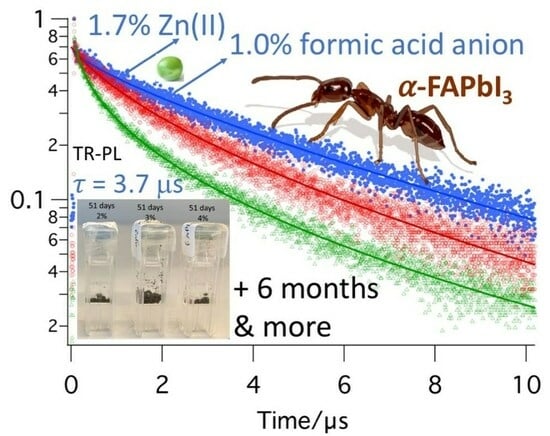
Synergistic Zinc(II) and Formate Doping of Perovskites: Thermal Phase Stabilization of α-FAPbI3 and Enhanced Photoluminescence Lifetime of FA0.8MA0.2PbI3 up to 3.7 µs
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction
2.2. Optical Properties of Doped FA0.8MA0.2PbI3 Thin Films
| Sample Composition | τ (ns) | Additives/ Specialties | Ref. |
|---|---|---|---|
| Cs0.05FA0.9MA0.05Pb(I0.9Br0.1)3 | 16,000 | Single crystal | [19] |
| MAPbI3 | 8830 | TOPO | [20] |
| FA0.8MA0.2PbI3 | 3700 | Zn(II), Fo− | This work |
| FA0.92MA0.08PbI3 | 2835 | PEAI, Cl− | [6] |
| Cs0.05FA0.85MA0.10Pb(I0.97Br0.03)3 | 2600 | BBAI | [18] |
| FA0.85MA0.15Pb(I0.95Br0.05)3 | 1547 | Zn(II), CPACl | [5] |
| FA0.95MA0.05Pb(I0.95Br0.05)3 | 1105 | (I3)− | [6] |
| FAPbI3 | 439 | HI | [16] |
| MAPbI3 | 920 | [20] |
2.3. Thermal Stability of Doped Perovskites
3. Experimental
3.1. FAPbI3 Synthesis
3.2. Precursor Solution Preparation for Spin Coating
3.3. Perovskite Powders
3.4. Thin Films for XRD and TR-PL (FA0.8MA0.2PbI3)
3.5. Doped Powder Preparation (FAPbI3)
3.6. Substrate Cleaning
3.7. Spin Coating
3.8. Time-Resolved Photoluminescence
3.9. XRD Measurements of Thin Films
3.10. Software Used
4. Conclusions and Future Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPACl | 3-chloropropylamine hydrochloride |
| DEE | diethyl ether |
| PL | photoluminescence |
| PTFE | polytetrafluoroethylene |
| DMF | dimethylformamide |
| DMSO | dimethylsulfoxide |
| GBL | gamma butyrolactone |
| lifetime of an excited state | |
| MABr | methylammonium bromide |
| MACl | methylammonium chloride |
| TR-PL | time-resolved photoluminescence |
| Fo | formate anion (HCO2)- |
| FAPbI3 | formamidinium lead tri-iodide |
| TOPO | tri-n-octylphosphine oxide |
| XRD | X-ray diffraction |
References
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosado, P. “Energy”. Published Online at OurWorldInData.org. 2022. Available online: https://ourworldindata.org/energy (accessed on 13 April 2023).
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- NREL. Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 13 April 2023).
