Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective
Abstract
1. Introduction
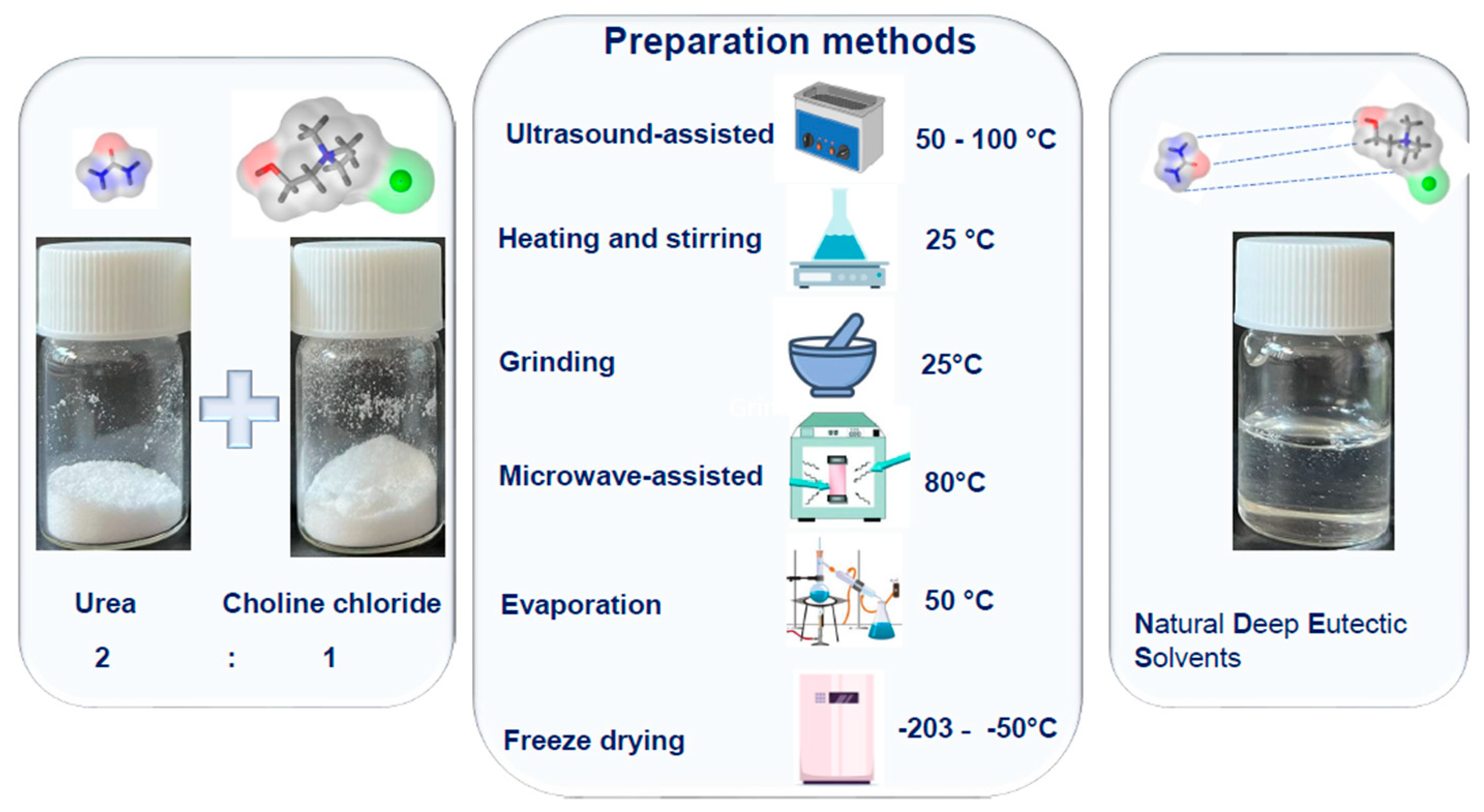
2. Natural Deep Eutectic Solvents: Composition and Preparation Methods
3. Properties and Applications of NADES
4. Cost of NADES Solvents
5. Solubility Challenges of NADES and the Role of Co-Solvents
6. Toxicity of NADES
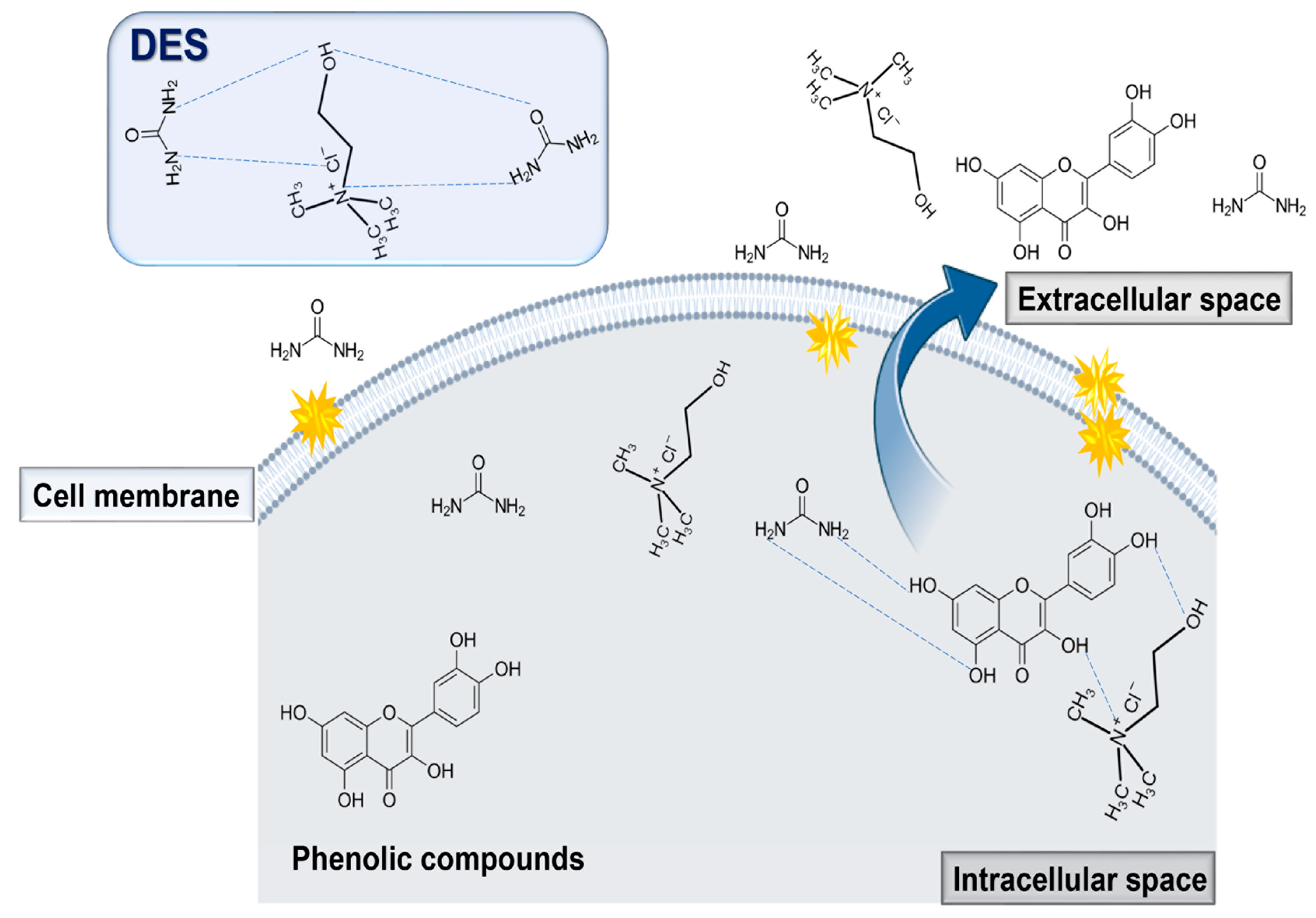
7. Mechanism of Extraction of Bioactives Using NADES
