Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels
Abstract
1. Introduction of Slow-Release Fertilizers
2. Development of Starch-Based Hydrogels
2.1. Grafting Copolymerization with Starches
2.2. Radiation In Situ Polymerization
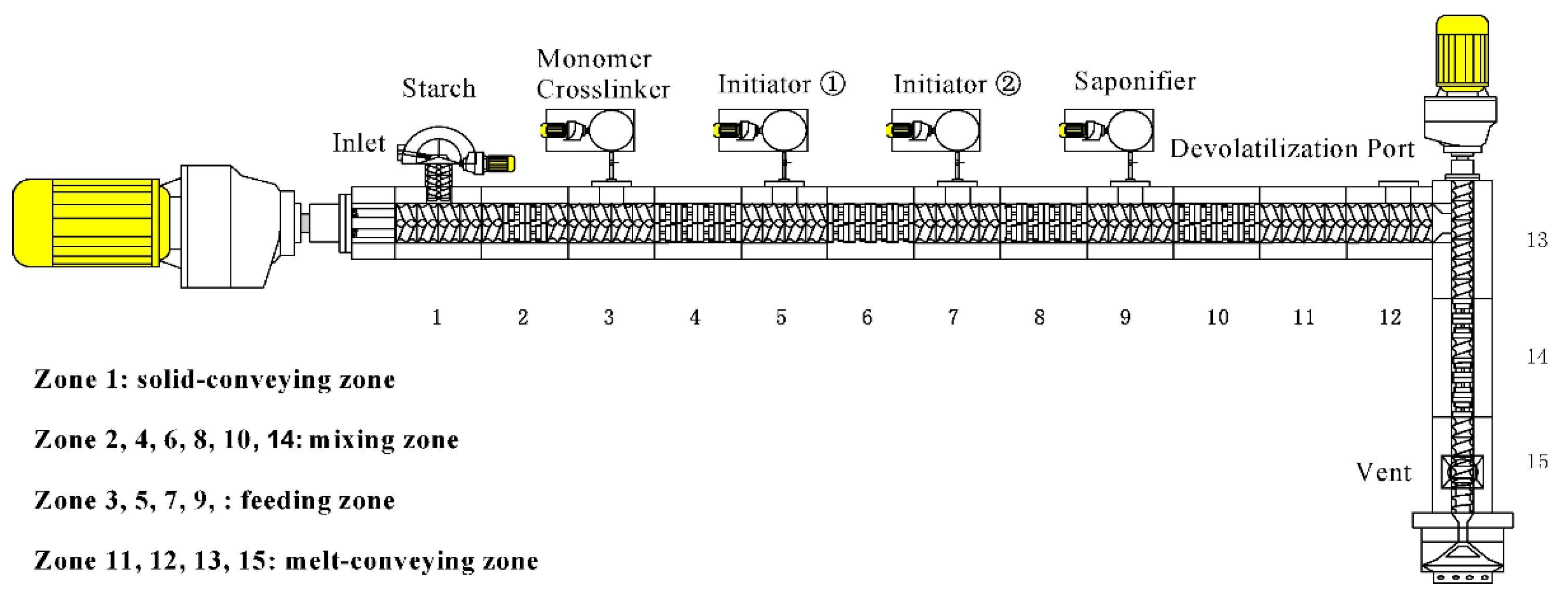
2.3. Application of Reactive Extrusion Technology
2.4. Characterization Techniques for Hydrogel Network Structure
3. Loading Fertilizer into Hydrogels
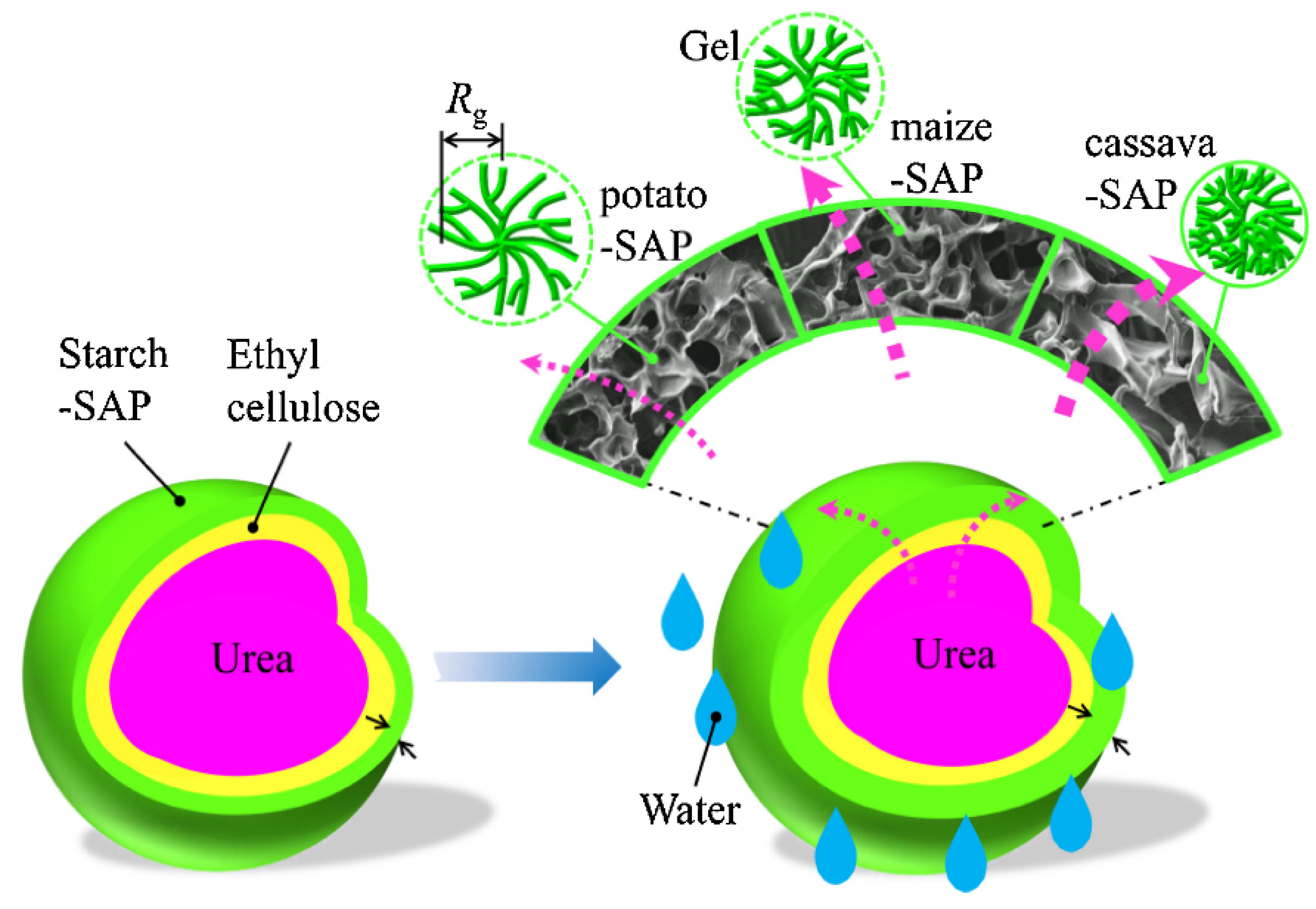
3.1. Coating by Starch
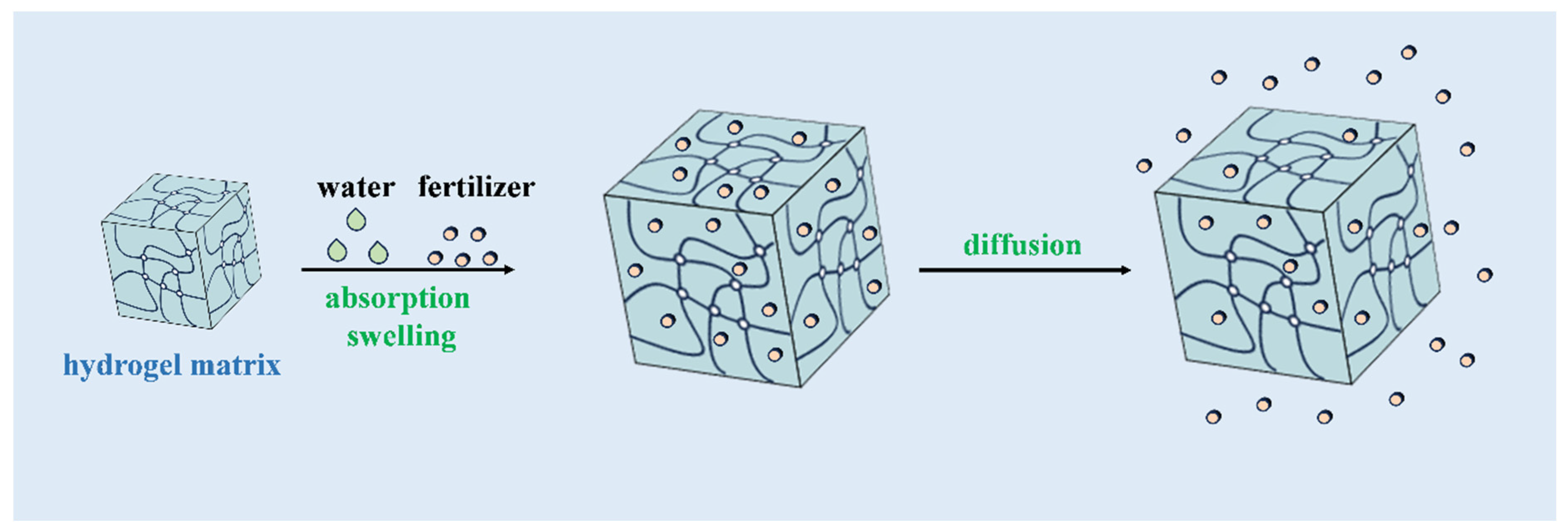
3.2. Swelling and Absorption Equilibrium Method
3.3. In Situ Polymerization Technology
3.4. Reactive Extrusion Technology
3.5. Compounding with Cellulose
3.6. Other Hydrogel Systems
4. Release Mechanisms and Kinetics
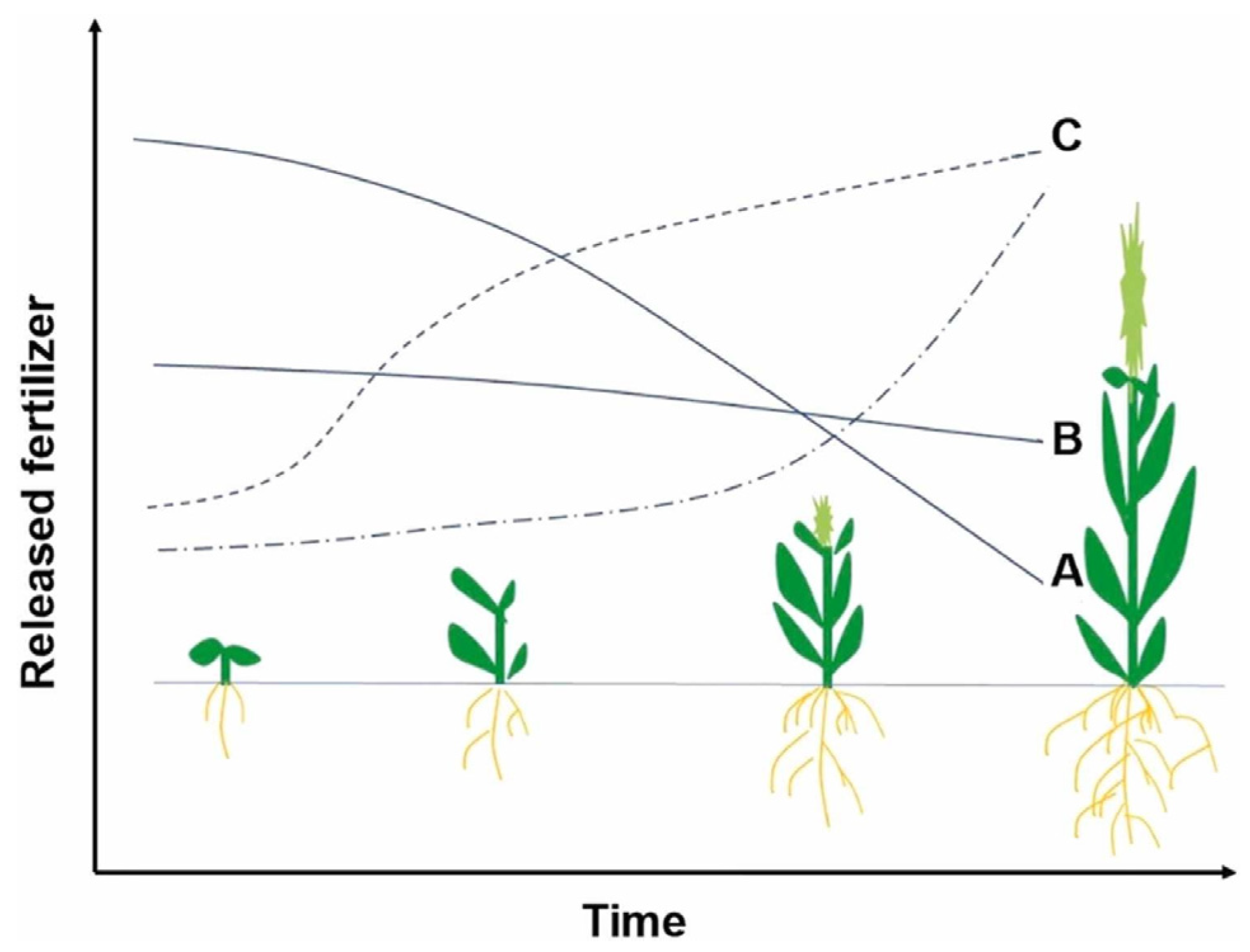
4.1. Release Mechanisms
4.2. Kinetic Models
5. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Association, I.F. Fertilizer Outlook 2019–2023. In Proceedings of the 87th IFA Annual Conference, Montreal, QC, Canada, 11–13 June 2019. [Google Scholar]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.; Maity, A.; Halli, H.M.; Sujayananad, G.K.; Vadivel, R.; Das, T.K.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortiz, R.; Naranjo, M.A.; Ruiz-Navarro, A.; Atares, S.; Garcia, C.; Zotarelli, L.; San Bautista, A.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. [Google Scholar] [CrossRef]
- Messiga, A.J.; Dyck, K.; Ronda, K.; van Baar, K.; Haak, D.; Yu, S.; Dorais, M. Nutrients Leaching in Response to Long-Term Fertigation and Broadcast Nitrogen in Blueberry Production. Plants 2020, 9, 1530. [Google Scholar] [CrossRef]
- Li, S.; Chen, G. Contemporary strategies for enhancing nitrogen retention and mitigating nitrous oxide emission in agricultural soils: Present and future. Environ. Dev. Sustain. 2019, 22, 2703–2741. [Google Scholar] [CrossRef]
- Li, T.; Gao, B.; Tong, Z.; Yang, Y.; Li, Y. Chitosan and graphene oxide nanocomposites as coatings for controlled-release fertilizer. Water Air Soil Pollut. 2019, 230, 1–9. [Google Scholar] [CrossRef]
- Wei, X.; Bao, X.; Yu, L.; Liu, H.; Lu, K.; Chen, L.; Bai, L.; Zhou, X.; Li, Z.; Li, W. Correlation Between Gel Strength of Starch-Based Hydrogel and Slow Release Behavior of Its Embedded Urea. J. Polym. Environ. 2020, 28, 863–870. [Google Scholar] [CrossRef]
- Duan, Q.; Jiang, S.; Chen, F.; Li, Z.; Ma, L.; Song, Y.; Yu, X.; Chen, Y.; Liu, H.; Yu, L. Fabrication, evaluation methodologies and models of slow-release fertilizers: A review. Ind. Crops Prod. 2023, 192, 116075. [Google Scholar] [CrossRef]
- Fu, J.; Wang, C.; Chen, X.; Huang, Z.; Chen, D. Classification research and types of slow controlled release fertilizers (SRFs) used—A review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.-H.; El Bouchtaoui, F.-Z.; Jaouahar, M.; El Achaby, M. Polymer coated slow/controlled release granular fertilizers: Fundamentals and research trends. Prog. Mater. Sci. 2024, 144, 101269. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating materials for slow release of nitrogen from urea fertilizer: A review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- El Assimi, T.; Blažic, R.; Vidović, E.; Raihane, M.; El Meziane, A.; Baouab, M.H.V.; Khouloud, M.; Beniazza, R.; Kricheldorf, H.; Lahcini, M. Polylactide/cellulose acetate biocomposites as potential coating membranes for controlled and slow nutrients release from water-soluble fertilizers. Prog. Org. Coat. 2021, 156, 106255. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Zhang, L.; Wei, Y.; Zhang, L.; Ma, Y.; Ruso, J.M.; Liu, Z. Engineering and slow-release properties of lignin-based double-layer coated fertilizer. Polym. Adv. Technol. 2023, 34, 2029–2043. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Pillai, S.S.; Aravind, M.; Salim, S.A.; Kuzhivilayil, S.J. Cassava starch-graft-poly (acrylonitrile)-coated urea fertilizer with sustained release and water retention properties. Adv. Polym. Technol. 2018, 37, 2687–2694. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, X.; Zhang, Y.; Zhang, X.; Zhu, W.; Huang, H.; Han, X.; Liu, Y.; Xu, C. Poly (vinyl alcohol)/sodium alginate polymer membranes as eco-friendly and biodegradable coatings for slow release fertilizers. J. Sci. Food Agric. 2023, 103, 3592–3601. [Google Scholar] [CrossRef]
- Andry, H.; Yamamoto, T.; Irie, T.; Moritani, S.; Inoue, M.; Fujiyama, H. Water retention, hydraulic conductivity of hydrophilic polymers in sandy soil as affected by temperature and water quality. J. Hydrol. 2009, 373, 177–183. [Google Scholar] [CrossRef]
