Adhesin Antibody-Grafted Mesoporous Silica Nanoparticles Suppress Immune Escape for Treatment of Fungal Systemic Infection
Abstract
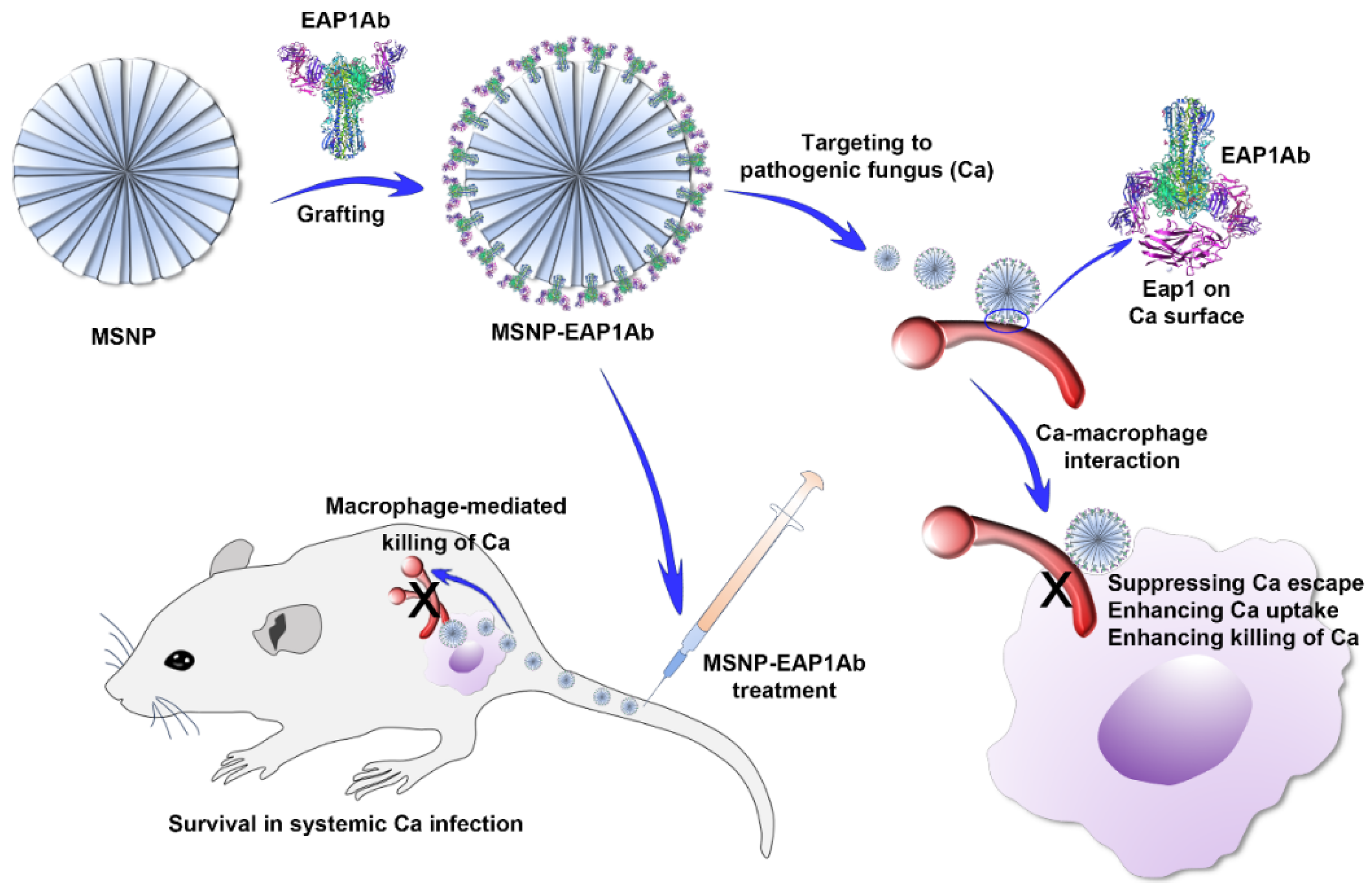
:1. Introduction
2. Results
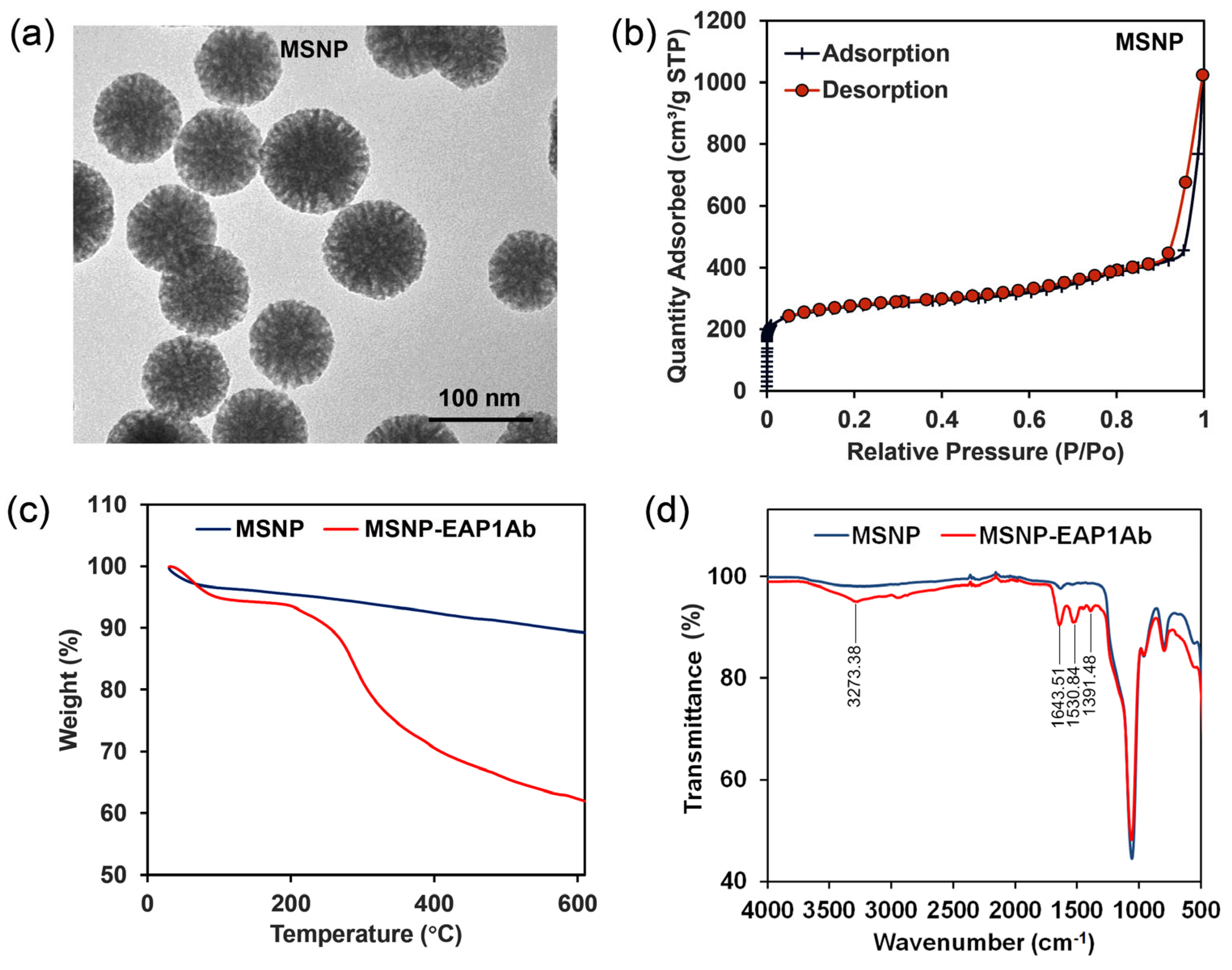
2.1. Characterization of MSNP and MSNP-EAP1Ab
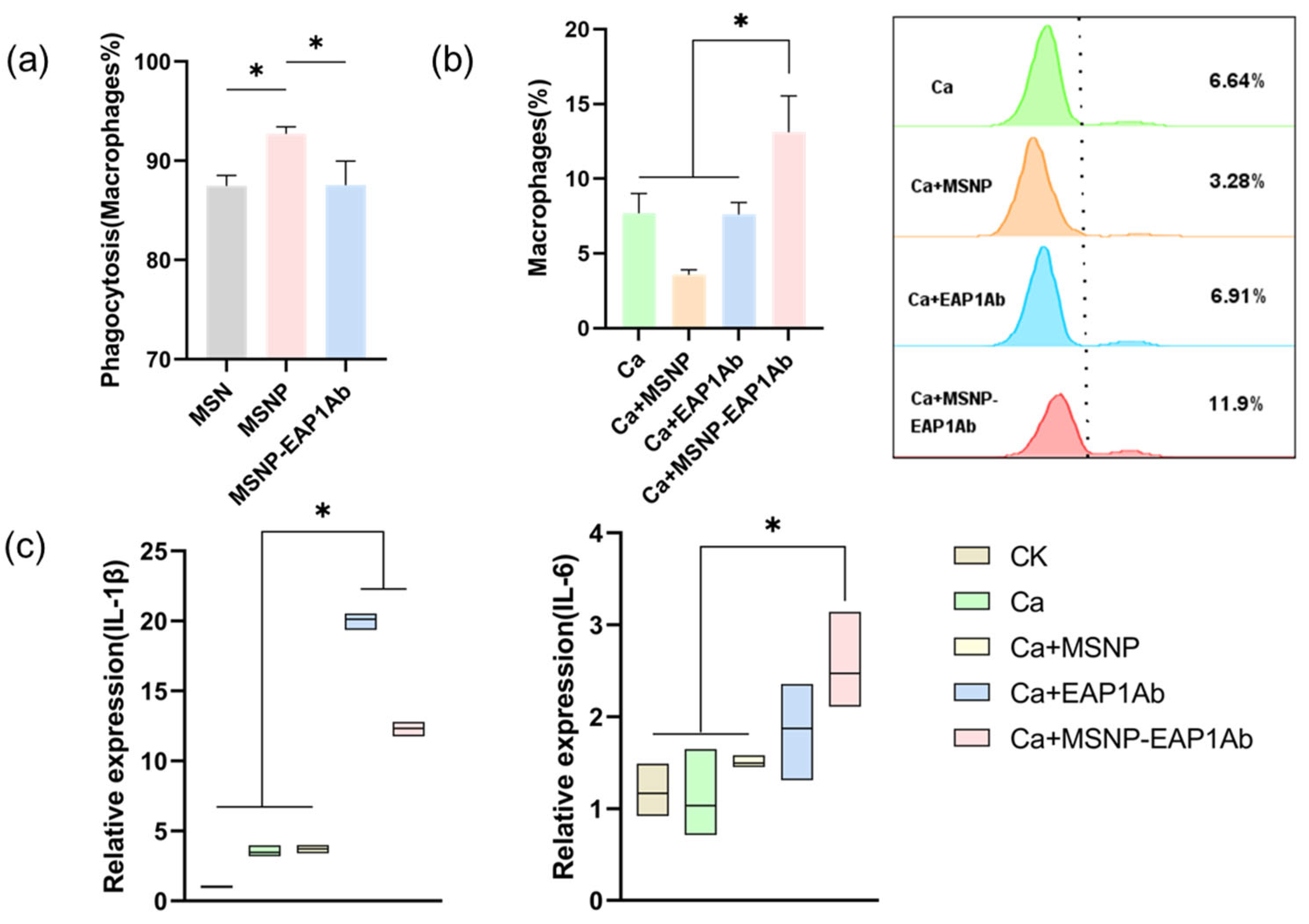
2.2. MSNP-EAP1Ab Enhances Both Phagocytosis and Cytokine Secretion in RAW264.7 Cells
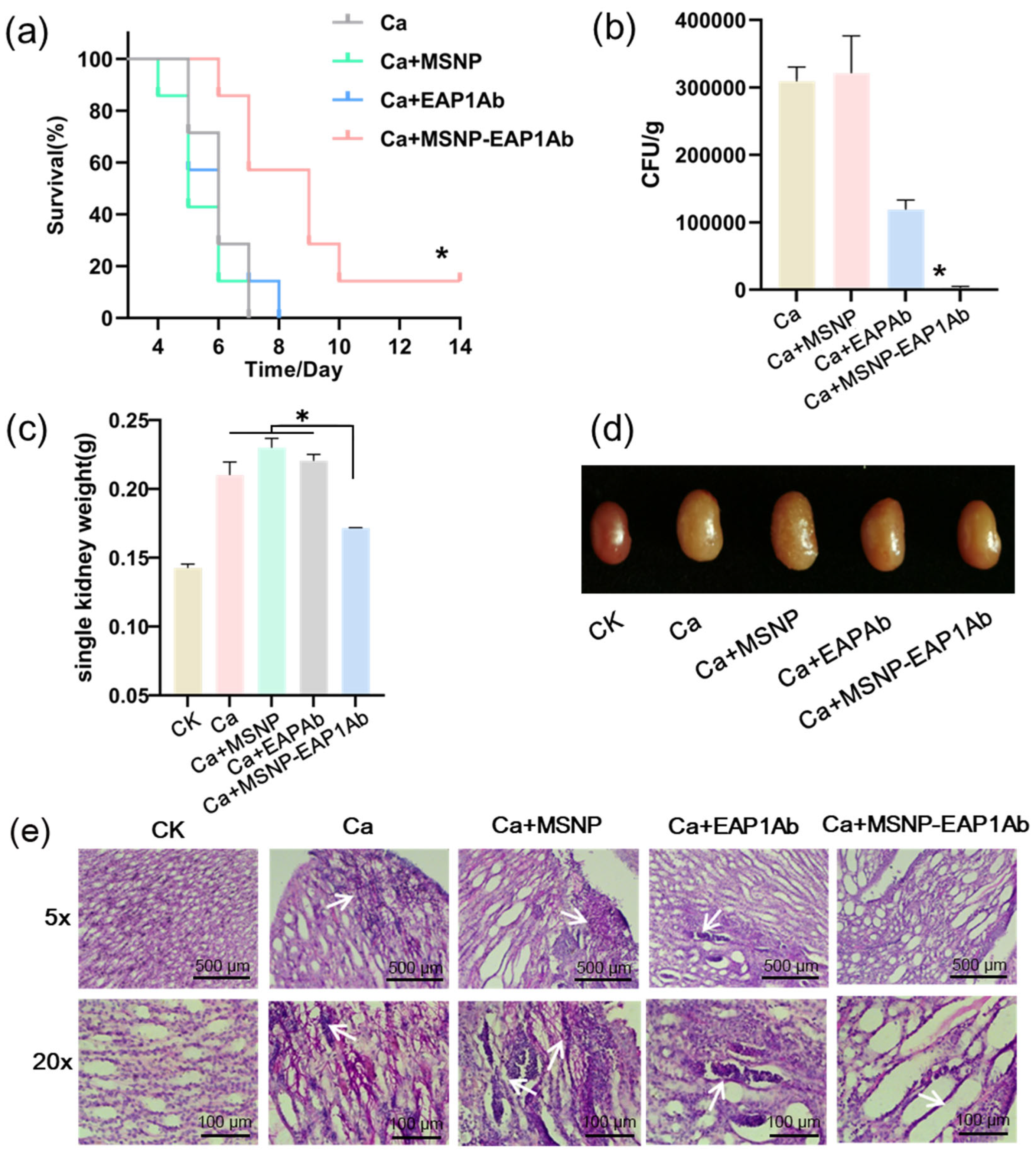
2.3. MSNP-EAP1Ab Suppresses Mouse Death and Kidney Damage Induced by Systemic C. albicans Infection
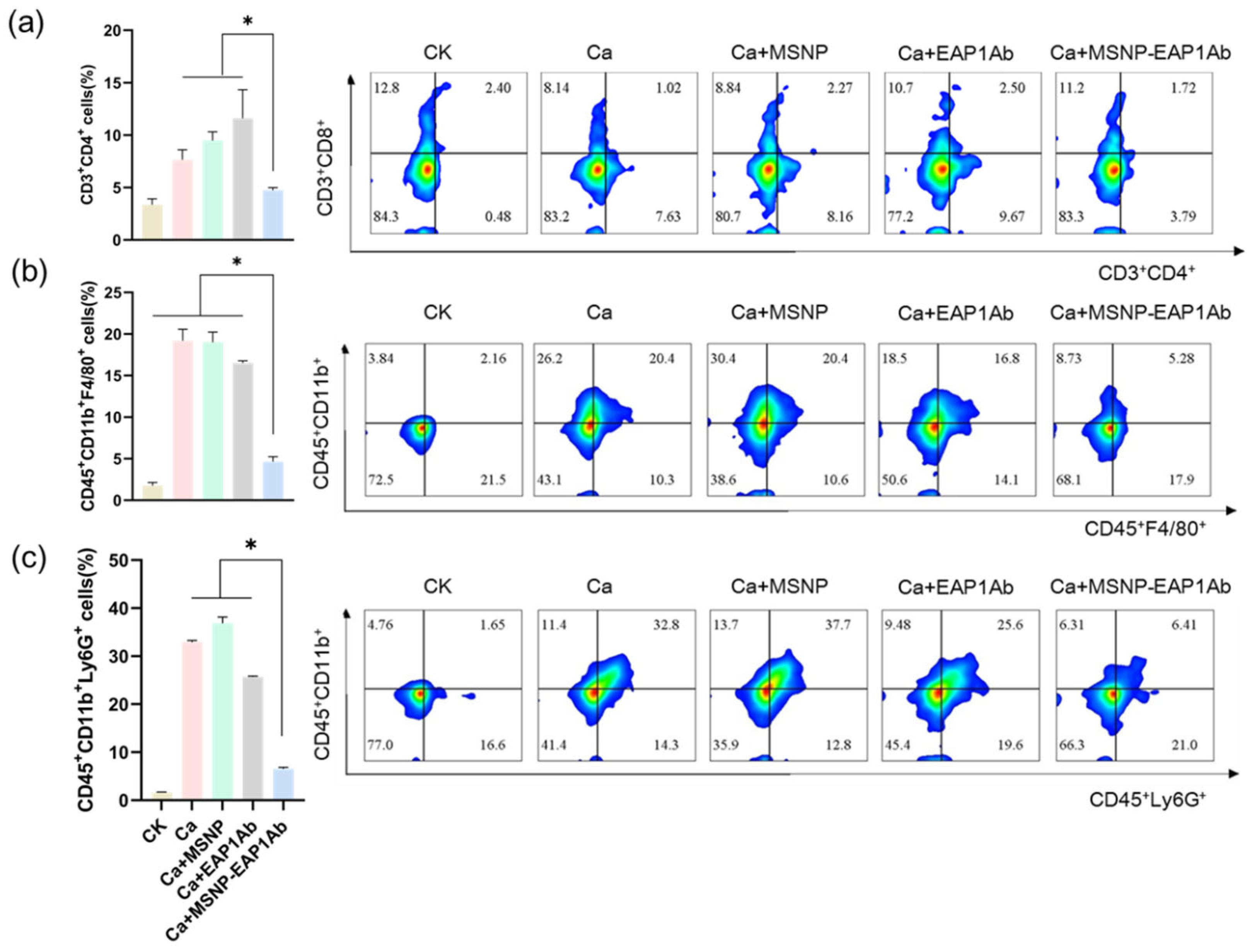
2.4. MSNP-EAP1Ab Enhances the Cytotoxicity of Renal Immune Cells without Inducing Excessive Inflammation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of the Nanoparticles
4.3. Animals
4.4. Cell Culture
4.5. Cellular Phagocytosis
4.6. MSN Endocytosis Efficiency
4.7. Reverse Transcriptase PCR
4.8. Systemic Infection
4.9. Flow Cytometry Assays
4.10. Paraffin Immunofluorescence
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Hong, T.; Jiang, Y.; Whiteway, M.; Zhang, S. Candidiasis: From Cutaneous to Systemic, New Perspectives of Potential Targets and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2023, 199, 114960. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson, G.R.; Ostrosky- Zeichner, L.; Govrins, M.A. Invasive Candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.; Leonhardt, I.; Hünniger, K.; Kurzai, O. Host Response to Candida Albicans Bloodstream Infection and Sepsis. Virulence 2015, 6, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Hebecker, B.; Vlaic, S.; Conrad, T.; Bauer, M.; Brunke, S.; Kapitan, M.; Linde, J.; Hube, B.; Jacobsen, I.D. Dual-Species Transcriptional Profiling during Systemic Candidiasis Reveals Organ-Specific Host-Pathogen Interactions. Sci. Rep. 2016, 6, 36055. [Google Scholar] [CrossRef]
- MacCallum, D.M.; Castillo, L.; Brown, A.J.P.; Gow, N.A.R.; Odds, F.C. Early-Expressed Chemokines Predict Kidney Immunopathology in Experimental Disseminated Candida Albicans Infections. PLoS ONE 2009, 4, e6420. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Lim, J.K.; Lee, C.-C.R.; Murphy, P.M. Organ-Specific Innate Immune Responses in a Mouse Model of Invasive Candidiasis. J. Innate Immun. 2011, 3, 180–199. [Google Scholar] [CrossRef]
- Chu, C.; Rung, S.; Wang, Y.; Qu, Y.; Man, Y. Comment on “In Situ Mannosylated Nanotrinity-Mediated Macrophage Remodeling Combats Candida Albicans Infection”. ACS Nano 2021, 15, 3541–3543. [Google Scholar] [CrossRef]
- Gazendam, R.P.; Van Hamme, J.L.; Tool, A.T.J.; Van Houdt, M.; Verkuijlen, P.J.J.H.; Herbst, M.; Liese, J.G.; Van De Veerdonk, F.L.; Roos, D.; Van Den Berg, T.K.; et al. Two Independent Killing Mechanisms of Candida Albicans by Human Neutrophils: Evidence from Innate Immunity Defects. Blood 2014, 124, 590–597. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Vergis, A.L.; Ye, H.; Bajwa, A.; Narayan, V.; Strieter, R.M.; Rosin, D.L.; Okusa, M.D. IL-17 Produced by Neutrophils Regulates IFN-γ–Mediated Neutrophil Migration in Mouse Kidney Ischemia-Reperfusion Injury. J. Clin. Investig. 