Pitfalls in Photochemical and Photoelectrochemical Reduction of CO2 to Energy Products
Abstract
:1. Introduction
2. Analysis of Potential Sources of False-Positive Results and Quantification of Their Relevance
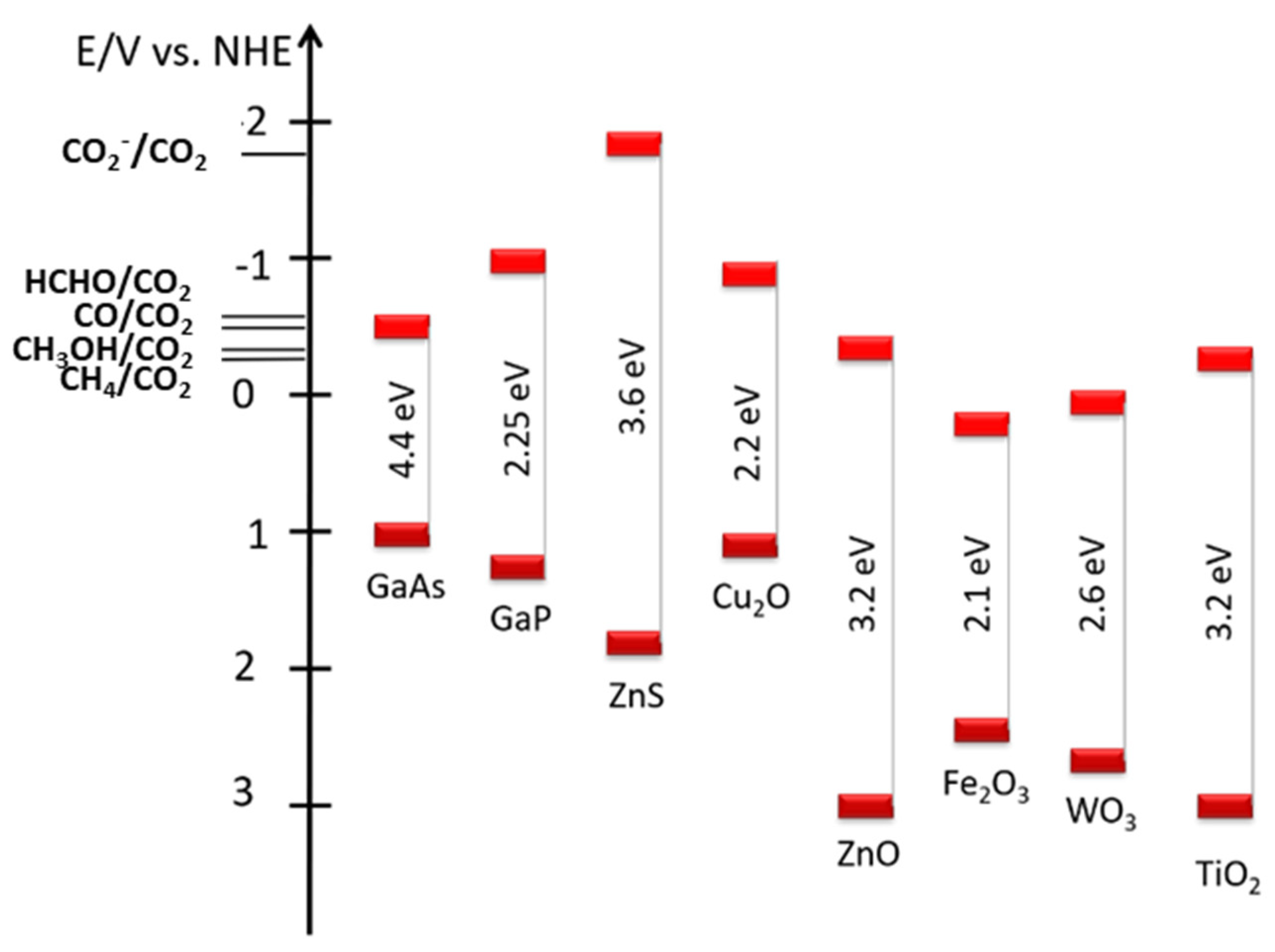
2.1. Degradation of Carbon-Containing Photocatalysts
2.2. Contaminants on a Photocatalyst
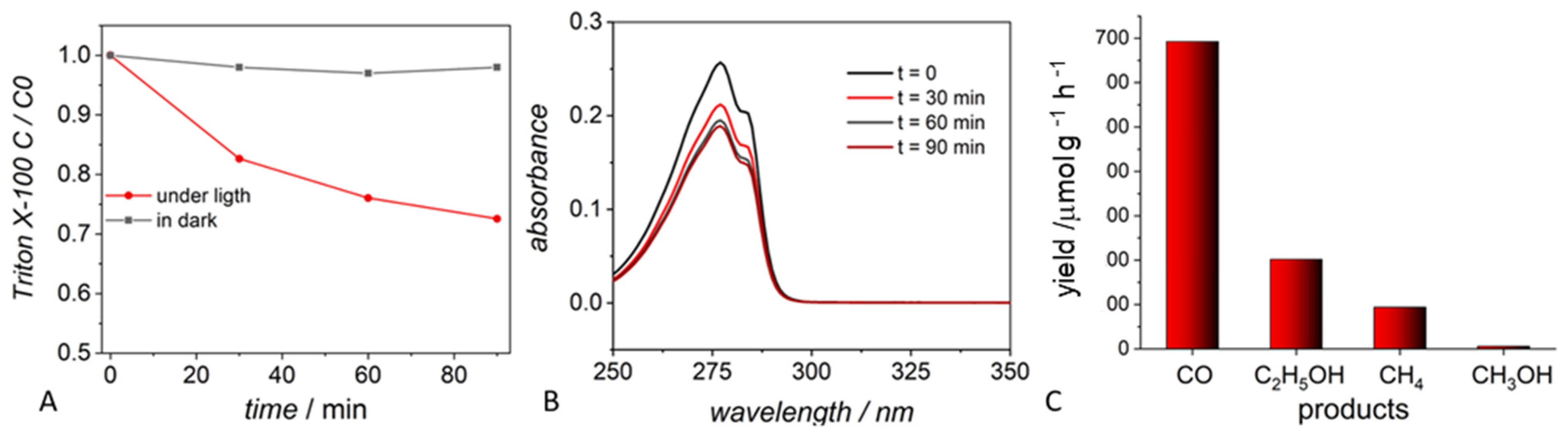
2.2.1. Surfactants
2.2.2. Ionic Liquids
2.2.3. Solvents
2.3. Decomposition of Sacrificial Reagents or Reaction Additives
2.4. Contaminants from Other System Components, Including the Photoreactor or Laboratory Glassware, Equipment, and Materials Used, Such as Pipettes, Syringes, Vials, and Filtration Materials
2.5. Airborne Contaminants within the Laboratory Environment
2.6. The Operator
3. Good Practices in CO2 Reduction
3.1. Detection and Elimination of Possible External Carbon Sources and Identification of Contaminants
- Acid or base etching.
