Synthesis and Biological Activity of Chromeno[3,2-c]Pyridines
Abstract
1. Introduction
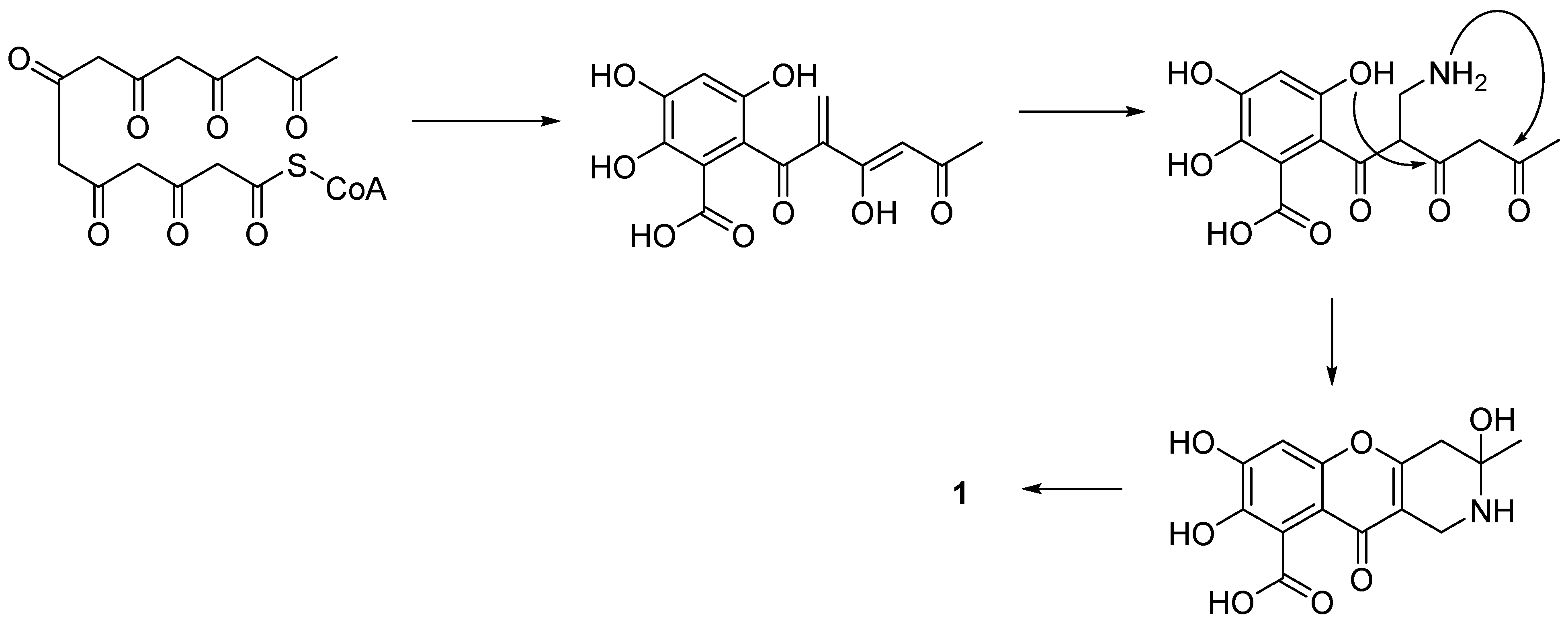
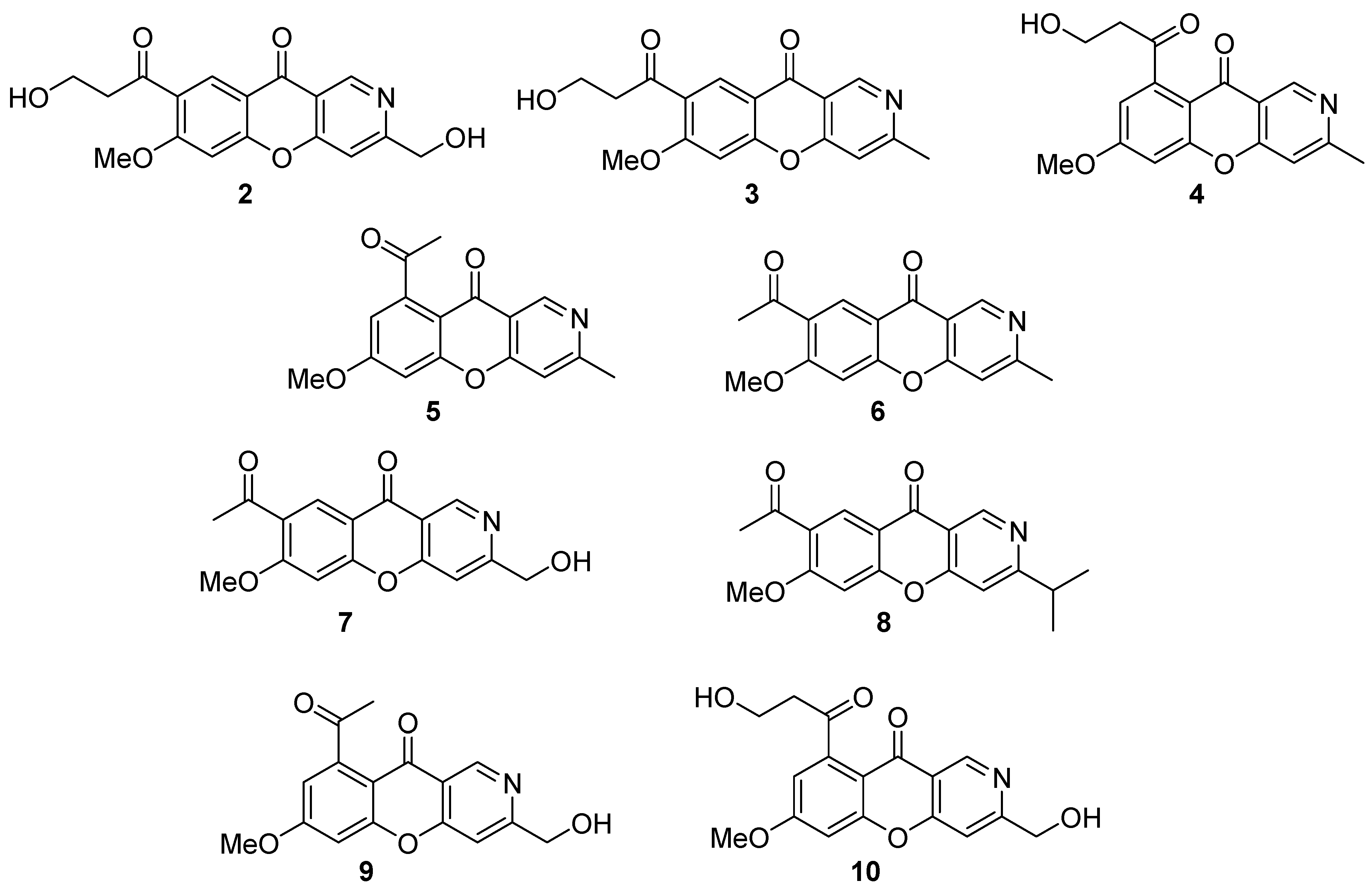
2. Recently Isolated Natural Chromeno[3,2-c]Pyridines and Their Bioactivities
3. Synthetic Ways to Chromeno[3,2-c]Pyridines and Chromeno[3,2-c]Quinolines
3.1. Construction of Chromene Fragment
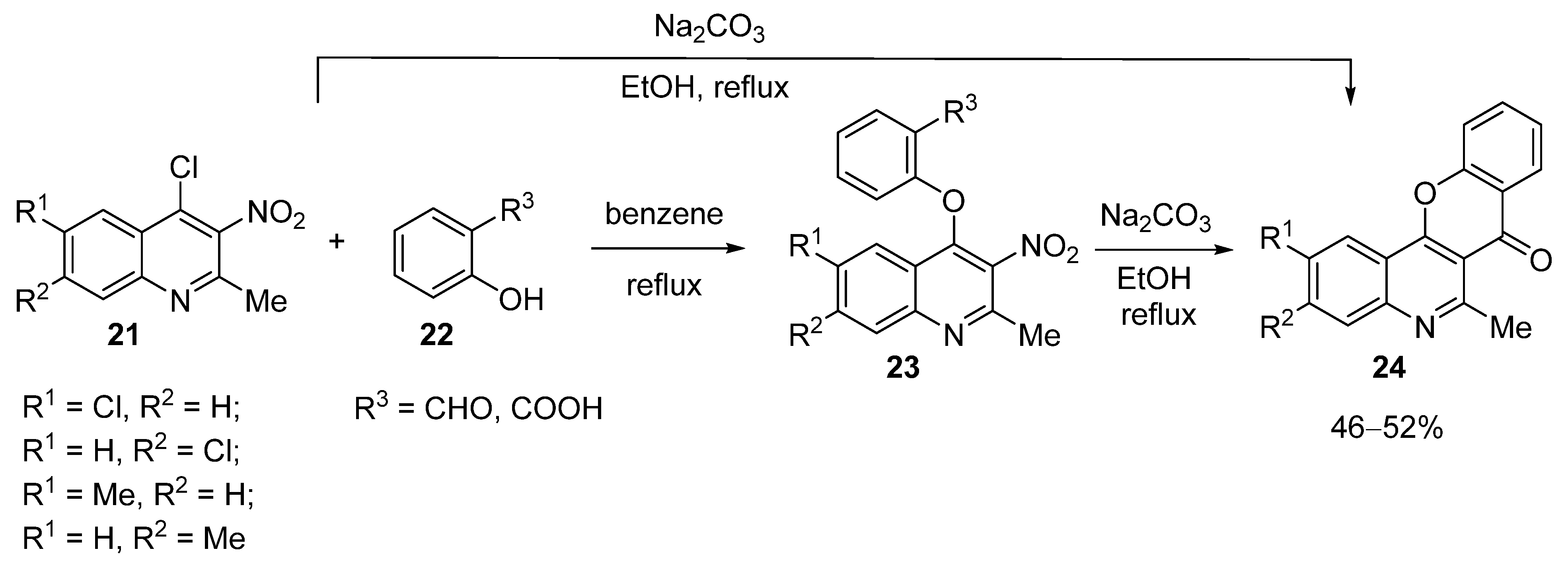
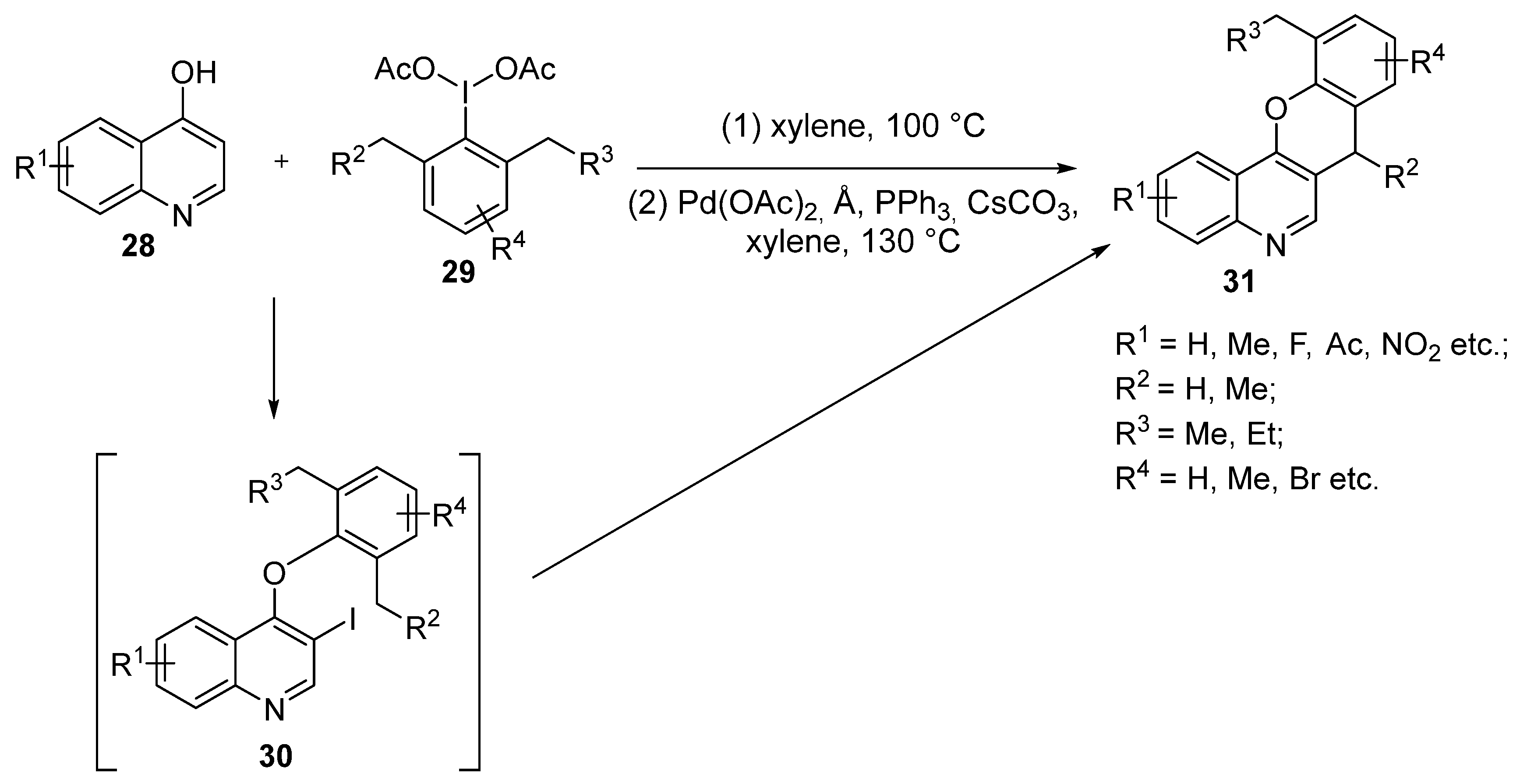
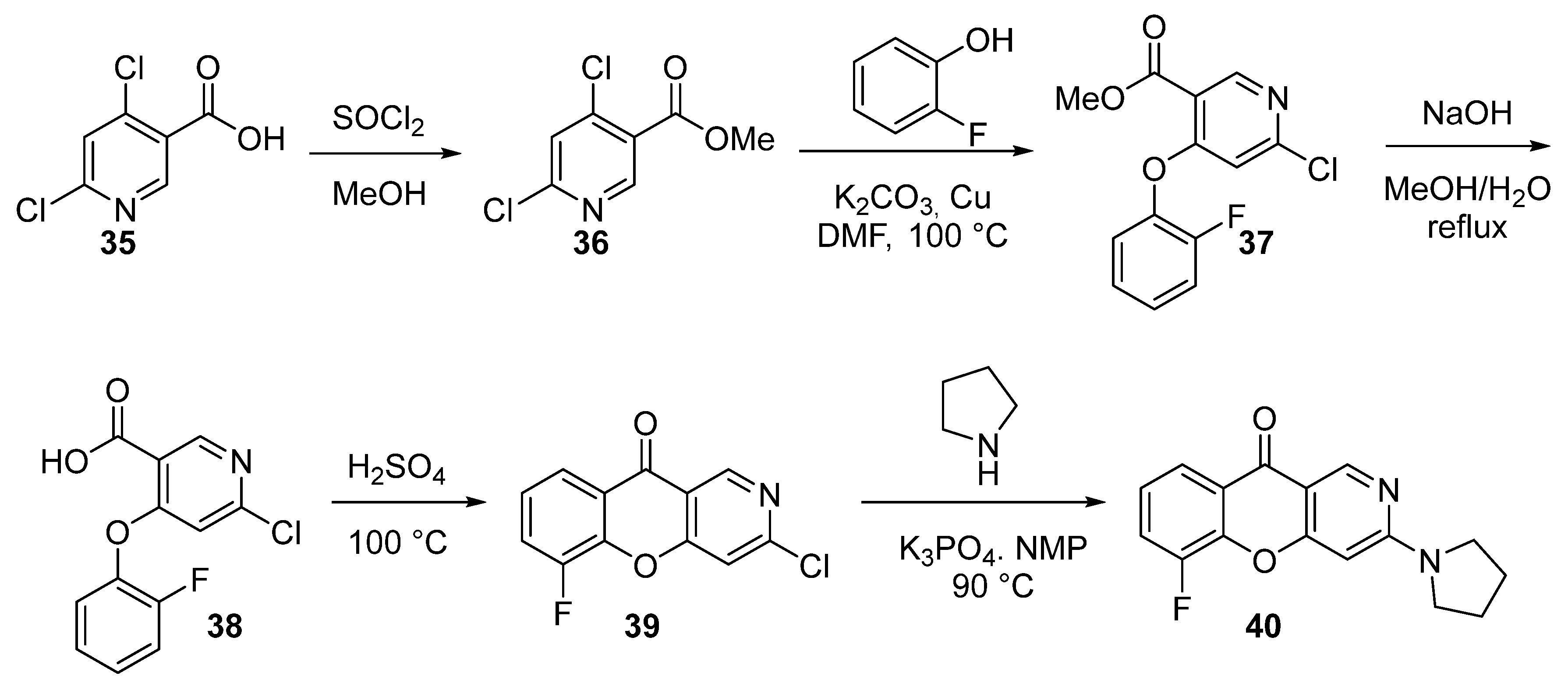
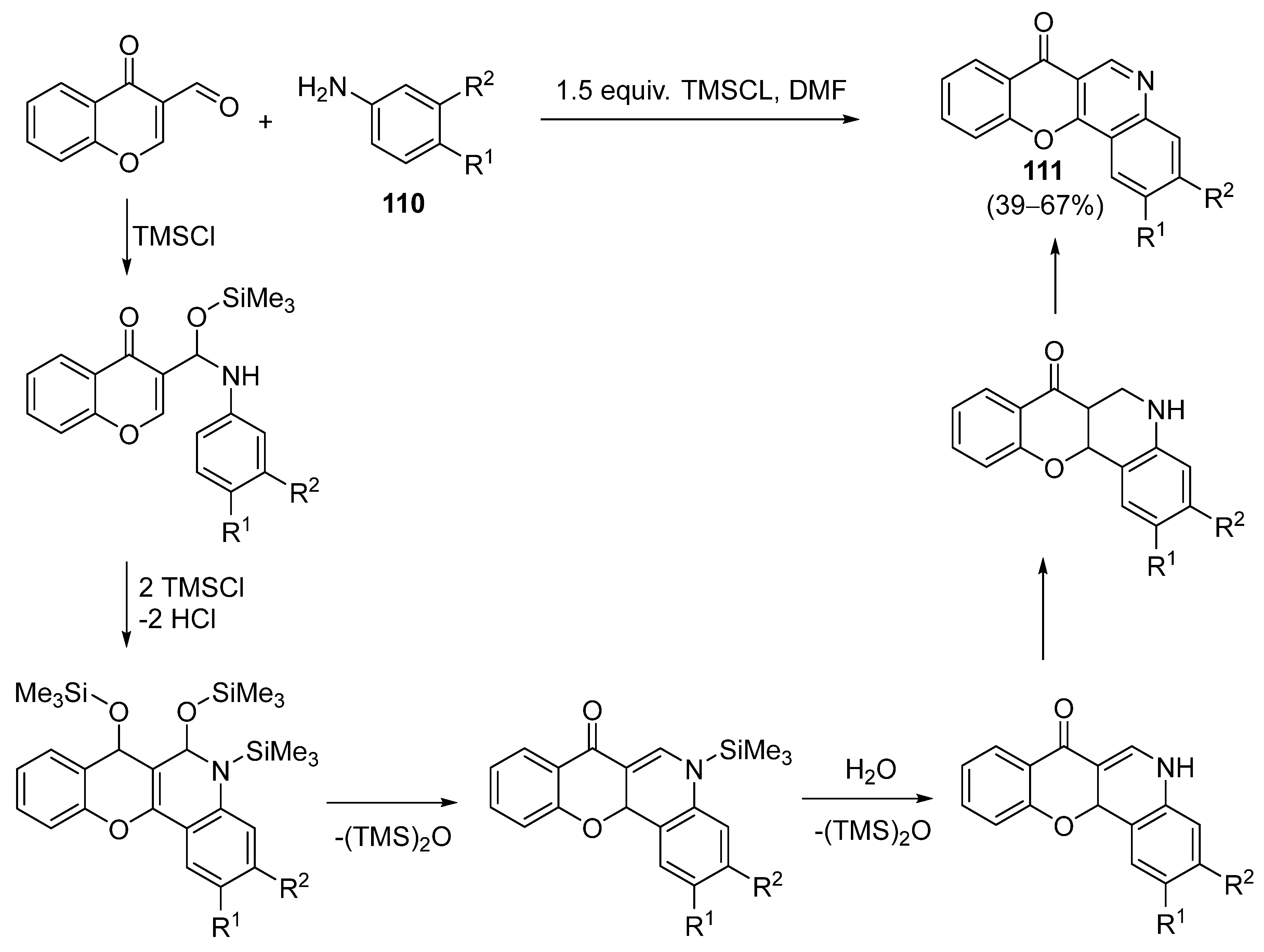
3.1.1. Synthesis Based on Cyclization of Pyridyl(Quinolyl) Phenyl Ethers
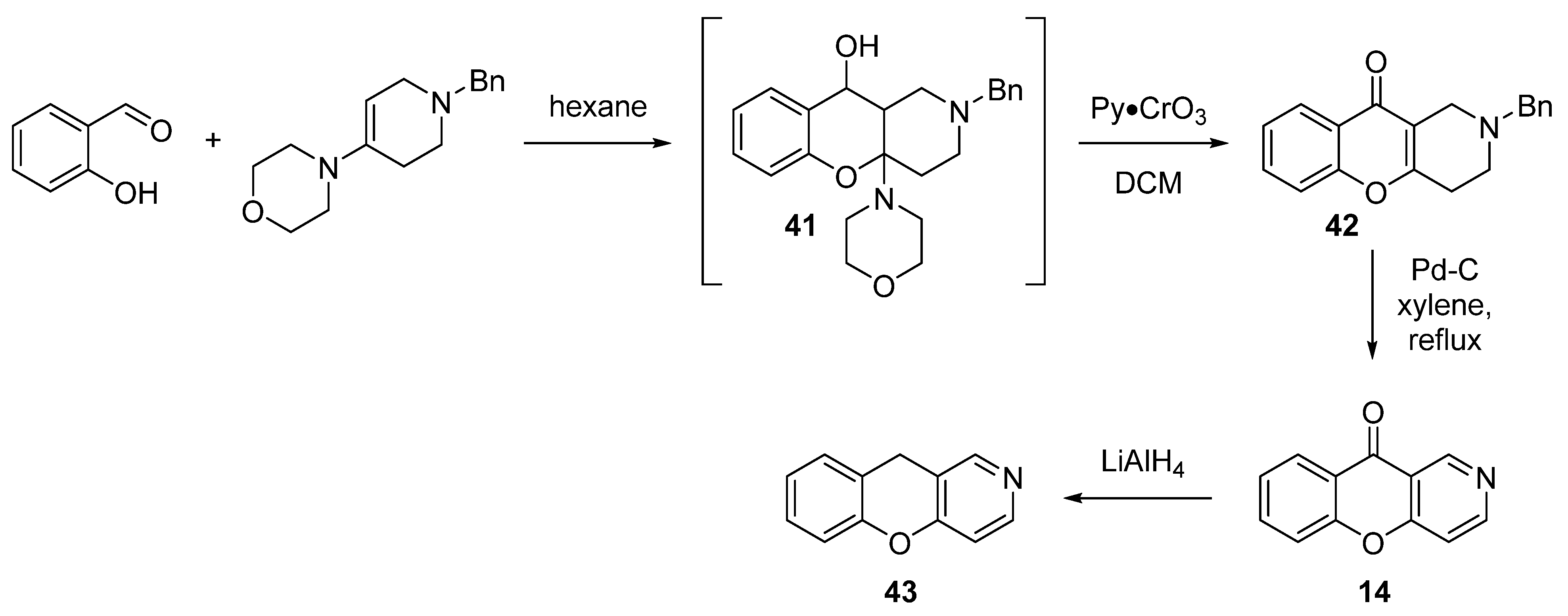
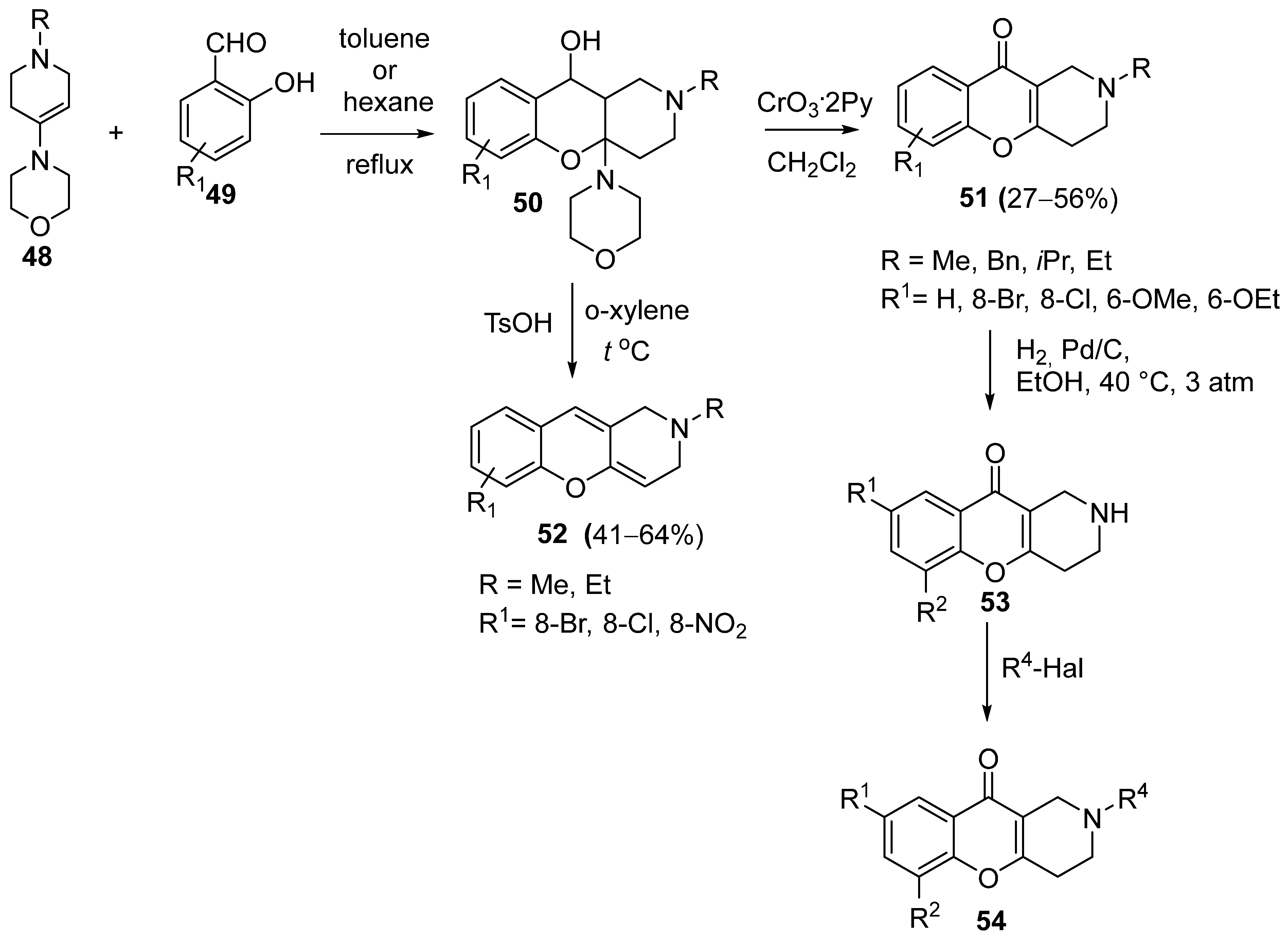
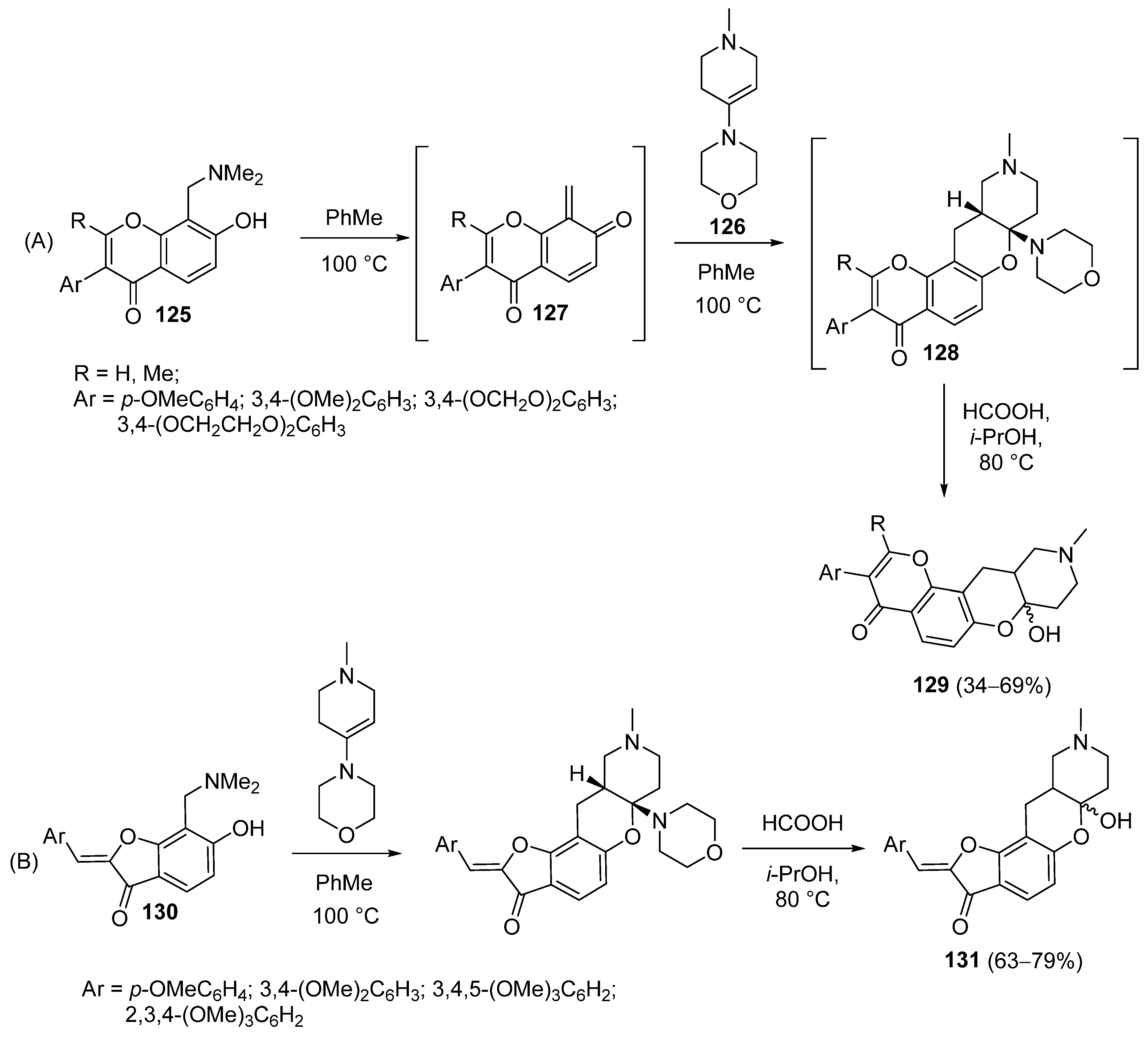
3.1.2. Synthesis Based on Morpholine Enamine
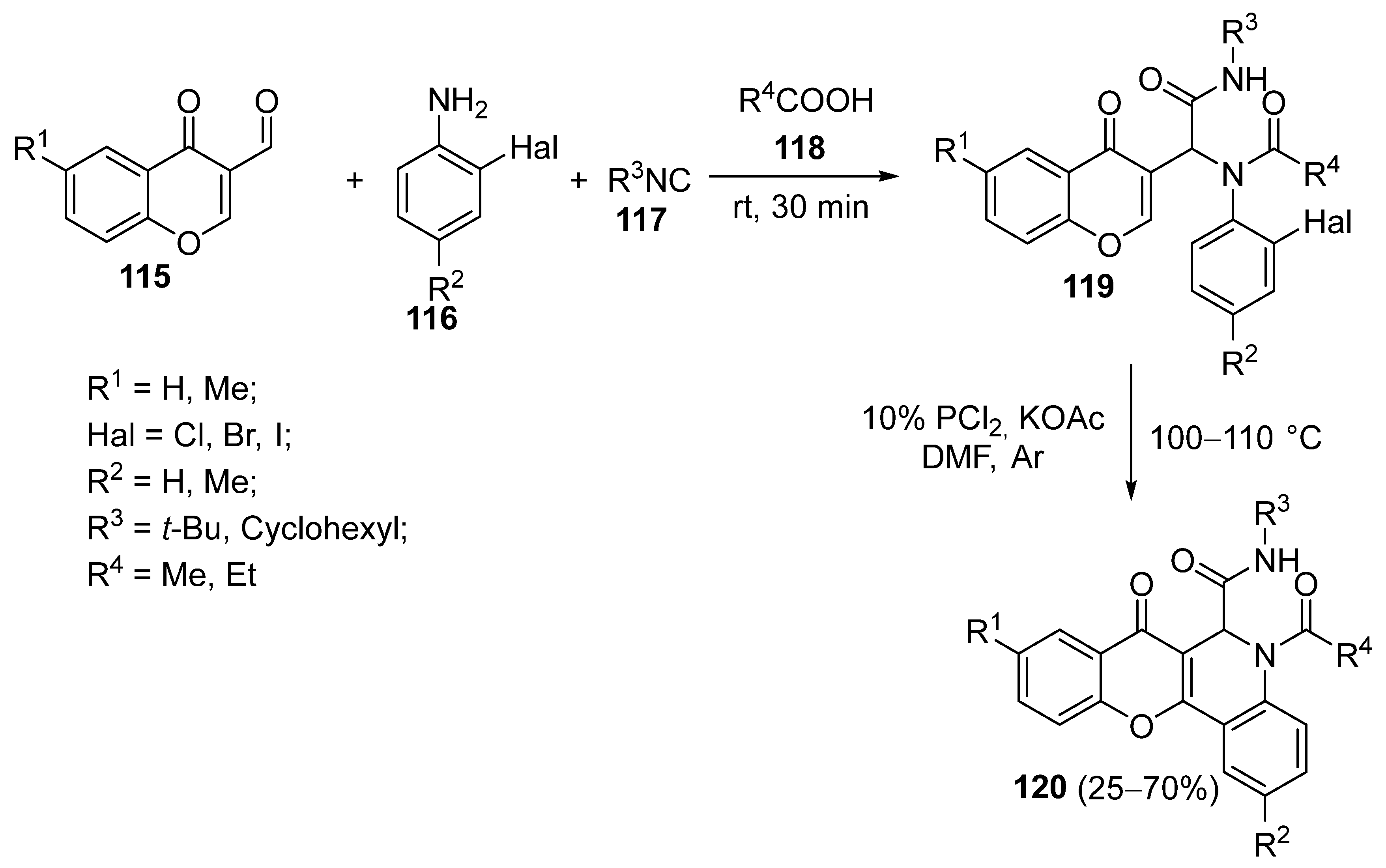
3.1.3. Synthesis Based on Intramolecular Cyclization via Nucleophilic Substitution of Halogen
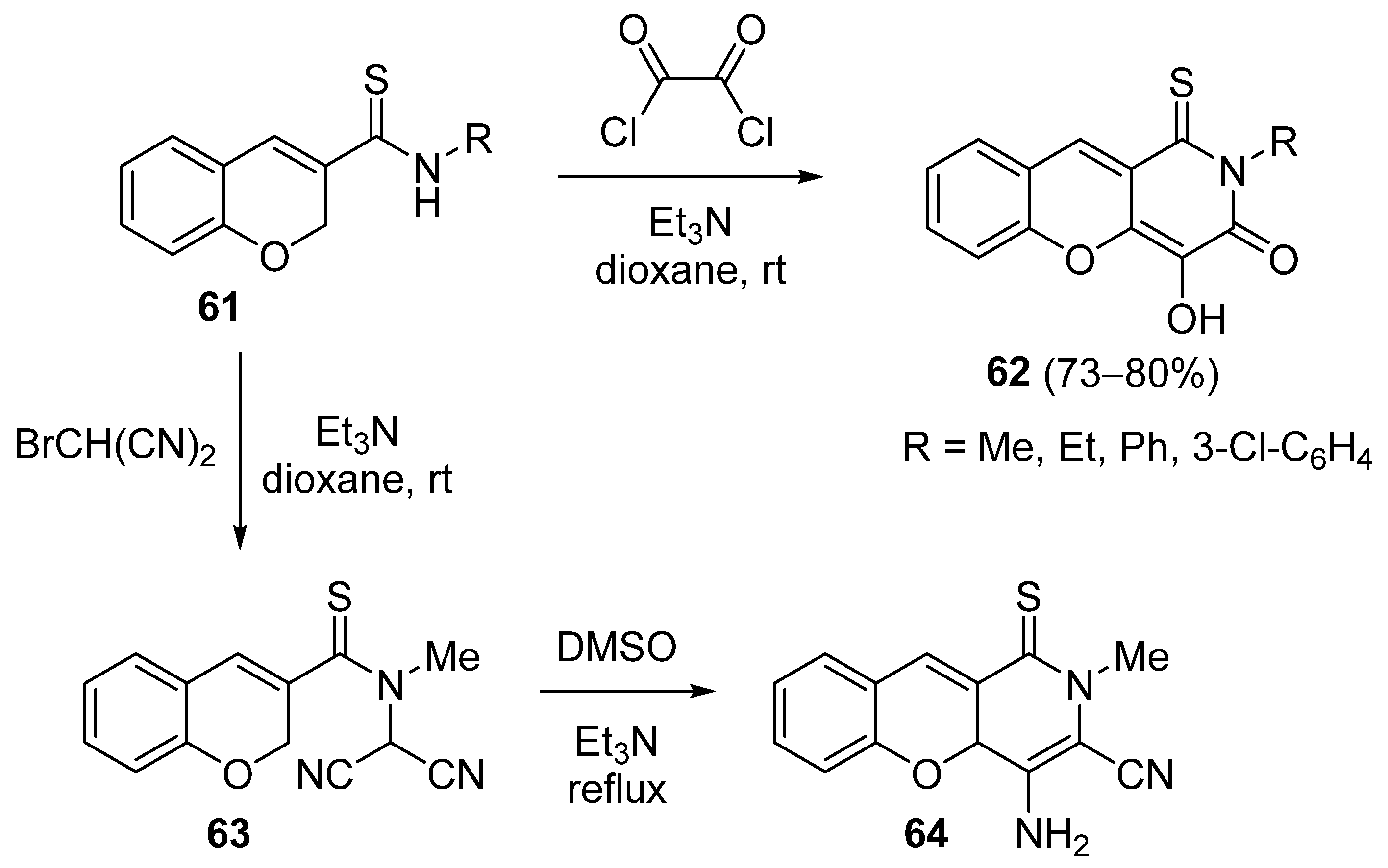
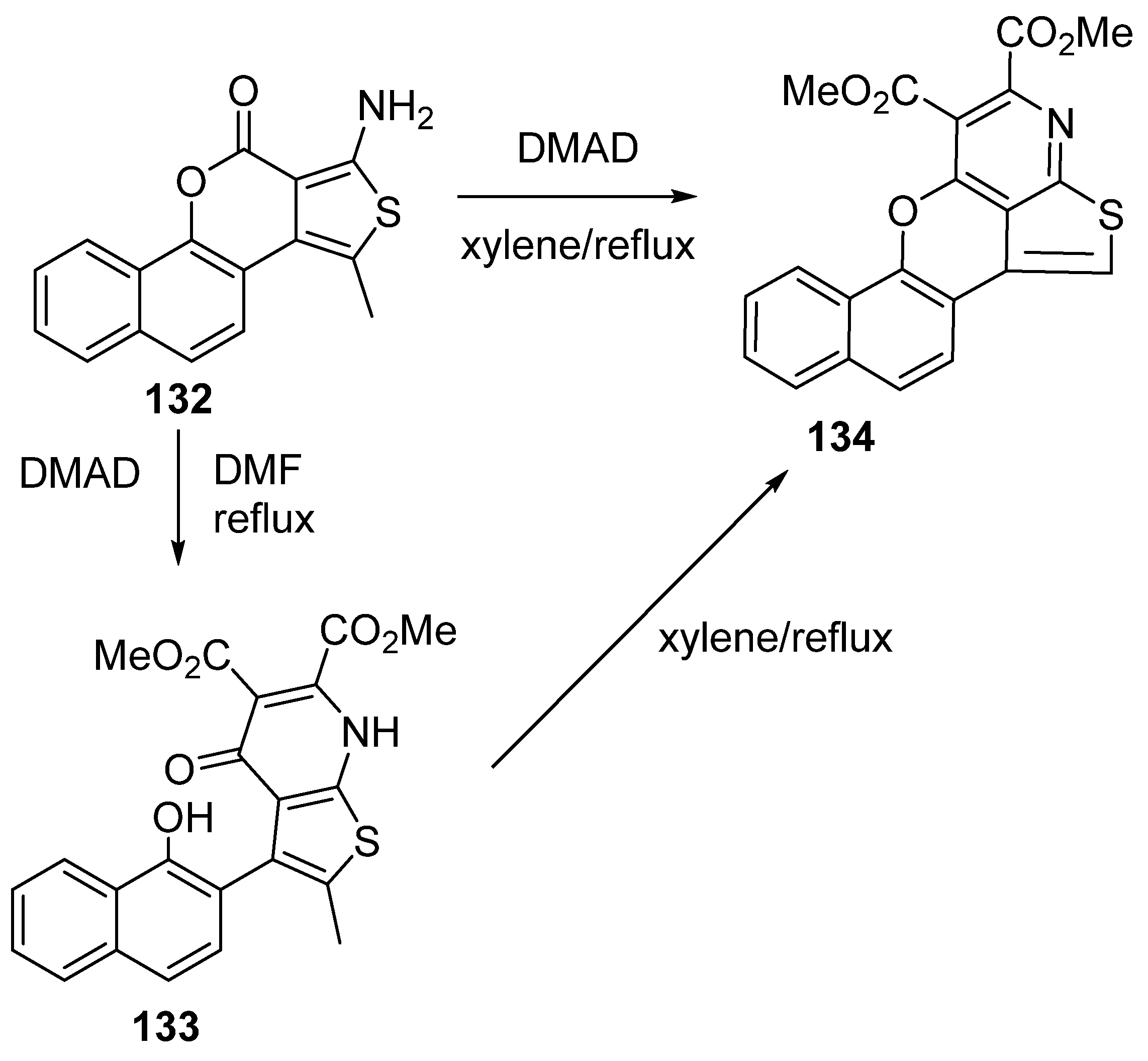
3.1.4. Synthesis Based on Chromene-3-Thiocarboxamide
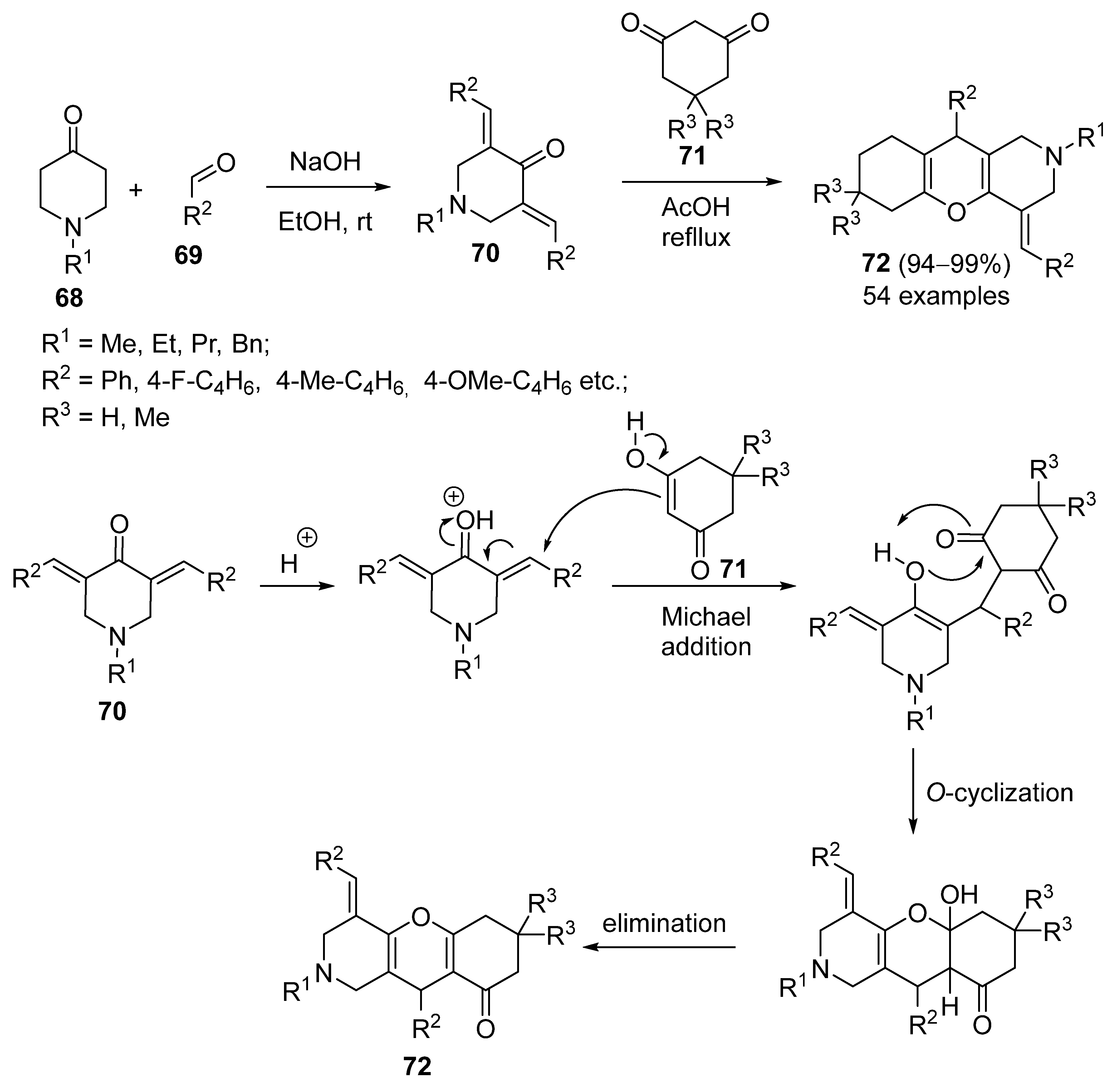
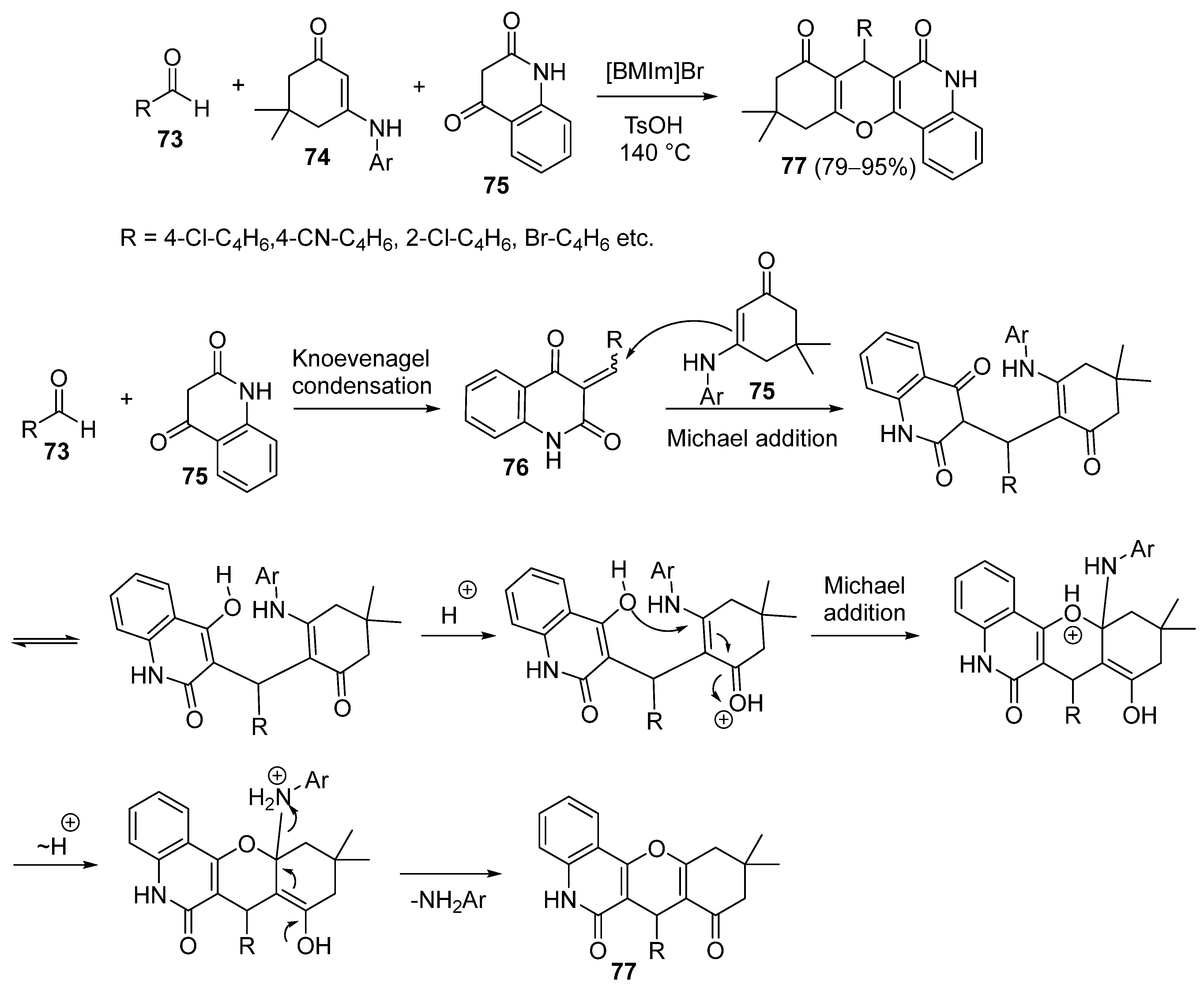
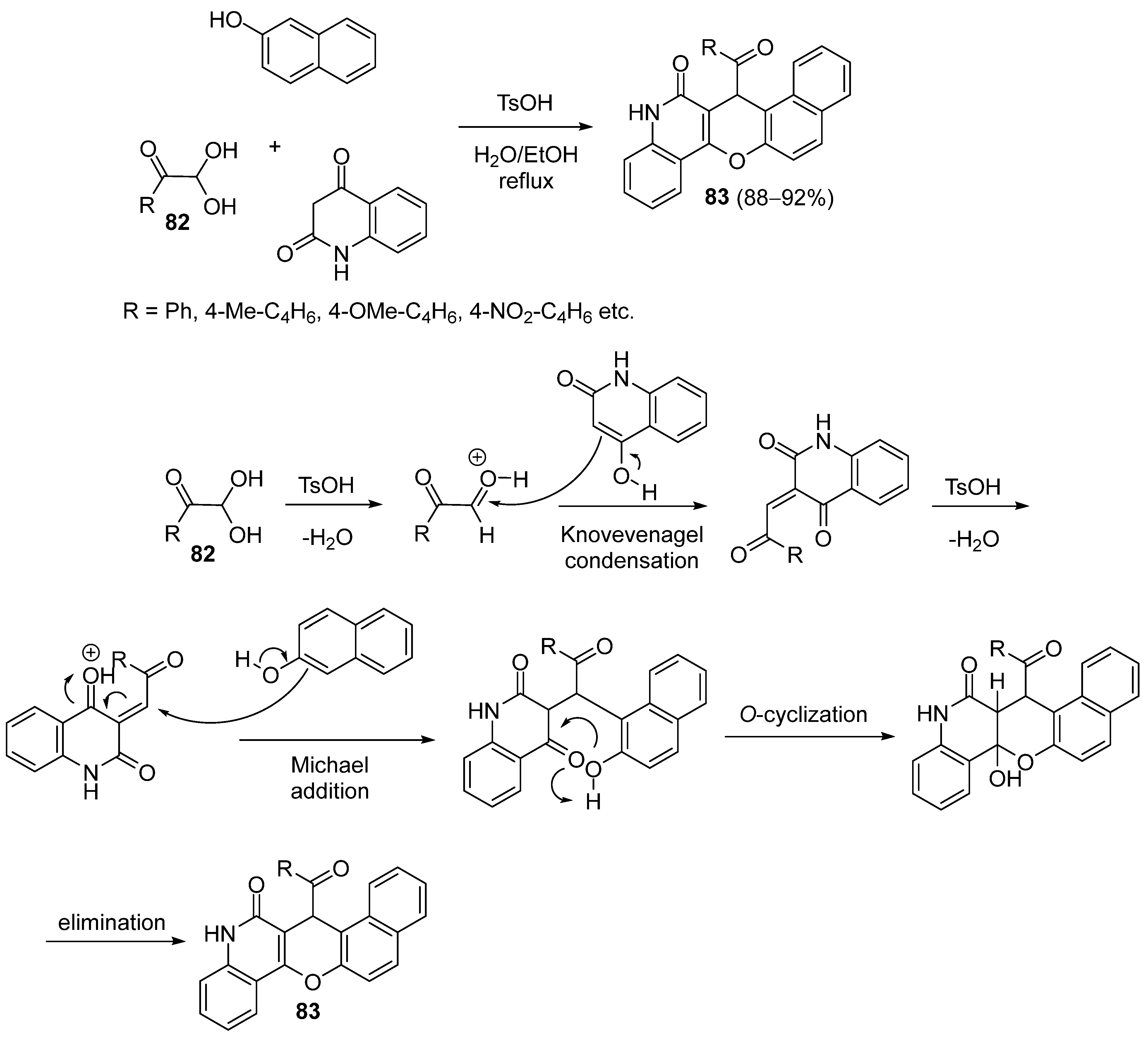
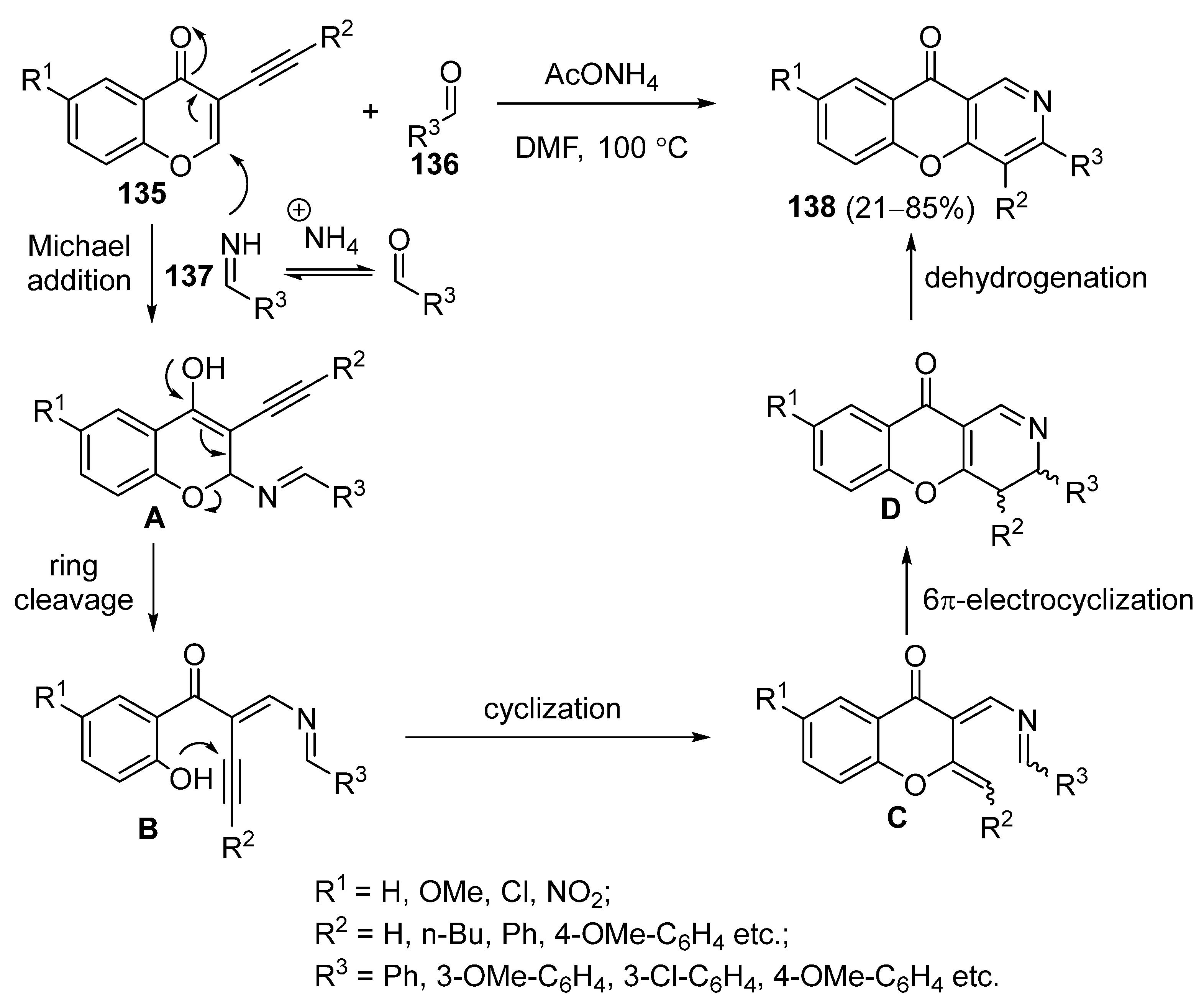
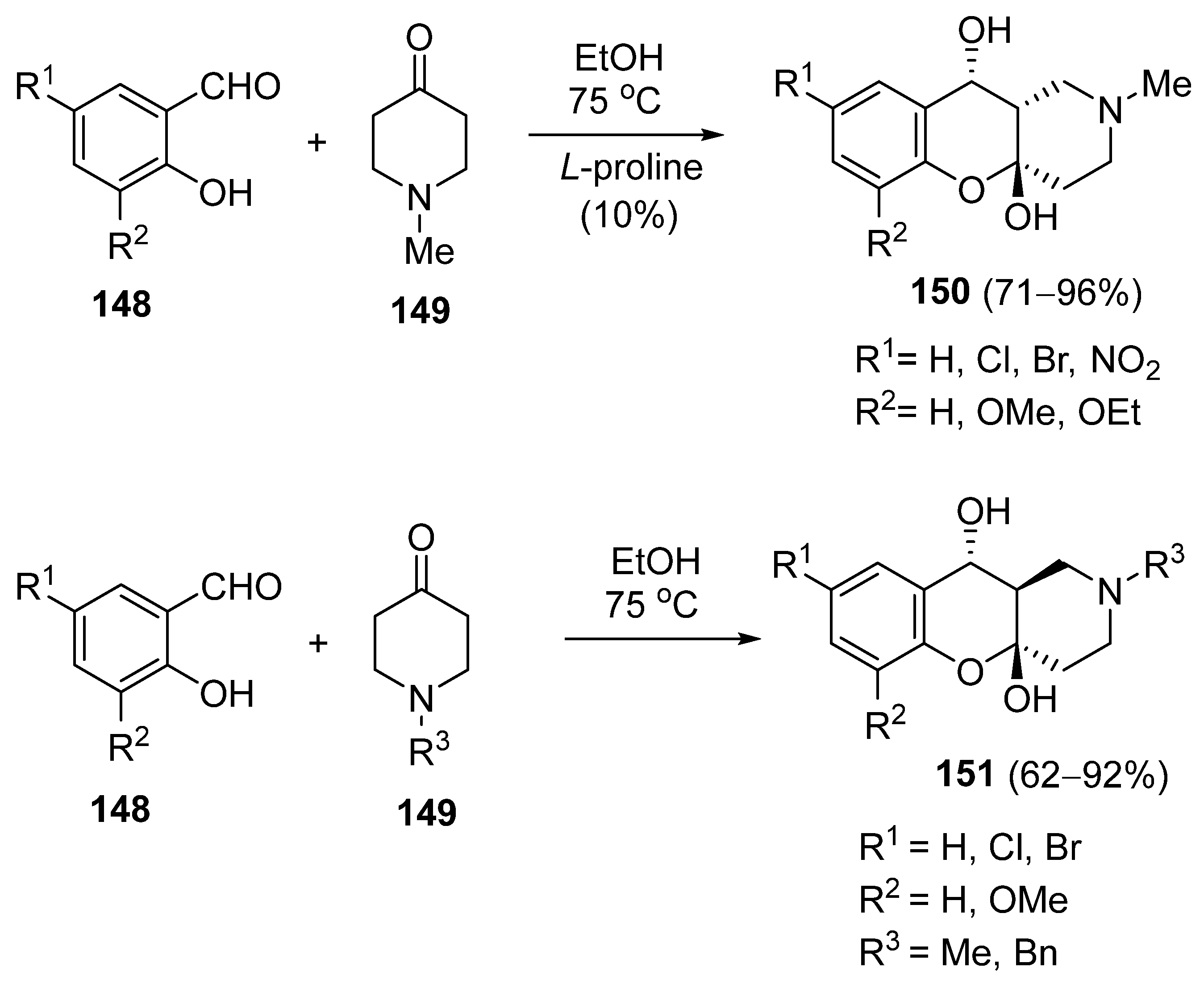
3.1.5. Synthesis Based on Knoevenagel Condensation/Michael Addition Sequence
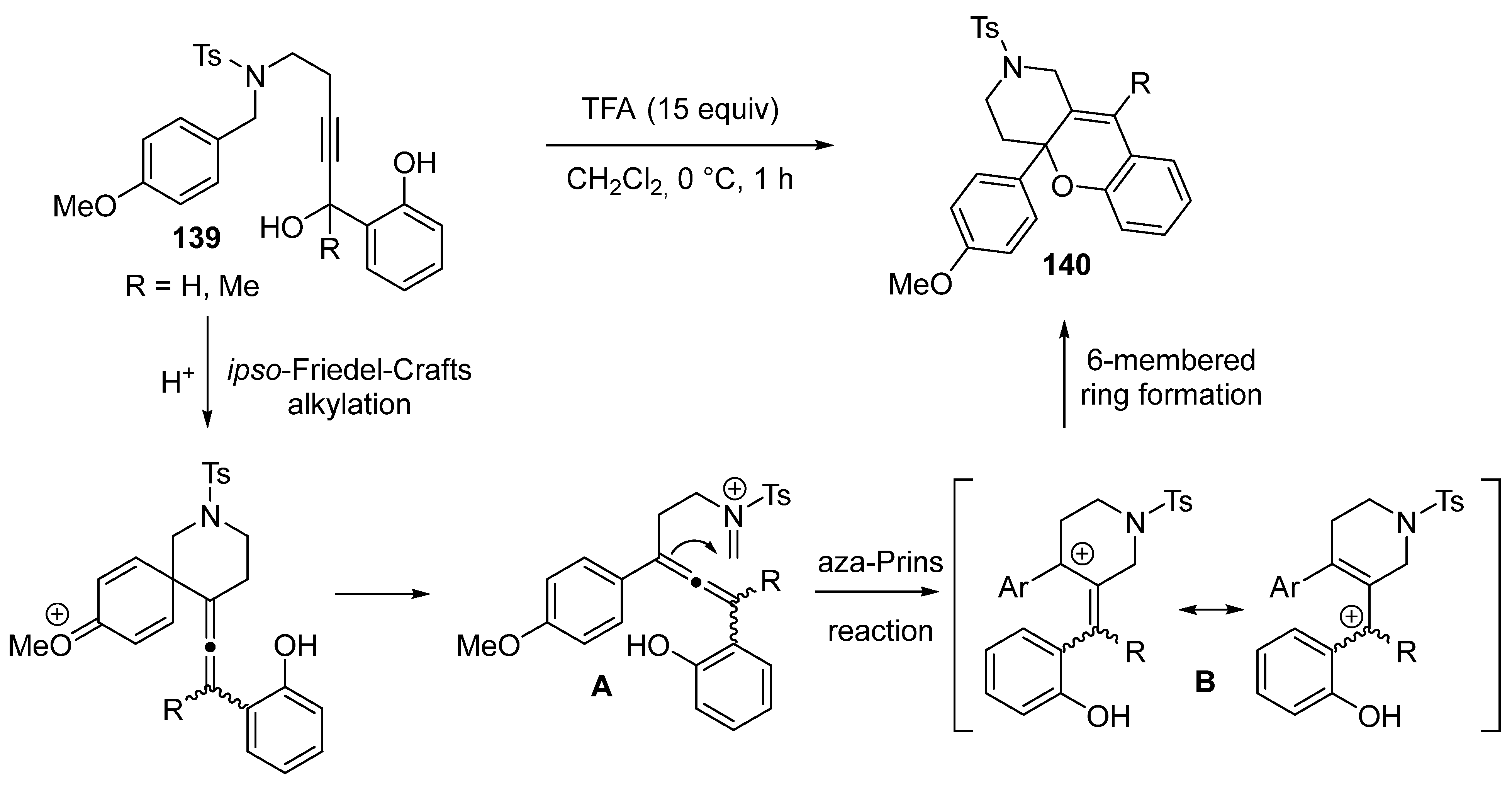
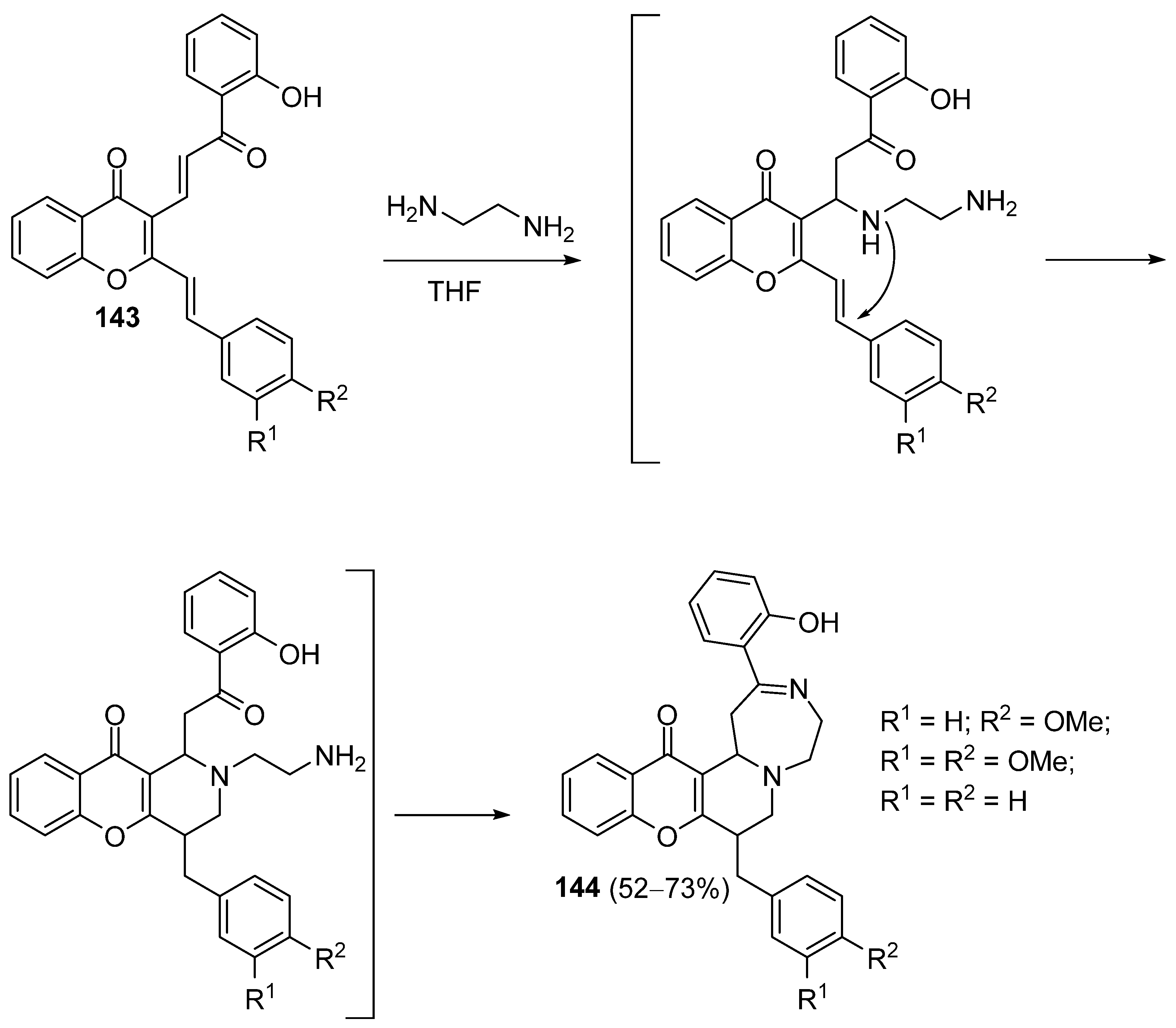
3.1.6. Miscellaneous
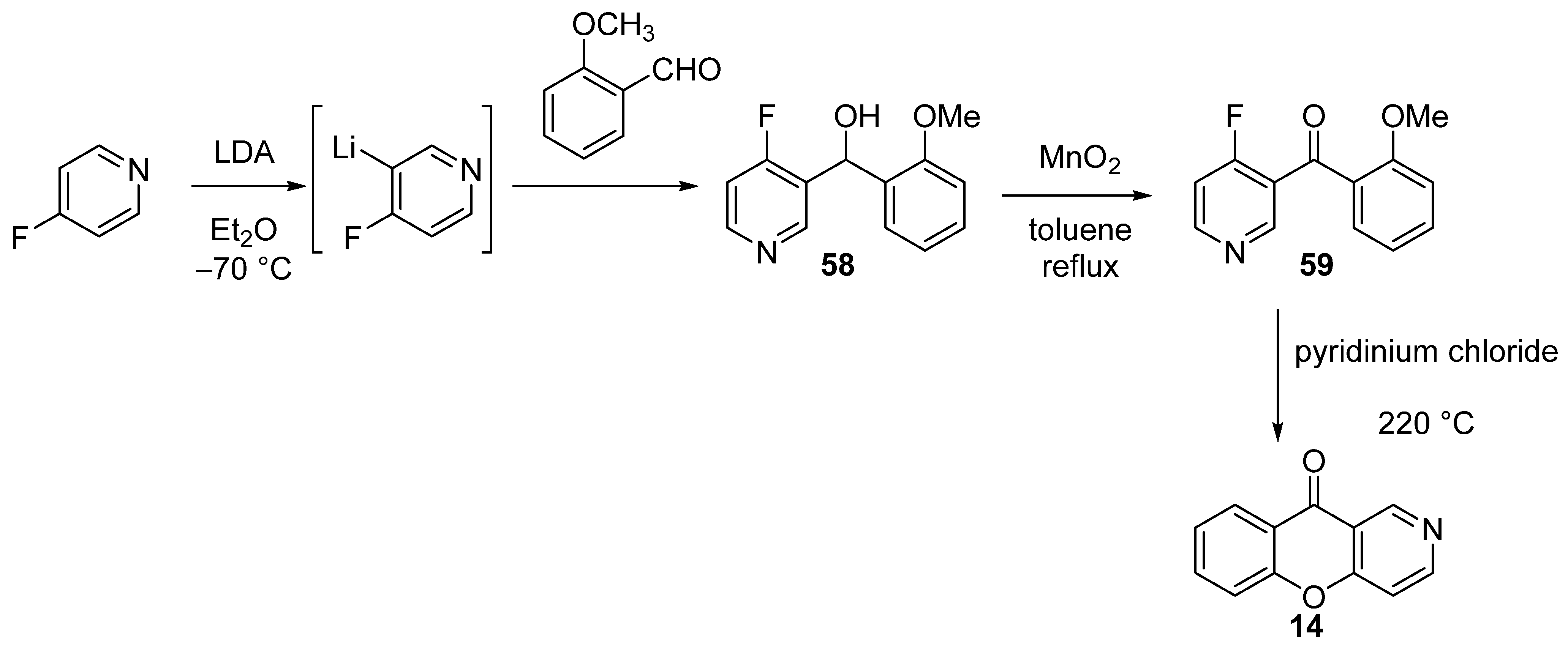
3.2. Construction of Pyridine Fragment
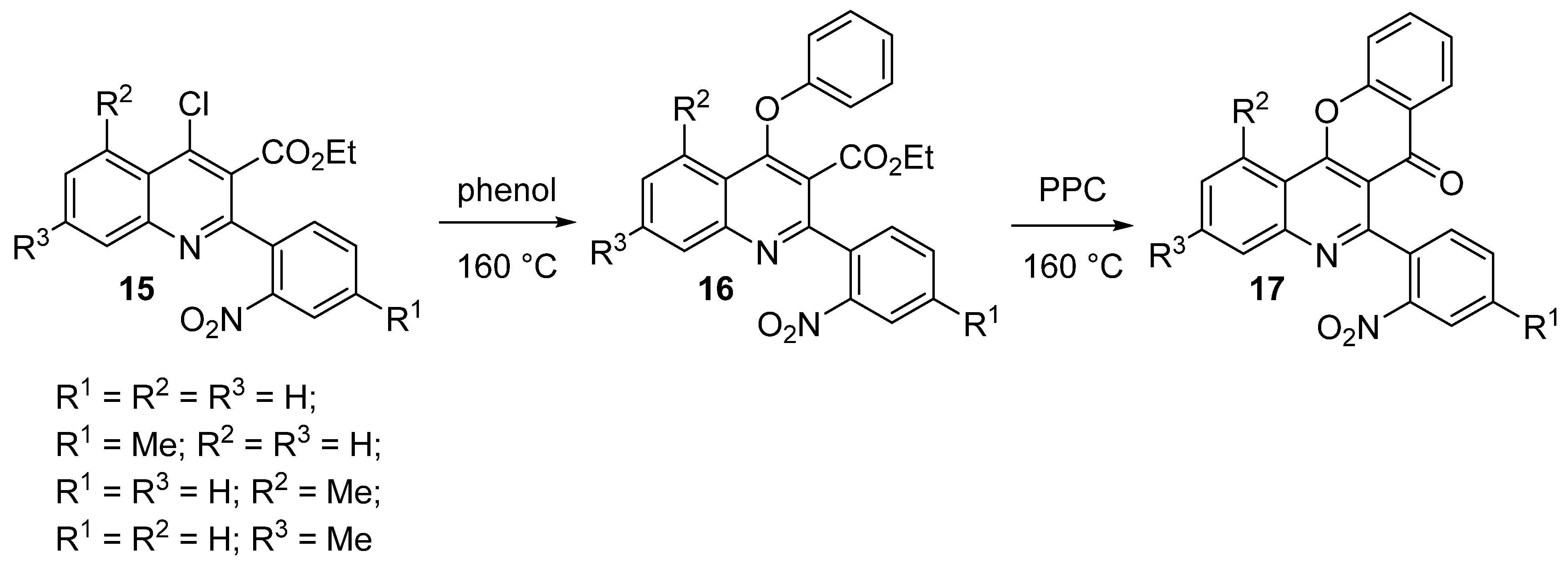
3.2.1. Synthesis Based on Ethyl Coumarin-3-Carboxylate
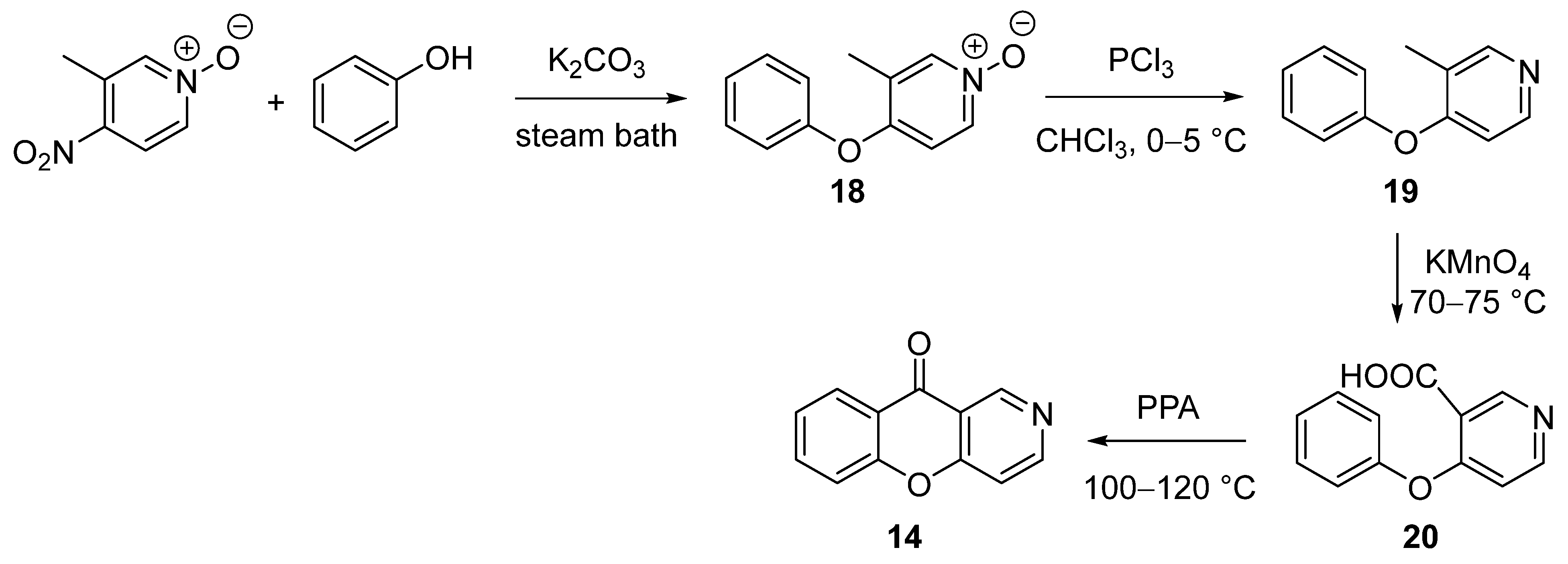
3.2.2. Synthesis Based on 3-Carbonylchromones
3.2.3. Synthesis Based on Diels-Alder Reaction
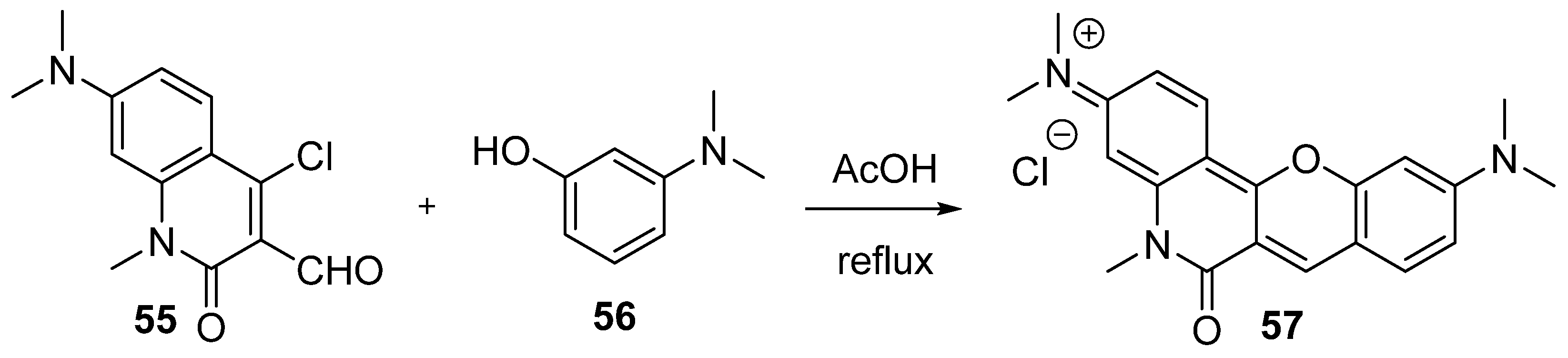
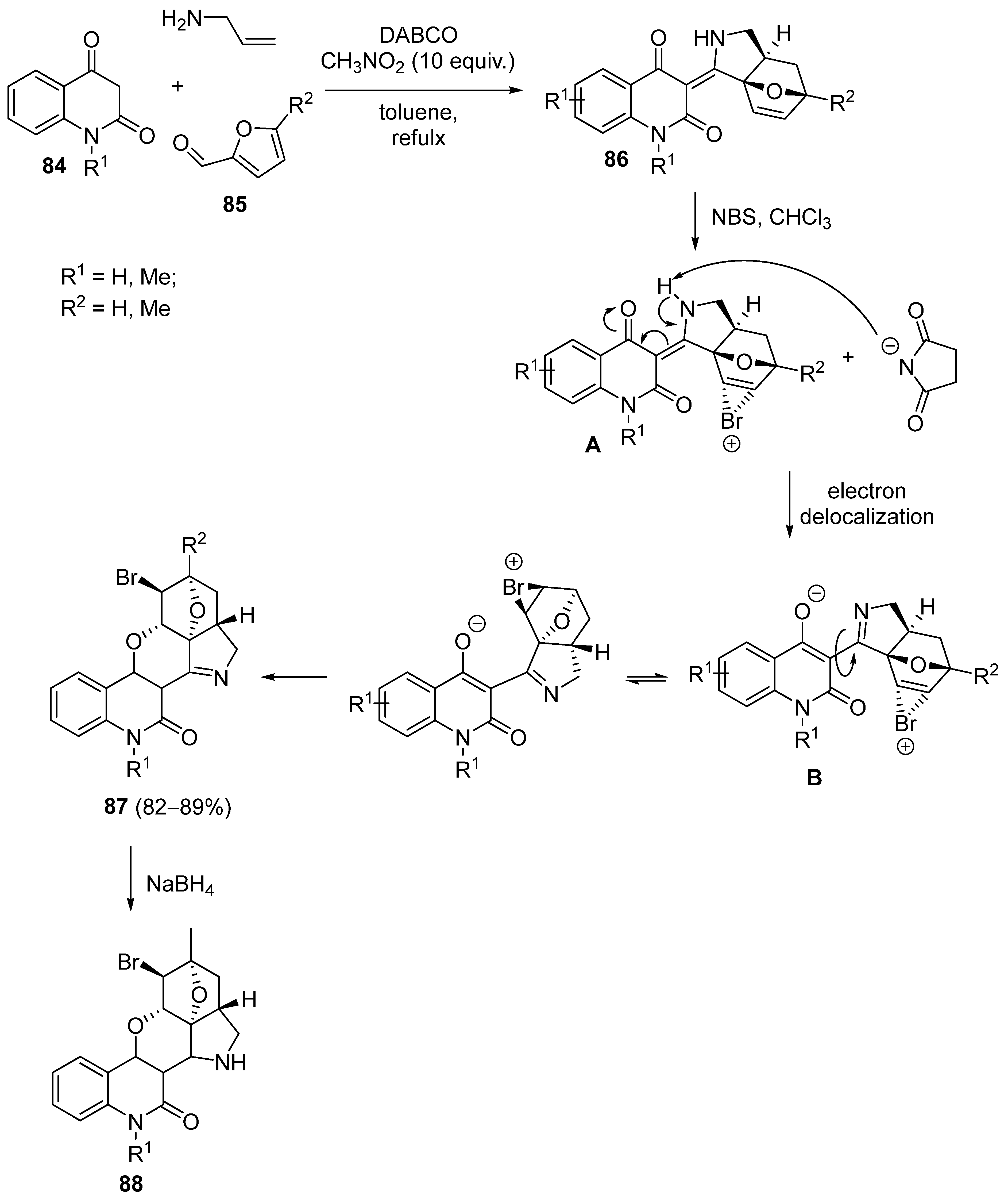
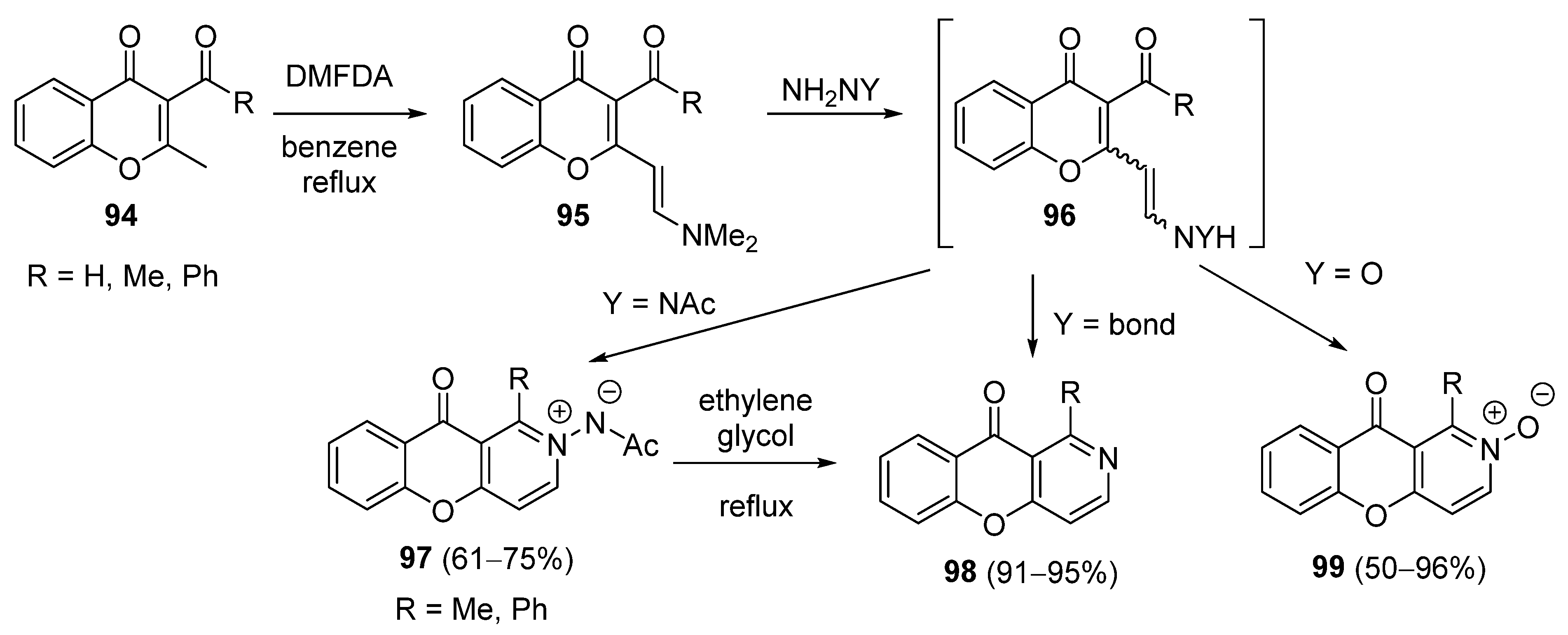
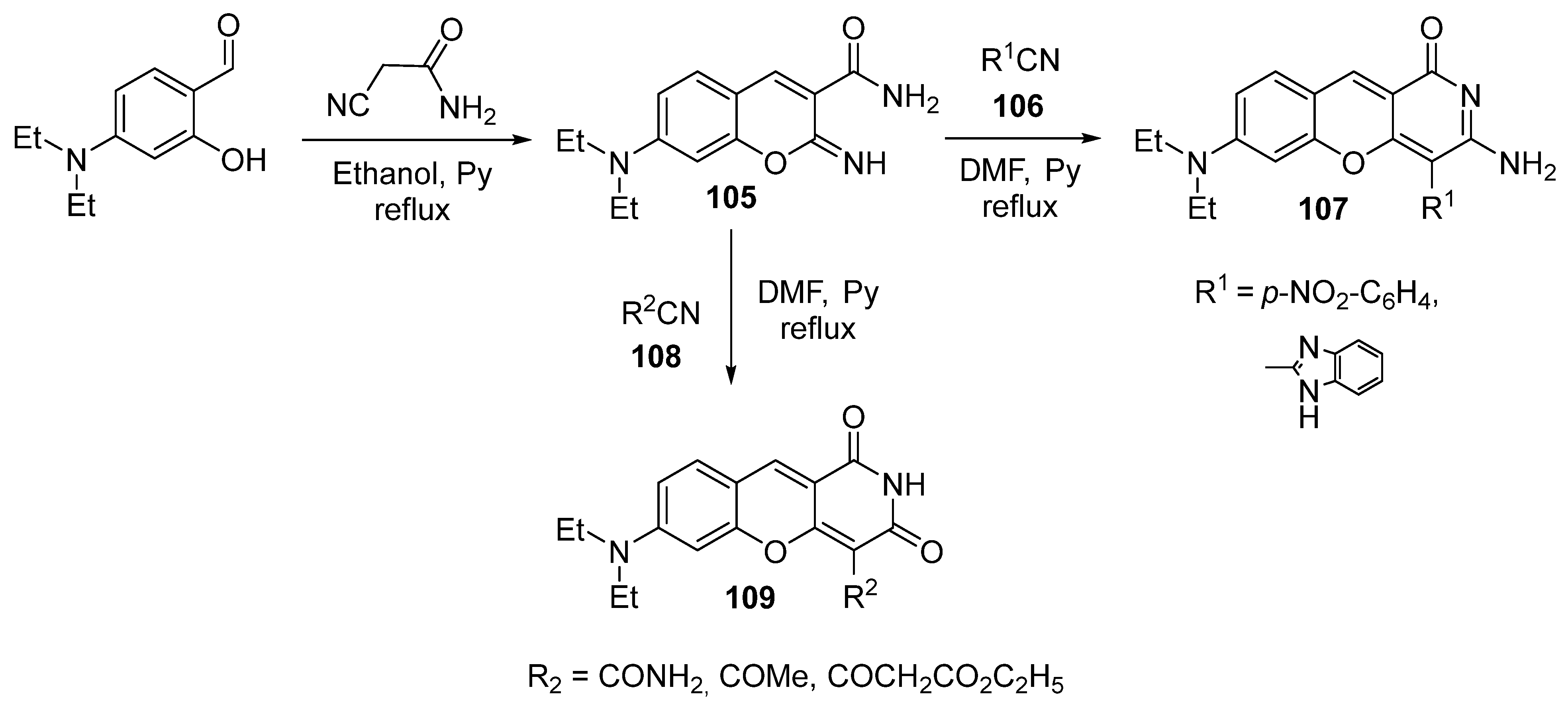
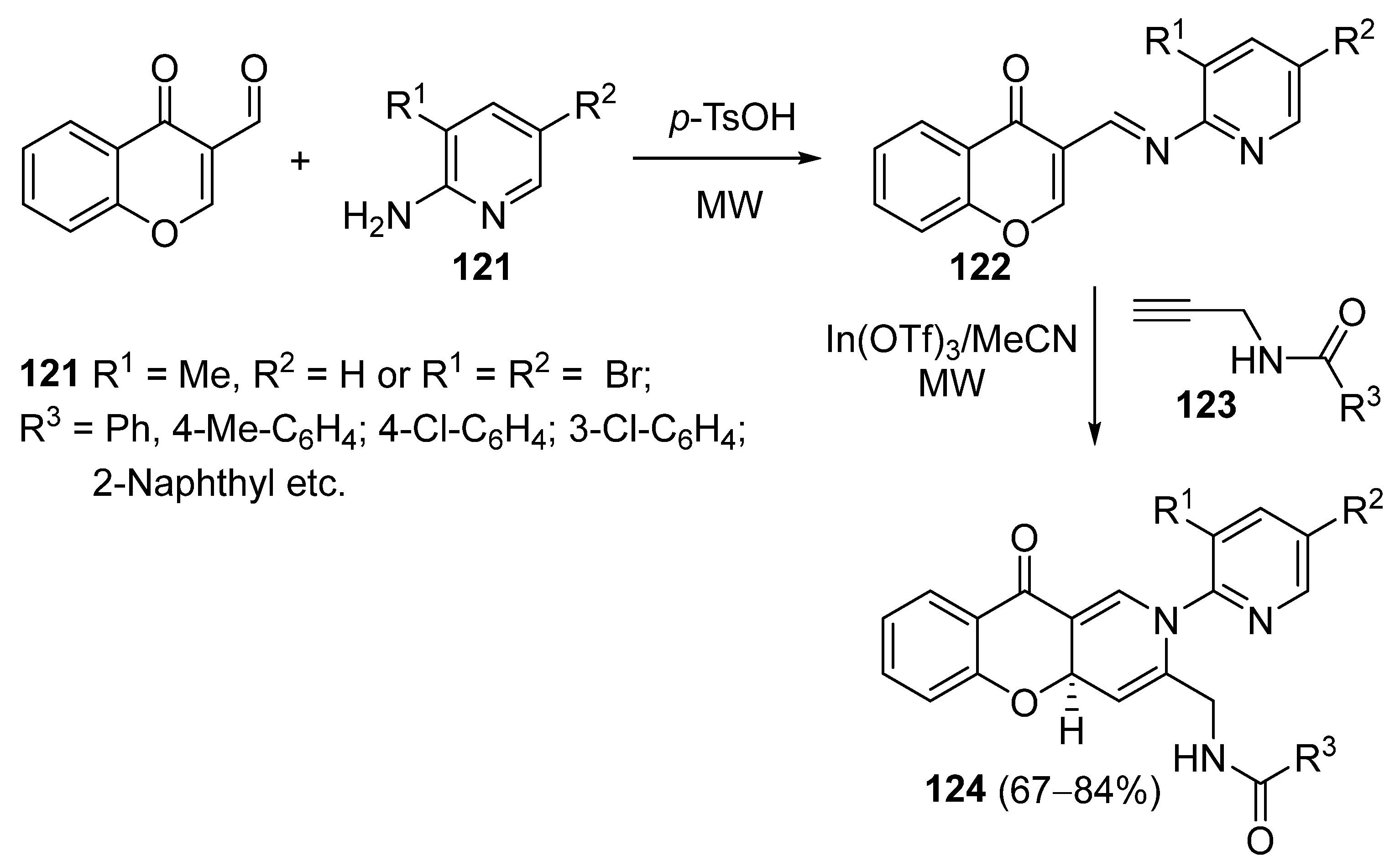
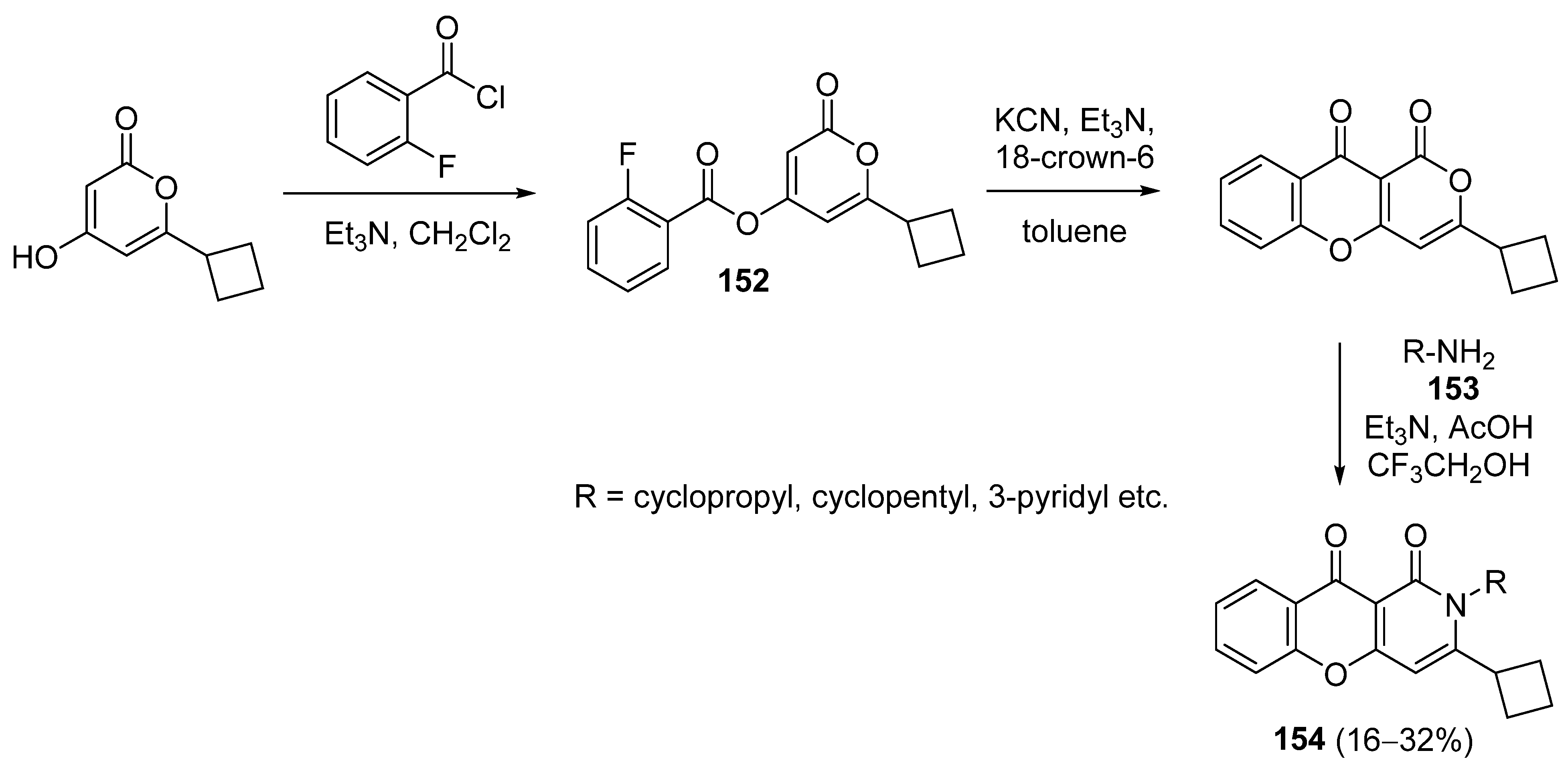
3.2.4. Miscellaneous
4. Biological Activity of Chromeno[3,2-c]Pyridines
| Chemical Structure | Clinical Use | Concentration of Compound | Year–Author–Lit |
|---|---|---|---|
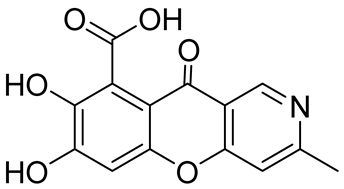 SB236049 | inhibitory activity towards the Bacillus cereus II and Bacteroides fragilis CfiA metallo-β-lactamases | IC50 values of 0.3 μM and 2 μM | In 2002, Paune et al. [33] isolated from the fungus Chaetomium funicola |
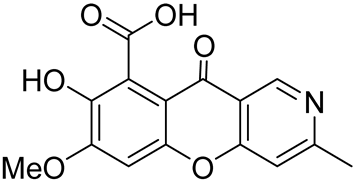 | the alkaloid displayed inhibitory activity against New Delhi metallo-β-lactamase | IC50 value of 87.9 μM | In 2013, Gan et al. [34] isolated cultures of Penicillium sp. I09F 484 |
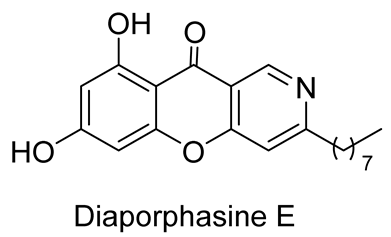 | cytotoxicity against the tested cell lines L929 and KB3.1 | IC50 values of 0.9 and 3.7 μM | In 2023, Phutthacharoen et al. [36] isolated by Phutthacharoen et al. from a mycelial extract of a saprotrophic fungus Lachnum sp. IW157 growing on the common reed grass Phragmites communis |
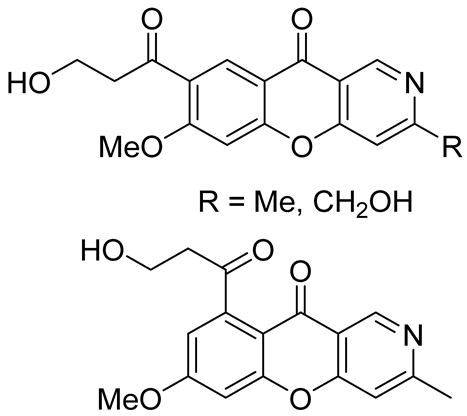 | antirotavirus activity | TI values of 18.3, 23.7, and 19.2 therapeutic index(TI)—CC50/EC50. CC50: mean (50%) value of cytotoxic concentration; EC50: mean (50%) value of effective concentration | In 2021–2022, Yang et al. [40] isolated from plants of Thalictrum scabrifolium |
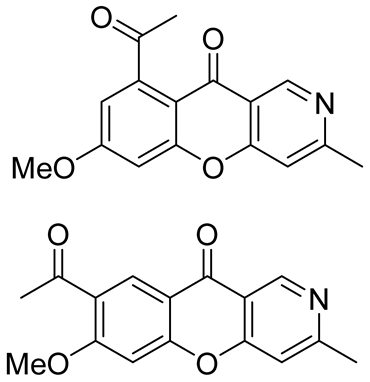 | antibacterial activity against 12 microbial strains isolated from the saliva of smokers | antibacterial activity in the range of 11.1 to 35.3 mm | In 2022, Yin et al. [41] isolated from plants of Thalictrum scabrifolium |
 | antirotavirus activity | TI values of 19.7 and 17.1 therapeutic index(TI)—CC50/EC50. CC50: mean (50%) value of cytotoxic concentration; EC50: mean (50%) value of effective concentration | In 2022, Hu et al. [42] isolated from plants of Thalictrum finetii |
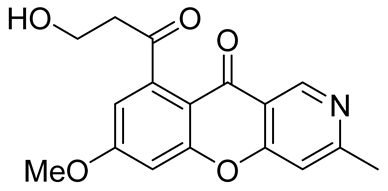 | anti-tobacco mosaic virus (anti-TMV) activity | IC50 value of 29.3 μM | In 2023, Wu et al. [44] plants of Thalictrum microgynum |
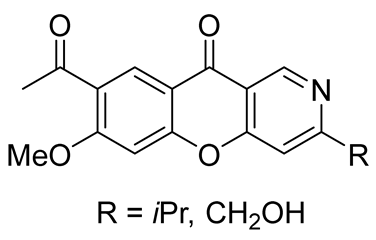
 | efficacy in significantly reducing chronic hepatitis B virus (HBV) antigens, DNA, and intrahepatic cccDNA levels | IC50 value of 0.51 μM | In 2022, Chen et al. [52] |
