In Vivo Wound Healing Potential and Molecular Pathways of Amniotic Fluid and Moringa Olifera-Loaded Nanoclay Films
Abstract
1. Introduction
2. Results
2.1. Physical Analysis of Films
2.2. Antioxidant Activity
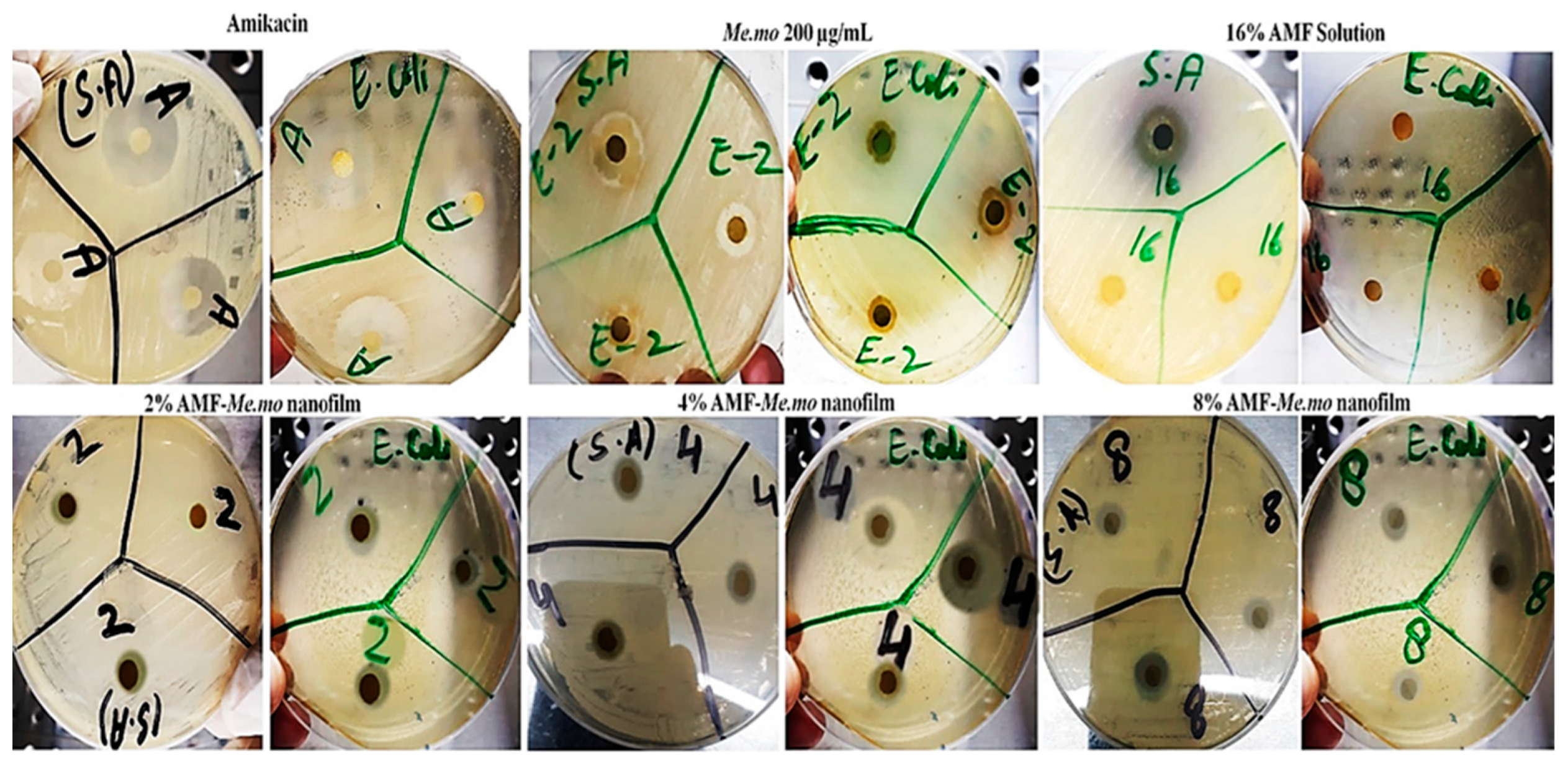
2.3. Antibacterial Activity
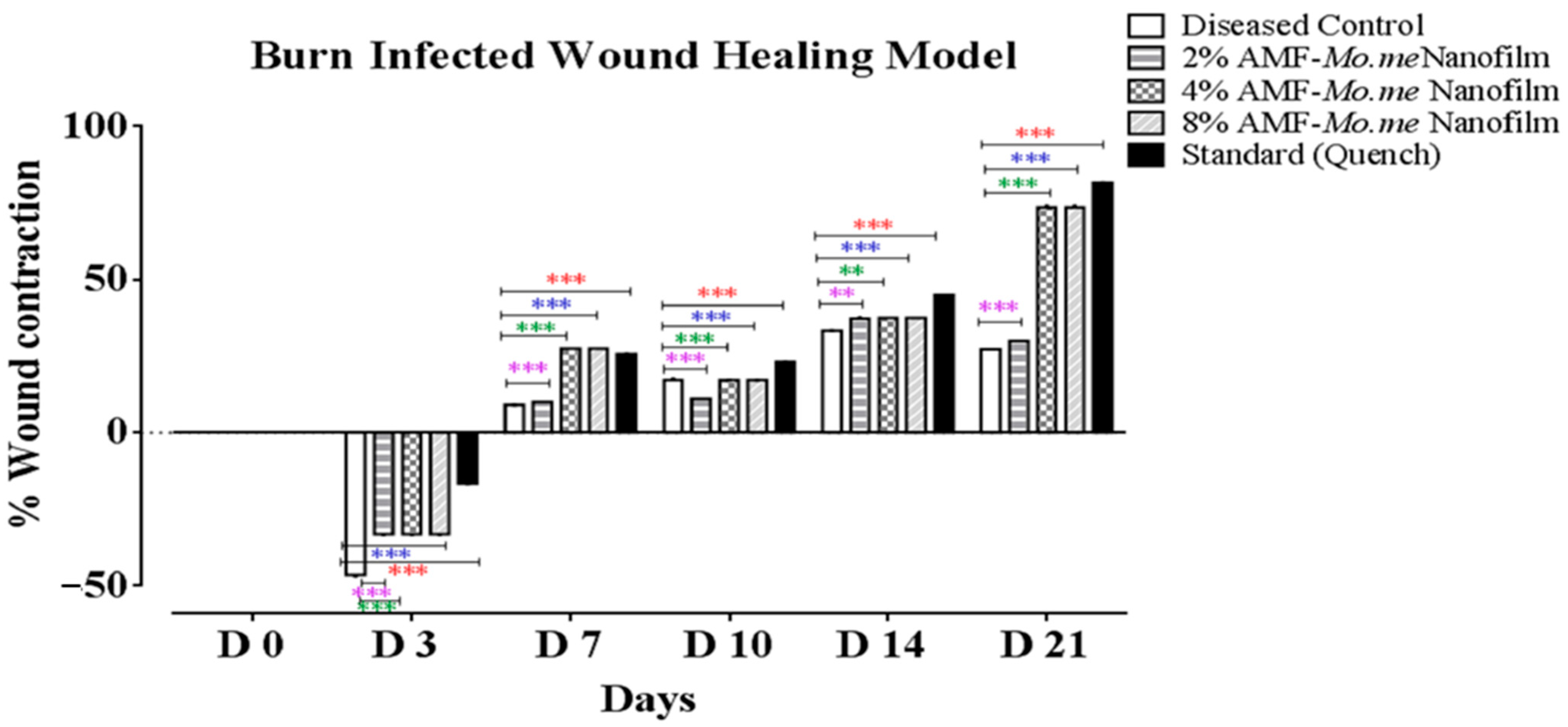
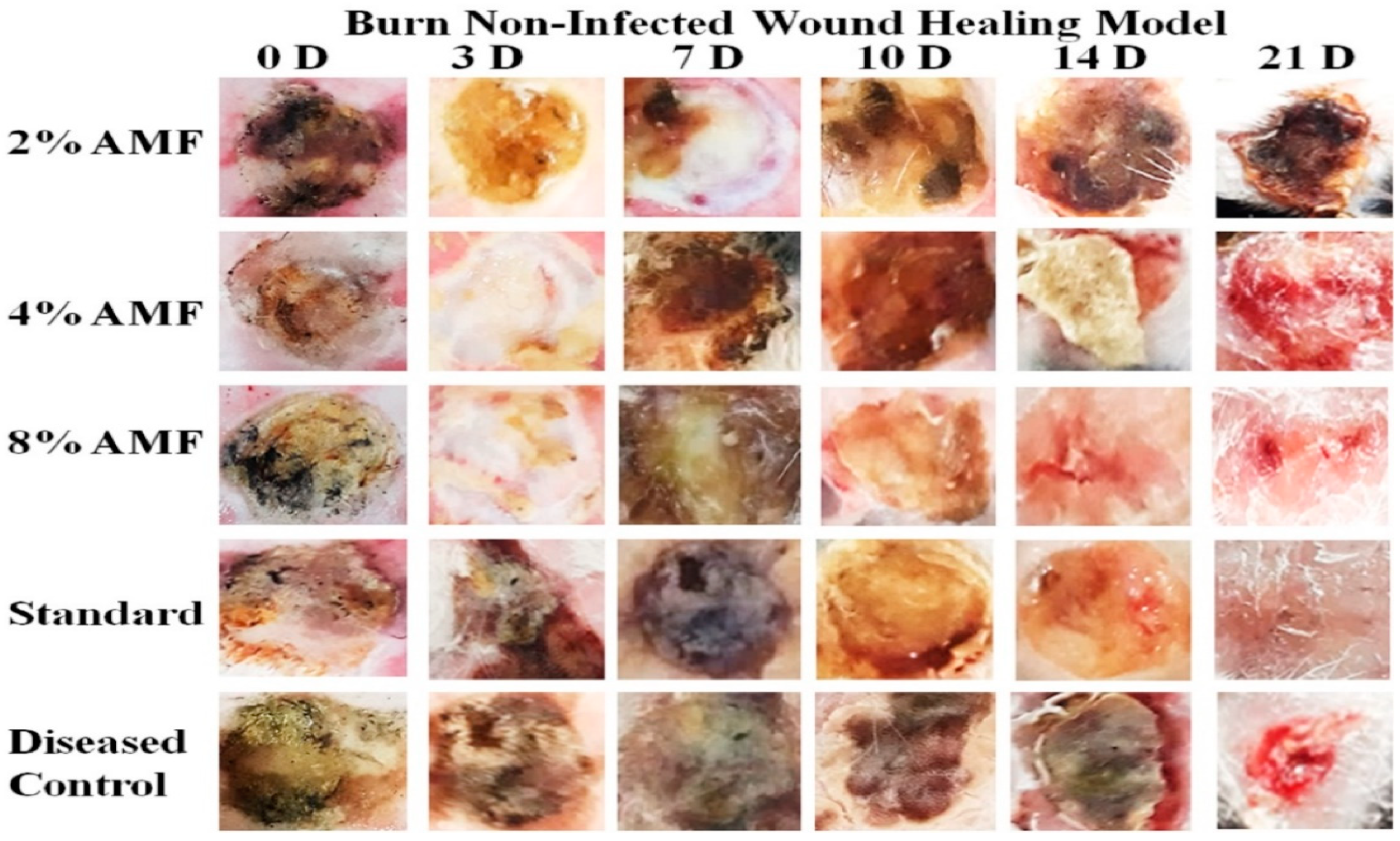
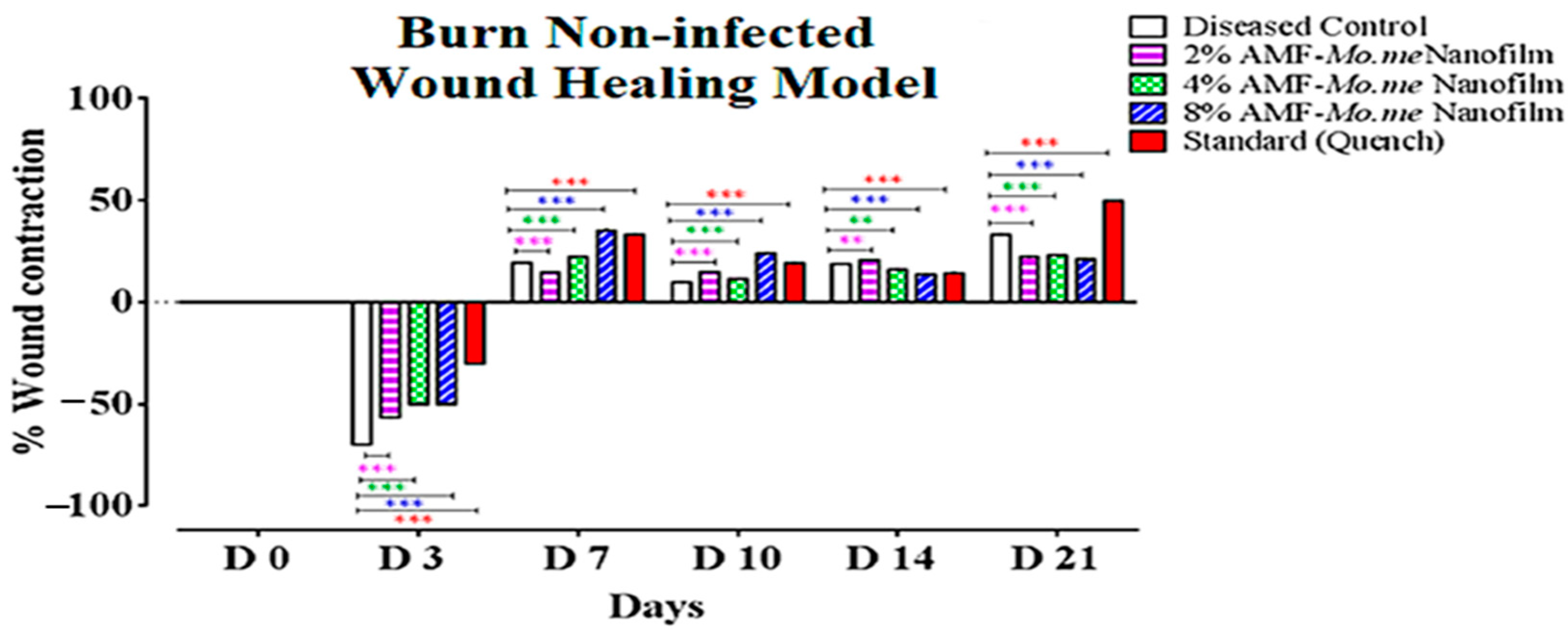
2.4. Wound Healing Activity
2.5. Measurement of TNF-α and TGF-ß1 and MMP Levels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Collection of Plant Material
4.3. Extract Preparation
4.4. Isolation and Preparation of Amniotic Membrane Fluid
4.5. Preparation of AMF- and Moringa olifera-Loaded Nanocomposites
4.6. Physical Analysis of Films
4.6.1. Films Thickness
4.6.2. Moisture Content
4.7. In Vitro Studies
4.7.1. Antibacterial Studies
4.7.2. Antioxidant Activity through 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
4.8. In Vivo Studies
4.8.1. Burn Wound Model
4.8.2. Wound Healing Activity
4.8.3. Histopathological Examination
4.8.4. Measurement of Inflammatory Mediators and Growth Factors with Real Time Polymerase Chain Reaction (RT-PCR)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridiandries, A.; Tan, J.; Bursill, C.A. The role of chemokines in wound healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Gonzalez, A.C.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Kaleem, S.; Khan, A.K.; Murtaza, G. Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Berberis lycium nanofibers. Green Process. Synth. 2022, 11, 229–237. [Google Scholar] [CrossRef]
