FFF/FDM 3D-Printed Solid Polymer Electrolytes Based on Acrylonitrile Copolymers for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Characterization of poly(AN-co-PEGMEA)
2.1.1. Elemental Analysis
2.1.2. FTIR Spectroscopy
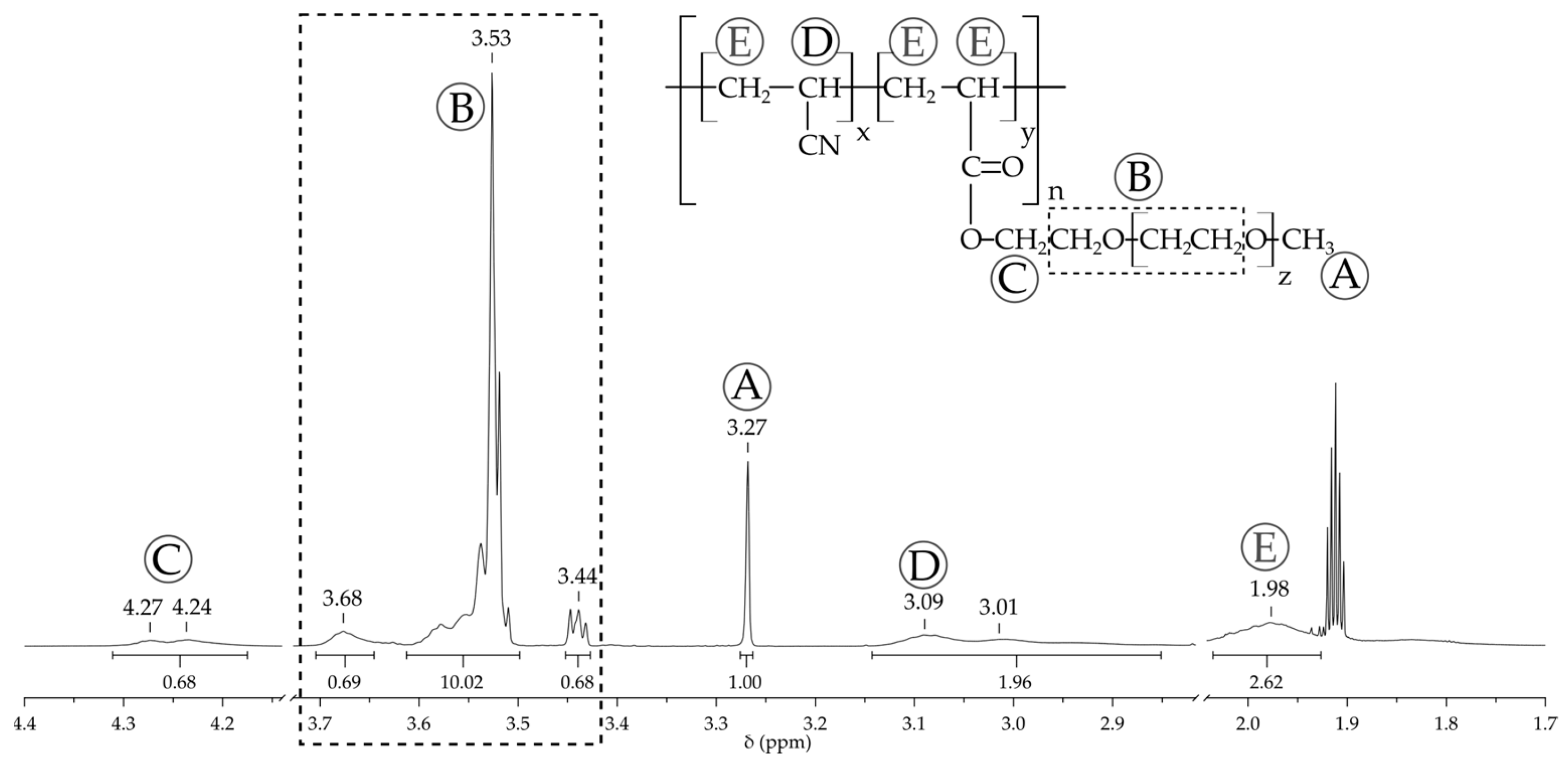
2.1.3. 1H NMR Spectroscopy
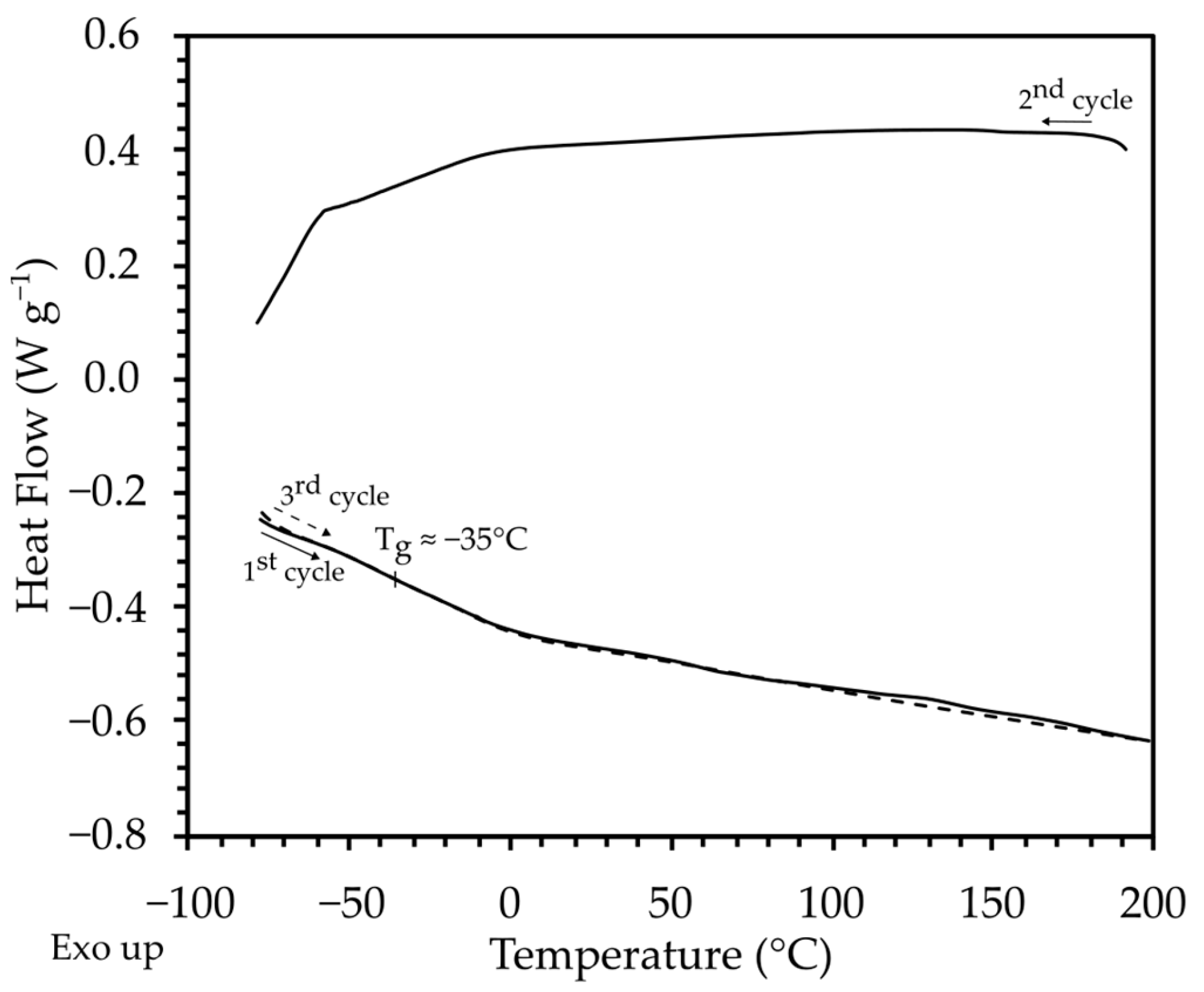
2.1.4. Differential Scanning Calorimetry (DSC)
2.2. Electrochemical Characterization of poly(AN-co-PEGMEA) Electrolytes
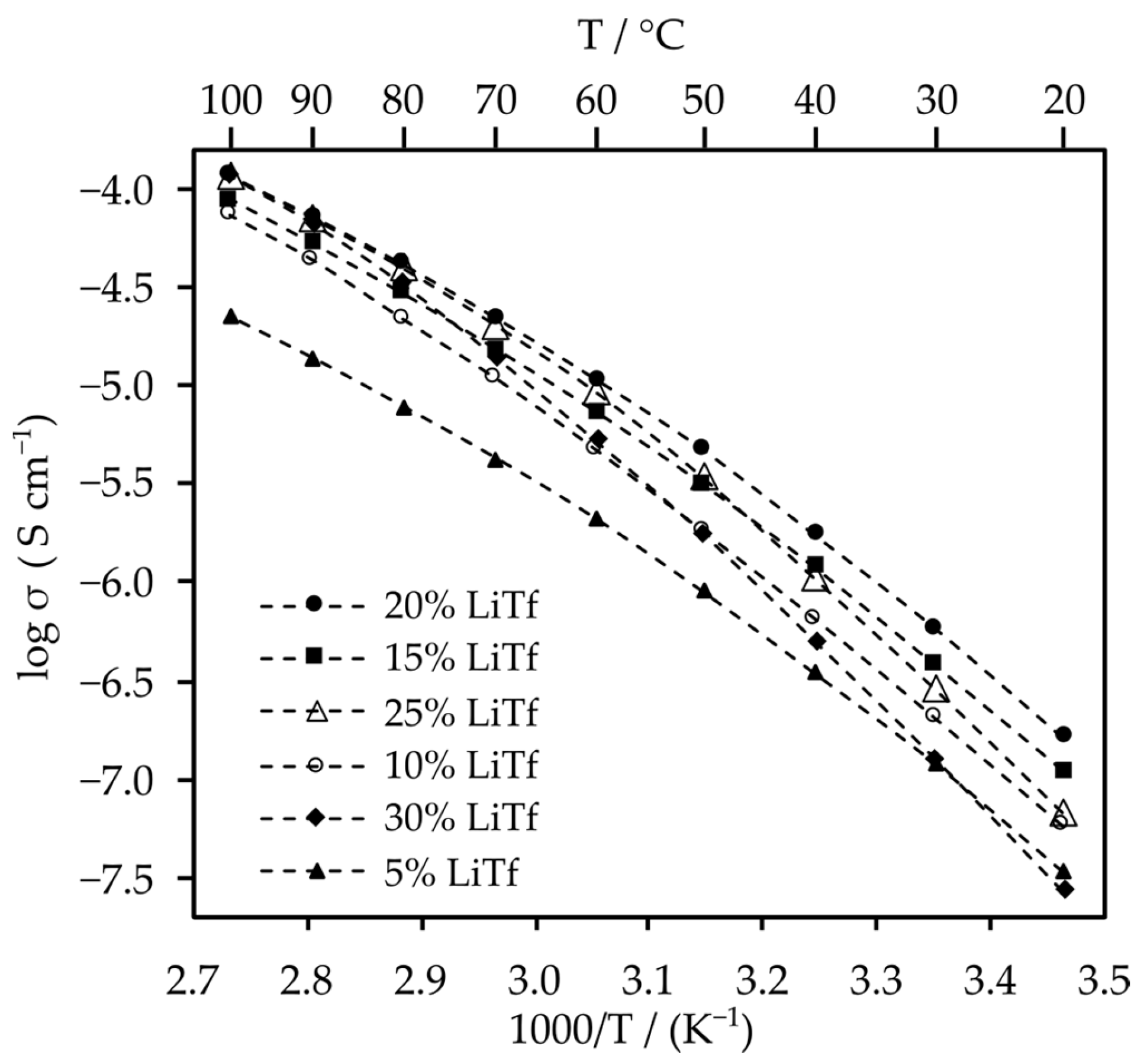
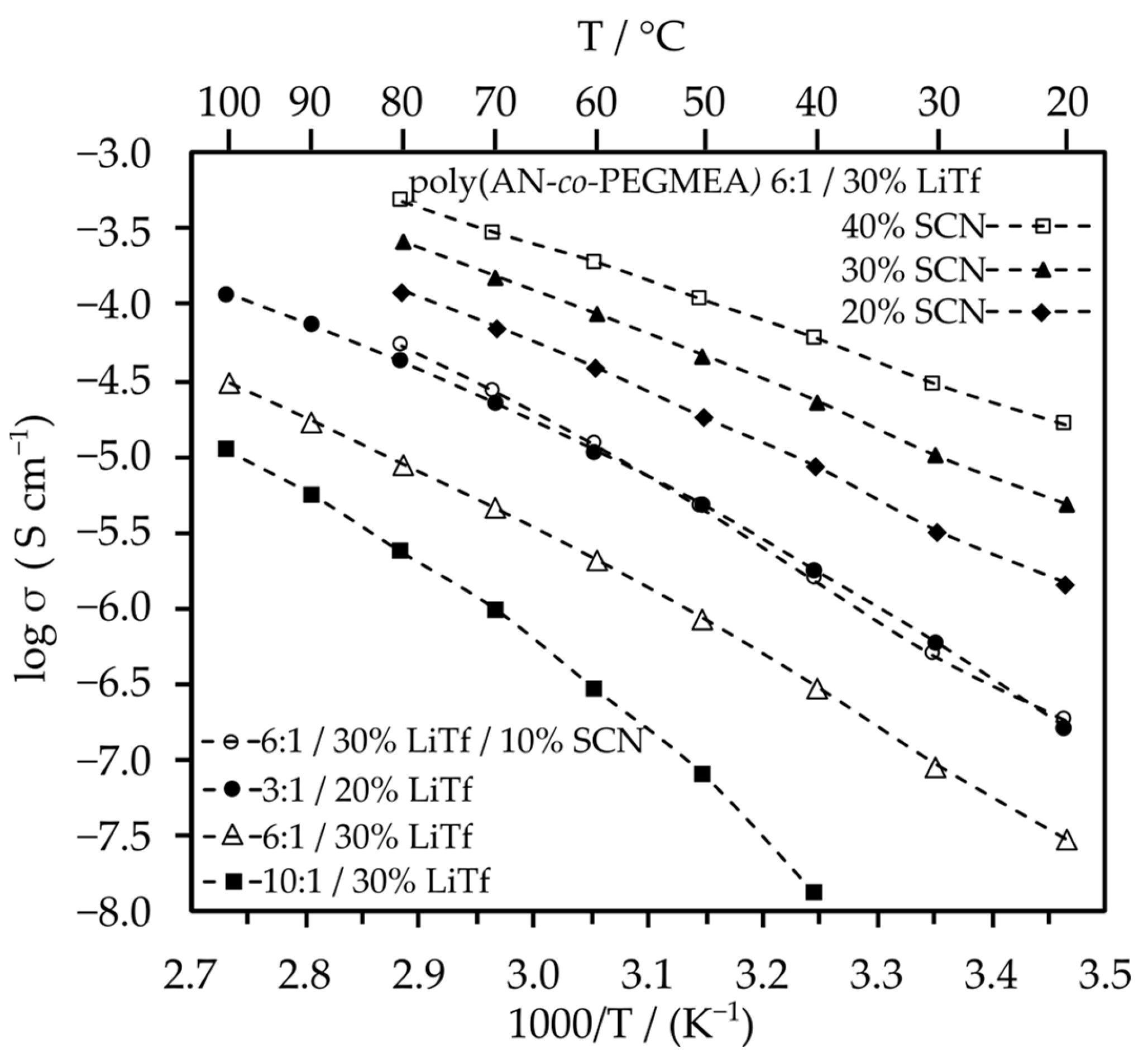
2.2.1. Ionic Conductivity
2.2.2. Lithium Cation Transference Number
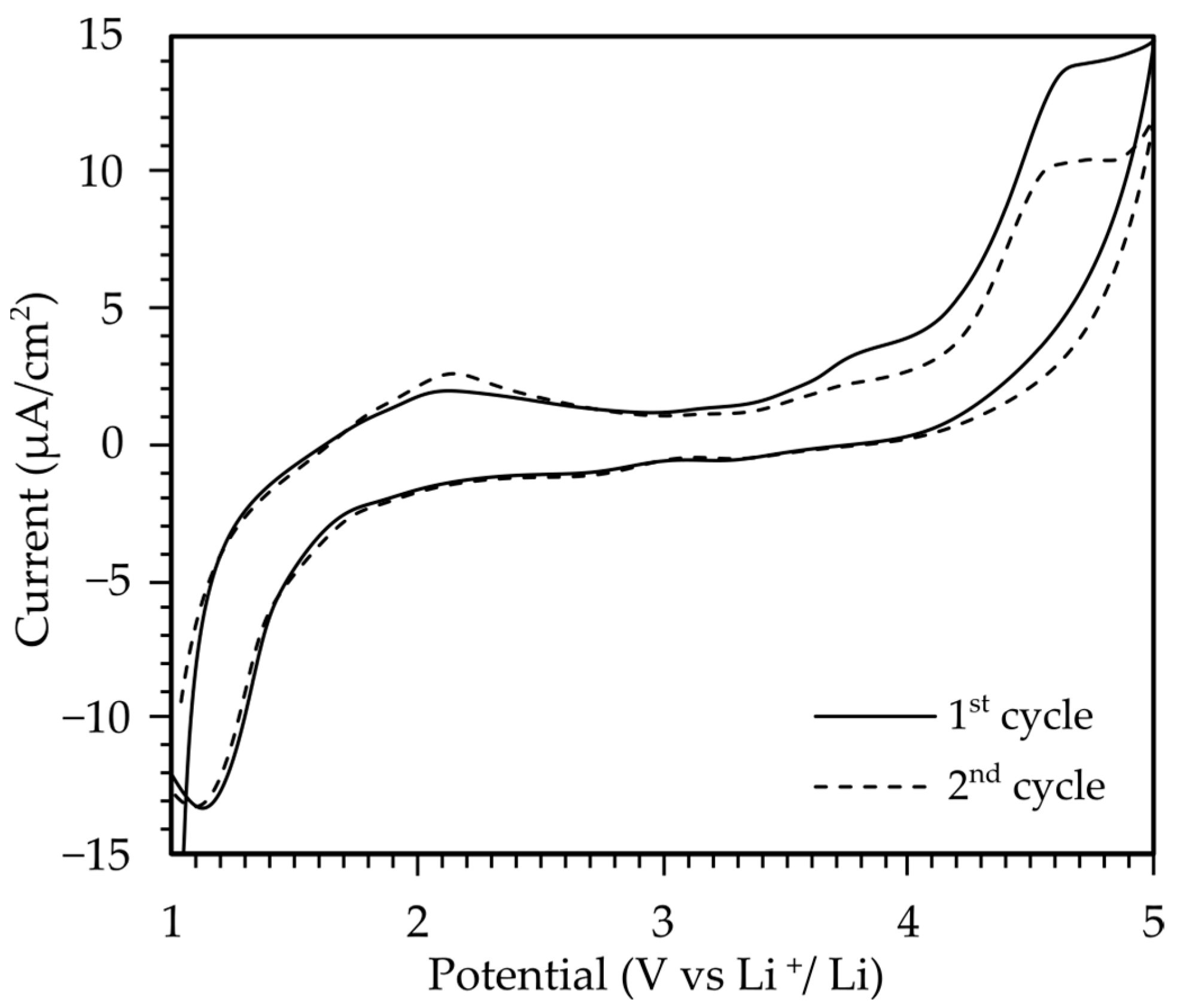
2.2.3. Cyclic Voltammetry
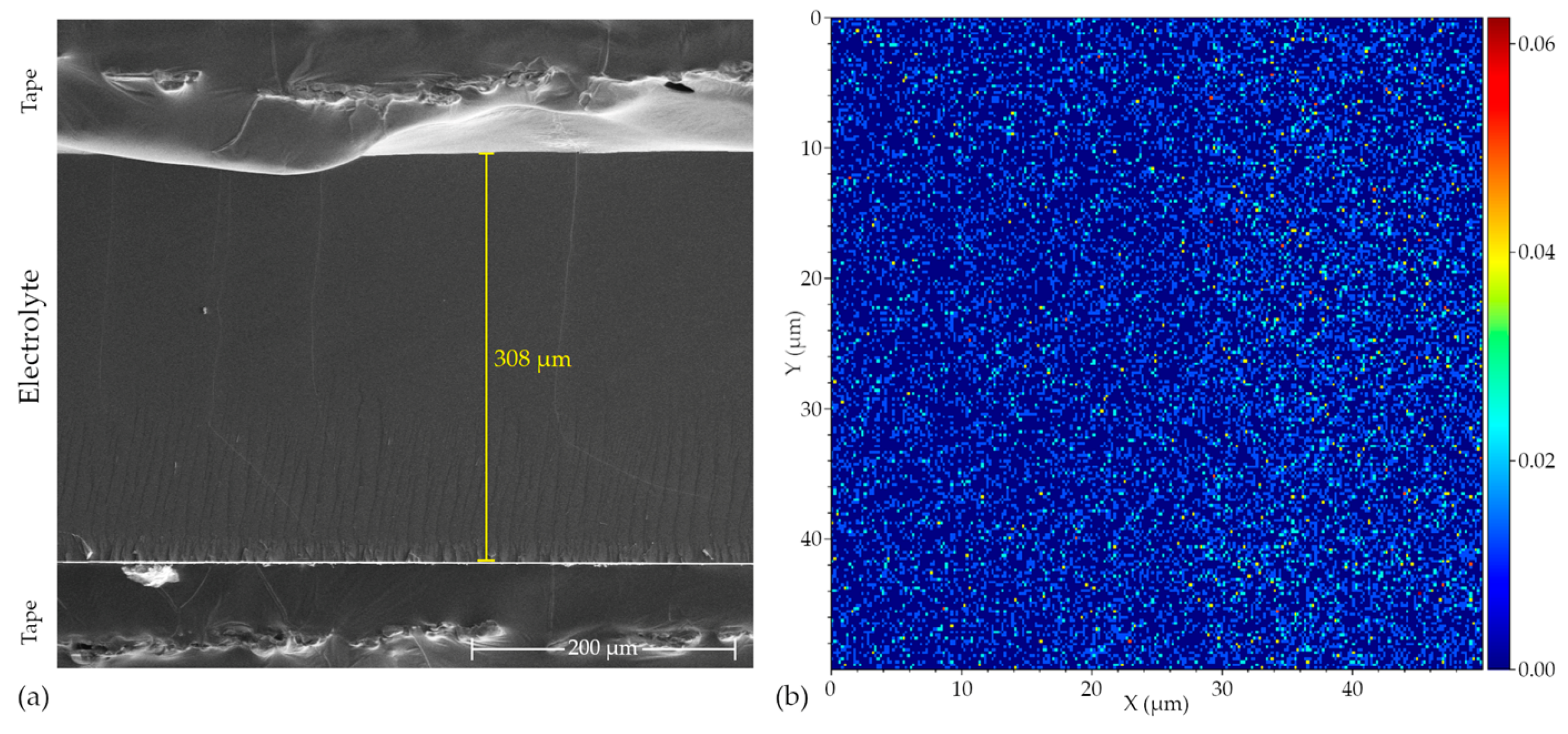
2.2.4. TOF-SIMS and EDS Spectroscopy
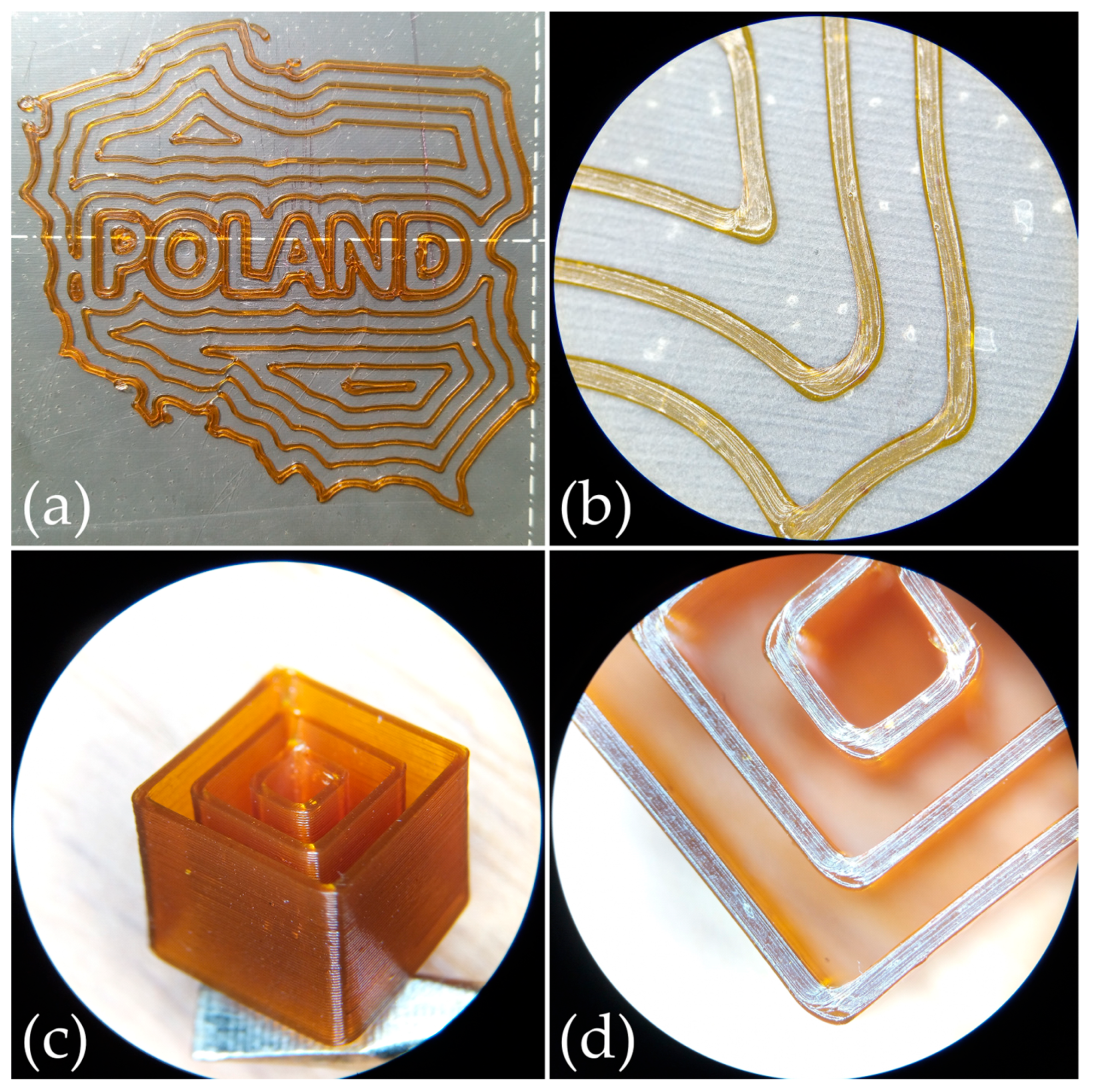
2.3. 3D Printing of poly(AN-co-PEGMEA)
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of Poly(AN-co-PEGMEA)
3.3. Preparation of Electrolytes
3.4. Experimental Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obayi, C.S.; Nnamchi, P.S.; Ezema, F.I. Lithium-Ion Batteries: From the Materials’ Perspective. In Electrode Materials for Energy Storage and Conversion; Kebede, M.A., Ezema, F.I., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–22. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Tidblad, A.A.; Edström, K.; Hernández, G.; de Meatza, I.; Landa-Medrano, I.; Jacas Biendicho, J.; Trilla, L.; Buysse, M.; Ierides, M.; Horno, B.P.; et al. Future Material Developments for Electric Vehicle Battery Cells Answering Growing Demands from an End-User Perspective. Energies 2021, 14, 4223. [Google Scholar] [CrossRef]
- Pellegrini, V.; Bodoardo, S.; Brandell, D.; Edström, K. Challenges and Perspectives for New Material Solutions in Batteries. Solid State Commun. 2019, 303, 113733. [Google Scholar] [CrossRef]