 | inhibitors of the human isoforms of MAO A | IC50 value of 4.4 μM | In 2020, Purgatorio et al. [57] |
 | inhibitors of the human isoforms of MAO B and AChE. | IC50 values of 2.23 μM and 3.22 μM | In 2020, Purgatorio et al. [57] |
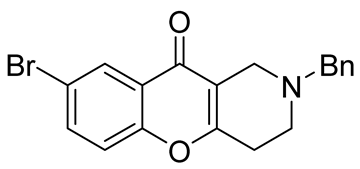 | inhibitors of the human isoforms of MAO B and AChE. | IC50 values of 4.98 μM and 17.3 μM | In 2020, Purgatorio et al. [57] |
 | inhibitors of the human isoforms of MAO B | IC50 value of 0.89 μM | In 2020, Purgatorio et al. [57] |
 | inhibitors of the human isoforms of MAO B and AChE | IC50 values of 1.15 μM and 23.0 μM | In 2020, Purgatorio et al. [57] |
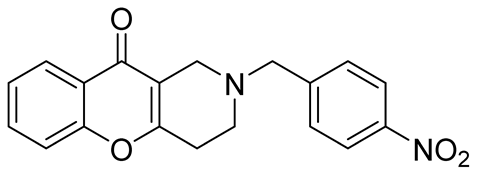 | inhibitors of the human isoforms of MAO A, MAO B, and AChE | IC50 values of 7.1 μM, 2.08 μM and 3.43 μM | In 2020, Purgatorio et al. [57] |
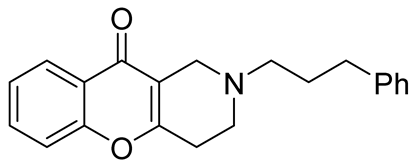 | inhibitors of the human isoforms of MAO B and BChE | IC50 values of 2.81 μM and 3.87 μM | In 2020, Purgatorio et al. [57] |
 | inhibitors of the human isoforms of MAO A, MAO B and AChE | IC50 values of 1.14 μM, 4.91 μM, and 2.05 μM | In 2020, Purgatorio et al. [57] |
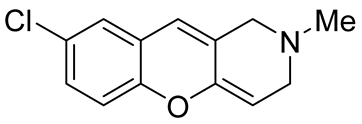 | inhibitors of the human isoforms of MAO A | IC50 value of 1.18 μM | In 2023, Kulikova et al. [58] |
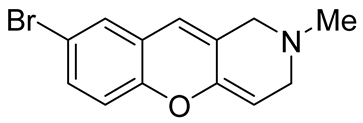 | inhibitors of the human isoforms of MAO A and B | IC50 values of 0.703 μM and 7.88 μM | In 2023, Kulikova et al. [58] |
 | inhibitors of the human isoforms of MAO B | IC50 value of 0.626 μM | In 2023, Kulikova et al. [58] |
 | inhibitors of the human isoforms of MAO B and ChE (AChE and BChE). | IC50 values of 0.510 μM, 6.78 μM, and 4.42 μM | In 2023, Kulikova et al. [58] |
 | inhibitors of the human isoforms of MAO B the tumor growth inhibitory activity assayed in three cell lines (i.e., MCF-7, HCT116, and SK-OV-3) | IC50 value of 7.3 μM IC50 value of 4.8 μM (MCF-7) IC50 value of 8.62 μM (HCT116) IC50 value of 14.7 μM (SK-OV-3) | In 2023, Kulikova et al. [58] |
 | inhibitors of the human isoforms of MAO B the tumor growth inhibitory activity assayed in three cell lines (i.e., MCF-7, HCT116 and SK-OV-3) | IC50 value of 4.72 μM IC50 value of 6.62 μM (MCF-7) IC50 value of 18.6 μM (HCT116) IC50 value of 22.3 μM (SK-OV-3) | In 2023, Kulikova et al. [58] |
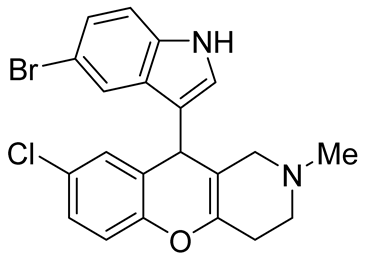 | inhibitors of the human isoforms of MAO B the tumor growth inhibitory activity assayed in three cell lines (i.e., MCF-7, HCT116 and SK-OV-3) | IC50 value of 3.51 μM IC50 value of 4.83 μM (MCF-7) IC50 value of 9.40 μM (HCT116) IC50 value of 11.3 μM (SK-OV-3) | In 2023, Kulikova et al. [58] |
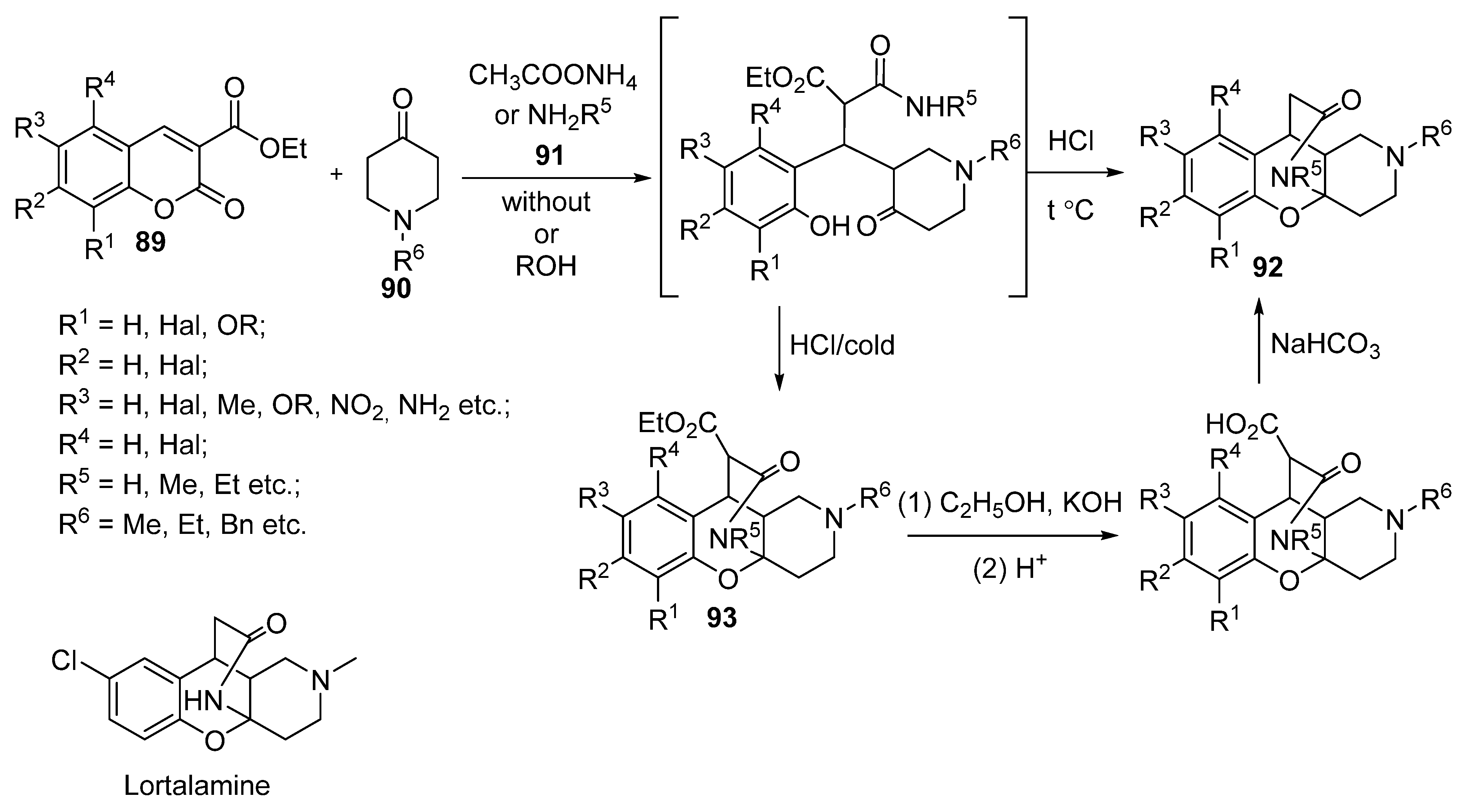
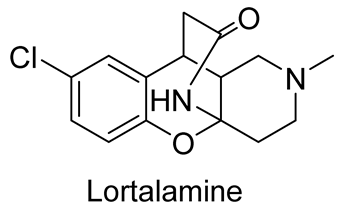 | antidepressant activity | Lortalamine is a potent NET inhibitor with a potency higher than imipramine (13 fold) and desipramine (5 fold) | In 1980, Briet et al. [69], in 1985, Depin et al. [104], in 2006, Ding et al. [105] |
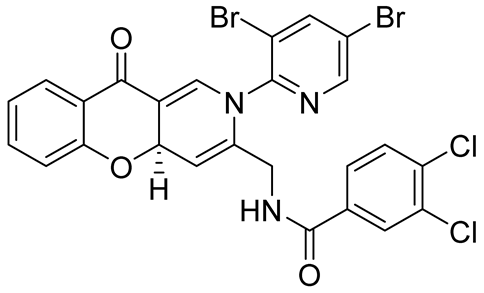 | activity against Mycobacterium tuberculosis H37Rv (MTB) and multidrug-resistant M. tuberculosis (MDR-TB). | IC50 value of 50.69 μM | In 2010, Sriram et al. [80] |
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Benny, A.T.; Arikkatt, S.D.; Vazhappilly, C.G.; Kannadasan, S.; Thomas, R.; Leelabaiamma, M.S.N.; Radhakrishnan, E.K.; Shanmugam, P. Chromone, A Privileged Scaffold in Drug Discovery: Developments in the Synthesis and Bioactivity. MRMC 2022, 22, 1030–1063. [Google Scholar] [CrossRef]
- Verma, N.; Sood, P.; Singh, J.; Jha, N.K.; Rachamalla, M.; Dua, K. Chromene and its Importance: Chemistry and Biology. In The Role of Chromenes in Drug Discovery and Development; Kumar Dash, A., Kumar, D., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023; pp. 1–16. [Google Scholar]
- Keri, R.S.; Budagumpi, S.; Pai, R.K.; Balakrishna, R.G. Chromones as a privileged scaffold in drug discovery: A review. Eur. J. Med. Chem. 2014, 78, 340–374. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Catto, M.; Muncipinto, G.; Nicolotti, O.; Carrieri, A.; Rullo, M.; Stefanachi, A.; Leonetti, F.; Altomare, C. A twenty-year journey exploring coumarin-based derivatives as bioactive molecules. Front. Chem. 2022, 10, 1002547. [Google Scholar] [CrossRef] [PubMed]
