β-Cyclodextrin-Modified Laser-Induced Graphene Electrode for Detection of N6-Methyladenosine in RNA
Abstract
:1. Introduction
2. Results and Discussions
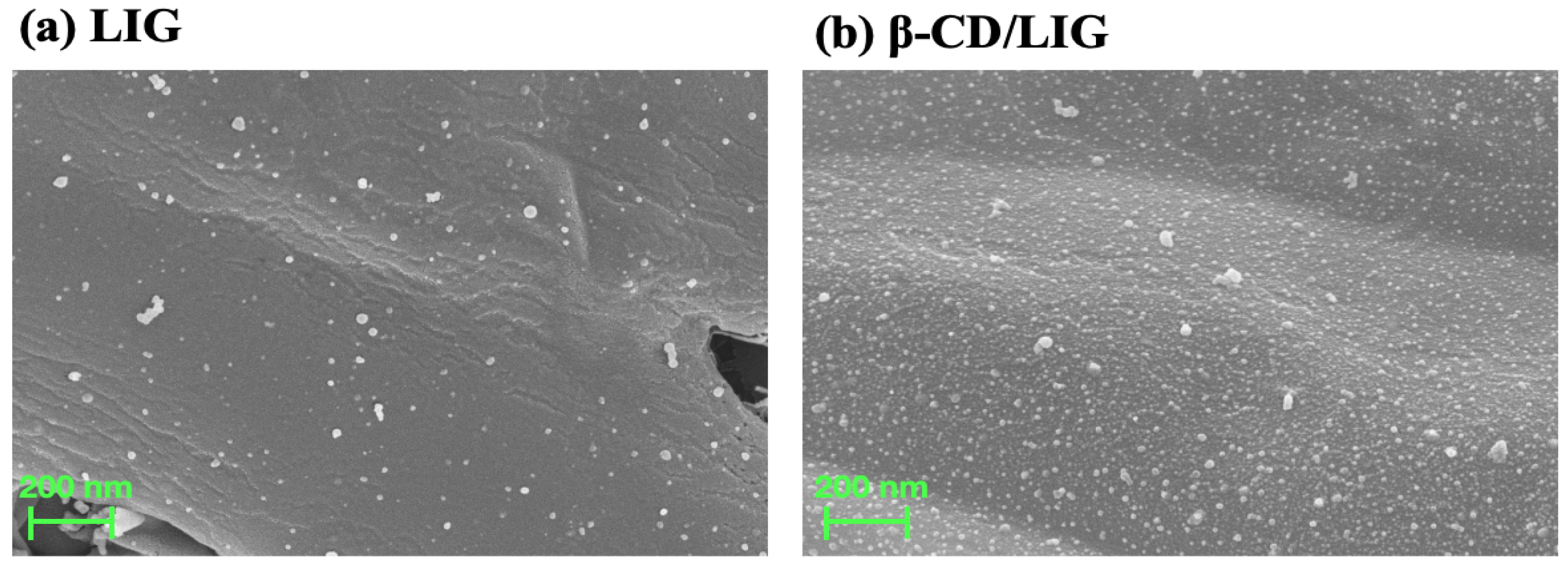
2.1. Characterization of Bare LIG and β-CD/LIG
2.2. Electrochemical Characterization
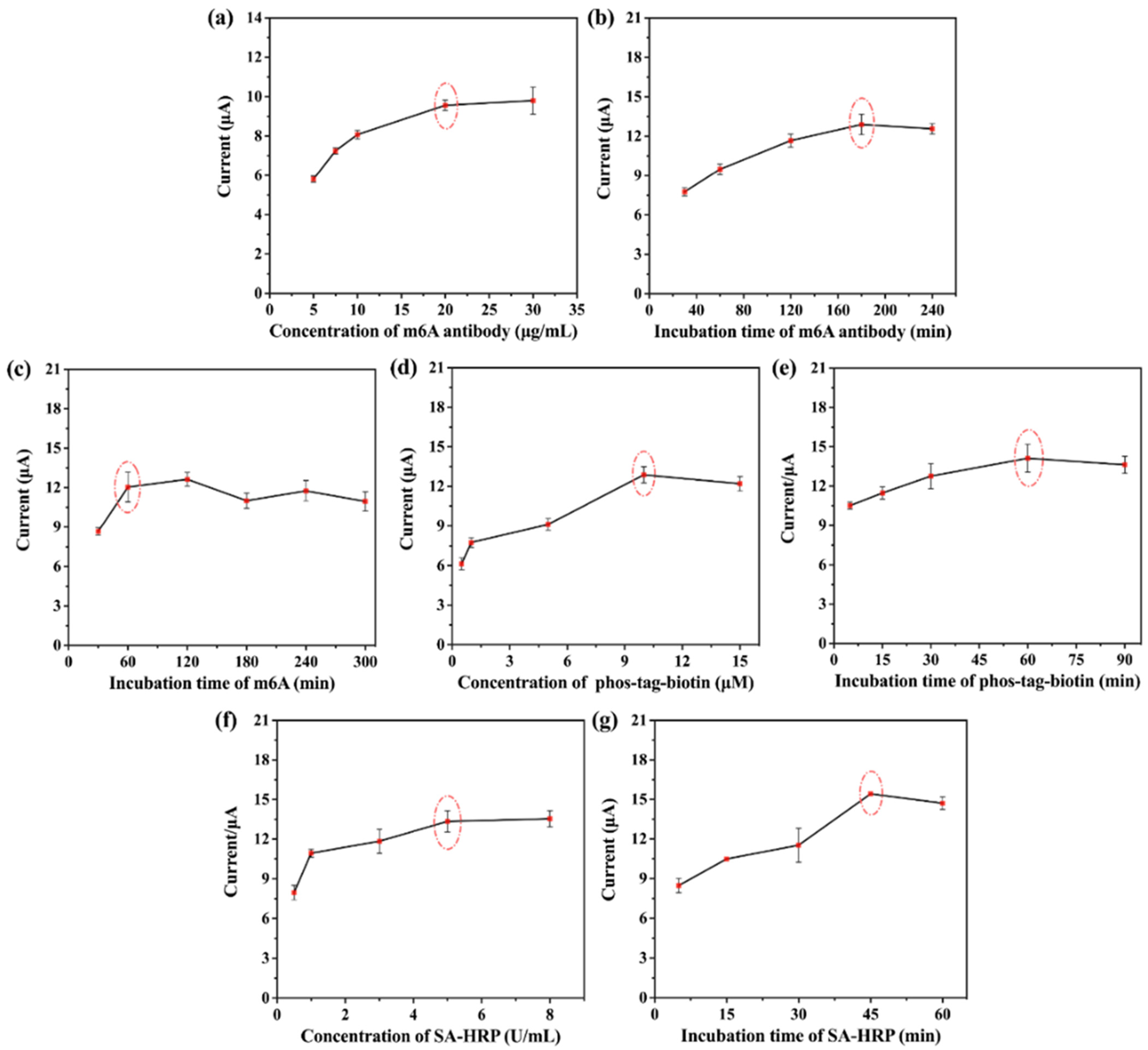
2.3. Optimization of Experimental Conditions
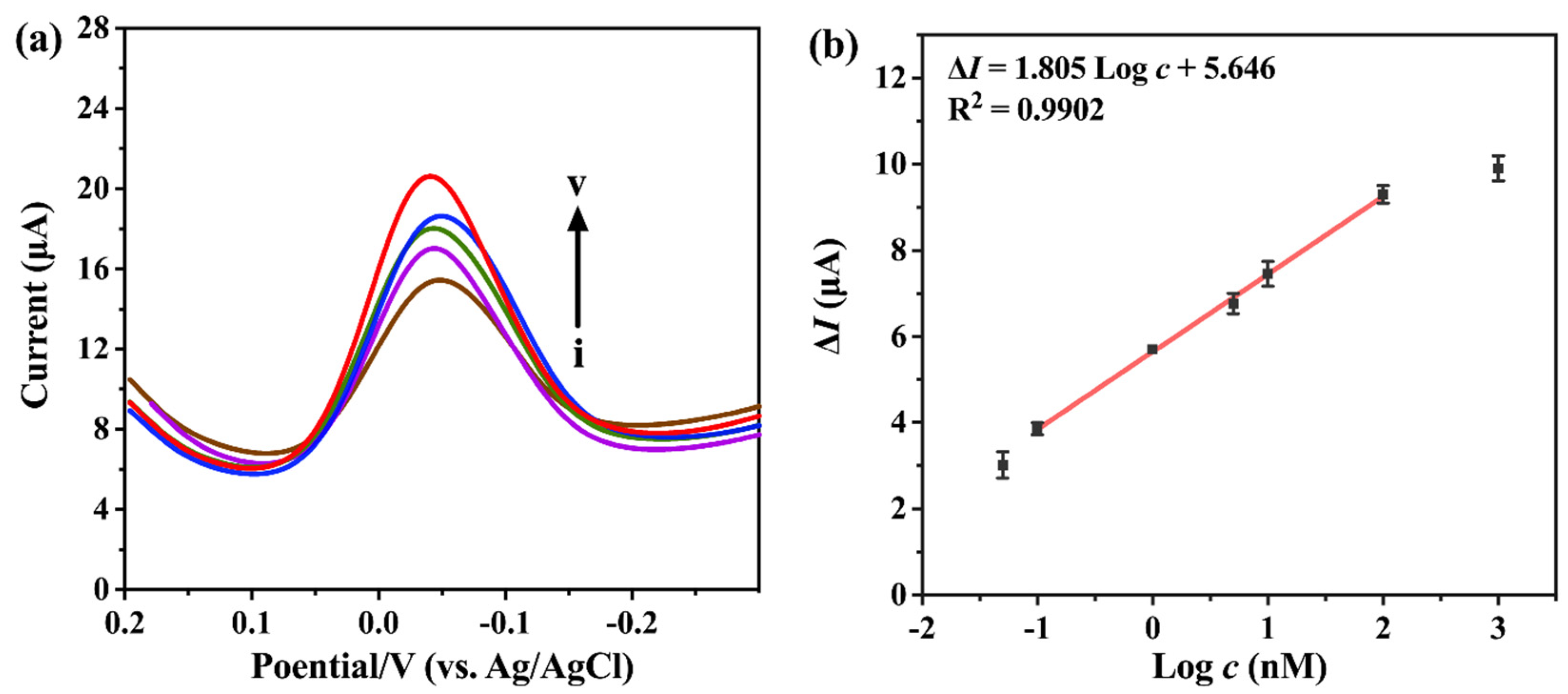
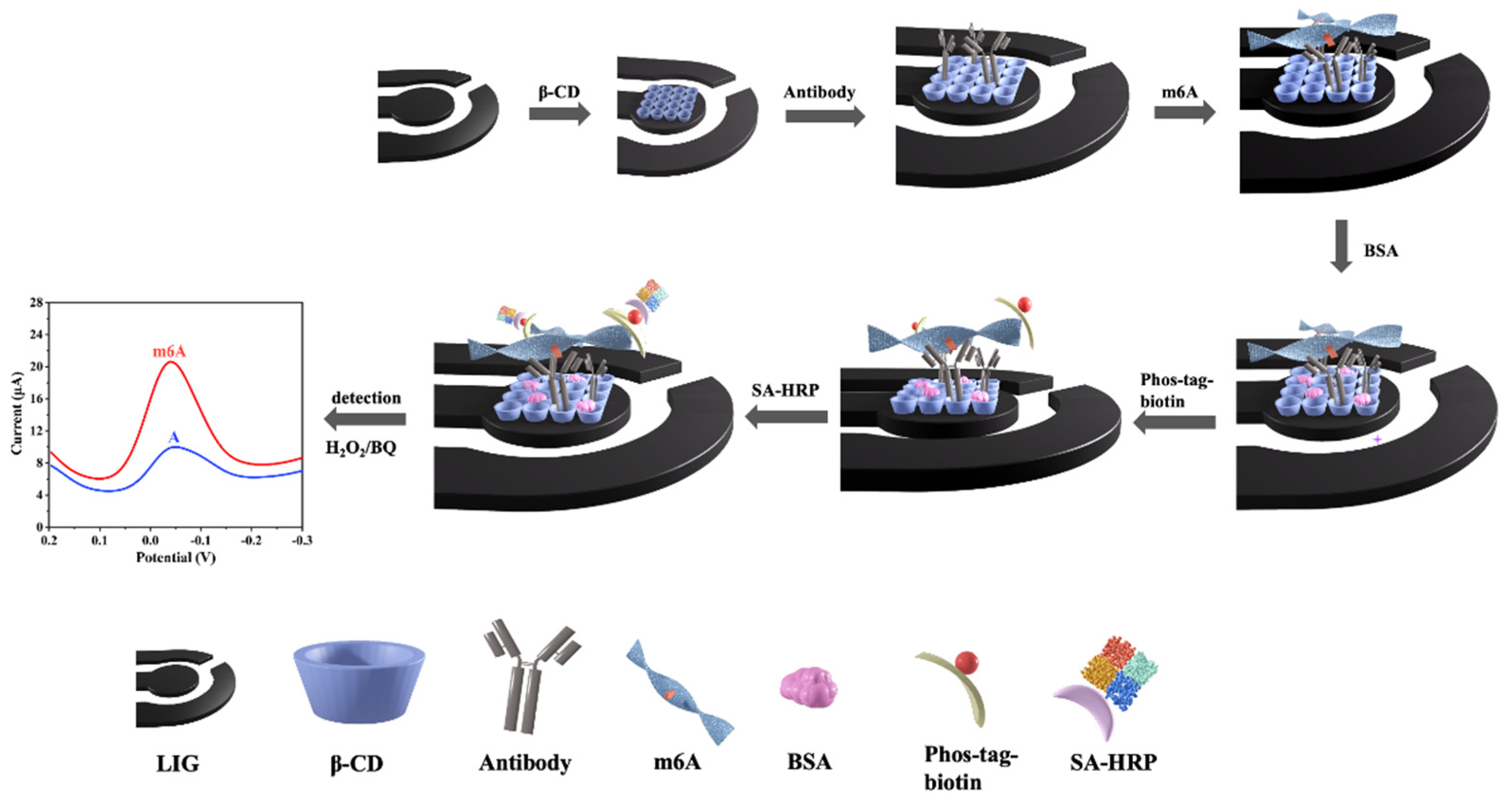
2.4. Electrochemical Analysis
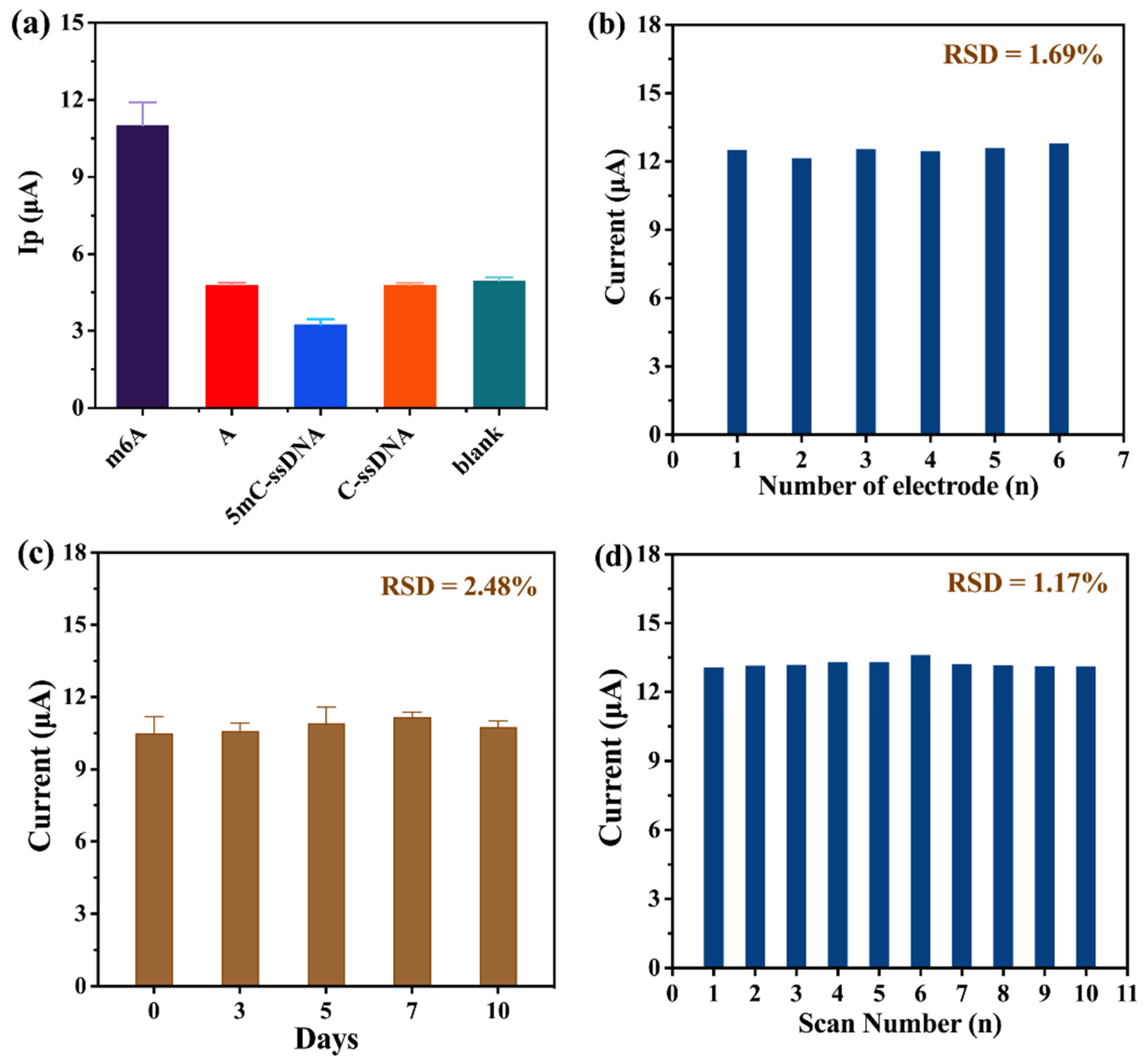
2.5. Specificity, Stability, and Repeatability
2.6. Applications in Spiked Samples
3. Experimental Section
3.1. Materials and Reagents
- m6A-RNA: 5′-GGACUGAGAGGAmCUGUCUGGGUGCCAAG-3′
- Unmethylated-RNA: 5′-GGACUGAGAGGACUGUCUGGGUGCCAAG-3′
3.2. Apparatus
3.3. Fabrication of LIG and β-CD/LIG
3.4. Assembly of Molecules on LIG
3.5. Electrochemical Detection of m6A
3.6. Total RNA Isolation and Purification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, J.; Peng, Z.W.; Liu, Y.Y.; Ruiz-Zepeda, F.; Ye, R.Q.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Lu, L.S.; Xie, Y.X.; Wang, W.T.; Lin, Z.R.; Tang, B.; Lin, N. Preparation of Laser-Induced Graphene Fabric from Silk and Its Application Examples for Flexible Sensor. Adv. Eng. Mater. 2021, 23, 2100195. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Shchadenko, S.; Murastov, G.; Lipovka, A.; Fatkullin, M.; Petrov, I.; Tran, T.H.; Khalelov, A.; Saqib, M.; Villa, N.E.; et al. Ultra-Robust Flexible Electronics by Laser-Driven Polymer-Nanomaterials Integration. Adv. Func. Mater. 2021, 31, 2008818. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, Y.; Zhang, P.P.; Liu, F.; Luo, S.D. Laser-Induced Freestanding Graphene Papers: A New Route of Scalable Fabrication with Tunable Morphologies and Properties for Multifunctional Devices and Structures. Small 2018, 14, 1802350. [Google Scholar] [CrossRef]
