Platinum/Platinum Sulfide on Sulfur-Doped Carbon Nanosheets with Multiple Interfaces toward High Hydrogen Evolution Activity
Abstract
:1. Introduction
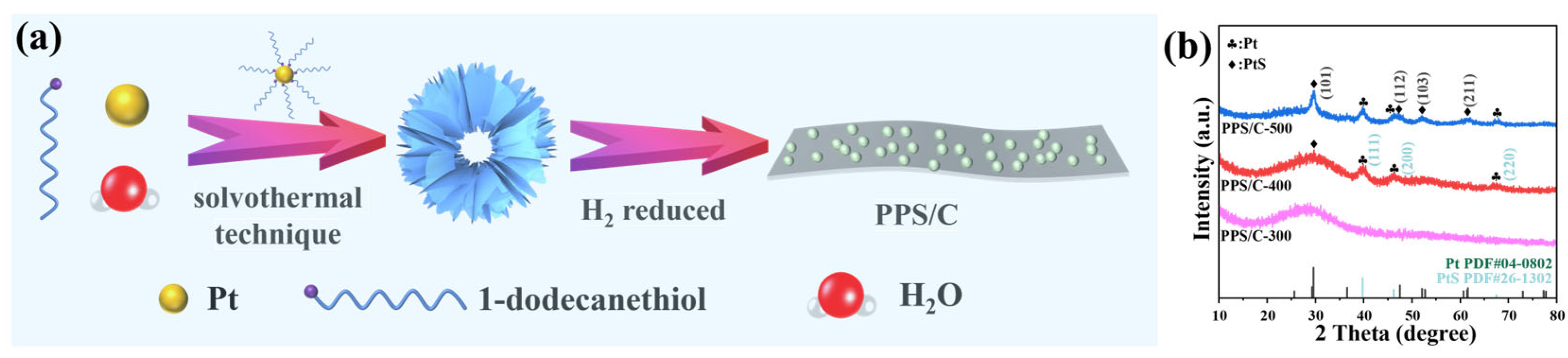
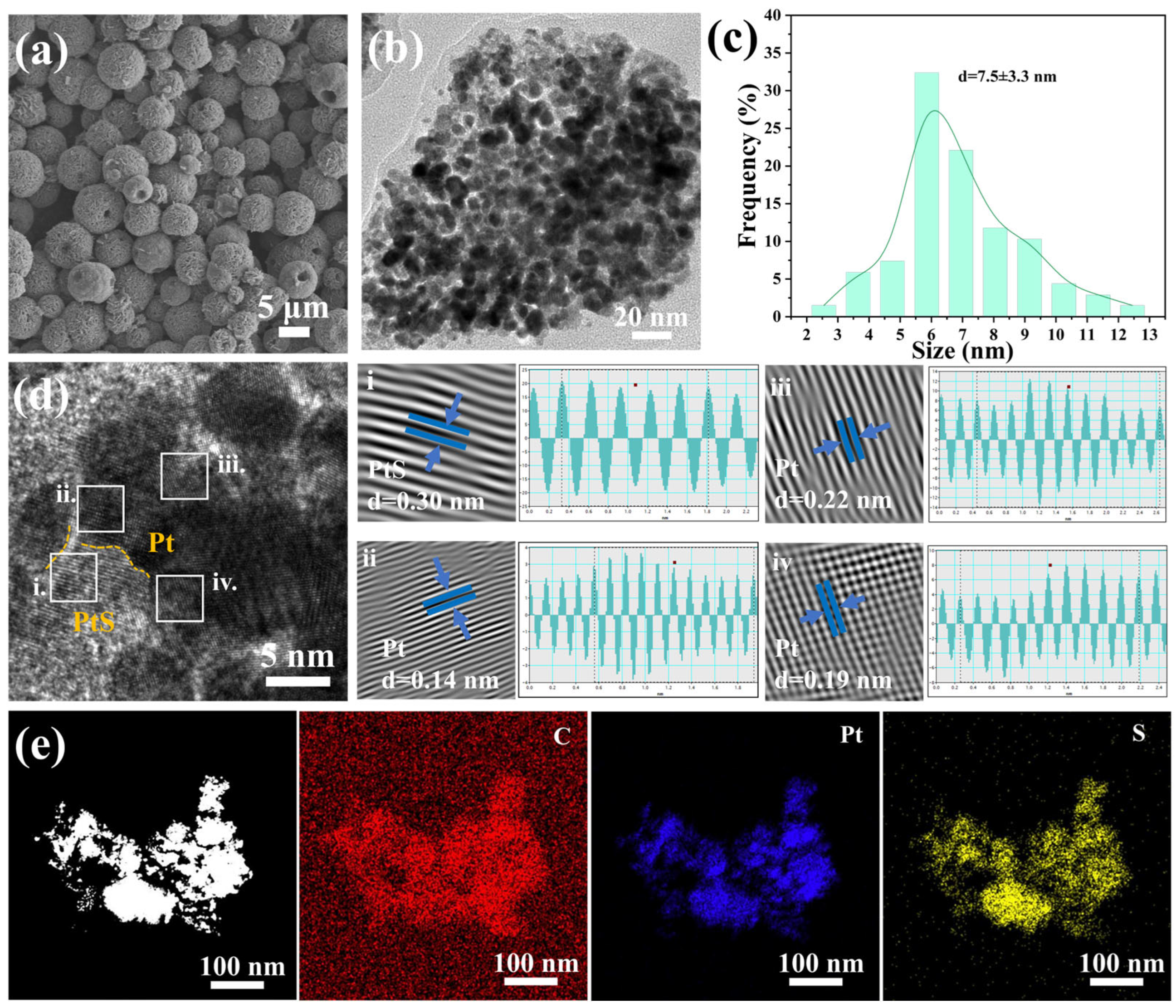
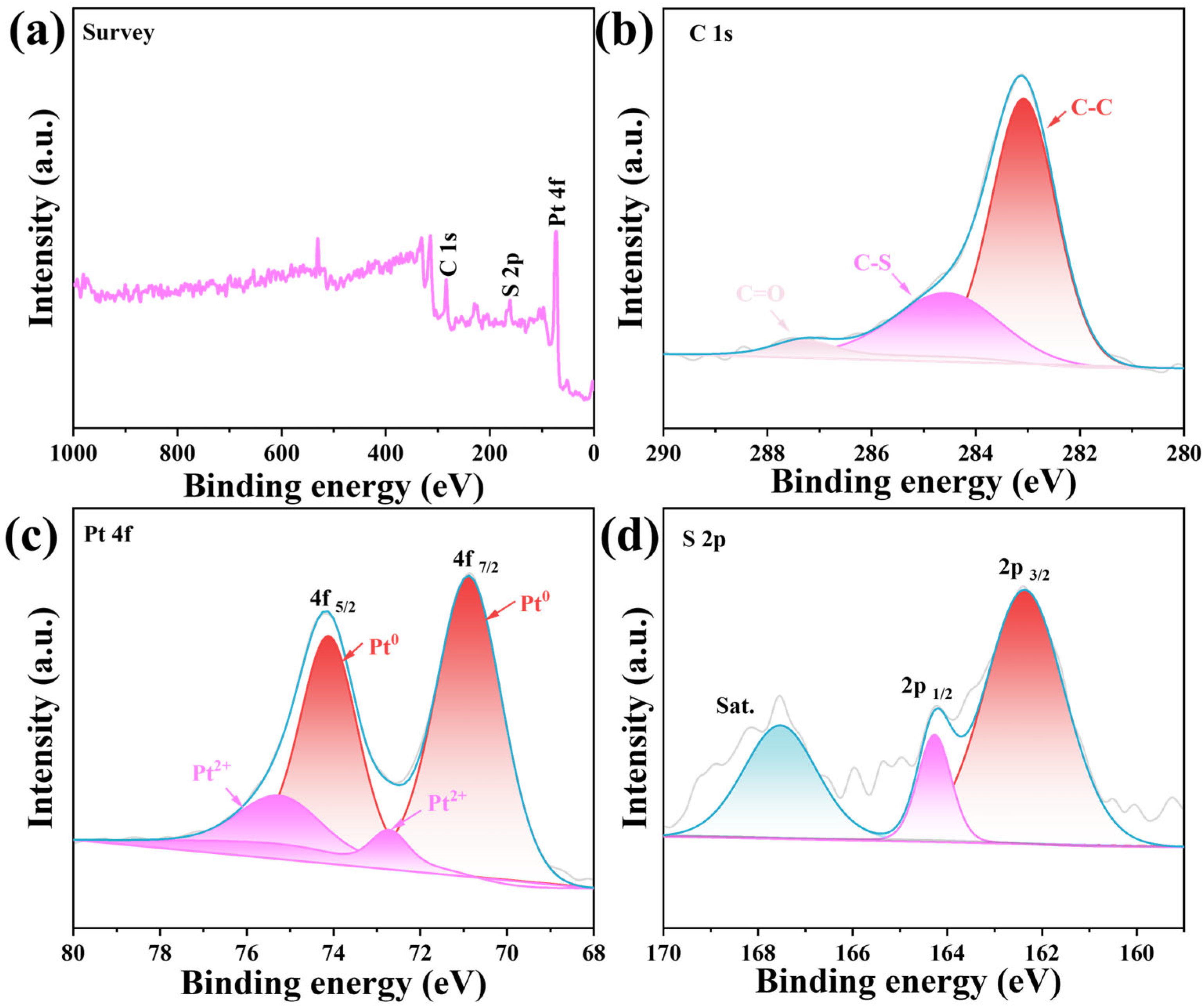
2. Results and Discussion
3. Experimental
3.1. Synthesis of Pt-1-Dodecanethiol Hollow Nanospheres
3.2. Synthesis of Pt/PtS/C
3.3. Characterizations
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; D‘OLimpio, G.; Huang, H.; Kuo, C.; Lue, C.S.; Nicotra, G.; Lin, F.; Boukhvalov, D.W.; Politano, A.; Liu, L. Self-Powered Hydrogen Production From Seawater Enabled by Trifunctional Exfoliated PtTe Nanosheet Catalysts. Adv. Funct. Mater. 2024, 34, 2403099. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N.; et al. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced electrochemical hydrogen evolution. Nat. Commun. 2019, 10, 982. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, Z.; Han, C.B.; Ke, X.; Wang, C.; Jin, Y.; Zhang, Q.; Liu, J.; Wang, H.; Yan, H. Platinum single-atom catalyst coupled with transition metal/metal oxide heterostructure for accelerating alkaline hydrogen evolution reaction. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Huang, J.; Li, T.; Chen, W.; Chen, G.; Zhang, Q.; Zhang, X.; Qian, Q.; Ostrikov, K. Multiphase nanosheet-nanowire cerium oxide and nickel-cobalt phosphide for highly-efficient electrocatalytic overall water splitting. Appl. Catal. B Environ. 2022, 316, 121678. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Qin, Y.; Lu, Q.; Gao, F. Waste leather-derived (Cr, N)-co-doped carbon cloth coupling with Mo2C nanoparticles as a self-supported electrode for highly active hydrogen evolution reaction performances. J. Power Sources 2020, 476, 