Layered Double Hydroxides as Systems for Capturing Small-Molecule Air Pollutants: A Density Functional Theory Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lattice Parameter Analysis
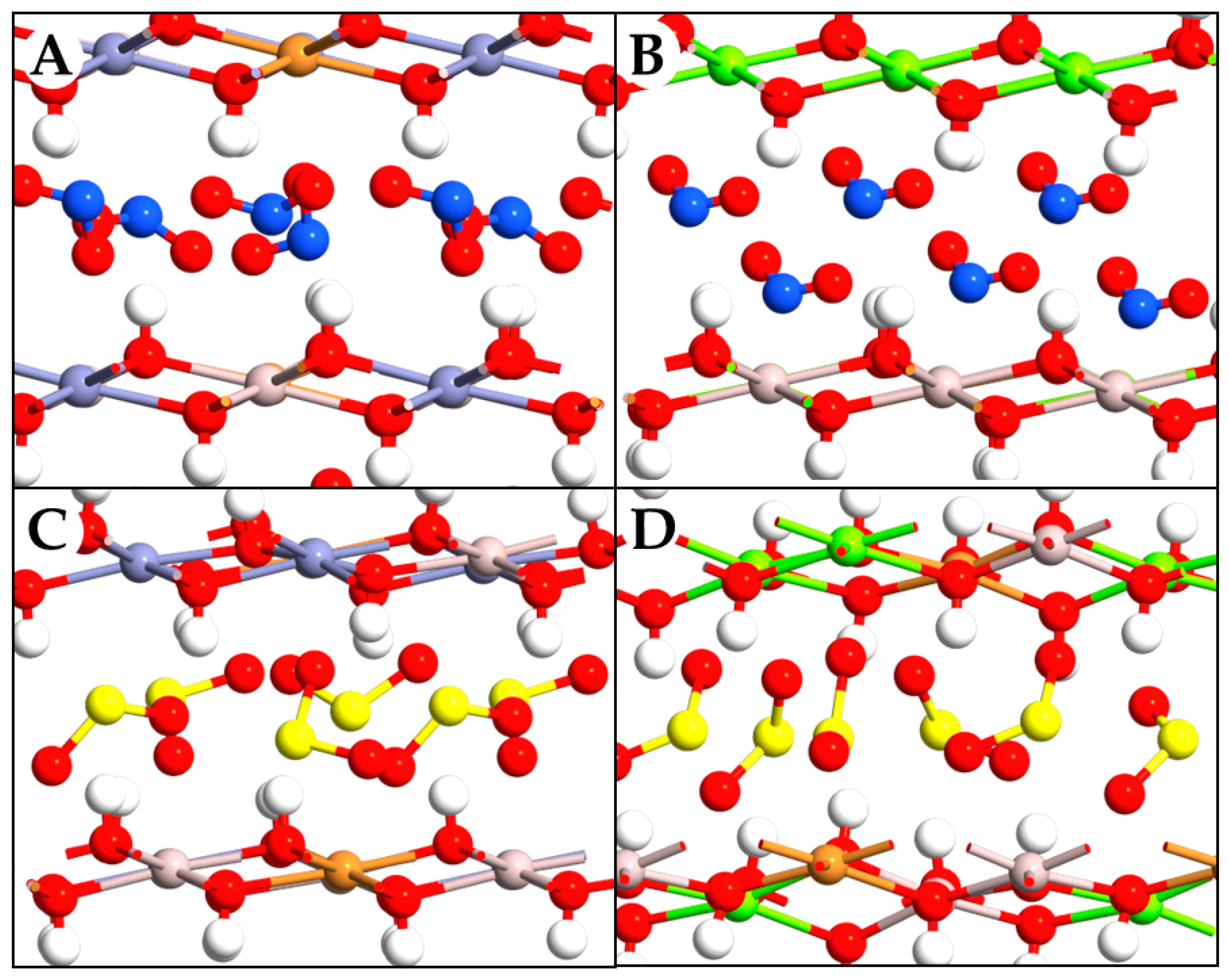
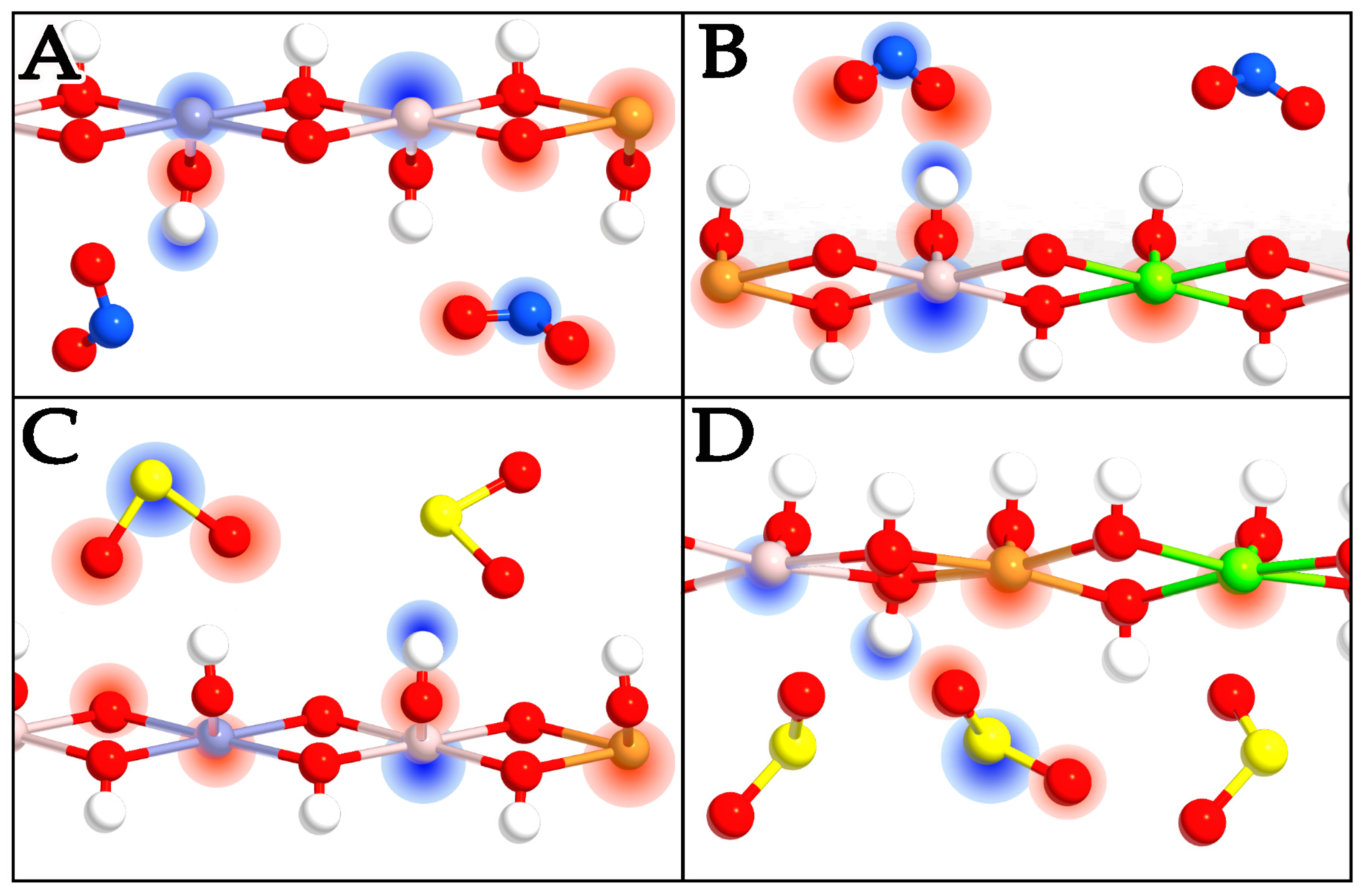