- Shi, X.Y.; Zhou, F.; Peng, J.X.; Wu, R.A.; Wu, Z.S.; Bao, X.H. One-Step Scalable Fabrication of Graphene-Integrated Micro-Supercapacitors with Remarkable Flexibility and Exceptional Performance Uniformity. Adv. Funct. Mater. 2019, 29, 1902860. [Google Scholar] [CrossRef]
- Lu, B.; Jin, X.T.; Han, Q.; Qu, L.T. Planar Graphene-Based Microsupercapacitors. Small 2021, 17, 2006827. [Google Scholar] [CrossRef]
- Lee, J.B.; Jang, J.N.; Zhou, H.Y.; Lee, Y.J.; In, J.B. Densified Laser-Induced Graphene for Flexible Microsupercapacitors. Energies 2020, 13, 6567. [Google Scholar] [CrossRef]
- Ren, M.Q.; Zhang, J.B.; Fan, M.M.; Ajayan, P.M.; Tour, J.M. Li-Breathing Air Batteries Catalyzed by MnNiFe/Laser-Induced Graphene Catalysts. Adv. Mater. Interfaces 2019, 6, 1901035. [Google Scholar] [CrossRef]
- Han, X.; Ye, R.Q.; Chyan, Y.; Wang, T.; Zhang, C.H.; Shi, L.L.; Zhang, T.; Zhao, Y.; Tour, J.M. Laser-Induced Graphene from Wood Impregnated with Metal Salts and Use in Electrocatalysis. ACS Appl. Nano Mater. 2018, 1, 5053–5061. [Google Scholar] [CrossRef]
- Alhajji, E.; Zhang, F.; Alshareef, H.N. Status and Prospects of Laser-Induced Graphene for Battery Applications. Energy Technol. 2021, 9, 2100454. [Google Scholar] [CrossRef]
- Yi, J.S.; Chen, J.H.; Yang, Z.; Dai, Y.; Li, W.M.; Cui, J.; Ciucci, F.; Lu, Z.H.; Yang, C.L. Facile Patterning of Laser-Induced Graphene with Tailored Li Nucleation Kinetics for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1901796. [Google Scholar] [CrossRef]
- Nasraoui, S.; Al-Hamry, A.; Teixeira, P.R.; Ameur, S.; Paterno, L.G.; Ben Ali, M.; Kanoun, O. Electrochemical sensor for nitrite detection in water samples using flexible laser-induced graphene electrodes functionalized by CNT decorated by Au nanoparticles. J. Electroanal. Chem. 2021, 880, 114893. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Hu, C.G. Laser-Scribed Graphene Sensors on Nail Polish with Tunable Composition for Electrochemical Detection of Nitrite and Glucose. Sens. Actuators B-Chem. 2022, 357, 131394. [Google Scholar] [CrossRef]