- Salgado, R.M.; Danzi, F.; Oliveira, J.E.; El-Azab, A.; Camanho, P.P.; Braga, M.H. The Latest Trends in Electric Vehicles Batteries. Molecules 2021, 26, 3188. [Google Scholar] [CrossRef] [PubMed]
- Lu, X. Polymer-Based Solid-State Electrolytes for High-Energy-Density Lithium-Ion Batteries-Review. Adv. Energy Mater. 2023, 13, 2301746. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, e2205315. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, Q.; Xu, X.; Lu, L. A high-performance solid-state polymer electrolyte for lithium-metal battery. Funct. Mater. Lett. 2023, 16, 2340002. [Google Scholar] [CrossRef]
- Nathan, M. Microbattery Technologies for Miniaturized Implantable Medical Devices. Curr. Pharm. Biotechnol. 2010, 11, 404–410. [Google Scholar] [CrossRef]
- Dinis, H.; Mendes, P.M. A Comprehensive Review of Powering Methods Used in State-of-the-Art Miniaturized Implantable Electronic Devices. Biosens. Bioelectron. 2021, 172, 112781. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Martinez-Hurtado, J.L.; Ünal, B.; Khademhosseini, A.; Butt, H. Wearables in Medicine. Adv. Mater. 2018, 30, e1706910. [Google Scholar] [CrossRef]
- Warner, J.T. Lithium-Ion Battery Chemistries: A Primer; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Wei, L.; Liu, S.-T.; Balaish, M.; Li, Z.; Zhou, X.-Y.; Rupp, J.L.M.; Guo, X. Customizable Solid-State Batteries toward Shape-Conformal and Structural Power Supplies. Mater. Today 2022, 58, 297–312. [Google Scholar] [CrossRef]