- Teot, L.; Ohura, N. Challenges and Management in Wound Care. Plast. Reconstr. Surg. 2021, 147, 9S–15S. [Google Scholar] [CrossRef]
- Clinton, A.; Carter, T. Chronic wound biofilms: Pathogenesis and potential therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial contribution in chronicity of wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Fukutake, M.; Ochiai, D.; Masuda, H.; Abe, Y.; Sato, Y.; Otani, T.; Sakai, S.; Aramaki-Hattori, N.; Shimoda, M.; Matsumoto, T.; et al. Human amniotic fluid stem cells have a unique potential to accelerate cutaneous wound healing with reduced fibrotic scarring like a fetus. Hum. Cell 2019, 32, 51–63. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet rich plasma: New insights for cutaneous wound healing management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Subhan, B.S.; Kwong, J.; Kuhn, J.F.; Monas, A.; Sharma, S.; Rabbani, P.S. Amniotic fluid-derived multipotent stromal cells drive diabetic wound healing through modulation of macrophages. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and opportunities in drug delivery for wound healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, M.; Ilyas, S.; Mir, S.; Khan, A.K.; Rashid, R.; Khan, M.Z.; Kanwal, Z.G.; Nawaz, A.; Shah, A.; Murtaza, G. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An. Acad. Bras. Ciências 2016, 88, 2303–2317. [Google Scholar] [CrossRef]