- Nam, K.H.; Abdulhafez, M.; Castagnola, E.; Tomaraei, G.N.; Cui, X.T.; Bedewy, M. Laser Direct Write of Heteroatom-Doped Graphene on Molecularly Controlled Polyimides for Electrochemical Biosensors with Nanomolar Sensitivity. Carbon 2022, 188, 209–219. [Google Scholar] [CrossRef]
- Prabhakaran, A.; Nayak, P. Surface Engineering of Laser-Scribed Graphene Sensor Enables Non-Enzymatic Glucose Detection in Human Body Fluids. ACS Appl. Nano Mater. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.J.; Wang, D.B.; Zhang, P.; Wang, Y.Y.; Lu, S.; Xu, R.Q.; Zhao, J. A Flexible Non-Enzymatic Glucose Sensor Based on Copper Nanoparticles Anchored on Laser-Induced Graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Yoon, H.S.; Nah, J.S.; Kim, H.S.; Ko, S.G.; Sharifuzzaman, M.; Barman, S.C.; Xuan, X.; Kim, J.Y.; Park, J.Y. A Chemically Modified Laser-Induced Porous Graphene Based Flexible and Ultrasensitive Electrochemical Biosensor for Sweat Glucose Detection. Sens. Actuator B-Chem. 2020, 311, 127866. [Google Scholar] [CrossRef]
- Rauf, S.; Lahcen, A.A.; Aljedaibi, A.; Beduk, T.; Ilton de Oliveira Filho, J.; Salama, K.N. Gold Nanostructured Laser-Scribed Graphene: A New Electrochemical Biosensing Platform for Potential Point-Of-Care Testing of Disease Biomarkers. Biosens. Bioelectron. 2021, 180, 113116. [Google Scholar] [CrossRef]
- Wan, Z.F.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.T.; Trinchi, A.; Shiddiky, M.J.A.; Gao, Y.S.; Li, Q. Laser Induced Self-N-Doped Porous Graphene as an Electrochemical Biosensor for Femtomolar miRNA Detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Li, Q.; Wang, W.J.; Wen, Y.P.; Ji, K.X.; Liu, X.X.; He, P.P.; Campos Janegitz, B.; Tang, K.J. The Fabrication of a Flexible and Portable Sensor Based on Home-Made Laser-Induced Porous Graphene Electrode for the Rapid Detection of Sulfonamides. Microchem. J. 2022, 182, 107898. [Google Scholar] [CrossRef]