- Burke, D.R.; Akay, G.; Bilsborrow, P.E. Development of novel polymeric materials for agroprocess intensification. J. Appl. Polym. Sci. 2010, 118, 3292–3299. [Google Scholar] [CrossRef]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Channab, B.-E.; El Idrissi, A.; Zahouily, M.; Essamlali, Y.; White, J.C. Starch-based controlled release fertilizers: A review. Int. J. Biol. Macromol. 2023, 238, 124075. [Google Scholar] [CrossRef]
- Matmin, J.; Ibrahim, S.I.; Mohd Hatta, M.H.; Ricky Marzuki, R.; Jumbri, K.; Nik Malek, N.A.N. Starch-Derived Superabsorbent Polymer in Remediation of Solid Waste Sludge Based on Water–Polymer Interaction. Polymers 2023, 15, 1471. [Google Scholar] [CrossRef]
- Azeem, B.; Elboughdiri, N.; KuShaari, K.; Jamoussi, B.; Ghernaout, D.; Ghareba, S.; Raza, S.; Gasmi, A. Valorization of almond shells’ lignocellulosic microparticles for controlled release urea production: Interactive effect of process parameters on longevity and kinetics of nutrient release. J. Coat. Technol. Res. 2021, 19, 643–660. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, X.; Zhang, L.; Chen, X.; Sun, S.; Sun, R.C. Research Progress in Lignin-Based Slow/Controlled Release Fertilizer. ChemSusChem 2020, 13, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Fertahi, S.; Ilsouk, M.; Zeroual, Y.; Oukarroum, A.; Barakat, A. Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J. Control. Release 2021, 330, 341–361. [Google Scholar] [CrossRef]
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Ford Versypt, A.N. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54. [Google Scholar] [CrossRef]
- Jennett, T.S.; Zheng, Y. Component characterization and predictive modeling for green roof substrates optimized to adsorb P and improve runoff quality: A review. Environ. Pollut. 2018, 237, 988–999. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled release fertilizers: A review on coating materials and mechanism of release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Wesołowska, M.; Rymarczyk, J.; Góra, R.; Baranowski, P.; Sławiński, C.; Klimczyk, M.; Supryn, G.; Schimmelpfennig, L. New slow-release fertilizers—Economic, legal and practical aspects: A Review. Int. Agrophys. 2021, 35, 11–24. [Google Scholar] [CrossRef]
- Yuan, S.; Cheng, L.; Tan, Z. Characteristics and preparation of oil-coated fertilizers: A review. J. Control. Release 2022, 345, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. Appl. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Mu, B.; Wang, Y.; Quan, Z.; Wang, A. Synthesis, characterization, and swelling behaviors of sodium carboxymethyl cellulose-g-poly(acrylic acid)/semi-coke superabsorbent. Polym. Bull. 2022, 79, 935–953. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Z.; Zhang, R.; Zhou, S.; Yang, H.; Chen, Y.; Zhang, J.; Yin, H.; Yu, D. Research Advances in Superabsorbent Polymers. Polymers 2024, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wen, G. Development history and synthesis of super-absorbent polymers: A review. J. Polym. Res. 2020, 27, 136. [Google Scholar] [CrossRef]
- Manuel, M.; Jennifer, A. A review on starch and cellulose-enhanced superabsorbent hydrogel. J. Chem. Rev. 2023, 5, 183–203. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.-E.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A comprehensive review on starch: Structure, modification, and applications in slow/controlled-release fertilizers in agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, H.-Q.; Xu, G.-X.; Cheng, J.-S.; Zhang, B. Preparation, characterization, and encapsulation capability of the hydrogel cross-linked by esterified tapioca starch. Int. J. Biol. Macromol. 2020, 155, 1–5. [Google Scholar] [CrossRef]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal Ul, A.; Ullah, R.S.; Khan, A.; et al. Advances in chemical modifications of starches and their applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Mongkolsawat, K.; Sonsuk, M. Synthesis and property characterization of cassava starch grafted poly [acrylamide-co-(maleic acid)] superabsorbent via γ-irradiation. Polymer 2002, 43, 3915–3924. [Google Scholar] [CrossRef]
- Czarnecka, E.; Nowaczyk, J. Semi-Natural Superabsorbents Based on Starch-g-poly(acrylic acid): Modification, Synthesis and Application. Polymers 2020, 12, 1794. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, E.; Nowaczyk, J. Synthesis and Characterization Superabsorbent Polymers Made of Starch, Acrylic Acid, Acrylamide, Poly(Vinyl Alcohol), 2-Hydroxyethyl Methacrylate, 2-Acrylamido-2-methylpropane Sulfonic Acid. Int. J. Mol. Sci. 2021, 22, 4325. [Google Scholar] [CrossRef]
