Upcycling Waste Polyethylene Terephthalate to Produce Nitrogen-Doped Porous Carbon for Enhanced Capacitive Deionization
Abstract
1. Introduction
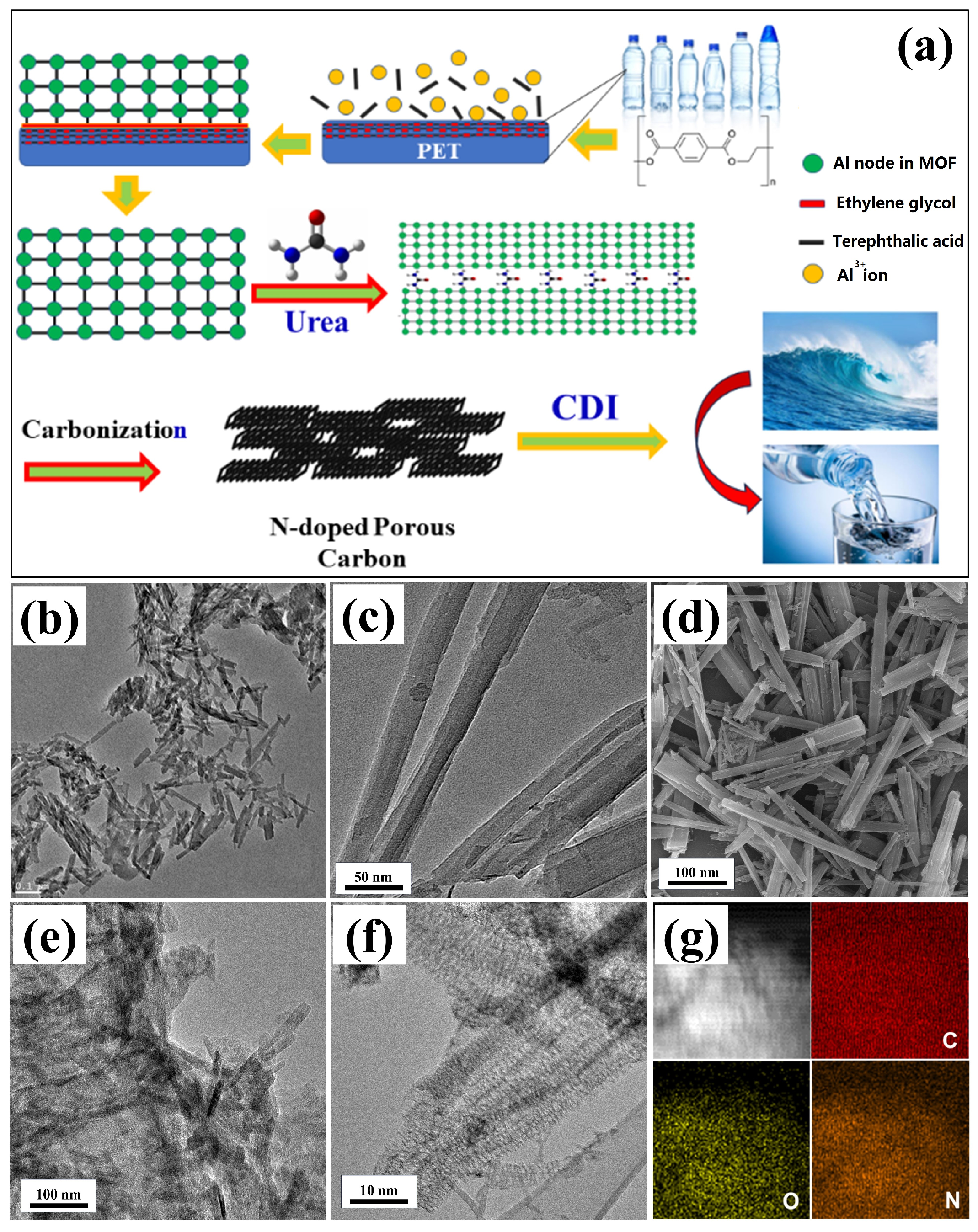
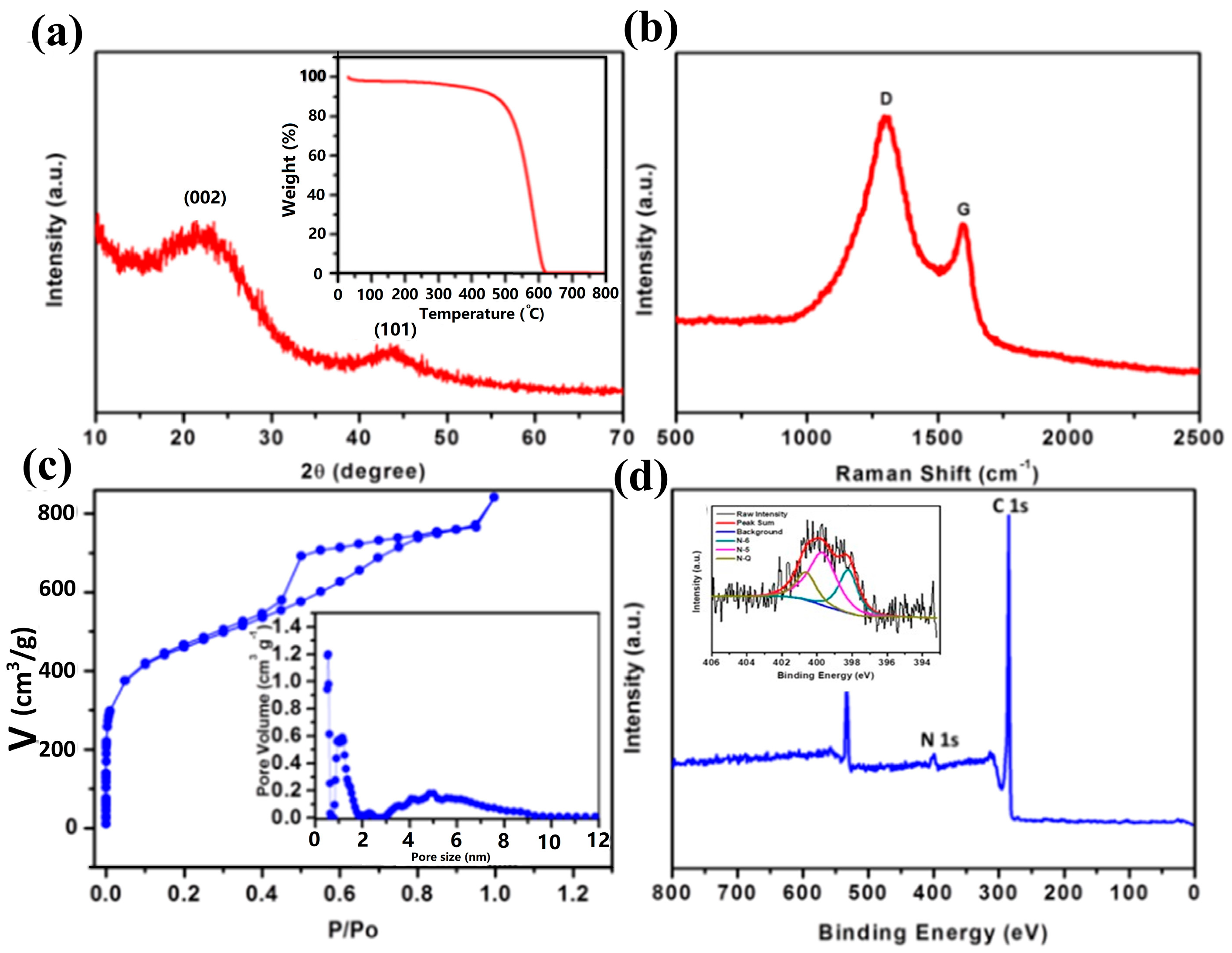
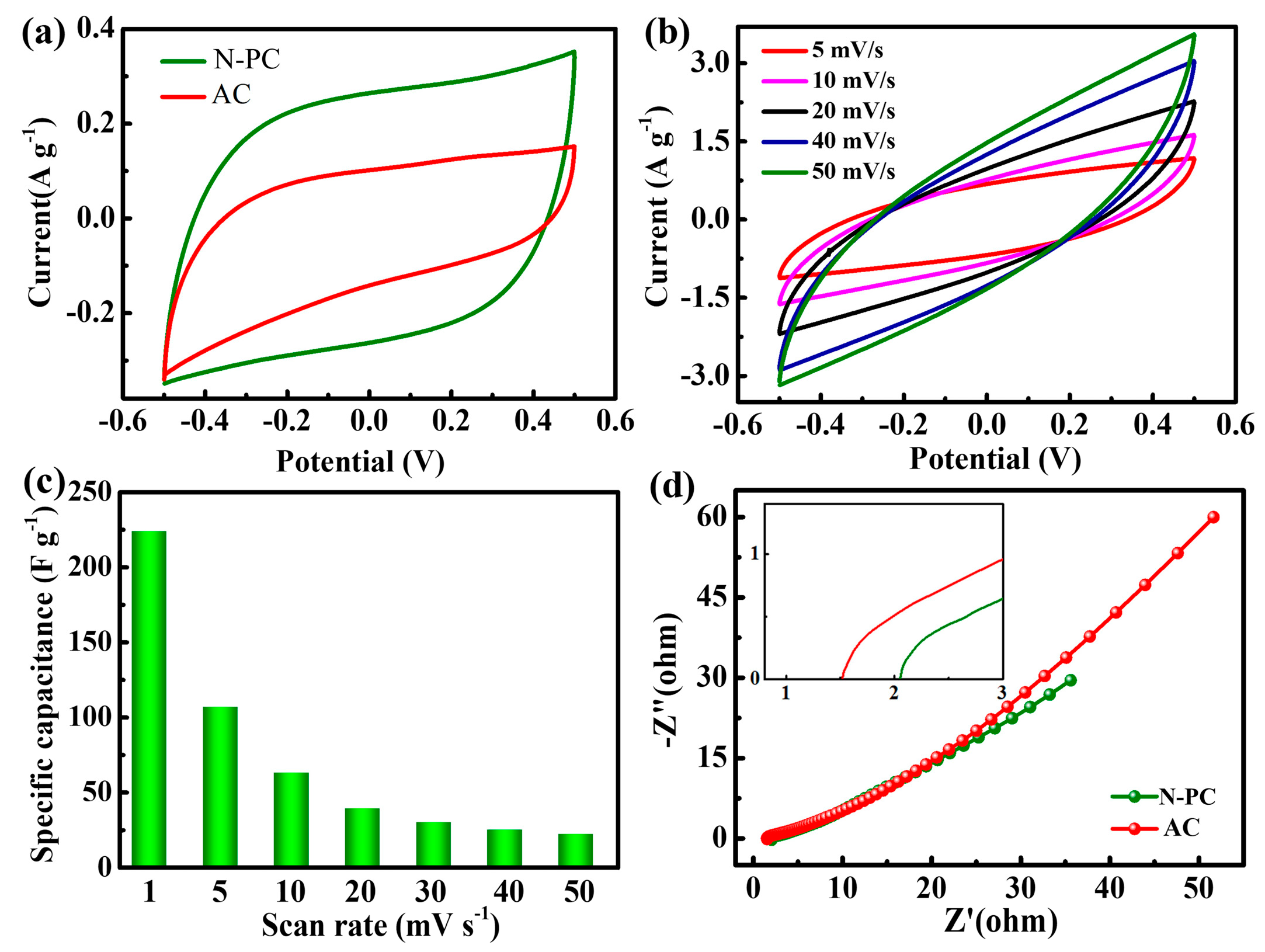
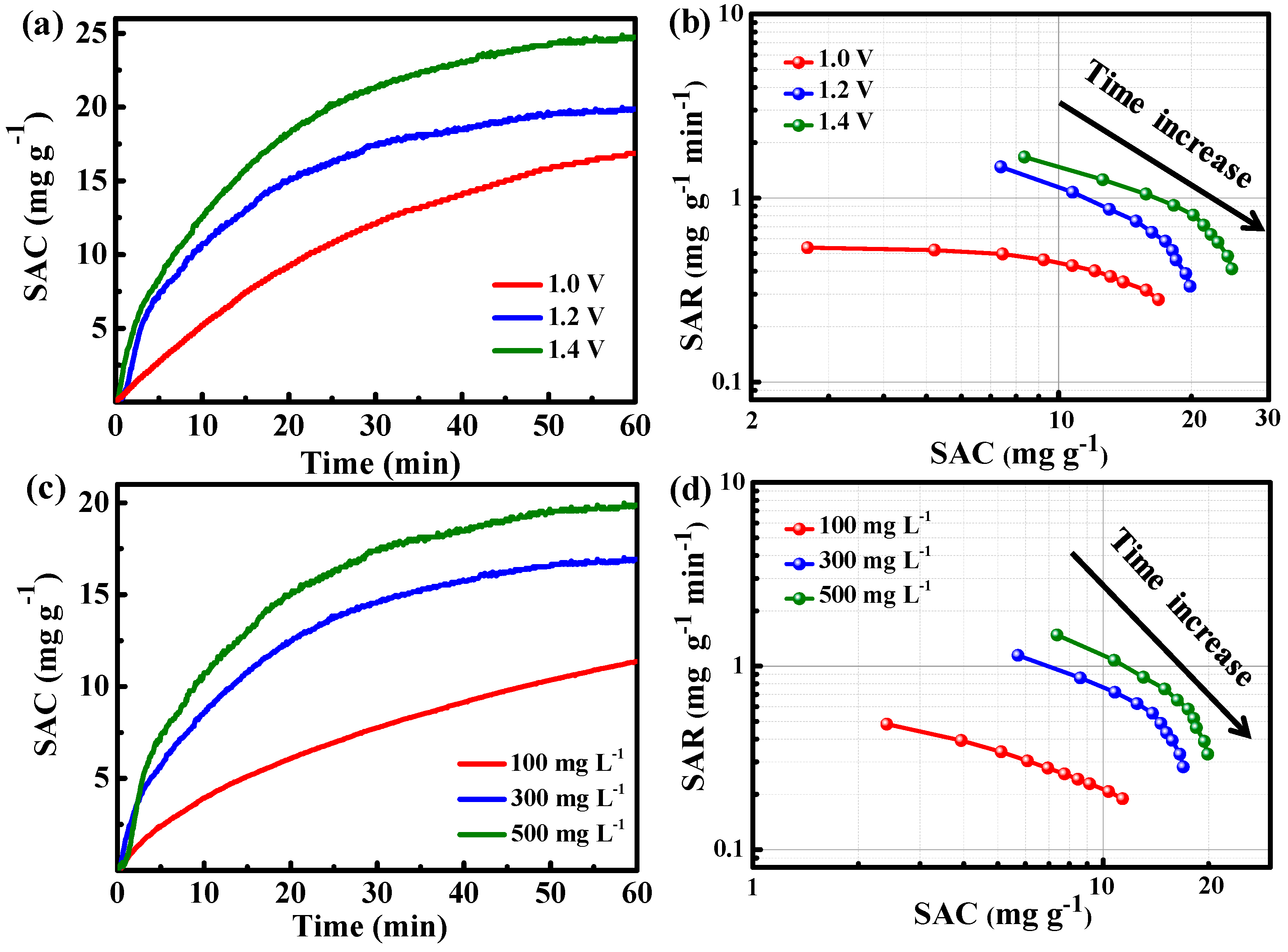
2. Results
| Entry | Component | NaCl Solution (mg/L) | Applied Voltage (V) | SAC (mg/g) | Ref. |
|---|---|---|---|---|---|
| 1 | NHCF-800 | 500 | 1.2 | 30.1 | [45] |
| 2 | N,B-NPC | 500 | 1.2 | 21.5 | [46] |
| 3 | A-HCMs | 400 | 1.0 | 14.64 | [47] |
| 4 | 3D-NPC | 500 | 1.34 | 19.4 | [48] |
| 5 | NPC | 500 | 1.2 | 19.5 | [49] |
| 6 | N-porous carbon | 500 | 1.2 | 14.63 | [50] |
| 7 | N-porous carbon | 500 | 1.2 | 12.56 | [51] |
| 8 | N-ALS | 500 | 1.2 | 24.79 | [52] |
| 9 | C(ZIF-8) | 58.44 | 1.2 | 8.52 | [53] |
| 10 | F-N-GPM | 100 | 1.8 | 21.8 | [54] |
| 11 | N-PC | 500 | 1.2/1.4 | 19.9/24.7 | This work |
3. Materials and Methods
3.1. Preparation of N-PC Materials
3.2. Characterization
3.3. CDI Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Semiat, R. Energy Issues in Desalination Processes. Environ. Sci. Technol. 2008, 42, 8193–8201. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Huang, Z. Carbon materials for water desalination by capacitive deionization. New Carbon Mater. 2023, 38, 405–431. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Yang, K.L.; Ying, T.Y.; Yiacoumi, S.; Tsouris, C.; Vittoratos, E.S. Electrosorption of Ions from Aqueous Solutions by Carbon Aerogel: An Electrical Double-Layer Model. Langmuir 2001, 17, 1961–1969. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Pan, L.; Sun, Z. Novel Graphene-Like Electrodes for Capacitive Deionization. Environ. Sci. Technol. 2010, 44, 8692–8697. [Google Scholar] [CrossRef]
