Fabrication of a Heptapeptide-Modified Poly(glycidyl Methac-Rylate) Nanosphere for Oriented Antibody Immobilization and Immunoassay
Abstract
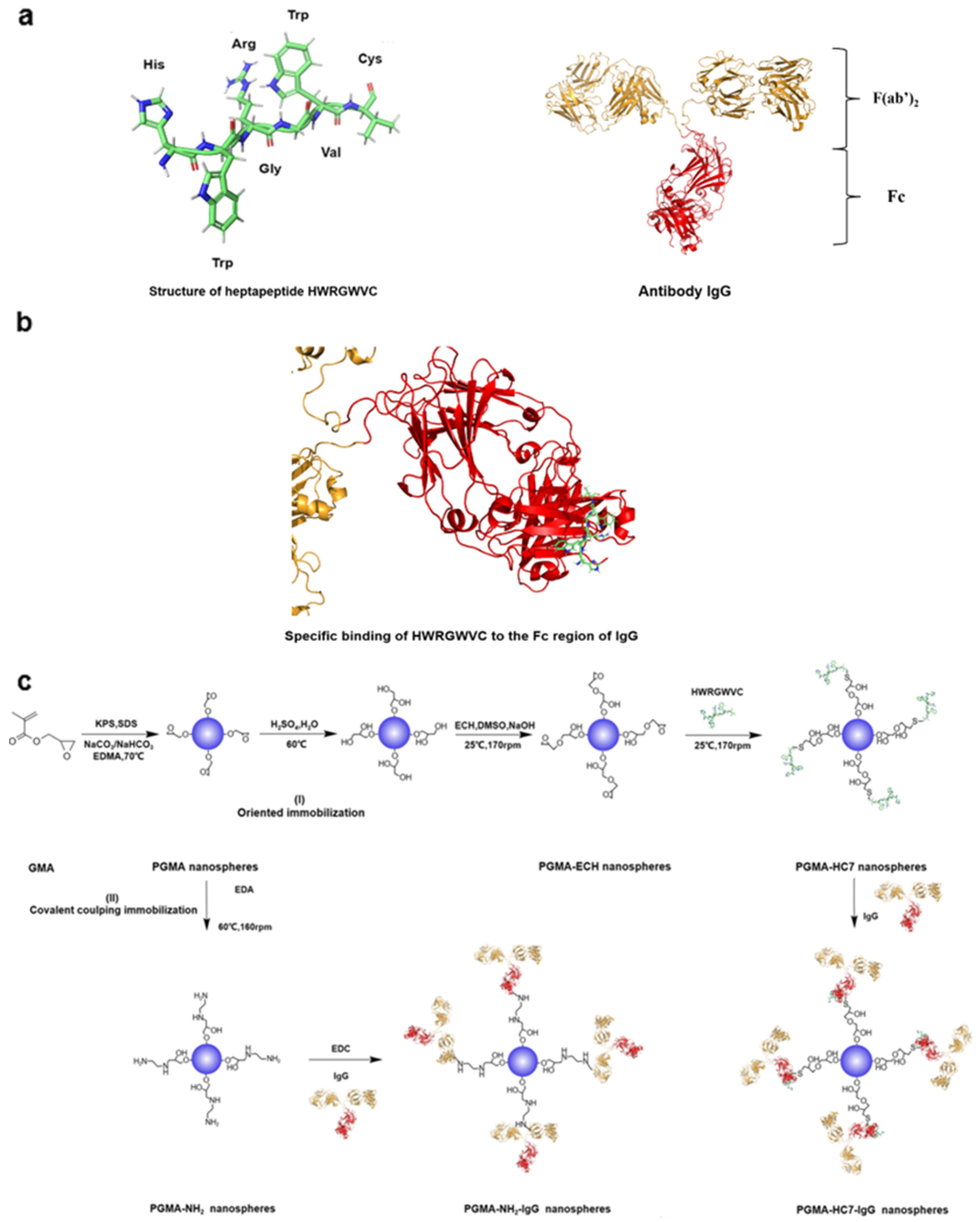
1. Introduction
2. Results and Discussion
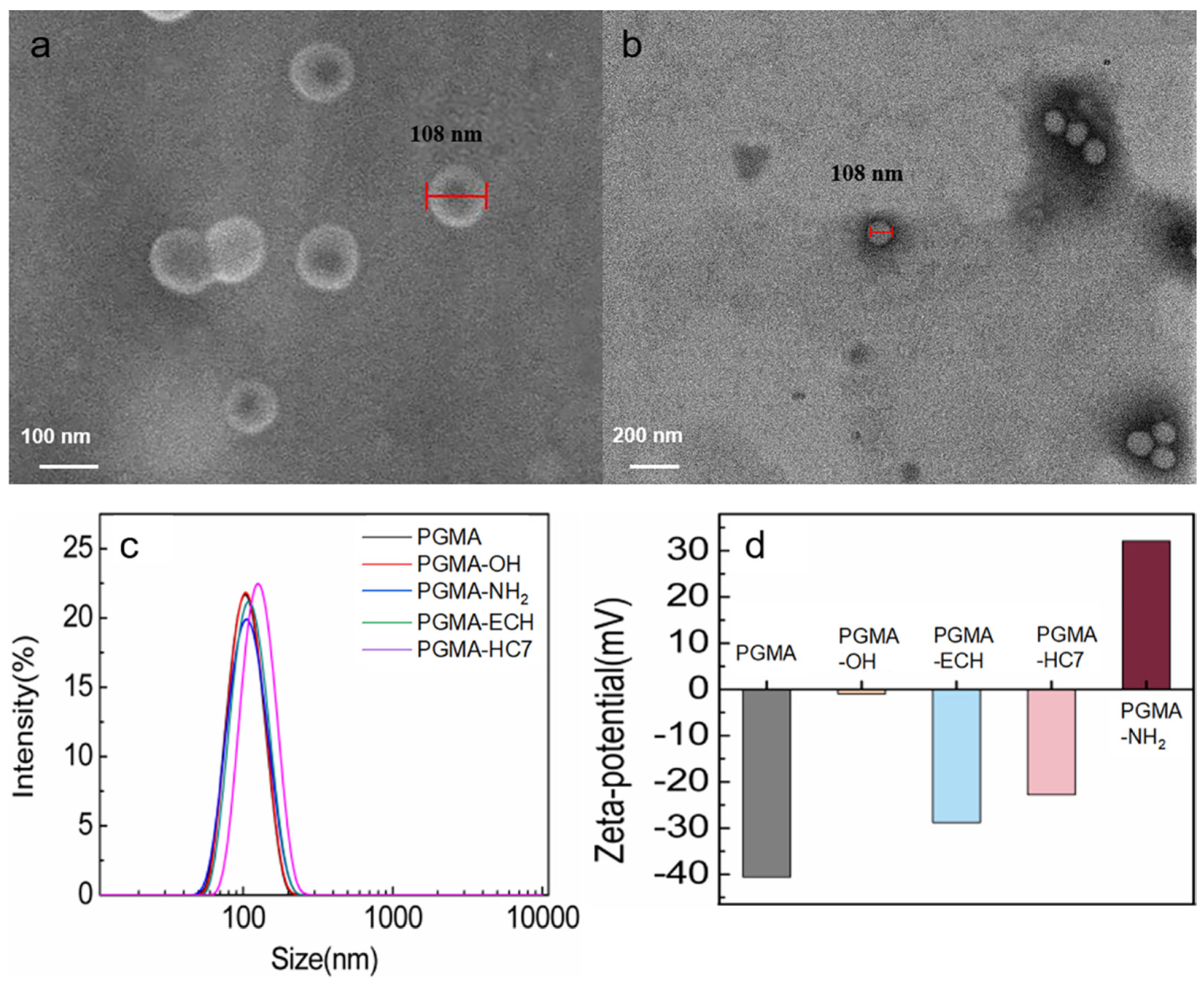
2.1. Characterization of PGMA Nanospheres
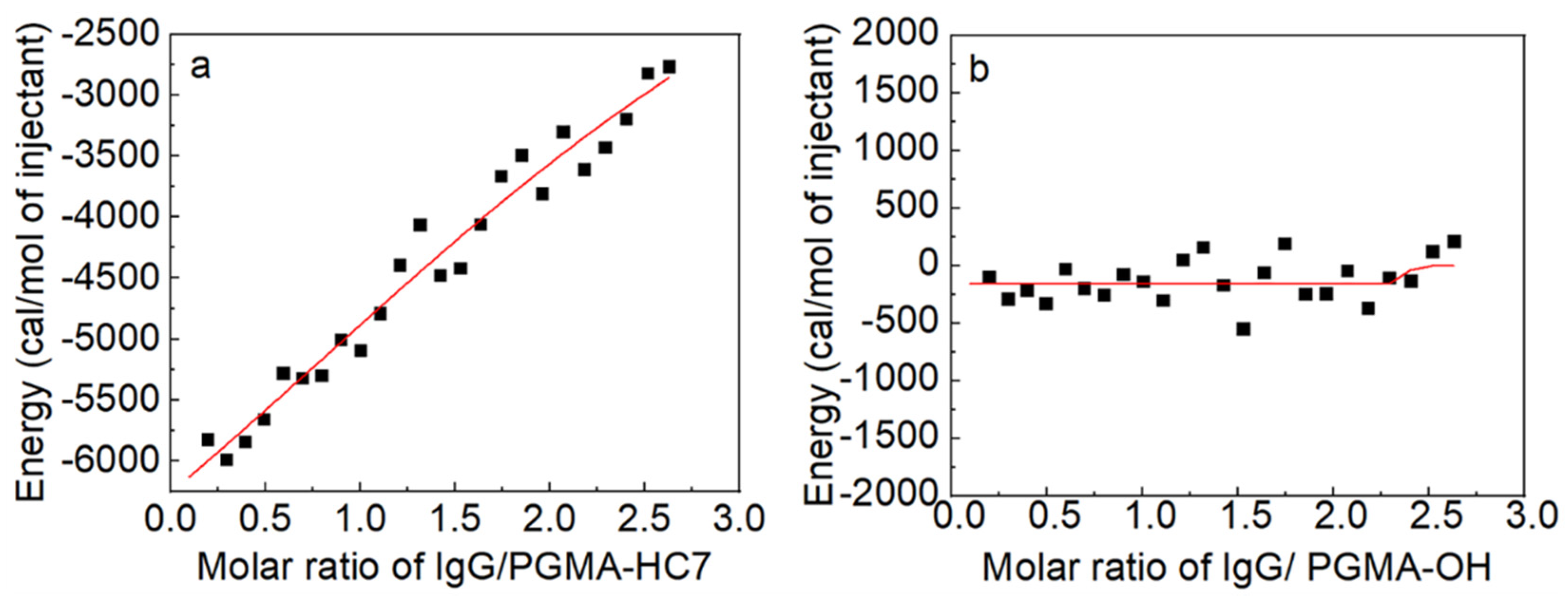
2.2. Thermodynamic Analysis of IgG Binding
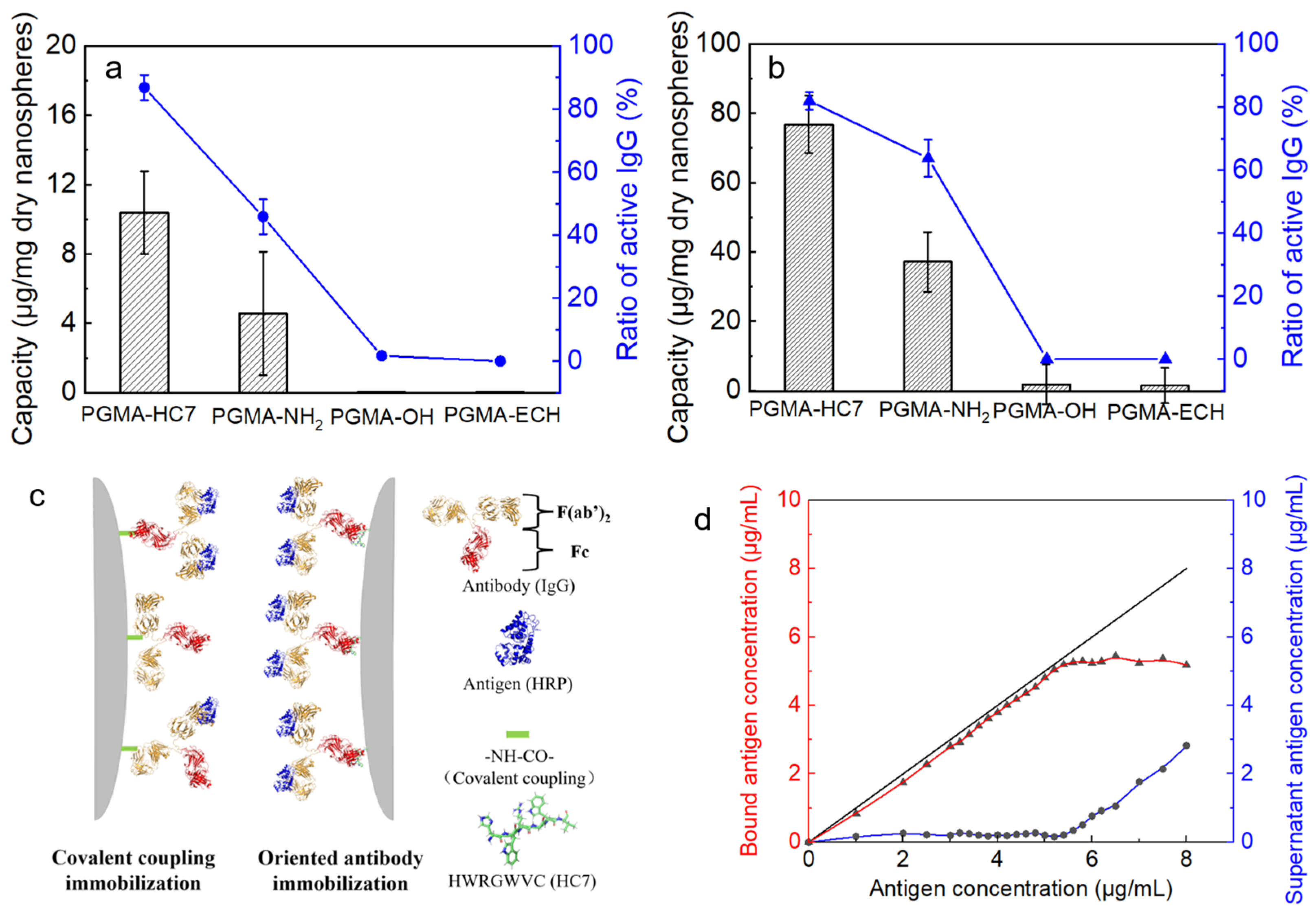
2.3. Batch Adsorption Behaviors of IgG onto PGMA Nanospheres
2.4. Oriented Antibody Immobilization Performance of PGMA-HC7 Nanospheres
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Mono-Sized PGMA Nanospheres
3.3. Surface Modification of PGMA Nanospheres
3.4. Characterization of PGMA Nanospheres
3.5. Isothermal Titration Calorimetry (ITC) Assay
3.6. Batch Binding Experiments
3.7. Antibody Immobilization for Antigen Recognition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Animesh, S.; Singh, Y.D. A Comprehensive Study on Aptasensors For Cancer Diagnosis. Curr. Pharm. Biotechnol. 2021, 22, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Jeong, J.Y.; Chung, B.H. Recent advances in immobilization methods of antibodies on solid supports. Analyst 2008, 133, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Danczyk, R.C.; Krieder, B.; North, A.; Webster, T.J.; Rundell, A. Comparison of antibody functionality using different immobilization methods. Biotechnol. Bioeng. 2003, 84, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Rusling, J.F.; Dixit, C.K. Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development. Methods 2017, 116, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Nam, U.; Suh, H.N.; Sung, S.-K.; Seo, C.; Lee, J.H.; Lee, J.Y.; Kim, S.; Lee, J. Rapid and High-Density Antibody Immobilization Using Electropolymerization of Pyrrole for Highly Sensitive Immunoassay. ACS Appl. Mater. Interfaces 2024, 16, 30611–30621. [Google Scholar] [CrossRef]