- Khan, I.U.; Mahmood, H.; Shahzad, Y.; Asghar, S.; Syed, H.K. Hydrogel Composite Films for Wound Healing; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Shah, A.; Buabeid, M.A.; Arafa, E.-S.A.; Hussain, I.; Li, L.; Murtaza, G. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Zeng, J.; Matsusaki, M. Layer-by-layer assembly of nanofilms to control cell functions. Polym. Chem. 2019, 10, 2960–2974. [Google Scholar] [CrossRef]
- Kshirsagar, T.; Jaiswal, N.; Chavan, G.; Zambre, K.; Ramkrushna, S.; Dinesh, D. Formulation & evaluation of fast dissolving oral film. World J. Pharm. Res. 2021, 10, 503–561. [Google Scholar]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H. Recent advances of nanocellulose in drug delivery systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm. Res. IJPR 2019, 18, 430. [Google Scholar]
- Salimi, F.; Mohammadipanah, F. Nanomaterials Versus The Microbial Compounds With Wound Healing Property. Front. Nanotechnol. 2021, 2, 21. [Google Scholar] [CrossRef]
- Ashok Kumar, N.; Pari, L. Antioxidant action of Moringa oleifera Lam. (drumstick) against antitubercular drugs induced lipid peroxidation in rats. J. Med. Food 2003, 6, 255–259. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Bardania, H.; Mahmoudi, R.; Bagheri, H.; Salehpour, Z.; Fouani, M.H.; Darabian, B.; Khoramrooz, S.S.; Mousavizadeh, A.; Kowsari, M.; Moosavifard, S.E.; et al. Facile preparation of a novel biogenic silver-loaded Nanofilm with intrinsic anti-bacterial and oxidant scavenging activities for wound healing. Sci. Rep. 2020, 10, 6129. [Google Scholar] [CrossRef]