- Ultrasonic cleaning.
- Solvent (water) washing or sequential washing using a series of different solvents to target a broader range of contaminants. Residual organic solvents must then be carefully eliminated.
- UV–ozone cleaning.
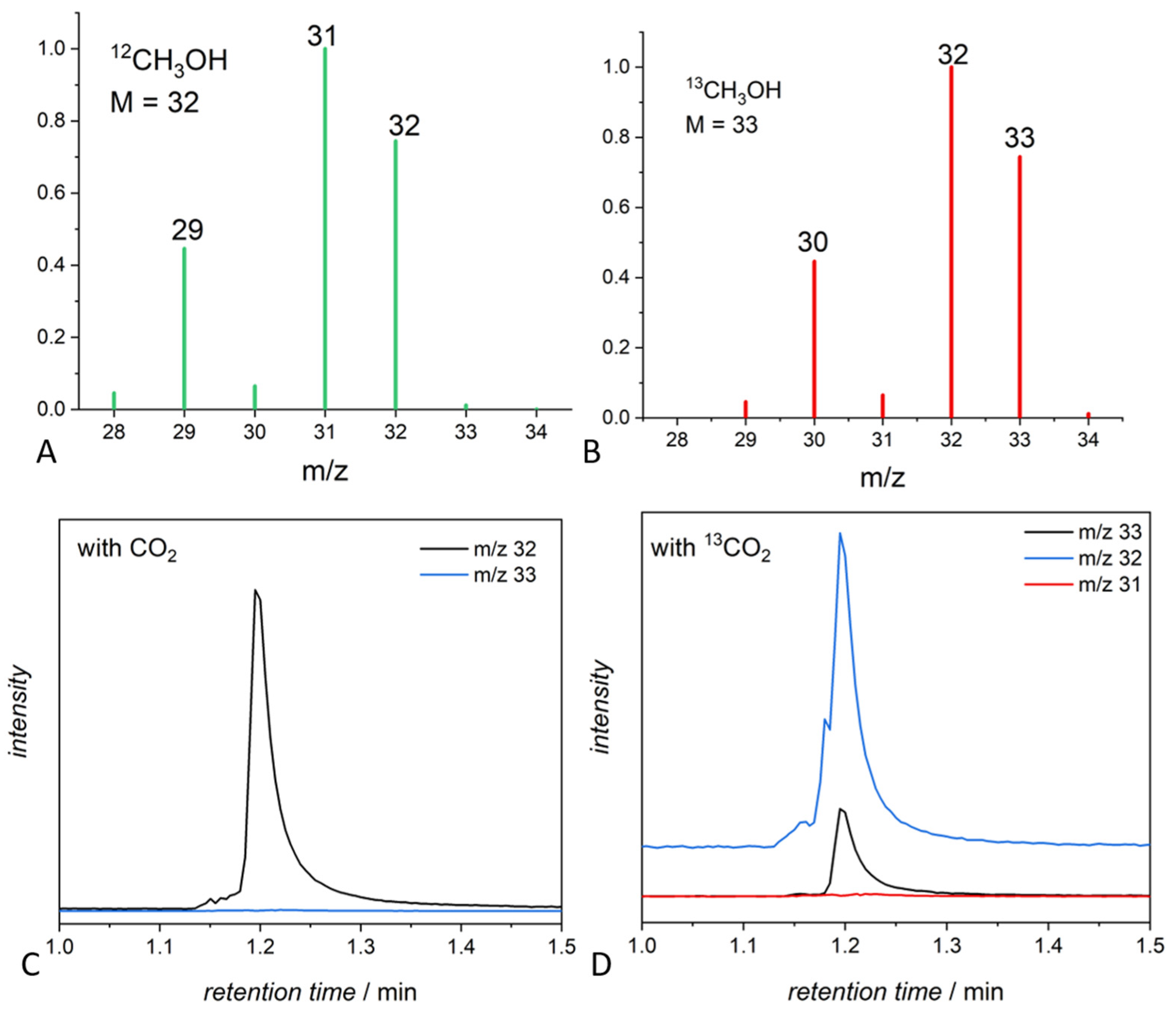
3.2. Experiments with Labeled 13CO2
3.3. Blank Test Importance
3.4. Good Practice in Analytical Procedures
- Ensuring clean carrier/makeup gas and delivery tubing.
- Using clean solvents.
- Properly washing the syringe with proper solvents to prevent cross-contamination between samples.
- Checking the inlet liner/septum, column, and detector plumbing and base weldment for cleanliness.
4. Materials and Methods
4.1. Synthesis of Materials
4.1.1. Synthesis of Rutin@TiO2
4.1.2. Synthesis of SrTiO3
4.1.3. Synthesis of ZnS Nanoparticles
4.1.4. Synthesis of the CuZnOx Photocatalyst
4.1.5. Preparation of g-C3N4 Thin Film on a Glass Plate
4.2. Photocatalytic Test
4.2.1. Degradation of Triton X-100
4.2.2. Blank Test on Photocatalysts in the Absence of CO2
4.2.3. Photocatalytic Degradation of an Ionic Liquid
4.2.4. Photoelectrochemical Tests
4.3. Materials Characterization, Irradiation Experiments, and GC Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A. Circular Economy and Carbon Dioxide Conversion. In The Carbon Dioxide Revolution: Challenges and Perspectives for a Global Society; Aresta, M., Dibenedetto, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 139–175. [Google Scholar] [CrossRef]
- Summary of Global Climate Action at COP 28. Available online: https://unfccc.int/sites/default/files/resource/Summary_GCA_COP28.pdf (accessed on 30 August 2024).