2010, 120, 331–342. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Shi, Z.; Zhu, H.; Li, M.; Yu, Q. Pathogen Infection-Responsive Nanoplatform Targeting Macrophage Endoplasmic Reticulum for Treating Life-Threatening Systemic Infection. Nano Res. 2022, 15, 6243–6255. [Google Scholar] [CrossRef]
- Tham, E.L.; Freeley, S.J.; Bearder, S.; Barros, F.F.; Cragg, M.S.; Mócsai, A.; Robson, M.G. VISTA Deficiency Protects from Immune Complex-Mediated Glomerulonephritis by Inhibiting Neutrophil Activation. J. Autoimmun. 2020, 113, 102501. [Google Scholar] [CrossRef] [PubMed]
- Kretschmar, M.; Hein, A.; Geginat, G.; Mueller, C.; Hof, H.; Nichterlein, T. Inefficient T Cell Memory in the Brain of Mice Infected with Candida Albicans. J. Neuroimmunol. 2000, 105, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Do Prado, A.F.; Gomes, M.S.; Pernomian, L.; Da Silva, C.H.T.P.; Gerlach, R.F.; De Oliveira, A.M. MAS Receptors Mediate Vasoprotective and Atheroprotective Effects of Candesartan upon the Recovery of Vascular Angiotensin-Converting Enzyme 2–Angiotensin-(1-7)–MAS Axis Functionality. Eur. J. Pharmacol. 2015, 764, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, Z.; Guo, Y.; Song, L.; Weatherhead, J.E.; Huang, X.; Zeng, Y.; Bimler, L.; Chang, C.-Y.; Knight, J.M.; et al. Candida Albicans Elicits Protective Allergic Responses via Platelet Mediated T Helper 2 and T Helper 17 Cell Polarization. Immunity 2021, 54, 2595–2610.e7. [Google Scholar] [CrossRef]