- Madhav, H.; Jameel, E.; Rehan, M.; Hoda, N. Recent advancements in chromone as a privileged scaffold towards the development of small molecules for neurodegenerative therapeutics. RSC Med. Chem. 2022, 13, 258–279. [Google Scholar] [CrossRef]
- Kumar Dash, A.; Kumar, D. The Role of Chromenes in Drug Discovery and Development; Bentham Science Publishers: Sharjah, United Arab Emirates, 2023. [Google Scholar]
- Carosati, E.; Ioan, P.; Micucci, M.; Broccatelli, F.; Cruciani, G.; Zhorov, B.S.; Chiarin, A.; Budriesi, R. 1,4-Dihydropyridine Scaffold in Medicinal Chemistry, The Story So Far And Perspectives (Part 2): Action in Other Targets and Antitargets. CMC 2012, 19, 4306–4323. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef]
- Bräse, S. Privileged Scaffolds in Medicinal Chemistry: Design, Synthesis, Evaluation; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Costantino, L.; Barlocco, D. Privileged Structures as Leads in Medicinal Chemistry. CMC 2006, 13, 65–85. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Iannazzo, D.; Romeo, R.; Giofrè, S.V.; Legnani, L. Pyridine and Pyrimidine Derivatives as Privileged Scaffolds in Biologically Active Agents. CMC 2020, 26, 7166–7195. [Google Scholar] [CrossRef]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Romano, R.; Mobilia, M.A.; Izzo, A.A.; Santini, A. Human health-related properties of chromones: An overview. Nat. Prod. Res. 2020, 34, 137–152. [Google Scholar] [CrossRef]
- Hammond, P.R.; Atkins, R.L. 2-Keto-4-trifluoromethyl-9-methyl-6,7,8,9-tetrahydro-2H-pyrano [3,2-g] quinoline, an efficient, stable laser dye. J. Heterocycl. Chem. 1975, 12, 1061. [Google Scholar] [CrossRef]
- Harnisch, H. Über 4-Chlor-7-dimethylamino-1-methyl-chinolon-(2)-aldehyd-(3), II. Justus Liebigs Ann. Chem. 1971, 751, 155–158. [Google Scholar] [CrossRef]
- Atkins, R.L.; Bliss, D.E. Substituted coumarins and azacoumarins. Synthesis and fluorescent properties. J. Org. Chem. 1978, 43, 1975–1980. [Google Scholar] [CrossRef]
- Fujimoto, A.; Sakurai, A.; Iwase, E. The Sensitization of Rare Earth Ion Luminescence in Dilute Solutions by [1]Benzopyrano[3,4-c]pyridine-4,5(3H)-dione Derivatives. Bull. Chem. Soc. Jpn. 1976, 49, 809–810. [Google Scholar] [CrossRef]
- Arden-Jacob, J.; Frantzeskos, J.; Kemnitzer, N.U.; Zilles, A.; Drexhage, K.H. New fluorescent markers for the red region. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2271–2283. [Google Scholar] [CrossRef]
- Mandal, T.K.; Kuznetsov, V.V.; Soldatenkov, A.T. Chemistry of pyrido[c]coumarins (review). Chem. Heterocycl. Compd. 1994, 30, 867–887. [Google Scholar] [CrossRef]
- Ramazani, A.; Kiani, M.T.; Rezayati, S. A Review on the Syntheses and Applications of the 5H-chromeno[2,3- b]pyridines. Lett. Org. Chem. 2023, 20, 28–53. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94. [Google Scholar] [CrossRef]
- Nunez-Vergara, J.L.; Squella, J.A.; Navarrete-Encina, A.P.; Vicente-García, E.; Preciado, S.; Lavilla, R. Chromenopyridines: Promising Scaffolds for Medicinal and Biological Chemistry. Curr. Med. Chem. 2011, 18, 4761–4785. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Durães, F.; Maia, M.; Sousa, E.; Pinto, M.M.M. Recent advances in the synthesis of xanthones and azaxanthones. Org. Chem. Front. 2020, 7, 3027–3066. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A.; Sioumis, A.A.; Wunderlich, J.A. Alkaloids of a new type from Elaeocarpus polydactylus Schl. (family elaeocarpaceae). Chem. Commun. 1968, 6, 290–291. [Google Scholar] [CrossRef]
- Johns, S.; Lamberton, J.; Sioumis, A.; Willing, R. Elaeocarpus alkaloids. I. The structures of (±)-elaeocarpine, (±)-isoelaeocarpine, and (±)-isoelaeocarpicine, three new indolizidine alkaloids from Elaeocarpus polydactylus. Aust. J. Chem. 1969, 22, 775. [Google Scholar] [CrossRef]
- Johns, S.; Lamberton, J.; Sioumis, A. Elaeocarpus alkaloids. II. (+)-Elaeocarpiline and (-)-isoelaeocarpiline, new indolizidine alkaloids from Elaeocarpus dolichostylis. Aust. J. Chem. 1969, 22, 793. [Google Scholar] [CrossRef]
- Johns, S.R.; Lamberton, J.A.; Sioumis, A.A.; Suares, H.; Willing, R.I. The structures and absolute configurations of seven alkaloids from Elaeocarpus sphaericus. J. Chem. Soc. D 1970, 13, 804–805. [Google Scholar] [CrossRef]