- Demilew, W.; Adinew, G.M.; Asrade, S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae). Evid. Based Complement. Altern. Med. 2018, 2018, 2047896. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- De Masi, E.C.D.J.; Campos, A.C.L.; De Masi, F.D.J.; Ratti, M.A.S.; Ike, I.S.; De Masi, R.D.J. The influence of growth factors on skin wound healing in rats☆. Braz. J. Otorhinolaryngol. 2016, 82, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.-M.; Lee, K.-W.; Moon, B.-K.; Hwang, I.-K. Antioxidant characteristics and preparation of chocolate added with sochungryong-tang (oriental medicinal plants extract). Korean J. Food Cook. Sci. 2005, 21, 585–590. [Google Scholar]
- Gholipourmalekabadi, M.; Farhadihosseinabadi, B.; Faraji, M.; Nourani, M.R. How preparation and preservation procedures affect the properties of amniotic membrane? How safe are the procedures? Burns 2020, 46, 1254–1271. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Ashames, A.A.; Buabeid, M.A.; Murtaza, G. Synthesis, in vitro characterization and antibacterial efficacy of moxifloxacin-loaded chitosan-pullulan-silver-nanocomposite films. J. Drug Deliv. Sci. Technol. 2020, 55, 101366. [Google Scholar] [CrossRef]
- Shah, A.; Hussain, I.; Murtaza, G. Chemical synthesis and characterization of chitosan/silver nanocomposites films and their potential antibacterial activity. Int. J. Biol. Macromol. 2018, 116, 520–529. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Bibi, S.; Khan, A.K.; Murtaza, G. Development and butyrylcholinesterase/monoamine oxidase inhibition potential of PVA-Moringa oleifera developed nanofibers9. J. Exp. Nanosci. 2022, 17, 34–46. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tchuenguem, R.T.; Iqbal, J.; Yameen, M.A.; Mannan, A.; Shahzadi, I.; Ismail, T.; Fatima, N.; Murtaza, G. Anticandidal activity of green synthesised silver nanoparticles and extract loaded chitosan nanoparticles of Euphorbia prostata. Artif. Cells Nanomed. Biotechnol. 2022, 50, 188–197. [Google Scholar] [CrossRef]
- Sobajima, S.; Shimer, A.L.; Chadderdon, R.C.; Kompel, J.F.; Kim, J.S.; Gilbertson, L.G.; Kang, J.D. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. Spine J. 2005, 5, 14–23. [Google Scholar] [CrossRef]


| Formulation Code | Film Thickness (mm) | Moisture Content (%) |
|---|---|---|
| 2% AMF-Mo.me | 0.5 ± 0.02 | 56.4 ± 0.12 |
| 4% AMF-Mo.me | 0.8 ± 0.01 | 40.5 ± 0.09 |
| 8% AMF-Mo.me | 0.6 ± 0.01 | 33.6 ± 0.05 |
| Formulation Code | IC50 |
|---|---|
| 2% AMF-Mo.me | 126.20 ± 0.11 |
| 4% AMF-Mo.me | 67.78 ± 0.07 |
| 8% AMF-Mo.me | 68.03 ± 0.13 |
| Ascorbic acid (standard) | 27.44 ± 0.02 |
| Staphylococcus aureus | ||||||
|---|---|---|---|---|---|---|
| Sr. No. | Zone of Inhibition (mm) | |||||
| Amikacin RS | 2% AMF | 4% AMF | 8% AMF | 16% AMF | Me.mo | |
| 01 | 20.0 | 0.3 | 0.6 | 0.9 | 10.0 | 0.8 |
| 02 | 21.0 | 0.4 | 0.5 | 0.8 | 11.0 | 0.9 |
| 03 | 20.0 | 0.0 | 0.6 | 0.6 | 0.0 | 0.8 |
| Average | 20.3 | 0.2 | 0.6 | 0.8 | 10.5 | 0.8 |
| Escherichia coli | ||||||
| Sr. No. | Zone of inhibition (mm) | |||||
| Amikacin RS | 2% AMF | 4% AMF | 8% AMF | 16% AMF | Me.mo | |
| 01 | 19.0 | 0.2 | 0.4 | 0.7 | 0.0 | 0.6 |
| 02 | 22.0 | 0.1 | 0.4 | 0.8 | 0.0 | 0.5 |
| 03 | 21.0 | 0.2 | 0.5 | 0.6 | 0.0 | 0.6 |
| Average | 20.6 | 0.16 | 0.43 | 0.7 | 0.0 | 0.56 |