- Sabri, M.A.; Al Jitan, S.; Bahamon, D.; Vega, L.F.; Palmisano, G. Current and future perspectives on catalytic-based integrated carbon capture and utilization. Sci. Total Environ. 2021, 790, 148081. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A. Merging the Green-H2 production with Carbon Recycling for stepping towards the Carbon Cyclic Economy. J. CO2 Util. 2024, 80, 102688. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Zinc sulfide functionalized with ruthenium nanoparticles for photocatalytic reduction of CO2. Appl. Catal. B Environ. 2015, 178, 170–176. [Google Scholar] [CrossRef]
- Baran, T.; Visibile, A.; Busch, M.; He, X.; Wojtyla, S.; Rondinini, S.; Minguzzi, A.; Vertova, A. Copper Oxide-Based Photocatalysts and Photocathodes: Fundamentals and Recent Advances. Molecules 2021, 26, 7271. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J.; Jaroniec, M. Carbon-based two-dimensional layered materials for photocatalytic CO2 reduction to solar fuels. Energy Storage Mater. 2016, 3, 24–35. [Google Scholar] [CrossRef]
- Neaţu, Ş.; Maciá-Agulló, J.A.; Garcia, H. Solar light photocatalytic CO2 reduction: General considerations and selected bench-mark photocatalysts. Int. J. Mol. Sci. 2014, 15, 5246–5262. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, P.; Khan, A.A.P.; Le, Q.V.; Nguyen, V.-H.; Thakur, S.; Raizada, P. CO2 photoreduction into solar fuels via vacancy engineered bismuth-based photocatalysts: Selectivity and mechanistic insights. Chem. Eng. J. 2022, 439, 135563. [Google Scholar] [CrossRef]
- Sohail, M.; Anwar, U.; Taha, T.A.; Qazi, H.I.A.; Al-Sehemi, A.G.; Ullah, S.; Algarni, H.; Ahmed, I.M.; Amin, M.A.; Palamanit, A.; et al. Nanostructured materials based on g-C3N4 for enhanced photocatalytic activity and potentials application: A review. Arab. J. Chem. 2022, 15, 104070. [Google Scholar] [CrossRef]
- Luo, T.; Gilmanova, L.; Kaskel, S. Advances of MOFs and COFs for photocatalytic CO2 reduction, H2 evolution and organic redox transformations. Coord. Chem. Rev. 2023, 490, 215210. [Google Scholar] [CrossRef]
- Sadanandan, A.M.; Yang, J.-H.; Devtade, V.; Singh, G.; Dharmarajan, N.P.; Fawaz, M.; Lee, J.M.; Tavakkoli, E.; Jeon, C.-H.; Kumar, P.; et al. Carbon nitride based nanoarchitectonics for nature-inspired photocatalytic CO2 reduction. Prog. Mater. Sci. 2024, 142, 101242. [Google Scholar] [CrossRef]
- Strunk, J. Separating fiction from fact for photocatalytic CO2 reduction. Nat. Chem. 2023, 15, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, Q.; Xu, C.; Zhao, D.; Zhu, Q.; Zhu, Z.; Wang, J.; Liu, C.; Yu, H.; Sun, C.; et al. Current dilemma in photocatalytic CO2 reduction: Real solar fuel production or false positive outcomings? Carbon Neutrality 2022, 1, 10. [Google Scholar] [CrossRef]
- Yang, C.-C.; Yu, Y.-H.; van der Linden, B.; Wu, J.C.S.; Mul, G. Artificial Photosynthesis over Crystalline TiO2-Based Catalysts: Fact or Fiction? J. Am. Chem. Soc. 2010, 132, 8398–8406. [Google Scholar] [CrossRef]
- Grigioni, I.; Dozzi, M.V.; Bernareggi, M.; Chiarello, G.L.; Selli, E. Photocatalytic CO2 reduction vs. H2 production: The effects of surface carbon-containing impurities on the performance of TiO2-based photocatalysts. Catal. Today 2017, 281, 214–220. [Google Scholar] [CrossRef]
- You, J.; Xiao, M.; Liu, S.; Lu, H.; Chen, P.; Jiang, Z.; Shangguan, W.; Wang, Z.; Wang, L. How carbon contamination on the photocatalysts interferes with the performance analysis of CO2 reduction. J. Mater. Chem. A 2023, 11, 10149–10154. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, D.; Xia, B.; Jaroniec, M.; Ran, J.; Qiao, S.-Z. Photocatalytic CO2 Reduction: Identification and Elimination of False-Positive Results. ACS Energy Lett. 2022, 7, 1611–1617. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, B.; Henzie, J.; Xu, F.; Liu, C.; Meng, X.; Zou, S.; Song, H.; Pan, Y.; Li, H.; et al. Designing reliable and accurate isotope-tracer experiments for CO2 photoreduction. Nat. Commun. 2023, 14, 2534. [Google Scholar] [CrossRef]