8. Extraction of Bioactive Compounds from Fruit Waste and By-Products
9. Extraction of Bioactive Compounds from Vegetable Wastes and By-Products
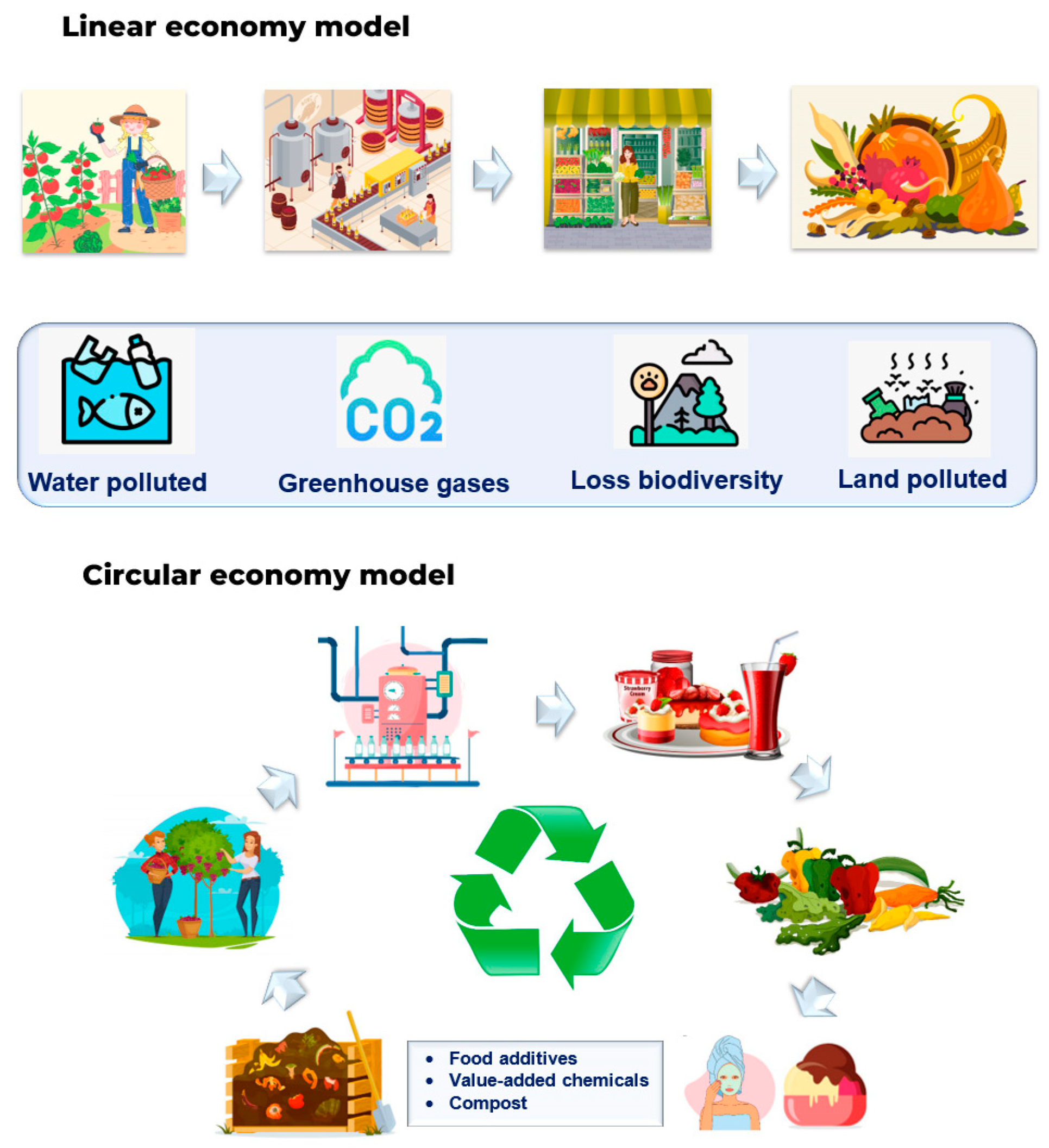
10. Circular Economy Concept Supported by Green Extraction of Bioactives
11. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurostat. Available online: https://food.ec.europa.eu/safety/food-waste_en (accessed on 29 July 2024).
