Behavioral and Biochemical Effects of an Arylhydrazone Derivative of 5-Methoxyindole-2-Carboxylic Acid in a Scopolamine-Induced Model of Alzheimer’s Type Dementia in Rats
Abstract
1. Introduction
2. Results
2.1. Behavioral Assessment of the Effects of 5MeO on Learning and Memory
2.1.1. Step-Through Inhibitory Avoidance
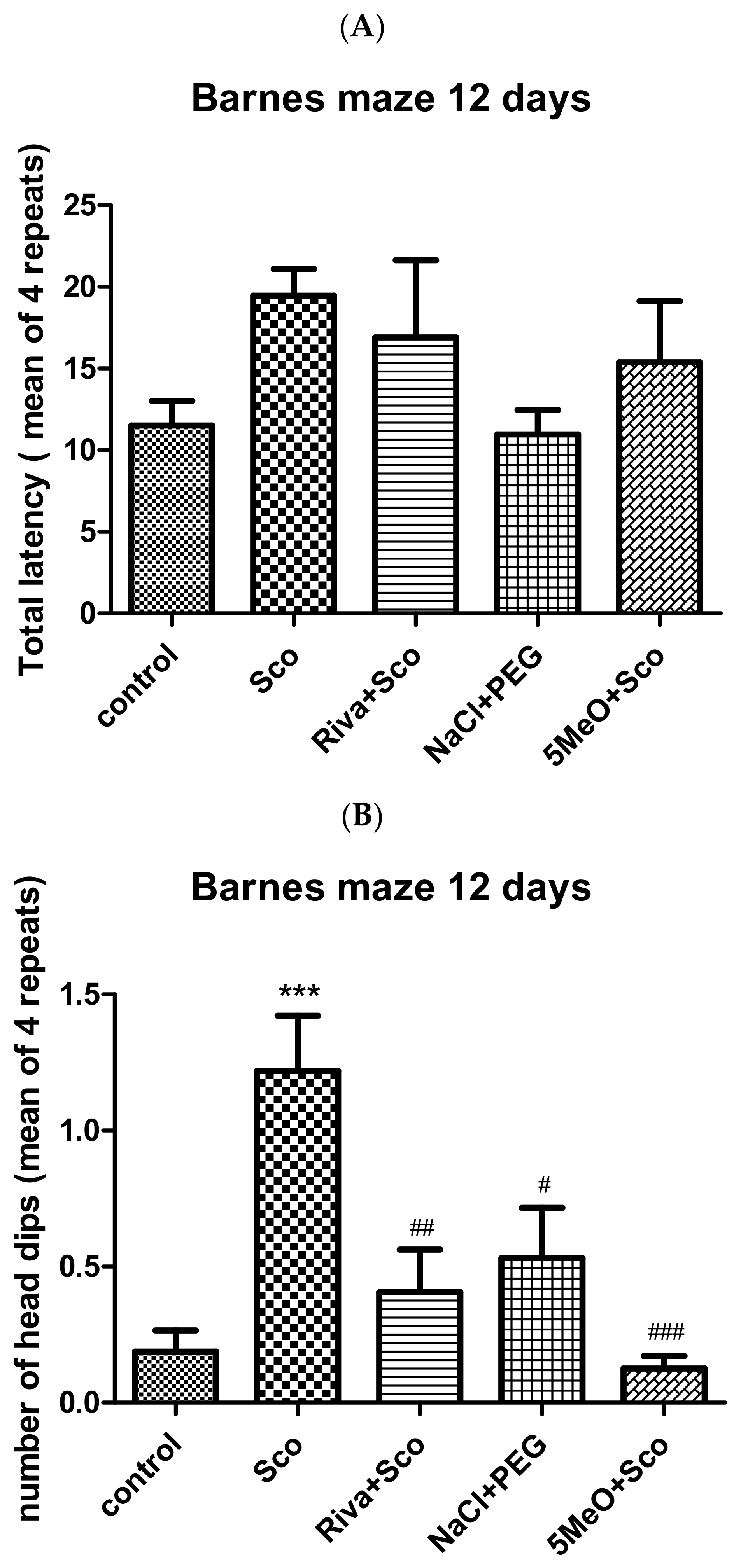
2.1.2. Barnes Maze
2.2. Biochemical Assessment of the Effects of 5MeO
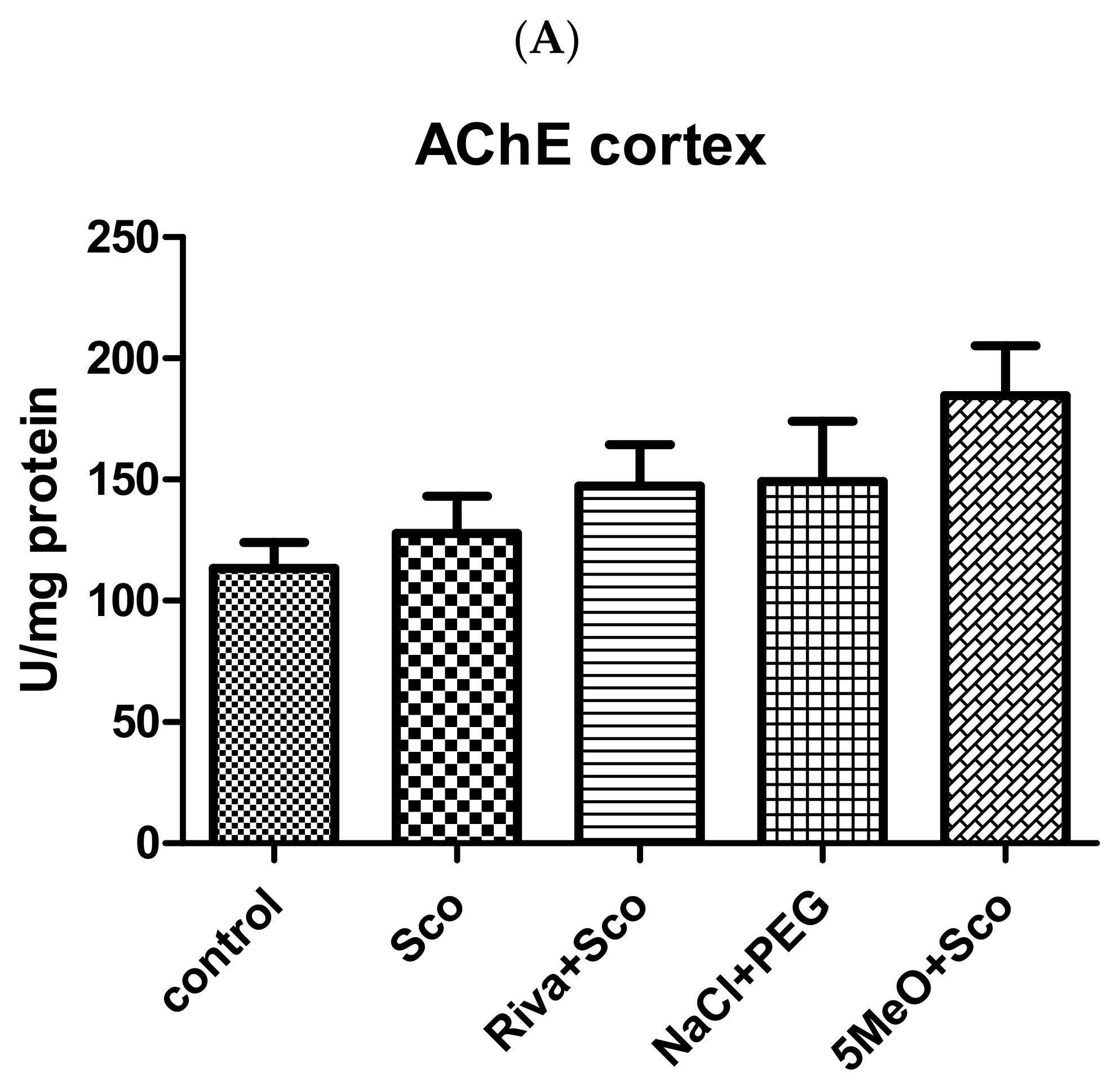
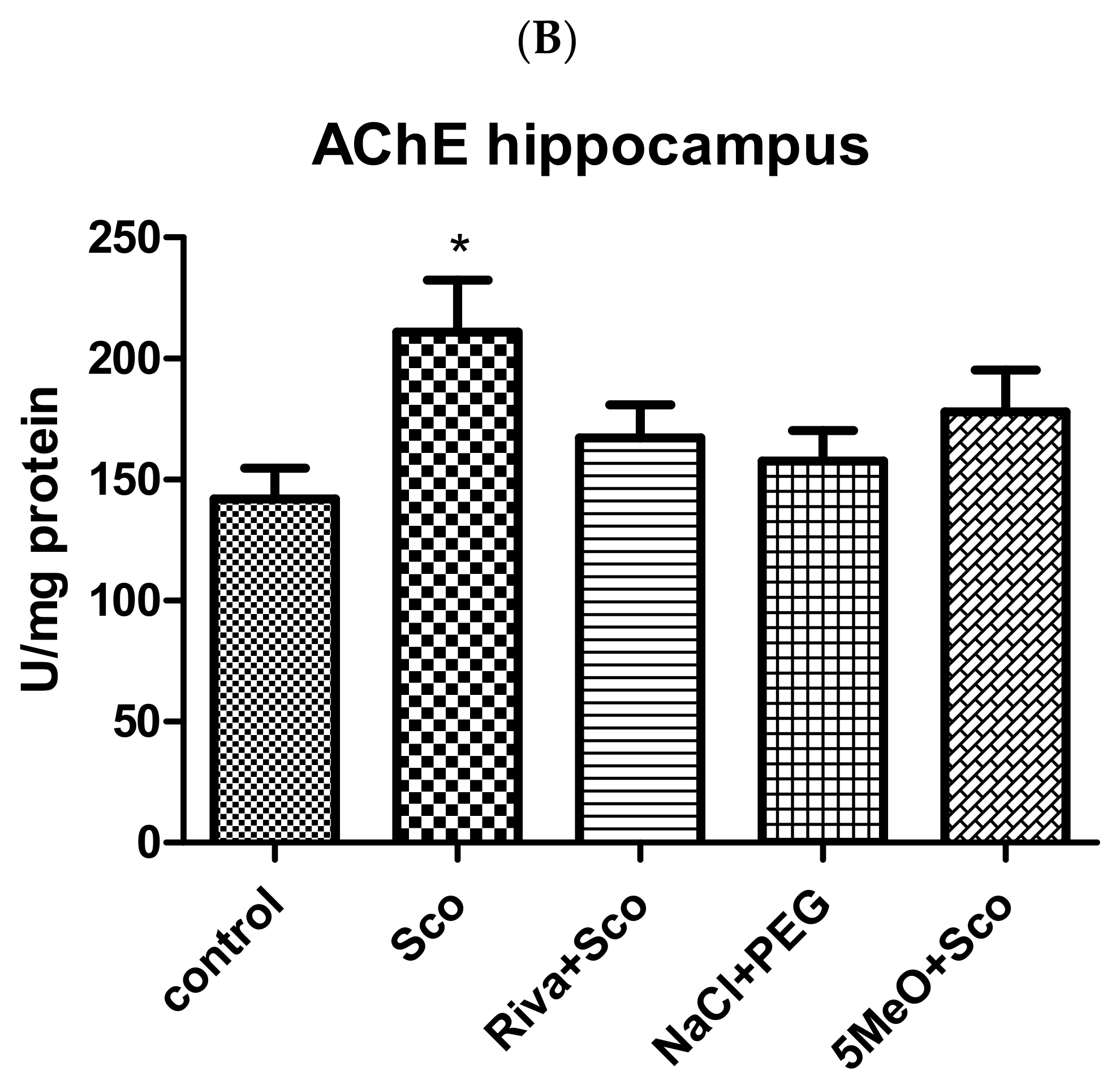
2.2.1. Effect of 5MeO on Brain AChE Activity
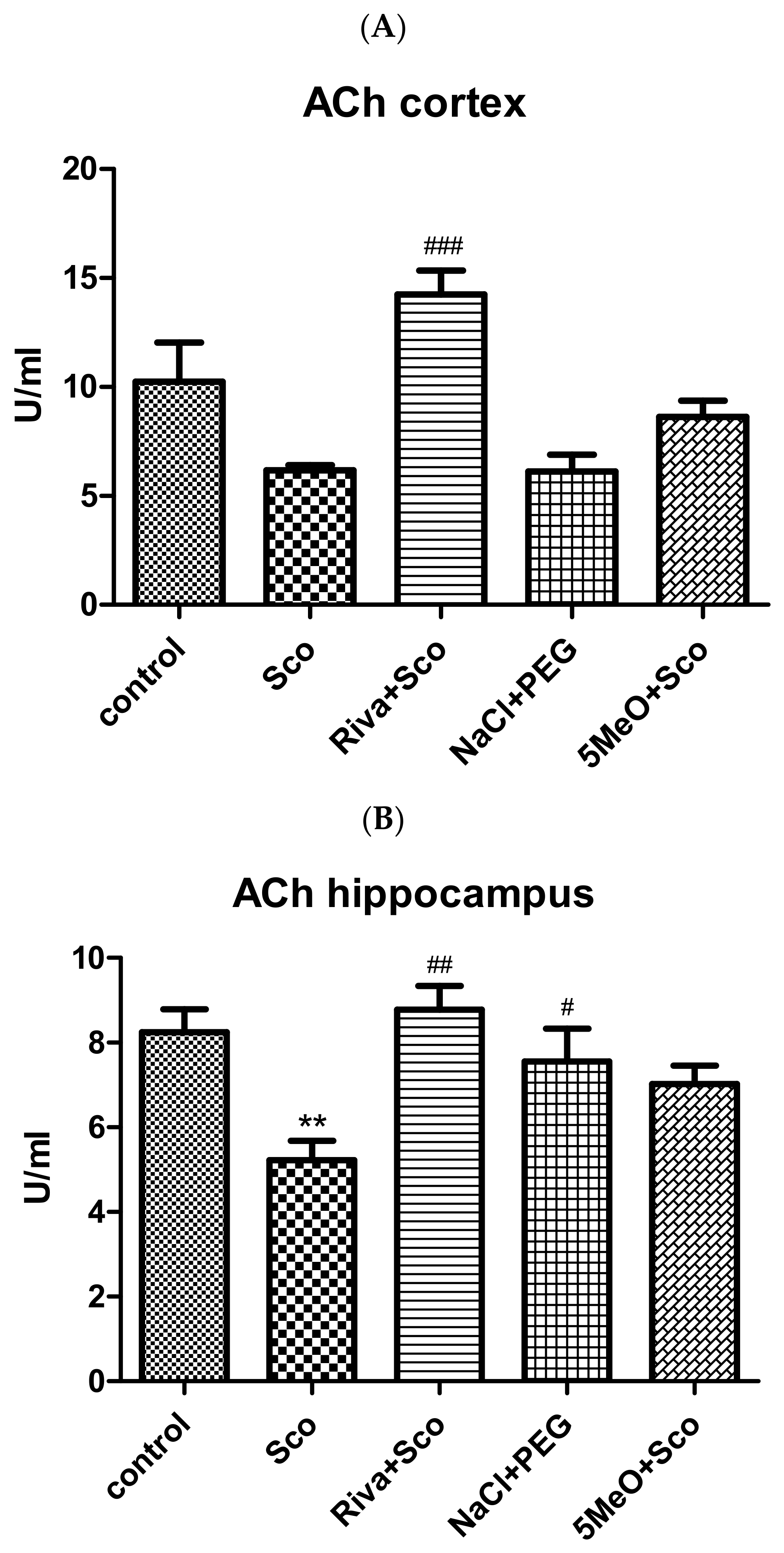
2.2.2. Effect of 5MeO on Brain ACh
2.2.3. Antioxidant Potential of 5MeO
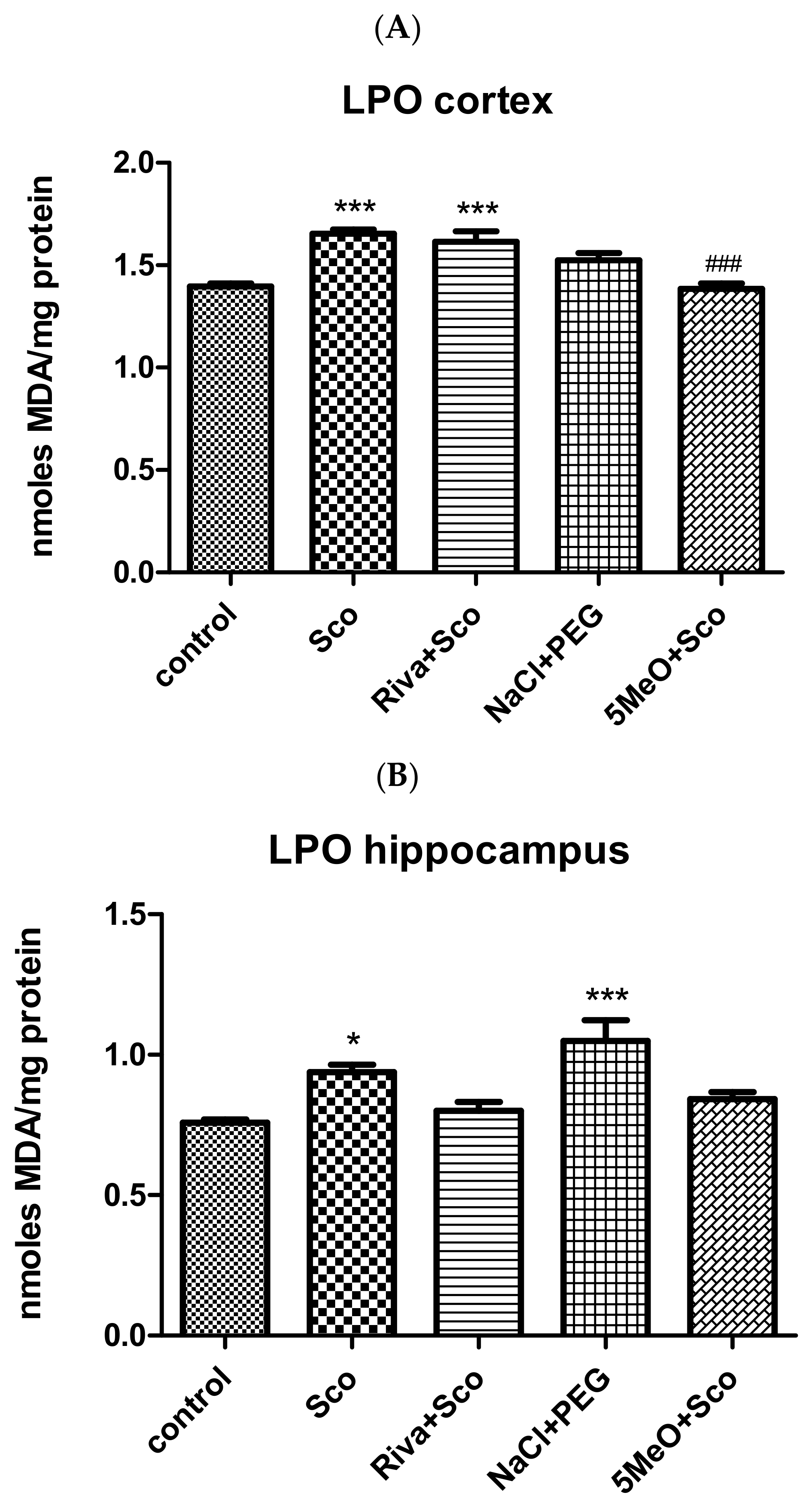
Effect of 5MeO on Brain Levels of MDA as a Measure of Lipid Peroxidation (LPO)
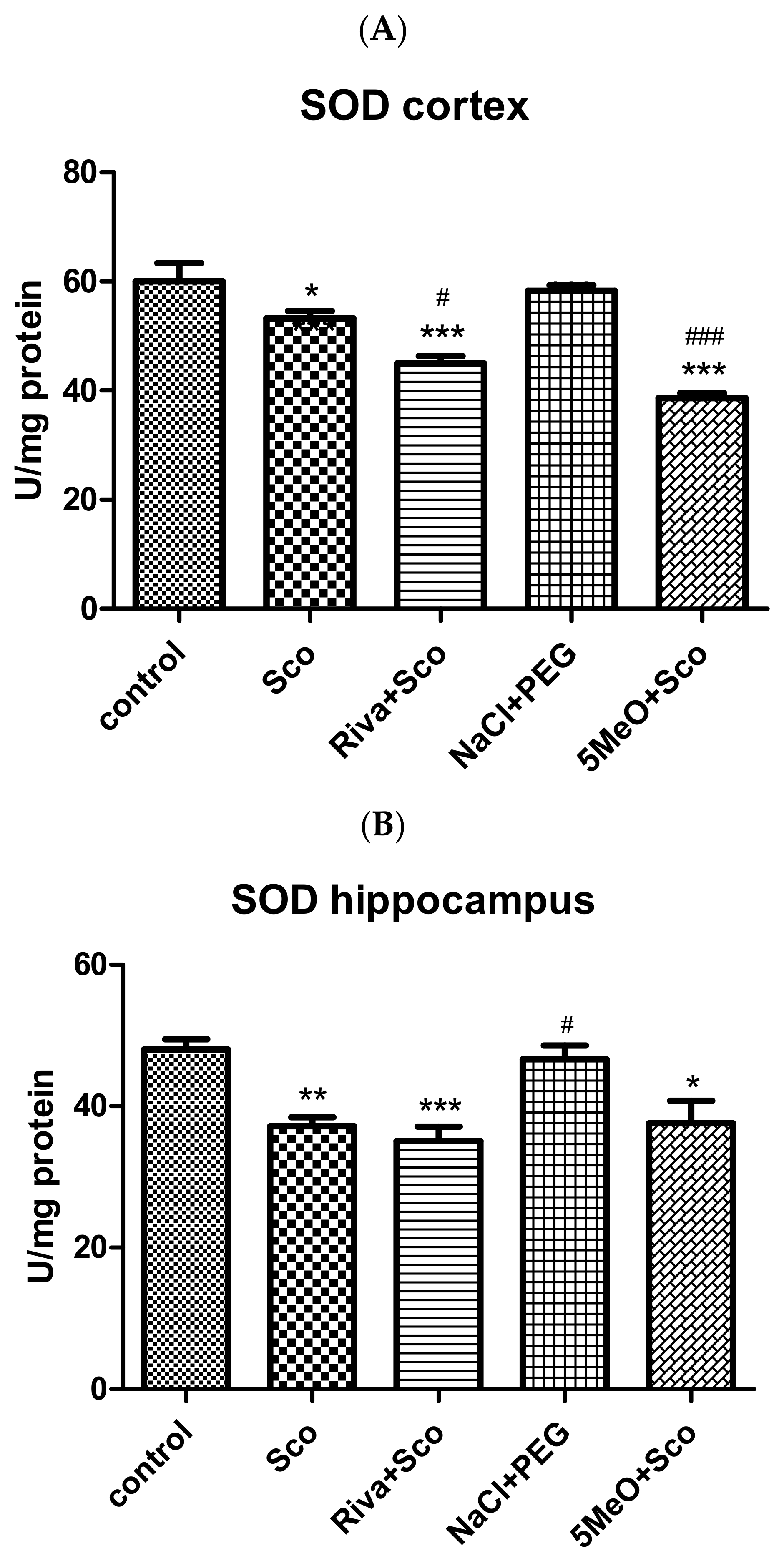
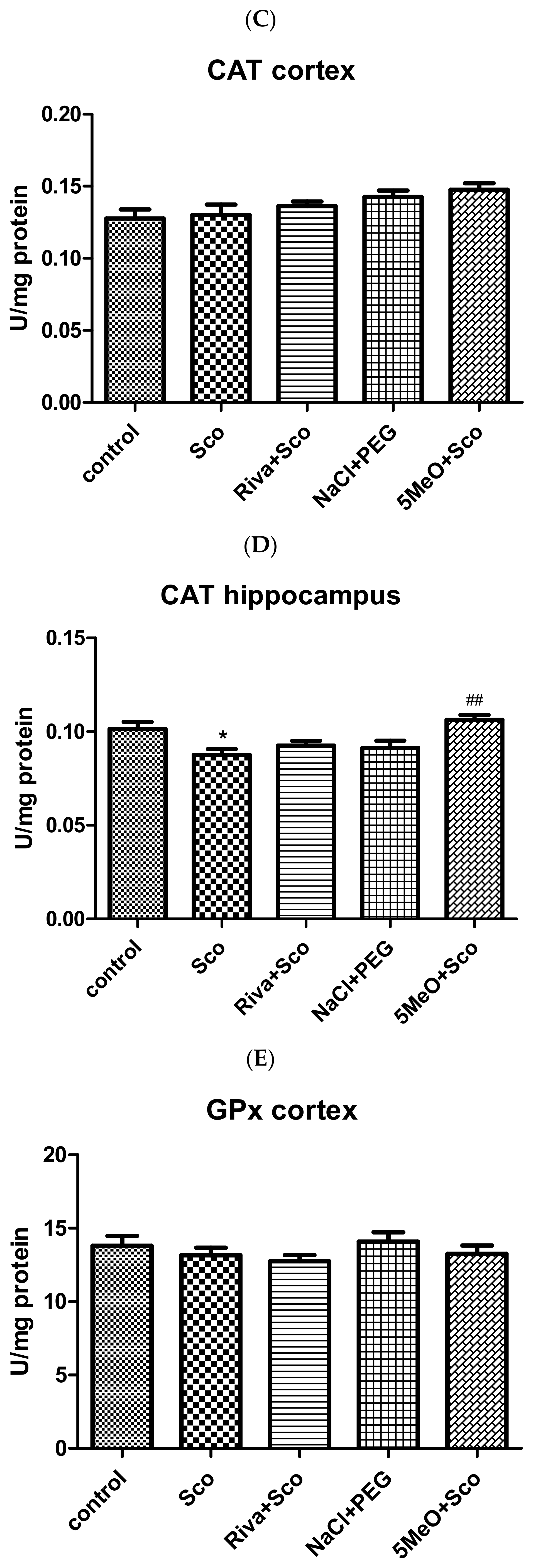
Effect of 5MeO on SOD, Catalase, and GPx Activity
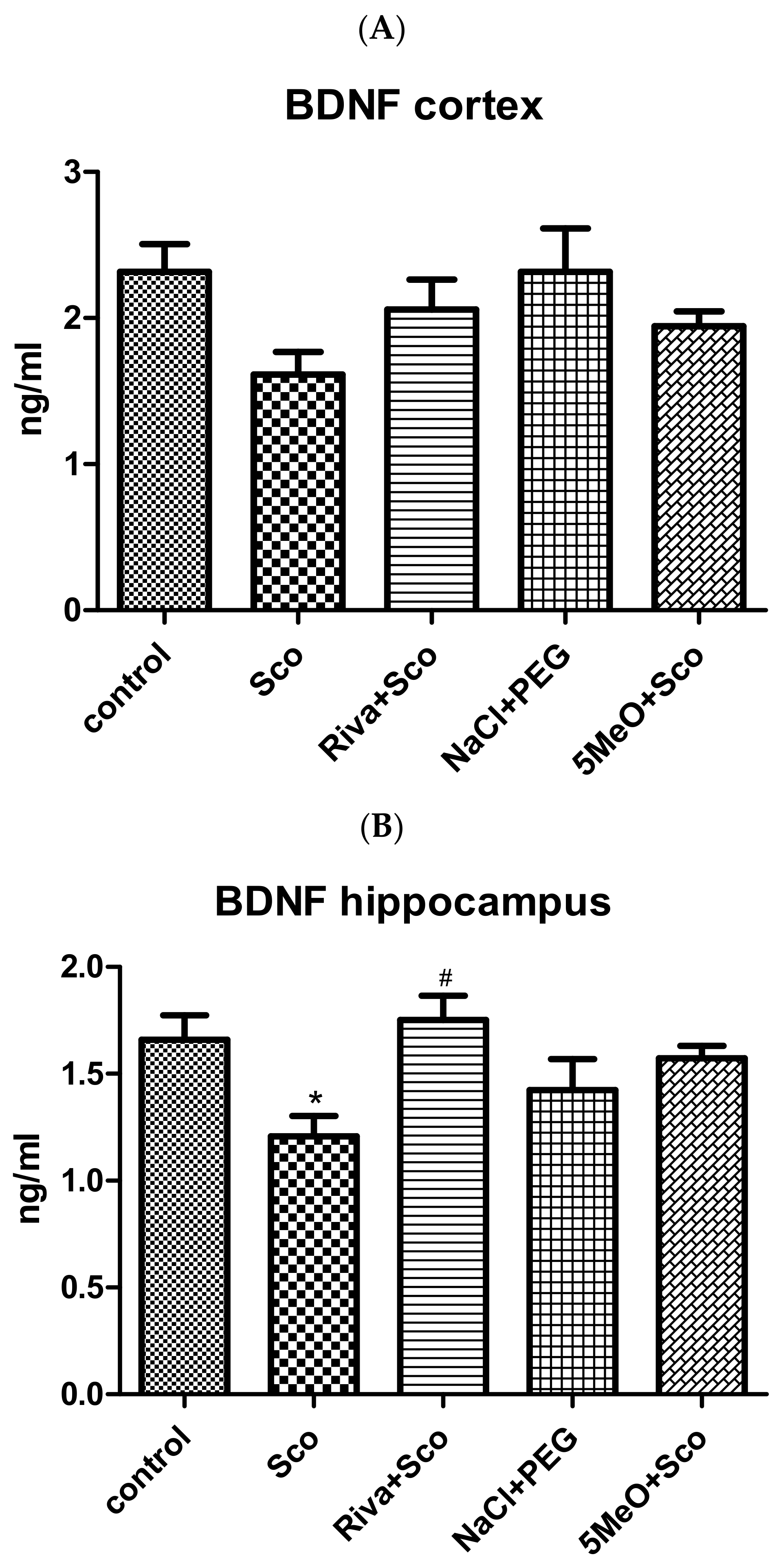
2.2.4. Effect of 5MeO on Brain BDNF
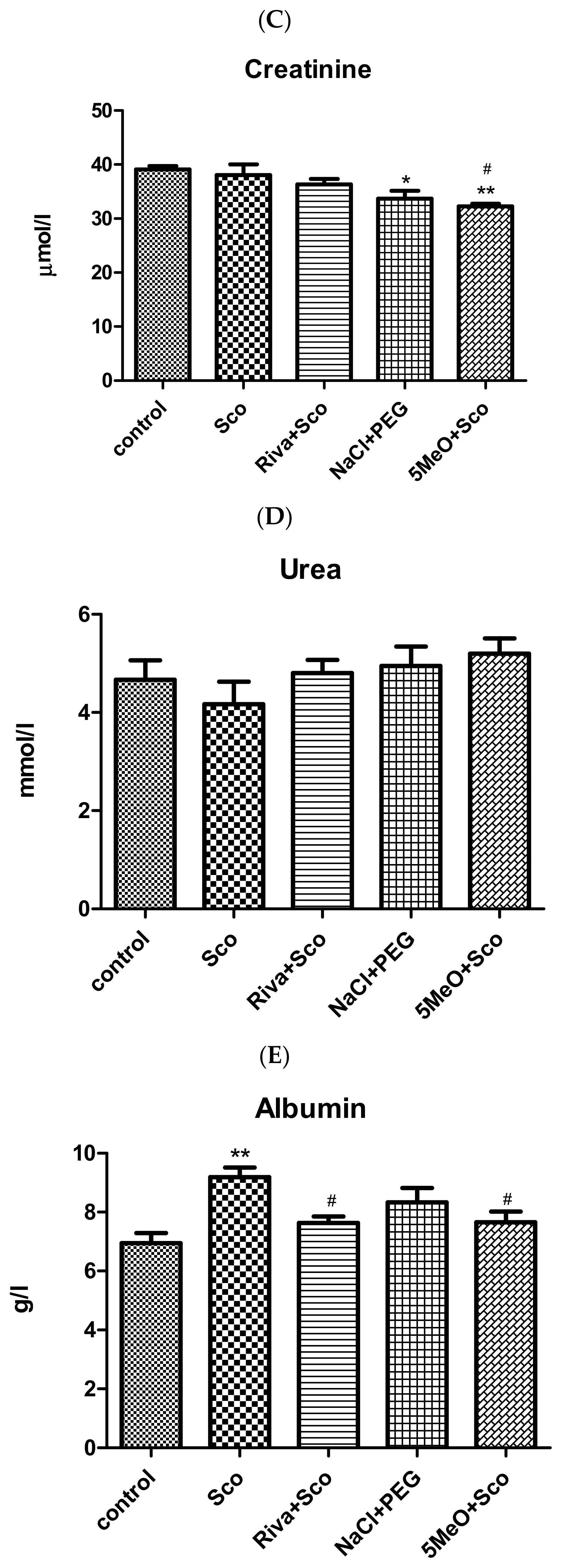
2.3. Biochemical Evaluation of Toxicity of 5MeO (ALAT, ASAT, Creatinine, Urea, Albumin, Globulin, and Total Protein Assays)
3. Discussion
4. Conclusions
5. Materials and Methods
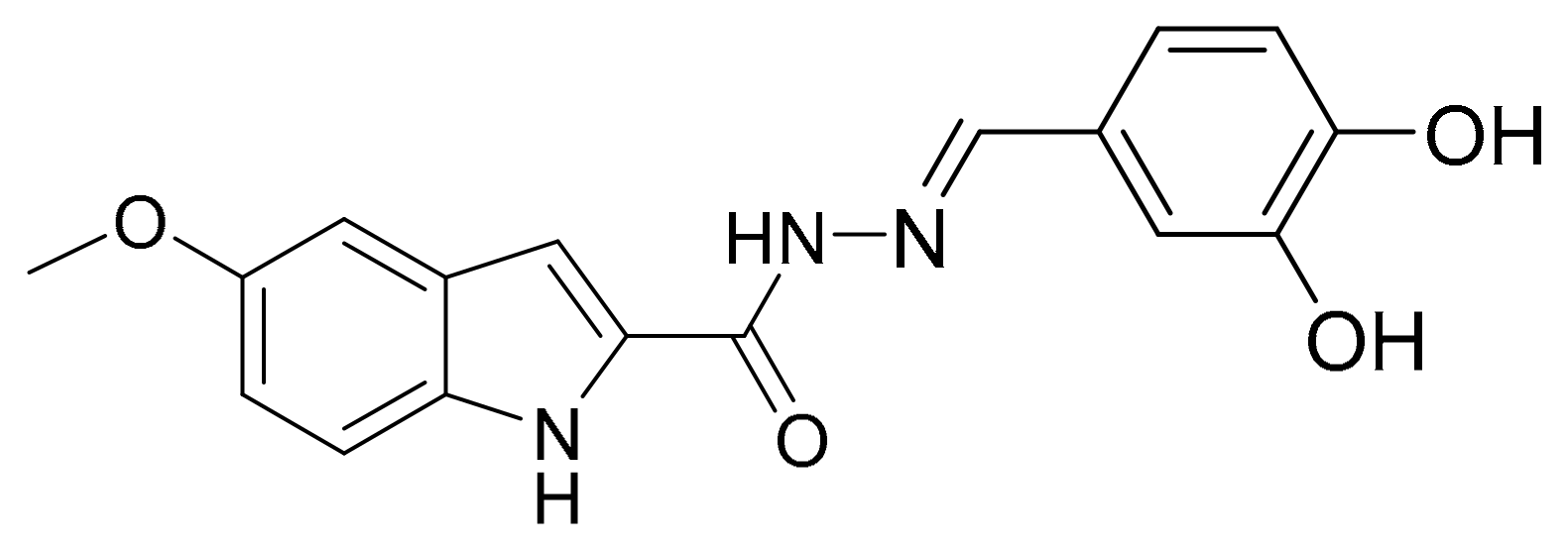
5.1. Synthesis
5.2. Laboratory Animals
5.3. Experimental Groups and Design
- -
- Control group (control)—receiving saline (0.5 mL/100 g, i.p.) for 11 days;
- -
- Scopolamine group (Sco)—receiving 2 mg/kg scopolamine (0.5 mL/100 g, i.p.) for 11 days;
- -
- Rivastigmine and scopolamine group (Riva + Sco)—receiving 2.5 mg/kg Rivastigmine (0.5 mL/100 g, i.p) and 2 mg/kg scopolamine (0.5 mL/100 g, i.p.) for 11 days;
- -
- Polyethylene glycol group (NaCl + PEG)—receiving Polyethylene glycol (30%) in 0.9% NaCl (0.5 mL/100 g, i.p.) for 11 days;
- -
- N′-(3,4-dihydroxybenzylidene)-5-methoxy-1H-indole-2-carbohydrazide) (5MeO) and scopolamine group (5MeO + Sco)—receiving 14.7 μM (4.86 mg/mL) 5MeO in 30% PEG in 0.9% NaC (0.5 mL/100 g, i.p.) and 2 mg/kg scopolamine (0.5 mL/100 g, i.p.) for 11 days.
5.4. Behavioral Experimental Methods
5.4.1. Step-Through Inhibitory (Passive) Avoidance
5.4.2. Barnes Maze
5.5. Biochemical Methods
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Chernoff, Y.O. Risk of Alzheimer’s Disease in Cancer Patients: Analysis of Mortality Data from the US SEER Population-Based Registries. Cancers 2020, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s Disease Hypothesis and Related Therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The Amyloid Cascade Hypothesis: Are We Poised for Success or Failure? J. Neurochem. 2016, 139 (Suppl. 2), 237–252. [Google Scholar] [CrossRef]
- Bhatia, S.; Rawal, R.; Sharma, P.; Singh, T.; Singh, M.; Singh, V. Mitochondrial Dysfunction in Alzheimer’s Disease: Opportunities for Drug Development. Curr. Neuropharmacol. 2022, 20, 675–692. [Google Scholar] [CrossRef]
- Conti Filho, C.E.; Loss, L.B.; Marcolongo-Pereira, C.; Rossoni Junior, J.V.; Barcelos, R.M.; Chiarelli-Neto, O.; da Silva, B.S.; Passamani Ambrosio, R.; Castro, F.C.A.Q.; Teixeira, S.F.; et al. Advances in Alzheimer’s Disease’s Pharmacological Treatment. Front. Pharmacol. 2023, 14, 1101452. [Google Scholar] [CrossRef]
- Mafi, J.N.; Leng, M.; Arbanas, J.C.; Tseng, C.H.; Damberg, C.L.; Sarkisian, C.; Landon, B.E. Estimated Annual Spending on Aducanumab in the US Medicare Program. JAMA Health Forum 2022, 1, e214495. [Google Scholar] [CrossRef]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef]