- Ali, S.; Flores, M.C.; Razzaq, A.; Sorcar, S.; Hiragond, C.B.; Kim, H.R.; Park, Y.H.; Hwang, Y.; Kim, H.S.; Kim, H.; et al. Gas Phase Photocatalytic CO2 Reduction, “A Brief Overview for Benchmarking”. Catalysts 2019, 9, 727. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Shoji, S.; Yamaguchi, A.; Sakai, E.; Miyauchi, M. Strontium Titanate Based Artificial Leaf Loaded with Reduction and Oxidation Cocatalysts for Selective CO2 Reduction Using Water as an Electron Donor. ACS Appl. Mater. Interfaces 2017, 9, 20613–20619. [Google Scholar] [CrossRef]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Garcia, H. Photocatalytic CO2 reduction using non-itanium metal oxides and sulfides. ChemSusChem 2013, 6, 562–577. [Google Scholar] [CrossRef]
- Baran, T.; Wojtyła, S.; Dibenedetto, A.; Aresta, M.; Macyk, W. Photocatalytic Carbon Dioxide Reduction at p-Type Copper(I) Iodide. ChemSusChem 2016, 9, 2933–2938. [Google Scholar] [CrossRef]
- Khaidar, D.M.; Isahak, W.N.R.W.; Ramli, Z.A.C.; Ahmad, K.N. Transition metal dichalcogenides-based catalysts for CO2 conversion: An updated review. Int. J. Hydrogen Energy 2024, 68, 35–50. [Google Scholar] [CrossRef]
- Alshamkhani, M.T.; Teong, L.K.; Putri, L.K.; Mohamed, A.R.; Lahijani, P.; Mohammadi, M. Effect of graphite exfoliation routes on the properties of exfoliated graphene and its photocatalytic applications. J. Environ. Chem. Eng. 2021, 9, 106506. [Google Scholar] [CrossRef]
- Domingo-Tafalla, B.; Martínez-Ferrero, E.; Franco, F.; Palomares-Gil, E. Applications of Carbon Dots for the Photocatalytic and Electrocatalytic Reduction of CO2. Molecules 2022, 27, 1081. [Google Scholar] [CrossRef]
- Sinopoli, A.; La Porte, N.T.; Wasielewski, M.R.; Sohail, M. Photosensitisers for CO2 photoreduction: From metal complexes to rylenes, an overview. ACS Symp. Ser. 2018, 1311, 82–124. [Google Scholar] [CrossRef]
- Bizzarri, C. Homogeneous Systems Containing Earth-Abundant Metal Complexes for Photoactivated CO2 Reduction: Recent Advances. Eur. J. Org. Chem. 2022, 2022, e202200185. [Google Scholar] [CrossRef]
- Cheong, H.-Y.; Kim, S.-Y.; Cho, Y.-J.; Cho, D.W.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Photosensitization Behavior of Ir(III) Complexes in Selective Reduction of CO2 by Re(I)-Complex-Anchored TiO2 Hybrid Catalyst. Inorg. Chem. 2017, 56, 12042–12053. [Google Scholar] [CrossRef]
- Kumagai, H.; Tamaki, Y.; Ishitani, O. Photocatalytic Systems for CO2 Reduction: Metal-Complex Photocatalysts and Their Hybrids with Photofunctional Solid Materials. Acc. Chem. Res. 2022, 55, 978–990. [Google Scholar] [CrossRef]
- Ozcan, O.; Yukruk, F.; Akkaya, E.U.; Uner, D. Dye sensitized CO2 reduction over pure and platinized TiO2. Top. Catal. 2007, 44, 523–528. [Google Scholar] [CrossRef]
- Shizuno, M.; Kato, K.; Nishioka, S.; Kanazawa, T.; Saito, D.; Nozawa, S.; Yamakata, A.; Ishitani, O.; Maeda, K. Effects of a Nanoparticulate TiO2 Modifier on the Visible-Light CO2 Reduction Performance of a Metal-Complex/Semiconductor Hybrid Photocatalyst. ACS Appl. Energy Mater. 2022, 5, 9479–9486. [Google Scholar] [CrossRef]
- Buchalska, M.; Kuncewicz, J.; Świętek, E.; Łabuz, P.; Baran, T.; Stochel, G.; Macyk, W. Photoinduced hole injection in semiconductor-coordination compound systems. Coord. Chem. Rev. 2013, 257, 767–775. [Google Scholar] [CrossRef]
- Labuz, P.; Sadowski, R.; Stochel, G.; Macyk, W. Visible light photoactive titanium dioxide aqueous colloids and coatings. Chem. Eng. J. 2013, 230, 188–194. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Baran, T.; Angelini, A.; Labuz, P.; Macyk, W. An integrated photocatalytic/enzymatic system for the reduction of CO2 to methanol in bioglycerol-water. Beilstein J. Org. Chem. 2014, 10, 2556–2565. [Google Scholar] [CrossRef]