- Du, Y.; Xu, C.-M.; Zhang, Y.-M.; Pan, Z.-X.; Wang, F.-S.; Yang, H.-M.; Tang, J.-B. Fabrication of cysteine-modified antibodies with Fc-specific conjugation for covalent and oriented immobilization of native antibodies. Int. J. Biol. Macromol. 2024, 276, 133962. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E. Solid Supports in Enzyme-Linked Immunosorbent Assay and Other Solid-Phase Immunoassays. Methods 2000, 22, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, Y.; Yoo, T.H. Methods to generate site-specific conjugates of antibody and protein. Biorg. Med. Chem. 2021, 30, 115946. [Google Scholar] [CrossRef]
- Nandy, S.; Crum, M.; Wasden, K.; Strych, U.; Goyal, A.; Maranholkar, V.; Mo, W.; Vu, B.; Kourentzi, K.; Willson, R.C. Protein A–Nanoluciferase fusion protein for generalized, sensitive detection of immunoglobulin G. Anal. Biochem. 2023, 660, 114929. [Google Scholar] [CrossRef]
- Brambilla, D.; Sola, L.; Damin, F.; Mussida, A.; Chiari, M. Immobilization of biotinylated antibodies through streptavidin binding aptamer. Talanta 2023, 265, 124847. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; Cadano, D.; Agüí, L.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Click chemistry-assisted antibodies immobilization for immunosensing of CXCL7 chemokine in serum. J. Electroanal. Chem. 2019, 837, 246–253. [Google Scholar] [CrossRef]