- Majer, O.; Bourgeois, C.; Zwolanek, F.; Lassnig, C.; Kerjaschki, D.; Mack, M.; Müller, M.; Kuchler, K. Type I Interferons Promote Fatal Immunopathology by Regulating Inflammatory Monocytes and Neutrophils during Candida Infections. PLoS Pathog. 2012, 8, e1002811. [Google Scholar] [CrossRef]
- Hu, L.; Bai, G.; Xu, Q.; Zhao, G.; Jiang, N.; Yao, H.; Liu, X.; Du, Z. Candidalysin Amplifies the Immune Inflammatory Response in Candida Albicans Keratitis through the TREM-1/DAP12 Pathway. Int. Immunopharmacol. 2023, 119, 110195. [Google Scholar] [CrossRef]
- Liu, J.; Lai, X.; Yu, R.; Ding, H.; Bai, H.; Yang, Z.; Yin, Y.; Xu, F.; Cao, J. Progranulin Aggravates Lethal Candida Albicans Sepsis by Regulating Inflammatory Response and Antifungal Immunity. PLoS Pathog. 2022, 18, e1010873. [Google Scholar] [CrossRef]
- Lv, Q.-Z.; Li, D.-D.; Han, H.; Yang, Y.-H.; Duan, J.-L.; Ma, H.-H.; Yu, Y.; Chen, J.-Y.; Jiang, Y.-Y.; Jia, X.-M. Priming with FLO8-Deficient Candida Albicans Induces Th1-Biased Protective Immunity against Lethal Polymicrobial Sepsis. Cell. Mol. Immunol. 2021, 18, 2010–2023. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, L.; Xu, Z.; Zhang, J.; Jiang, Y.; Cao, Y.; Yan, T. Innate Immune Cell Response upon Candida Albicans Infection. Virulence 2016, 7, 512–526. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.; Pan, W.; Zheng, H.; Wang, M.; Peng, X.; Dai, S.; Tang, Y.; Zeng, K.; Huang, X. Zinc Oxide Nanoparticles Prime a Protective Immune Response in Galleria Mellonella to Defend Against Candida Albicans. Front. Microbiol. 2021, 12, 766138. [Google Scholar] [CrossRef]