- Muscarella, L.A.; Petrova, D.; Jorge Cervasio, R.; Farawar, A.; Lugier, O.; McLure, C.; Slaman, M.J.; Wang, J.; Ehrler, B.; von Hauff, E.; et al. Air-Stable and Oriented Mixed Lead Halide Perovskite (FA/MA) by the One-Step Deposition Method Using Zinc Iodide and an Alkylammonium Additive. ACS Appl. Mater. Interfaces 2019, 11, 17555–17562. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, A.; Muscarella, L.A.; Williams, R.M. Perovskite Thin Film Materials Stabilized and Enhanced by Zinc(II) Doping. Appl. Sci. 2019, 9, 1678. [Google Scholar] [CrossRef]
- Ye, S.; Rao, H.; Feng, M.; Xi, L.; Yen, Z.; Seng, D.H.L.; Xu, Q.; Boothroyd, C.; Chen, B.; Guo, Y.; et al. Expanding the low-dimensional interface engineering toolbox for efficient perovskite solar cells. Nat. Energy 2023, 8, 284–293. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-Halide Anion Engineering for α-FAPbI3 Perovskite Solar Cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yeh, H.; Chiang, Y.; Jeng, U.; Su, C.; Shiu, H.; Hsu, Y.; Kosugi, N.; Ohigashi, T.; Chen, Y.; et al. Highly Efficient 2D/3D Hybrid Perovskite Solar Cells via Low-Pressure Vapor-Assisted Solution Process. Adv. Mater. 2018, 30, 1801401. [Google Scholar] [CrossRef]
- Zhou, T.; Lai, H.; Liu, T.; Lu, D.; Wan, X.; Zhang, X.; Liu, Y.; Chen, Y. Highly Efficient and Stable Solar Cells Based on Crystalline Oriented 2D/3D Hybrid Perovskite. Adv. Mater. 2019, 31, 1901242. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-Efficiency Solution-Processed Perovskite Solar Cells with Millimeter-Scale Grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef]
- Huang, J.; Tan, S.; Lund, P.D.; Zhou, H. Impact of H2O on Organic-Inorganic Hybrid Perovskite Solar Cells. Energy Environ. Sci. 2017, 10, 2284–2311. [Google Scholar] [CrossRef]
- Muscarella, L.A.; Petrova, D.; Cervasio, R.J.; Farawar, A.; Lugier, O.; McLure, C.; Slaman, M.J.; Wang, J.; von Hauff, E.; Williams, R.M. Enhanced Grain-Boundary Emission Lifetime and Additive Induced Crystal Orientation in One-Step Spin-Coated Mixed Cationic (FA/MA) Lead Perovskite Thin Films Stabilized by Zinc Iodide Doping. ChemRxiv 2017. [Google Scholar] [CrossRef]
- Bi, D.; El-Zohry, A.M.; Hagfeldt, A.; Boschloo, G. Unraveling the Effect of PbI2 Concentration on Charge Recombination Kinetics in Perovskite Solar Cells. ACS Photonics 2015, 2, 589–594. [Google Scholar] [CrossRef]
- Handa, T.; Tex, D.M.; Shimazaki, A.; Wakamiya, A.; Kanemitsu, Y. Charge Injection Mechanism at Heterointerfaces in CH3NH3PbI3 Perovskite Solar Cells Revealed by Simultaneous Time-Resolved Photoluminescence and Photocurrent Measurements. J. Phys. Chem. Lett. 2017, 8, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.-H.; Wang, F.; Adjokatse, S.; Zhao, N.; Even, J.; Loi, M.A. Photoexcitation Dynamics in Solution-Processed Formamidinium Lead Iodide Perovskite Thin Films for Solar Cell Applications. Light. Sci. Appl. 2016, 5, e16056. [Google Scholar] [CrossRef] [PubMed]