- Yang, H.; Gurgel, P.V.; Williams, D.K.; Bobay, B.G.; Cavanagh, J.; Muddiman, D.C.; Carbonell, R.G. Binding site on human immunoglobulin G for the affinity ligand HWRGWV. J. Mol. Recognit. 2009, 23, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kang, H.J.; Lee, J.M.; Jung, S.O.; Yun, W.S.; Chung, S.J.; Chung, B.H. Controlled antibody immobilization onto immunoanalytical platforms by synthetic peptide. Anal. Biochem. 2008, 374, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gurgel, P.V.; Carbonell, R.G. Purification of human immunoglobulin G via Fc-specific small peptide ligand affinity chromatography. J. Chromatogr. A 2009, 1216, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kish, W.S.; Naik, A.D.; Menegatti, S.; Carbonell, R.G. Peptide-Based Affinity Adsorbents with High Binding Capacity for the Purification of Monoclonal Antibodies. Ind. Eng. Chem. Res. 2013, 52, 8800–8811. [Google Scholar] [CrossRef]
- Janu, L.; Stanisavljevic, M.; Krizkova, S.; Sobrova, P.; Vaculovicova, M.; Kizek, R.; Adam, V. Electrophoretic study of peptide-mediated quantum dot-human immunoglobulin bioconjugation. Electrophoresis 2013, 34, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gurgel, P.V.; Carbonell, R.G. Hexamer peptide affinity resins that bind the Fc region of human immunoglobulin G. J. Pept. Res. 2005, 66, 120–137. [Google Scholar] [CrossRef]
- Sun, X.; Weaver, J.; Wickramasinghe, S.R.; Qian, X. Identification and Characterization of Novel Fc-Binding Heptapeptides from Experiments and Simulations. Polymers 2018, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-B.; Yang, H.-M.; Gao, X.-Y.; Zeng, X.-Z.; Wang, F.-S. Directional immobilization of antibody onto magnetic nanoparticles by Fc-binding protein-assisted photo-conjugation for high sensitivity detection of antigen. Anal. Chim. Acta 2021, 1184, 339054. [Google Scholar] [CrossRef] [PubMed]