- Johns, S.; Lamberton, J.; Sioumis, A.; Suares, H.; Willing, R.I. Elaeocarpus alkaloids. IV. The alkaloids of Elaeocarpus sphaericus. Aust. J. Chem. 1971, 24, 1679. [Google Scholar] [CrossRef]
- Ray, A.B.; Chand, L.; Pandey, V.B. Rudrakine, a new alkaloid from Elaeocarpus ganitrus. Phytochemistry 1979, 18, 700–701. [Google Scholar] [CrossRef]
- Katavic, P.L.; Venables, D.A.; Forster, P.I.; Guymer, G.; Carroll, A.R. Grandisines C−G, Indolizidine Alkaloids from the Australian Rainforest Tree Elaeocarpus grandi s. J. Nat. Prod. 2006, 69, 1295–1299. [Google Scholar] [CrossRef]
- Katavic, P.L.; Venables, D.A.; Rali, T.; Carroll, A.R. Indolizidine Alkaloids with δ-Opioid Receptor Binding Affinity from the Leaves of Elaeocarpus fuscoides. J. Nat. Prod. 2007, 70, 872–875. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Mo, J.; Zhang, J.; Gan, L.S. Optical Resolution and Structure Determination of New Indolizidine Alkaloids from Elaeocarpus sphaericus. Helv. Chim. Acta 2011, 94, 347–354. [Google Scholar] [CrossRef]
- Hong, W.; Zhang, Y.; Yang, J.; Xia, M.Y.; Luo, J.F.; Li, X.N.; Wang, Y.H.; Wang, J.S. Alkaloids from the Branches and Leaves of Elaeocarpus angustifolius. J. Nat. Prod. 2019, 82, 3221–3226. [Google Scholar] [CrossRef]
- Payne, D.J.; Hueso-Rodríguez, J.A.; Boyd, H.; Concha, N.O.; Janson, C.A.; Gilpin, M.; Bateson, J.H.; Cheever, C.; Niconovich, N.L.; Pearson, S.; et al. Identification of a Series of Tricyclic Natural Products as Potent Broad-Spectrum Inhibitors of Metallo-β-Lactamases. Antimicrob. Agents Chemother. 2002, 46, 1880–1886. [Google Scholar] [CrossRef]
- Gan, M.; Liu, Y.; Bai, Y.; Guan, Y.; Li, L.; Gao, R.; He, W.; You, X.; Li, Y.; Yu, L.; et al. Polyketides with New Delhi Metallo-β-lactamase 1 Inhibitory Activity from Penicillium sp. J. Nat. Prod. 2013, 76, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yu, J.; Chen, S.; Ding, M.; Huang, X.; Yuan, J.; She, Z. Alkaloids from the mangrove endophytic fungus Diaporthe phaseolorum SKS019. Bioorg. Med. Chem. Lett. 2017, 27, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Phutthacharoen, K.; Khalid, S.J.; Schrey, H.; Ding, M.; Huang, X.; Yuan, J.; She, Z. Diaporphasines E and F: New Polyketides from the Saprotrophic Fungus Lachnum sp. IW157 Growing on the Reed Grass Phragmites communis. ACS Omega 2023, 8, 41689–41695. [Google Scholar] [CrossRef]
- Ding, B.; Wang, Z.; Xia, G.; Huang, X.; Xu, F.; Chen, W.; She, Z. Three New Chromone Derivatives Produced by Phomopsis sp. HNY29-2B from Acanthus ilicifolius Linn. Chin. J. Chem. 2017, 35, 1889–1893. [Google Scholar] [CrossRef]
- Wei, C.; Sun, C.; Feng, Z.; Zhang, X.; Xu, J. Four New Chromones from the Endophytic Fungus Phomopsis asparagi DHS-48 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2021, 19, 348. [Google Scholar] [CrossRef]
- Xing, D.-X.; Song, X.-S.; Pan, W.-C.; Cui, H.; Zhao, Z.X. New chromone compounds from the marine derived fungus Diaporthe sp. XW12-1. Fitoterapia 2023, 164, 105384. [Google Scholar] [CrossRef]
- Hu, Q.F.; Wu, F.; Zhou, T.; Zhou, M.; Zhu, Y.N.; Cai, B.B.; Liu, M.X.; Li, M.F.; Yang, G.Y.; Li, Y.K. Three New Anti-Rotavirus Chromeno[3,2-c]pyridines from the Whole Plant of Thalictrum scabrifolium. Heterocycles 2021, 102, 1810. [Google Scholar] [CrossRef]
- Yin, G.-Y.; Zhu, Y.-N.; Wu, F.; Mi, Q.L.; Shi, J.Q.; Gao, Q.; Zhu, L.C.; Zhou, T.; Li, J.; Liu, X.; et al. Two New Antibacterial Chromeno[3,2-c]Pyridine Alkaloids from Whole Plants of Thalictrum scabrifolium. Chem. Nat. Compd. 2022, 58, 506–510. [Google Scholar] [CrossRef]
- Hu, Q.-F.; Zhang, L.-F.; Liu, M.-X.; Cai, B.-B.; Li, Y.; Zhou, T.; Li, M.-F.; Wang, H.-S.; Xu, Y.; Kong, W.-S.; et al. Two New Chromeno[3,2-c]Pyridine Derivatives from the Whole Plants of Thalictrum finetii and Their Antirotavirus Activity. Chem. Nat. Compd. 2022, 58, 511–515. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Lin, Z.-L.; Zhao, G.-K.; Zhou, M.; Yao, H.; Zhang, G.H.; Li, W.; Yang, G.Y.; Li, Y.K.; Hu, Q.F.; et al. Two New Anti-Tobacco Mosaic Virus Alkaloids from the Whole Plants of Thalictrum microgynum. Chem. Nat. Compd. 2022, 58, 699–703. [Google Scholar] [CrossRef]
