The Neuroprotective Role of Cyanobacteria with Focus on the Anti-Inflammatory and Antioxidant Potential: Current Status and Perspectives
Abstract
1. Introduction
2. Cyanobacteria
3. The Beneficial Role of Cyanobacterial Natural Products in Inflammation and Oxidative Stress
3.1. Polysaccharides
3.2. Lipids
3.3. Peptides
3.4. Ultraviolet Absorbing Compounds
3.5. Phycobiliproteins
3.6. Carotenoids and Phenolic Compounds
4. Arthrospira Studies of Success
5. Opportunities, Prospects, and Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-d.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 107. [Google Scholar] [CrossRef]
- Castaneda, A.; Ferraz, R.; Vieira, M.; Cardoso, I.; Vasconcelos, V.; Martins, R. Bridging Cyanobacteria to Neurodegenerative Diseases: A New Potential Source of Bioactive Compounds against Alzheimer’s Disease. Mar. Drugs 2021, 19, 343. [Google Scholar] [CrossRef]
- Gholami, A.; Minai-Tehrani, D.; Eriksson, L.A. In silico and in vitro studies confirm Ondansetron as a novel acetylcholinesterase and butyrylcholinesterase inhibitor. Sci. Rep. 2023, 13, 643. [Google Scholar] [CrossRef]
- Athar, T.; Al Balushi, K.; Khan, S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, B. New Possibilities in the Therapeutic Approach to Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 8902. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Ren, J. Basic information about memantine and its treatment of Alzheimer’s disease and other clinical applications. Ibrain 2023, 9, 340–348. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- McColgan, P.; Tabrizi, S.J. Huntington’s disease: A clinical review. Eur. J. Neurol. 2018, 25, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Rüb, U.; Seidel, K.; Heinsen, H.; Vonsattel, J.P.; den Dunnen, W.F.; Korf, H.W. Huntington’s disease (HD): The neuropathology of a multisystem neurodegenerative disorder of the human brain. Brain Pathol. 2016, 26, 726–740. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Morea, V.; Bidollari, E.; Colotti, G.; Fiorillo, A.; Rosati, J.; De Filippis, L.; Squitieri, F.; Ilari, A. Glucose transportation in the brain and its impairment in Huntington disease: One more shade of the energetic metabolism failure? Amino Acids 2017, 49, 1147–1157. [Google Scholar] [CrossRef]
- Rawlins, M.D.; Wexler, N.S.; Wexler, A.R.; Tabrizi, S.J.; Douglas, I.; Evans, S.J.; Smeeth, L. The Prevalence of Huntington’s Disease. Neuroepidemiology 2016, 46, 144–153. [Google Scholar] [CrossRef]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.A.S.; Pinho, B.R.; Oliveira, J.M.A. Swimming against ALS: How to model disease in zebrafish for pathophysiological and behavioral studies. Neurosci. Biobehav. Rev. 2023, 148, 105138. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, A.; Albertson, R.M.; Noto, J.; Simmons, Z. Ten years of riluzole use in a tertiary ALS clinic. Muscle Nerve 2022, 65, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Shefner, J.; Heiman-Patterson, T.; Pioro, E.P.; Wiedau-Pazos, M.; Liu, S.; Zhang, J.; Agnese, W.; Apple, S. Long-term edaravone efficacy in amyotrophic lateral sclerosis: Post-hoc analyses of Study 19 (MCI186-19). Muscle Nerve 2020, 61, 218–221. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fang, T.; De Marchi, F.; Neel, D.; Van Weehaeghe, D.; Berry, J.D.; Paganoni, S. Pharmacotherapy for Amyotrophic Lateral Sclerosis: A Review of Approved and Upcoming Agents. Drugs 2022, 82, 1367–1388. [Google Scholar] [CrossRef]