- Jiménez-López, C.; Lorenz, M.C. Fungal Immune Evasion in a Model Host–Pathogen Interaction: Candida Albicans versus Macrophages. PLoS Pathog. 2013, 9, e1003741. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles in Nanomedicine Applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ellis, C.M.; Davis, J.J. Mesoporous Silica Nanoparticles in Bioimaging. Materials 2020, 13, 3795. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Hu, J.; Han, X.; Liu, H.; Hu, Y. Transferrin Gated Mesoporous Silica Nanoparticles for Redox-Responsive and Targeted Drug Delivery. Colloids Surf. B Biointerfaces 2017, 152, 77–84. [Google Scholar] [CrossRef]
- De La Torre, C.; Coll, C.; Ultimo, A.; Sancenón, F.; Martínez-Máñez, R.; Ruiz-Hernández, E. In Situ-Forming Gels Loaded with Stimuli-Responsive Gated Mesoporous Silica Nanoparticles for Local Sustained Drug Delivery. Pharmaceutics 2023, 15, 1071. [Google Scholar] [CrossRef]
- Sha, L.; Zhao, Q.; Wang, D.; Li, X.; Wang, X.; Guan, X.; Wang, S. “Gate” Engineered Mesoporous Silica Nanoparticles for a Double Inhibition of Drug Efflux and Particle Exocytosis to Enhance Antitumor Activity. J. Colloid Interface Sci. 2019, 535, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Zhang, Y.; Cheng, M.; Wu, Q.; Yuan, Z. “Three-in-One” Multifunctional Gatekeeper Gated Mesoporous Silica Nanoparticles for Intracellular pH-Activated Targeted Cancer Therapy. ACS Appl. Bio Mater. 