- Maramai, S.; Benchekroun, M.; Gabr, M.T.; Yahiaoui, S. Multitarget Therapeutic Strategies for Alzheimer’s Disease: Review on Emerging Target Combinations. BioMed Res. Int. 2020, 2020, 5120230. [Google Scholar] [CrossRef]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid Peroxidation and Protein Oxidation in Alzheimer’s Disease Brain: Potential Causes and Consequences Involving Amyloid Beta-Peptide-Associated Free Radical Oxidative Stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Boyd-Kimball, D.; Poon, H.F.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Markesbery, W.R.; Butterfield, D.A. Redox Proteomics Identification of Oxidized Proteins in Alzheimer’s Disease Hippocampus and Cerebellum: An Approach to Understand Pathological and Biochemical Alterations in AD. Neurobiol. Aging 2006, 27, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; Lovell, M. Brain Regional Correspondence Between Alzheimer’s Disease Histopathology and Biomarkers of Protein Oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Yatin, S.M.; Varadarajan, S.; Koppal, T. Amyloid Beta-Peptide-Associated Free Radical Oxidative Stress, Neurotoxicity, and Alzheimer’s Disease. Methods Enzymol. 1999, 309, 746–768. [Google Scholar]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative Stress in Alzheimer’s Disease: A Review on Emergent Natural Polyphenolic Therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Sehgal, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bungau, S.; Bumbu, A.G. Role of Monoamine Oxidase Activity in Alzheimer’s Disease: An Insight into the Therapeutic Potential of Inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef]
- Schedin-Weiss, S.; Inoue, M.; Hromadkova, L.; Teranishi, Y.; Yamamoto, N.G.; Wiehager, B.; Bogdanovic, N.; Winblad, B.; Sandebring-Matton, A.; Frykman, S.; et al. Monoamine Oxidase B is Elevated in Alzheimer Disease Neurons, is Associated with γ-Secretase and Regulates Neuronal Amyloid β-Peptide Levels. Alzheimers Res. Ther. 2017, 9, 57. [Google Scholar] [CrossRef]
- Anastassova, N.; Aluani, D.; Kostadinov, A.; Rangelov, M.; Todorova, N.; Hristova-Avakumova, N.; Argirova, M.; Lumov, N.; Kondeva-Burdina, M.; Tzankova, V.; et al. Evaluation of the combined activity of benzimidazole arylhydrazones as new anti-Parkinsonian agents: Monoamine oxidase-B inhibition, neuroprotection and oxidative stress modulation. Neural Regen. Res. 2021, 16, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Anastassova, N.; Stefanova, D.; Hristova-Avakumova, N.; Georgieva, I.; Kondeva-Burdina, M.; Rangelov, M.; Todorova, N.; Hristova-Avakumova, N.; Argirova, M.; Lumov, N.; et al. New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors. Antioxidants 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Anastassova, N.; Aluani, D.; Hristova-Avakumova, N.; Tzankova, V.; Kondeva-Burdina, M.; Rangelov, M.; Todorova, N.; Yancheva, D. Study on the Neuroprotective, Radical-Scavenging and MAO-B Inhibiting Properties of New Benzimidazole Arylhydrazones as Potential Multi-target Drugs for the Treatment of Parkinson’s Disease. Antioxidants 2022, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Bespyatykh, A.Y.; Burlakova, O.V.; Golichenkov, V.A. Melatonin as an antioxidant: The main functions and properties. Biol. Bull. Rev. 2011, 1, 143–150. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Distelmaier, F.; Valsecchi, F.; Forkink, M.; van Emst-de Vries, S.; Swarts, H.G.; Rodenburg, R.J.T.; Verwiel, E.T.P.; Smeitink, J.A.M. Trolox-sensitive reactive oxygen species regulate mitochondrial morphology, oxidative phosphorylation. Antioxid. Redox Signal. 2012, 17, 1652–1669. [Google Scholar] [CrossRef]