- Mottaghi, M.; Pearce, J.M. A Review of 3D Printing Batteries. Batteries 2024, 10, 110. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Armand, M.; Fleutot, B.; Courty, M.; Prashantha, K.; Davoisne, C.; Tortajada, H.; Panier, S.; Dupont, L. Overview on Lithium-Ion Battery 3D-Printing by Means of Material Extrusion. ECS Trans. 2020, 98, 3–21. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Panagiotopoulos, A.; Mattevi, C. Direct Ink Writing of Energy Materials. Mater. Adv. 2021, 2, 540–563. [Google Scholar] [CrossRef]
- Polychronopoulos, N.D.; Brouzgou, A. Direct Ink Writing for Electrochemical Device Fabrication: A Review of 3D-Printed Electrodes and Ink Rheology. Catalysts 2024, 14, 110. [Google Scholar] [CrossRef]
- Zhang, M.; Mei, H.; Chang, P.; Cheng, L. 3D Printing of Structured Electrodes for Rechargeable Batteries. J. Mater. Chem. A Mater. Energy Sustain. 2020, 8, 10670–10694. [Google Scholar] [CrossRef]
- Soares, D.M.; Ren, Z.; Mujib, S.B.; Mukherjee, S.; Martins Real, C.G.; Anstine, M.; Zanin, H.; Singh, G. Additive Manufacturing of Electrochemical Energy Storage Systems Electrodes. Adv. Energy Sustain. Res. 2021, 2, 2000111. [Google Scholar] [CrossRef]
- Batista, M. Design and Additive Manufacturing of Optimized Electrodes for Energy Storage Applications. Carbon 2023, 205, 262–269. [Google Scholar] [CrossRef]
- Pinilla, S.; Ryan, S.; McKeon, L.; Lian, M.; Vaesen, S.; Roy, A.; Schmitt, W.; Coleman, J.N.; Nicolosi, V. Additive Manufacturing of Li-ion Batteries: A Comparative Study between Electrode Fabrication Processes. Adv. Energy Mater. 2023, 13. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y. 3D-Printed Solid-State Electrolytes for Electrochemical Energy Storage Devices. J. Mater. Res. 2021, 36, 4547–4564. [Google Scholar] [CrossRef]
- Ramesh, A.; Reddy, B. The Promise of 3D Printed Solid Polymer Electrolytes for Developing Sustainable Batteries: A Techno-Commercial Perspective. Int. J. Precis. Eng. Manuf. -Green Technol. 2024, 11, 321–352. [Google Scholar] [CrossRef]