- Ramanathan, S.; Thamilselvan, A.; Radhika, N.; Padmanabhan, D.; Durairaj, A.; Obadiah, A.; Lydia, I.S.; Vasanthkumar, S. Development of rutin-rGO/TiO2 nanocomposite for electrochemical detection and photocatalytic removal of 2,4-DCP. J. Iran. Chem. Soc. 2021, 18, 2457–2472. [Google Scholar] [CrossRef]
- Marchal, C.; Behr, M.; Vigneron, F.; Caps, V.; Keller, V. Au/TiO2 photocatalysts prepared by solid grinding for artificial solar-light water splitting. New J. Chem. 2016, 40, 4428–4435. [Google Scholar] [CrossRef]
- Tsunoji, N.; Ide, Y.; Yagenji, Y.; Sadakane, M.; Sano, T. Design of Layered Silicate by Grafting with Metal Acetylacetonate for High Activity and Chemoselectivity in Photooxidation of Cyclohexane. ACS Appl. Mater. Interfaces 2014, 6, 4616–4621. [Google Scholar] [CrossRef]
- Habibi, M.H.; Askari, E. Spectrophotometric studies of photo-induced degradation of Tertrodirect Light Blue (TLB) using a nanostructure zinc zirconate composite. J. Ind. Eng. Chem. 2013, 19, 1400–1405. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, G.; Zhang, S. Fate and implication of acetylacetone in photochemical processes for water treatment. Water Res. 2016, 101, 233–240. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Ruan, Q.; Ohemeng, P.O.; Luo, B.; Jing, D.; Godin, R.; Tang, J. Polymer Photoelectrodes for Solar Fuel Production: Progress and Challenges. Chem. Rev. 2022, 122, 11778–11829. [Google Scholar] [CrossRef]
- Hidalgo, D.; Bocchini, S.; Fontana, M.; Saracco, G.; Hernández, S. Green and low-cost synthesis of PANI–TiO2 nanocomposite mesoporous films for photoelectrochemical water splitting. RSC Adv. 2015, 5, 49429–49438. [Google Scholar] [CrossRef]
- Mohammed, A.M.; Mohtar, S.S.; Aziz, F.; Aziz, M.; Ul-Hamid, A. Cu2O/ZnO-PANI ternary nanocomposite as an efficient photocatalyst for the photodegradation of Congo Red dye. J. Environ. Chem. Eng. 2021, 9, 105065. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Xie, A.; Qiu, L.; Li, S.; Wang, Y. Novel structure CuI/PANI nanocomposites with bifunctions: Superhydrophobicity and photocatalytic activity. J. Mater. Chem. 2011, 21, 9641. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Zhang, L.; Sun, S. Bi2WO6/PANI: An efficient visible-light-induced photocatalytic composite. Catal. Today 2014, 224, 147–153. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Shi, H.; Yu, K. Facile synthesis of p-type Cu2O/n-type ZnO nano-heterojunctions with novel photoluminescence properties, enhanced field emission and photocatalytic activities. Nanoscale 2012, 4, 7817–7824. [Google Scholar] [CrossRef]
- Bai, X.; Luan, J.; Song, T.; Sun, H.; Yan, B.; Dai, Y.; Yu, J. Polyvinyl alcohol/polyethyleneimine grafted carbon oxynitride composite nanofiber membranes with the synergistical enhanced photocatalytic degradation and bactericidal performance. J. Appl. Polym. Sci. 2024, 141, e55699. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Scotognella, F.; Waiskopf, N.; Kriegel, I.; Conte, S.D.; Cerullo, G.; Banin, U. Effect of Surface Coating on the Photocatalytic Function of Hybrid CdS–Au Nanorods. Small 2015, 11, 462–471. [Google Scholar] [CrossRef]
- Coralli, I.; Fabbri, D.; Facchin, A.; Torri, C.; Stevens, L.A.; Snape, C.E. Analytical pyrolysis of polyethyleneimines. J. Anal. Appl. Pyrolysis 2023, 169, 105838. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, X.; Zeng, G.; Liu, Z.; Tang, L.; Shao, B.; Zeng, Z.; Zhang, W.; Liu, Y.; Cheng, M.; et al. Surfactant-assisted synthesis of photocatalysts: Mechanism, synthesis, recent advances and environmental application. Chem. Eng. J. 2019, 372, 429–451. [Google Scholar] [CrossRef]