- Noordergraaf, I.-W.; Witono, J.R.; Heeres, H.J. Grafting Starch with Acrylic Acid and Fenton’s Initiator: The Selectivity Challenge. Polymers 2024, 16, 255. [Google Scholar] [CrossRef]
- Aniagor, C.O.; Afifi, M.A.; Hashem, A. Rapid and efficient uptake of aqueous lead pollutant using starch-based superabsorbent hydrogel. Polym. Bull. 2022, 79, 6373–6388. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P.A. Starch-derived superabsorbent polymers in agriculture applications: An overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Khan, M.A.; Bhattacharia, S.K.; Kader, M.A.; Bahari, K. Preparation and characterization of ultra violet (UV) radiation cured bio-degradable films of sago starch/PVA blend. Carbohydr. Polym. 2006, 63, 500–506. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Synthesis, characterization and swelling behaviour of superabsorbent polymers from cassava starch-graft-poly(acrylamide). Starch Starke 2012, 64, 207–218. [Google Scholar] [CrossRef]
- Amiri, F.; Kabiri, K.; Bouhendi, H.; Abdollahi, H.; Najafi, V.; Karami, Z. High gel-strength hybrid hydrogels based on modified starch through surface cross-linking technique. Polym. Bull. 2019, 76, 4047–4068. [Google Scholar] [CrossRef]
- Ma, D.; Zhu, B.; Cao, B.; Wang, J.; Zhang, J. The Microstructure and Swelling Properties of Poly Acrylic Acid-Acrylamide Grafted Starch Hydrogels. J. Macromol. Sci. Part B 2016, 55, 1124–1133. [Google Scholar] [CrossRef]
- Zamani-Babgohari, F.; Irannejad, A.; Kalantari Pour, M.; Khayati, G.R. Synthesis of carboxymethyl starch co (polyacrylamide/ polyacrylic acid) hydrogel for removing methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2024, 269, 132053. [Google Scholar] [CrossRef] [PubMed]
- Bazoubandi, B.; Soares, J.B.P. Amylopectin-graft-polyacrylamide for the flocculation and dewatering of oil sands tailings. Miner. Eng. 2020, 148, 106196. [Google Scholar] [CrossRef]
- Mahmoodi-Babolan, N.; Nematollahzadeh, A.; Heydari, A.; Merikhy, A. Bioinspired catecholamine/starch composites as superadsorbent for the environmental remediation. Int. J. Biol. Macromol. 2019, 125, 690–699. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Zhang, C.; Aluko, R.E.; Yuan, J.; Ju, X.; He, R. Structural and functional characterization of rice starch-based superabsorbent polymer materials. Int. J. Biol. Macromol. 2020, 153, 1291–1298. [Google Scholar] [CrossRef]
- Siyamak, S.; Laycock, B.; Luckman, P. Synthesis of starch graft-copolymers via reactive extrusion and their ammonium sorption properties. Chem. Eng. J. 2020, 398, 124291. [Google Scholar] [CrossRef]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]
- Bao, X.; Yu, L.; Simon, G.P.; Shen, S.; Xie, F.; Liu, H.; Chen, L.; Zhong, L. Rheokinetics of graft copolymerization of acrylamide in concentrated starch and rheological behaviors and microstructures of reaction products. Carbohydr. Polym. 2018, 192, 1–9. [Google Scholar] [CrossRef]
- Bao, X.; Yu, L.; Shen, S.; Simon, G.P.; Liu, H.; Chen, L. How rheological behaviors of concentrated starch affect graft copolymerization of acrylamide and resultant hydrogel. Carbohydr. Polym. 2019, 219, 395–404. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Medha; Thakur, S.; Kaith, B.S.; Sharma, N.; Ansar, S.; Pandey, S.; Kuma, V. Biopolymer starch-gelatin embedded with silver nanoparticle–based hydrogel composites for antibacterial application. Biomass Convers. Biorefinery 2022, 12, 5363–5384. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and swelling behavior of environmentally friendly starch-based superabsorbent hydrogels reinforced with natural char nano/micro particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Olad, A.; Doustdar, F.; Gharekhani, H. Fabrication and characterization of a starch-based superabsorbent hydrogel composite reinforced with cellulose nanocrystals from potato peel waste. Colloids Surf. A 2020, 601, 124962. [Google Scholar] [CrossRef]
- Li, A.; Zhang, J.; Wang, A. Utilization of starch and clay for the preparation of superabsorbent composite. Bioresour. Technol. 2007, 98, 327–332. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; Abdel-Aal, S.E.; Badway, N.A.; Abo Farha, S.A.; Alshafei, E.A. Radiation synthesis and characterization of starch-based hydrogels for removal of acid dye. Starch Stärke 2014, 66, 400–408. [Google Scholar] [CrossRef]
- Relleve, L.S.; Aranilla, C.T.; Barba, B.J.D.; Gallardo, A.K.R.; Cruz, V.R.C.; Ledesma, C.R.M.; Nagasawa, N.; Abad, L.V. Radiation-synthesized polysaccharides/polyacrylate super water absorbents and their biodegradabilities. Radiat. Phys. Chem. 2020, 170, 108618. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Saif, M.J.; Naveed, M.; Asif, H.M.; Akhtar, R. Irradiation applications for polymer nano-composites: A state-of-the-art review. J. Ind. Eng. Chem. 2018, 60, 218–236. [Google Scholar] [CrossRef]