- Camps, P.; Muñoz-Torrero, D. Cholinergic drugs in pharmacotherapy of Alzheimer’s disease. Mini Rev. Med. Chem. 2002, 2, 11–25. [Google Scholar] [CrossRef]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomized controlled trial. Br. Med. J. 1999, 318, 633–638, Erratum in Br. Med. J. 2001, 322, 1456. [Google Scholar] [CrossRef]
- Takeda, A.; Loveman, E.; Clegg, A.; Kirby, J.; Picot, J.; Payne, E.; Green, C. A systematic review of the clinical effectiveness of donepezil, rivastigmine, and galantamine on cognition, quality of life, and adverse events in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2006, 21, 17–28. [Google Scholar] [CrossRef]
- Zuin, M.; Cherubini, A.; Volpato, S.; Ferrucci, L.; Zuliani, G. Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia. Sci. Rep. 2022, 12, 12214. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Pai, M.C.; Senanarong, V.; Looi, I.; Ampil, E.; Park, K.W.; Karanam, A.K.; Christopher, S. Rivastigmine: The advantages of dual inhibition of acetylcholinesterase and butyrylcholinesterase and its role in subcortical vascular dementia and Parkinson’s disease dementia. Clin. Interv. Aging 2017, 12, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, S.; Rao, J. Rivastigmine in Parkinson’s disease dementia. Expert Opin. Drug Metab. Toxicol. 2009, 5, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of cholinergic signaling in Alzheimer’s disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative stress in Alzheimer’s disease: Current knowledge of signaling pathways and therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Hritcu, L.; Stoica, B.; Bild, W.; Stefanescu, C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2010, 469, 6–10. [Google Scholar] [CrossRef]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef]
- Moawad, M.H.E.; Serag, I.; Alkhawaldeh, I.M.; Abbas, A.; Sharaf, A.; Alsalah, S.; Sadeq, M.A.; Shalaby, M.M.M.; Hefnawy, M.T.; Abouzid, M.; et al. Exploring the mechanisms and therapeutic approaches of mitochondrial dysfunction in Alzheimer’s disease: An educational literature review. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; Imbimbo, B.P.; Grasso, M.; Fidilio, A.; Emanuele, E.; Minoretti, P.; López-Ortiz, S.; Martín-Hernández, J.; Gabelle, A.; Caruso, G.; et al. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: A strategy for individualized therapeutic approaches? J. Neuroinflamm. 2024, 21, 187. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Arciniega-Martínez, I.M.; García-Marín, I.D.; Correa-Basurto, J.; Rosales-Hernández, M.C. Chronic administration of scopolamine increased GSK3βP9, beta secretase, amyloid beta, and oxidative stress in the hippocampus of Wistar rats. Mol. Neurobiol. 2020, 57, 3979–3988. [Google Scholar] [CrossRef]
- Liskowsky, W.; Schliebs, R. Muscarinic acetylcholine receptor inhibition in transgenic Alzheimer-like Tg2576 mice by scopolamine favours the amyloidogenic route of processing of amyloid precursor protein. Int. J. Dev. Neurosci. 2006, 24, 149–156. [Google Scholar] [CrossRef]
- Wako, M.; Ohara, K.; Hasegawa, Y. Sulfated polysaccharides isolated from nacre extract suppress chronic scopolamine administration-induced amyloid-beta deposition. Appl. Sci. 2024, 14, 7830. [Google Scholar] [CrossRef]
- Safar, H.H.; Arab, S.M.; Rizk, S.A.; El-Maraghy, S.M. Bone marrow-derived endothelial progenitor cells protect against scopolamine-induced Alzheimer-like pathological aberrations. Mol. Neurobiol. 2016, 53, 1403–1418. [Google Scholar] [CrossRef]
- Medeiros, R.; Kitazawa, M.; Caccamo, A.; Baglietto-Vargas, D.; Estrada-Hernandez, T.; Cribbs, D.H.; Fisher, A.; LaFerla, F.M. Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am. J. Pathol. 2011, 179, 980–991. [Google Scholar] [CrossRef]
