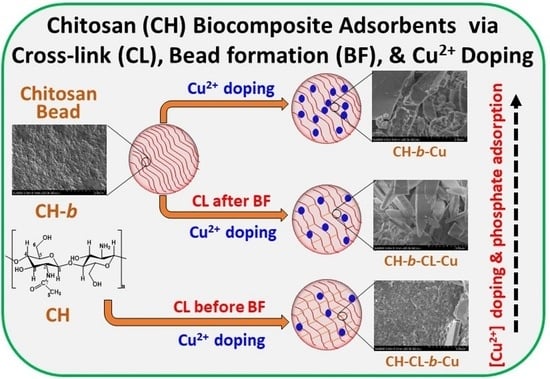
Chitosan Biocomposites with Variable Cross-Linking and Copper-Doping for Enhanced Phosphate Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization Studies
2.1.1. FTIR Spectroscopy
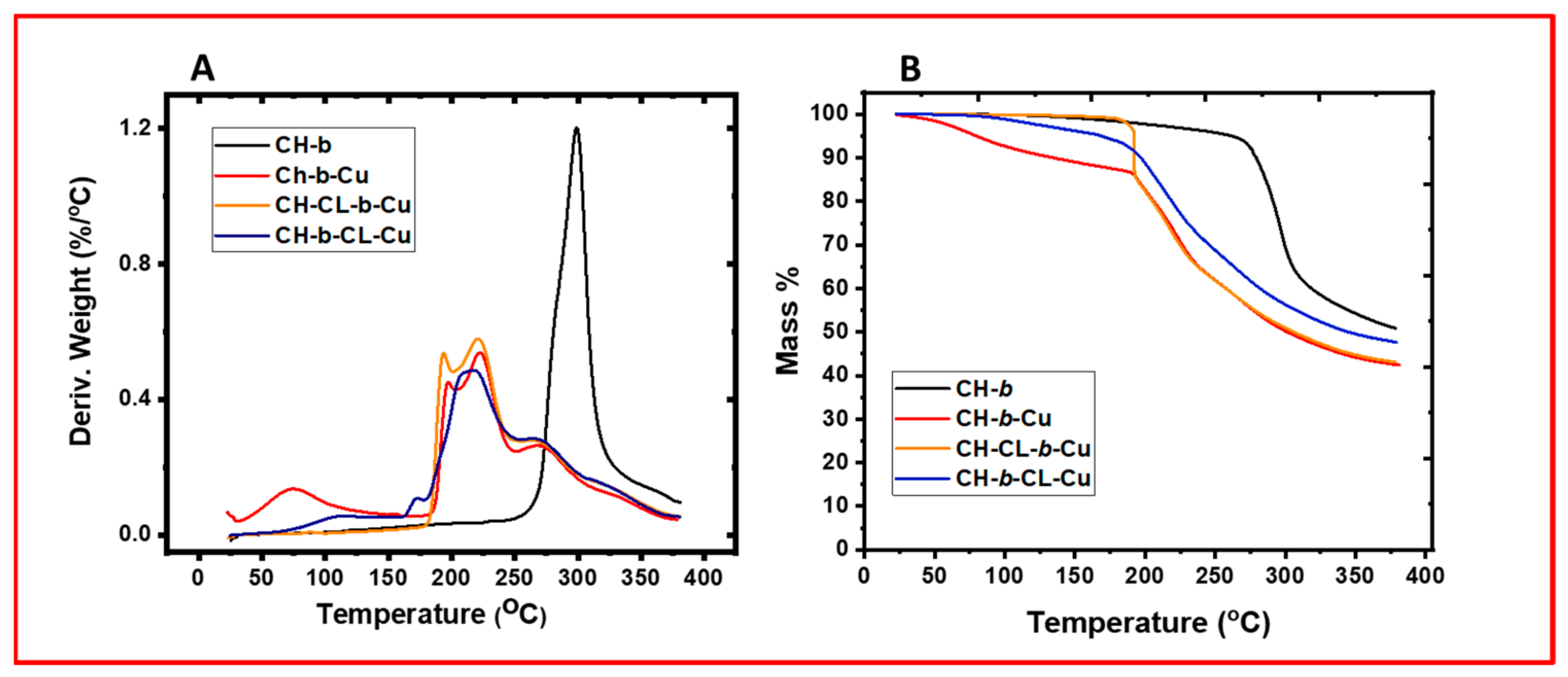
2.1.2. Thermal Gravimetry Analysis (TGA)
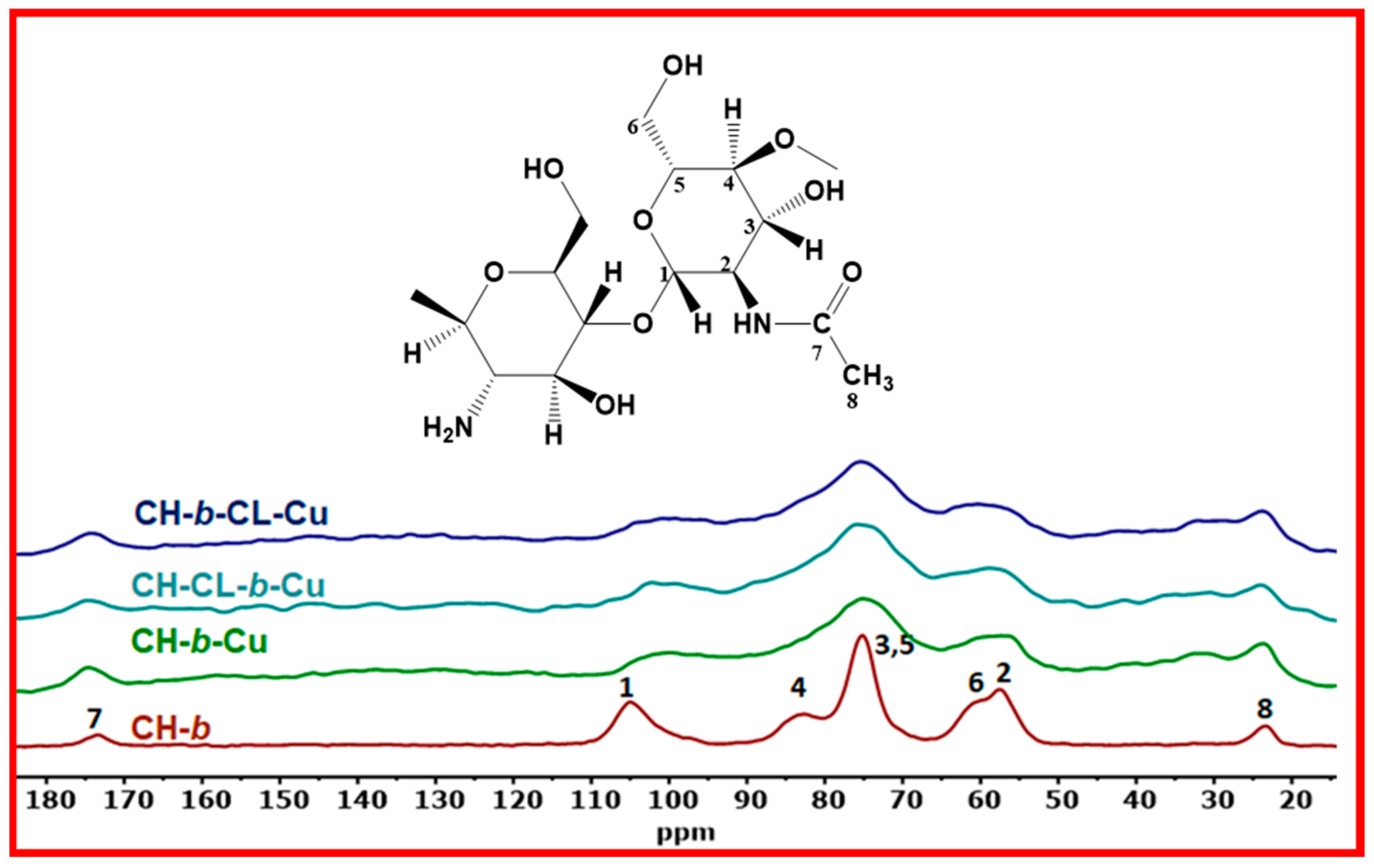
2.1.3. Solid State 13C NMR Spectroscopy
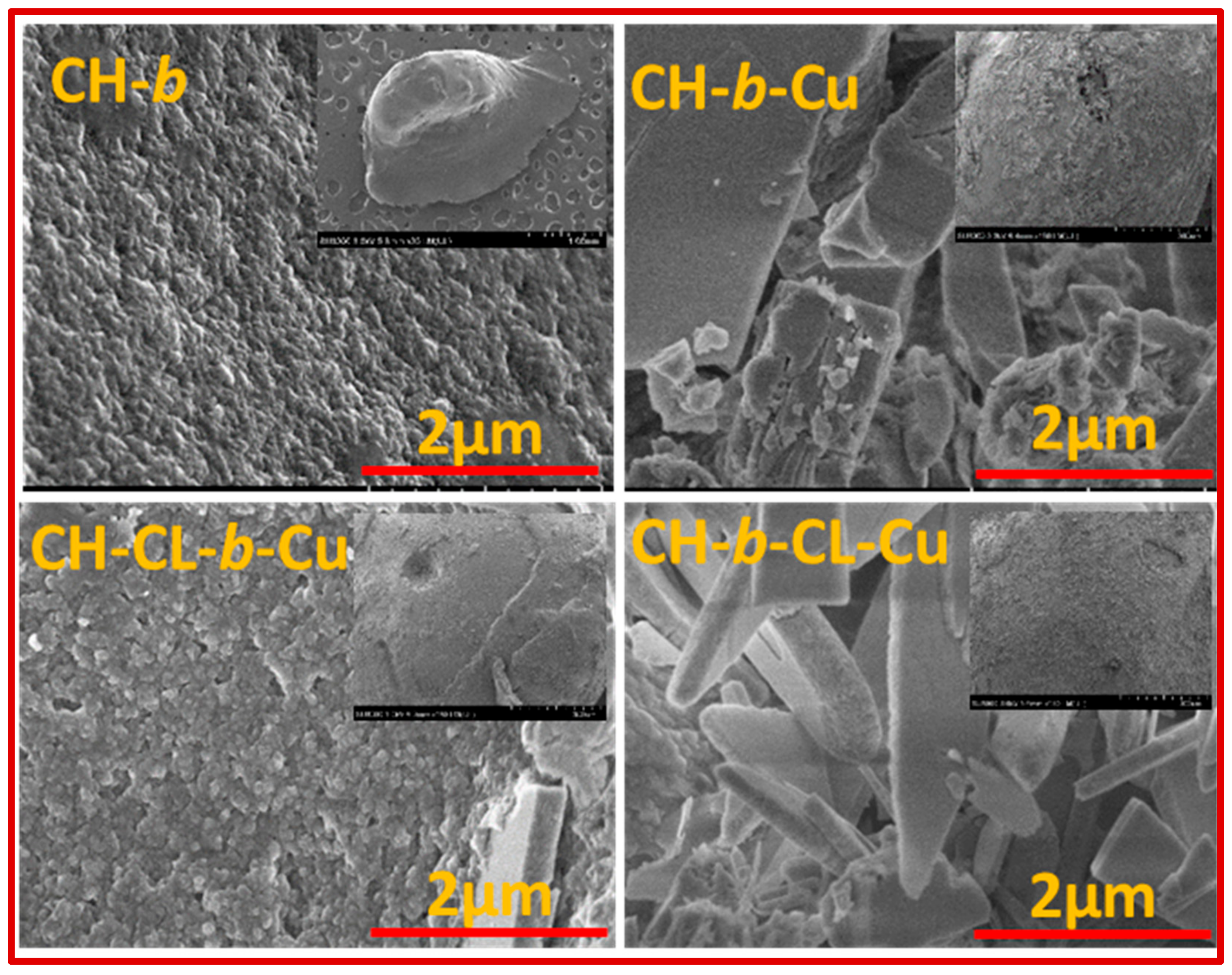
2.1.4. Scanning Electron Microscopy (SEM)
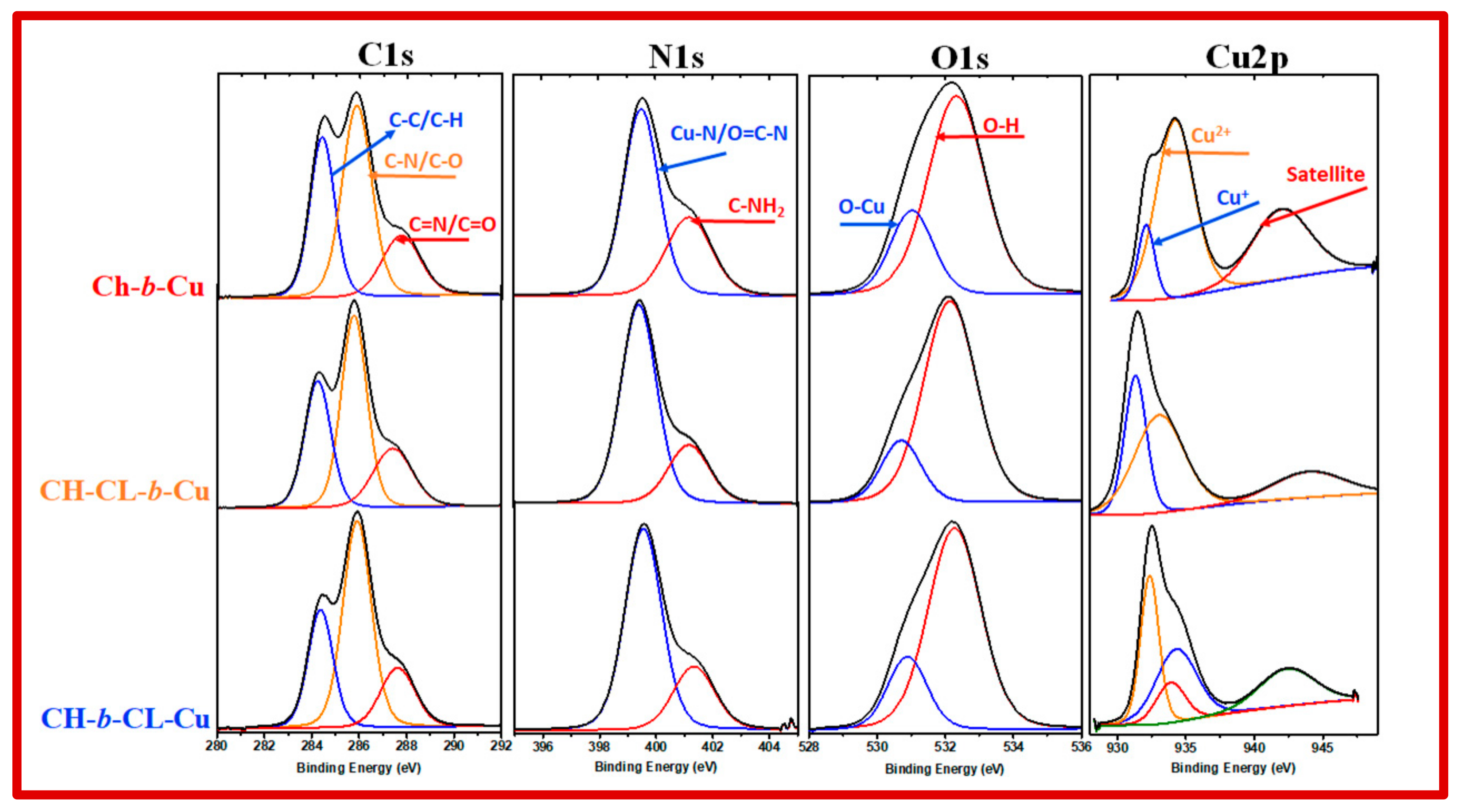
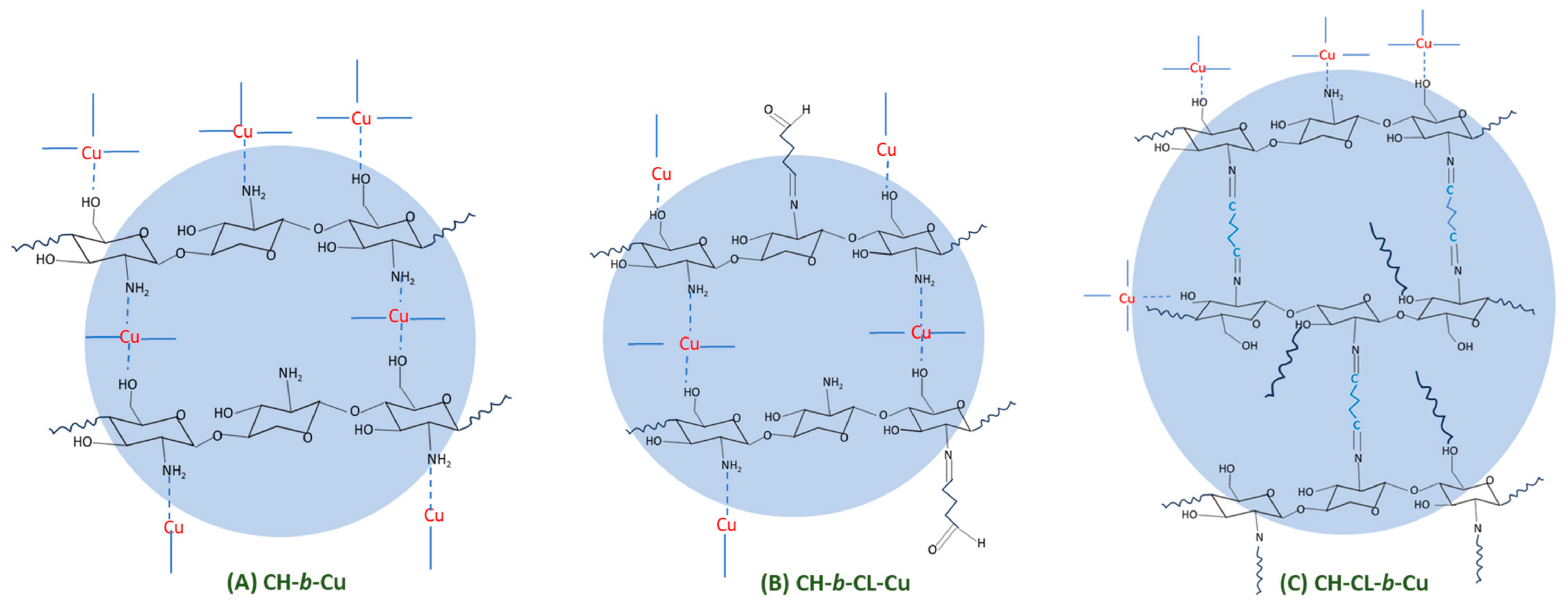