- Zheng, S.M.; Yuan, Z.H.; Dionysios, D.D.; Zhong, L.B.; Zhao, F.; Yang, J.C.E.; Zheng, Y.M. Silkworm cocoon waste-derived nitrogen-doped hierarchical porous carbon as robust electrode materials for efficient capacitive desalination. Chem. Eng. J. 2023, 458, 141471. [Google Scholar] [CrossRef]
- Tsouris, C.; Mayes, R.; Kiggans, J.; Sharma, K.; Yiacoumi, S.; DePaoli, D.; Dai, S. Mesoporous Carbon for Capacitive Deionization of Saline Water. Environ. Sci. Technol. 2011, 45, 10243–10249. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef]
- Han, L.; Karthikeyan, K.G.; Anderson, M.A.; Gregory, K.B. Exploring the impact of pore size distribution on the performance of carbon electrodes for capacitive deionization. J. Colloid Interf. Sci. 2014, 430, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Wang, M.; Yang, X.; Zhu, C.; Lu, T.; Zhao, R.; Pan, L. Design and fabrication of mesoporous graphene via carbothermal reaction for highly efficient capacitive deionization. Electrochim. Acta 2016, 188, 406–413. [Google Scholar] [CrossRef]
- Shi, W.; Li, H.; Cao, X.; Leong, Z.Y.; Zhang, J.; Chen, T.; Zhang, H.; Yang, H.Y. Ultrahigh Performance of Novel Capacitive Deionization Electrodes based on A Three-Dimensional Graphene Architecture with Nanopores. Sci. Rep. 2016, 6, 18966–18975. [Google Scholar] [CrossRef]
- Xu, X.; Tang, H.; Wang, M.; Liu, Y.; Li, Y.; Lu, T.; Pan, L. Carbon spheres with hierarchical micro/mesopores for water desalination by capacitive deionization. J. Mater. Chem. A 2016, 4, 16094–16100. [Google Scholar] [CrossRef]