- Naz, S.; Beach, J.; Heckert, B.; Tummala, T.; Pashchenko, O.; Banerjee, T.; Santra, S. Cerium oxide nanoparticles: A ‘radical’ approach to neurodegenerative disease treatment. Nanomedicine 2017, 12, 545–553. [Google Scholar] [CrossRef]
- Chen, J.-C.; Liu, K.S.; Yang, T.-J.; Hwang, J.-H.; Chan, Y.-C.; Lee, I.-T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef]
- Pérez-Juárez, A.; Chamorro, G.; Alva-Sánchez, C.; Paniagua-Castro, N.; Pacheco-Rosado, J. Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm. Biol. 2016, 54, 1408–1412. [Google Scholar] [CrossRef]
- Pathak, N.; Vimal, S.K.; Tandon, I.; Agrawal, L.; Hongyi, C.; Bhattacharyya, S. Neurodegenerative Disorders of Alzheimer, Parkinsonism, Amyotrophic Lateral Sclerosis and Multiple Sclerosis: An Early Diagnostic Approach for Precision Treatment. Metab. Brain Dis. 2022, 37, 67–104. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.T.D.; Herath, K.H.I.N.M.; Sanjeewa, K.K.A.; Jayawardena, T.U. Recent Reports on Bioactive Compounds from Marine Cyanobacteria in Relation to Human Health Applications. Life 2023, 13, 1411. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Żymańczyk-Duda, E.; Samson, S.O.; Brzezińska-Rodak, M.; Klimek-Ochab, M. Versatile Applications of Cyanobacteria in Biotechnology. Microorganisms 2022, 10, 2318. [Google Scholar] [CrossRef] [PubMed]
- Morone, J.; Lopes, G.; Oliveira, B.; Vasconcelos, V.; Martins, R. Chapter 9—Cyanobacteria in Cosmetics: A Natural Alternative for Anti-Aging Ingredients. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–286. [Google Scholar] [CrossRef]
- Strunecký, O.; Ivanova, A.P.; Mareš, J. An updated classification of cyanobacterial orders and families based on phylogenomic and polyphasic analysis. J. Phycol. 2023, 59, 12–51. [Google Scholar] [CrossRef]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal Models of Inflammation for Screening of Anti-inflammatory Drugs: Implications for the Discovery and Development of Phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Koronyo-Hamaoui, M.; Gaire, B.P.; Frautschy, S.A.; Alvarez, J.I. Editorial: Role of Inflammation in Neurodegenerative Diseases. Front. Immunol. 2022, 13, 958487. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders—A Review. Curr. Neurol. Neurosci. Rep 2017, 17, 25. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Zhang, Z.; Zhang, R.; Zhang, Z.; Liu, Y.; Ma, B. Translocator Protein 18 kDa (TSPO) as a Novel Therapeutic Target for Chronic Pain. Neural Plast. 2022, 2022, e8057854. [Google Scholar] [CrossRef]
- Ching, A.S.C.; Kuhnast, B.; Damont, A.; Roeda, D.; Tavitian, B.; Dollé, F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 2012, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Tremolanti, C.; Germelli, L.; Barresi, E.; Da Pozzo, E.; Simorini, F.; Castellano, S.; Taliani, S.; Da Settimo, F.; Martini, C.; Costa, B. Translocator Protein 18-kDa: A Promising Target to Treat Neuroinflammation- related Degenerative Diseases. Curr. Med. Chem. 2022, 29, 4831–4861. [Google Scholar] [CrossRef]
- Baweja, G.S.; Gupta, S.; Kumar, B.; Patel, P.; Asati, V. Recent updates on structural insights of MAO-B inhibitors: A review on target-based approach. Mol. Divers. 2023, 28, 1823–1845. [Google Scholar] [CrossRef]
- Almeida, J.R.; Freitas, M.; Cruz, S.; Leão, P.N.; Vasconcelos, V.; Cunha, I. Acetylcholinesterase in Biofouling Species: Characterization and Mode of Action of Cyanobacteria-Derived Antifouling Agents. Toxins 2015, 7, 2739–2756. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Mendonça, J.d.S.; Guimarães, R.d.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.d.C.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef] [PubMed]