2018, 1, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Palecek, S.P. EAP1, a Candida Albicans Gene Involved in Binding Human Epithelial Cells. Eukaryot. Cell 2003, 2, 1266–1273. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Li, M.; Yu, Q. Construction of Candida Albicans Adhesin-Exposed Synthetic Cells for Preventing Systemic Fungal Infection. Vaccines 2023, 11, 1521. [Google Scholar] [CrossRef]
- Onyishi, C.U.; Desanti, G.E.; Wilkinson, A.L.; Lara-Reyna, S.; Frickel, E.-M.; Fejer, G.; Christophe, O.D.; Bryant, C.E.; Mukhopadhyay, S.; Gordon, S.; et al. Toll-like Receptor 4 and Macrophage Scavenger Receptor 1 Crosstalk Regulates Phagocytosis of a Fungal Pathogen. Nat. Commun. 2023, 14, 4895. [Google Scholar] [CrossRef] [PubMed]
- Navarathna, D.H.M.L.P.; Pathirana, R.U.; Lionakis, M.S.; Nickerson, K.W.; Roberts, D.D. Candida Albicans ISW2 Regulates Chlamydospore Suspensor Cell Formation and Virulence In Vivo in a Mouse Model of Disseminated Candidiasis. PLoS ONE 2016, 11, e0164449. [Google Scholar] [CrossRef]
- Nguyen, N.Z.N.; Tran, V.G.; Baek, J.; Kim, Y.; Youn, E.H.; Na, S.W.; Park, S.J.; Seo, S.-K.; Kwon, B. IL-33 Coordinates Innate Defense to Systemic Candida Albicans Infection by Regulating IL-23 and IL-10 in an Opposite Way. J. Immunol. 2022, 208, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Davies, L.C.; Liao, C.-T.; Da Fonseca, D.M.; Griffiths, J.S.; Andrews, R.; Jones, A.V.; Clement, M.; Brown, G.D.; Humphreys, I.R.; et al. The Protective Effect of Inflammatory Monocytes during Systemic, C. Albicans Infection Is Dependent on Collaboration between C-Type Lectin-like Receptors. PLoS Pathog. 2019, 15, e1007850. [Google Scholar] [CrossRef] [PubMed]