- Ögren, S.O.; Stiedl, O. Passive avoidance. In Encyclopedia of Psychopharmacology; Stolerman, I.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Izquierdo, I.; da Cunha, C.; Rosat, R.; Jerusalinsky, D.; Ferreira, M.B.; Medina, J.H. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav. Neural Biol. 1992, 58, 16–26. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Fox, G.B.; Fan, L.; LeVasseur, R.A.; Fadden, A.I. Effect of traumatic brain injury on mouse spatial and non-spatial learning in the Barnes circular maze. J. Neurotrauma 1998, 15, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N. Learning impairments of hippocampal-lesioned mice in a paddling pool. Behav. Neurosci. 2002, 116, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, J.S.; Mayes, A.R.; Cezayirli, E.; Isaac, C.L.; Aggleton, J.P.; Roberts, N. A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia 2000, 38, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Broadbent, N.J.; Squire, L.R. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus 2005, 15, 340–346. [Google Scholar] [CrossRef][Green Version]
- Janis, L.S.; Glasier, M.M.; Fulop, Z.; Stein, D.G. Intraseptal injections of 192 IgG saporin produce deficits for strategy selection in spatial-memory tasks. Behav. Brain Res. 1998, 90, 23–34. [Google Scholar] [CrossRef]
- Johnson, D.A.; Zambon, N.J.; Gibbs, R.B. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Res. 2002, 943, 132–141. [Google Scholar] [CrossRef]
- Ukai, M.; Shinkai, N.; Kameyama, T. Cholinergic receptor agonists inhibit pirenzepine-induced dysfunction of spontaneous alternation performance in the mouse. Gen. Pharmacol. 1995, 26, 1529–1532. [Google Scholar] [CrossRef]
- George, A.; Ng, C.P.; O’Callaghan, M.; Jensen, G.S.; Wong, H.J. In vitro and ex-vivo cellular antioxidant protection and cognitive enhancing effects of an extract of Polygonum minus Huds (Lineminus™) demonstrated in a Barnes Maze animal model for memory and learning. BMC Complement. Altern. Med. 2014, 14, 161. [Google Scholar] [CrossRef]
- Malikowska-Racia, N.; Podkowa, A.; Sałat, K. Phencyclidine and scopolamine for modeling amnesia in rodents: Direct comparison with the use of Barnes maze test and contextual fear conditioning test in mice. Neurotox. Res. 2018, 34, 431–441. [Google Scholar] [CrossRef]
- Khurana, K.; Kumar, M.; Bansal, N. Lacidipine prevents scopolamine-induced memory impairment by reducing brain oxido-nitrosative stress in mice. Neurotox. Res. 2021, 39, 1087–1102. [Google Scholar] [CrossRef]
- Giovannini, M.G.; Lana, D.; Pepeu, G. The integrated role of ACh, ERK, and mTOR in the mechanisms of hippocampal inhibitory avoidance memory. Neurobiol. Learn. Mem. 2015, 119, 18–33. [Google Scholar] [CrossRef]
- Himmelheber, A.M.; Sarter, M.; Bruno, J.P. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res. Cogn. Brain Res. 2000, 9, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Arnold, H.M.; Burk, J.A.; Hodgson, E.M.; Sarter, M.; Bruno, J.P. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 2002, 114, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Snyder, P.J.; Pietrzak, R.H.; Jackson, C.E.; Bednar, M.; Maruff, P. Specific impairments in visuospatial working and short-term memory following low-dose scopolamine challenge in healthy older adults. Neuropsychologia 2008, 46, 2476–2484. [Google Scholar] [CrossRef]
- Croxson, P.L.; Kyriazis, D.A.; Baxter, M.G. Cholinergic modulation of a specific memory function of prefrontal cortex. Nat. Neurosci. 2011, 14, 1510–1512. [Google Scholar] [CrossRef]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef]