- Soutinho, S.M.A. Avaliação Dos Compostos Fenólicos E Da Actividade Antioxidante De Frutos Vermelhos Produzidos Em Modo Biológico. 2012. Available online: http://hdl.handle.net/10400.19/1770 (accessed on 15 April 2024).
- Silva, M.L.C.; Costa, R.S.; Santana, A.D.S.; Koblitz, M.G.B. Compostos fenólicos, carotenóides e atividade antioxidante em produtos vegetais. Semina Ciênc. Agrár. 2010, 31, 669. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, R.M.; Adessi, A.; Caldara, F.; Codato, A.; Furlan, M.; Rampazzo, C.; De Philippis, R.; La Rocca, N.; Dalla Valle, L. Anti-Inflammatory Activity of Exopolysaccharides from Phormidium sp. ETS05, the Most Abundant Cyanobacterium of the Therapeutic Euganean Thermal Muds, Using the Zebrafish Model. Biomolecules 2020, 10, 582. [Google Scholar] [CrossRef]
- Quan, Y.; Yang, S.; Wan, J.; Su, T.; Zhang, J.; Wang, Z. Optimization for the extraction of polysaccharides from Nostoc commune and its antioxidant and antibacterial activities. J. Taiwan Inst. Chem. Eng. 2015, 52, 14–21. [Google Scholar] [CrossRef]
- Belhaj, D.; Frikha, D.; Athmouni, K.; Jerbi, B.; Ahmed, M.B.; Bouallagui, Z.; Kallel, M.; Maalej, S.; Zhou, J.; Ayadi, H. Box-Behnken design for extraction optimization of crude polysaccharides from Tunisian Phormidium versicolor cyanobacteria (NCC 466): Partial characterization, in vitro antioxidant and antimicrobial activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef]
- Trabelsi, L.; Mnari, A.; Abdel-Daim, M.M.; Abid-Essafi, S.; Aleya, L. Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement. Altern. Med. 2016, 16, 515. [Google Scholar] [CrossRef]
- Li, J.; Shen, S.G.; Han, C.F.; Liu, S.T.; Chen, N.; Jia, S.R.; Han, P.P. Nostoc flagelliforme capsular polysaccharides from different culture conditions improve hyperlipidemia and regulate intestinal flora in C57BL/6J mice to varying degrees. Int. J. Biol. Macromol. 2022, 202, 224–233. [Google Scholar] [CrossRef]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H.; Zhao, D.X.; Han, P.P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Hashim, I.I.A.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory Effects of Novel Polysaccharide Sacran Extracted from Cyanobacterium Aphanothece sacrum in Various Inflammatory Animal Models. Biol. Pharm. Bull. 2016, 39, 1172–1178. [Google Scholar] [CrossRef]
- Doi, M.; Sagawa, Y.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Defensive Effects of a Unique Polysaccharide, Sacran, to Protect Keratinocytes against Extracellular Stimuli and Its Possible Mechanism of Action. Biol. Pharm. Bull. 2018, 41, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Sagawa, Y.; Momose, S.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Topical treatment with sacran, a sulfated polysaccharide from Aphanothece sacrum, improves corneocyte-derived parameters. J. Dermatol. 2017, 44, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Tena Pérez, V.; Apaza Ticona, L.; Cabanillas, A.H.; Maderuelo Corral, S.; Rosero Valencia, D.F.; Quintana, A.M.; Ortega Domenech, M.; Rumbero Sánchez, Á. Anti-inflammatory activity of glycolipids isolated from cyanobacterium Nodularia harveyana. Nat. Prod. Res. 2021, 35, 6204–6209. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, F.H.; Simon, E.F.; Liu, N.; Ratnayake, R.; Paul, V.J.; Luesch, H. Discovery and Anti-Inflammatory Activity of a Cyanobacterial Fatty Acid Targeting the Keap1/Nrf2 Pathway. Mar. Drugs 2023, 21, 553. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Kumar Saha, S. Anti-inflammatory and antithrombotic properties of polar lipid extracts, rich in unsaturated fatty acids, from the Irish marine cyanobacterium Spirulina subsalsa. J. Funct. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Amaro, H.M.; Barros, R.; Tavares, T.; Almeida, R.; Pinto, I.S.; Malcata, F.X.; Guedes, A.C. Gloeothece sp.—Exploiting a New Source of Antioxidant, Anti-Inflammatory, and Antitumor Agents. Mar. Drugs 2021, 19, 623. [Google Scholar] [CrossRef]