- Baloch, A.A.B.; Alharbi, F.H.; Grancini, G.; Hossain, M.I.; Nazeeruddin, K.; Tabet, N. Analysis of Photocarrier Dynamics at Interfaces in Perovskite Solar Cells by Time-Resolved Photoluminescence. J. Phys. Chem. C 2018, 122, 26805–26815. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, Y.; Eickemeyer, F.T.; Pan, L.; Ren, D.; Ruiz-Preciado, M.A.; Carlsen, B.; Yang, B.; Dong, X.; Wang, Z.; et al. Tailored Amphiphilic Molecular Mitigators for Stable Perovskite Solar Cells with 23.5% Efficiency. Adv. Mater. 2020, 32, 1907757. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tan, Y.-Y.; Chen, Z.-X.; Wang, T.; Hu, S.; Nan, Z.-A.; Xie, L.-Q.; Hui, Y.; Huang, J.-X.; Zhan, C.; et al. Toward Long-Term Stability: Single-Crystal Alloys of Cesium-Containing Mixed Cation and Mixed Halide Perovskite. J. Am. Chem. Soc. 2019, 141, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- De Quilettes, D.W.; Koch, S.; Burke, S.; Paranji, R.K.; Shropshire, A.J.; Ziffer, M.E.; Ginger, D.S. Photoluminescence Lifetimes Exceeding 8 μs and Quantum Yields Exceeding 30% in Hybrid Perovskite Thin Films by Ligand Passivation. ACS Energy Lett. 2016, 1, 438–444. [Google Scholar] [CrossRef]
- Tong, G.; Son, D.-Y.; Ono, L.K.; Kang, H.-B.; He, S.; Qiu, L.; Zhang, H.; Liu, Y.; Hieulle, J.; Qi, Y. Removal of Residual Compositions by Powder Engineering for High Efficiency Formamidinium-Based Perovskite Solar Cells with Operation Lifetime over 2000 h. Nano Energy 2021, 87, 106152. [Google Scholar] [CrossRef]
- Tang, G.; You, P.; Tai, Q.; Wu, R.; Yan, F. Performance Enhancement of Perovskite Solar Cells Induced by Lead Acetate as an Additive. Sol. RRL 2018, 2, 1800066. [Google Scholar] [CrossRef]
- Zhao, J.; Fürer, S.O.; McMeekin, D.P.; Lin, Q.; Lv, P.; Ma, J.; Tan, W.L.; Wang, C.; Tan, B.; Chesman, A.S.R.; et al. Efficient and Stable Formamidinium–Caesium Perovskite Solar Cells and Modules from Lead Acetate-Based Precursors. Energy Environ. Sci. 2023, 16, 138–147. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Zhuang, Q.; He, D.; Liu, B.; Chen, C.; Zhang, B.; Pauporté, T.; Zang, Z.; Chen, J. Stabilizing Perovskite Precursor by Synergy of Functional Groups for NiOx-Based Inverted Solar Cells with 23.5 % Efficiency. Angew. Chem. Int. Ed. 2022, 61, e202206914. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.; Hassan, H.; Spooner, E. Spin Coating: Complete Guide to Theory and Techniques. Ossila. Available online: https://www.ossila.com/en-eu/pages/spin-coating (accessed on 15 September 2022).
- Marchioro, A.; Teuscher, J.; Friedrich, D.; Kunst, M.; van de Krol, R.; Moehl, T.; Grätzel, M.; Moser, J.-E. Unravelling the Mechanism of Photoinduced Charge Transfer Processes in Lead Iodide Perovskite Solar Cells. Nat. Photonics 2014, 8, 250–255. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Welcome to Python.org. Available online: https://www.python.org/ (accessed on 25 November 2022).