- Davidson, P.S.R.; Karpov, G.; Giguère, L.; Castro, A.W.; Tremblay, F. Older adults’ episodic memory is related to a neurophysiological marker of brain cholinergic activity. Exp. Brain Res. 2022, 240, 2269–2276. [Google Scholar] [CrossRef]
- Easton, A.; Barros, M.; Lever, C. Acetylcholine and spontaneous recognition memory in rodents and primates. In Behavioral Pharmacology of the Cholinergic System; Shoaib, M., Wallace, T., Eds.; Current Topics in Behavioral Neurosciences; Springer Nature: Berlin, Germany, 2020; Volume 45, pp. 29–45. [Google Scholar] [CrossRef]
- Robinson, L.; Platt, B.; Riedel, G. Involvement of the cholinergic system in conditioning and perceptual memory. Behav. Brain Res. 2011, 221, 443–465. [Google Scholar] [CrossRef]
- Prado, V.F.; Janickova, H.; Al-Onaizi, M.A.; Prado, M.A.M. Cholinergic circuits in cognitive flexibility. Neuroscience 2017, 345, 130–141. [Google Scholar] [CrossRef]
- Knight, R.; Khondoker, M.; Magill, N.; Stewart, R.; Landau, S. A systematic review and meta-analysis of the effectiveness of acetylcholinesterase inhibitors and memantine in treating the cognitive symptoms of dementia. Dement. Geriatr. Cogn. Disord. 2018, 45, 131–151. [Google Scholar] [CrossRef]
- Giacobini, E.; Spiegel, R.; Enz, A.; Veroff, A.E.; Cutler, N.R. Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: Correlation with cognitive benefit. J. Neural Transm. 2002, 109, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.A.; Mufson, E.J.; Gómez-Ramos, P. Colocalization of cholinesterases with beta amyloid protein in aged and Alzheimer’s brains. Acta Neuropathol. 1993, 85, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Perez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Opazo, C.; Alarcón, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-beta peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef]
- Alvarez, A.; Alarcón, R.; Opazo, C.; Campos, E.O.; Muñoz, F.J.; Calderón, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; De’Mello, F.G.; et al. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer’s fibrils. J. Neurosci. 1998, 18, 3213–3223. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Sagal, J.P.; Quintanilla, R.A.; Godoy, J.A.; Arrázola, M.S.; Inestrosa, N.C. Amyloid-beta-acetylcholinesterase complexes potentiate neurodegenerative changes induced by the Abeta peptide: Implications for the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 4. [Google Scholar] [CrossRef]
- Zueva, I.V.; Vasilieva, E.A.; Gaynanova, G.A.; Moiseenko, A.V.; Burtseva, A.D.; Boyko, K.M.; Zakharova, L.Y.; Petrov, K.A. Can activation of acetylcholinesterase by β-amyloid peptide decrease the effectiveness of cholinesterase inhibitors? Int. J. Mol. Sci. 2023, 24, 16395. [Google Scholar] [CrossRef]
- Hu, W.; Gray, N.W.; Brimijoin, S. Amyloid-beta increases acetylcholinesterase expression in neuroblastoma cells by reducing enzyme degradation. J. Neurochem. 2003, 86, 470–478. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Xu, Y.J.; Yang, C.; Tang, Y.; Li, L.; Cai, H.B.; Hou, B.N.; Chen, H.F.; Wang, Q.; Shi, X.G.; et al. Sodium tanshinone IIA sulfonate attenuates scopolamine-induced cognitive dysfunctions via improving cholinergic system. BioMed Res. Int. 2016, 2016, 9852536. [Google Scholar] [CrossRef]
- Schiller, D.; Eichenbaum, H.; Buffalo, E.A.; Davachi, L.; Foster, D.J.; Leutgeb, S.; Ranganath, C. Memory and Space: Towards an Understanding of the Cognitive Map. J. Neurosci. 2015, 35, 13904–13911. [Google Scholar] [CrossRef]