- Choi, H.; Mascuch, S.J.; Villa, F.A.; Byrum, T.; Teasdale, M.E.; Smith, J.E.; Preskitt, L.B.; Rowley, D.C.; Gerwick, L.; Gerwick, W.H. Honaucins A–C, Potent Inhibitors of Eukaryotic Inflammation and Bacterial Quorum Sensing: Synthetic Derivatives and Structure-Activity Relationships. Chem. Biol. 2012, 19, 589–598. [Google Scholar] [CrossRef]
- Malloy, K.L.; Villa, F.A.; Engene, N.; Matainaho, T.; Gerwick, L.; Gerwick, W.H. Malyngamide 2, an Oxidized Lipopeptide with Nitric Oxide Inhibiting Activity from a Papua New Guinea Marine Cyanobacterium. J. Nat. Prod. 2011, 74, 95–98. [Google Scholar] [CrossRef]
- Kapuścik, A.; Hrouzek, P.; Kuzma, M.; Bártová, S.; Novák, P.; Jokela, J.; Pflüger, M.; Eger, A.; Hundsberger, H.; Kopecký, J. Novel Aeruginosin-865 from Nostoc sp. as a Potent Anti-inflammatory Agent. ChemBioChem 2013, 14, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Faltermann, S.; Hutter, S.; Christen, V.; Hettich, T.; Fent, K. Anti-Inflammatory Activity of Cyanobacterial Serine Protease Inhibitors Aeruginosin 828A and Cyanopeptolin 1020 in Human Hepatoma Cell Line Huh7 and Effects in Zebrafish (Danio rerio). Toxins 2016, 8, 219. [Google Scholar] [CrossRef]
- Engene, N.; Choi, H.; Esquenazi, E.; Byrum, T.; Villa, F.A.; Cao, Z.; Murray, T.F.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Phylogeny-Guided Isolation of Ethyl Tumonoate A from the Marine Cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ryu, B.; Kim, S.-K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.R.; Jo, S.A.; Lee, H.; Yoon, Y.D.; Kwon, J.-H.; Yang, J.-W.; Choi, B.J.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Inhibition of Skin Inflammation by Scytonemin, an Ultraviolet Sunscreen Pigment. Mar. Drugs 2020, 18, 300. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. The high-energy radiation protectant extracellular sheath pigment scytonemin and its reduced counterpart in the cyanobacterium Scytonema sp. R77DM. Bioresour. Technol. 2014, 171, 396–400. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Lopes, G.; Sousa-Pinto, I.; Vasconcelos, V.; Guedes, A.C. Bioactive potential of Cyanobium sp. pigment-rich extracts. J. Appl. Phycol. 2020, 32, 3031–3040. [Google Scholar] [CrossRef]
- Alzokaky, A.A.; Abdelkader, E.M.; El-Dessouki, A.M.; Khaleel, S.A.; Raslan, N.A. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: Role of HMGB1/NLRP3/NF-κB pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 265–277. [Google Scholar] [CrossRef]
- Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Marín-Prida, J.; Pavón-Fuentes, N.; Falcon-Cama, V.; Piniella-Matamoros, B.; Camacho-Rodríguez, H.; Fernández-Massó, J.R.; Valenzuela-Silva, C.; Raíces-Cruz, I.; et al. Beneficial effects of oral administration of C-Phycocyanin and Phycocyanobilin in rodent models of experimental autoimmune encephalomyelitis. Life Sci. 2018, 194, 130–138. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Wang, L.; Pan, S.; Liu, Y.; Li, S.; Wang, D. C-phycocyanin Mitigates Cognitive Impairment in Doxorubicin-Induced Chemobrain: Impact on Neuroinflammation, Oxidative Stress, and Brain Mitochondrial and Synaptic Alterations. Neurochem. Res. 2021, 46, 149–158. [Google Scholar] [CrossRef]
