Biosynthesis of Iron Oxide Nanoparticles by Marine Streptomyces sp. SMGL39 with Antibiofilm Activity: In Vitro and In Silico Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Marine Sample Collection and Isolation of Marine Actinobacteria
2.2. Preparation of Actinomycetes Filtrate and Screening Their Ability to Biosynthesis Iron Oxide Nanoparticles
2.3. Selection of a Potent Strain for Biosynthesis of Iron Oxide Nanoparticles
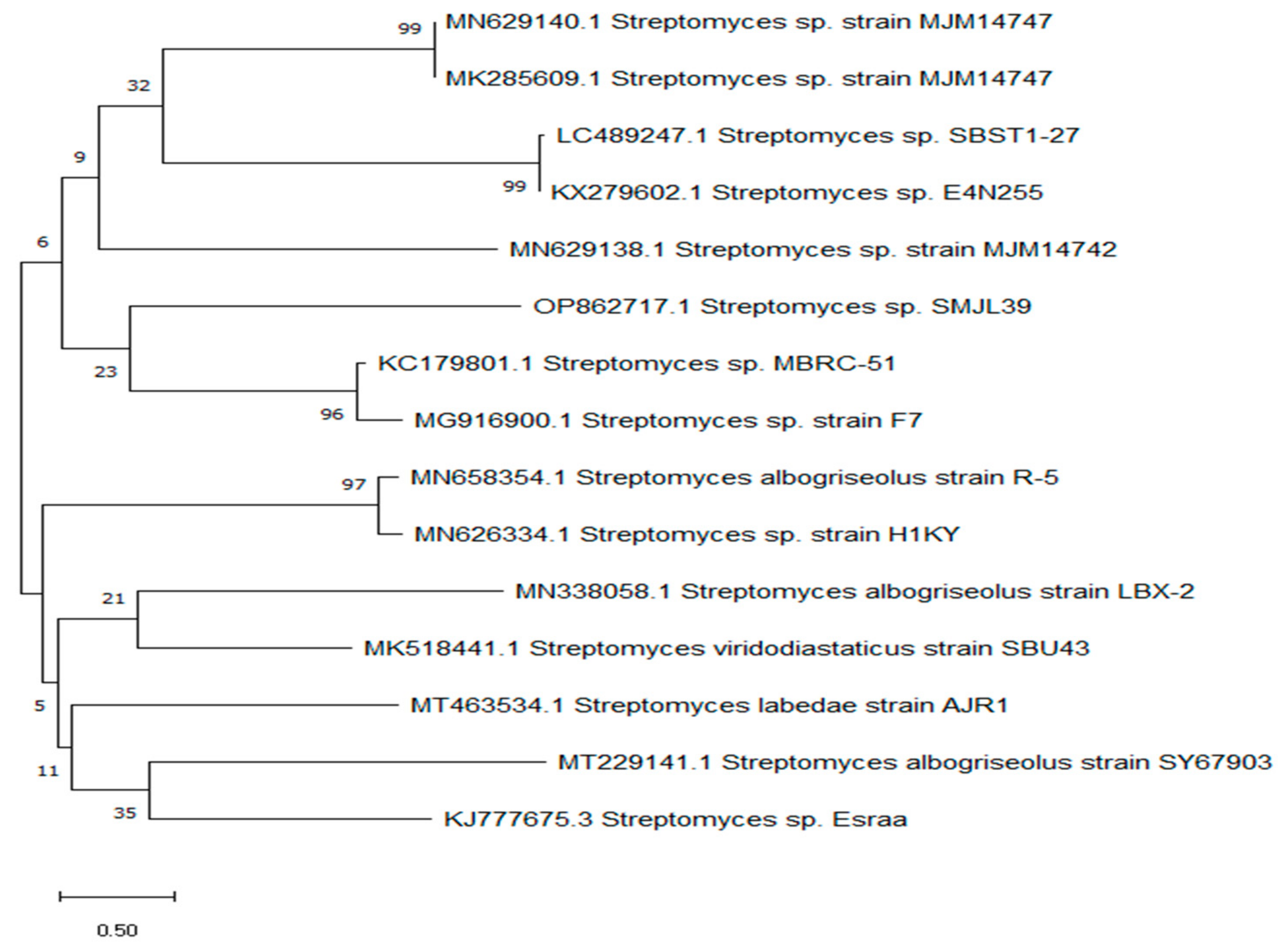
2.4. Genetic Identification of the Most Potent Actinomycete Isolate
2.5. Biosynthesis of Iron Oxide Nanoparticles Using Streptomyces sp. SMGL39
2.6. Characterization of Biosynthesized FeNPs
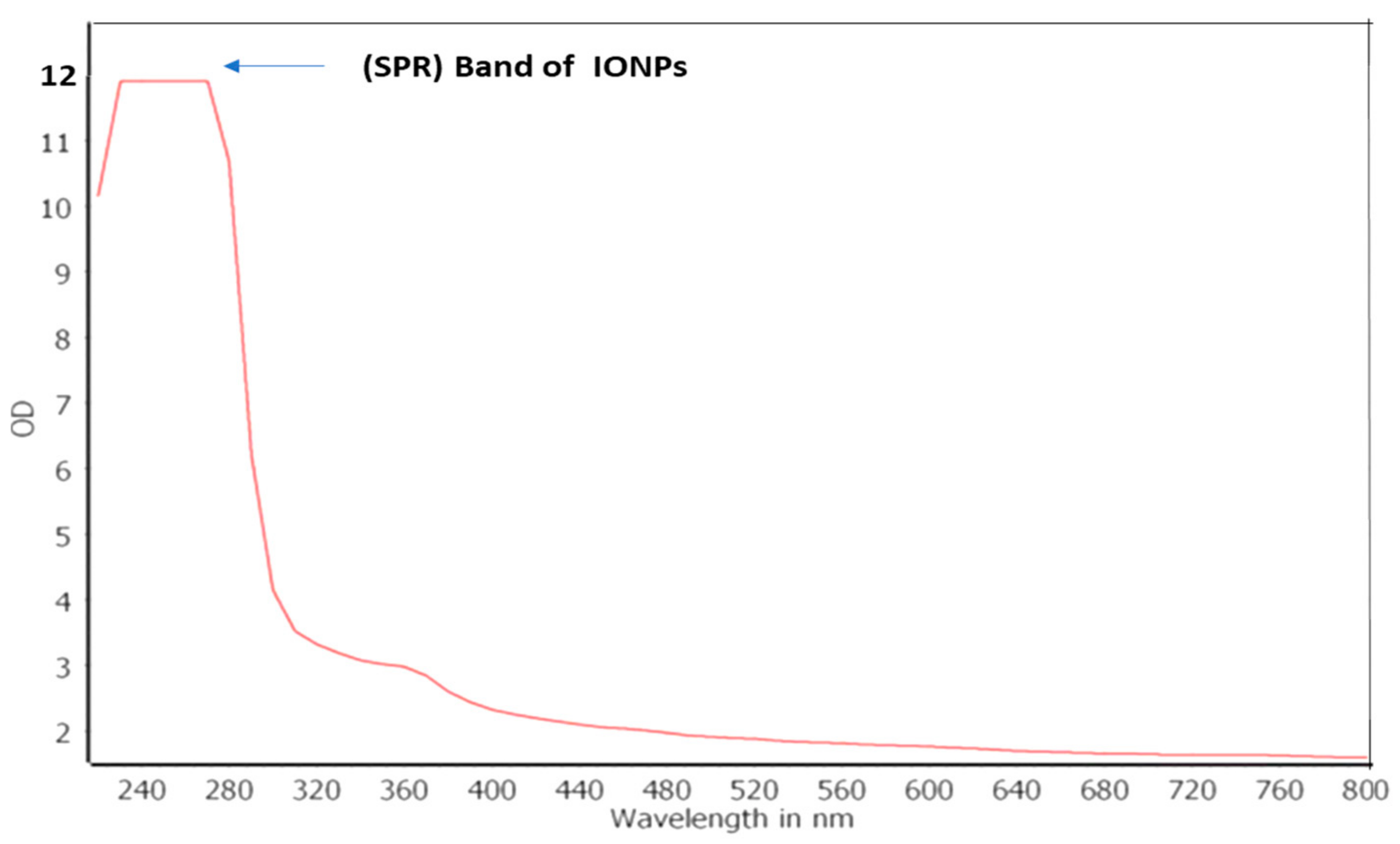
2.6.1. Ultraviolet–Visible (UV–Vis) Spectral Analysis
2.6.2. Transmission Electron Microscopy (TEM) Analysis
2.6.3. X-ray Diffraction (XRD) Analysis
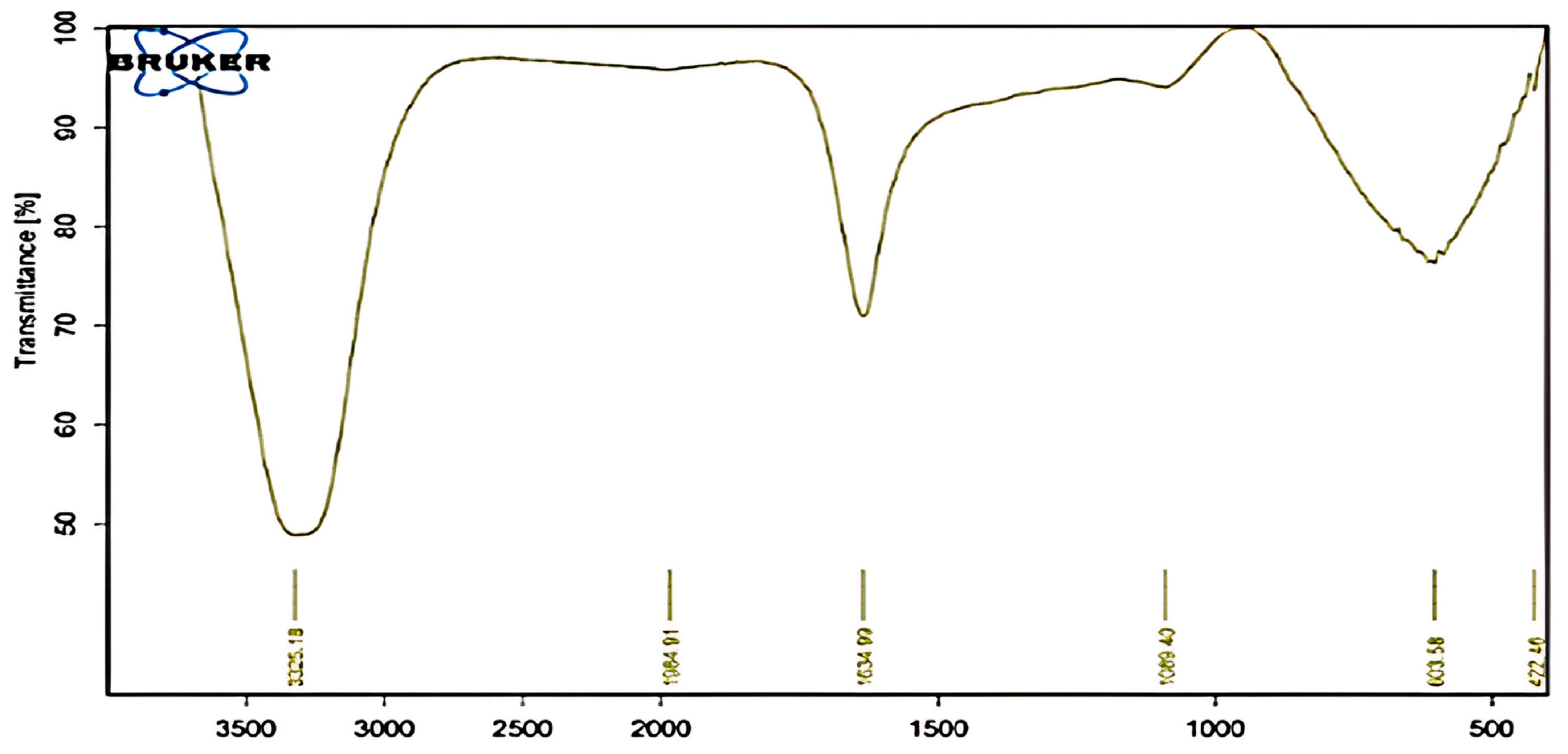
2.6.4. Fourier Transform Infra-Red (FTIR) Spectroscopic Analysis
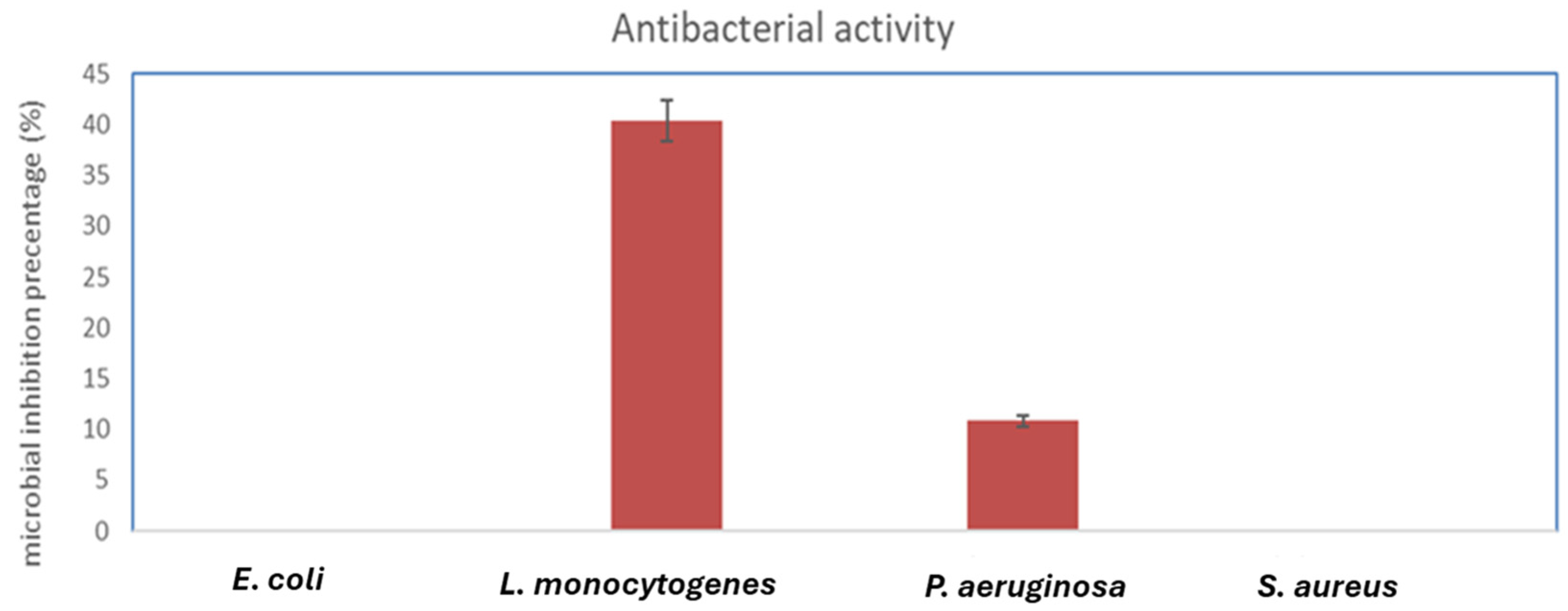
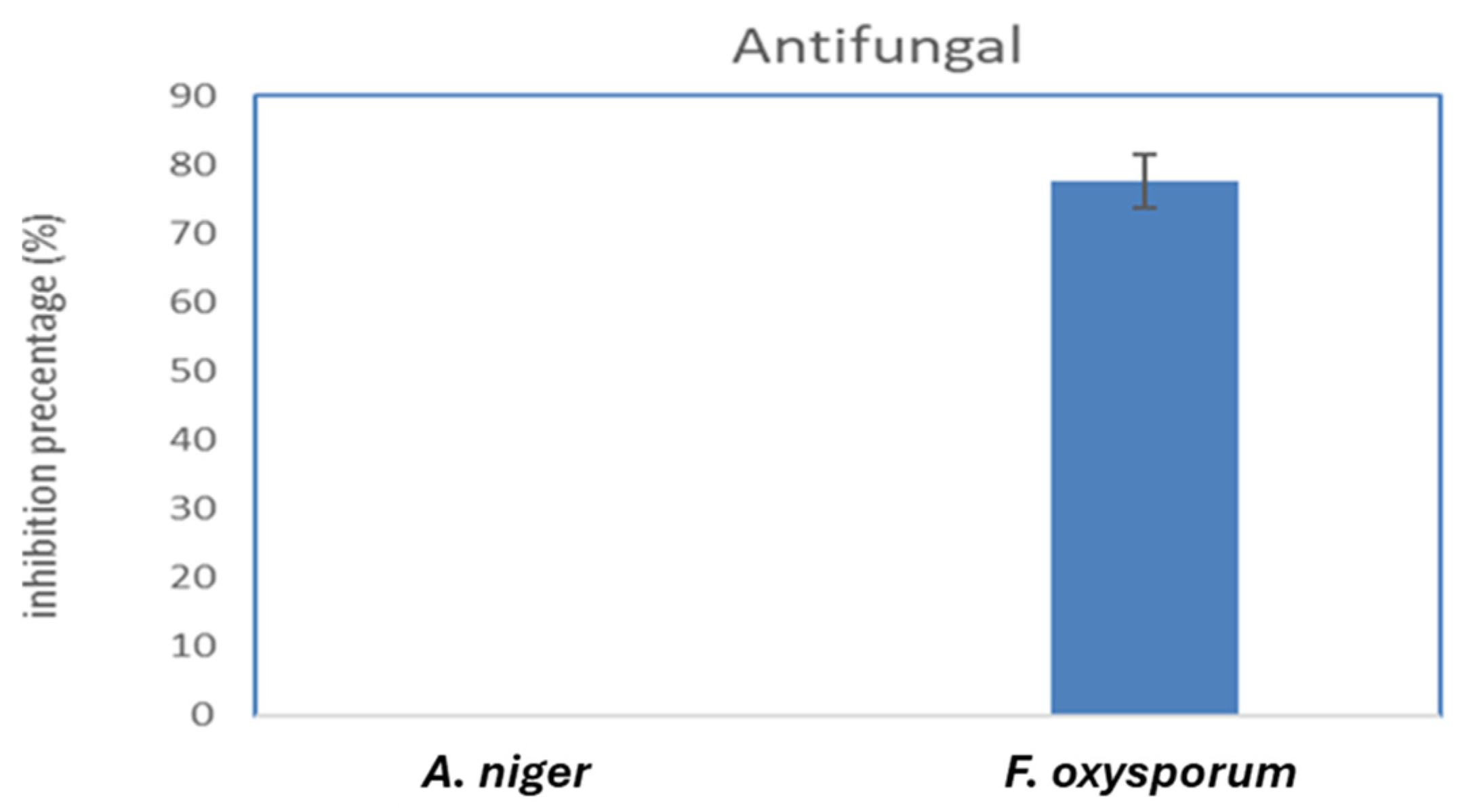
2.7. Antimicrobial Activity of Biosynthesized FeNPs
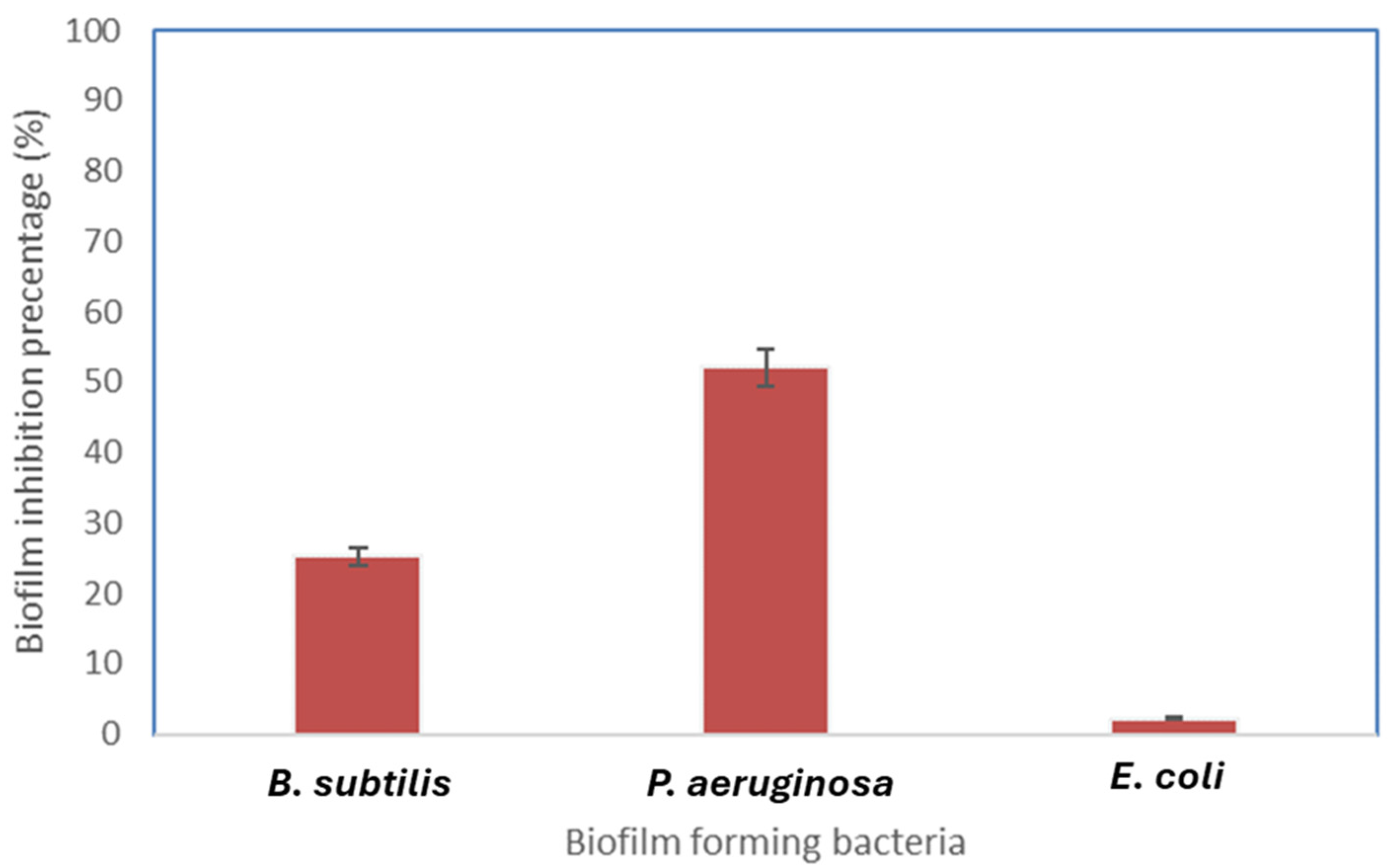
2.8. Antibiofilm Activity of Biosynthesized FeNPs
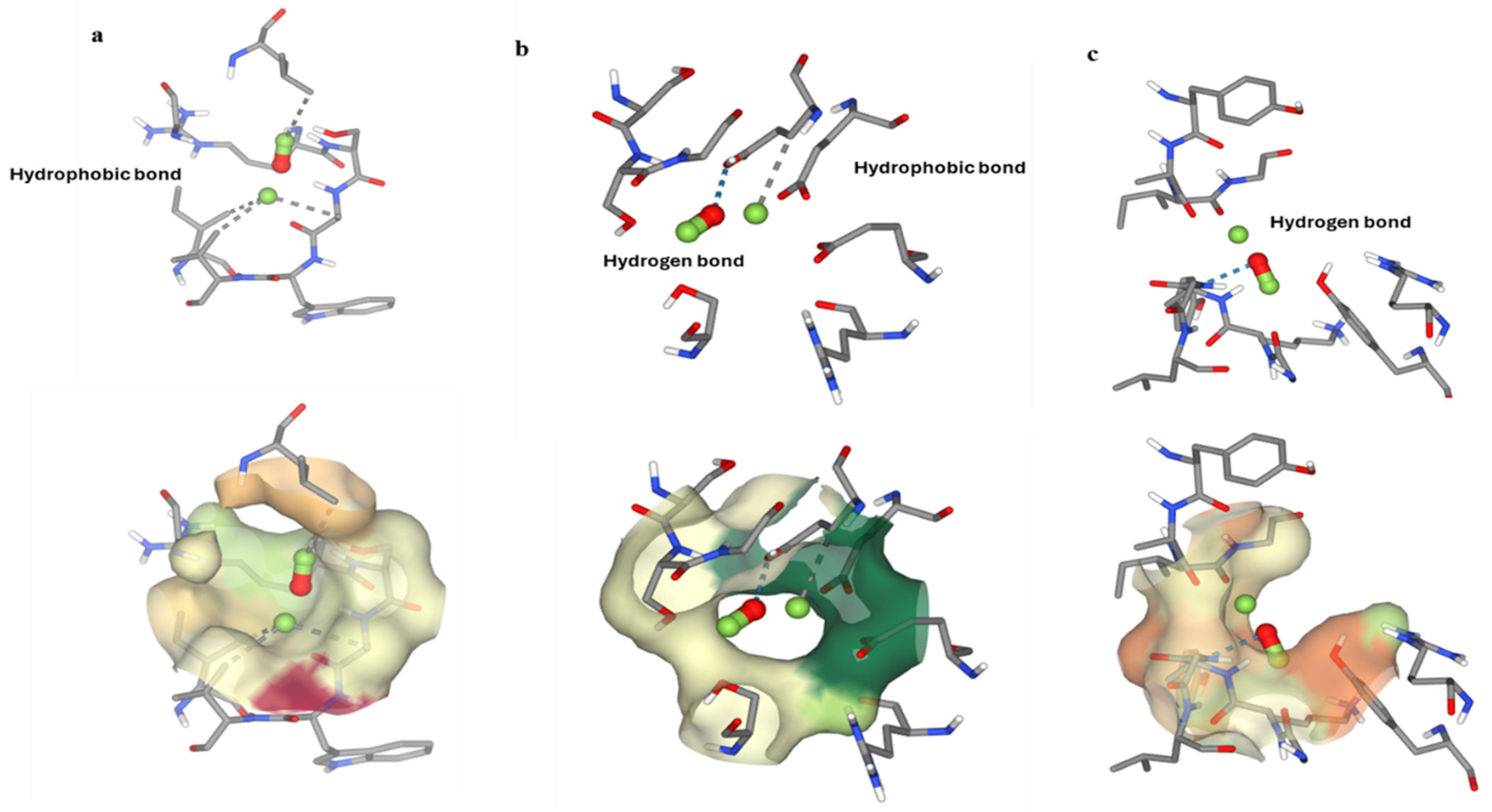
2.9. In Silico Studies
3. Materials and Methods
3.1. Collection of Marine Samples and Isolation of Marine Actinobacteria
3.2. Actinomycete Cell-Free Extract Production
