, K.W.; Williams, P.; Chan, K.G.; Heeb, S.; Chan, W.C. Actinomadura graeca sp. nov.: A novel producer of the macrocyclic antibiotic zelkovamycin. PLoS ONE 2021, 16, e0260413. [Google Scholar] [CrossRef]
- Hao, X.M.; Yu, J.Q.; Wang, Y.J.; Connolly, J.A.; Liu, Y.F.; Zhang, Y.Q.; Yu, L.Y.; Cen, S.; Goss, R.J.M.; Gan, M.L. Zelkovamycins B-E, Cyclic Octapeptides Containing Rare Amino Acid Residues from an Endophytic Kitasatospora sp. Org. Lett. 2020, 22, 9346–9350. [Google Scholar] [CrossRef]
- Hao, X.M.; Li, S.S.; Wang, G.Y.; Li, J.R.; Peng, Z.G.; Zhang, Y.Q.; Yu, L.Y.; Gan, M.L. Zelkovamycins F and G, Cyclopeptides with Cα-Methyl-Threonine Residues, from an Endophytic Kitasatospora sp. J. Nat. Prod. 2022, 85, 1715–1722. [Google Scholar] [CrossRef]
- Krahn, D.; Heilmann, G.; Vogel, F.C.E.; Papadopoulos, C.; Zweerink, S.; Kaschani, F.; Meyer, H.; Roesch, A.; Kaiser, M. Zelkovamycin is an OXPHOS Inhibitory Member of the Argyrin Natural Product Family. Chem. Eur. J. 2020, 26, 8524–8531. [Google Scholar] [CrossRef]
- Sasse, F.; Steinmetz, H.; Schupp, T.; Petersen, F.; Memmert, K.; Hofmann, H.; Heusser, C.; Brinkmann, V.; Matt, P.V.; Höfle, G.; et al. Argyrins, Immunosuppressive Cyclic Peptides from Myxobacteria I. Production, isolation, physico-chemical and biological properties. J. Antibiot. 2002, 55, 543–551. [Google Scholar] [CrossRef]
- Vollbrecht, L.; Steinmetz, H.; Höfle, G. Argyrins, Immunosuppressive Cyclic Peptides from Myxobacteria II. Structure Elucidation and Stereochemistry. J. Antibiot. 2002, 55, 715–721. [Google Scholar] [CrossRef]
- Pogorevc, D.; Tang, Y.; Hoffmann, M.; Zipf, G.; Bernauer, H.S.; Popoff, A.; Steinmetz, H.; Wenzel, S.C. Biosynthesis and Heterologous Production of Argyrins. ACS Synth. Biol. 2019, 8, 1121–1133. [Google Scholar] [CrossRef]
- Nickeleit, I.; Zender, S.; Sasse, F.; Geffers, R.; Brandes, G.; Sörensen, I.; Steinmetz, H.; Kubicka, S.; Carlomagno, T.; Menche, D.; et al. Argyrin A Reveals a Critical Role for the Tumor Suppressor Protein p27kip1 in Mediating Antitumor Activities in Response to Ptoteasome Inhibition. Cancer Cell 2008, 14, 23–35. [Google Scholar] [CrossRef]
- Stauch, B.; Simon, B.; Basile, T.; Schneider, G.; Malek, N.P.; Kalesse, M.; Priv-Doz, T.C. Elucidation of the Structure and Intermolecular Interaction of a Reversible Cyclic-Peptide Inhibitor of the Proteasome by NMR Spectroscopy and Molecular Modeling. Angew. Chem. Int. Ed. 2010, 49, 3934–3938. [Google Scholar] [CrossRef]
- Allardyce, D.J.; Bell, C.M.; Loizidou, E.Z. Argyrin B, a non-competitive inhibitor of the human immunoproteasome exhibiting preference for β1i. Chem. Biol. Drug Des. 2019, 94, 1556–1567. [Google Scholar] [CrossRef]