- Kruljec, N.; Molek, P.; Hodnik, V.; Anderluh, G.; Bratkovič, T. Development and Characterization of Peptide Ligands of Immunoglobulin G Fc Region. Bioconjug. Chem. 2018, 29, 2763–2775. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, J.; Zhu, L.; Gong, X.; Yu, L.; Sun, Y. Characterization of a heptapeptide-modified microsphere for oriented antibody immobilization. J. Pept. Sci. 2022, 28, e3411. [Google Scholar] [CrossRef]
- Crivianu-Gaita, V.; Thompson, M. Immobilization of Fab’ fragments onto substrate surfaces: A survey of methods and applications. Biosens. Bioelectron. 2015, 70, 167–180. [Google Scholar] [CrossRef]
- Susini, V.; Ferraro, G.; Fierabracci, V.; Ursino, S.; Sanguinetti, C.; Caponi, L.; Romiti, N.; Rossi, V.L.; Sanesi, A.; Paolicchi, A.; et al. Orientation of capture antibodies on gold nanoparticles to improve the sensitivity of ELISA-based medical devices. Talanta 2023, 260, 124650. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Sung, D.; Chang, J.H. Highly efficient antibody purification with controlled orientation of protein A on magnetic nanoparticles. MedChemComm 2018, 9, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Tripathi, K.; Okyem, S.; Driskell, J.D. pH Impacts the Orientation of Antibody Adsorbed onto Gold Nanoparticles. Bioconjug. Chem. 2019, 30, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, L.; Feng, R.; Ma, H.; Fan, D.; Yan, T.; Feng, R.; Du, B.; Wei, Q. MnCO3 as a New Electrochemiluminescence Emitter for Ultrasensitive Bioanalysis of β-Amyloid1–42 Oligomers Based on Site-Directed Immobilization of Antibody. ACS Appl. Mater. Interfaces 2019, 11, 7157–7163. [Google Scholar] [CrossRef] [PubMed]
- Beloglazkina, E.K.; Majouga, A.G.; Romashkina, R.B.; Zyk, N.V.; Zefirov, N.S. Gold nanoparticles modified with coordination compounds of metals: Synthesis and application. Russ. Chem. Rev. 2012, 81, 65–90. [Google Scholar] [CrossRef]