3.3. Screening the Ability of Isolated Actinobacteria to Biosynthesize Iron Oxide Nanoparticles
3.4. Genetic Identification of the Most Potent Bacterial Strain
3.5. Characterization of Biosynthesized Iron Oxide Nanoparticles
3.5.1. Ultraviolet–Visible (UV–Vis) Spectral Analysis
3.5.2. Transmission Electron Microscopy (TEM) Analysis
3.5.3. X-ray Diffraction (XRD) Analysis
3.5.4. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
3.6. Assessment of Biological Activities
3.6.1. Antimicrobial Assay
3.6.2. Anti-Biofilm Assay
3.7. In Silico Studies
Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Nagórniewicz, B.; Storm, G.; Prakash, J. Relaxin-coated superparamagnetic iron-oxide nanoparticles as a novel theranostic approach for the diagnosis and treatment of liver fibrosis. J. Hepatol. 2017, 1, S43. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.-J.; Zhang, G.-B.; Ling, D.; Wang, M.-Q.; Zhou, Y.; Wu, Y.-D.; Wu, T.; Hackett, M.J.; Kim, B.H.; et al. Iron oxide nanoclusters for T 1 magnetic resonance imaging of non-human primates. Nat. Biomed. Eng. 2017, 1, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Barabadi, H.; Jounaki, K.; Pishgahzadeh, E.; Morad, H.; Vahidi, H. Penicillium species as an innovative microbial platform for bioengineering of biologically active nanomaterials. In Mycosynthesis of Nanomaterials: Perspectives and Challenges; CRC Press: Boca Raton, FL, USA, 2023; pp. 50–80. [Google Scholar]