| Groups | 3 D | 7 D | 10 D | 14 D | 21 D |
|---|---|---|---|---|---|
| Diseased Control | −76.2 ± 0.5 | 20.9 ± 0.1 | 26.1 ± 0.1 | 35.2 ± 0.2 | 25.0 ± 0.0 |
| 2% AMF-Me.mo Nanofilms | −46.3 ± 0.3 *** | 9.1 ± 0.0 *** | 17.3 ± 0.3 *** | 33.3 ± 0.1 ** | 27.3 ± 0.0 *** |
| 4% AMF-Me.mo Nanofilms | −33.2 ± 0.2v *** | 10.0 ± 0.0 *** | 11.1 ± 0.0 *** | 37.3 ± 0.3 ** | 30.0 ± 0.0 *** |
| 8% AMF-Me.mo Nanofilms | −33.2 ± 0.2 *** | 27.5 ± 0.0 *** | 17.2 ± 0.0 *** | 37.5 ± 0.0 *** | 73.7 ± 0.3 *** |
| Standard (Quench®) | −16.6 ± 0.1 *** | 25.7 ± 0.0 *** | 23.0 ± 0.0 *** | 45.0 ± 0.0 *** | 81.8 ± 0.1 *** |
| Groups | 3 D | 7 D | 10 D | 14 D | 21 D |
|---|---|---|---|---|---|
| Diseased Control | −70.0 ± 0.0 | 19.6 ± 0.0 | 9.7 ± 0.0 | 18.9 ± 0.0 | 33.2 ± 0.2 |
| 2% AMF-Me.mo Nanofilms | −56.6 ± 0.0 *** | 14.9 ± 0.0 *** | 15.0 ± 0.0 *** | 20.6 ± 0.0 *** | 22.2 ± 0.1 *** |
| 4% AMF-Me.mo Nanofilms | −50.0 ± 0.0 *** | 22.2 ± 0.0 *** | 11.4 ± 0.0 *** | 16.1 ± 0.0 ** | 23.1 ± 0.0 *** |
| 8% AMF-Me.mo Nanofilms | −50.0 ± 0.0 *** | 35.3 ± 0.3 *** | 24.1 ± 0.1 *** | 13.6 ± 0.0 ** | 21.0 ± 0.0 *** |
| Standard (Quench®) | −30.0 ± 0.0 *** | 33.2 ± 0.2 *** | 19.2 ± 0.0 *** | 14.3 ± 0.0 *** | 50.0 ± 0.0 *** |
| Groups | TNF-α | VEGF | MMP |
|---|---|---|---|
| Diseased Control | 32.8 ± 0.1 | 32.6 ± 0.1 | 31.2 ± 0.1 |
| 2% AMF-Moringa Nanofilms | 32.5 ± 0.1 | 32.8 ± 0.0 | 31.1 ± 0.1 |
| 4% AMF-Moringa Nanofilms | 32.4 ± 0.1 | 33.1 ± 0.1 | 32.2 ± 0.1 |
| 8% AMF-Moringa Nanofilms | 32.1 ± 0.1 | 33.6 ± 0.1 | 33.0 ± 0.0 |
| Standard (Quench) | 31.4 ± 0.0 | 35.2 ± 0.1 | 33.8 ± 0.1 |