228706. [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, S.R.; Zhang, G.F.; Yu, X.; Zhao, Z.Y.; Zhang, Y.P.; Shi, Y.; Zhu, H.B.; Xiao, X. Efficient electrocatalytic overall water splitting of porous 3D CoPt3/a-FCWO-NS heterostructures: Morphology modulation and interfacial engineering for the for-mation of crystalline-amorphous nanosheets. Appl. Catal. B Environ. 2024, 342, 123387. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Author Correction: Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2019, 4, 249. [Google Scholar] [CrossRef]
- Bai, S.; Wang, C.M.; Deng, M.S.; Gong, M.; Bai, Y.; Jiang, J.; Xiong, Y.J. Surface polarization matters: Enhancing the hydro-gen-evolution reaction by shrinking Pt shells in Pt-Pd-graphene stack structures. Angew. Chem. Int. Ed. 2014, 53, 12120–12124. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, L.L.; Wu, X.C.; Tao, Y.R.; Xiong, W.W. Ta3N5 Nanobelt-Loaded Ru Nanoparticle Hybrids’ Electrocatalysis for Hydrogen Evolution in Alkaline Media. Molecules 2023, 28, 1100. [Google Scholar] [CrossRef]
- Kwon, I.S.; Kwak, I.H.; Kim, J.Y.; Lee, S.J.; Sial, Q.A.; Ihsan, J.; Lee, K.; Yoo, S.J.; Park, J.; Kang, H.S. 2H–2M Phase Control of WSe2 Nanosheets by Se Enrichment Toward Enhanced Electrocatalytic Hydrogen Evolution Reaction. Adv. Mater. 2023, 36, e2307867. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liang, J.Y.; Song, D.; Li, X.Y.; Li, H. Modulation of charge redistribution in heterogeneous NiO-Ni3Se4 nanosheet arrays for advanced water electrolysis. Adv. Funct. Mater. 2024, 34, 2308345. [Google Scholar] [CrossRef]