- Pomoni, D.I.; Koukou, M.K.; Vrachopoulos, M.G.; Vasiliadis, L. Circular Economy: A Multilevel Approach for Natural Resources and Wastes under an Agri-Food Perspective. Water-Energy Nexus 2024, 7, 103–123. [Google Scholar] [CrossRef]
- Jaglo, K.; Kenny, S.K.; Stephenson, J.S. Part 1 From Farm to Kitchen: The Environmental Impacts of U.S. Food Waste; 2021; U.S. Environmental Protection Agency: Washington, DC, USA. [Google Scholar]
- Liu, Z.; de Souza, T.S.P.; Holland, B.; Dunshea, F.; Barrow, C.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Dou, Z.; Dierenfeld, E.S.; Wang, X.; Chen, X.; Shurson, G.C. A Critical Analysis of Challenges and Opportunities for Upcycling Food Waste to Animal Feed to Reduce Climate and Resource Burdens. Resour. Conserv. Recycl. 2024, 203, 107418. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Turiel, E.; Díaz-Álvarez, M.; Martín-Esteban, A. Natural Deep Eutectic Solvents as Sustainable Alternative for the Ultrasound-Assisted Extraction of Triazines from Agricultural Soils. Microchem. J. 2024, 196, 109675. [Google Scholar] [CrossRef]
- Santana-Mayor, Á.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.Á. Deep Eutectic Solvents. The New Generation of Green Solvents in Analytical Chemistry. TrAC Trends Anal. Chem. 2021, 134, 116108. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Koh, Q.Q.; Kua, Y.L.; Gan, S.; Tan, K.W.; Lee, T.Z.E.; Cheng, W.K.; Lau, H.L.N. Sugar-Based Natural Deep Eutectic Solvent (NADES): Physicochemical Properties, Antimicrobial Activity, Toxicity, Biodegradability and Potential Use as Green Extraction Media for Phytonutrients. Sustain. Chem. Pharm. 2023, 35, 101218. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the Relationships between Physicochemical Properties, Solvent Performance, and Applications of Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef] [PubMed]
- Xin, R.; Qi, S.; Zeng, C.; Khan, F.I.; Yang, B.; Wang, Y. A Functional Natural Deep Eutectic Solvent Based on Trehalose: Structural and Physicochemical Properties. Food Chem. 2017, 217, 560–567. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine:Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef] [PubMed]
- Benfica, J.; Morais, E.S.; Miranda, J.S.; Freire, M.G.; de Cássia Superbi de Sousa, R.; Coutinho, J.A.P. Aqueous Solutions of Organic Acids as Effective Solvents for Levodopa Extraction from Mucuna Pruriens Seeds. Sep. Purif. Technol. 2021, 274, 119084. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Strieder, M.M.; Pizani, R.S.; de Souza Mesquita, L.M.; González-Miquel, M.; Rostagno, M.A. Revisiting Natural Deep Eutectic Solvents (NADES) as Extraction Media and Ready-to-Use Purposes. TrAC Trends Anal. Chem. 2024, 175, 117726. [Google Scholar] [CrossRef]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V. V Bioavailability and Biological Effects of Bioactive Compounds Extracted with Natural Deep Eutectic Solvents and Ionic Liquids: Advantages over Conventional Organic Solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M.; Harun@Kamaruddin, A. Hydrophilic Natural Deep Eutectic Solvent: A Review on Physicochemical Properties and Extractability of Bioactive Compounds. J. Mol. Liq. 2021, 339, 116923. [Google Scholar] [CrossRef]
- Lazović, M.; Cvijetić, I.; Jankov, M.; Milojković-Opsenica, D.; Trifković, J.; Ristivojević, P. COSMO-RS in Prescreening of Natural Eutectic Solvents for Phenolic Extraction from Teucrium Chamaedrys. J. Mol. Liq. 2023, 387, 122649. [Google Scholar] [CrossRef]
- Ivkovic, D.; Cvijetic, I.; Radoicic, A.; Stojkovic-Filipovic, J.; Trifkovic, J.; Krstic Ristivojevic, M.; Ristivojevic, P. NADES-Based Extracts of Selected Medicinal Herbs as Promising Formulations for Cosmetic Usage. Processes 2024, 12, 992. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.M.; Frank, O.; Dawid, C.; Hofmann, T.F. A New Inert Natural Deep Eutectic Solvent (NADES) as a Reaction Medium for Food-Grade Maillard-Type Model Reactions. Foods 2023, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Kalhor, P.; Xu, J.; Ashraf, H.; Cao, B.; Yu, Z.-W. Structural Properties and Hydrogen-Bonding Interactions in Binary Mixtures Containing a Deep-Eutectic Solvent and Acetonitrile. J. Phys. Chem. B 2020, 124, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.I.B.; Craveiro, R.; Silva, J.M.; Reis, R.L.; Paiva, A.C.; Duarte, A.R. Natural Deep Eutectic Systems as Alternative Nontoxic Cryoprotective Agents. Cryobiology 2018, 83, 15–26. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable Synthesis of Natural Deep Eutectic Solvents (NADES) by Different Methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2022, 12, 56. [Google Scholar] [CrossRef]