- Goganian, A.M.; Hamishehkar, H.; Arsalani, N.; Khiabani, H.K. Microwave-Promoted Synthesis of Smart Superporous Hydrogel for the Development of Gastroretentive Drug Delivery System. Adv. Polym. Technol. 2015, 34, 21490. [Google Scholar] [CrossRef]
- Naikwadi, A.T.; Sharma, B.K.; Bhatt, K.D.; Mahanwar, P.A. Gamma Radiation Processed Polymeric Materials for High Performance Applications: A Review. Front. Chem. 2022, 10, 837111. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rehim, H.; Hegazy, E.-S.A.; Diaa, D. Radiation synthesis of eco-friendly water reducing sulfonated starch/acrylic acid hydrogel designed for cement industry. Radiat. Phys. Chem. 2013, 85, 139–146. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R.; Duţă, D. Electron-beam processed corn starch: Evaluation of physicochemical and structural properties and technical-economic aspects of the processing. Braz. J. Chem. Eng. 2013, 30, 847–856. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Sánchez-Moreno, V.E.; Sandoval-Pauker, C.; Aldas, M.; Ciobotă, V.; Luna, M.; Vargas Jentzsch, P.; Muñoz Bisesti, F. Synthesis of inulin hydrogels by electron beam irradiation: Physical, vibrational spectroscopic and thermal characterization and arsenic removal as a possible application. J. Polym. Res. 2020, 27, 184. [Google Scholar] [CrossRef]
- More, A.P.; Chapekar, S. Irradiation assisted synthesis of hydrogel: A Review. Polym. Bull. 2024, 81, 5839–5908. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Hegazy, E.A.; El-Nesr, E.M.; El-Wahab, M.A. Synthesis, characterization and properties of radiation-induced Starch/(EG-co-MAA) hydrogels. Arab. J. Chem. 2016, 9, S1627–S1635. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Chandra Dafader, N.; Hara, K.; Okabe, H.; Hidaka, Y.; Rahman, M.M.; Mizanur Rahman Khan, M.; Rahman, N. Synthesis of Potato Starch-Acrylic-Acid Hydrogels by Gamma Radiation and Their Application in Dye Adsorption. Int. J. Polym. Sci. 2016, 2016, 9867859. [Google Scholar] [CrossRef]
- Chen, F.; Miao, C.; Duan, Q.; Jiang, S.; Liu, H.; Ma, L.; Li, Z.; Bao, X.; Lan, B.; Chen, L.; et al. Developing slow release fertilizer through in-situ radiation-synthesis of urea-embedded starch-based hydrogels. Ind. Crops Prod. 2023, 191, 115971. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem. Cent. J. 2017, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Finkenstadt, V.L.; Willett, J.L. Reactive Extrusion of Starch-Polyacrylamide Graft Copolymers: Effects of Monomer/Starch Ratio and Moisture Content. Macromol. Chem. Phys. 2005, 206, 1648–1652. [Google Scholar] [CrossRef]
- Willett, J.L.; Finkenstadt, V.L. Initiator effects in reactive extrusion of starch-polyacrylamide graft copolymers. J. Appl. Polym. Sci. 2006, 99, 52–58. [Google Scholar] [CrossRef]
- Willett, J.L.; Finkenstadt, V.L. Reactive Extrusion of Starch–Polyacrylamide Graft Copolymers Using Various Starches. J. Polym. Environ. 2006, 14, 125–129. [Google Scholar] [CrossRef]
- Willett, J.L.; Finkenstadt, V.L. Comparison of Cationic and Unmodified Starches in Reactive Extrusion of Starch–Polyacrylamide Graft Copolymers. J. Appl. Polym. Sci. 2009, 17, 248–253. [Google Scholar] [CrossRef]
- Willett, J.L.; Finkenstadt, V.L. Starch-poly (acrylamide-co-2-acrylamido-2-methylpropanesulfonic acid) graft copolymers prepared by reactive extrusion. J. Appl. Polym. Sci. 2015, 132, 42405. [Google Scholar] [CrossRef]
- Li, T.-T.; Feng, L.-F.; Gu, X.-P.; Zhang, C.-L.; Wang, P.; Hu, G.-H. Intensification of Polymerization Processes by Reactive Extrusion. Ind. Eng. Chem. Res. 2021, 60, 2791–2806. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- McGauran, T.; Harris, M.; Dunne, N.; Smyth, B.M.; Cunningham, E. Development and optimisation of extruded bio-based polymers from poultry feathers. Eur. Polym. J. 2021, 158, 110678. [Google Scholar] [CrossRef]
- Moad, G. Chemical modification of starch by reactive extrusion. Prog. Polym. Sci. 2011, 36, 218–237. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Liu, H.; Chen, L. Starch Modification Using Reactive Extrusion. Starch Starke 2006, 58, 131–139. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; BeMiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Siyamak, S.; Laycock, B.; Luckman, P. Synthesis of starch graft-copolymers via reactive extrusion: Process development and structural analysis. Carbohydr. Polym. 2020, 227, 115066. [Google Scholar] [CrossRef]