- Wu, Y.-P.; Zhao, G.-K.; Liu, Z.-Y.; Tan, T.; Li, Z.M.; Zhou, M.; Yao, H.; Li, Y.K.; Wang, W.G.; Hu, Q.F.; et al. Antiviral Chromone Alkaloids from the Cigar Tobacco Leaves Derived Endophytic Fungus Aspergillus lentulus. Chem. Nat. Compd. 2023, 59, 721–725. [Google Scholar] [CrossRef]
- Kruger, S.; Mann, F.G. Xanthones and thioxanthones. Part VI. The preparation and properties of 9-thia-3-aza-anthrone. J. Chem. Soc. 1955, 2755–2763. [Google Scholar] [CrossRef]
- Bloomfield, D.G.; Partridge, M.W.; Vipond, H.J. Cyclic amidines. Part XXIII. Dibenzo[b,h][1]benzopyrano[2,3,4-de][1,6]naphthyridines and their molecular orientation in carcinogenesis. J. Chem. Soc. C 1970, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Villani, F.J.; Mann, T.A.; Wefer, E.A.; Hannon, J.; Larca, L.L.; Landon, M.J.; Spivak, W.; Vashi, D.; Tozzi, S. Benzopyranopyridine derivatives. 1. Aminoalkyl derivatives of the azaxanthenes as bronchodilating agents. J. Med. Chem. 1975, 18, 1–8. [Google Scholar] [CrossRef]
- Khodair, A.I.; Abbasi, Μ.M.A.; Ibrahim, E.-S.I.; Soliman, A.H.; El-Ashry, E.S.H. Synthesis of substituted quinolines and heterocyclo[x,y-c]quinolines by the nucleophilic substitution and rearrangements of 4-chloro-2-methyl-3-nitroquinolines. Heterocycl. Commun. 1999, 5, 577–584. [Google Scholar] [CrossRef]
- Okada, E.; Hatakenaka, M.; Kuratani, M.; Mori, T.; Ashida, T. A Facile and Convenient Synthetic Method for Fluorine-Containing Dibenzo[b,h][1,6]naphthyridines, Thiochromeno[3,2-c]quinolines, and Chromeno[3,2-c]quinolines. Heterocycles 2014, 88, 799. [Google Scholar] [CrossRef]
- Hong, F.; Lu, N.; Lu, B.; Cheng, J. Synthesis of Fused Heterocycles via One-pot Oxidative O-Arylation, Pd-Catalyzed C(sp3)-H Arylation. Adv. Synth. Catal. 2017, 359, 3299–3303. [Google Scholar] [CrossRef]
- Kardile, R.A.; Sarkate, A.P.; Borude, A.S.; Mane, R.S.; Lokwani, D.K.; Tiwari, S.V.; Azad, R.; Burra, P.V.L.S.; Thopate, S.R. Design and synthesis of novel conformationally constrained 7,12-dihydrodibenzo[b,h][1,6] naphthyridine and 7H-Chromeno[3,2-c] quinoline derivatives as topoisomerase I inhibitors: In vitro screening, molecular docking and ADME predictions. Bioorg. Chem. 2021, 115, 105174. [Google Scholar] [CrossRef]
- Chen, D.; Tan, X.; Chen, W.; Liu, Y.; Li, C.; Wu, J.; Zheng, J.; Shen, H.C.; Zhang, M.; Wu, W.; et al. Discovery of Novel cccDNA Reducers toward the Cure of Hepatitis B Virus Infection. J. Med. Chem. 2022, 65, 10938–10955. [Google Scholar] [CrossRef]
- Sliwa, H.; Cordonnier, C. Synthesis of new fundamental heterocycles. Part VII. Synthesis of 2-azaxanthene. J. Heterocycl. Chem. 1977, 14, 169–170. [Google Scholar] [CrossRef]
- Cordonnier, G.; Sliwa, H. A new route to 6-azachromones. An improved synthesis of 2-azaxanthone. J. Heterocycl. Chem. 1987, 24, 111–115. [Google Scholar] [CrossRef]
- Kulikova, L.N.; Borisov, R.S.; Voskressensky, L.G. Ring opening in 1,2,3,4-tetrahydrochromeno[3,2-c]pyridines under the action of electron-deficient alkynes. Mendeleev Commun. 2017, 27, 640–641. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Boltneva, N.P.; Lushchekina, S.V.; Rudakova, E.V.; Serebryakova, O.G.; Kulikova, L.N.; Beloglazkin, A.A.; Borisov, R.S.; Richardson, R.J. Synthesis, molecular docking, and biological activity of 2-vinyl chromones: Toward selective butyrylcholinesterase inhibitors for potential Alzheimer’s disease therapeutics. Bioorg. Med. Chem. 2018, 26, 4716–4725. [Google Scholar] [CrossRef]
- Purgatorio, R.; Kulikova, L.N.; Pisani, L.; Catto, M.; Candia, M.; Carrieri, A.; Cellamare, S.; De Palma, A.; Beloglazkin, A.A.; Raesi, G.R.; et al. Scouting around 1,2,3,4-Tetrahydrochromeno[3,2-c]pyridin-10-ones for Single- and Multitarget Ligands Directed towards Relevant Alzheimer’s Targets. ChemMedChem 2020, 15, 1947–1955. [Google Scholar] [CrossRef]
- Kulikova, L.N.; Purgatorio, R.; Beloglazkin, A.A.; Tafeenko, V.A.; Reza, R.G.; Levickaya, D.D.; Sblano, S.; Boccarelli, A.; de Candia, M.; Catto, M.; et al. Chemical and Biological Evaluation of Novel 1H-Chromeno[3,2-c]pyridine Derivatives as MAO Inhibitors Endowed with Potential Anticancer Activity. Int. J. Mol. Sci. 2023, 24, 7724. [Google Scholar] [CrossRef]