- Duff, M.C.; Covington, N.V.; Hilverman, C.; Cohen, N.J. Semantic Memory and the Hippocampus: Revisiting, Reaffirming, and Extending the Reach of Their Critical Relationship. Front. Hum. Neurosci. 2020, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Todorova, R.; Tang, W.; Oliva, A.; Fernandez-Ruiz, A. Associative and predictive hippocampal codes support memory-guided behaviors. Science 2023, 382, eadi8237. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Irving, G.; Jiang, L.; Wang, H.; Li, M.; Wang, X.; Han, W.; Xu, Y.; Yang, Y.; Zeng, T.; et al. Oxidative Stress Mediated Hippocampal Neuron Apoptosis Participated in Carbon Disulfide-Induced Rats Cognitive Dysfunction. Neurochem. Res. 2017, 42, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.R.; Kumar, R.; Gupta, A.; Meena, R.C.; Nanda, S.; Mishra, K.P.; Singh, S.B. Heat stress induced oxidative damage and perturbation in BDNF/ERK1/2/CREB axis in hippocampus impairs spatial memory. Behav. Brain Res. 2021, 396, 112895. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, J.; Yang, H.; Gao, J.; Chen, H.; Liu, C.; Gao, W. Prunella vulgaris L., an Edible and Medicinal Plant, Attenuates Scopolamine-Induced Memory Impairment in Rats. J. Agric. Food Chem. 2017, 65, 291–300. [Google Scholar] [CrossRef]
- Ionita, R.; Postu, P.A.; Beppe, G.J.; Mihasan, M.; Petre, B.A.; Hancianu, M.; Cioanca, O.; Hritcu, L. Cognitive-enhancing and antioxidant activities of the aqueous extract from Markhamia tomentosa (Benth.) K. Schum. stem bark in a rat model of scopolamine. Behav. Brain Funct. 2017, 13, 5. [Google Scholar] [CrossRef]
- Zhang, S.J.; Luo, D.; Li, L.; Tan, R.R.; Xu, Q.Q.; Qin, J.; Zhu, L.; Luo, N.C.; Xu, T.T.; Zhang, R.; et al. Ethyl Acetate Extract Components of Bushen-Yizhi Formula Provides Neuroprotection against Scopolamine-induced Cognitive Impairment. Sci. Rep. 2017, 7, 9824. [Google Scholar] [CrossRef]
- Hancianu, M.; Cioanca, O.; Mihasan, M.; Hritcu, L. Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 2013, 20, 446–452. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, K.; Rajakumar, S.; Sarkar, M.N.; Nachiappan, V. Glutathione peroxidase 3 of Saccharomyces cerevisiae protects phospholipids during cadmium-induced oxidative stress. Antonie van Leeuwenhoek 2011, 99, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.; Albrecht, J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem. Int. 2015, 88, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Ghosh, A.; Carnahan, J.; Greenberg, M.E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994, 263, 1618–1623. [Google Scholar] [CrossRef]
- Knüsel, B.; Winslow, J.W.; Rosenthal, A.; Burton, L.E.; Seid, D.P.; Nikolics, K.; Hefti, F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc. Natl. Acad. Sci. USA 1991, 88, 961–965. [Google Scholar] [CrossRef]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef]
- Gray, J.; Yeo, G.S.; Cox, J.J.; Morton, J.; Adlam, A.L.; Keogh, J.M.; Yanovski, J.A.; El Gharbawy, A.; Han, J.C.; Tung, Y.L.; et al. Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 2006, 55, 3366–3371. [Google Scholar] [CrossRef]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AD): A systematic review and meta-analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, C.; Kuroda, K.; Yanagawa, T.; Okamura, T.; Yoneda, H. Serum BDNF, TNF-alpha, and IL-1beta levels in dementia patients: Comparison between Alzheimer’s disease and vascular dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 402–406, Erratum in Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 406. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Cao, C.; Cawley, N.X.; Liu, T.T.; Yuan, J.; Loh, Y.P.; Cheng, Y. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: A meta-analysis study (N = 7277). Mol. Psychiatry 2017, 22, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Pláteník, J.; Fišar, Z.; Buchal, R.; Jirák, R.; Kitzlerová, E.; Zvěřová, M.; Raboch, J. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 83–93. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Diniz, B.S.; Teixeira, A.L.; Radanovic, M.; Talib, L.L.; Rocha, N.P.; Gattaz, W.F. Lower cerebrospinal fluid concentration of brain-derived neurotrophic factor predicts progression from mild cognitive impairment to Alzheimer’s disease. Neuromol. Med. 2015, 17, 326–332. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef]