- Hegazy, M.B.Z.; Berber, M.R.; Yamauchi, Y.; Pakdel, A.; Cao, R.; Apfel, U.-P. Synergistic Electrocatalytic Hydrogen Evolution in Ni/NiS Nanoparticles Wrapped in Multi-Heteroatom-Doped Reduced Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 34043–34052. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Yu, H.Z.; Huang, H.M.; Huang, W.C.; Li, S.; Cao, Y.H.; Lu, H.F.; Li, G.M.; Li, Y.J.; Li, X.K.; et al. Boosting electrocatalytic activity of Ru for hydrogen evolution through engineering Ru on multiple interfaces of 1T-MoS2 and carbon. Chem. Eng. J. 2024, 489, 151295. [Google Scholar] [CrossRef]
- Tang, K.; Wang, X.F.; Li, Q.; Yan, C.L. High edge selectivity of in situ electrochemical Pt deposition on edge-rich layered WS2. Nanosheets. Adv. Mater. 2018, 30, 1704779. [Google Scholar] [CrossRef] [PubMed]
- Mosallanezhad, A.; Wei, C.; Koudakan, P.A.; Fang, Y.; Niu, S.; Bian, Z.; Liu, B.; Huang, T.; Pan, H.; Wang, G. Interfacial synergies between single-atomic Pt and CoS for enhancing hydrogen evolution reaction catalysis. Appl. Catal. B Environ. 2022, 315, 121534. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; He, W.; Li, Y.; Mao, J.; Zheng, X.; Chen, C.; Cui, C.; Hao, Q. Interfacial electronic modulation of Ni3S2 nanosheet arrays decorated with Au nanoparticles boosts overall water splitting. Appl. Catal. B Environ. 2022, 304, 120935. [Google Scholar] [CrossRef]
- Li, H.; Ding, X.; Su, M.; Zhang, C.; Gao, F.; Lu, Q. Induced Diffusion Effect Realizes Heterogeneous Separation/Aggregation for In Situ Fabrication of Heterostructure with Enhanced Catalytic Activity. Adv. Funct. Mater. 2024, 34, 2405339. [Google Scholar] [CrossRef]
- Huang, Y.P.; Zhang, X.; Li, L.F.; Humayun, M.; Zhang, H.M.; Xu, X.F.; Anthony, S.P.; Chen, Z.H.; Zeng, J.R.; Shtansky, D.V.; et al. Mott–Schottky barrier enabling high-performance hydrazine-assisted hydrogen generation at ampere-level current densities. Adv. Funct. Mater. 2024, 34, 2401011. [Google Scholar] [CrossRef]