- Yang, L.; Fan, D.; Zhang, Y.; Ding, C.; Wu, D.; Wei, Q.; Ju, H. Ferritin-Based Electrochemiluminescence Nanosurface Energy Transfer System for Procalcitonin Detection Using HWRGWVC Heptapeptide for Site-Oriented Antibody Immobilization. Anal. Chem. 2019, 91, 7145–7152. [Google Scholar] [CrossRef]
- Xue, J.; Yang, L.; Jia, Y.; Zhang, Y.; Wu, D.; Ma, H.; Hu, L.; Wei, Q.; Ju, H. Dual-quenching electrochemiluminescence resonance energy transfer system from Ru–In2S3 to α-MoO3-Au based on protect of protein bioactivity for procalcitonin detection. Biosens. Bioelectron. 2019, 142, 111524. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xue, J.; Jia, Y.; Zhang, Y.; Wu, D.; Ma, H.; Wei, Q.; Ju, H. Construction of well-ordered electrochemiluminescence sensing interface using peptide-based specific antibody immobilizer and N-(aminobutyl)-N-(ethylisoluminol) functionalized ferritin as signal indicator for procalcitonin analysis. Biosens. Bioelectron. 2019, 142, 111562. [Google Scholar] [CrossRef] [PubMed]
- Zasonská, B.; Čadková, M.; Kovářová, A.; Bílková, Z.; Korecká, L.; Horák, D. Thionine-Modified Poly(glycidyl methacrylate) Nanospheres as Labels of Antibodies for Biosensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 24926–24931. [Google Scholar] [CrossRef] [PubMed]
- Tocchio, A.; Horák, D.; Babič, M.; Trchová, M.; Veverka, M.; Beneš, M.J.; Šlouf, M.; Fojtík, A. Magnetic poly(glycidyl methacrylate) particles prepared in the presence of surface-modified γ-Fe2O3. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4982–4994. [Google Scholar] [CrossRef]
- Fukuda, T.; Kohara, N.; Onogi, Y.; Inagaki, H. Swelling of poly(glycidyl methacrylate) gel particles by organic solvents. J. Appl. Polym. Sci. 1991, 43, 2201–2205. [Google Scholar] [CrossRef]