- Yang, Z. Toxicity and Biodegradability of Deep Eutectic Solvents and Natural Deep Eutectic Solvents. In Deep Eutectic Solvents; Wiley: Hoboken, NJ, USA, 2019; pp. 43–60. [Google Scholar]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Kranz, M.; Hofmann, T. Food-Grade Synthesis of Maillard-Type Taste Enhancers Using Natural Deep Eutectic Solvents (NADES). Molecules 2018, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Voelker, A.L.; Miller, J.; Running, C.A.; Taylor, L.S.; Mauer, L.J. Chemical Stability and Reaction Kinetics of Two Thiamine Salts (Thiamine Mononitrate and Thiamine Chloride Hydrochloride) in Solution. Food Res. Int. 2018, 112, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research Progress on the Preparation and Action Mechanism of Natural Deep Eutectic Solvents and Their Application in Food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Ferreira, W.H.; Goycoolea, F.M.; Murray, B.S.; Andrade, C.T. Improved Antioxidant and Mechanical Properties of Food Packaging Films Based on Chitosan/Deep Eutectic Solvent, Containing Açaí-Filled Microcapsules. Molecules 2023, 28, 1507. [Google Scholar] [CrossRef]
- Shishov, A.; Bulatov, A.; Locatelli, M.; Carradori, S.; Andruch, V. Application of Deep Eutectic Solvents in Analytical Chemistry. A Review. Microchem. J. 2017, 135, 33–38. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Słupek, E.; Makoś, P.; Gębicki, J. Theoretical and Economic Evaluation of Low-Cost Deep Eutectic Solvents for Effective Biogas Upgrading to Bio-Methane. Energies 2020, 13, 3379. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, J.; Huang, Z.; Guo, Y. Sustainable Recovery and Recycling of Natural Deep Eutectic Solvent for Biomass Fractionation via Industrial Membrane-Based Technique. Ind. Crops Prod. 2023, 194, 116351. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Ye, Z.; Chai, M.; Yuan, J.; Xiong, Y.; Yang, H.; Yao, L. Effective fractionation of lignocellulose components and lignin valorization by combination of deep eutectic solvent with ethanol. Front. Bioeng. Biotechnol. 2023, 10, 1115469. [Google Scholar] [CrossRef]
- Huber, V.; Muller, L.; Hioe, J.; Degot, P.; Touraud, D.; Kunz, W. Improvement of the Solubilization and Extraction of Curcumin in an Edible Ternary Solvent Mixture. Molecules 2021, 26, 7702. [Google Scholar] [CrossRef]
- Huber, V.; Muller, L.; Degot, P.; Touraud, D.; Kunz, W. NADES-based surfactant-free microemulsions for solubilization and extraction of curcumin from Curcuma Longa. Food Chem. 2021, 355, 129624. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.J.; Oliveira, F.; Jesus, A.R.; Paiva, A.; Duarte, A.R. Current methodologies for the assessment of deep eutectic systems toxicology: Challenges and perspectives. J. Mol. Liq. 2022, 362, 119675. [Google Scholar] [CrossRef]
- Ferreira, J.I.; Meneses, L.; Paiva, A.; Diniz, M.; Ana Rita, C.; Duarte, C.C.R. Assessment of deep eutectic solvents toxicity in zebrafish (Danio rerio). Chemosphere 2022, 299, 134415. [Google Scholar] [CrossRef]
- García, C.B.; Concha, J.; Culleré, L.; Lomba, L.; Sangüesa, E.; Ribate, M.P. Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed? Appl. Sci. 2023, 13, 5980. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.H. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, J.E.; Díaz, I.; Rodríguez, M. Hydrophobic eutectic solvents for extraction of natural phenolic antioxidants from winery wastewater. Sep. Purif. Technol. 2021, 254, 117590. [Google Scholar] [CrossRef]
- de Oliveira, J.A.R.; de Paula Menezes Barbosa, P.; Macêdo, G.A. High Concentrate Flavonoids Extract from Citrus Pomace Using Enzymatic and Deep Eutectic Solvents Extraction. Foods 2022, 11, 3205. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.C.A.; Plepis, A.M.G.; Bogusz, S. Utilization of Pomegranate Peel Waste: Natural Deep Eutectic Solvents as a Green Strategy to Recover Valuable Phenolic Compounds. J. Clean. Prod. 2021, 327, 129471. [Google Scholar] [CrossRef]