- Nyfeler, B.; Hoepfner, D.; Palestrant, D.; Kiiby, C.A.; Whitehead, L.; Yu, R.; Deng, D.; Cauhglan, R.E.; Woods, A.L.; Jones, A.K.; et al. Identification of Elongation Factor G as the Conserved Cellular Target of Argyrin B. PLoS ONE 2012, 7, e42657. [Google Scholar] [CrossRef]
- Bieleck, P.; Hüsecken, P.L.K.; Dötsch, A.; Steinmetz, H.; Hartmann, R.W.; Müller, R.; Häussler, S. Mutation in Elongation Factor G Confers Resistance to the Antibiotic Argyrin in the Opportunistic Pathogen Pseudomonas aeruginosa. ChemBioChem 2012, 13, 2339–2345. [Google Scholar] [CrossRef]
- Romano, G.; Almeida, M.; Coelho, A.V.; Cutignano, A.; Gonçalves, L.G.; Hansen, E.; Khnykin, D.; Mass, T.; Ramsak, A.; Rocha, M.S.; et al. Biomaterials and Bioactive Natural Products from Marine Invertebrates: From Basic Research to Innovative Applications. Mar. Drugs 2022, 20, 219–263. [Google Scholar] [CrossRef]
- Kigoshi, H.; Yamada, S. Synthesis of Dolastatin I, a Cytotoxic Cyclic Hexapeptide from the Sea Hare Dolabella Auricularia. Tetrahedron 1999, 55, 12301–12308. [Google Scholar] [CrossRef]
- Lajoie, G.; Lepine, F.; Maziak, L.; Belleau, B. Facile Regioselective Formation of Thiopeptide Linkages from Oligopeptides with New Thionation Reagents. Tetrahedron Lett. 1983, 24, 3815–3818. [Google Scholar] [CrossRef]
- Aguilar, E.; Meyers, A.I. Reinvestigation of a Modified Hantzsch Thiazole Synthesis. Tetrahedron Lett. 1994, 35, 2473–2476. [Google Scholar] [CrossRef]
- Erik, S.; Robert-Franz-Xaver, K.; Nediljko, B.; Markus, K. Painting argyrins blue: Negishi cross-coupling for synthesis of deep-blue tryptophan analogue β-(1-azulenyl)-L alanine and its incorporation into argyrin C. Bioorgan. Med. Chem. 2018, 26, 5259–5269. [Google Scholar]
- Merrifield, R.B. Solid phase peptide synthesis I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Atherthon, E.; Fox, H.; Harkiss, D.; Logan, C.J.; Sheppard, R.C.; Williams, B.J. A mild procedure for solid phase peptide synthesis: Use of fluorenylmethyloxycarbonylamino-acids. J. Chem. Soc. Chem. Commun. 1978, 537–539. [Google Scholar] [CrossRef]
- Barlos, K.; Chatzi, O.; Gatos, D.; Stavropoulos, G. 2-chlorotrityl chloride resin. Studies on anchoring of Fmoc-amino acids and peptide cleavage. Int. J. Pep. Protein Res. 1991, 37, 513–520. [Google Scholar]
- Lopez, J.; Beck, J.; Bucher, C.; Berthelmann, A.; Markos, S.; Eissler, S. Missing Link: Enabling Loading of 2-Chloride Resin in N-Butylpyrrolidinone as a Green Solvent. Org. Process Res. Dev. 2022, 26, 1450–1457. [Google Scholar] [CrossRef]