- Farhan, A.; Qayyum, W.; Fatima, U.; Nawaz, S.; Balčiūnaitė, A.; Kim, T.H.; Srivastava, V.; Vakros, J.; Frontistis, Z.; Boczkaj, G. Powering the future by iron sulfide type material (FexSy) based electrochemical materials for water splitting and energy storage applications: A review. Small 2024, 20, 2402015. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, D.-J.; Tong, Y.J. Mechanistic Insight into Sulfide-Enhanced Oxygen Reduction Reaction Activity and Stability of Commercial Pt Black: An in Situ Raman Spectroscopic Study. ACS Catal. 2016, 6, 5000–5004. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Wu, C.; Li, H.; Cao, Y.; Li, S.; Xia, H. Synthesis of S-doped AuPbPt alloy nanowire-networks as superior catalysts towards the ORR and HER. J. Mater. Chem. A 2020, 8, 23906–23918. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, R.; Liu, K. Integrating PtCo nanoparticles on N, S doped pore carbon nanosheets as high-performance bi-functional catalysts for oxygen reduction and hydrogen evolution reactions. J. Colloid Interface Sci. 2024, 654, 1186–1198. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Wang, Y.R.; Zhu, B.C.; Xia, F.J.; Tung, C.W.; Wu, J.S.; Chen, H.M.; Yu, J.G. Pt Single atoms supported on N-doped mesoporous hollow carbon spheres with enhanced electrocatalytic H2-evolution activity. Adv. Mater. 2021, 33, 2008599. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, X.; Chen, J.; Wang, X.; Chang, H.; Li, B.; Song, D.; Li, J.; Li, H.; Wang, N. Ultrasmall Ru Nanoparticles Highly Dispersed on Sulfur-Doped Graphene for HER with High Electrocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 48591–48597. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Ma, X.-Y.; Wu, W.-Z.; He, H.-B.; Wang, N.-N.; Zheng, R.-J.; Ma, S.-J.; Zhu, Y.-Q.; Shen, P.-K.; Zhu, J.-L. MnS–MnO heterogeneous nanocube@N, S-doped carbon as a highly efficient bifunctional water splitting electrocatalyst. Rare Met. 2024, 43, 1977–1988. [Google Scholar] [CrossRef]