2.1.5. X-ray Photoelectron Spectroscopy (XPS)
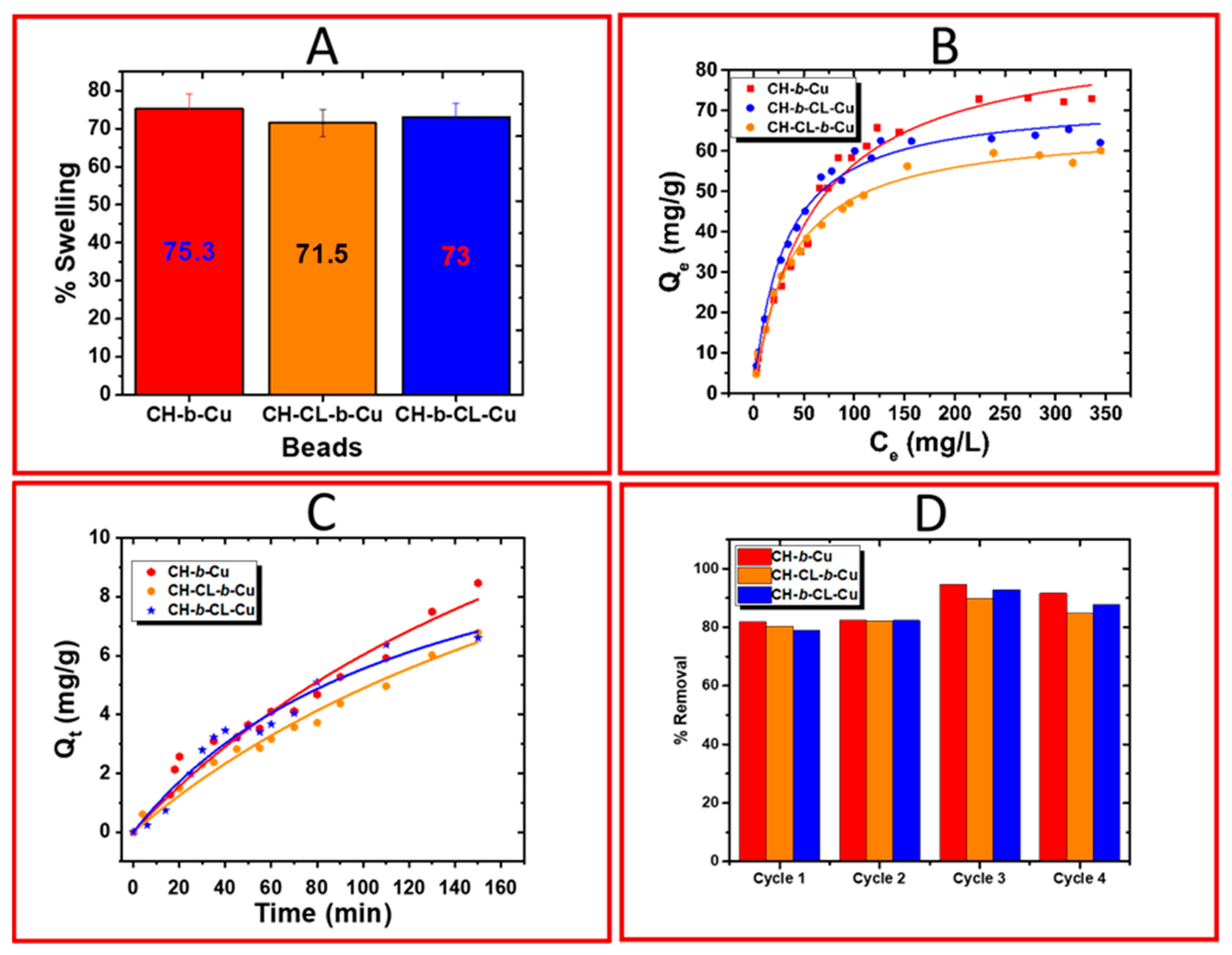
2.1.6. Equilibrium Swelling Studies
2.2. Adsorption Studies
2.2.1. Equilibrium Uptake Studies
2.2.2. Kinetic Uptake Studies
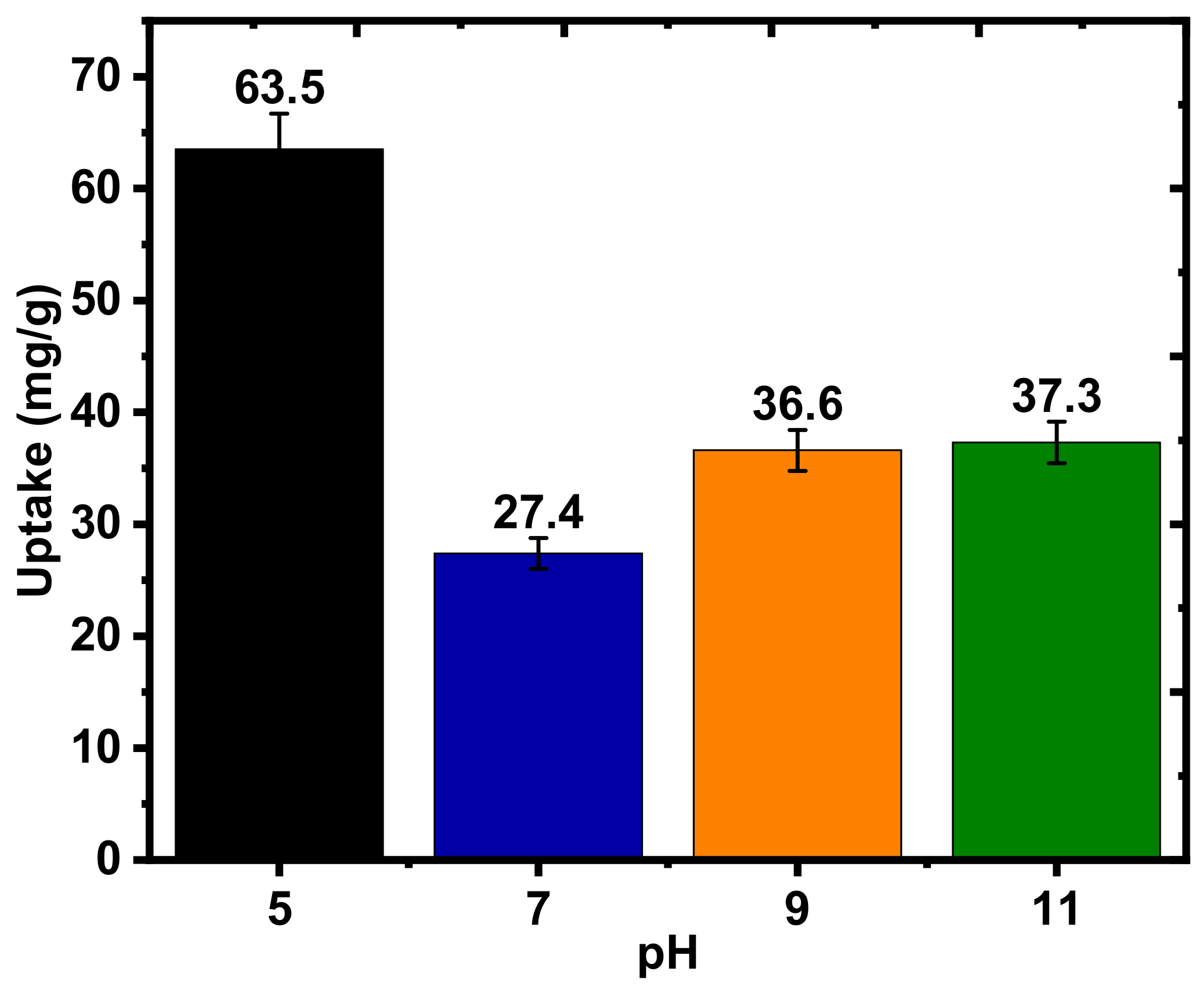
2.2.3. Effects of pH
2.2.4. Regeneration Studies
2.3. Comparative Studies of Other Chitosan-Based Adsorbents
3. Experimental
3.1. Materials
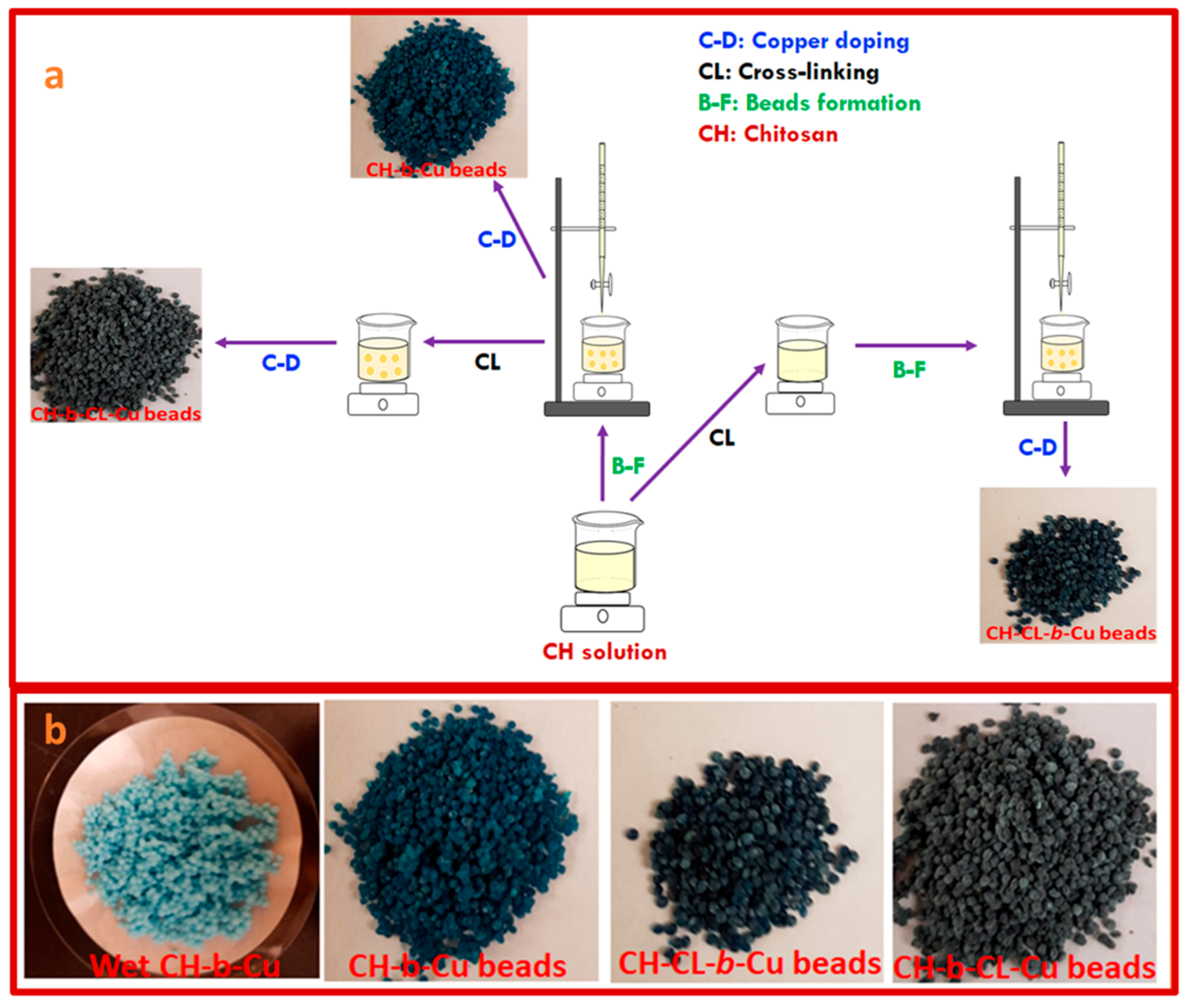
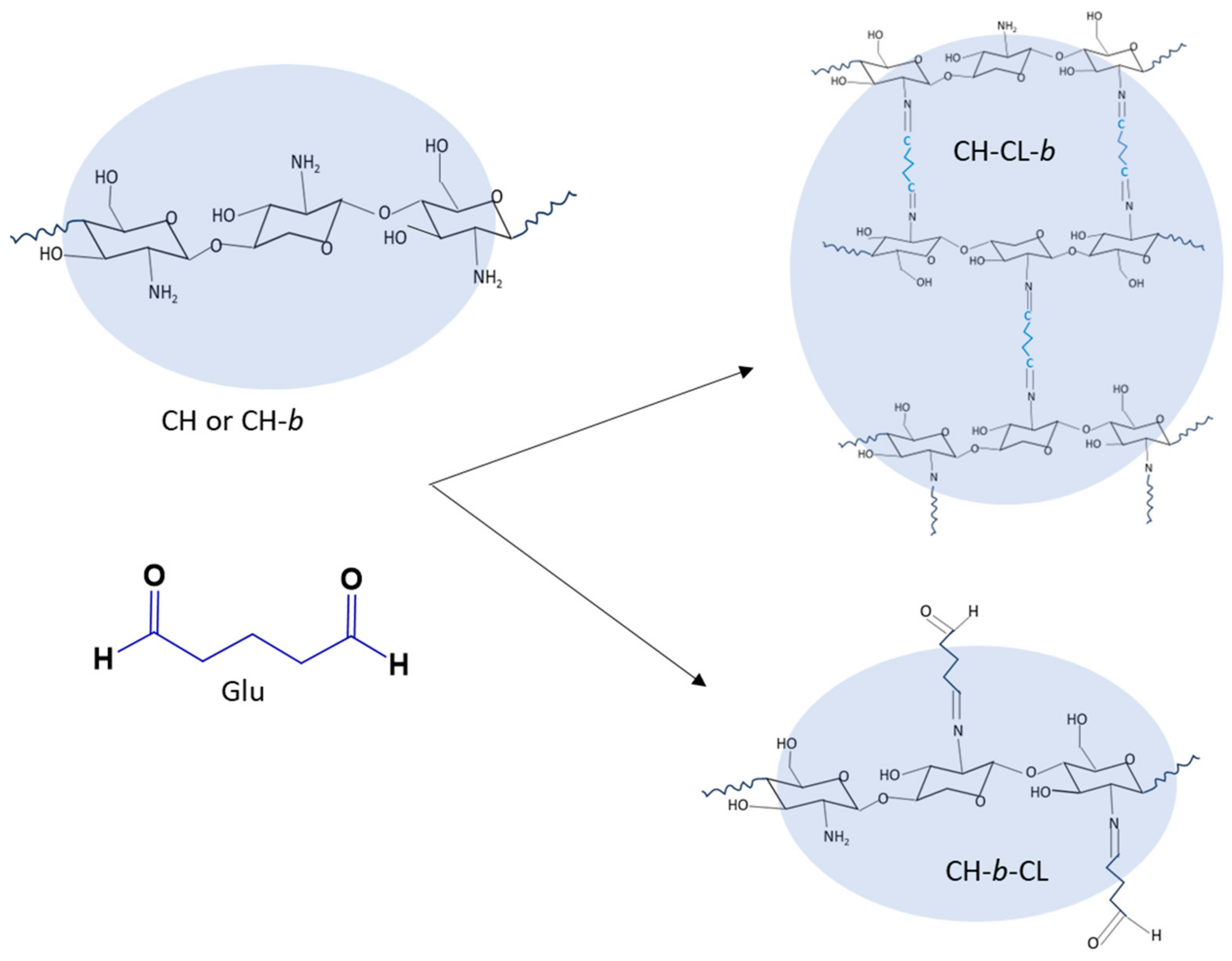
3.2. Synthesis of Copper-Imbibed Chitosan Beads
3.3. Characterization
3.3.1. Thermogravimetric Analysis (TGA)