- Wanjari, V.P.; Reddy, A.S.; Duttagupta, S.P.; Singh, S.P. Laser-induced graphene-based electrochemical biosensors for environmental applications: A perspective. Environ. Sci. Pollut. Res. Int. 2023, 30, 42643–42657. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Nguyen, N.-T.; Gao, Y.; Li, Q. Laser induced graphene for biosensors. Sustain. Mater. Technol. 2020, 25, e00205. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.K.; Zhao, Y.L.; Yang, Y.; Yang, Y.G. RNA Methylations in Human Cancers. Semin. Cancer Biol. 2021, 75, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhao, R.; Meng, W.; Liao, Y. Effects and translatomics characteristics of a small-molecule inhibitor of METTL3 against non-small cell lung cancer. J. Pharm. Anal. 2023, 13, 625–639. [Google Scholar] [CrossRef]
- Yang, B.C.; Wang, J.Q.; Yao, T.; Yuan, R.Z.; Chen, Z.S.; Zou, C. RNA Methylation and Cancer Treatment. Pharmacol. Res. 2021, 174, 105937. [Google Scholar] [CrossRef]
- Huang, H.L.; Weng, H.Y.; Sun, W.J.; Qin, X.; Shi, H.L.; Wu, H.Z.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N(6)-Methyladenosine by IGF2BP Proteins Enhances mRNA Stability and Translation. Nat. Cell Biol. 2018, 20, 285–295. [Google Scholar] [CrossRef]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.S.; Hao, Y.J.; Sun, B.F.; Sun, H.Y.; Li, A.; Ping, X.L.; Lai, W.Y.; et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, B.F.; Xiao, W.; Yang, X.; Sun, H.Y.; Zhao, Y.L.; Yang, Y.G. Dynamic m6A Modification and its Emerging Regulatory Role in mRNA Splicing. Sci. Bull. 2015, 60, 21–32. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.K.; Han, D.L.; Ma, H.H.; Weng, X.C.; Chen, K.; Shi, H.L.; He, C. N(6)-Methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef]