- Sun, L.H.; Li, Q.Y.; Zhang, S.N.; Xu, D.; Xue, Z.H.; Su, H.; Lin, X.; Zhai, G.Y.; Gao, P.; Hirano, S.I.; et al. Heterojunc-tion-based electron donators to dtabilize and activate ultrafine Pt nanoparticles for efficient hydrogen atom dissociation and gas evolution. Angew. Chem. Int. Ed. 2021, 60, 25766–25770. [Google Scholar] [CrossRef]
- He, X.; Du, P.; Yu, G.; Wang, R.; Long, Y.; Deng, B.; Yang, C.; Zhao, W.; Zhang, Z.; Huang, K.; et al. High-Performance Hydrogen Evolution Reaction Catalytic Electrodes by Liquid Joule-Heating Growth. Small Methods 2023, 7, e2300544. [Google Scholar] [CrossRef]
- Li, Y.R.; Du, H.M.; Su, Y.F.; Zhao, J.S.; Qu, K.G.; Zhang, X.X.; Zhang, Y.; Dong, Y.Y.; Guo, Z.R. Construction of heterostructured NiS/NiSe2 and their application in electrocatalytic water splitting. Int. J. Hydrogen Energy 2024, 66, 286–293. [Google Scholar] [CrossRef]
- Huang, J.Z.; Han, J.C.; Wu, T.; Feng, K.; Yao, T.; Wang, X.J.; Liu, S.W.; Zhong, J.; Zhang, Z.H.; Zhang, Y.M.; et al. Boosting hy-drogen transfer during Volmer reaction at oxides/metal nanocomposites for efficient alkaline hydrogen evolution. ACS Energy Lett. 2019, 4, 3002–3010. [Google Scholar] [CrossRef]
- Farahani, F.S.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Hassani, N.; Neek-Amal, M.; Kaner, R.B.; Mousavi, M.F. Trilayer Metal–Organic Frameworks as Multifunctional Electrocatalysts for Energy Conversion and Storage Applications. J. Am. Chem. Soc. 2022, 144, 3411–3428. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiang, D.; Li, Z.; Liu, X.; Tang, Z.; Zhang, X.; Zhao, L.; Huang, M.; Liu, H.; Song, K.; et al. Laser Synthesis of PtMo Single-Atom Alloy Electrode for Ultralow Voltage Hydrogen Generation. Adv. Mater. 