- Zhuang, Y.; Saadatkhah, N.; Morgani, M.S.; Xu, T.; Martin, C.; Patience, G.S.; Ajji, A. Experimental methods in chemical engineering: Reactive extrusion. Can. J. Chem. Eng. 2023, 101, 59–77. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, F.; Duan, Q.; Bao, X.; Jiang, S.; Liu, H.; Chen, L.; Yu, L. Designing and application of reactive extrusion with twice initiations for graft copolymerization of acrylamide on starch. Eur. Polym. J. 2022, 165, 111008. [Google Scholar] [CrossRef]
- McKenna, G.B. Deformation and flow of matter: Interrogating the physics of materials using rheological methods. J. Rheol. 2012, 56, 113–158. [Google Scholar] [CrossRef]
- Serrero, A.; Trombotto, S.; Cassagnau, P.; Bayon, Y.; Gravagna, P.; Montanari, S.; David, L. Polysaccharide Gels Based on Chitosan and Modified Starch: Structural Characterization and Linear Viscoelastic Behavior. Biomacromolecules 2010, 11, 1534–1543. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic properties of poly (vinyl alcohol) hydrogels having permanent and transient cross-links studied by microrheology, classical rheometry, and dynamic light scattering. Macromolecules 2013, 46, 4174–4183. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterisation of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Wetton, R.E.; Marsh, R.D.L.; Van-de-Velde, J.G. Theory and application of dynamic mechanical thermal analysis. Thermochim. Acta 1991, 175, 1–11. [Google Scholar] [CrossRef]
- Sha, H.; Zhang, X.; Harrison, I.R. A dynamic mechanical thermal analysis (DMTA) study of polyethylenes. Thermochim. Acta 1991, 192, 233–242. [Google Scholar] [CrossRef]
- Badia, J.-D.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A. Dynamic Mechanical Thermal Analysis of Polymer Blends. In Characterization of Polymer Blends; Wiley: Hoboken, NJ, USA, 2014; pp. 365–392. [Google Scholar]
- Ramli, R.A. Slow release fertilizer hydrogels: A review. Polym. Chem. 2019, 10, 6073–6090. [Google Scholar] [CrossRef]
- Kalita, A.; Elayarajan, M.; Janaki, P.; Suganya, S.; Sankari, A.; Parameswari, E. Organo-monomers coated slow-release fertilizers: Current understanding and future prospects. Int. J. Biol. Macromol. 2024, 274, 133320. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhong, S.; Meng, Q.; Li, Y.; Wang, J.; Gao, Y.; Cui, X. Advances in controlled-release fertilizer encapsulated by organic-inorganic composite membranes. Particuology 2024, 84, 236–248. [Google Scholar] [CrossRef]
- Ahmed Khan, T.; Zakaria, M.E.T.; Kim, H.J.; Ghazali, S.; Jamari, S.S. Carbonaceous microsphere-based superabsorbent polymer as filler for coating of NPK fertilizer: Fabrication, properties, swelling, and nitrogen release characteristics. J. Appl. Polym. Sci. 2020, 137, 48396. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Zhuang, X.; Chen, X.; Jing, X. Controlled release of urea encapsulated by starch-g-poly(l-lactide). Carbohydr. Polym. 2008, 72, 342–348. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and polyvinyl alcohol encapsulated biodegradable nanocomposites for environment friendly slow release of urea fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Boetje, L.; Lan, X.; Silvianti, F.; van Dijken, J.; Polhuis, M.; Loos, K. A more efficient synthesis and properties of saturated and unsaturated starch esters. Carbohydr. Polym. 2022, 292, 119649. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Safari, M.; Motesharezadeh, B. Synthesis of urea slow-release fertilizer using a novel starch-g-poly (styrene-co-butylacrylate) nanocomposite latex and its impact on a model crop production in greenhouse. J. Cleaner Prod. 2021, 322, 129082. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Gao, C.; Wang, X.; Xu, X.; Bai, X.; Gao, N.; Feng, C.; Wei, Y.; Wu, L.; Liu, M. Synthesis of a starch derivative and its application in fertilizer for slow nutrient release and water-holding. RSC Adv. 2014, 4, 51208–51214. [Google Scholar] [CrossRef]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Bandyopadhyay, K.K.; Saha, M.; Chawla, G.; Saha, J.K.; et al. Preparation of novel biodegradable starch/poly(vinyl alcohol)/bentonite grafted polymeric films for fertilizer encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; González, J.; Piña, C.; Muñoz-Bonilla, A.; Fernandez-García, M. Environmentally Friendly Fertilizers Based on Starch Superabsorbents. Materials 2019, 12, 3493. [Google Scholar] [CrossRef]