- Xu, X.; Pan, L.; Liu, Y.; Lu, T.; Sun, Z. Enhanced capacitive deionization performance of graphene by nitrogen doping. J. Colloid Interf. Sci. 2015, 445, 143–150. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.; Wang, J.; Liu, N.L.; Bando, Y.; Alshehri, A.A.; Yamauchi, Y.; Hou, C.H.; Wu, K.C.W. High performance capacitive deionization using modified ZIF-8-derived, N-doped porous carbon with improved conductivity. Nanoscale 2018, 10, 14852–14859. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, T.; Wang, H.; Chen, G.; Huang, L.; Zhang, J.; Shi, L.; Zhang, D. High capacity and high rate capability of nitrogen-doped porous hollow carbon spheres for capacitive deionization. Appl. Surf. Sci. 2016, 369, 460–469. [Google Scholar] [CrossRef]
- Yan, T.; Liu, J.; Lei, H.; Shi, L.; An, Z.; Park, H.S.; Zhang, D. Capacitive deionization of saline water using sandwich-like nitrogen-doped graphene composites via a self-assembling strategy. Environ. Sci. Nano 2018, 5, 2722–2730. [Google Scholar] [CrossRef]
- Han, J.; Shi, L.; Yan, T.; Zhang, J.; Zhang, D. Removal of ions from saline water using N, P co-doped 3D hierarchical carbon architectures via capacitive deionization. Environ. Sci. Nano 2018, 5, 2337–2345. [Google Scholar] [CrossRef]
- Xu, X.; Allah, A.E.; Wang, C.; Tan, H.; Farghali, A.A.; Khedr, M.H.; Malgras, V.; Yang, T.; Yamauchi, Y. Capacitive deionization using nitrogen-doped mesostructured carbons for highly efficient brackish water desalination. Chem. Eng. J. 2019, 362, 887–896. [Google Scholar] [CrossRef]