2023, 36, e2305375. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.T.; González-Gil, R.M.; Gómez-Romero, P.; Huguenin, F. Platinum Electrodeposited onto MoSx for Hydrogen Evolution Reaction: A Kinetic Model under Diffusive-Convective Transport Influence. J. Phys. Chem. C 2024, 128, 7483–7495. [Google Scholar] [CrossRef]
- Liu, S.X.; Cao, W.; Wu, J.; Hu, E.L.; Zhang, J.; Gao, X.H.; Chen, Z.W. Integrated PtxCoy-hierarchical carbon matrix electrocatalyst for efficient hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2024, 16, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, J.; Mao, X.; Chu, S.; Zhang, X.; Luo, Z.; Li, J.; Wang, B.; Jia, C.; Qian, D. Experimental and Theoretical Insights into Enhanced Hydrogen Evolution over PtCo Nanoalloys Anchored on a Nitrogen-Doped Carbon Matrix. J. Phys. Chem. Lett. 2022, 13, 5195–5203. [Google Scholar] [CrossRef]
- Wang, J.; Zang, W.; Liu, X.; Sun, J.; Xi, S.; Liu, W.; Kou, Z.; Shen, L.; Wang, J. Switch Volmer-Heyrovsky to Volmer-Tafel Pathway for Efficient Acidic Electrocatalytic Hydrogen Evolution by Correlating Pt Single Atoms with Clusters. Small 2024, 20, e2309427. [Google Scholar] [CrossRef]
- Li, M.X.; Zhu, Y.; Song, N.; Wang, C.; Lu, X.F. Fabrication of Pt nanoparticles on nitrogen-doped carbon/Ni nanofibers for im-proved hydrogen evolution activity. J. Colloid Interface Sci. 2018, 514, 199–207. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jia, J.; Wang, A.; Zhao, L.; Xiong, T.; Liu, H.; Zhou, W. Confined distribution of platinum clusters on MoO2 hexagonal nanosheets with oxygen vacancies as a high-efficiency electrocatalyst for hydrogen evolution reaction. Nano Energy 2019, 62, 127–135. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liang, C.; Liang, Z.; Fan, C.; Wang, F.; Lei, J. Low-Pt-loading electrocatalyst derived from the reduction of hydrogenated MoO3 for highly efficient hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 51, 701–708. [Google Scholar] [CrossRef]