- Perez, J.J.; Francois, N.J. Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohydr. Polym. 2016, 148, 134–142. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Aparicio-Saguilán, A.; Mata-Mata, J.L.; González-García, G.; Hernández-Mendoza, H.; Gutiérrez-Fuentes, A.; Báez-García, E. Chemical modification of banana starch by the in situ polymerization of ϵ-caprolactone in one step. Starch Starke 2017, 69, 1600197. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, Y.; Hu, C.; Wu, H. Superabsorbent fabric: In situ polymerisation of macroporous starch–sodium alginate–polyacrylate hydrogel on fibre surface. Cellulose 2023, 30, 7113–7128. [Google Scholar] [CrossRef]
- Salimi, M.; Motamedi, E.; Motesharezedeh, B.; Hosseini, H.M.; Alikhani, H.A. Starch-g-poly(acrylic acid-co-acrylamide) composites reinforced with natural char nanoparticles toward environmentally benign slow-release urea fertilizers. J. Environ. Chem. Eng. 2020, 8, 103765. [Google Scholar] [CrossRef]
- Bolt, R.R.; Leitch, J.A.; Jones, A.C.; Nicholson, W.I.; Browne, D.L. Continuous flow mechanochemistry: Reactive extrusion as an enabling technology in organic synthesis. Chem. Soc. Rev. 2022, 51, 4243–4260. [Google Scholar] [CrossRef]
- Dong, Q.; Xian, B.; Hong, L.; Xing, L.; Ling, C.; Long, Y.; Xiao, Z.; Pei, C. Preparation of cassava starch-based superabsorbent polymer using a twin-roll mixer as reactor. Chin. J. Polym. Sci. 2014, 32, 1348–1356. [Google Scholar] [CrossRef]
- Qiao, D.; Ma, H.; Yu, L.; Liu, H.; Zou, W.; Chen, L.; Chen, P. Synthesis and characteristics of graft copolymerization of starch-G-PAM using A twin-roll mixer as reactor for cornstarch with different amylose/amylopectin ratios. Int. Polym. Proc. 2014, 29, 252–259. [Google Scholar] [CrossRef]
- Qiao, D.; Tu, W.; Wang, Z.; Yu, L.; Zhang, B.; Bao, X.; Jiang, F.; Lin, Q. Influence of crosslinker amount on the microstructure and properties of starch-based superabsorbent polymers by one-step preparation at high starch concentration. Int. J. Biol. Macromol. 2019, 129, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Bhaladhare, S.; Das, D. Cellulose: A fascinating biopolymer for hydrogel synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef]
- Tan, X.; Lv, Q.; Dong, G.; Zhang, Z.; Chai, D.-f.; Zhao, M.; Zhang, W.; Li, J. Insight into the preparation and improved properties of cellulose citrate ester hydrogel slow-release fertilizer. Ind. Crops Prod. 2024, 222, 119517. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M. Progress in the preparation of stimulus-responsive cellulose hydrogels and their application in slow-release fertilizers. Polymers 2023, 15, 3643. [Google Scholar] [CrossRef]
- González, K.; Guaresti, O.; Palomares, T.; Alonso-Varona, A.; Eceiza, A.; Gabilondo, N. The role of cellulose nanocrystals in biocompatible starch-based clicked nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 143, 265–272. [Google Scholar] [CrossRef]
- Moharrami, P.; Motamedi, E. Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour. Technol. 2020, 313, 123661. [Google Scholar] [CrossRef]
- Bahadoran Baghbadorani, N.; Behzad, T.; Karimi Darvanjooghi, M.H.; Etesami, N. Modelling of water absorption kinetics and biocompatibility study of synthesized cellulose nanofiber-assisted starch-graft-poly(acrylic acid) hydrogel nanocomposites. Cellulose 2020, 27, 9927–9945. [Google Scholar] [CrossRef]
- Bora, A.; Sarmah, D.; Karak, N. Cellulosic wastepaper modified starch/itaconic acid/acrylic acid-based biodegradable hydrogel as a sustain release of NPK fertilizer vehicle for agricultural applications. Int. J. Biol. Macromol. 2023, 253, 126555. [Google Scholar] [CrossRef] [PubMed]
- Vudjung, C.; Saengsuwan, S. Synthesis and properties of biodegradable hydrogels based on cross-linked natural rubber and cassava starch. J. Elastomers Plast. 2017, 49, 574–594. [Google Scholar] [CrossRef]
- Vudjung, C.; Saengsuwan, S. Biodegradable IPN hydrogels based on pre-vulcanized natural rubber and cassava starch as coating membrane for environment-friendly slow-release urea fertilizer. J. Polym. Environ. 2018, 26, 3967–3980. [Google Scholar] [CrossRef]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency enhancement of slow release of fertilizer using nanozeolite–chitosan/sago starch-based biopolymer composite. J. Coat. Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Binti Mansor, N.; Man, Z. Lignin loading effect on biodegradability and nitrogen release properties of urea modified tapioca starch in wet soil. Key Eng. Mater. 2014, 594, 798–802. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Cai, Y.; Jiang, P.; Yu, B. Co-incorporation of hydrotalcite and starch into biochar-based fertilizers for the synthesis of slow-release fertilizers with improved water retention. Biochar 2023, 5, 44. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; He, J.; Yang, Y.; Yu, X.; Yue, G. Preparation and properties of a degradable interpenetrating polymer networks based on starch with water retention, amelioration of soil, and slow release of nitrogen and phosphorus fertilizer. J. Appl. Polym. Sci. 2013, 128, 407–415. [Google Scholar] [CrossRef]