- Ben-Barak, I.; Ragones, H.; Golodnitsky, D. 3D printable solid and quasi-solid electrolytes for advanced batteries. Electrochem. Sci. Adv. 2022, 2, e2100167. [Google Scholar] [CrossRef]
- Bourseau, F.; Grugeon, S.; Lafont, U.; Dupont, L. 3D Printing of Solid Polymer Electrolytes by Fused Filament Fabrication: Challenges towards in-Space Manufacturing. J. Phys. Energy 2024, 6, 012001. [Google Scholar] [CrossRef]
- McOwen, D.W.; Xu, S.; Gong, Y.; Wen, Y.; Godbey, G.L.; Gritton, J.E.; Hamann, T.R.; Dai, J.; Hitz, G.T.; Hu, L.; et al. 3D-printing Electrolytes for Solid-state Batteries. Adv. Mater. 2018, 30, 1707132. [Google Scholar] [CrossRef]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-Dimensional Printing of a Complete Lithium Ion Battery with Fused Filament Fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Lyu, Z.; Lim, G.J.H.; Koh, J.J.; Li, Y.; Ma, Y.; Ding, J.; Wang, J.; Hu, Z.; Wang, J.; Chen, W.; et al. Design and Manufacture of 3D-Printed Batteries. Joule 2021, 5, 89–114. [Google Scholar] [CrossRef]
- Fonseca, N.; Thummalapalli, S.V.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Patil, D.; Thippanna, V.; Ramanathan, A.; Xu, W.; Guo, S.; et al. 3D Printing-Enabled Design and Manufacturing Strategies for Batteries: A Review. Small 2023, 19, e2302718. [Google Scholar] [CrossRef]
- Choi, K.-H.; Ahn, D.B.; Lee, S.-Y. Current Status and Challenges in Printed Batteries: Toward Form Factor-Free, Monolithic Integrated Power Sources. ACS Energy Lett. 2018, 3, 220–236. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest Advances in the Manufacturing of 3D Rechargeable Lithium Microbatteries. J. Power Sources 2015, 286, 25–46. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, M.; Yang, X.; Sun, R.; Zhang, J.; Sun, X. Emerging Application of 3D-Printing Techniques in Lithium Batteries: From Liquid to Solid. Mater. Today 2022, 59, 161–181. [Google Scholar] [CrossRef]