- Ma, Y.; Fang, T.; Xu, Y.; Liu, S.; Liu, B.; Zhang, K.; Li, H. Electrochemical Water Splitting by Mo-Based Metal–Organic Framework-Derived MoO2/NC/Pt Nanostructures in a Wide pH Range. ACS Appl. Nano Mater. 2024, 7, 17364–17372. [Google Scholar] [CrossRef]
- Fang, Y.Q.; Wang, S.S.; Lin, G.X.; Wang, X.; Huang, F.Q. A new compound Pt3Bi2S2 with superior performance for the hydrogen evolution reaction. Chem. Commun. 2021, 57, 7946–7949. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Wan, L.L.; Wang, J.; Cheng, L.; Chen, R.S.; Ni, H.W. Binary electrocatalyst composed of Mo2C nanocrystals with ul-tra-low Pt loadings anchored in TiO2 nanotube arrays for hydrogen evolution reaction. Appl. Surface Sci. 2020, 509, 144679. [Google Scholar] [CrossRef]
- Kim, D.; Surendran, S.; Jeong, Y.; Lim, Y.; Im, S.; Park, S.; Kim, J.Y.; Kim, S.; Kim, T.; Koo, B.; et al. Electrosynthesis of Low Pt-Loaded High Entropy Catalysts for Effective Hydrogen Evolution with Improved Acidic Durability. Adv. Mater. Technol. 2022, 8, 2200882. [Google Scholar] [CrossRef]
- Yang, M.; Li, Y.; Luo, D.; Wang, Q.; Zhang, X.; Liu, J.; Li, L.; Zhao, J.; Deng, G. A novel sea urchin like nanostructure as a bifunctional catalyst for overall water splitting. Electrochim. Acta 2024, 475, 143599. [Google Scholar] [CrossRef]
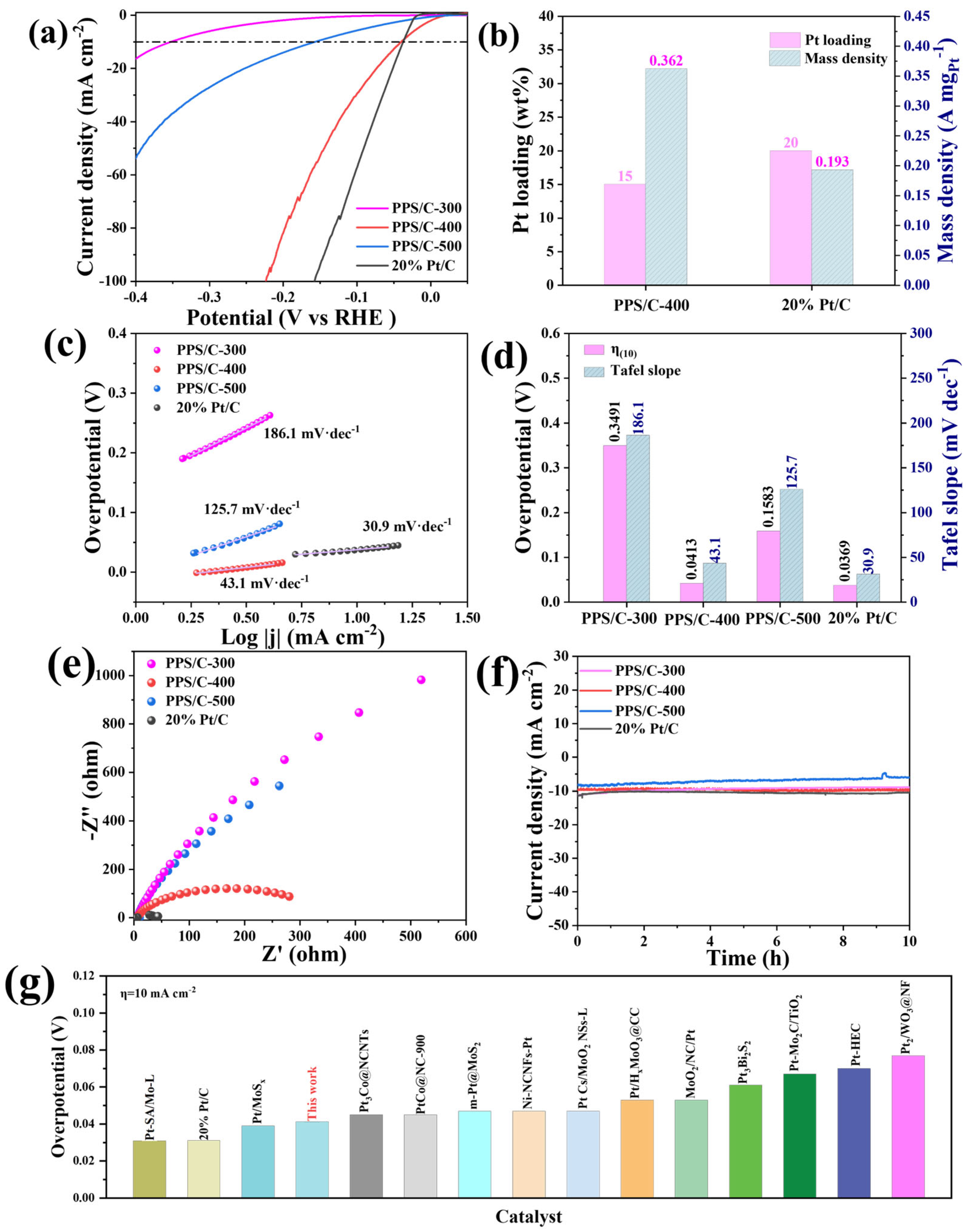
| Catalysts | Overpotential (mV) | Reference |
|---|---|---|
| Pt-SA/Mo-L | 31 | Adv. Mater. 2024, 36, 2305375 [32] |
| 20% Pt/C | 36.9 | / |
| Pt-MoSx | 39 | J. Phys. Chem. C 2024, 128, 7483–7495 [33] |