- Marchant, C.; Williams, R.M. Perovskite/Silicon Tandem Solar Cells—Compositions for Improved Stability and Power Conversion Efficiency. Photochem. Photobiol. Sci. 2023. [Google Scholar] [CrossRef]
- Aydin, E.; Allen, T.G.; De Bastiani, M.; Razzaq, A.; Xu, L.; Ugur, E.; Liu, J.; De Wolf, S. Pathways toward Commercial Perovskite/Silicon Tandem Photovoltaics. Science 2024, 383, eadh3849. [Google Scholar] [CrossRef]










| Sample Code | Peak (°) | % | Peak Amplitude | FWHM (°) | Designation |
|---|---|---|---|---|---|
| 1 | 12.7 | 13.6 | 23 | - | PbI2 |
| 13.9 | 100 | 169 | 0.1263 | α-phase | |
| 24.3 | 16.6 | 28 | - | α-phase | |
| 28.1 | 37 | 63 | 0.1273 | α-phase | |
| 2 | 12.7 | 39 | 52 | 0.1415 | PbI2 |
| 13.94 | 100 | 133 | 0.1425 | α-phase | |
| 24.3 | 14 | 19 | - | α-phase | |
| 28.1 | 31.5 | 42 | 0.1727 | α-phase | |
| 3 | 12.36 | 7.7 | 20 | - | PbI2 |
| 12.7 | 85 | 221 | 0.153 | PbI2 | |
| 13.96 | 100 | 260 | 0.118 | α-phase | |
| ~24 | 7.7 | 20 | - | α-phase | |
| 28.1 | 39 | 102 | 0.0809 | α-phase |
| Sample Code | % Zn | % Fo | % Total | τ (ns) | β |
|---|---|---|---|---|---|
| 1 | 1.7 | 1.0 | 2.7 | 3700 | 0.77 |
| 2 | 2.6 | 2.0 | 4.6 | 610 | 0.46 |
| 3 | 3.8 | 3.0 | 6.8 | 1420 | 0.58 |
| Sample Code | Peak (°) | % | Peak Amplitude | FWHM (°) | Designation |
|---|---|---|---|---|---|
| 1 | 12.7 | 13.6 | 23 | - | PbI2 |
| 13.9 | 100 | 169 | 0.1263 | α-phase | |
| 24.3 | 16.6 | 28 | - | α-phase | |
| 28.1 | 37 | 63 | 0.1273 | α-phase | |
| 1 (aged) | 12.7 | 20 | 29 | 0.1783 | PbI2 |
| 13.9 | 100 | 146 | 0.1356 | α-phase | |
| 24.3 | 12 | 18 | - | α-phase | |
| 28.1 | 34 | 50 | 0.1941 | α-phase |
| Sample Code | FAPbI3 (mg) | ZnFo2 (mg) | ZnI2 (mg) | MACl mg (mol%) | % Zn | % Fo | % Total |
|---|---|---|---|---|---|---|---|
| 1 | 800.2 | 0.9 | 5.2 | 18.5 (21.7) | 1.7 | 1.0 | 2.7 |
| 2 | 806.5 | 2.0 | 6.9 | 21.2 (24.6) | 2.6 | 2.0 | 4.6 |
| 3 | 806.1 | 3.0 | 9.3 | 19.9 (23.1) | 3.8 | 3.0 | 6.8 |
| Sample Code | FAPbI3 (mg) | ZnFo2 (mg) | % Zn | % Fo | % Total |
|---|---|---|---|---|---|
| 0.0% | 295.6 | 0.0 | 0.00 | 0.00 | 0.00 |
| 0.5% | 299.0 | 0.3 | 0.41 | 0.82 | 1.23 |
| 1.0% | 297.8 | 0.7 | 0.96 | 1.92 | 2.88 |
| 2.0% | 297.3 | 1.7 | 2.33 | 4.66 | 6.99 |
| 3.0% | 297.0 | 2.2 | 3.02 | 6.04 | 9.06 |
| 4.0% | 298.2 | 2.9 | 3.96 | 7.92 | 11.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoeksma, M.M.; Williams, R.M. Synergistic Zinc(II) and Formate Doping of Perovskites: Thermal Phase Stabilization of α-FAPbI3 and Enhanced Photoluminescence Lifetime of FA0.8MA0.2PbI3 up to 3.7 µs. Molecules 2024, 29, 516. https://doi.org/10.3390/molecules29020516
Hoeksma MM, Williams RM. Synergistic Zinc(II) and Formate Doping of Perovskites: Thermal Phase Stabilization of α-FAPbI3 and Enhanced Photoluminescence Lifetime of FA0.8MA0.2PbI3 up to 3.7 µs. Molecules. 2024; 29(2):516. https://doi.org/10.3390/molecules29020516
Chicago/Turabian StyleHoeksma, Merk M., and René M. Williams. 2024. "Synergistic Zinc(II) and Formate Doping of Perovskites: Thermal Phase Stabilization of α-FAPbI3 and Enhanced Photoluminescence Lifetime of FA0.8MA0.2PbI3 up to 3.7 µs" Molecules 29, no. 2: 516. https://doi.org/10.3390/molecules29020516