- Han, D.; Yang, H.; Zhu, C.; Wang, F. Controlled synthesis of CuO nanoparticles using TritonX-100-based water-in-oil reverse micelles. Powder Technol. 2008, 185, 286–290. [Google Scholar] [CrossRef]
- Lu, H.; Ju, H.; Yang, Q.; Li, Z.; Ren, H.; Xin, X.; Xu, G. Synthesis of Ag@SiO2 hybrid nanoparticles templated by a Triton X-100)/1-hexanol/cyclohexane/H2O water-in-oil microemulsion. CrystEngComm 2013, 15, 6511–6517. [Google Scholar] [CrossRef]
- Matejka, P.; Vlckova, B.; Vohlidal, J.; Pancoska, P.; Baumruk, V. The role of triton X-100 as an adsorbate and a molecular spacer on the surface of silver colloid: A surface-enhanced Raman scattering study. J. Phys. Chem. 1992, 96, 1361–1366. [Google Scholar] [CrossRef]
- Rahman, F.; Hossain, J.; Kuddus, A.; Moon, M.A.; Ismail, A.B. Effect of Triton X-100 surfactant on thiol-amine cosolvents assisted facile synthesized CdS thin films on glass substrate by spin coating method. SN Appl. Sci. 2020, 2, 590. [Google Scholar] [CrossRef]
- Salili, S.M.; Worden, M.; Nemati, A.; Miller, D.W.; Hegmann, T. Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents. Nanomaterials 2017, 7, 211. [Google Scholar] [CrossRef]
- Saien, J.; Ojaghloo, Z.; Soleymani, A.R.; Rasoulifard, M.H. Homogeneous and heterogeneous AOPs for rapid degradation of Triton X-100 in aqueous media via UV light, nano titania hydrogen peroxide and potassium persulfate. Chem. Eng. J. 2011, 167, 172–182. [Google Scholar] [CrossRef]
- Corchero, R.; Rodil, R.; Soto, A.; Rodil, E. Nanomaterial Synthesis in Ionic Liquids and Their Use on the Photocatalytic Degradation of Emerging Pollutants. Nanomaterials 2021, 11, 411. [Google Scholar] [CrossRef]
- Hammond, O.S.; Mudring, A.-V. Ionic liquids and deep eutectics as a transformative platform for the synthesis of nanomaterials. Chem. Commun. 2022, 58, 3865–3892. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. [EMIM] BF4 ionic liquid-mediated synthesis of TiO2 nanoparticles using Vitex negundo Linn extract and its antibacterial activity. J. Mol. Liq. 2016, 221, 986–992. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids: A review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Klähn, M.; Stüber, C.; Seduraman, A.; Wu, P. What Determines the Miscibility of Ionic Liquids with Water? Identification of the Underlying Factors to Enable a Straightforward Prediction. J. Phys. Chem. B 2010, 114, 2856–2868. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Sun, Y.; Cui, Y.; Liang, M.; Zhao, J.; Li, N. Phase-manipulable synthesis of Cu-based nanomaterials using ionic liquid 1-butyl-3-methyl-imidazole tetrafluoroborate. Cryst. Res. Technol. 2010, 45, 398–404. [Google Scholar] [CrossRef]
- Baran, T. Efficiency of volatile organic compound degradation in air using doped strontium titanate photocatalysts. Quenching experiments towards understanding of doping mechanisms. React. Kinet. Mech. Catal. 2023, 136, 3243–3256. [Google Scholar] [CrossRef]
- Yadav, M.; Gyulavári, T.; Kiss, J.; Ábrahámné, K.B.; Efremova, A.; Szamosvölgyi, Á.; Pap, Z.; Sápi, A.; Kukovecz, Á.; Kónya, Z. Noble metal nanoparticles and nanodiamond modified strontium titanate photocatalysts for room temperature CO production from direct hydrogenation of CO2. J. CO2 Util. 2023, 78, 102621. [Google Scholar] [CrossRef]
- Burek, B.O.; Timm, J.; Bahnemann, D.W.; Bloh, J.Z. Kinetic effects and oxidation pathways of sacrificial electron donors on the example of the photocatalytic reduction of molecular oxygen to hydrogen peroxide over illuminated titanium dioxide. Catal. Today 2019, 335, 354–364. [Google Scholar] [CrossRef]