| PPS/C-400 | 41 | This work |
| Pt3Co@NCNTs | 45 | ACS Appl. Mater. Interfaces 2024, 16, 520–529 [34] |
| PtCo@NC-900 | 45 | J. Phys. Chem. Lett. 2022, 13, 5195–5203 [35] |
| m-Pt@MoS2 | 47 | Small 2024, 20, 2309427 [36] |
| Ni-NCNFs-Pt | 47 | J. Colloid Interface Sci. 2018, 514, 199–207 [37] |
| Pt Cs/MoO2 NSs-L | 47 | Nano Energy 2019, 62, 127–135 [38] |
| Pt/HxMoO3@CC | 53 | Int. J. Hydrogen Energy 2024, 51, 701–708 [39] |
| MoO2/NC/Pt | 53 | ACS Appl. Nano Mater. 2024, 7, 17364–17372 [40] |
| Pt3Bi2S2 | 61 | Chem. Commun. 2021, 57, 7946–7949 [41] |
| Pt-Mo2C/TiO2 | 67 | Appl. Surface Sci. 2020, 509, 144679 [42] |
| Pt-HEC | 70 | Adv. Mater. Tech. 2023, 8, 2200882 [43] |
| Pt2/WO3@NF | 77 | Electrochim. Acta 2024, 475, 143599 [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Su, M.; Zhang, C.; Gao, F.; Lu, Q. Platinum/Platinum Sulfide on Sulfur-Doped Carbon Nanosheets with Multiple Interfaces toward High Hydrogen Evolution Activity. Molecules 2024, 29, 4570. https://doi.org/10.3390/molecules29194570
Zhang M, Su M, Zhang C, Gao F, Lu Q. Platinum/Platinum Sulfide on Sulfur-Doped Carbon Nanosheets with Multiple Interfaces toward High Hydrogen Evolution Activity. Molecules. 2024; 29(19):4570. https://doi.org/10.3390/molecules29194570
Chicago/Turabian StyleZhang, Mou, Mengfei Su, Chunyan Zhang, Feng Gao, and Qingyi Lu. 2024. "Platinum/Platinum Sulfide on Sulfur-Doped Carbon Nanosheets with Multiple Interfaces toward High Hydrogen Evolution Activity" Molecules 29, no. 19: 4570. https://doi.org/10.3390/molecules29194570
APA StyleZhang, M., Su, M., Zhang, C., Gao, F., & Lu, Q. (2024). Platinum/Platinum Sulfide on Sulfur-Doped Carbon Nanosheets with Multiple Interfaces toward High Hydrogen Evolution Activity. Molecules, 29(19), 4570. https://doi.org/10.3390/molecules29194570