- Cheng, M.; Jiang, Y.; Yao, W.; Yuan, Y.; Deivanayagam, R.; Foroozan, T.; Huang, Z.; Song, B.; Rojaee, R.; Shokuhfar, T.; et al. Elevated-Temperature 3D Printing of Hybrid Solid-State Electrolyte for Li-Ion Batteries. Adv. Mater. 2018, 30, e1800615. [Google Scholar] [CrossRef] [PubMed]
- Vlad, A.; Singh, N.; Galande, C.; Ajayan, P.M. Design Considerations for Unconventional Electrochemical Energy Storage Architectures. Adv. Energy Mater. 2015, 5, 1402115. [Google Scholar] [CrossRef]
- Lee, K.; Shang, Y.; Bobrin, V.A.; Kuchel, R.; Kundu, D.; Corrigan, N.; Boyer, C. 3D Printing Nanostructured Solid Polymer Electrolytes with High Modulus and Conductivity. Adv. Mater. 2022, 34, e2204816. [Google Scholar] [CrossRef] [PubMed]
- Church, R.B.; Hart, A.J. Solid and Polymer Electrolyte Materials and Related Processing Methods Suitable for Three-Dimensional Battery Architectures. J. Electrochem. Soc. 2024, 171, 040512. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, e1904205. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Hou, W.; Yang, Y. Nanomaterials for Implantable Batteries to Power Cardiac Devices. Mater. Today Nano 2020, 9, 100070. [Google Scholar] [CrossRef]
- Zhou, B.; He, D.; Hu, J.; Ye, Y.; Peng, H.; Zhou, X.; Xie, X.; Xue, Z. A Flexible, Self-Healing and Highly Stretchable Polymer Electrolyte via Quadruple Hydrogen Bonding for Lithium-Ion Batteries. J. Mater. Chem. A Mater. Energy Sustain. 2018, 6, 11725–11733. [Google Scholar] [CrossRef]
- Ahmed, M.; Dipu, K.M. Polymer Electrolyte Design Strategies for High-Performance and Safe Lithium-Ion Batteries: Recent Developments and Future Prospects. Mater. Eng. Res. 2023, 5, 245–255. [Google Scholar] [CrossRef]