- Chang, L.; Li, J.; Duan, X.; Liu, W. Porous carbon derived from Metal-organic framework (MOF) for capacitive deionization electrode. Electrochim. Acta 2015, 176, 956–964. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Liu, Y.; Li, Y.; Lu, T.; Pan, L. From metal-organic frameworks to porous carbons: A promising strategy to prepare high-performance electrode materials for capacitive deionization. Carbon 2016, 108, 433–439. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Fang, J.; Shi, L.; Zhang, D. Nitrogen-doped porous carbon derived from a bimetallic metal-organic framework as highly efficient electrodes for flow-through deionization capacitors. J. Mater. Chem. A 2016, 4, 10858–10868. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, T.; Shi, L.; Zhang, D. In Situ Expanding Pores of Dodecahedron-like Carbon Frameworks Derived from MOFs for Enhanced Capacitive Deionization. ACS Appl. Mater. Interfaces 2017, 9, 15068–15078. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, J.; Han, J.; Yan, T.; Shi, L.; Zhang, D. N, P, S co-doped hollow carbon polyhedra derived from MOF-based core–shell nanocomposites for capacitive deionization. J. Mater. Chem. A 2018, 6, 15245–15252. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Hua, Y.; Lin, Y.; Wen, X.; Mijowska, E.; Tang, T.; Chen, X.; Ruoff, R.S. Facile synthesis of accordion-like porous carbon from waste PET bottles-based MIL-53(Al) and its application for high-performance Zn-ion capacitor. Green Energy Environ. 2024, 9, 1138–1150. [Google Scholar] [CrossRef]
- Liu, P.; Wang, H.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J. Mater. Chem. A 2016, 4, 5303–5313. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.; Zeng, W.; Chen, G.; Wu, S.; Zhao, Y.; Ni, K.; Tao, Z.; Ikram, M.; Ji, H.; et al. A Hierarchical Carbon Derived from Sponge-Templated Activation of Graphene Oxide for High-Performance Supercapacitor Electrodes. Adv. Mater. 2016, 28, 5222. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.; Jiang, Y.; Xie, K.; Wang, X.; Wu, Q.; Yang, L.; Jin, Z.; Ma, Y.; et al. Hydrophilic Hierarchical Nitrogen-Doped Carbon Nanocages for Ultrahigh Supercapacitive Performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, D.; Yan, T.; Wen, X.; Zhang, J.; Shi, L.; Zhong, Q. Three dimensional macroporous graphene architectures as high performance electrodes for capacitive deionization. J. Mater. Chem. A 2013, 1, 11778–11789. [Google Scholar] [CrossRef]
- Singh, D.K.; Krishna, K.S.; Harish, S.; Sampath, S.; Eswaramoorthy, M. No More HF: Teflon-Assisted Ultrafast Removal of Silica to Generate High-Surface-Area Mesostructured Carbon for Enhanced CO2 Capture and Supercapacitor Performance. Angew. Chem. Int. Ed. 2016, 55, 2032. [Google Scholar] [CrossRef] [PubMed]
- HongSun, T.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599. [Google Scholar]
- Zhao, J.; Jiang, Y.; Fan, H.; Liu, M.; Zhuo, O.; Wang, X.; Wu, Q.; Yang, L.; Ma, Y.; Hu, Z. Porous 3D Few-Layer Graphene-like Carbon for Ultrahigh-Power Supercapacitors with Well-Defined Structure-Performance Relationship. Adv. Mater. 2017, 29, 1604569–1604575. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Han, C.; Deng, J.; Wang, J.; Li, M.; Tang, M.; Jin, H.; Wang, Y. Asymmetric Flasklike Hollow Carbonaceous Nanoparticles Fabricated by the Synergistic Interaction between Soft Template and Biomass. J. Am. Chem. Soc. 2017, 139, 2657–2663. [Google Scholar] [CrossRef]