- León, O.; Soto, D.; Muñoz-Bonilla, A.; Fernández-García, M. Amylose Modified Starches as Superabsorbent Systems for Release of Potassium Fertilizers. J. Polym. Environ. 2022, 30, 2314–2328. [Google Scholar] [CrossRef]
- Dudu, T.E.; Alpaslan, D.; Aktas, N. Synthesis of controlled release hydrogels from dimethylacrylamide/maleic acid/starch and its application in lettuce cultivation. J. Polym. Res. 2022, 29, 524. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Chang, F.; Zhu, H.; Tian, C.; Jia, F.; Ke, Y.; Dai, J. Slow-Release Urea Fertilizer with Water Retention and Photosensitivity Properties Based on Sodium Alginate/Carboxymethyl Starch Sodium/Polydopamine. Processes 2024, 12, 842. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, H.; Zhang, Q.; Liu, Z.; Zhang, M.; Wang, J. Preparation of urea-containing starch-castor oil superabsorbent polyurethane coated urea and investigation of controlled nitrogen release. Carbohydr. Polym. 2021, 253, 117240. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Xiao, T.; Dong, G.; Tan, X.; Zhang, Z.; Zhao, M.; Zhu, M.; Li, J.; Zhang, W. Preparation and characterization of starch carbamate modified natural sodium alginate composite hydrogel blend formulation and its application for slow-release fertilizer. Int. J. Biol. Macromol. 2024, 278, 134713. [Google Scholar] [CrossRef]
- Mu, Z.; Zhang, W.; Chai, D.-f.; Lv, Q.; Tan, X.; Yuan, R.; Dong, G. Preparation and characterization of slow-release urea fertilizer encapsulated by a blend of starch derivative and polyvinyl alcohol with desirable biodegradability and availability. Int. J. Biol. Macromol. 2024, 271, 132693. [Google Scholar] [CrossRef]
- Ee Huey, C.; Zaireen Nisa Yahya, W.; Mansor, N. Allicin incorporation as urease inhibitor in a chitosan/starch based biopolymer for fertilizer application. Mater. Today Proc. 2019, 16, 2187–2196. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Priya, E.; Sarkar, S.; Maji, P.K. A review on slow-release fertilizer: Nutrient release mechanism and agricultural sustainability. J. Environ. Chem. Eng. 2024, 12, 113211. [Google Scholar] [CrossRef]
- Firmanda, A.; Fahma, F.; Syamsu, K.; Mahardika, M.; Suryanegara, L.; Munif, A.; Gozan, M.; Wood, K.; Hidayat, R.; Yulia, D. Biopolymer-based slow/controlled-release fertilizer (SRF/CRF): Nutrient release mechanism and agricultural sustainability. J. Environ. Chem. Eng. 2024, 12, 112177. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Yang, X.; Liu, H. Temperature-responsive hydrogel prepared from carboxymethyl cellulose-stabilized N-vinylcaprolactam with potential for fertilizer delivery. Carbohydr. Polym. 2023, 313, 120875. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and response of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/graphene oxide hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Salave, S.; Vitore, J.; Bachra, Y.; Jadhav, R.; Kommineni, N.; Karouach, F.; Paiva-Santos, A.C.; Varma, R.S.; Berrada, M. Properties and valuable applications of superabsorbent polymers: A comprehensive review. Polym. Bull. 2024, 81, 6671–6701. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Liu, S.; Chen, J.; Chen, R.; He, X.; Ma, G.; Lei, Z. Factors affecting the properties of superabsorbent polymer hydrogels and methods to improve their performance: A review. J. Mater. Sci. 2021, 56, 16223–16242. [Google Scholar] [CrossRef]
- de Lima, I.S.; Sousa, H.R.; Silva, A.S.; de Oliveira, L.H.; Muniz, E.C.; Osajima, J.A.; Silva-Filho, E.C. Superabsorbent hydrogel from cassava gum (Manihot esculenta) to release water and macronutrients. Ind. Crops Prod. 2024, 219, 119045. [Google Scholar] [CrossRef]
- Al Rohily, K.; El-Hamshary, H.; Ghoneim, A.; Modaihsh, A. Controlled Release of Phosphorus from Superabsorbent Phosphate-Bound Alginate-Graft-Polyacrylamide: Resistance to Soil Cations and Release Mechanism. ACS Omega 2020, 5, 32919–32929. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Alhaj Hamoud, Y.; Xu, X.; Wang, S.; Liu, H. A pH-responsive/sustained release nitrogen fertilizer hydrogel based on aminated cellulose nanofiber/cationic copolymer for application in irrigated neutral soils. J. Cleaner Prod. 2022, 368, 133098. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. [Google Scholar] [CrossRef]