- Maia, B.A.; Magalhães, N.; Cunha, E.; Braga, M.H.; Santos, R.M.; Correia, N. Designing Versatile Polymers for Lithium-Ion Battery Applications: A Review. Polymers 2022, 14, 403. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lim, D.-A.; Jeong, B.; Kim, H.-M.; Kim, D.-W. Chemically Cross-Linked Gel Polymer Electrolytes for Highly Safe Lithium-Ion Batteries with Good Cycling Stability. Meet. Abstr. 2021, MA2021-01, 134. [Google Scholar] [CrossRef]
- Kim, D.-W. CHAPTER 5: Gel Polymer Electrolytes. In Future Lithium-Ion Batteries; Eftekhari, A., Ed.; The Royal Society of Chemistry: Croydon, UK, 2019; pp. 102–129. [Google Scholar] [CrossRef]
- Chen, R.; Qu, W.; Guo, X.; Li, L.; Wu, F. The Pursuit of Solid-State Electrolytes for Lithium Batteries: From Comprehensive Insight to Emerging Horizons. Mater. Horiz. 2016, 3, 487–516. [Google Scholar] [CrossRef]
- Seki, S.; Watanabe, M. Polymer and Ionic Liquid Electrolytes for Advanced Lithium Batteries. In Nanoscale Technology for Advanced Lithium Batteries; Springer: New York, NY, USA, 2014; pp. 51–61. ISBN 9781461486749. [Google Scholar] [CrossRef]
- Murali, A.; Sakar, M.; Priya, S.; Vijayavarman, V.; Pandey, S.; Sai, R.; Katayama, Y.; Abdul Kader, M.; Ramanujam, K. Insights into the Emerging Alternative Polymer-Based Electrolytes for All Solid-State Lithium-Ion Batteries: A Review. Mater. Lett. 2022, 313, 131764. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid Polymer Electrolytes: Materials Designing and All-Solid-State Battery Applications: An Overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Isikli, S.; Ryan, K.M. Recent Advances in Solid-State Polymer Electrolytes and Innovative Ionic Liquids Based Polymer Electrolyte Systems. Curr. Opin. Electrochem. 2020, 21, 188–191. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling. Sci. Rep. 2019, 9, 18031. [Google Scholar] [CrossRef]