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Solid State 13C NMR Spectroscopy
3.3.5. X-ray Photoelectron Spectroscopy (XPS)
3.3.6. Equilibrium Swelling Studies
3.4. Adsorption Studies
3.4.1. Equilibrium Sorption of Phosphate Anions
3.4.2. Kinetic Uptake Studies
3.5. Regeneration Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akpor, O.B.; Momba, M.N.B.; Okonkwo, J. Effect of Nutrient/Carbon Supplements on Biological Phosphate and Nitrate Uptake by Protozoan Isolates. J. Appl. Sci. 2008, 8, 489–495. [Google Scholar] [CrossRef]
- İrdemez, Ş.; Demircioğlu, N.; Yildiz, Y.Ş. The Effects of PH on Phosphate Removal from Wastewater by Electrocoagulation with Iron Plate Electrodes. J. Hazard Mater. 2006, 137, 1231–1235. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface and groundwater. In Encyclopedia of the Anthropocene; Dellasala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; pp. 225–240. ISBN 978-0-12-813576-1. [Google Scholar]
- Zahed, M.A.; Salehi, S.; Tabari, Y.; Farraji, H.; Ataei-Kachooei, S.; Zinatizadeh, A.A.; Kamali, N.; Mahjouri, M. Phosphorus Removal and Recovery: State of the Science and Challenges. Environ. Sci. Pollut. Res. 2022, 29, 58561–58589. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W. Eutrophication and Recovery in Experimental Lakes: Implications for Lake Management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Agbovi, H. Biopolymer Flocculant Systems and Their Chemically Modified Forms for Aqueous Phosphate and Kaolinite Removal. Ph.D. Thesis, University of Saskatchewan, Saskatoon, Canada, 2020. Available online: https://harvest.usask.ca/items/f7c3d7b4-bc84-49c6-b0b3-c85eabc5a386 (accessed on 4 January 2024).