- Pellegrin, Y.; Odobel, F. Sacrificial electron donor reagents for solar fuel production. Comptes Rendus Chim. 2017, 20, 283–295. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Stufano, P.; Macyk, W.; Baran, T.; Fragale, C.; Costa, M.; Aresta, M. Hybrid technologies for an enhanced carbon recycling based on the enzymatic reduction of CO2 to methanol in water: Chemical and photochemical NADH regeneration. ChemSusChem 2012, 5, 373–378. [Google Scholar] [CrossRef]
- Johne, P.; Kisch, H. Photoreduction of carbon dioxide catalysed by free and supported zinc and cadmium sulphide powders. J. Photochem. Photobiol. A Chem. 1997, 111, 223–228. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2009, 37, D603–D610. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct, in situ determination of pH and solute concentrations in formic acid dehydrogenation and CO2 hydrogenation in pressurised aqueous solutions using 1H and 13C NMR spectroscopy. Dalton Trans. 2013, 42, 4353–4356. [Google Scholar] [CrossRef]
- Wojtyła, S.; Klama, P.; Baran, T. Is 3D printing safe? Analysis of the thermal treatment of thermoplastics: ABS, PLA, PET, and nylon. J. Occup. Environ. Hyg. 2017, 14, D80–D85. [Google Scholar] [CrossRef]
- Wojtyła, S.; Klama, P.; Śpiewak, K.; Baran, T. 3D printer as a potential source of indoor air pollution. Int. J. Environ. Sci. Technol. 2019, 17, 207–218. [Google Scholar] [CrossRef]
- Jones, N.R.; de Jersey, A.M.; Lavers, J.L.; Rodemann, T.; Rivers-Auty, J. Identifying laboratory sources of microplastic and nanoplastic contamination from the air, water, and consumables. J. Hazard. Mater. 2024, 465, 133276. [Google Scholar] [CrossRef]
- McDonald, G.R.; Hudson, A.L.; Dunn, S.M.J.; You, H.; Baker, G.B.; Whittal, R.M.; Martin, J.W.; Jha, A.; Edmondson, D.E.; Holt, A. Bioactive Contaminants Leach from Disposable Laboratory Plasticware. Science 2008, 322, 917. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Unterkofler, K.; Amann, A. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst 2013, 138, 1405–1418. [Google Scholar] [CrossRef]
- Idris, S.A.A.; Hanafiah, M.M.; Ismail, M.; Abdullah, S.; Khan, M.F. Laboratory air quality and microbiological contamination in a university building. Arab. J. Geosci. 2020, 13, 580. [Google Scholar] [CrossRef]
- Yau, Y.H.; Chew, B.T.; Saifullah, A.Z.A. Studies on the indoor air quality of Pharmaceutical Laboratories in Malaysia. Int. J. Sustain. Built Environ. 2012, 1, 110–124. [Google Scholar] [CrossRef]
- Seseña, S.; Rodríguez, A.M.; Palop, M.L. Indoor air quality analysis in naturally ventilated university training laboratories: A health risk assessment. Air Qual. Atmos. Health 2022, 15, 1817–1837. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef]
- Wasse, S.; Baran, T.; Mesto, E.; Norici, A.; Dibenedetto, A.; Aresta, M. Formation of faceted [111, 110] Cu2O induced by Frustules and their activity as photo(electro)catalysts for the coprocessing of CO2&H2O to energy products under Visible-light irradiation. In Proceedings of the 20th International Conference on Carbon Dioxide Utilization (ICCDU), Bari, Italy, 25–29 June 2023. [Google Scholar]
- Donose, B.C.; Taran, E.; Vakarelski, I.U.; Shinto, H.; Higashitani, K. Effects of cleaning procedures of silica wafers on their friction characteristics. J. Colloid Interface Sci. 2006, 299, 233–237. [Google Scholar] [CrossRef]
- Barecka, M.H.; Kovalev, M.K.; Muhamad, M.Z.; Ren, H.; Ager, J.W.; Lapkin, A.A. CO2 electroreduction favors carbon isotope 12C over 13C and facilitates isotope separation. iScience 2023, 26, 107834. [Google Scholar] [CrossRef]
- Roth, J.P.; Klinman, J.P. Kinetic Isotope Effects. In Encyclopedia of Biological Chemistry; Lennarz, W.J., Lane, M.D., Eds.; Elsevier: New York, NY, USA, 2004; pp. 522–527. [Google Scholar] [CrossRef]