- Marsais, F.; Trécourt, F.; Bréant, P.; Quéguiner, G. Directed lithiation of 4-halopyridines: Chemoselectivity, regioselectivity and application to synthesis. J. Heterocycl. Chem. 1988, 25, 81–87. [Google Scholar] [CrossRef]
- Marquise, N.; Harford, P.J.; Chevallier, F.; Roisnel, T.; Dorcet, V.; Gagez, A.-L.; Sablé, S.; Picot, L.; Thiéry, V.; Wheatley, A.E.H.; et al. Synthesis of azafluorenones and related compounds using deprotocupration–aroylation followed by intramolecular direct arylation. Tetrahedron 2013, 69, 10123–10133. [Google Scholar] [CrossRef]
- El-sayed, A.M.; Abd Allah, O.A. Synthetic and biological studies on coumarin hydrazone derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2001, 170, 75–86. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Synthesis of Some New Fused and Substituted Chromenes. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2709–2723. [Google Scholar] [CrossRef]
- Sumesh, R.V.; Malathi, A.; Ranjith Kumar, R. A facile tandem Michael addition/O-cyclization/elimination route to novel chromeno[3,2-c]pyridines. Mol. Divers. 2015, 19, 233–249. [Google Scholar] [CrossRef]
- Shen, J.-C.; Jin, R.-Z.; Yuan, K.; Zhang, M.M.; Wang, X.S. A Green Synthesis of Fused Polycyclic 5H-Chromeno[3,2-c]quinoline-6,8(7H,9H)-dione Derivatives Catalyzed by TsOH in Ionic Liquids. Polycycl. Aromat. Compd. 2016, 36, 758–772. [Google Scholar] [CrossRef]
- Kamali, M.; Keramat Pirolghor, F. One-pot three-component synthesis of novel chromeno[3,2-c]pyridine-1,9(2H)-diones by using SNCL2·2H2O as catalyst. J. Heterocycl. Chem. 2022, 59, 655–663. [Google Scholar] [CrossRef]
- Orang, N.S.; Soltani, H.; Ghiamirad, M.; Sabegh, M.A. Para toluenesulfonic acid-catalyzed one-pot, three-component synthesis of benzo[5,6]chromeno[3,2-c]quinoline compounds in aqueous medium. Heterocycl. Commun. 2021, 27, 90–99. [Google Scholar] [CrossRef]
- Sadeghpour Orang, N.; Ghiami Rad, M.; Soltani, H.; Ahmadi Sabegh, M. Synthesis and evaluation of the antibacterial activity of benzo [5,6] chromeno[3,2-c] quinoline derivatives. Int. J. Mol. Clin. Microbiol. 2022, 12, 1713–1721. [Google Scholar] [CrossRef]
- Chithanna, S.; Yang, D.-Y. Intramolecular Diels–Alder Cycloaddition of Furan-Derived β-Enamino Diketones: An Entry to Diastereoselective Synthesis of Polycyclic Pyrano[3,2- c ]quinolin-5-one Derivatives. J. Org. Chem. 2022, 87, 5178–5187. [Google Scholar] [CrossRef] [PubMed]
- Briet, P.; Berthelon, J.-J.; Depin, J.-C. Antidepressant Substituted Hexahydro Benzopyrano [3,2-c] Pyridines. U.S. Patent 4,201,783, 6 May 1980. [Google Scholar]
- Lin, K.-S.; Ding, Y.-S. Synthesis and C-11 labeling of three potent norepinephrine transporter selective ligands ((R)-nisoxetine, lortalamine, and oxaprotiline) for comparative PET studies in baboons. Bioorg. Med. Chem. 2005, 13, 4658–4666. [Google Scholar] [CrossRef]
- Munari, I.; Traldi, P.; Biala, J.; Czarnocki, Z. Characterization of stereoisomeric alkaloids by electrospray ionization tandem mass spectrometry. Rapid Comm. Mass. Spectrom. 2001, 15, 889–892. [Google Scholar] [CrossRef]
- Biała, J.; Czarnocki, Z.; Maurin, J.K. Diastereoselective synthesis of lortalamine analogs. Tetrahedron Asymmetry 2002, 13, 1021–1023. [Google Scholar] [CrossRef]
- Pawłowska, J.; Krawczyk, K.K.; Wojtasiewicz, K.; Maurin, J.K.; Czarnocki, Z. (S)-(−)-α-Methylbenzylamine as chiral auxiliary in the synthesis of (+)-lortalamine. Monatsh. Chem. 2009, 140, 83–86. [Google Scholar] [CrossRef]
- Ghosh, C.K.; Pal, C.; Maiti, J.; Sarkar, M. Benzopyrans. Part 23. Nitrogen heterocycles fused with or linked to 1-benzopyran from 3-acyl-2-methyl-1-benzopyran-4-one. J. Chem. Soc. Perkin Trans. 1988, 1, 1489. [Google Scholar] [CrossRef]
- Ghosh, C.K.; Karak, S.K.; Patra, A. Benzopyrans. Part 47. Reactions of 3-(β-Dimethylaminoacryloyl)-1-benzopyran-4-one with Some Nitrogen Nucleophiles. ChemInform 2005, 36, chin.200517040. [Google Scholar] [CrossRef]
- Rajagopal, R.; Shenoy, V.U.; Padmanabhan, S.; Sequeira, S.; Seshadri, S. Synthesis of fluorescent 2, 3-fused coumarin derivatives. Dye. Pigment. 1990, 13, 167–175. [Google Scholar] [CrossRef]
- Plaskon, A.S.; Ryabukhin, S.V.; Volochnyuk, D.M.; Gavrilenko, K.S.; Shivanyuk, A.N.; Tolmachev, A.A. Synthesis of Quinolines from 3-Formylchromone. J. Org. Chem. 2008, 73, 6010–6013. [Google Scholar] [CrossRef]