- Choi, J.H.; Ieong, K.W.; Demirci, H.; Chen, J.; Petrov, A.; Prabhakar, A.; O’Leary, S.E.; Dominissini, D.; Rechavi, G.; Soltis, S.M.; et al. N(6)-Methyladenosine in mRNA Disrupts tRNA Selection and Translation-Elongation Dynamics. Nat. Struct. Mol. Biol. 2016, 23, 110–115. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the Human and Mouse M6A RNA Methylomes Revealed by M6A-Seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Chen, X.; Xia, L.P.; Zhang, J.X.; Pan, Z.Z.; Ma, X.D.; Han, K.; Chen, J.W.; Judde, J.G.; Deas, O.; et al. N(6)-Methyladenosine Modification of circNSUN2 Facilitates Cytoplasmic Export and Stabilizes HMGA2 to Promote Colorectal Liver Metastasis. Nat. Commun. 2019, 10, 4695. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.P.; Pickering, B.F.; Cheng, Y.M.; Zaccara, S.; Nguyen, D.; Minuesa, G.; Chou, T.; Chow, A.; Saletore, Y.; MacKay, M.; et al. The N(6)-Methyladenosine (m(6)A)-forming Enzyme METTL3 Controls Myeloid Differentiation of Normal Hematopoietic and Leukemia Cells. Nat. Med. 2017, 23, 1369–1376. [Google Scholar] [CrossRef]
- Lin, X.Y.; Chai, G.S.; Wu, Y.M.; Li, J.X.; Chen, F.; Liu, J.Z.; Luo, G.Z.; Tauler, J.; Du, J.; Lin, S.B.; et al. RNA m(6)A Methylation Regulates the Epithelial Mesenchymal Transition of Cancer Cells and Translation of Snail. Nat. Commun. 2019, 10, 2065. [Google Scholar] [CrossRef]
- Li, Y.K.; Niu, C.X.; Wang, N.; Huang, X.; Cao, S.R.; Cui, S.J.; Chen, T.Y.; Huo, X.R.; Zhou, R.M. The Role of m(6)A Modification and m(6)A Regulators in Esophageal Cancer. Cancers 2022, 14, 5139. [Google Scholar] [CrossRef]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3′ UTRs and Near Stop Codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef]