- Kämpfer, P.; Genus, I. Streptomyces Waksman and Henrici 1943, 339AL emend. Witt and Stackebrandt 1990, 370 emend. Wellington, Stackebrandt, Sanders, Wolstrup and Jorgensen 1992, 159. Bergey’s Man. Syst. Bacteriol. 2012, 5, 1455–1767. [Google Scholar]
- Subathra Devi, C.; Merlyn Keziah, S.; Jemimah Naine, S.; Mohanasrinivasan, V. Actinomycetes: Microbiology to systems biology. In Actinobacteria: Microbiology to Synthetic Biology; Springer: Cham, Switzerland, 2022; pp. 1–35. [Google Scholar]
- Comanescu, C. Recent advances in surface functionalization of magnetic nanoparticles. Coatings 2023, 13, 1772. [Google Scholar] [CrossRef]
- Shankar, M.; Kesavan, S.S.; Biswas, K. Exploring the potentials of magnetic nanoscale material for different biomedical applications: A review. BioNanoScience 2023, 13, 1558–1581. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Lee, D.-J.; Tay, J.-H.; Zhang, Y.; Wan, C.-L.; Chen, X.-F. Recent advances on biosorption by aerobic granular sludge. J. Hazard. Mater. 2018, 357, 253–270. [Google Scholar] [CrossRef]
- Mathur, P.; Saini, S.; Paul, E.; Sharma, C.; Mehtani, P. Endophytic fungi mediated synthesis of iron nanoparticles: Characterization and application in methylene blue decolorization. Curr. Res. Green Sustain. Chem. 2021, 4, 100053. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sensors Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Sihem, L.; Hanine, D.; Faiza, B. Antibacterial activity of α-Fe2O3 and α-Fe2O3@ Ag nanoparticles prepared by Urtica leaf extract. Nanotechnologies Russ. 2020, 15, 198–203. [Google Scholar] [CrossRef]