- Santos-Martín, M.; Cubero-Cardoso, J.; González-Domínguez, R.; Cortés-Triviño, E.; Sayago, A.; Urbano, J.; Fernández-Recamales, Á. Ultrasound-Assisted Extraction of Phenolic Compounds from Blueberry Leaves Using Natural Deep Eutectic Solvents (NADES) for the Valorization of Agrifood Wastes. Biomass Bioenergy 2023, 175, 106882. [Google Scholar] [CrossRef]
- Gómez-Urios, C.; Viñas-Ospino, A.; Puchades-Colera, P.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Sustainable Development and Storage Stability of Orange By-Products Extract Using Natural Deep Eutectic Solvents. Foods 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Šafranko, S.; Jakovljević, M.; Cikoš, A.-M.; Kajić, N.; Kolarević, F.; Babić, J.; Molnar, M. Sustainable Green Procedure for Extraction of Hesperidin from Selected Croatian Mandarin Peels. Processes 2019, 7, 469. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Palaiologou, E.; Papadakis, E.N.; Makris, D.P.; Biliaderis, C.G.; Mourtzinos, I. Insights on the Impact of Deep Eutectic Solvents on the Composition of the Extracts from Lemon (Citrus limon L.) Peels Analyzed by a Novel RP-LC–QTOF-MS/MS Method. Eur. Food Res. Technol. 2022, 248, 2913–2927. [Google Scholar] [CrossRef]
- Moni Bottu, H.; Mero, A.; Husanu, E.; Tavernier, S.; Pomelli, C.S.; Dewaele, A.; Bernaert, N.; Guazzelli, L.; Brennan, L. The Ability of Deep Eutectic Solvent Systems to Extract Bioactive Compounds from Apple Pomace. Food Chem. 2022, 386, 132717. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Niu, D.; Wang, R.; Xu, F.-Y.; Chen, B.-R.; Lin, J.-W.; Tang, Z.-S.; Zeng, X.-A. Efficient and Green Strategy Based on Pulsed Electric Field Coupled with Deep Eutectic Solvents for Recovering Flavonoids and Preparing Flavonoid Aglycones from Noni-Processing Wastes. J. Clean. Prod. 2022, 368, 133019. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.-Q.; Liang, Z.; Wang, R.; Zhao, W.; Zeng, X.-A.; Wang, L. Pulsed Electric Field Combined with Deep Eutectic Solvents Enhanced the Antiglycation Activity of Noni Flavonoid Extracts by Directionally Regulating Their Glycoside and Aglycone Proportions. ACS Sustain. Chem. Eng. 2024, 12, 6355–6365. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High Voltage Electrical Discharges Followed by Deep Eutectic Solvents Extraction for the Valorization of Pomegranate Seeds (Punica granatum L.). Innov. Food Sci. Emerg. Technol. 2022, 79, 103055. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Microwave-Assisted Extraction for Recovery of Polyphenolic Antioxidants from Ripe Mango (Mangifera Indica L.) Peel Using Lactic Acid/Sodium Acetate Deep Eutectic Mixtures. Food Sci. Technol. Int. 2020, 26, 78–92. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural Deep Eutectic Solvent as a Unique Solvent for Valorisation of Orange Peel Waste by the Integrated Biorefinery Approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Radošević, K.; Cvjetko Bubalo, M.; Ganić, K.K.; Redovniković, I.R. COSMOtherm as an Effective Tool for Selection of Deep Eutectic Solvents Based Ready-To-Use Extracts from Graševina Grape Pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Plaza, M.; Domínguez-Rodríguez, G.; Sahelices, C.; Marina, M.L. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2021, 11, 5625. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative Process of Polyphenol Recovery from Pomegranate Peels by Combining Green Deep Eutectic Solvents and a New Infrared Technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use Natural Deep Eutectic Solvents as Efficient Green Reagents to Extract Procyanidins and Anthocyanins from Cranberry Pomace and Predictive Modeling by RSM and Artificial Neural Networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Fernández-Prior, Á.; Bermúdez Oria, A.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of Strawberry and Raspberry Waste for the Extraction of Bioactive Compounds by Deep Eutectic Solvents. LWT 2020, 130, 109645. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Ping-Kou; Jiang, Y.W.; Wang, L.-T.; Niu, L.-J.; Liu, Z.-M.; Fu, Y.-J. Natural Deep Eutectic Solvents Couple with Integrative Extraction Technique as an Effective Approach for Mulberry Anthocyanin Extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural Deep Eutectic Solvent Enhanced Pulse-Ultrasonication Assisted Extraction as a Multi-Stability Protective and Efficient Green Strategy to Extract Anthocyanin from Blueberry Pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Đorđević, B.; Todorović, Z.; Troter, D.; Stanojević, L.; Veljković, V. The Extraction of Quercetin from Waste Onion (Allium cepa L.): Tunic by the Aqueous Solutions of Different Deep Eutectic Solvents. Adv. Technol. 2018, 7, 5–10. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Vasyliev, G. Extraction of Phenolic Compounds from Tomato Pomace Using Choline Chloride–Based Deep Eutectic Solvents. J. Food Meas. Charact. 2022, 16, 1087–1104. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Barouh, N.; Baréa, B.; Villeneuve, P.; Bourlieu-Lacanal, C.; Ferreira, M.S.L.; Durand, E. Sequential One-Pot NaDES Assisted Extraction and Biotransformation of Rice Bran: A New Strategy to Boost Antioxidant Activity of Natural Extracts. Process Biochem. 2022, 117, 110–116. [Google Scholar] [CrossRef]
- Caviglia, D.; Russo, E.; Preda, S.; Robustelli della Cuna, F.S.; Villa, C. In Situ NADES Microwave-mediated Extraction of Bioactive Compounds from Beta vulgaris L. Var. Rubra Waste. Int. J. Food Sci. Technol. 2024, 59, 3271–3282. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ali Redha, A.; Koca, I. Enhanced Ultrasonically Assisted Extraction of Bitter Melon (Momordica Charantia) Leaf Phenolic Compounds Using Choline Chloride-Acetic Acid–Based Natural Deep Eutectic Solvent: An Optimization Approach and in Vitro Digestion. Biomass Convers. Biorefinery 2024, 14, 11491–11503. [Google Scholar] [CrossRef]
- Hong, J.; Deng, M.; Zhao, L. Natural Deep Eutectic Solvent Combined with Ultrasonic Enhancement: A Green Extraction Strategy for Solanesol in Tobacco Leaves. Ind. Crops Prod. 2022, 187, 115355. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Tsiouras, A.; Mourtzinos, I. Extraction of Lycopene from Tomato Using Hydrophobic Natural Deep Eutectic Solvents Based on Terpenes and Fatty Acids. Foods 2022, 11, 2645. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguirre, O.A.; Muro, C.; Hernández-Acosta, E.; Alvarado, Y.; del Díaz-Nava, M.C. Extraction and Stabilization of Betalains from Beetroot (Beta Vulgaris) Wastes Using Deep Eutectic Solvents. Molecules 2021, 26, 6342. [Google Scholar] [CrossRef]
- Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Marinaccio, L.; Zengin, G.; Bender, O.; Cichelli, A.; Novellino, E.; Stefanucci, A.; Mollica, A. Ultrasound Assisted Lycopene Extraction from Tomato Skin Waste by Volatile Natural Deep Eutectic Solvent. Food Chem. Adv. 2024, 4, 100656. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced Extraction of Natural Pigments from Curcuma longa L. Using Natural Deep Eutectic Solvents. Ind. Crops Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the Extraction of Quercetin from Vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Ni, W.; Xin, K.; Yu, Q.; Han, L. Green Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction of Phenolic Compounds from Waste Broccoli Leaves: Optimization, Identification, Biological Activity, and Structural Characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient Removal of Lignin from Vegetable Wastes by Ultrasonic and Microwave-Assisted Treatment with Ternary Deep Eutectic Solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
| Natural Source | Extraction Technique | NADES | Analytes | References | |
|---|---|---|---|---|---|
| 1. | Citrus pomace with and without pectin | Enzymatic extraction | B-LA = 1:1ChC-CA = 2:1, ChCl-LA = 1:2, ChCl-Mal = 1:2, ChCl-Gly = 1:2, B-Gly = 1:2, ChCl-OA = 1:2 | Flavonoids | [56] |
| 2. | Pomegranate peels | UAE | ChCl with different HBDs in a 1:1 ratio: Suc, Gly, LA, CA, and Glu | Phenolic compounds | [57] |