- Duan, H.; Yan, T.; Chen, G.; Zhang, J.; Shi, L.; Zhang, D. A facile strategy for the fast construction of porous graphene frameworks and their enhanced electrosorption performance. Chem. Commun. 2017, 53, 7465–7468. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Zhao, R.; Satpradit, O.; Rijnaarts, H.H.M.; Biesheuvel, P.M.; Van der Wal, A. Optimization of salt adsorption rate in membrane capacitive deionization. Water Res. 2013, 47, 1941–1952. [Google Scholar] [CrossRef]
- Duranoglu, D.; Al-Aghbari, M. Desalination Performance of a Unique Capacitive Deionization Cell Optimized with ANSYS Flow Simulation. Korean J. Chem. Eng. 2024, 41, 1151. [Google Scholar] [CrossRef]
- Długołęcki, P.; Wal, A.V.D. Energy Recovery in Membrane Capacitive Deionization. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Wang, M.; Zhu, C.; Lu, T.; Zhao, R.; Pan, L. Hierarchical hybrids with microporous carbon spheres decorated three-dimensional graphene frameworks for capacitive applications in supercapacitor and deionization. Electrochim. Acta 2016, 193, 88–95. [Google Scholar] [CrossRef]
- Wu, H.; Sui, X.; Lei, Y.; Liu, L.; Xu, W.; Liang, G.; Li, C.; Li, X. Compositing of Co3O4 with boron nitride to promote the catalytic performance for methane oxidation. Fuel 2024, 369, 131786. [Google Scholar] [CrossRef]
- Sun, S.; Li, G.; Zhu, S.; Meng, W.; Xu, L.; Jiang, J.; Wang, F.; Li, X. A judicious injection and abstraction of a hetero-dopant in a Co3O4 catalyst for efficient methane oxidation. J. Mater. Chem. A 2024, 12, 17463–17470. [Google Scholar] [CrossRef]
- Gong, C.; Chen, Z.; Geng, W.; Fu, Z.; Chen, C.; Zhang, Y.; Wang, G. Controlled fabrication of nitrogen-doped porous carbon foam with refined hierarchical architectures for desalination via capacitive deionization. J. Colloid Interface Sci. 2023, 643, 516–527. [Google Scholar] [CrossRef]