- Li, D.; Shen, M.; Xia, J.; Shi, X. Recent developments of cancer nanomedicines based on ultrasmall iron oxide nanoparticles and nanoclusters. Nanomedicine 2021, 16, 609–612. [Google Scholar] [CrossRef] [PubMed]
- AlMatar, M.; Makky, E.A.; Var, I.; Koksal, F. The role of nanoparticles in the inhibition of multidrug-resistant bacteria and biofilms. Curr. Drug Deliv. 2018, 15, 470–484. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Zare, M.; Kiyanpour, S.; Berenjian, A.; Niknezhad, S.V.; Ghasemi, Y. Biosynthesis of xanthangum-coated INPs by using Xanthomonas campestris. IET Nanobiotechnology 2018, 12, 254–258. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.Y.; Lee, W.I.; Nelson, K.L.; Yoon, J.; Sedlak, D.L. Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ. Sci. Technol. 2008, 42, 4927–4933. [Google Scholar] [CrossRef]
- Jayanthi, S.A.; Sukanya, D.; Pragasam, A.J.A.; Sagayaraj, P. The influence of PEG 20,000 concentration on the size control and magnetic properties of functionalized bio-compatible magnetic nanoparticles. Der Pharma Chem. 2013, 5, 90–102. [Google Scholar]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “Protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Brindhadevi, K.; LewisOscar, F.; Mylonakis, E.; Shanmugam, S.; Verma, T.N.; Pugazhendhi, A. Biofilm and quorum sensing mediated pathogenicity in Pseudomonas aeruginosa. Process. Biochem. 