- Parasana, N.; Shah, M.; Unnarkat, A. Recent Advances in Developing Innovative Sorbents for Phosphorus Removal—Perspective and Opportunities. Environ. Sci. Pollut. Res. 2022, 29, 38985–39016. [Google Scholar] [CrossRef]
- Barquet, K.; Järnberg, L.; Rosemarin, A.; Macura, B. Identifying Barriers and Opportunities for a Circular Phosphorus Economy in the Baltic Sea Region. Water Res. 2020, 171, 115433. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Xu, Y.; Guo, T.; Dai, H.; Lu, X. Mining Phosphorus from Waste Streams at Wastewater Treatment Plants: A Review of Enrichment, Extraction, and Crystallization Methods. Environ. Sci. Pollut. Res. 2023, 30, 28407–28421. [Google Scholar] [CrossRef]
- Wang, Y.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically Mediated Precipitation of Phosphate Minerals for Phosphorus Removal and Recovery: Progress and Perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Pechsiri, J.S.; Thomas, J.-B.E.; Bahraoui, N.E.; Fernandez, F.G.A.; Chaouki, J.; Chidami, S.; Tinoco, R.R.; Martin, J.P.; Gomez, C.; Combe, M.; et al. Comparative Life Cycle Assessment of Conventional and Novel Microalgae Production Systems and Environmental Impact Mitigation in Urban-Industrial Symbiosis. Sci. Total Environ. 2023, 854, 158445. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhao, Y.; Bo, S.; Yang, L.; Xiao, Z.; An, Q.; Zhai, S. Magnetic Aminated Lignin/CeO2/Fe3O4 Composites with Tailored Interfacial Chemistry and Affinity for Selective Phosphate Removal. Sci. Total Environ. 2021, 796, 148984. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Steiger, B.G.K.; Wilson, L.D. Pyridinium-Furfuryl-Modified Granular Agro-Waste Adsorbent for Orthophosphate Recovery. RSC Sustain. 2023, 1, 1540–1546. [Google Scholar] [CrossRef]
- Rajeswari, A.; Amalraj, A.; Pius, A. Removal of Phosphate Using Chitosan-Polymer Composites. J. Environ. Chem. Eng. 2015, 3, 2331–2341. [Google Scholar] [CrossRef]
- Yang, H.-R.; Li, S.-S.; Shan, X.-C.; Yang, C.; An, Q.-D.; Zhai, S.-R.; Xiao, Z.-Y. Hollow Polyethyleneimine/Carboxymethyl Cellulose Beads with Abundant and Accessible Sorption Sites for Ultra-Efficient Chromium (VI) and Phosphate Removal. Sep. Purif. Technol. 2021, 278, 119607. [Google Scholar] [CrossRef]
- Wan Yusof, W.R.; Awang, N.Y.F.; Azhar Laile, M.A.; Azizi, J.; Awang Husaini, A.A.S.; Seeni, A.; Wilson, L.D.; Sabar, S. Chemically Modified Water-Soluble Chitosan Derivatives: Modification Strategies, Biological Activities, and Applications. Polym. Plast. Technol. Mater. 2023, 62, 2182–2220. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of Various Pollutants from Water and Wastewater by Modified Chitosan Adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Fractionation of Carboxylate Anions from Aqueous Solution Using Chitosan Cross-Linked Sorbent Materials. RSC Adv. 2015, 5, 82065–82077. [Google Scholar] [CrossRef]
- Quinlan, P.J.; Grishkewich, N.; Tam, K.C. Removal of 2-Naphthoxyacetic Acid from Aqueous Solution Using Quaternized Chitosan Beads. Can. J. Chem. Eng. 2017, 95, 21–32. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Faye, O.; Wilson, L.D. Adsorption of Phosphate Dianions by Hybrid Inorganic–Biopolymer Polyelectrolyte Complexes: Experimental and Computational Studies. ACS Appl. Polym. Mater. 2020, 2, 899–910. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-Assembled and Cross-Linked Animal and Plant-Based Polysaccharides: Chitosan-Cellulose Composites and Their Anion Uptake Properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef] [PubMed]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Hybrid Zipper-Like Chitosan-Carboxymethyl Cellulose-Ferric Adsorbents for Tunable Anion Adsorption. Carbohydr. Polym. Technol. Appl. 2023, 6, 100335. [Google Scholar] [CrossRef]
- Hassan, M.M.; Mohamed, M.H.; Udoetok, I.A.; Steiger, B.G.K.; Wilson, L.D. Sequestration of Sulfate Anions from Groundwater by Biopolymer-Metal Composite Materials. Polymers 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Filipkowska, U.; Jóźwiak, T. Application of Chemically-Cross-Linked Chitosan for the Removal of Reactive Black 5 and Reactive Yellow 84 Dyes from Aqueous Solutions. J. Polym. Eng. 2013, 33, 735–747. [Google Scholar] [CrossRef]
- Dai, J.; Yang, H.; Yan, H.; Shangguan, Y.; Zheng, Q.; Cheng, R. Phosphate Adsorption from Aqueous Solutions by Disused Adsorbents: Chitosan Hydrogel Beads after the Removal of Copper(II). Chem. Eng. J. 2011, 166, 970–977. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, P.; Wang, J.; Zhang, L.; Huang, R. Phosphate Adsorption from Aqueous Solutions by Zirconium (IV) Loaded Cross-Linked Chitosan Particles. J. Taiwan Inst. Chem. Eng. 2016, 59, 311–319. [Google Scholar] [CrossRef]
- Jung, K.-Y.; An, B.; Choi, J.-W.; Park, C.; Lee, S. Separation of Phosphate from Wastewater Using an Ion Exchanger Based on Chitosan. Glob. Environ. Res. 2015, 19, 35–42. [Google Scholar]
- An, B.; Jung, K.Y.; Lee, S.H.; Lee, S.; Choi, J.W. Effective Phosphate Removal from Synthesized Wastewater Using Copper-Chitosan Bead: Batch and Fixed-Bed Column Studies. Water Air Soil Pollut. 2014, 225, 2050. [Google Scholar] [CrossRef]
- Sowmya, A.; Meenakshi, S. A Novel Quaternized Chitosan–Melamine–Glutaraldehyde Resin for the Removal of Nitrate and Phosphate Anions. Int. J. Biol. Macromol. 2014, 64, 224–232. [Google Scholar] [CrossRef]