- Renugadevi, K.; Valli Nachiyar, C.; Sowmiya, P.; Sunkar, S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- Patel, S.N.; Sonani, R.R.; Jakharia, K.; Bhastana, B.; Patel, H.M.; Chaubey, M.G.; Singh, N.K.; Madamwar, D. Antioxidant activity and associated structural attributes of Halomicronema phycoerythrin. Int. J. Biol. Macromol. 2018, 111, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.F.; Ratnayake, R.R.; Meerajini, K.; Wasantha Kumara, K.L. Antioxidant properties in some selected cyanobacteria isolated from fresh water bodies of Sri Lanka. Food Sci. Nutr. 2016, 4, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Patias, L.D. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kelman, D.; Ben-Amotz, A.; Berman-Frank, I. Carotenoids provide the major antioxidant defence in the globally significant N2-fixing marine cyanobacterium Trichodesmium. Environ. Microbiol. 2009, 11, 1897–1908. [Google Scholar] [CrossRef]
- Piovan, A.; Battaglia, J.; Filippini, R.; Dalla Costa, V.; Facci, L.; Argentini, C.; Pagetta, A.; Giusti, P.; Zusso, M. Pre- and Early Post-treatment With Arthrospira platensis (Spirulina) Extract Impedes Lipopolysaccharide-triggered Neuroinflammation in Microglia. Front. Pharmacol. 2021, 12, 724993. [Google Scholar] [CrossRef]
- Sagara, T.; Nishibori, N.; Kishibuchi, R.; Itoh, M.; Morita, K. Non-protein components of Arthrospira (Spirulina) platensis protect PC12 cells against iron-evoked neurotoxic injury. J. Appl. Phycol. 2015, 27, 849–855. [Google Scholar] [CrossRef]
- Kumar, A.; Christian, P.K.; Panchal, K.; Guruprasad, B.R.; Tiwari, A.K. Supplementation of Spirulina (Arthrospira platensis) Improves Lifespan and Locomotor Activity in Paraquat-Sensitive DJ-1βΔ93 Flies, a Parkinson’s Disease Model in Drosophila melanogaster. J. Diet. Suppl. 2017, 14, 573–588. [Google Scholar] [CrossRef]
- Koh, E.-J.; Kim, K.-J.; Choi, J.; Kang, D.-H.; Lee, B.-Y. Spirulina maxima extract prevents cell death through BDNF activation against amyloid beta 1-42 (Aβ 1-42 ) induced neurotoxicity in PC12 cells. Neurosci. Lett. 2018, 673, 33–38. [Google Scholar] [CrossRef]
- Koh, E.-J.; Seo, Y.-J.; Choi, J.; Lee, H.Y.; Kang, D.-H.; Kim, K.-J.; Lee, B.-Y. Spirulina maxima Extract Prevents Neurotoxicity via Promoting Activation of BDNF/CREB Signaling Pathways in Neuronal Cells and Mice. Molecules 2017, 22, 1363. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abdou, H.M.; Abd Elkader, H.T.A.; Hussein, H.K.; Abou Samra, W.E.M. Neuroprotective Potential of Spirulina Platensis Against Aluminium Chloride-Induced Neural Degeneration. Curr. Top. Nutraceutical Res. 2019, 18, 310–318. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-inflammatory Activity of Bioactive Compounds from Microalgae and Cyanobacteria by Focusing on the Mechanisms of Action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Debnath, S.; Muthuraj, M.; Bandyopadhyay, T.K.; Bobby, M.N.; Vanitha, K.; Tiwari, O.N.; Bhunia, B. Engineering strategies and applications of cyanobacterial exopolysaccharides: A review on past achievements and recent perspectives. Carbohydr. Polym. 2024, 328, 121686. [Google Scholar] [CrossRef]