2020, 96, 49–57. [Google Scholar] [CrossRef]
- Hamed, A.A.; Kabary, H.; Khedr, M.; Emam, A.N. Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Adv. 2020, 10, 10361–10367. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Y.M.; Azzam, A.M.; Amin, B.H.; Safwat, N.A. Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity. Afr. J. Biotechnol. 2015, 14, 1234–1241. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rzhetsky, A.; Nei, M. A Simple Method for Estimating and Testing Minimum-Evolution Trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar] [CrossRef]
- Hifnawy, M.S.; Hassan, H.M.; Mohammed, R.; Fouda, M.M.; Sayed, A.M.; Hamed, A.A.; AbouZid, S.F.; Rateb, M.E.; Alhadrami, H.A.; Abdelmohsen, U.R. Induction of antibacterial metabolites by co-cultivation of two red-sea-sponge-associated actinomycetes Micromonospora sp. UR56 and Actinokinespora sp. EG49. Mar. Drugs 2020, 18, 243. [Google Scholar] [CrossRef]
- Elkhouly, H.I.; Hamed, A.A.; El Hosainy, A.M.; Ghareeb, M.A.; Sidkey, N.M. Bioactive secondary metabolite from endophytic Aspergillus tubenginses ASH4 isolated from Hyoscyamus muticus: Antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn. J. 2020, 13, 434–442. [Google Scholar] [CrossRef]