- Holback, S.; Adlerz, L.; Iverfeldt, K. Increased processing of APLP2 and APP with concomitant formation of APP intracellular domains in BDNF and retinoic acid-differentiated human neuroblastoma cells. J. Neurochem. 2005, 95, 1059–1068. [Google Scholar] [CrossRef]
- Nigam, S.M.; Xu, S.; Kritikou, J.S.; Marosi, K.; Brodin, L.; Mattson, M.P. Exercise and BDNF reduce Aβ production by enhancing α-secretase processing of APP. J. Neurochem. 2017, 142, 286–296. [Google Scholar] [CrossRef]
- Kitiyanant, N.; Kitiyanant, Y.; Svendsen, C.N.; Thangnipon, W. BDNF-, IGF-1-, and GDNF-secreting human neural progenitor cells rescue amyloid β-induced toxicity in cultured rat septal neurons. Neurochem. Res. 2012, 37, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Silhol, M.; Moulière, F.; Meffre, J.; Höllinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Matrone, C.; Ciotti, M.T.; Mercanti, D.; Marolda, R.; Calissano, P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 13139–13144. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signaling mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB neurotrophic activity stimulates δ-secretase by upregulating C/EBPβ in Alzheimer’s disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef]
- Bharani, K.L.; Ledreux, A.; Gilmore, A.; Carroll, S.L.; Granholm, A.-C. Serum proBDNF levels correlate with phospho-tau staining in Alzheimer’s disease. Neurobiol. Aging 2020, 87, 49–59. [Google Scholar] [CrossRef]
- Ishola, I.O.; Jacinta, A.A.; Adeyemi, O.O. Cortico-hippocampal memory enhancing activity of hesperetin on scopolamine-induced amnesia in mice: Role of antioxidant defense system, cholinergic neurotransmission, and expression of BDNF. Metab. Brain Dis. 2019, 34, 979–989. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J. Fucoidan ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats via activation of cholinergic system and regulation of cAMP-response element-binding protein and brain-derived neurotrophic factor expressions. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 711–720. [Google Scholar] [CrossRef]
- Eun, C.S.; Lim, J.S.; Lee, J.; Lee, S.P.; Yang, S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef]
- Patel, D.; Roy, A.; Raha, S.; Kundu, M.; Gonzalez, F.J.; Pahan, K. Upregulation of BDNF and hippocampal functions by a hippocampal ligand of PPARα. JCI Insight 2020, 5, e136654. [Google Scholar] [CrossRef]
- Weber, D.K.; Danielson, K.; Wright, S.; Foley, J.E. Hematology and serum biochemistry values of dusky-footed wood rat (Neotoma fuscipes). J. Wildlife Dis. 2002, 38, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.; Lundblad, A. Creatinine concentration in plasma from dog, rat, and mouse: A comparison of 3 different methods. Vet. Clin. Pathol. 