- Nguyen, S.H.; Berek, D. Adsorption and desorption of macromolecules on solid surfaces studied by on-line size exclusion chromatography 2. Preferential adsorption and exchange processes. Colloid Polym. Sci. 1999, 277, 1179–1185. [Google Scholar] [CrossRef]
- Canpolat, C.; Tatlisoz, M.M. Protein adsorption on a nanoparticle with a nanostructured surface. Electrophoresis 2022, 43, 2324–2333. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Yu, L.-L.; Dong, X.-Y.; Sun, Y. Mono-sized microspheres modified with poly(ethylenimine) facilitate the refolding of like-charged lysozyme. React. Funct. Polym. 2012, 72, 889–896. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-L.; Tao, S.-P.; Dong, X.-Y.; Sun, Y. Protein adsorption to poly(ethylenimine)-modified Sepharose FF: I. A critical ionic capacity for drastically enhanced capacity and uptake kinetics. J. Chromatogr. A 2013, 1305, 76–84. [Google Scholar] [CrossRef]
- Yu, L.-L.; Sun, Y. Protein adsorption to poly(ethylenimine)-modified Sepharose FF: II. Effect of ionic strength. J. Chromatogr. A 2013, 1305, 85–93. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Liu, Y.; Sun, Y. Implications from the grafting density and ionic capacity effects on protein adsorption to poly (N,N-dimethylaminopropyl acrylamide)-grafted sepharose FF. Biochem. Eng. J. 2020, 157, 107546. [Google Scholar] [CrossRef]
- Reese, H.R.; Xiao, X.; Shanahan, C.C.; Chu, W.; Van Den Driessche, G.A.; Fourches, D.; Carbonell, R.G.; Hall, C.K.; Menegatti, S. Novel peptide ligands for antibody purification provide superior clearance of host cell protein impurities. J. Chromatogr. A 2020, 1625, 461237. [Google Scholar] [CrossRef]