- Ragones, H.; Vinegrad, A.; Ardel, G.; Goor, M.; Kamir, Y.; Dorfman, M.M.; Gladkikh, A.; Golodnitsky, D. On the Road to a Multi-Coaxial-Cable Battery: Development of a Novel 3D-Printed Composite Solid Electrolyte. J. Electrochem. Soc. 2020, 167, 070503. [Google Scholar] [CrossRef]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly (Ethylene Oxide)− LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [Google Scholar] [CrossRef]
- Vinegrad, A.; Ragones, H.; Jayakody, N.; Ardel, G.; Goor, M.; Kamir, Y.; Dorfman, M.M.; Gladkikh, A.; Burstein, L.; Horowitz, Y.; et al. Plasticized 3D-Printed Polymer Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 110549. [Google Scholar] [CrossRef]
- Huang, B. Lithium Ion Conduction in Polymer Electrolytes Based on PAN. Solid State Ion. 1996, 85, 79–84. [Google Scholar] [CrossRef]
- Croce, F.; Brown, S.D.; Greenbaum, S.G.; Slane, S.M.; Salomon, M. Lithium-7 NMR and Ionic Conductivity Studies of Gel Electrolytes Based on Polyacrylonitrile. Chem. Mater. 1993, 5, 1268–1272. [Google Scholar] [CrossRef]
- Whba, R.; Su’ait, M.S.; Whba, F.; Ahmad, A. Research progress on polyacrylonitrile-based polymer electrolytes for electrochemical devices: Insight into electrochemical performance. J. Power Sources 2024, 606, 234539. [Google Scholar] [CrossRef]
- Yu, J.; Miller, G.C.; Riffle, J.S.; Baird, D.G. Identifying Melt Processing Conditions for a Polyacrylonitrile Copolymer Plasticized with Water, Acetonitrile and Their Mixtures. Int. Polym. Process. 2019, 34, 307–313. [Google Scholar] [CrossRef]


| Assumed AN:PEGMEA Ratio | %N | %C | %H | Calculated AN:PEGMEA Ratio (%N) | %C Calculated | %H Calculated | 1H NMR AN:PEGMEA Ratio |