- Huang, W.; Qi, C.B.; Lv, S.W.; Xie, M.; Feng, Y.Q.; Huang, W.H.; Yuan, B.F. Determination of DNA and RNA Methylation in Circulating Tumor Cells by Mass Spectrometry. Anal. Chem. 2016, 88, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.H.; Wang, Y.G.; Hu, Y.N.; Feng, Y.; Dai, C.G.; Wu, J.X.; Wu, D.M.; Zhang, F.; Zhai, Q.W. A High-Throughput Quantitative Approach Reveals More Small RNA Modifications in Mouse Liver and Their Correlation with Diabetes. Anal. Chem. 2013, 85, 12173–12181. [Google Scholar] [CrossRef]
- Linder, B.; Grozhik, A.V.; Olarerin-George, A.O.; Meydan, C.; Mason, C.E.; Jaffrey, S.R. Single-Nucleotide-Resolution Mapping of m6A and m6Am Throughout the Transcriptome. Nat. Methods 2015, 12, 767–772. [Google Scholar] [CrossRef]
- Dai, T.; Pu, Q.L.; Guo, Y.C.; Zuo, C.; Bai, S.L.; Yang, Y.J.; Yin, D.; Li, Y.; Sheng, S.C.; Tao, Y.Y.; et al. Analogous Modified DNA Probe and Immune Competition Method-Based Electrochemical Biosensor for RNA Modification. Biosens. Bioelectron. 2018, 114, 72–77. [Google Scholar] [CrossRef]
- Pu, Q.L.; Ye, Y.Y.; Hu, J.; Xie, C.; Zhou, X.; Yu, H.Y.; Liao, F.L.; Jiang, S.; Jiang, L.S.; Xie, G.M.; et al. XNA Probe and CRISPR/Cas12a-Powered Flexible Fluorescent and Electrochemical Dual-Mode Biosensor for Sensitive Detection of m6A Site-Specific RNA Modification. Talanta 2023, 252, 123754. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, B.C.; Yin, H.S.; Zhang, Q.H.; Wang, H.Y.; Fan, H.; Ai, S.Y. Electrochemical Immunosensor Based on Hairpin DNA Probe for Specific Detection of N6-Methyladenosine RNA. J. Electroanal. Chem. 2017, 804, 192–198. [Google Scholar] [CrossRef]
- Povedano, E.; Gamella, M.; Torrente-Rodriguez, R.M.; Ruiz-Valdepenas Montiel, V.; Montero-Calle, A.; Solis-Fernandez, G.; Navarro-Villoslada, F.; Pedrero, M.; Pelaez-Garcia, A.; Mendiola, M.; et al. Multiplexed Magnetic Beads-Assisted Amperometric Bioplatforms for Global Detection of Methylations in Nucleic Acids. Anal. Chim. Acta 2021, 1182, 338946. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Wang, Y.F.; Tang, J.; Wang, F.; Chen, Z.L. End-Labeling-Based Electrochemical Strategy for Detection of Adenine Methylation in Nucleic Acid by Differential Pulse Voltammetry. Microchim. Acta 2021, 188, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Zhu, L.; Li, J.; Liu, Y.; Luo, J.; Xie, T.; Chen, D. Tumor cell membrane-coated continuous electrochemical sensor for GLUT1 inhibitor screening. J. Pharm. Anal. 2023, 13, 673–682. [Google Scholar] [CrossRef]
- Yin, H.S.; Zhou, Y.L.; Yang, Z.Q.; Guo, Y.L.; Wang, X.X.; Ai, S.Y.; Zhang, X.S. Electrochemical Immunosensor for N6-Methyladenosine RNA Modification Detection. Sens. Actuators B-Chem. 2015, 221, 1–6. [Google Scholar] [CrossRef]
- Yin, H.S.; Wang, H.Y.; Jiang, W.J.; Zhou, Y.L.; Ai, S.Y. Electrochemical Immunosensor for N6-Methyladenosine Detection in Human Cell Lines Based on Biotin-Streptavidin System and Silver-SiO2 Signal Amplification. Biosens. Bioelectron. 2017, 90, 494–500. [Google Scholar] [CrossRef]
- Ou, X.Y.; Pu, Q.L.; Sheng, S.C.; Dai, T.; Gou, D.; Yu, W.; Yang, T.Y.; Dai, L.; Yang, Y.J.; Xie, G.M. Electrochemical Competitive Immunodetection of Messenger RNA Modified with N6-Methyladenosine by Using DNA-Modified Mesoporous PtCo Nanospheres. Microchim. Acta 2020, 187, 31. [Google Scholar] [CrossRef]
- Kinoshita, E.; Kinoshita-Kikuta, E.; Koike, T. History of Phos-tag Technology for Phosphoproteomics. J. Proteom. 2022, 252, 104432. [Google Scholar] [CrossRef]
- Wang, F.C.; Wang, K.D.; Dong, X.; Mei, X.S.; Zhai, Z.Y.; Zheng, B.X.; Lv, J.; Duan, W.Q.; Wang, W.J. Formation of Hierarchical Porous Graphene Films with Defects Using a Nanosecond Laser on Polyimide Sheet. Appl. Surf. Sci. 2017, 419, 893–900. [Google Scholar] [CrossRef]
- Castillo, G.; Lamberti, I.; Mosiello, L.; Hianik, T. Impedimetric DNA Aptasensor for Sensitive Detection of Ochratoxin A in Food. Electroanalysis 2012, 24, 512–520. [Google Scholar] [CrossRef]