- Carpino, N.; Naseem, S.; Frank, D.M.; Konopka, J.B. Modulating Host Signaling Pathways to Promote Resistance to Infection by Candida Albicans. Front. Cell. Infect. Microbiol. 2017, 7, 481. [Google Scholar] [CrossRef]
- Swidergall, M.; Khalaji, M.; Solis, N.V.; Moyes, D.L.; Drummond, R.A.; Hube, B.; Lionakis, M.S.; Murdoch, C.; Filler, S.G.; Naglik, J.R. Candidalysin Is Required for Neutrophil Recruitment and Virulence During Systemic Candida Albicans Infection. J. Infect. Dis. 2019, 220, 1477–1488. [Google Scholar] [CrossRef]
- Massahi Khosrowshahi, F.; Ebrahimi-Hosseinzadeh, B.; Hatamian-Zarmi, A. Sumac (Rhus Coriaria) Extract Loaded-Mesoporous Silica Nanoparticle Efficiently as a Controlled Drug Delivery System for the Treatment of Atherosclerosis. J. Inorg. Organomet. Polym. Mater. 2024, 34, 793–803. [Google Scholar] [CrossRef]
- Adnane, F.; Soliman, S.M.A.; ElZayat, E.; Abdelsalam, E.M.; Fahmy, H.M. Evaluation of Chlorophyll-Loaded Mesoporous Silica Nanoparticles for Photodynamic Therapy on Cancer Cell Lines. Lasers Med. Sci. 2024, 39, 45. [Google Scholar] [CrossRef]
- Xu, C.; Amna, N.; Shi, Y.; Sun, R.; Weng, C.; Chen, J.; Dai, H.; Wang, C. Drug-Loaded Mesoporous Silica Nanoparticles Enhance Antitumor Immunotherapy by Regulating MDSCs. Molecules 2024, 29, 2436. [Google Scholar] [CrossRef]