- Islam, T.; Naik, A.D.; Hashimoto, Y.; Menegatti, S.; Carbonell, R.G. Optimization of Sequence, Display, and Mode of Operation of IgG-Binding Peptide Ligands to Develop Robust, High-Capacity Affinity Adsorbents That Afford High IgG Product Quality. Int. J. Mol. Sci. 2019, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, J.; Li, J.; Wang, Y.; Shi, G. Oriented immobilization of antibodies through peptide ligands for ultrasensitive detection of gastrin 17 in human serum via lateral flow immunoassay. Microchem. J. 2024, 202, 110819. [Google Scholar] [CrossRef]
- Muyldermans, S. Applications of Nanobodies. Annu. Rev. Anim. Biosci. 2021, 9, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Barakat, S.; Berksöz, M.; Zahedimaram, P.; Piepoli, S.; Erman, B. Nanobodies as molecular imaging probes. Free Radic. Bio Med. 2022, 182, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-P.; Wang, C.; Sun, Y. Coating of nanoparticles on cryogel surface and subsequent double-modification for enhanced ion-exchange capacity of protein. J. Chromatogr. A 2014, 1359, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cui, P.; Wang, D.; Tang, C.; Dong, L.; Zhang, C.; Duan, H.; Yang, V.C. Preparation and characterization of lysine-immobilized poly(glycidyl methacrylate) nanoparticle-coated capillary for the separation of amino acids by open tubular capillary electrochromatography. J. Chromatogr. A 2014, 1323, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Zhang, R.; Ge, J.; Ye, P.; Song, C.; Zhu, K.; Na, X.; Huang, Y.; Zhao, L.; Zhou, W.; et al. Rapid and high-capacity loading of IgG monoclonal antibodies by polymer brush and peptides functionalized microspheres. J. Chromatogr. A 2021, 1640, 461948. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Sun, Y.; Dong, X. Design of Gallic Acid–Glutamine Conjugate and Chemical Implications for Its Potency Against Alzheimer’s Amyloid-β Fibrillogenesis. Bioconjug. Chem. 2022, 33, 677–690. [Google Scholar] [CrossRef]