- Elkhouly, H.I.; Sidkey, N.M.; Ghareeb, M.A.; El Hosainy, A.M.; Hamed, A.A. Bioactive secondary metabolites from endophytic Aspergillus terreus AH1 isolated from Ipomoea carnea growing in Egypt. Egypt. J. Chem. 2021, 64, 7611–7620. [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Devi, H.S.; Boda, M.A.; Shah, M.A.; Parveen, S.; Wani, A.H. Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for anti-fungal activity. Green Process. Synth. 2019, 8, 38–45. [Google Scholar] [CrossRef]
- Abdeen, S.; Isaac, R.R.; Geo, S.; Sornalekshmi, S.; Rose, A.; Praseetha, P.K. Evaluation of antimicrobial activity of biosynthesized iron and silver nanoparticles using the fungi Fusarium oxysporum and Actinomycetes sp. on human pathogens. Nano Biomed. Eng. 2013, 5, 39–45. [Google Scholar] [CrossRef]
- Saranya, S.; Vijayarani, K.; Pavithra, S. Green synthesis of iron nanoparticles using aqueous extract of Musa ornata flower sheath against pathogenic bacteria. Indian J. Pharm. Sci. 2017, 79, 688. [Google Scholar] [CrossRef]
- Batool, F.; Iqbal, M.S.; Khan, S.U.D.; Khan, J.; Ahmed, B.; Qadir, M.I. Biologically synthesized iron nanoparticles (FeNPs) from Phoenix dactylifera have anti-bacterial activities. Sci. Rep. 2021, 11, 22132. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, J.; Baltrusaitis, J.; Chen, H.; Stebounova, L.; Wu, C.