- Mladenović, M.; Jarić, S.; Mundžić, M.; Pavlović, A.; Bobrinetskiy, I.; Knežević, N.Ž. Biosensors for Cancer Biomarkers Based on Mesoporous Silica Nanoparticles. Biosensors 2024, 14, 326. [Google Scholar] [CrossRef]
- Kovtareva, S.; Kusepova, L.; Tazhkenova, G.; Mashan, T.; Bazarbaeva, K.; Kopishev, E. Surface Modification of Mesoporous Silica Nanoparticles for Application in Targeted Delivery Systems of Antitumour Drugs. Polymers 2024, 16, 1105. [Google Scholar] [CrossRef]

| Primer | Sequence | |
|---|---|---|
| Sense | Antisense | |
| Mouse-TNFα | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| Mouse-IL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| Mouse-IL-6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| Mouse-β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.; Liu, S.; Zhu, M.; Li, M.; Yu, Q. Adhesin Antibody-Grafted Mesoporous Silica Nanoparticles Suppress Immune Escape for Treatment of Fungal Systemic Infection. Molecules 2024, 29, 4547. https://doi.org/10.3390/molecules29194547
Cheng M, Liu S, Zhu M, Li M, Yu Q. Adhesin Antibody-Grafted Mesoporous Silica Nanoparticles Suppress Immune Escape for Treatment of Fungal Systemic Infection. Molecules. 2024; 29(19):4547. https://doi.org/10.3390/molecules29194547
Chicago/Turabian StyleCheng, Mengjuan, Suke Liu, Mengsen Zhu, Mingchun Li, and Qilin Yu. 2024. "Adhesin Antibody-Grafted Mesoporous Silica Nanoparticles Suppress Immune Escape for Treatment of Fungal Systemic Infection" Molecules 29, no. 19: 4547. https://doi.org/10.3390/molecules29194547