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071. [Google Scholar] [CrossRef]
- Vo, P.T.; Nguyen, H.T.; Trinh, H.T.; Nguyen, V.M.; Le, A.-T.; Tran, H.Q.; Nguyen, T.T.T. The nitrogen slow-release fertilizer based on urea incorporating chitosan and poly(vinyl alcohol) blend. Environ. Technol. Innov. 2021, 22, 101528. [Google Scholar] [CrossRef]
- Pogorzelski, D.; Filho, J.F.L.; Matias, P.C.; Santos, W.O.; Vergütz, L.; Melo, L.C.A. Biochar as composite of phosphate fertilizer: Characterization and agronomic effectiveness. Sci. Total Environ. 2020, 743, 140604. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Duan, Q.; Ma, L.; Song, Y.; Xie, H.; Liu, H.; Chen, L.; Yu, L. Preparation and characterization of slow-release fertilizer through coating acrylate epoxidized soybean oil. Environ. Technol. Innov. 2024, 34, 103626. [Google Scholar] [CrossRef]
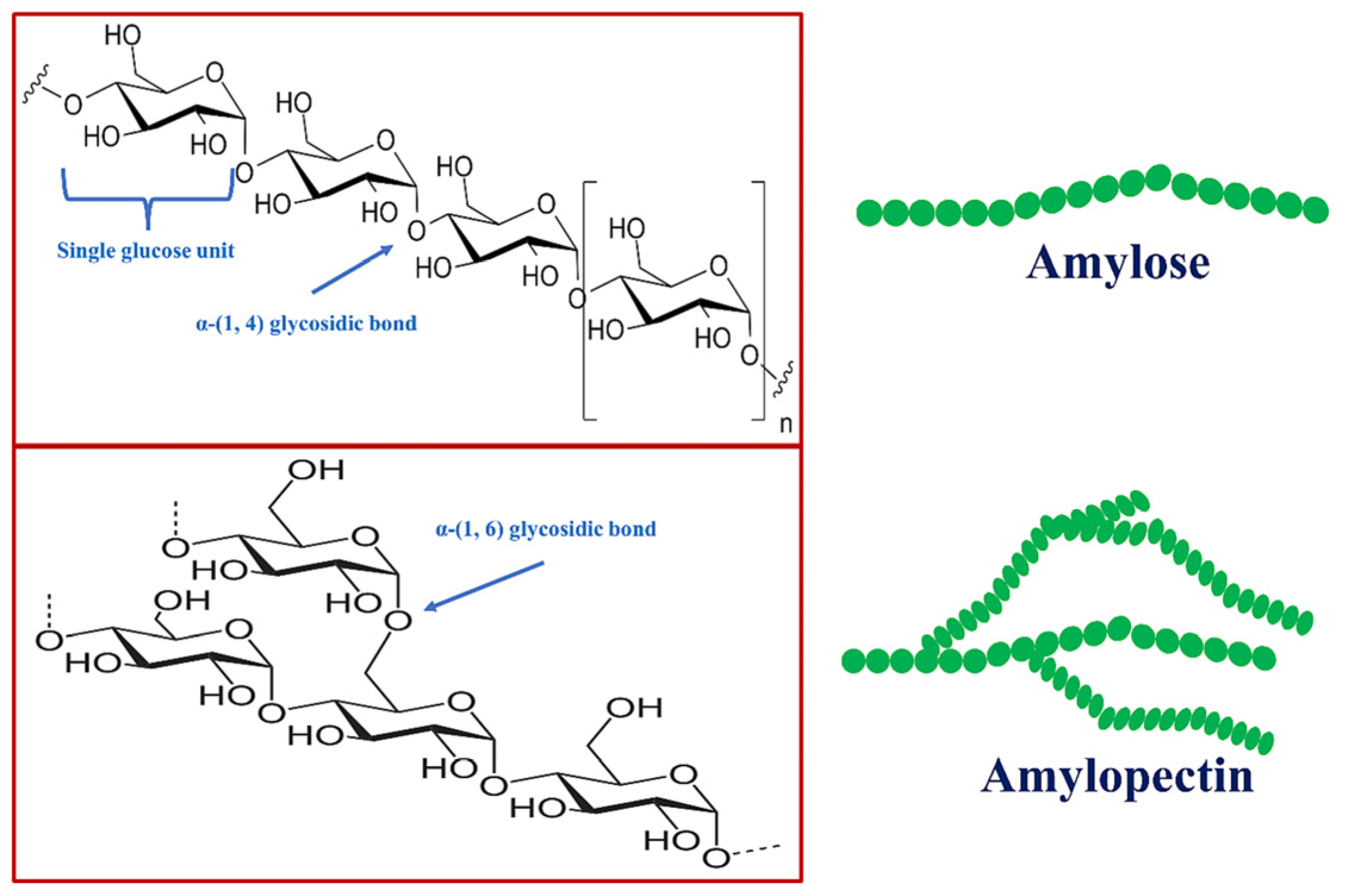
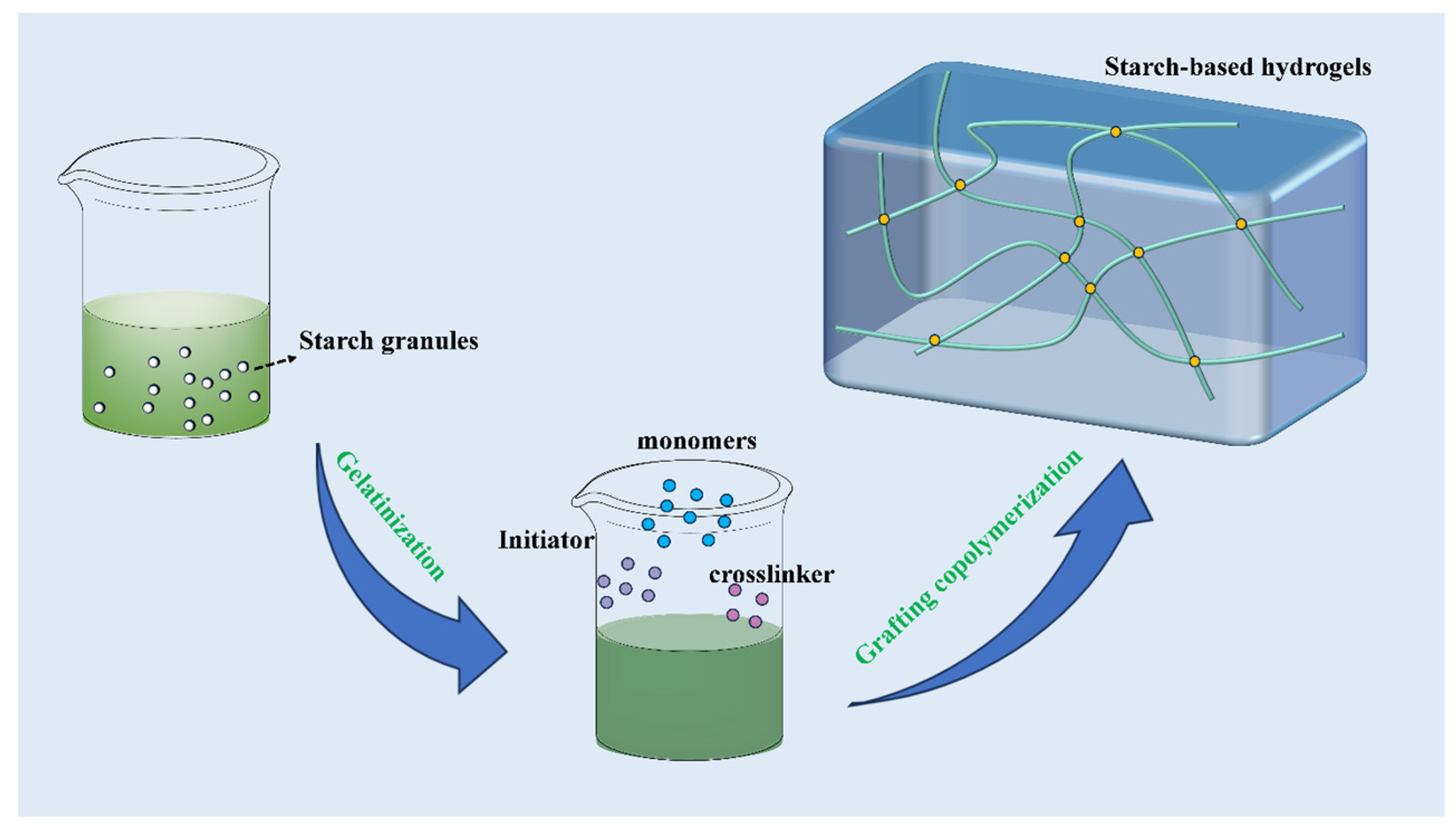

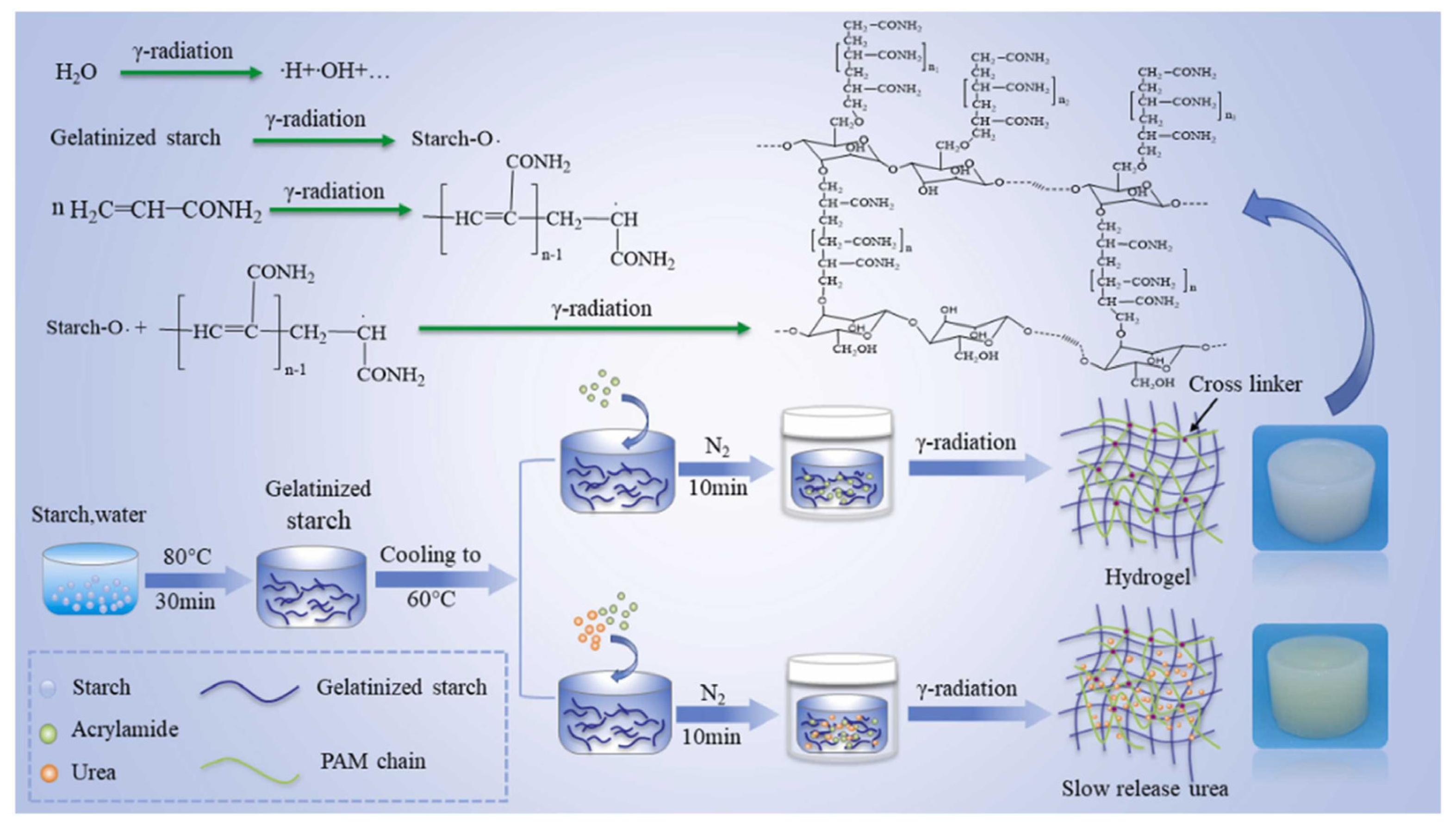
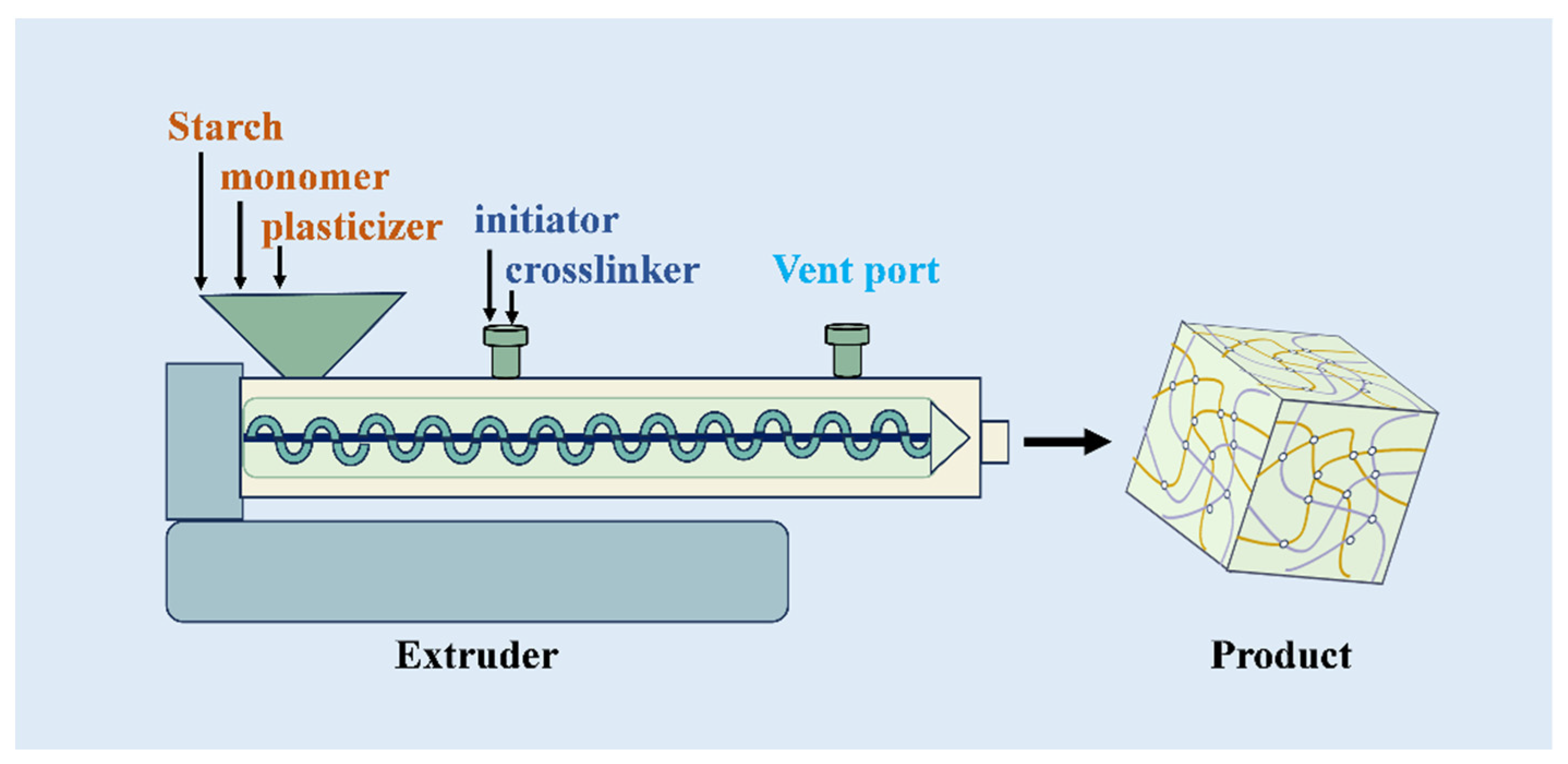


| Starch/Additives | Fertilizer | Modification | Performance | References |
|---|---|---|---|---|
| Cassava starch–PAA/natural rubber/PVA | Urea | CSt-g-PAA networks and linear NR/PVA blends | In water: hydrogel wax coated urea (BHWCU) <60% within 24 h, in 7 days, 89.1%, 68.6%, and 41.5% in BHWCU/2:8, BHWCU/6:4, and BHWCU/9:1 formulations. In soil: hydrogel wax coated urea <68% in 20 days | [141] |
| Cassava starch-g-poly(acrylonitrile) | Urea | Crosslinking of cassava starch and acrylonitrile using MBA applied as a coating | Uncoated urea released 100% after 28 days in soil, coated urea 70% in 108 days | [15] |
| Corn starch–Poly (AA-co-AM) | Diammonium phosphate | Crosslinking of starch, AA, and AM using MBA | 60% N release during 30 days in soil | [142] |
| High-amylose maize starch-g-poly(sodium acid maleate-disodium maleate) | Potassium | High-amylose maize starch graft copolymerization with disodium maleate, using KMnO4-NaHSO3 redox system, and covalently crosslinked with MBA | The release percentages of KNO3 and KH2PO4 were between 92 and 67% and 89 and 61% in 320 h | [143] |
| Poly (N, N-dimethylacrylamide –maleic acid)–starch | Urea | Crosslinking of starch, N, N-dimethylacrylamide, and maleic acid using MBA | Urea released from hydrogels DMSt1, DMSt2, and DMSt3 after 14,000 min was found as 80.2%, 45.5%, and 67.7% in well water and 81.5%, 46.6%, and 68.9% in tap water | [144] |
| Sodium alginate–carboxymethyl starch sodium–polydopamine | Urea | Sodium alginate and carboxymethyl starch sodium were compounded, and polydopamine (PDA) film was formed on its surface by self-polymerization | In soil: >25 days | [145] |
| Corn starch–castor oil superabsorbent–polyurethane | Urea | Starch–castor oil SAP–polyurethane-coated urea in smooth rotating drum | Nitrogen-controlled release period of 60–150 days in water | [146] |
| Starch carbamate–sodium alginate–SRF | Urea | Starch carbamate and sodium alginate through cationic ion crosslinking | In water: 61.6% within 10 h and almost completely release >16 h. In soil: 58.5% of urea released within 25 days and exceeded 50 days for complete release | [147] |
| Starch phosphate carbamate–PVA–stearic acid | Urea | Stearic acid as inner coating layer and starch phosphate carbamate–PVA film crosslinked with citric acid as external layer | In water: 50.3% within 15 h, nearly complete release over 25 h. In soil: 46.6% was released within 20 d, extending to approximately 30 d | [148] |
| Chitosan–starch | Urea | Crosslinking of starch and chitosan using glutaraldehyde | 63.71% N release during seven days in soil | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Ma, L.; Duan, Q.; Xie, H.; Dong, X.; Zhang, H.; Yu, L. Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels. Molecules 2024, 29, 4835. https://doi.org/10.3390/molecules29204835
Song Y, Ma L, Duan Q, Xie H, Dong X, Zhang H, Yu L. Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels. Molecules. 2024; 29(20):4835. https://doi.org/10.3390/molecules29204835
Chicago/Turabian StyleSong, Yue, Litao Ma, Qingfei Duan, Huifang Xie, Xinyi Dong, Huaran Zhang, and Long Yu. 2024. "Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels" Molecules 29, no. 20: 4835. https://doi.org/10.3390/molecules29204835
APA StyleSong, Y., Ma, L., Duan, Q., Xie, H., Dong, X., Zhang, H., & Yu, L. (2024). Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels. Molecules, 29(20), 4835. https://doi.org/10.3390/molecules29204835