| 3. | Blueberry leaves | UAE | CA:Glu:water = 1:2:7.5, LA:Glu:water = 6:1:6, LA:Gln:water = 3:1:3, LA:NaAce:water = 3:1:2, ChCl:U = 1:2, ChCl:U:water = 1:2:1, ChCl: Gly = 1:2, ChCl: Gly:water = 1:2:1, ChCl:EG = 1:2, ChCl:EG:water = 1:2:1, ChCl:LA = 1:2, ChCl:OA = 1:1, ChCl:1,4-BD = 1:6, B:Gly:water = 1:2:1, B: EG:water = 1:2:1, Pro:LA = 1:1, Pro:OA = 1:1, Pro:Gly = 2:5 | Phenolic compounds | [58] |
| 4. | Orange by-products | Magnetic stirring and heating | ChCl:Glu = 2:1, ChCl:Fru = 1.9:1, ChCl:Xyl = 2:1, ChCl:Gly = 1:2, ChCl:MA = 1:1, ChCl:TA = 2:1, ChCl:LA = 1:3, ChCl:CA = 2:1, ChCl:Pro:MA = 1:1:1, LA:Glu = 5:1, MA:Glu = 1:1, Pro:MA = 1:1, B:CA = 1:1, B:MA = 1:1 | Phenolic compounds | [59] |
| 5. | Mandarin peels | Stirring at a temperature of 50 °C for 30 min. | ChCl:Acm = 1:2, ChCl: B˗1,4˗D = 1:2, ChCl:CA = 1:1, ChCl:EG = 1:1, ChCl:Gly = 1:2, ChCl:LA = 1:1, ChCl:LVA = 1:1, ChCl:MLA = 1:1, ChCl:MA = 1:1, ChCl: NMU = 1:3, ChCl:OA = 1:1, ChCl:Sol = 1:1, ChCl:ThU = 1:1, ChCl:U = 1:1, ChCl:Xyl = 1:1 | Hesperidin | [60] |
| 6. | Dried lemon peel wastes | Stirring at a temperature of 50 °C for 30 min. | Gly: ChCl = 3:1 | Phenolic compounds | [61] |
| 7. | Apple pomace | Stirring at a temperature of 50 °C for 30 min. | ChCl:OA = 1:1, ChCl: EG = 1:4 | Bioactive compounds | [62] |
| 8. | Noni-processing wastes | Pulsed electric field | ChCl:OA = 1:1, ChCl:MA = 1:1, ChCl:CA = 1:1, ChCl:LVA = 1:2, ChCl:Glz = 1:2, ChCl:TEG = 1:4, ChCl:EG = 1:2, ChCl:D-Sol = 1:1, ChCl:U = 1:2, ChCl:Glu:water = 5:2:5, ChCl:Suc:water = 5:2:5, ChCl:Fru:water = 5:2:5, B:OA = 1:1, B:TEG = 1:4, B = EG = 1:2, Pro:OA = 1:1, Pro:TEG = 1:4, Pro:EG = 1:2 | Flavonoids and preparing flavonoid aglycones | [63] |
| 9. | Noni fruit pomace | Pulsed electric field | ChCl:OxA = 1:1 | Rutin and quercetin | [64] |
| 10. | Pomegranate seeds | High-voltage electrical discharges | ChCl was employed as hydrogen bond acceptor (HBA) and CA, acetic acid (AA), LA, Gly and Glu were used as hydrogen bond donors (HBDs). HBA and HBD were mixed in a 1:1 ratio | Proteins and polyphenols | [65] |
| 12. | Grapefruit peels | High-voltage electrical discharges | LA: ChCl = 3:1, LA:NaAce = 3:1, LA:Gln = 3:1, LA:NH4Ace = 3:1, ChCl:TA = 2:1, LA:Glu = 5:1 | Polyphenols | [66] |
| 11. | Mango (Mangifera indica L.) peel | Microwave-assisted extraction (MAE) | ChCl:U = 1:2, ChCl:Sol = 3:1, ChCl:Suc = 1:1, ChCl:Gly = 1:3, NaAce:Gly = 1:3, ChCl:LA = 1:3, NaAce:LA = 1:1, 1:2, 1:3, and 1:4, ChCl:MA = 1.5:1 | Polyphenolic antioxidants | [67] |
| 13 | Orange peel waste | Stabilizing effect on the hydrolytic enzymes | ChCl:Glu = 1:1, ChCl:Gly = 1:2, ChCl:EG = 1:2, Glu:Fru:Suce = 1:1:1, Glu:EG = 1:2, sol: EG = 1:2, Glu: Gly = 1:2 | Bioactive compounds | [68] |
| 14. | Grape pomace | UMAE | ChCl:CA = 2:1, ChCl:MA = 1:1, ChCl: Pro:MA = 1:1:1, Pro:MA = 1:1, B:MA = 1:1, B:CA = 1:1, MA:Glu:Gly = 1:1:1, MA:Glu = 1:1 | Anthocyanins | [69] |
| 15. | Grape pomace | Stirring at a temperature of 50 °C for 30 min | B:CA = 1:1, B:EG = 1:2, and B:U = 1:2 were prepared by mixing betaine as HBA and three different HBDs at the appropriate stoichiometric ratio | Anthocyanins | [70] |
| 16. | Grape pomace | Solid–liquid extraction | B:Gly = 1:2, Ch:Gly = 1:2, B:EG = 1:2, Ch:EG = 1:2, Ch:U = 1:2, B:Xyl = 1:1, Ch:Xyl = 2:1, EG:Xyl = 2:1, Me:C8 = 1:1, Ty:C8 = 1:3, Me:C10 = 1:1, Ty:C10 = 1:1, B:Scu = 1:1, Ch:Scu = 2:1, CA:Scu = 1:1, MA:Scu = 1:1, B:CA = 1:1, B:MA = 1:1, Ch:CA = 1:1, Ch:MA = 1:1, Ch:OxA = 1:1, Pro:MA = 1:1, EG:Sol2:1, B:Glc = 1:1, Ch:Glc = 1:1, CA:Glc = 1:1, MA:Gl = 1:1, EG:Glc = 2:1, Gly:Glc = 2:1, Ch:Fru = 1:1, CA:Fru = 1:1, MA:Fru = 1:1, EG:Fru = 2:1, Glc:Fru = 1:1, Ch:Sor = 1:1, CA:Sor = 2:3, EG:Sor = 2:1, Me:Cam = 1:1, Me:SA = 4:1, Me:Ty = 3:2, Ty:Cou = 3:2, Ch:Xyol = 5:2, Ch:Sol = 1:1 | Polyphenols | [71] |
| 17. | Grape seeds and skin | UAE | ChCl:CA = 2:1, ChCl:Glu = 1:1 | Polyphenols | [72] |
| 18. | Mangosteen peels | Stirring at a temperature of 50 °C for 30 min | ChCl:Gly = 1:2, ChCl:EG = 1:2, ChCl:U = 1:2, ChCl:Sol = 1:1, ChCl:LA = 1:2, ChCl:CA = 2:1, ChCl:FA = 1:2 | Polyphenols | [73] |
| 19. | Pomegranate peels | Infrared-assisted extraction and UAE | LA:ChCl = 3:1, MA:Suc = 1:1, Gly:Gln = 3:1, ChCl:Fru = 1.9:1, Glu:TA = 1:1, Gly:U = 1:1, MA:Glu:Gly = 1:1:1, LA:Gly = 3:1 | Polyphenols | [74] |