- Kong, W.; Ge, X.; Zhang, Q.; Wang, Y.; Wang, Y.; Lu, J.; Zhang, M.; Kong, D.; Feng, Y. Ultrahigh content Boron and nitrogen codoped hierarchically porous carbon obtained from biomass byproduct okara for capacitive deionization. ACS Omega 2022, 7, 48282–48290. [Google Scholar] [CrossRef]
- Wang, P.; Ma, W.; Xue, S.; Wang, L.; Chen, Y.; Wang, Y. N-doped carbon nanosheets assembled microspheres for more effective capacitive deionization. Sep. Purif. Technol. 2021, 276, 119336. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, X.; Zhao, C.; Wang, H.; Lu, H.; Kang, S.; Zhou, H.; Zhang, H.; Zhao, H.; Wang, G. Three-Dimensiona lN-doped Porous Carbon Derived from Monosodium Glutamate for Capacitive Deionization and the Oxygen Reduction Reaction. ChemElectroChem 2018, 5, 3873–3880. [Google Scholar] [CrossRef]
- Konga, W.; Ge, X.; Zhang, M.; Mi, M.; Kong, D.; Feng, Y. Nitrogen-doped hierarchical porous carbon obtained from silk cocoon for capacitive deionization. Diam. Relat. Mater. 2022, 129, 109388. [Google Scholar] [CrossRef]
- Men, L.; Chen, C.; Liu, A.; Yu, S.; Zhou, L.; Xie, Y.; Ju, D. N-doped porous carbon-based capacitive deionization electrode materials loaded with activated carbon fiber for water desalination applications. J. Environ. Chem. Eng. 2022, 10, 107943. [Google Scholar] [CrossRef]
- Liu, R.; Yao, S.; Shen, Y.; Tian, Y.; Zhang, Q. Preparation of N-Doped Layered Porous Carbon and Its Capacitive Deionization Performance. Materials 2023, 16, 1435. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, A.; Fei, C.; Hui, B.; Li, Y.; Guan, D.; Ju, D. High-performance nitrogen-doped porous carbon electrode materials for capacitive deionization of Industrial salt-contaminated wastewater. Desalination 2023, 565, 116863. [Google Scholar] [CrossRef]