- Banu, H.T.; Meenakshi, S. One Pot Synthesis of Chitosan Grafted Quaternized Resin for the Removal of Nitrate and Phosphate from Aqueous Solution. Int. J. Biol. Macromol. 2017, 104, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Bozorgpour, F.; Ramandi, H.F.; Jafari, P.; Samadi, S.; Yazd, S.S.; Aliabadi, M. Removal of Nitrate and Phosphate Using Chitosan/Al2O3/Fe3O4 Composite Nanofibrous Adsorbent: Comparison with Chitosan/Al2O3/Fe3O4 Beads. Int. J. Biol. Macromol. 2016, 93, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the Nature of the Metal Ions on the Complexation with Chitosan.: Application to the Treatment of Liquid Waste. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the Study of the Complexation of Copper by Chitosan and Oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Zhao, Y.; Yang, C.-P.; Jing, S.; Wu, Q.; Brozena, A.; Miller, J.; Libretto, N.; Wu, T.; et al. A High-Performance Hydroxide Exchange Membrane Enabled by Cu2+-Crosslinked Chitosan. Nat. Nanotechnol. 2022, 17, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Del Gamboa-Solana, C.C.; Chuc-Gamboa, M.G.; Aguilar-Pérez, F.J.; Cauich-Rodríguez, J.V.; Vargas-Coronado, R.F.; Aguilar-Pérez, D.A.; Herrera-Atoche, J.R.; Pacheco, N. Zinc Oxide and Copper Chitosan Composite Films with Antimicrobial Activity. Polymers 2021, 13, 3861. [Google Scholar] [CrossRef]
- Olanipekun, E.O.; Ayodele, O.; Olatunde, O.C.; Olusegun, S.J. Comparative Studies of Chitosan and Carboxymethyl Chitosan Doped with Nickel and Copper: Characterization and Antibacterial Potential. Int. J. Biol. Macromol. 2021, 183, 1971–1977. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Mohamed, H.I.; Al-Subaie, A.M.; Al-Ohali, A.I.; Mahmoud, N.M.R. Investigation of the Antimicrobial Activity and Hematological Pattern of Nano-Chitosan and Its Nano-Copper Composite. Sci. Rep. 2021, 11, 9540. [Google Scholar] [CrossRef]
- Syame, S.M.; Mohamed, W.S.; Mahmoud, R.K.; Omara, S.T. Synthesis of Copper-Chitosan Nanocomposites and Their Applications in Treatment of Local Pathogenic Isolates Bacteria. Orient. J. Chem. 2017, 33, 2959–2969. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Wilson, L.D. Modular Cross-Linked Chitosan Beads with Calcium Doping for Enhanced Adsorptive Uptake of Organophosphate Anions. Ind. Eng. Chem. Res. 2016, 55, 11706–11715. [Google Scholar] [CrossRef]
- Li, B.; Shan, C.-L.; Zhou, Q.; Fang, Y.; Wang, Y.-L.; Xu, F.; Han, L.-R.; Ibrahim, M.; Guo, L.-B.; Xie, G.-L.; et al. Synthesis, Characterization, and Antibacterial Activity of Cross-Linked Chitosan-Glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.; Arun, T.; Manickam, S.T.D. A Review on Applications of Chitosan-Based Schiff Bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, J.; Abu Naim, A.; Wan Ibrahim, W.A. New Crosslinked-Chitosan Graft Poly(N-Vinyl-2-Pyrrolidone) for the Removal of Cu(II) Ions from Aqueous Solutions. Int. J. Biol. Macromol. 2018, 107, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, A.M. Green Synthesis of Copper-Chitosan Nanoparticles and Study of Its Antibacterial Activity. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Rogina, A.; Lončarević, A.; Antunović, M.; Marijanović, I.; Ivanković, M.; Ivanković, H. Tuning Physicochemical and Biological Properties of Chitosan through Complexation with Transition Metal Ions. Int. J. Biol. Macromol. 2019, 129, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Cárdenas, G. Chitosan-Copper Paint Types as Antifouling. J. Chil. Chem. Soc. 2014, 59, 2415–2419. [Google Scholar] [CrossRef]
- Xue, C. Investigation of Chitosan-Based Materials for Sorptive Uptake of Urea. Master’s Thesis, University of Saskatchewan, Saskatoon, Canada, 2015. Available online: https://harvest.usask.ca/items/ba9c7300-55c6-4602-9c53-b39373462afc (accessed on 14 January 2024).
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium Studies of the Sorption of Cu(II) Ions onto Chitosan. J. Colloid Interface Sci. 2002, 255, 64–74. [Google Scholar] [CrossRef]
- Filipkowska, U.; Jóźwiak, T.; Szymczyk, P. Application of Cross-Linked Chitosan for Phosphate Removal from Aqueous Solutions. Prog. Chem. Appl. Chitin Deriv. 2014, XIX, 5–14. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Rodziewicz, J.; Nowosad, E. Effect of Cross-Linking with Glutaraldehyde on Adsorption Capacity of Chitosan Beads. Prog. Chem. Appl. Chitin Deriv. 2013, 18, 35–48. [Google Scholar]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef]
- Dambies, L.; Guimon, C.; Yiacoumi, S.; Guibal, E. Characterization of Metal Ion Interactions with Chitosan by X-Ray Photoelectron Spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2001, 177, 203–214. [Google Scholar] [CrossRef]
- Modrzejewska, Z. Sorption Mechanism of Copper in Chitosan Hydrogel. React Funct. Polym. 2013, 73, 719–729. [Google Scholar] [CrossRef]
- Vieira, R.S.; Oliveira, M.L.M.; Guibal, E.; Rodríguez-Castellón, E.; Beppu, M.M. Copper, Mercury and Chromium Adsorption on Natural and Crosslinked Chitosan Films: An XPS Investigation of Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2011, 374, 108–114. [Google Scholar] [CrossRef]
- Duffy, C.; O’Riordan, D.; O’Sullivan, M.; Jacquier, J. In Vitro Evaluation of Chitosan Copper Chelate Gels as a Multimicronutrient Feed Additive for Cattle. J. Sci. Food Agric. 2018, 98, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Sritweesinsub, W.; Charuchinda, S. Alginate/Carboxymethyl Cellulose Hydrogel Films in Relation to Crosslinking with Glutaraldehyde and Copper Sulfate. MATEC Web Conf. 2015, 30, 02005. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. Controlled Drug Release Properties of Ionically Cross-Linked Chitosan Beads: The Influence of Anion Structure. Int. J. Pharm. 2002, 233, 217–225. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, P.; Luo, S.; Tu, X.; Cao, Q.; Shu, M. Synthesis of Novel Nanocomposite Fe3O4/ZrO2/Chitosan and Its Application for Removal of Nitrate and Phosphate. Appl. Surf. Sci. 2013, 284, 942–949. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L. Removal of Phosphate Anions Using the Modified Chitosan Beads: Adsorption Kinetic, Isotherm and Mechanism Studies. Powder Technol. 2015, 277, 112–119. [Google Scholar] [CrossRef]