- Manning, S.R. Microalgal lipids: Biochemistry and biotechnology. Curr. Opin. Biotechnol. 2022, 74, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paul-André, S.; Norio, M. (Eds.) Lipids in Photosynthesis: Structure, Function and Genetics, vol. 6; Advances in Photosynthesis and Respiration, vol. 6; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Pérez Gallego, R.; Bale, N.J.; Sinninghe Damste, J.S.; Villanueva, L. Developing a genetic approach to target cyanobacterial producers of heterocyte glycolipids in the environment. Front. Microbiol. 2023, 14, 1257040. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Ogawa, H.; Nguyen, K.A.; Suenaga, K. Jahanyne, an apoptosis-inducing lipopeptide from the marine cyanobacterium Lyngbya sp. Org. Lett. 2015, 17, 652–655. [Google Scholar] [CrossRef]
- Mascuch, S.J.; Boudreau, P.D.; Carland, T.M.; Pierce, N.T.; Olson, J.; Hensler, M.E.; Choi, H.; Campanale, J.; Hamdoun, A.; Nizet, V.; et al. Marine Natural Product Honaucin A Attenuates Inflammation by Activating the Nrf2-ARE Pathway. J. Nat. Prod. 2018, 81, 506–514. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural Products from Cyanobacteria: Focus on Beneficial Activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, P.; Steven, A.N.; Chan, L.-W.; Rahmat, Z.; Jamaluddin, H.; Mohd, N.I. Noh, Bioactive Metabolites Produced by Cyanobacteria for Growth Adaptation and Their Pharmacological Properties. Biology 2021, 10, 1061. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.L.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Li, X.; Lai, X.; Yang, M.; Liu, F.; Luan, F.; Meng, X. Polysaccharides from Spirulina platensis: Extraction methods, structural features and bioactivities diversity. Int. J. Biol. Macromol. 2023, 231, 123211. [Google Scholar] [CrossRef]
- Chei, S.; Oh, H.J.; Song, J.H.; Seo, Y.J.; Lee, K.; Kim, K.J.; Lee, B.Y. Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling. Sci. Rep. 2020, 10, 2075. [Google Scholar] [CrossRef]
- Bigagli, E.; D’Ambrosio, M.; Cinci, L.; Pieraccini, G.; Romoli, R.; Biondi, N.; Niccolai, A.; Rodolfi, L.; Tredici, M.R.; Luceri, C. A comparative study of metabolites profiles, anti-inflammatory and antioxidant activity of methanolic extracts from three Arthrospira strains in RAW 264.7 macrophages. Algal Res. 2023, 73, 103171. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef]
- Deepika, C.; Wolf, J.; Roles, J.; Ross, I.; Hankamer, B. Sustainable Production of Pigments from Cyanobacteria. Adv. Biochem. Eng. Biotechnol. 2023, 183, 171–251. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. United States Am. 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Ramos, V.; Reis, M.; Ferreira, L.; Silva, A.M.; Ferraz, R.; Vieira, M.; Vasconcelos, V.; Martins, R. Stalling the Course of Neurodegenerative Diseases: Could Cyanobacteria Constitute a New Approach toward Therapy? Biomolecules 2023, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, M.; Barupal, T.; Swapnil, P.; Sharma, K. Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 2020, 22, 100737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xu, J.; Adhikari, B.; Lv, W.; Chen, H. Nostoc sphaeroides Cyanobacteria: A review of its nutritional characteristics and processing technologies. Crit. Rev. Food Sci. Nutr. 2023, 63, 8975–8991. [Google Scholar] [CrossRef] [PubMed]
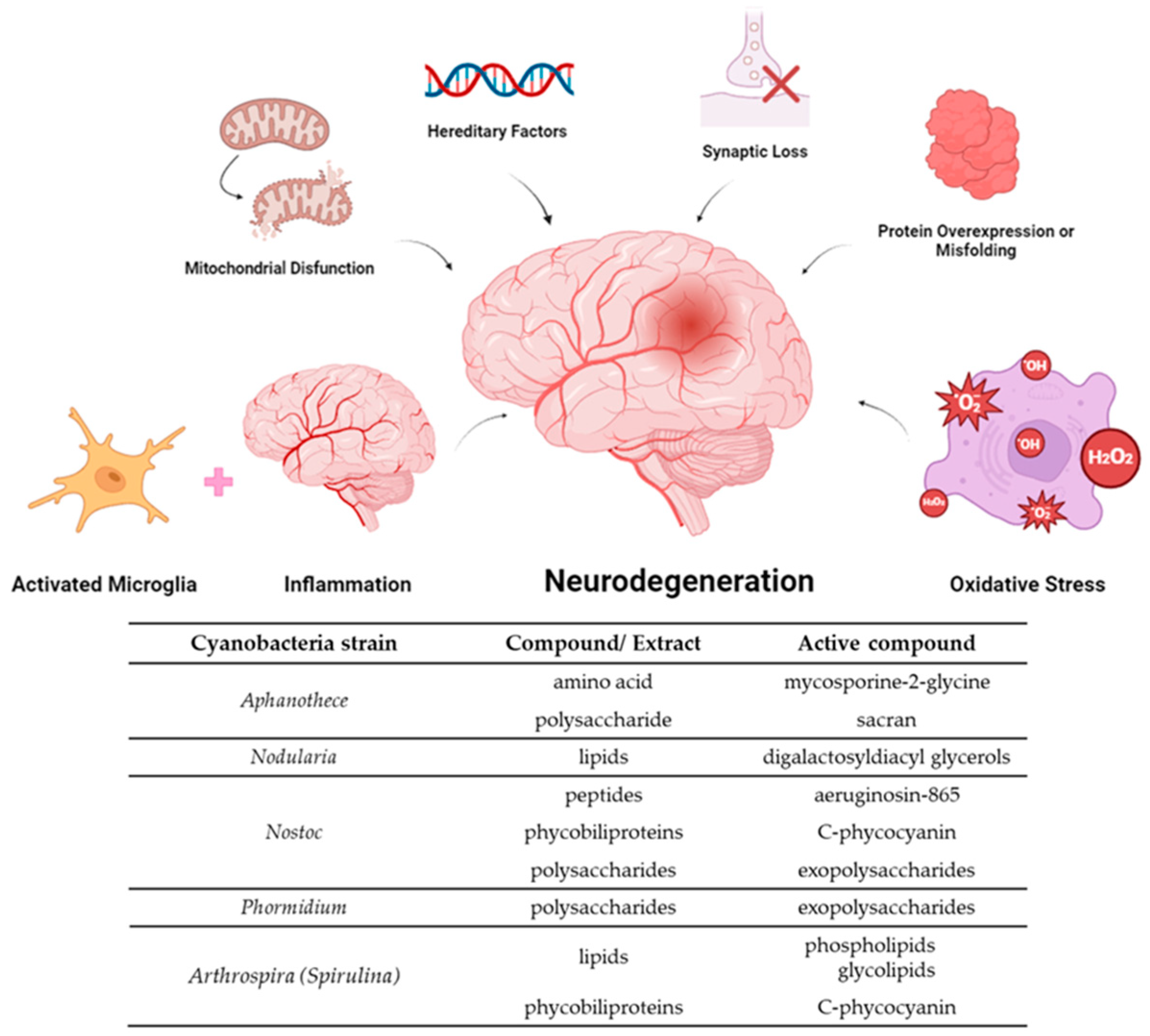
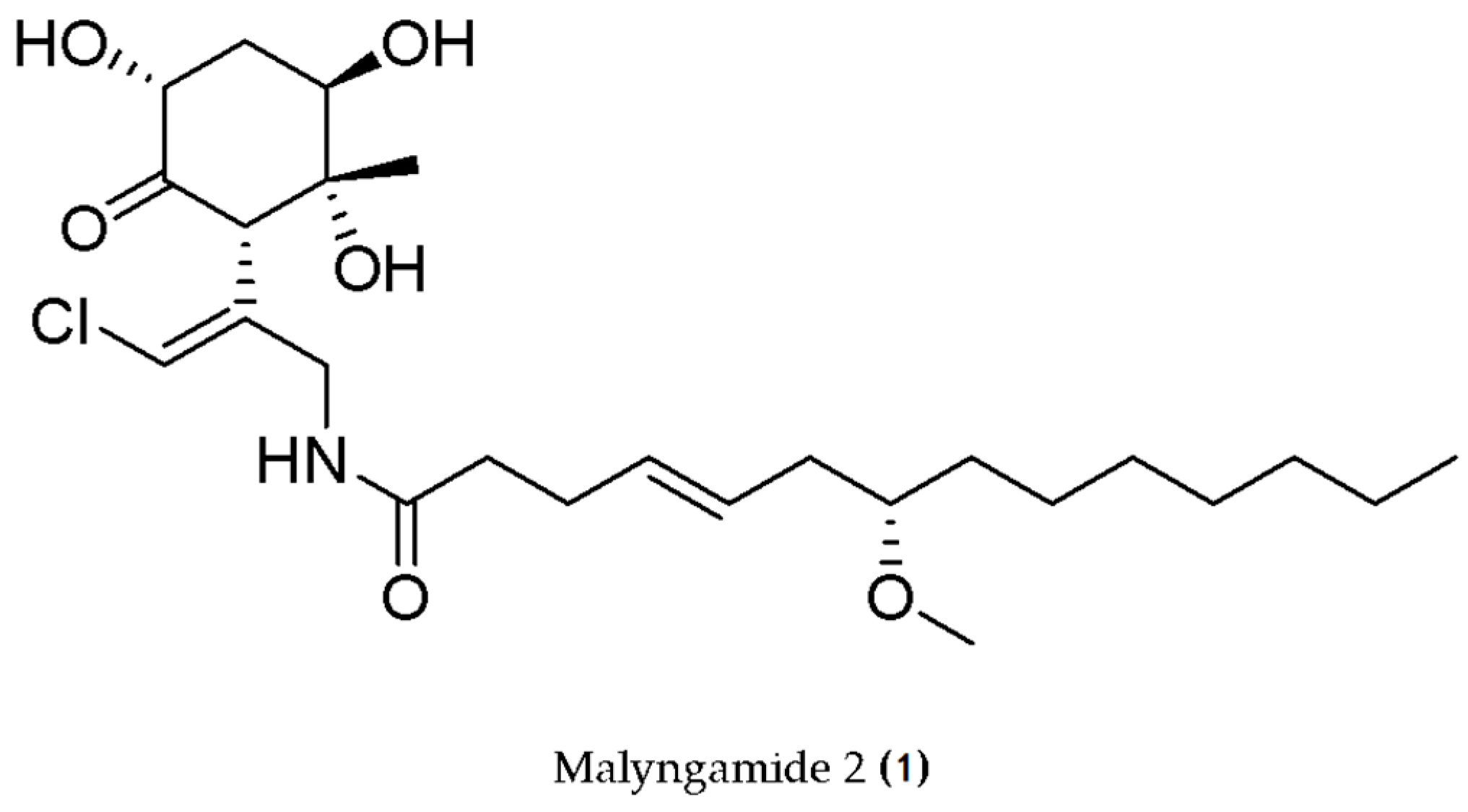

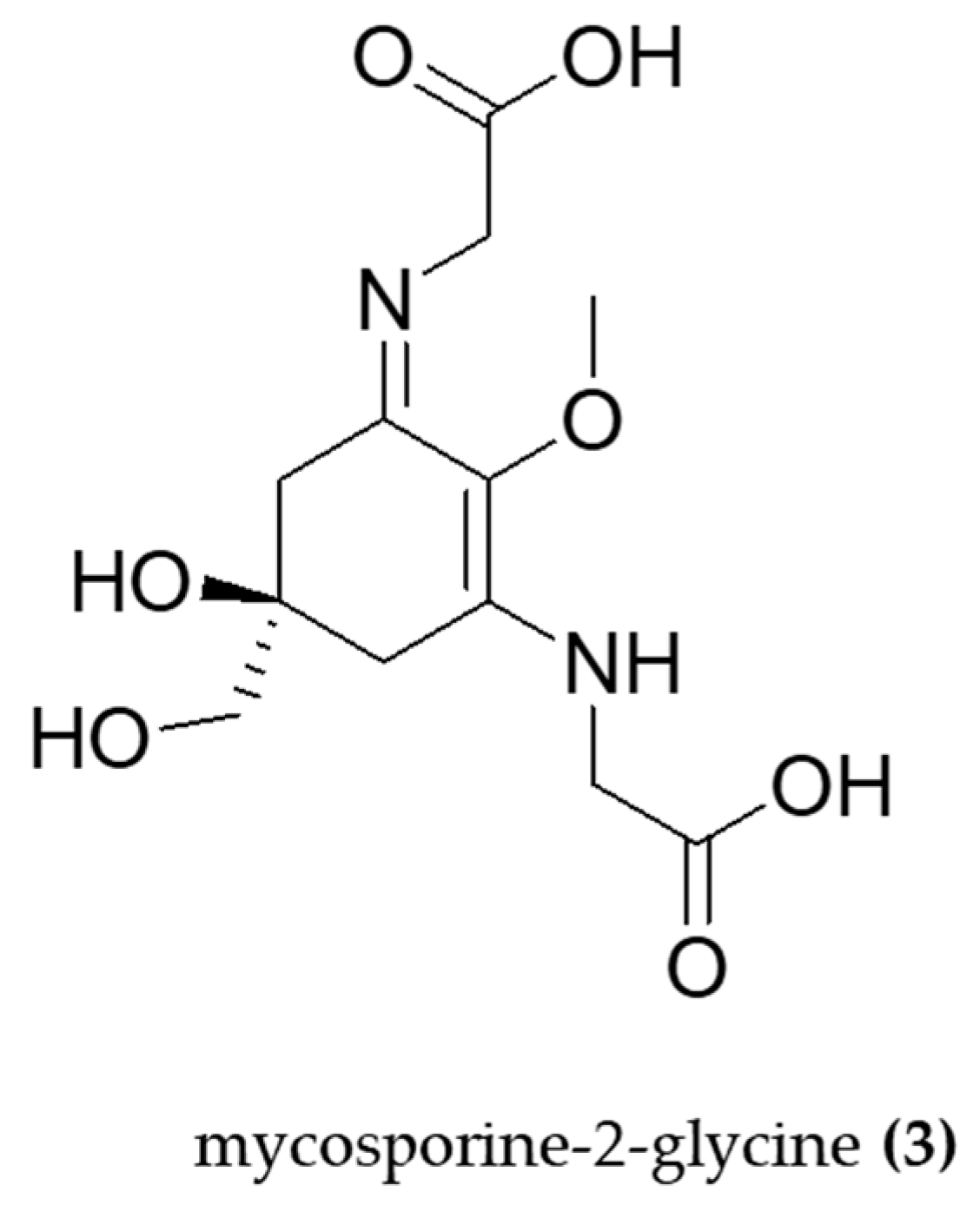
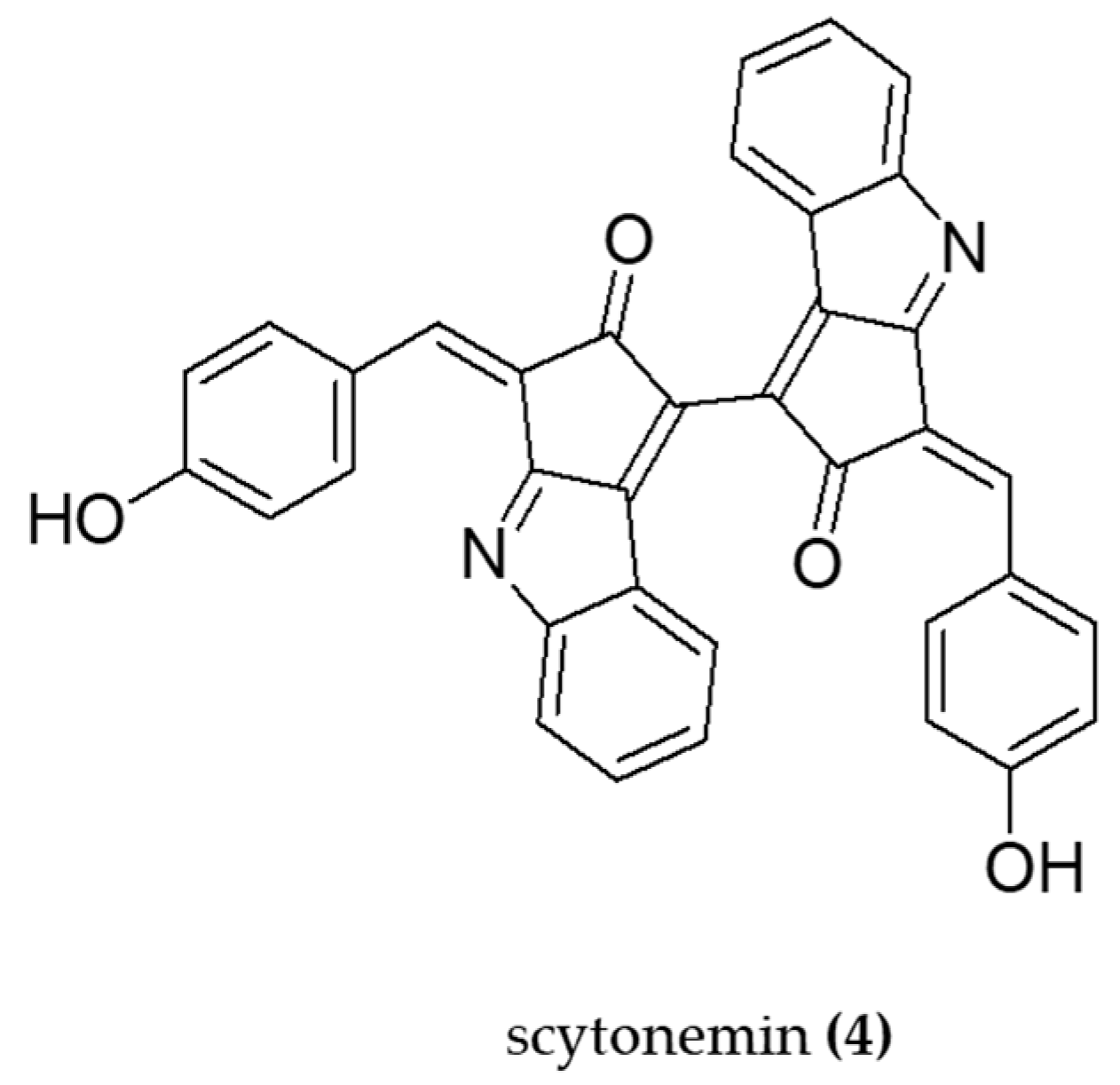
| Genus/Species/Code | Compound/Extract | Mechanism/Effect | In Vitro Assays | In Vivo Assays | Reference |
|---|---|---|---|---|---|
| Phormidium sp. ETS05 | Exopolysaccharides (EPS) | Anti-inflammatory | Human skin fibroblasts (HSF) exposed to EPS | Chemical (copper and DSS) and injury (amputation) zebrafish larvae | [56] |