| Disc Name | Pectin (%) | Sericin (%) | Nanoclay Polymer Concentration (Phr) | Amniotic Fluid (10% of Total Polymer Weight) | Moringa olifera Extract (10% of Total Polymer Weight) | Glycerol (80% of Total Polymer Weight) |
|---|---|---|---|---|---|---|
| 2% AMF-Mo.me | 50% | 50% | 3 | 2 | 20 | 60 |
| 4% AMF-Mo.me | 50% | 50% | 3 | 4 | 20 | 60 |
| 8% AMF-Mo.me C | 50% | 50% | 3 | 8 | 20 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashames, A.; Ijaz, M.; Buabeid, M.; Yasin, H.; Yaseen, S.; Bhandare, R.R.; Murtaza, G. In Vivo Wound Healing Potential and Molecular Pathways of Amniotic Fluid and Moringa Olifera-Loaded Nanoclay Films. Molecules 2024, 29, 729. https://doi.org/10.3390/molecules29030729
Ashames A, Ijaz M, Buabeid M, Yasin H, Yaseen S, Bhandare RR, Murtaza G. In Vivo Wound Healing Potential and Molecular Pathways of Amniotic Fluid and Moringa Olifera-Loaded Nanoclay Films. Molecules. 2024; 29(3):729. https://doi.org/10.3390/molecules29030729
Chicago/Turabian StyleAshames, Akram, Munaza Ijaz, Manal Buabeid, Haya Yasin, Sidra Yaseen, Richie R. Bhandare, and Ghulam Murtaza. 2024. "In Vivo Wound Healing Potential and Molecular Pathways of Amniotic Fluid and Moringa Olifera-Loaded Nanoclay Films" Molecules 29, no. 3: 729. https://doi.org/10.3390/molecules29030729
APA StyleAshames, A., Ijaz, M., Buabeid, M., Yasin, H., Yaseen, S., Bhandare, R. R., & Murtaza, G. (2024). In Vivo Wound Healing Potential and Molecular Pathways of Amniotic Fluid and Moringa Olifera-Loaded Nanoclay Films. Molecules, 29(3), 729. https://doi.org/10.3390/molecules29030729