- Yazdani, M.R.; Virolainen, E.; Conley, K.; Vahala, R. Chitosan-Zinc(II) Complexes as a Bio-Sorbent for the Adsorptive Abatement of Phosphate: Mechanism of Complexation and Assessment of Adsorption Performance. Polymers 2017, 10, 25. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Potential of Phosphate Ion Removal Using an Al3+-Cross-Linked Chitosan-g-Poly(Acrylic Acid)/Vermiculite Ionic Hybrid. Adsorpt. Sci. Technol. 2010, 28, 89–99. [Google Scholar] [CrossRef]
- Kumar, I.A.; Viswanathan, N. Preparation and Testing of a Tetra-Amine Copper(II) Chitosan Bead System for Enhanced Phosphate Remediation. Carbohydr. Polym. 2018, 183, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, A.; Meenakshi, S. Zr(IV) Loaded Cross-Linked Chitosan Beads with Enhanced Surface Area for the Removal of Nitrate and Phosphate. Int. J. Biol. Macromol. 2014, 69, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Mahaninia, M. Preparation and Characterization of Cross-Linked Chitosan Beads for Phosphate Adsorption in Aqueous Solution. Master’s Thesis, University of Saskatchewan, Saskatoon, Canada, 2016. [Google Scholar]
- An, B.; Choi, J.-W. An Experimental Application of Four Types of Chitosan Bead for Removal of Cationic and Anionic Pollutants. Water Air Soil Pollut. 2019, 230, 314. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D. Kinetic Uptake Studies of Powdered Materials in Solution. Nanomaterials 2015, 5, 969–980. [Google Scholar] [CrossRef]

| Elements/Beads | Copper (Cu2p) | Carbon (C1s) | Oxygen (O1s) | Nitrogen (N1s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position | Area | % | Position | Area | % | Position | Area | % | Position | Area | % | |
| CH-b-Cu | 932.1 | 2385.3 | 10.5 | 284.4 | 10,688 | 33.3 | 531.0 | 13,528 | 24.4 | 399.5 | 2670.7 | 65.0 |
| 934.2 | 12,756.7 | 56 | 285.9 | 15,177 | 47.3 | 532.3 | 42,009 | 75.6 | 401.1 | 1409 | 35.0 | |
| 942 | 7635.4 | 33.5 | 287.7 | 6244.8 | 19.4 | NA | ||||||
| CH-CL-b-Cu | 930.7 | 4545.6 | 31.3 | 284.2 | 10,941 | 31.7 | 530.7 | 10,909 | 18.8 | 399.4 | 4083.3 | 74.9 |
| 932.1 | 7263.3 | 49.9 | 285.8 | 16,186 | 46.7 | 532.1 | 47,251 | 81.2 | 400.2 | 1371.7 | 25.1 | |
| 940.9 | 2740.1 | 18.8 | 287.4 | 3794.5 | 21.7 | NA | ||||||
| CH-b-CL-Cu | 932.4 | 5881.9 | 30.5 | 284.4 | 8586.4 | 27.2 | 530.9 | 12,203 | 21.3 | 399.5 | 3978.2 | 73.8 |
| 933.9 | 2546.1 | 13.3 | 285.9 | 17,011 | 53.8 | 532.3 | 45,216 | 78.7 | 400.3 | 1413.2 | 26.2 | |
| 934.3 | 6384.6 | 33.1 | 287.6 | 5954.8 | 18.8 | NA | ||||||
| 942.4 | 4456.7 | 23.1 | NA | |||||||||
| Adsorbent | pH | Qm (mg/g) | Ref | |
|---|---|---|---|---|
| 1 | Glutaraldehyde/copper cross-linked chitosan beads | 7.5–8.0 | 42.8 | [31] |
| 2 | Copper cross-linked chitosan beads | 7.5–8.0 | 53.6 | [31] |
| 3 | Epichlorohydrin cross-linked chitosan beads | 3 | 139 | [27] |
| 4 | Glutaraldehyde cross-linked chitosan beads | 3 | 108 | [27] |
| 5 | Chitosan Zn II complex | 4 | 6.55 | [62] |
| 6 | Zirconium-modified chitosan beads | 4 | 60.6 | [61] |
| 7 | PEG chitosan composite | 3 | 74.9 | [17] |
| 8 | PVA chitosan composite | 3 | 46.2 | [17] |
| 9 | Fe3O4/ZrO2/chitosan | 3 | 79.5 * | [60] |
| 10 | Tetra amine Cu(II) chitosan | 7 | 41.4 | [64] |
| 11 | Quaternized chitosan–melamine–glutaraldehyde resin beads | 3–10 | 113 | [32] |
| 12 | Al3+ cross-linked chitosan g-poly (acrylic acid) and vermiculite ionic hybrid | 3–6 | 22.6 | [63] |
| 13 | Zr(IV)-loaded chitosan beads | 4–8 | 43.9 | [65] |
| 14 | Cross-linked chitosan bead with EP | 8.5 | 52.1 | [66] |
| 15 | Hydrogel chitosan beads (HCB) | 3–5 | 4.19 | [67] |
| 16 | Cross-linked HCB | 3–5 | 251 | [67] |
| 17 | Dried chitosan bead (DCB) | 3–5 | 232 | [67] |
| 18 | Cross-linked DCB | 3–5 | 58.4 | [67] |
| 19 | Copper cross-linked chitosan (CH-b-Cu) beads | 8.5 | 133 ± 45 | This study |
| 20 | Glutaraldehyde/Cu(II) cross-linked chitosan (CH-CL-b-Cu) beads | 8.5 | 80.2 ± 5.4 | This study |
| 21 | Glutaraldehyde/Cu(II) cross-linked chitosan (CH-b-CL-Cu) beads | 8.5 | 83.9 ± 11.8 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udoetok, I.A.; Karoyo, A.H.; Mohamed, M.H.; Wilson, L.D. Chitosan Biocomposites with Variable Cross-Linking and Copper-Doping for Enhanced Phosphate Removal. Molecules 2024, 29, 445. https://doi.org/10.3390/molecules29020445
Udoetok IA, Karoyo AH, Mohamed MH, Wilson LD. Chitosan Biocomposites with Variable Cross-Linking and Copper-Doping for Enhanced Phosphate Removal. Molecules. 2024; 29(2):445. https://doi.org/10.3390/molecules29020445
Chicago/Turabian StyleUdoetok, Inimfon A., Abdalla H. Karoyo, Mohamed H. Mohamed, and Lee D. Wilson. 2024. "Chitosan Biocomposites with Variable Cross-Linking and Copper-Doping for Enhanced Phosphate Removal" Molecules 29, no. 2: 445. https://doi.org/10.3390/molecules29020445
APA StyleUdoetok, I. A., Karoyo, A. H., Mohamed, M. H., & Wilson, L. D. (2024). Chitosan Biocomposites with Variable Cross-Linking and Copper-Doping for Enhanced Phosphate Removal. Molecules, 29(2), 445. https://doi.org/10.3390/molecules29020445