- Wittstein, K.; García, A.; Schürmann, M.; Kumar, K. Exploring α-Chromonyl Nitrones as 1,5-Dipoles. Synlett 2012, 2012, 227–232. [Google Scholar] [CrossRef][Green Version]
- Ghosh, J.; Biswas, P.; Maiti, S.; Sarkar, T.; Drew, M.G.B.; Bandyopadhyay, C. Ligand-free palladium-catalyzed intramolecular arylation of chromones: An expedient synthesis of 1-benzopyrano[3,2-c]quinolines. Tetrahedron Lett. 2013, 54, 2221–2225. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Dinakaran, M.; Banerjee, D.; Bhat, P.; Gadhwal, S. Discovery of novel antitubercular 2,10-dihydro-4aH-chromeno[3,2-c]pyridin-3-yl derivatives. Eur. J. Med. Chem. 2010, 45, 120–123. [Google Scholar] [CrossRef]
- Popova, A.V.; Mrug, G.P.; Kondratyuk, K.M.; Bondarenko, S.P.; Frasinyuk, M.S. New Heterocyclic Pyrano[2′,3′:5,6]Chromeno[3,2-c]Pyridin-4-Ones and Furo[2′,3′:5,6]Chromeno[3,2-c]Pyridin-3(2H)-Ones Synthesized Via a Hetero-Diels–Alder Reaction. Chem. Nat. Compd. 2016, 52, 1000–1004. [Google Scholar] [CrossRef]
- Abdelkhalik, M.M.; Negm, A.M.; Elkhouly, A.I.; Elnagdi, M.H. Studies with condensed amino-thiophenes: Further investigation of reactivity of amino-thieno-coumarines and amino-thieno-benzo[h]coumarines toward electron-poor olefins and acetylenes. Heteroat. Chem. 2004, 15, 502–507. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, M.; Hu, F.; Hu, Y. Direct Synthesis of Functional Azaxanthones by Using a Domino Three-Component Reaction. Org. Lett. 2012, 14, 3206–3209. [Google Scholar] [CrossRef]
- Yokosaka, T.; Shiga, N.; Nemoto, T.; Hamada, Y. Construction of Divergent Fused Heterocycles via an Acid-Promoted Intramolecular ipso-Friedel–Crafts Alkylation of Phenol Derivatives. J. Org. Chem. 2014, 79, 3866–3875. [Google Scholar] [CrossRef]
- Kim, Y.; Moon, Y.; Kang, D.; Hong, S. Synthesis of heterocyclic-fused benzopyrans via the Pd(II)-catalyzed C–H alkenylation/C–O cyclization of flavones and coumarins. Org. Biomol. Chem. 2014, 12, 3413–3422. [Google Scholar] [CrossRef]
- Bouchama, A.; Hassaine, R.; Talhi, O.; Taibi, N.; Mendes, R.F.; Paz, F.A.A.; Bachari, K.; Silva, A.M.S. Diastereoselective One-Pot Tandem Synthesis of Chromenopyridodiazepinones through 1,4- and 1,6-Aza-Conjugate Additions/Heterocyclizations. Synlett 2018, 29, 885–889. [Google Scholar] [CrossRef]
- Kumar, T.U.; Roy, D.; Bhattacharya, A. Iron(III) catalyzed direct C–H functionalization at the C-3 position of chromone for the synthesis of fused chromeno-quinoline scaffolds. Tetrahedron Lett. 2019, 60, 1895–1898. [Google Scholar] [CrossRef]
- Kawai, S.; Takashima, S.; Ando, M.; Shintaku, S.; Takeda, S.; Otake, K.; Ito, Y.; Fukui, M.; Yamamoto, M.; Shoji, Y.; et al. Discovery of Novel Chromenopyridine Derivatives as Readthrough-Inducing Drugs. Chem. Pharm. Bull. 2023, 71, 859–878. [Google Scholar] [CrossRef] [PubMed]
- Unangst, P.C.; Capiris, T.; Connor, D.T.; Heffner, T.G.; MacKenzie, R.G.; Miller, S.R.; Pugsley, T.A.; Wise, L.D. Chromeno[3,4-c]pyridin-5-ones: Selective Human Dopamine D4 Receptor Antagonists as Potential Antipsychotic Agents. J. Med. Chem. 1997, 40, 2688–2693. [Google Scholar] [CrossRef] [PubMed]
- Oset-Gasque, M.J.; González, M.P.; Pérez-Peña, J.; García-Font, N.; Romero, A.; del Pino, J.; Ramos, E.; Hadjipavlou-Litina, D.; Soriano, E.; Chioua, M.; et al. Toxicological and pharmacological evaluation, antioxidant, ADMET and molecular modeling of selected racemic chromenotacrines {11-amino-12-aryl-8,9,10,12-tetrahydro-7H-chromeno[2,3-b]quinolin-3-ols} for the potential prevention and treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 74, 491–501. [Google Scholar]
- Dawane, B.S.; Konda, S.G.; Bodade, R.G.; Bhosale, R.B. An efficient one-pot synthesis of some new 2,4-diaryl pyrido[3,2-c]coumarins as potent antimicrobial agents. J. Heterocycl. Chem. 2010, 47, 237–241. [Google Scholar] [CrossRef]
- Patel, A.A.; Lad, H.B.; Pandya, K.R.; Patel, C.V.; Brahmbhatt, D.I. Synthesis of a new series of 2-(2-oxo-2H-chromen-3-yl)-5H-chromeno[4,3-b]pyridin-5-ones by two facile methods and evaluation of their antimicrobial activity. Med. Chem. Res. 2013, 22, 4745–4754. [Google Scholar] [CrossRef]