|---|---|---|---|---|---|---|---|
| 1.01 | 3.105 | 55.865 | 8.705 | 1.21 | 56.32 | 8.35 | 1.11 |
| 3.01 | 6.305 | 52.285 | 7.255 | 2.84 | 57.91 | 7.98 | 2.85 |
| 6.02 | 10.355 | 60.085 | 8.110 | 5.84 | 59.93 | 7.52 | 5.88 |
| 10.08 | 13.510 | 60.145 | 7.460 | 9.48 | 61.50 | 7.15 | 9.51 |
| t+ | |
|---|---|
| poly(AN-co-PEGMEA) 3:1/30wt.% LiTf | 0.29 |
| poly(AN-co-PEGMEA) 6:1/30wt.% LiTf | 0.16 |
| poly(AN-co-PEGMEA) 6:1/30wt.% LiTf/10wt.% SCN | 0.73 |
| poly(AN-co-PEGMEA) 6:1/30wt.% LiTf/20wt.% SCN | 0.85 |
| poly(AN-co-PEGMEA) 6:1/30wt.% LiTf/30wt.% SCN | 0.69 |
| poly(AN-co-PEGMEA) 6:1/30wt.% LiTf/40wt.% SCN | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwiński, A.; Słojewska, M.; Jurczak, J.; Dębowski, M.; Zygadło-Monikowska, E. FFF/FDM 3D-Printed Solid Polymer Electrolytes Based on Acrylonitrile Copolymers for Lithium-Ion Batteries. Molecules 2024, 29, 4526. https://doi.org/10.3390/molecules29194526
Czerwiński A, Słojewska M, Jurczak J, Dębowski M, Zygadło-Monikowska E. FFF/FDM 3D-Printed Solid Polymer Electrolytes Based on Acrylonitrile Copolymers for Lithium-Ion Batteries. Molecules. 2024; 29(19):4526. https://doi.org/10.3390/molecules29194526
Chicago/Turabian StyleCzerwiński, Arkadiusz, Magdalena Słojewska, Justyna Jurczak, Maciej Dębowski, and Ewa Zygadło-Monikowska. 2024. "FFF/FDM 3D-Printed Solid Polymer Electrolytes Based on Acrylonitrile Copolymers for Lithium-Ion Batteries" Molecules 29, no. 19: 4526. https://doi.org/10.3390/molecules29194526
APA StyleCzerwiński, A., Słojewska, M., Jurczak, J., Dębowski, M., & Zygadło-Monikowska, E. (2024). FFF/FDM 3D-Printed Solid Polymer Electrolytes Based on Acrylonitrile Copolymers for Lithium-Ion Batteries. Molecules, 29(19), 4526. https://doi.org/10.3390/molecules29194526