-M.; Rubasinghege, G.; Mudunkotuwa, I.A.; Caraballo, J.C.; Zabner, J.; Grassian, V.H.; et al. Iron oxide nanoparticles induce Pseudomonas aeruginosa growth, induce biofilm formation, and inhibit antimicrobial peptide function. Environ. Sci. Nano 2014, 1, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bandyopadhyay, A.; Sarkar, K. Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J. Nanobiotechnology 2011, 9, 34. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Vranish, J.N.; Ancona, M.G.; Oh, E.; Susumu, K.; Lasarte Aragones, G.; Breger, J.C.; Walper, S.A.; Medintz, I.L. Enhancing coupled enzymatic activity by colocalization on nanoparticle surfaces: Kinetic evidence for directed channeling of intermediates. ACS Nano 2018, 12, 7911–7926. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S.R.; Mandale, A.B.; Rao, M.; Sastry, M. Pepsin–gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron oxide nanozyme: A multifunctional enzyme mimetic for biomedical applications. Theranostics 2017, 7, 3207. [Google Scholar] [CrossRef]
- Akagi, T.; Kaneko, T.; Kida, T.; Akashi, M. Multifunctional conjugation of proteins on/into bio-nanoparticles prepared by amphiphilic poly(γ-glutamic acid). J. Biomater. Sci. Polym. Ed. 2006, 17, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Miranda, O.R.; Li, X.; Garcia-Gonzalez, L.; Zhu, Z.-J.; Yan, B.; Bunz, U.H.F.; Rotello, V.M. Colorimetric bacteria sensing using a supramolecular enzyme–nanoparticle biosensor. J. Am. Chem. Soc. 2011, 133, 9650–9653. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attea, S.A.; Ghareeb, M.A.; Kelany, A.K.; Elhakim, H.K.A.; Allemailem, K.S.; Bukhari, S.I.; Rashidi, F.B.; Hamed, A.A. Biosynthesis of Iron Oxide Nanoparticles by Marine Streptomyces sp. SMGL39 with Antibiofilm Activity: In Vitro and In Silico Study. Molecules 2024, 29, 4784. https://doi.org/10.3390/molecules29194784
Attea SA, Ghareeb MA, Kelany AK, Elhakim HKA, Allemailem KS, Bukhari SI, Rashidi FB, Hamed AA. Biosynthesis of Iron Oxide Nanoparticles by Marine Streptomyces sp. SMGL39 with Antibiofilm Activity: In Vitro and In Silico Study. Molecules. 2024; 29(19):4784. https://doi.org/10.3390/molecules29194784
Chicago/Turabian StyleAttea, Sara A., Mosad A. Ghareeb, Ayda K. Kelany, Heba K. A. Elhakim, Khaled S. Allemailem, Sarah I. Bukhari, Fatma B. Rashidi, and Ahmed A. Hamed. 2024. "Biosynthesis of Iron Oxide Nanoparticles by Marine Streptomyces sp. SMGL39 with Antibiofilm Activity: In Vitro and In Silico Study" Molecules 29, no. 19: 4784. https://doi.org/10.3390/molecules29194784
APA StyleAttea, S. A., Ghareeb, M. A., Kelany, A. K., Elhakim, H. K. A., Allemailem, K. S., Bukhari, S. I., Rashidi, F. B., & Hamed, A. A. (2024). Biosynthesis of Iron Oxide Nanoparticles by Marine Streptomyces sp. SMGL39 with Antibiofilm Activity: In Vitro and In Silico Study. Molecules, 29(19), 4784. https://doi.org/10.3390/molecules29194784