- Patel, M.A.; Bhila, V.G.; Patel, N.H.; Patel, A.K.; Brahmbhatt, D.I. Synthesis, characterization and biological evaluation of some pyridine and quinoline fused chromenone derivatives. Med. Chem. Res. 2012, 21, 4381–4388. [Google Scholar] [CrossRef]
- Li, Z.; Mu, G.; Chen, W.; Gao, L.; Jhanji, V.; Wang, L. Comparative Evaluation of Topical Pranoprofen and Fluorometholone in Cases with Chronic Allergic Conjunctivitis. Cornea 2013, 32, 579. [Google Scholar] [CrossRef]
- Makino, H.; Saijo, T.; Ashida, Y.; Kuriki, H.; Maki, Y. Mechanism of Action of an Antiallergic Agent, Amlexanox (AA-673), in Inhibiting Histamine Release from Mast Cells: Acceleration of cAMP Generation and Inhibition of Phosphodiesterase. Int. Arch. Allergy Appl. Immunol. 2009, 82, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Tsuruoka, S.; Kanai, Y.; Endou, H.; Saito, K.; Miyamoto, E.; Fujimura, A. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur. J. Pharmacol. 2008, 596, 166–172. [Google Scholar] [CrossRef]
- Banerjee, S.; Wang, J.; Pfeffer, S.; Ma, D.; Pfeffer, L.M.; Patil, S.A.; Li, W.; Miller, D.D. Design, Synthesis and Biological Evaluation of Novel 5H-Chromenopyridines as Potential Anti-Cancer Agents. Molecules 2015, 20, 17152–17165. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, S.; Pontes, O.; Lopes, D.; Sampaio-Marques, B.; Costa, M.D.; Carvalho, L.; Gonçalves, C.S.; Costa, B.M.; Maciel, P.; Ludovico, P.; et al. Unravelling the anticancer potential of functionalized chromeno[2,3-b]pyridines for breast cancer treatment. Bioorg. Chem. 2020, 100, 103942. [Google Scholar] [CrossRef] [PubMed]
- Mulakayala, N.; Rambabu, D.; Raja, M.R.; Chaitanya, M.; Kumar, C.S.; Kalle, A.M.; Krishna, G.R.; Reddy, C.M.; Rao, M.V.B.; Pal, M. Ultrasound mediated catalyst free synthesis of 6H-1-benzopyrano[4,3-b]quinolin-6-ones leading to novel quinoline derivatives: Their evaluation as potential anti-cancer agents. Bioorg. Med. Chem. 2012, 20, 759–768. [Google Scholar] [CrossRef]
- Thapa, P.; Jun, K.-Y.; Kadayat, T.M.; Park, C.; Zheng, Z.; Magar, T.B.T.; Bist, G.; Shrestha, A.; Na, Y.; Kwon, Y.; et al. Design and synthesis of conformationally constrained hydroxylated 4-phenyl-2-aryl chromenopyridines as novel and selective topoisomerase II-targeted antiproliferative agents. Bioorg. Med. Chem. 2015, 23, 6454–6466. [Google Scholar] [CrossRef]
- Hamama, W.S.; Ibrahim, M.E.; Metwalli, A.E.; Zoorob, H.H. New synthetic approach to coumarino[4,3-b]pyridine systems and potential cytotoxic evaluation. Med. Chem. Res. 2014, 23, 2615–2621. [Google Scholar] [CrossRef]
- Tian, S.; Huang, Z.; Meng, Q.; Liu, Z. Multi-target drug design of anti-alzheimer’s disease based on tacrine. Mini Rev. Med. Chem. 2021, 21, 2039–2064. [Google Scholar] [CrossRef]
- Sameem, B.; Saeedi, M.; Mahdavi, M.; Shafiee, A. A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 128, 332–345. [Google Scholar] [CrossRef]
- Depin, J.C.; Betbeder-Matibet, A.; Bonhomme, Y.; Muller, A.J.; Berthelon, J.-J. Pharmacology of lortalamine, a new potent non-tricyclic antidepressant. Arzneim. Forsch. 1985, 35, 1655–1662. [Google Scholar]
- Ding, Y.S.; Lin, K.S.; Logan, J. PET imaging of norepinephrine transporters. Curr. Pharm. Des. 2006, 12, 3831–3845. [Google Scholar] [CrossRef] [PubMed]






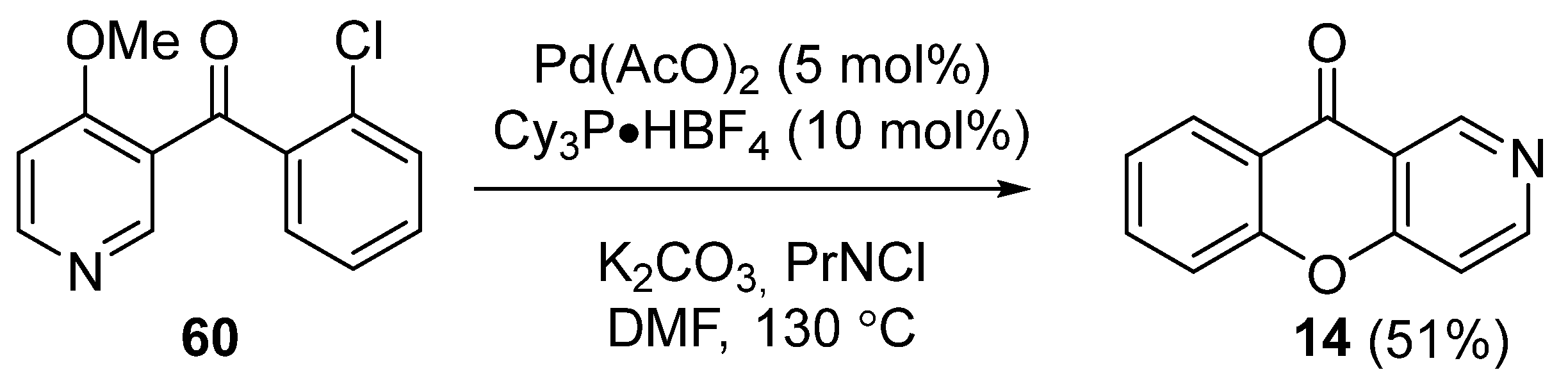




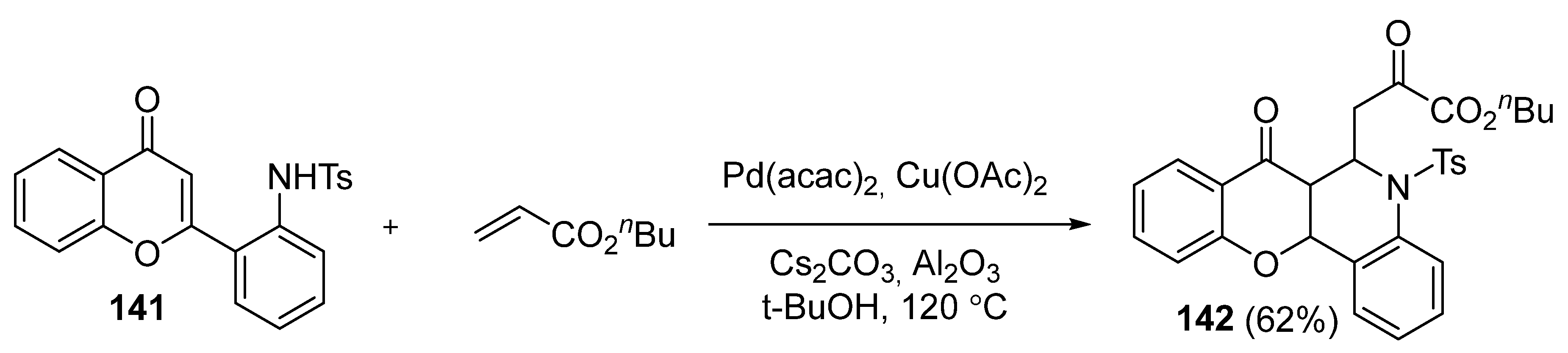

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Listratova, A.V.; Borisov, R.S.; Polovkov, N.Y.; Kulikova, L.N. Synthesis and Biological Activity of Chromeno[3,2-c]Pyridines. Molecules 2024, 29, 4997. https://doi.org/10.3390/molecules29214997
Listratova AV, Borisov RS, Polovkov NY, Kulikova LN. Synthesis and Biological Activity of Chromeno[3,2-c]Pyridines. Molecules. 2024; 29(21):4997. https://doi.org/10.3390/molecules29214997
Chicago/Turabian StyleListratova, Anna V., Roman S. Borisov, Nikolay Yu. Polovkov, and Larisa N. Kulikova. 2024. "Synthesis and Biological Activity of Chromeno[3,2-c]Pyridines" Molecules 29, no. 21: 4997. https://doi.org/10.3390/molecules29214997
APA StyleListratova, A. V., Borisov, R. S., Polovkov, N. Y., & Kulikova, L. N. (2024). Synthesis and Biological Activity of Chromeno[3,2-c]Pyridines. Molecules, 29(21), 4997. https://doi.org/10.3390/molecules29214997