| 20. | Cranberry pomace | UAE | ChCl:LVA:EG = 1:1:2, ChCl:B:LVA = 1:1:2, ChCl: P-1,2-D:LA = 1:1:2, ChCl:LA = 1:2, ChCl: B˗1,4˗D = 1:4, ChCl:B:EG = 1:1:2, ChCl:Pro:MA = 1:1:1, ChCl:Gly = 1:2, Glu:LA = 1:5, CA:mal = 4:1 | polyphenols | [75] |
| 21. | Strawberry and raspberry waste | Stirring | ChCl:Gly = 1:2, ChCl:Suc = 1:2, ChCl: B˗1,4˗D = 1:5, ChCl: P-1,2-D = 1:1, B:Suc = 2:1, B:LVA = 1:2, ChCl: GlyA OA = 1:1.7 | Bioactives | [76] |
| 22. | Sour cherry pomace | Ultrafast MAE | ChCl:MA = 1:1, ChCl:U = 1:1, ChCl:Fru = 1:1 | Polyphenols | [77] |
| 26. | Mulberry | HSH and CBE | ChCl:CA:Glu = 1:1:1 | Anthocyanin | [78] |
| 25. | Blueberry pomace | Pulse-ultrasonication assisted extraction | ChCl:MA = 3:2, ChCl:OA = 1:1, ChCl:LA = 1:1, ChCl:CA = 1:1, ChCl:SA = 1:1, ChCl:TA = 2:1, ChCl: PG = 1:2, ChCl:Gly = 1:2, ChCl:Bnd = 2:2, ChCl:Mal = 4:1, ChCl:Glu = 1:1, ChCl:Suc = 1:1 | Anthocyanin | [79] |
| No. | Vegetable Waste and By-Products | Extraction Technique | NADES Composition | Target Analyte | Reference |
|---|---|---|---|---|---|
| 1. | Waste onion | Stirring with a magnetic stirrer | ChCl:U = 1:2, ChCl:Gly = 1:2, CA:Glu = 1:1, CA:Fru = 1:1, Le:U = 1:2, Le:Gly = 1:2. | Quercetin | [80] |
| 2. | Tomato pomace | UAE | ChCl:1,2- P-1,2-D = 1:2, ChCl:LA = 1:2 | Phenolic compounds | [81] |
| 3. | Rice bran | One-pot extraction | ChCl: P-1,2-D: water = 1:1:1, ChCl:LA = 1:10, ChCl:Xyl:water = 1:1:1 | Phenolics | [82] |
| 4. | Beta vulgaris L. var. rubra waste | MAE | ChCl:Fru:water = 2:5:5, ChCl:Gly = 1:2, ChCl:CA:water = 2:1:6, ChCl:U = 1:2, B:Fru:water = 1:1:5, B:Gly = 1:2, B:CA:water = 2:1:6, B:U = 1:2 | Bioactives | [83] |
| 5. | Olive pomace | HAE, MAE, UAE, or HHPAE | ChCl:CA, ChCl:LA, ChCl:Mal, ChCl:Gly, all in 1:2 ratio | Phenolic compounds | [84] |
| 6. | Bitter melon (Momordica charantia) | UAE | ChCl: Ace = 1:4.35 | Phenolic compounds (including gallic acid, chlorogenic acid, vanillic acid, epicatechin, and quercetin-3-glucoside) | [85] |
| 7. | Tobacco leaves | UAE | ChCl:U = 1:2 | [86] | |
| 8. | Tomato | Heated at 50 °C upon stirring (750 rpm) | Men: CapA, Me: LauA, Thy:CapA, Thy: LauA, CapA: LauA in ratios 1:1, 1:2, and 2:1 | Lycopene | [87] |
| 9. | Beetroot (Beta vulgaris) | Heated at 50 °C upon stirring (750 rpm) | MgCl2·6H2O and U in 1:1 and 2:1 ratios | Betalains | [88] |
| 10. | Tomato by-products | Solvent:solid 25:1, 90 min, 50 °C | Me: HeA = 2:1 | Carotenoids | [89] |
| 11. | Tomato skin | UAE | Me:Thy = 1:1 | Lycopene | [90] |
| 12. | Curcuma longa L | Constant stirring for 40 min | CA:Glu, MA:Glu, LA:Glu, all in 1:1 ratio | Curcumin | [91] |
| 13. | Onion and broccoli | UAE solid–liquid method | (Ery, Rib, Xyl, fuc, ChCl–Glul, Man, Gala, ChCl–Mal, and ChCl–GluA), and B-based NADESs with (B–Ery, rib, xyl, B–Fuc, B–Glu, B–Man, B–Gal, B–Mal, and B–GluA) | Quercetin | [92] |
| 14. | Waste broccoli leaves | UAE solid–liquid method | ChCl:MA = 1.5:1, ChCl:LA = 1:1, ChCl:Glu = 2:1, ChCl:OA = 1:1, ChCl: P-1,2-D = 1:2, ChCl: 1,3-B = 1:2, ChCl:Gly = 1:2, ChCl:CA = 3:1, ChCl:D-Sol = 1:1, ChCl:U = 1:2 | Phenolic compounds | [93] |
| 15. | Vegetable wastes | UAE and MAE | ChCl and Gly were prepared by stirring (200 rpm) the mixture of ChCl and Gly (mole ratio1:1, 1:2, 2:1) | Lignin | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristivojević, P.; Krstić Ristivojević, M.; Stanković, D.; Cvijetić, I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules 2024, 29, 4717. https://doi.org/10.3390/molecules29194717
Ristivojević P, Krstić Ristivojević M, Stanković D, Cvijetić I. Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules. 2024; 29(19):4717. https://doi.org/10.3390/molecules29194717
Chicago/Turabian StyleRistivojević, Petar, Maja Krstić Ristivojević, Dalibor Stanković, and Ilija Cvijetić. 2024. "Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective" Molecules 29, no. 19: 4717. https://doi.org/10.3390/molecules29194717
APA StyleRistivojević, P., Krstić Ristivojević, M., Stanković, D., & Cvijetić, I. (2024). Advances in Extracting Bioactive Compounds from Food and Agricultural Waste and By-Products Using Natural Deep Eutectic Solvents: A Circular Economy Perspective. Molecules, 29(19), 4717. https://doi.org/10.3390/molecules29194717