- Carpino, L.A.; EI-Faham, A. Effect of Tertiary Bases on O-Benzotriazolyluronium Salt-Induced Peptide Segment Coupling. J. Org. Chem. 1994, 59, 695–698. [Google Scholar] [CrossRef]
- Qiu, S.; Gussem, E.D.; Tehrani, K.A.; Sergeyev, S.; Bultinck, P.; Herrebout, W. Stereochemistry of the Tadalafil Diastereoisomers: A Critical Assessment of Vibrational Circular Dichroism, Electronic Circular Dichroism, and Optical Rotatory Dispersion. J. Med. Chem. 2013, 56, 8903–8914. [Google Scholar] [CrossRef]


| No. | Amino Acid Residue | Natural (150 MHz) | Synthetic (100 MHz) | Δδ |
|---|---|---|---|---|
| 1 | Gly | 42.0 | 41.8 | −0.2 |
| 2 | 171.6 | 171.3 | −0.3 | |
| 3 | Ala | 51.3 | 51.1 | −0.2 |
| 4 | 15.4 | 14.1 | 1.3 | |
| 6 | 169.6 | 169.8 | 0.2 | |
| 7 | Dhb | 130.6 | 130.4 | −0.2 |
| 8 | 110.5 | 110.0 | −0.5 | |
| 9 | 11.4 | 11.4 | 0 | |
| 10 | 169.0 | 169.0 | 0 | |
| 11 | Sar | 37.4 | 37.6 | 0.2 |
| 12 | 51.1 | 48.5 | −2.6 | |
| 13 | 167.1 | 167.1 | 0 | |
| 14 | Ala-Tzl | 45.4 | 45.5 | 0.1 |
| 15 | 20.9 | 20.7 | −0.2 | |
| 16 | 171.2 | 171.2 | 0 | |
| 17 | 122.8 | 122.8 | 0 | |
| 18 | 150.3 | 150.2 | −0.1 | |
| 19 | 160.0 | 160.0 | 0 | |
| 20 | 4-Meo-Trp | 52.6 | 52.5 | −0.1 |
| 21 | 28.1 | 28.2 | 0.1 | |
| 22 | 125.0 | 124.8 | −0.2 | |
| 23 | 106.3 | 106.4 | 0.1 | |
| 24 | 116.3 | 116.3 | 0 | |
| 25 | 153.3 | 153.4 | 0.1 | |
| 26 | 100.0 | 99.8 | −0.2 | |
| 27 | 122.5 | 122.6 | 0.1 | |
| 28 | 105.9 | 105.8 | −0.1 | |
| 29 | 136.7 | 136.6 | −0.1 | |
| 30 | 55.6 | 57.7 | 2.1 | |
| 31 | 171.7 | 171.7 | 0 | |
| 32 | Abu | 54.5 | 55.5 | 1.0 |
| 34 | 21.1 | 21.4 | 0.3 | |
| 35 | 10.4 | 11.3 | 0.9 | |
| 36 | 169.4 | 169.4 | 0 |
| Compound | IC50 (24 h) |
|---|---|
| 21 | 56.2 |
| 22 | 62.8 |
| 23 | 75.7 |
| 5-fluorouracil | 15.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Huang, H.; Jaiswal, Y.S.; Su, S.; Yang, L.; Fan, Y.; Guan, Y.; Williams, L.L.; Bian, H. Synthesis and Anticancer Activity Assessment of Zelkovamycin Analogues. Molecules 2024, 29, 4483. https://doi.org/10.3390/molecules29184483
Xie X, Huang H, Jaiswal YS, Su S, Yang L, Fan Y, Guan Y, Williams LL, Bian H. Synthesis and Anticancer Activity Assessment of Zelkovamycin Analogues. Molecules. 2024; 29(18):4483. https://doi.org/10.3390/molecules29184483
Chicago/Turabian StyleXie, Xinrong, Hongshun Huang, Yogini S. Jaiswal, Shaoyang Su, Linxia Yang, Yu Fan, Yifu Guan, Leonard L. Williams, and Hedong Bian. 2024. "Synthesis and Anticancer Activity Assessment of Zelkovamycin Analogues" Molecules 29, no. 18: 4483. https://doi.org/10.3390/molecules29184483