| Nostoc commune | Polysaccharides | Antioxidant Antibacterial | H2O2, O2•−, DPPH•, RP | - | [57] |
| Phormidium versicolor | Polysaccharides | Antioxidant Antimicrobial | DPPH, FRAP, β-carotene bleaching, HO• assays | - | [58] |
| Leptolyngbya sp. | Polysaccharides | Antioxidant | DPPH, HO•, Fe2+ | - | [59] |
| Nostoc flagelliforme | Capsular polysaccharides | Antioxidant Anti-inflammatory | - | Male C57BL/6J mice | [60] |
| Nostoc flagelliforme | Polysaccharides | Antioxidant | DPPH, ABTS+, HO• assays | - | [61] |
| Aphanothece sacrum | Sacran | Anti-inflammatory. | HaCaT human keratinocyte cells. WST-1 method | Male Wistar rats and female BALB/c mice with paw edema induced by carrageenan, kaolin and dextran and ear edema induced by TPA. Histological analysis. | [62] |
| Aphanothece sacrum | Sacran | Anti-inflammatory. Antioxidant | HaCaT keratinocytes cells. ROS induced by SLS and IL-1α. | - | [63] |
| Aphanothece sacrun | Polysaccharide | Anti-inflammatory | - | Topical treatment with sacrun-containing serum in human volunteer (size, percentage of thick abrasion, ratio of SH to SS groups, ratio of IL-1 receptor antagonist to IL-1a, and carbonylated protein level). | [64] |
| Nodularia harveyana | Glycolipids | Anti-inflammatory Downregulation of TNF-α and NF-κB | LPS-stimulated leukemic monocyte cells (THP-1) | - | [65] |
| VPFK21-7 | Monounsaturated fatty acid 7(E)-9-keto-hexadec-7-enoic acid | Anti-inflammatory | HEK293 ARE-luc cells (ARE-Luciferase Reporter Assay) RAW264.7 cells (•NO levels) | - | [66] |
| Arthrospira subsalsa | Lipid fractions | Anti-inflammatory | hPRP (anti-PAF, antohrombotic activities) | - | [67] |
| Gloethece sp. | Lipidic extracts | Antioxidant Anti-inflammatory Anti-tumor | ABTS•+, DPPH•, •NO, O2•− HRBC, COX-2 TUNEL in AGS cancer cells | - | [68] |
| Leptolyngbya crossbyana | Lipopetides (Honaucins A-C) | Anti-inflammatory | LPS-stimulated RAW264.7 cell line (•NO production) | - | [69] |
| Lyngbya sordida | Lipopeptide (Malyngamide) | Anti-inflammatory | LPS-induced RAW 264.7 cell line (•NO production) | - | [70] |
| Nostoc | Aeruginosin-865 | Anti-inflammatory NF-κB inhibition | hTNF-α–stimulated HLMVECs cells (AlphaLISA assay). IL-8 and ICAM-1 levels | - | [71] |
| Planktothrix rubescens | Aeruginosin 828A and cyanopeptolin 1020 | Anti-inflammatory | Human hepatoma cell line Huh7 (IL-8, TNF-α) | Zebrafish AG 828A expoused | [72] |
| Oscillatoria margaritifera | Ethyl tumonoate A | Anti-inflammatory | Mouse cell line RAW264.7 (•NO assay) Neocortical neurons from Swiss webster mice (intracellular Ca2+ monitoritation) | - | [73] |
| Arthrospira maxima | Peptides (LDAVNR and MMLDF) | Anti-inflammatory | Antigen-stimulated RBL-2H3 mast cells. Histamine-stimulated EA.hy926 endhothelial cells | - | [74] |
| Aphanothece halophytica | Mycosporine-2-glycine | Anti-inflammatory Antioxidant | LPS- induced macrophages (RAW 264.7). iNOS, NF-κB, COX-2, H2O2. | - | [75] |
| - | Scytonemin | Anti-inflammatory | LPS-stimulated RAW 264.7 cells (TNF-α and NF-κB) | BALC/c mice with TPA-induced ear edema (TNF-α, iNOS) | [76] |
| Scytonema sp. R77DM | Scytonemin | Antioxidant | Cyanobacterial cells treated with scytonemin (ROS production) | - | [77] |
| Cyanobium sp. | Phycobiliproteins and carotenoids | Anti-inflammatory Antioxidant | ABTS•+, •NO, O2•− scavenging COX inhibition | [78] | |
| Arthrospira maxima | C-phycocyanin | Anti-inflammatory Anti-ulcerogenic | - | Male Wistar rats with ethanol-induced gastric ulcers (MDA, GSH, SOD, CAT, TNF-α, NF-κB) | [79] |