- Liu, N.; Dutta, S.; Salunkhe, R.; Ahamad, T.; Alshehri, S.; Yamauchi, Y.; Hou, C.; Wu, K. ZIF-8 Derived, Nitrogen-Doped Porous Electrodes of Carbon Polyhedron Particles for High-Performance Electrosorption of Salt Ions. Sci. Rep. 2016, 6, 28847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, W.; Chen, Z.; Long, J.; Xu, C. Freestanding N-doped graphene membrane electrode with interconnected porous architecture for efficient capacitive deionization. Carbon 2022, 187, 86–96. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Duan, H.; Chen, L.; Zhu, W.; Baranowska, D.; Hua, Y.; Zhang, D.; Chen, X. Upcycling Waste Polyethylene Terephthalate to Produce Nitrogen-Doped Porous Carbon for Enhanced Capacitive Deionization. Molecules 2024, 29, 4934. https://doi.org/10.3390/molecules29204934
Yu H, Duan H, Chen L, Zhu W, Baranowska D, Hua Y, Zhang D, Chen X. Upcycling Waste Polyethylene Terephthalate to Produce Nitrogen-Doped Porous Carbon for Enhanced Capacitive Deionization. Molecules. 2024; 29(20):4934. https://doi.org/10.3390/molecules29204934
Chicago/Turabian StyleYu, Hui, Haiyan Duan, Liang Chen, Weihua Zhu, Daria Baranowska, Yumeng Hua, Dengsong Zhang, and Xuecheng Chen. 2024. "Upcycling Waste Polyethylene Terephthalate to Produce Nitrogen-Doped Porous Carbon for Enhanced Capacitive Deionization" Molecules 29, no. 20: 4934. https://doi.org/10.3390/molecules29204934
APA StyleYu, H., Duan, H., Chen, L., Zhu, W., Baranowska, D., Hua, Y., Zhang, D., & Chen, X. (2024). Upcycling Waste Polyethylene Terephthalate to Produce Nitrogen-Doped Porous Carbon for Enhanced Capacitive Deionization. Molecules, 29(20), 4934. https://doi.org/10.3390/molecules29204934