- Sardaremelli, S.; Hasanzadeh, M.; Razmi, H. Chemical binding of horseradish peroxidase enzyme with poly beta-cyclodextrin and its application as molecularly imprinted polymer for the monitoring of H2O2 in human plasma samples. J. Mol. Recognit. 2021, 34, e2884. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Oliveira, A.E.F.; Bettio, G.B. β-Cyclodextrin electropolymerization: Mechanism, electrochemical behavior, and optimization. Chem. Pap. 2019, 73, 1795–1804. [Google Scholar] [CrossRef]
- Wang, L.K.; Shuman, S. Domain Structure and Mutational Analysis of T4 Polynucleotide Kinase. J. Biol. Chem. 2001, 276, 26868–26874. [Google Scholar] [CrossRef]


| Added (ng/µL) | Acquired (ng/µL) | Recovery (%) | RSD (%, N = 3) |
|---|---|---|---|
| 0.05 | 0.05 ± 0.002 | 101.59 | 4.87 |
| 0.25 | 0.25 ± 0.025 | 100.42 | 9.84 |
| 0.5 | 0.52 ± 0.038 | 104.01 | 7.23 |
| 0.75 | 0.78 ± 0.074 | 104.08 | 9.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Zhao, M.; Kuang, X.; Chen, Z.; Wang, F. β-Cyclodextrin-Modified Laser-Induced Graphene Electrode for Detection of N6-Methyladenosine in RNA. Molecules 2024, 29, 4718. https://doi.org/10.3390/molecules29194718
Guo J, Zhao M, Kuang X, Chen Z, Wang F. β-Cyclodextrin-Modified Laser-Induced Graphene Electrode for Detection of N6-Methyladenosine in RNA. Molecules. 2024; 29(19):4718. https://doi.org/10.3390/molecules29194718
Chicago/Turabian StyleGuo, Jingyi, Mei Zhao, Xia Kuang, Zilin Chen, and Fang Wang. 2024. "β-Cyclodextrin-Modified Laser-Induced Graphene Electrode for Detection of N6-Methyladenosine in RNA" Molecules 29, no. 19: 4718. https://doi.org/10.3390/molecules29194718
APA StyleGuo, J., Zhao, M., Kuang, X., Chen, Z., & Wang, F. (2024). β-Cyclodextrin-Modified Laser-Induced Graphene Electrode for Detection of N6-Methyladenosine in RNA. Molecules, 29(19), 4718. https://doi.org/10.3390/molecules29194718