| Arthrospira platensis | C-Phycocyanin | Antioxidant Anti-inflammatory Neuronal protection | - | EAE indued male Lewis rats and female C57BL/6 mice: MDA assay. PP assay. FRA assay. ELISA (IL-17, IL-6, IFN-γ). | [80] |
| Nostoc sphaeroides | C-phycocyanin | Anti-inflammatory Antioxidant Mitochondria protection Synapse protection | - | DOX + CP- induced C57BL/6 male mice (MWM, TNF-α, IL-1β, IL-6, MDA, GSH and SOD levels) | [81] |
| Geitlerinema sp. TRV57 | Phycocyanin | Antioxidant | Phosphomolybdenum, DPPH•, H2O2, FRAP, Antilipid peroxidation assays | - | [82] |
| Halomicronema sp. R31DM | Phycoerythrin | Antioxidant | DPPH, FRAP, RP | N2 Bristol wild type C. elegans (ROS levels) | [83] |
| Lyngbya sp. and Oscillatoria sp. | Phycobiliproteins, Phenolic and Flavonoids compounds | Antioxidant | TPC, TFC, PBPs, FRAP and DPPH• | - | [84] |
| Aphanothece microscopica Nageli | Carotenoids | Antioxidant | ROO• scavenger capacity | - | [85] |
| Trichodesmium sp. | Carotenoids | Anti-inflammatory | COX-1 and COX-2 inhibition | - | [86] |
| Arthrospira platensis | Acetonic extract | Anti-inflammatory | Primary microglia (IL-1β, TNF-α, iNOS, Nrf2, HO-1) | [87] | |
| Arthrospira platensis | Non-protein extract | Antioxidant | PC 12 cells (Ferric- reducing antioxidant activity and DPPH•) | - | [88] |
| Arthrospira platensis | Diet supplementation (5 and 10% w/v) | Antioxidant Reduced cellular stress | - | DJ-1β∆93 Drosophila Melanogaster exposed to paraquat: Survival assay. Locomotor assay. Enzymatic assays (SOD and CAT). | [89] |
| Arthrospira maxima | 70% ethanol extract (SM70EE) | Neuroprotection Antioxidant | Aβ1–42- induced PC12 cells (MTT, PARP, LDH, GSH levels, western blot) | - | [90] |
| Arthrospira maxima | 70% ethanol extract (SM70EE) | Antioxidant AChE inhibition | TMT- induced HT-22 cells (MTT, PARP, western blotting) | Scopolamine-induced ICR mice (MWM, Passive avoidance test) | [91] |
| Arthrospira platensis | Diet supplementation (1500 mg/kg, tablets) | Antioxidant Anti-inflammatory Neuronal morphology protection | - | Wistar rats induced with AlCl3: GSH content assay. Total thiol content assay. TAC assay. ELISA (TNF-α). Histology. Immunofluorescence (Aβ). | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, F.; Reis, M.; Ferreira, L.; Grosso, C.; Ferraz, R.; Vieira, M.; Vasconcelos, V.; Martins, R. The Neuroprotective Role of Cyanobacteria with Focus on the Anti-Inflammatory and Antioxidant Potential: Current Status and Perspectives. Molecules 2024, 29, 4799. https://doi.org/10.3390/molecules29204799
Rodrigues F, Reis M, Ferreira L, Grosso C, Ferraz R, Vieira M, Vasconcelos V, Martins R. The Neuroprotective Role of Cyanobacteria with Focus on the Anti-Inflammatory and Antioxidant Potential: Current Status and Perspectives. Molecules. 2024; 29(20):4799. https://doi.org/10.3390/molecules29204799
Chicago/Turabian StyleRodrigues, Flávia, Mariana Reis, Leonor Ferreira, Clara Grosso, Ricardo Ferraz, Mónica Vieira, Vitor Vasconcelos, and Rosário Martins. 2024. "The Neuroprotective Role of Cyanobacteria with Focus on the Anti-Inflammatory and Antioxidant Potential: Current Status and Perspectives" Molecules 29, no. 20: 4799. https://doi.org/10.3390/molecules29204799
APA StyleRodrigues, F., Reis, M., Ferreira, L., Grosso, C., Ferraz, R., Vieira, M., Vasconcelos, V., & Martins, R. (2024). The Neuroprotective Role of Cyanobacteria with Focus on the Anti-Inflammatory and Antioxidant Potential: Current Status and Perspectives. Molecules, 29(20), 4799. https://doi.org/10.3390/molecules29204799