2005, 34, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Al Awabdh, S.; Coumoul, X.; Chauvet, C. The SH-SY5Y human neuroblastoma cell line, a relevant in vitro cell model for investigating neurotoxicology in human: Focus on organic pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Preis, L.; Villringer, K.; Brosseron, F.; Düzel, E.; Jessen, F.; Petzold, G.C.; Ramirez, A.; Spottke, A.; Fiebach, J.B.; Peters, O. Assessing blood-brain barrier dysfunction and its association with Alzheimer’s pathology, cognitive impairment and neuroinflammation. Alzheimers Res. Ther. 2024, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.A.; Bernardes, C.; Bernardo-Castro, S.; Lino, M.; Albino, I.; Ferreira, L.; Brás, J.; Guerreiro, R.; Tábuas-Pereira, M.; Baldeiras, I.; et al. Reconsidering the role of blood-brain barrier in Alzheimer’s disease: From delivery to target. Front. Aging Neurosci. 2023, 15, 1102809. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Kotzur, R.; Richter, F. Blood–brain barrier alterations and their impact on Parkinson’s disease pathogenesis and therapy. Transl. Neurodegener. 2024, 13, 37. [Google Scholar] [CrossRef]
- Watanabe, T.; Dohgu, S.; Takata, F.; Nishioku, T.; Nakashima, A.; Futagami, K.; Yamauchi, A.; Kataoka, Y. Paracellular barrier and tight junction protein expression in the immortalized brain endothelial cell lines bEND.3, bEND.5 and mouse brain endothelial cell. Biol. Pharm. Bull. 2013, 36, 492–495. [Google Scholar] [CrossRef]
- Anastassova, N.; Hristova-Avakumova, N.; Rusew, R.; Shivachev, B.; Yancheva, D. Two 5-Methoxyindole Carboxylic Acid-Derived Hydrazones of Neuropharmacological Interest: Synthesis, Crystal Structure, and Chemiluminescent Study of Radical Scavenging Properties. Crystals 2024, 14, 396. [Google Scholar] [CrossRef]
- Tancheva, L.; Lazarova, M.; Velkova, L.; Dolashki, A.; Uzunova, D.; Minchev, B.; Petkova-Kirova, P.; Hassanova, Y.; Gavrilova, P.; Tasheva, K.; et al. Beneficial Effects of Snail Helix aspersa Extract in an Experimental Model of Alzheimer’s Type Dementia. J. Alzheimers Dis. 2022, 88, 155–175. [Google Scholar] [CrossRef]
- Jarvik, M.E.; Kopp, R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967, 21, 221–224. [Google Scholar] [CrossRef]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Storm, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova-Kirova, P.; Anastassova, N.; Minchev, B.; Uzunova, D.; Grigorova, V.; Tsvetanova, E.; Georgieva, A.; Alexandrova, A.; Stefanova, M.; Yancheva, D.; et al. Behavioral and Biochemical Effects of an Arylhydrazone Derivative of 5-Methoxyindole-2-Carboxylic Acid in a Scopolamine-Induced Model of Alzheimer’s Type Dementia in Rats. Molecules 2024, 29, 5711. https://doi.org/10.3390/molecules29235711
Petkova-Kirova P, Anastassova N, Minchev B, Uzunova D, Grigorova V, Tsvetanova E, Georgieva A, Alexandrova A, Stefanova M, Yancheva D, et al. Behavioral and Biochemical Effects of an Arylhydrazone Derivative of 5-Methoxyindole-2-Carboxylic Acid in a Scopolamine-Induced Model of Alzheimer’s Type Dementia in Rats. Molecules. 2024; 29(23):5711. https://doi.org/10.3390/molecules29235711
Chicago/Turabian StylePetkova-Kirova, Polina, Neda Anastassova, Borislav Minchev, Diamara Uzunova, Valya Grigorova, Elina Tsvetanova, Almira Georgieva, Albena Alexandrova, Miroslava Stefanova, Denitsa Yancheva, and et al. 2024. "Behavioral and Biochemical Effects of an Arylhydrazone Derivative of 5-Methoxyindole-2-Carboxylic Acid in a Scopolamine-Induced Model of Alzheimer’s Type Dementia in Rats" Molecules 29, no. 23: 5711. https://doi.org/10.3390/molecules29235711
APA StylePetkova-Kirova, P., Anastassova, N., Minchev, B., Uzunova, D., Grigorova, V., Tsvetanova, E., Georgieva, A., Alexandrova, A., Stefanova, M., Yancheva, D., Kalfin, R., & Tancheva, L. (2024). Behavioral and Biochemical Effects of an Arylhydrazone Derivative of 5-Methoxyindole-2-Carboxylic Acid in a Scopolamine-Induced Model of Alzheimer’s Type Dementia in Rats. Molecules, 29(23), 5711. https://doi.org/10.3390/molecules29235711