| Nanosphere | Average Size (nm) | PDI | HC7 Density (μmol/g Dry Nanospheres) |
|---|---|---|---|
| PGMA | 102.6 | 0.033 | 0 |
| PGMA-OH | 107.3 | 0.027 | 0 |
| PGMA-ECH | 110.1 | 0.039 | 0 |
| PGMA-HC7 | 119.3 | 0.021 | 60 |
| PGMA-NH2 | 108.8 | 0.049 | 0 |
| Immobilization Carrier | Equilibrium Time (min) | qm (mg/mg) | Kd (mg/mL) | |
|---|---|---|---|---|
| Nanosphere | PGMA-HC7 | 5 | 2.76 ± 0.16 | 0.87 ± 0.12 |
| Microsphere | pGMA-HC7-25 a | 30 | (3.33 ± 0.28) × 10−3 | 0.13 ± 0.06 |
| pGMA-HCH-75 a | 30 | (2.44 ± 0.10) × 10−3 | 0.03 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Zhang, J.; Zhu, L.; Bai, S.; Yu, L.; Sun, Y. Fabrication of a Heptapeptide-Modified Poly(glycidyl Methac-Rylate) Nanosphere for Oriented Antibody Immobilization and Immunoassay. Molecules 2024, 29, 4635. https://doi.org/10.3390/molecules29194635
Gong X, Zhang J, Zhu L, Bai S, Yu L, Sun Y. Fabrication of a Heptapeptide-Modified Poly(glycidyl Methac-Rylate) Nanosphere for Oriented Antibody Immobilization and Immunoassay. Molecules. 2024; 29(19):4635. https://doi.org/10.3390/molecules29194635
Chicago/Turabian StyleGong, Xiaoxing, Jie Zhang, Liyan Zhu, Shu Bai, Linling Yu, and Yan Sun. 2024. "Fabrication of a Heptapeptide-Modified Poly(glycidyl Methac-Rylate) Nanosphere for Oriented Antibody Immobilization and Immunoassay" Molecules 29, no. 19: 4635. https://doi.org/10.3390/molecules29194635
APA StyleGong, X., Zhang, J., Zhu, L., Bai, S., Yu, L., & Sun, Y. (2024). Fabrication of a Heptapeptide-Modified Poly(glycidyl Methac-Rylate) Nanosphere for Oriented Antibody Immobilization and Immunoassay. Molecules, 29(19), 4635. https://doi.org/10.3390/molecules29194635