- Bard, A.J. Encyclopedia of Electrochemistry of the Elements, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Wojtyła, S.; Baran, T. Electrochemically prepared copper/indium oxides photocathode for efficient photoelectrochemical hydrogen production. Sol. Energy Mater. Sol. Cells 2020, 206, 110262. [Google Scholar] [CrossRef]
- Wojtyła, S.; Baran, T. Copper zinc oxide heterostructure nanoflowers for hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 27343–27353. [Google Scholar] [CrossRef]
- Wojtyła, S.; Szmit, K.; Baran, T. Type II Heterostructures: The Way Towards Improved Photoelectrochemical Activity of Graphitic Carbon Nitride. J. Inorg. Organomet. Polym. 2018, 28, 492–499. [Google Scholar] [CrossRef]





 | |||
|---|---|---|---|
| Products | Percentage (%) | Products | Percentage (%) |
| Acetic acid | 35 | Acetaldehyde | 2 |
| Pyruvic acid | 4 | Carbon monoxide | 20 |
| Lactic acid | 4 | Formic acid | 4 |
| Formaldehyde | 9 | Others | 22 |
| Conditions of Drying | Residual Concentration of Isopropanol on Materials |
|---|---|
| Room temperature for 12 h | 895 ppm |
| 80 °C for 2 h | 574 ppm |
| 150 °C for 2 h | 18 ppm |
| Catalyst/Material/Source of Error | Origin of C Products | Detected Amount (False positive + CO2RP) | Real CO2RP Amount Clean Conditions |
|---|---|---|---|
| Rutin@TiO2 | Rutin—organic sensitizer of photocatalyst | CO (88 mmol g−1) in a 3 h test | CO (9 mmol g−1) in a 3 h test |
| Polyetheneimine PEI | PEI used in the preparation of electrodes/materials | C1 and Cn carboxylic acids, aldehydes and ketones, amines, and imines | Absence of Cn products and N-derivatives |
| ZnS + Triton | Triton—surfactant used for the synthesis of photocatalyst | CO (693 mmol g−1 h−1), CH4 (95 mmol g−1 h−1), CH3OH (7 mmol g−1 h−1), C2H5OH (203 mmol g−1 h−1) | Not detected if no sacrificial electron donor |
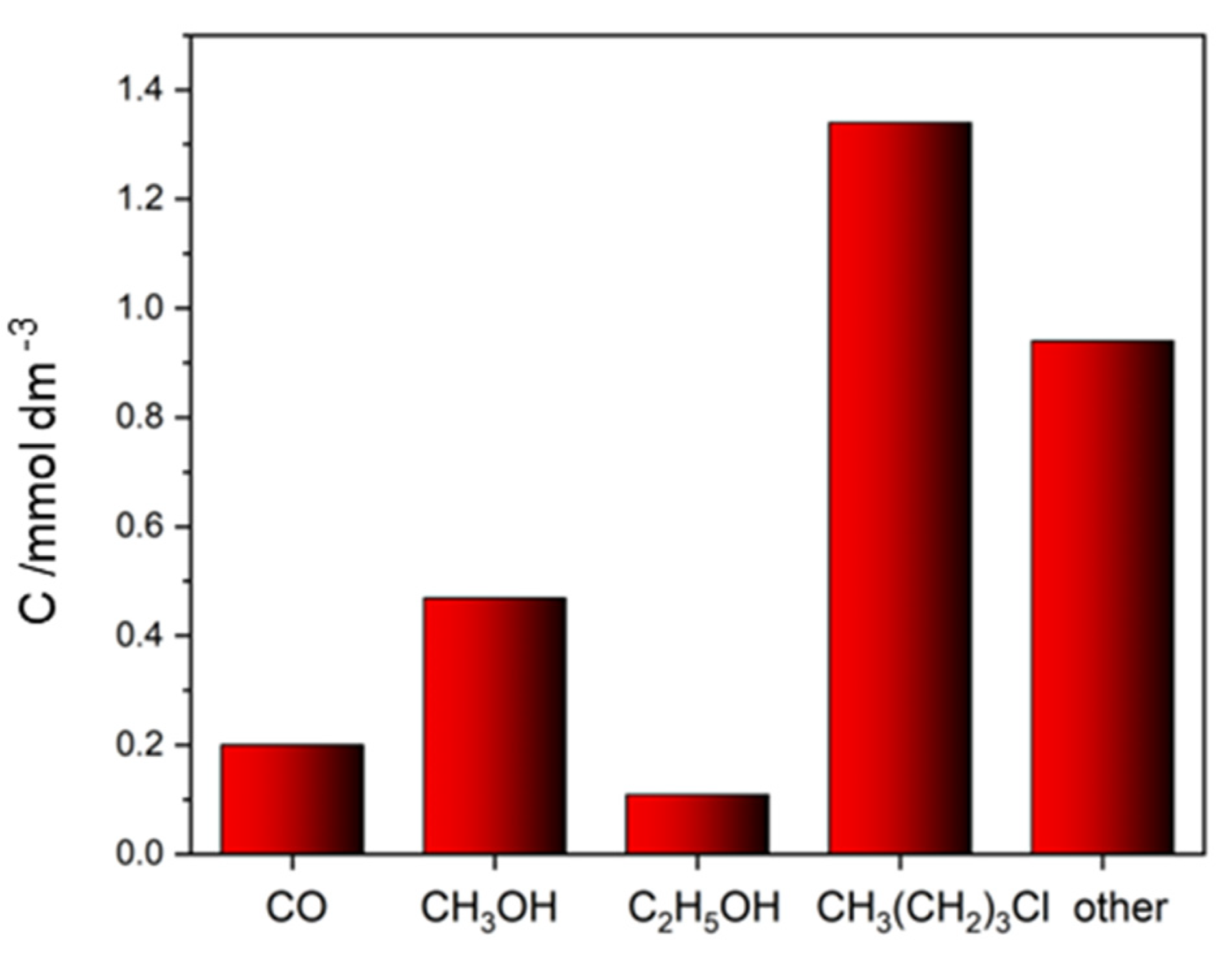
| 1-butyl-3-methylimidazolium/CuO/ZnO | 1-butyl-3-methylimidazolium chloride—ionic liquid | CO (20 mmol g−1), CH3OH (46 mmol g−1), C2H5OH (11 mmol g−1), chlorobutane (134 mmol g−1) | CO (1.9 mmol g−1) |
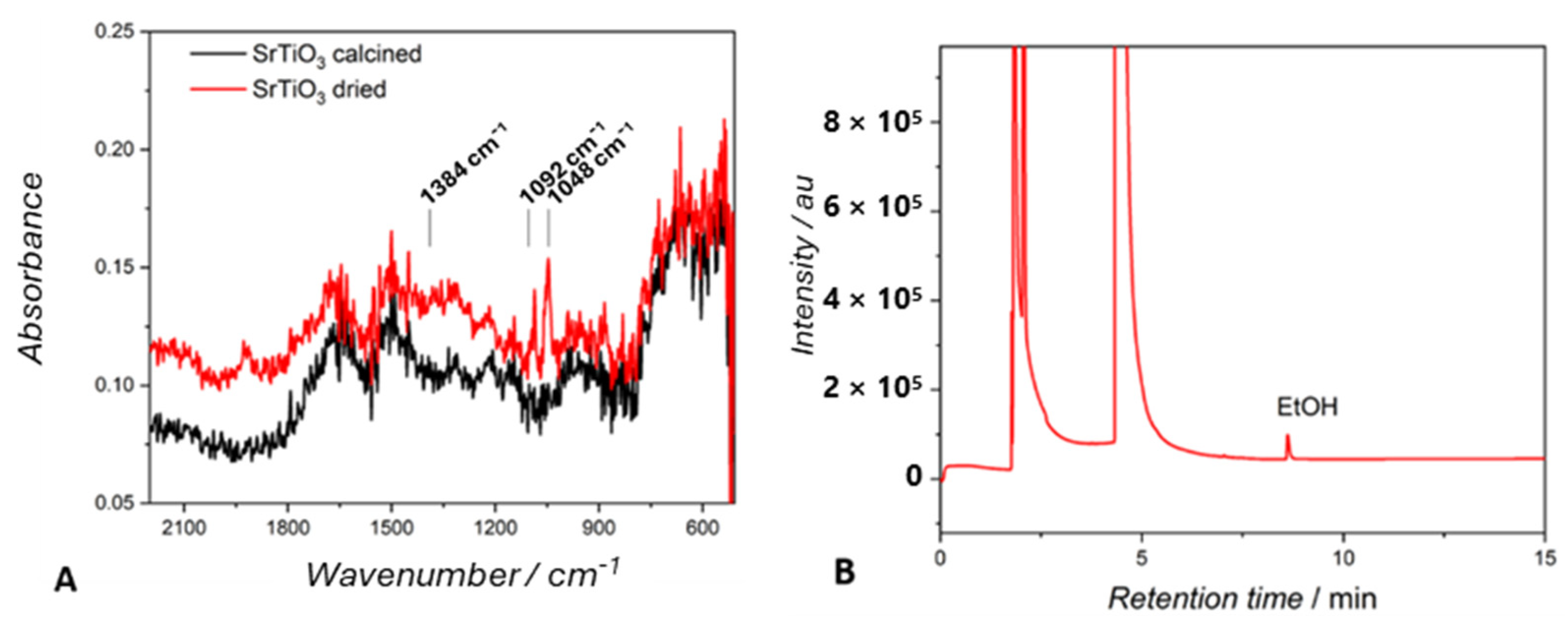
| SrTiO3 | Ethanol—solvent | C2H5OH (200 mmol g−1 h−1) | C2H5OH (0.6 mmol g−1 h−1) |
| g-C3N4 | Nafion solution in isopropanol | Isopropanol from 14 to 0.3 mmol g−1 (depending on drying temperature) | Not detected |
| ZnS | Glycerol—e-donor | Formic acid (only qualitative analysis) | Formic acid (only minor) |
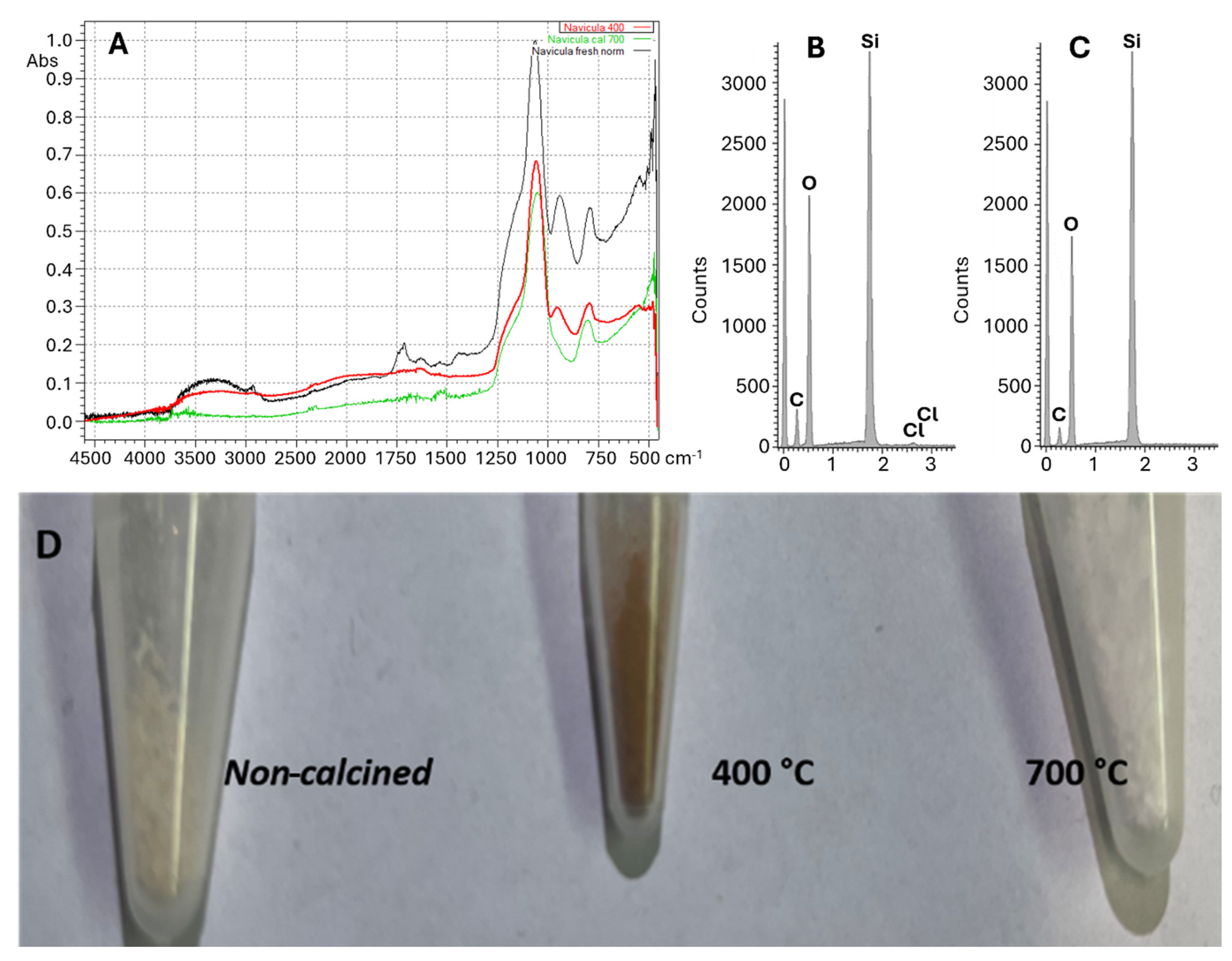
| Frustule | Organic contaminations | - | - |
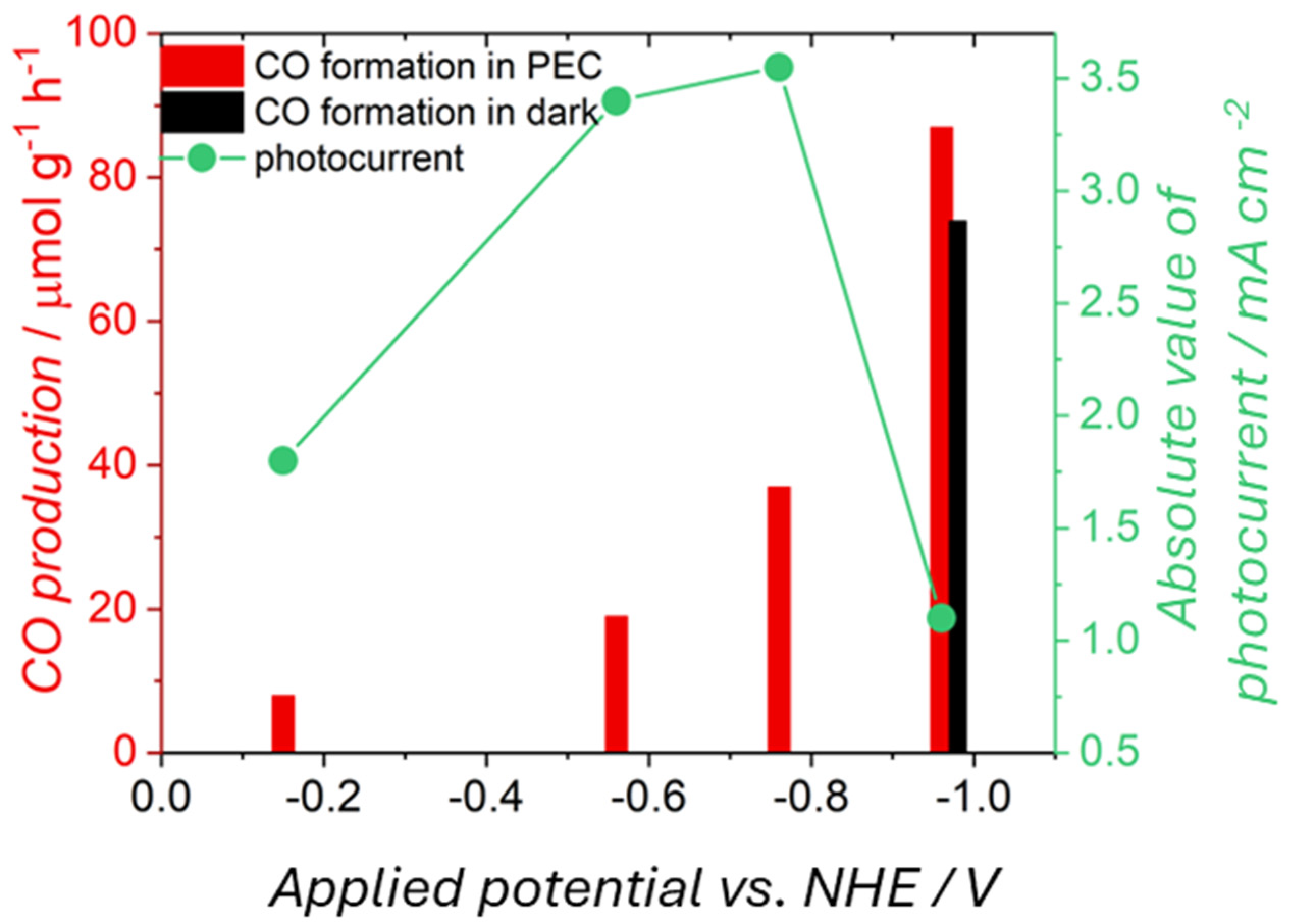
| CuO@In2O3 | Organic contaminations | CO formed in PEC (light + bias) 86 mmol g−1 h−1 CO formed in EC (bias) 73 mmol g−1 h−1 | |
| Operator | Breath | Acetone, ethanol, methanol: concentrations up to 30 ppm were measured depending on the exposure time and health conditions of the operator. This amount is often well above (two or three times) the level of CO2RR acetone under controlled conditions. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, T.; Caringella, D.; Dibenedetto, A.; Aresta, M. Pitfalls in Photochemical and Photoelectrochemical Reduction of CO2 to Energy Products. Molecules 2024, 29, 4758. https://doi.org/10.3390/molecules29194758
Baran T, Caringella D, Dibenedetto A, Aresta M. Pitfalls in Photochemical and Photoelectrochemical Reduction of CO2 to Energy Products. Molecules. 2024; 29(19):4758. https://doi.org/10.3390/molecules29194758
Chicago/Turabian StyleBaran, Tomasz, Domenico Caringella, Angela Dibenedetto, and Michele Aresta. 2024. "Pitfalls in Photochemical and Photoelectrochemical Reduction of CO2 to Energy Products" Molecules 29, no. 19: 4758. https://doi.org/10.3390/molecules29194758
APA StyleBaran, T., Caringella, D., Dibenedetto, A., & Aresta, M. (2024). Pitfalls in Photochemical and Photoelectrochemical Reduction of CO2 to Energy Products. Molecules, 29(19), 4758. https://doi.org/10.3390/molecules29194758