2.2. Intercalation Effects of NO2 and SO2
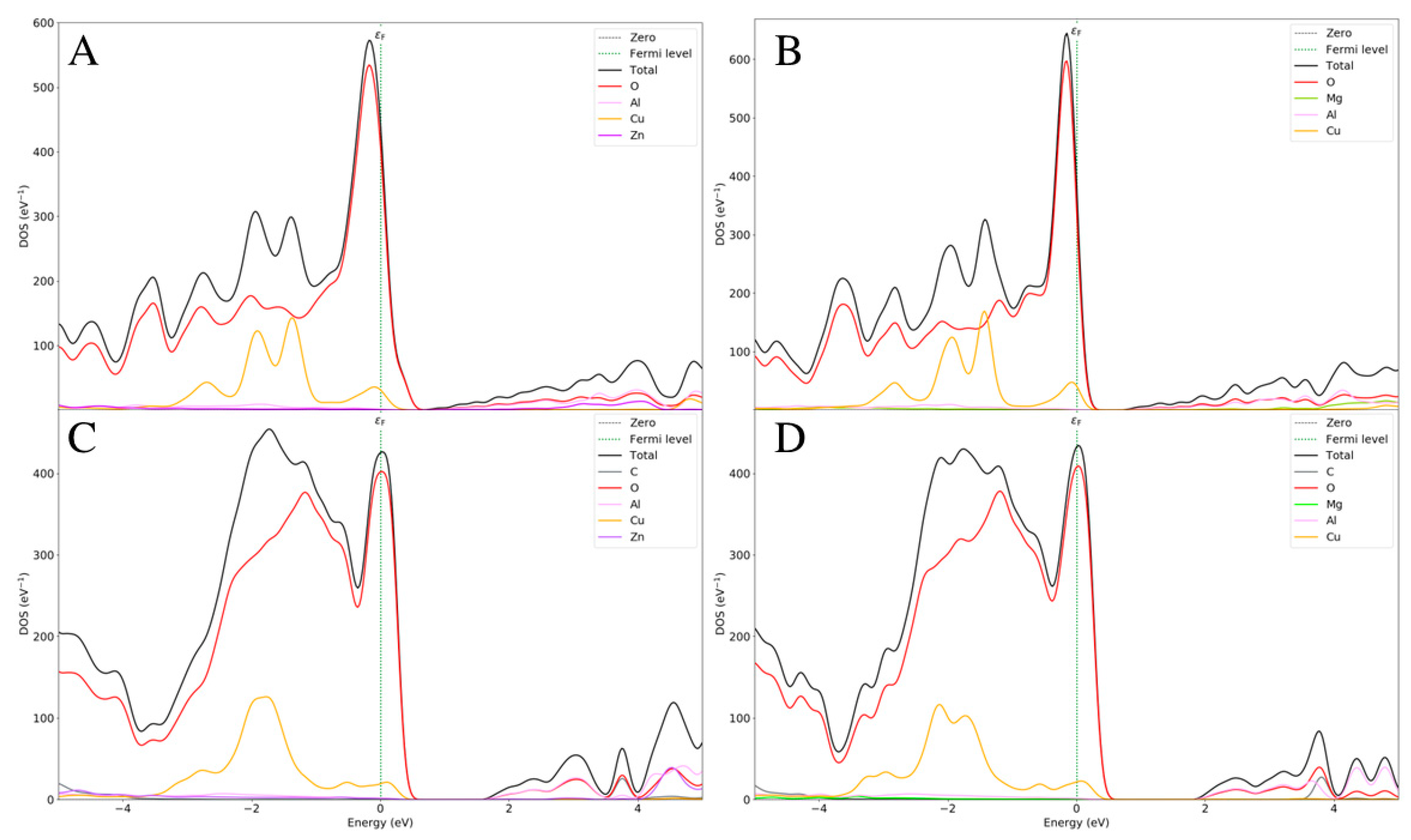
2.3. Electronic Properties
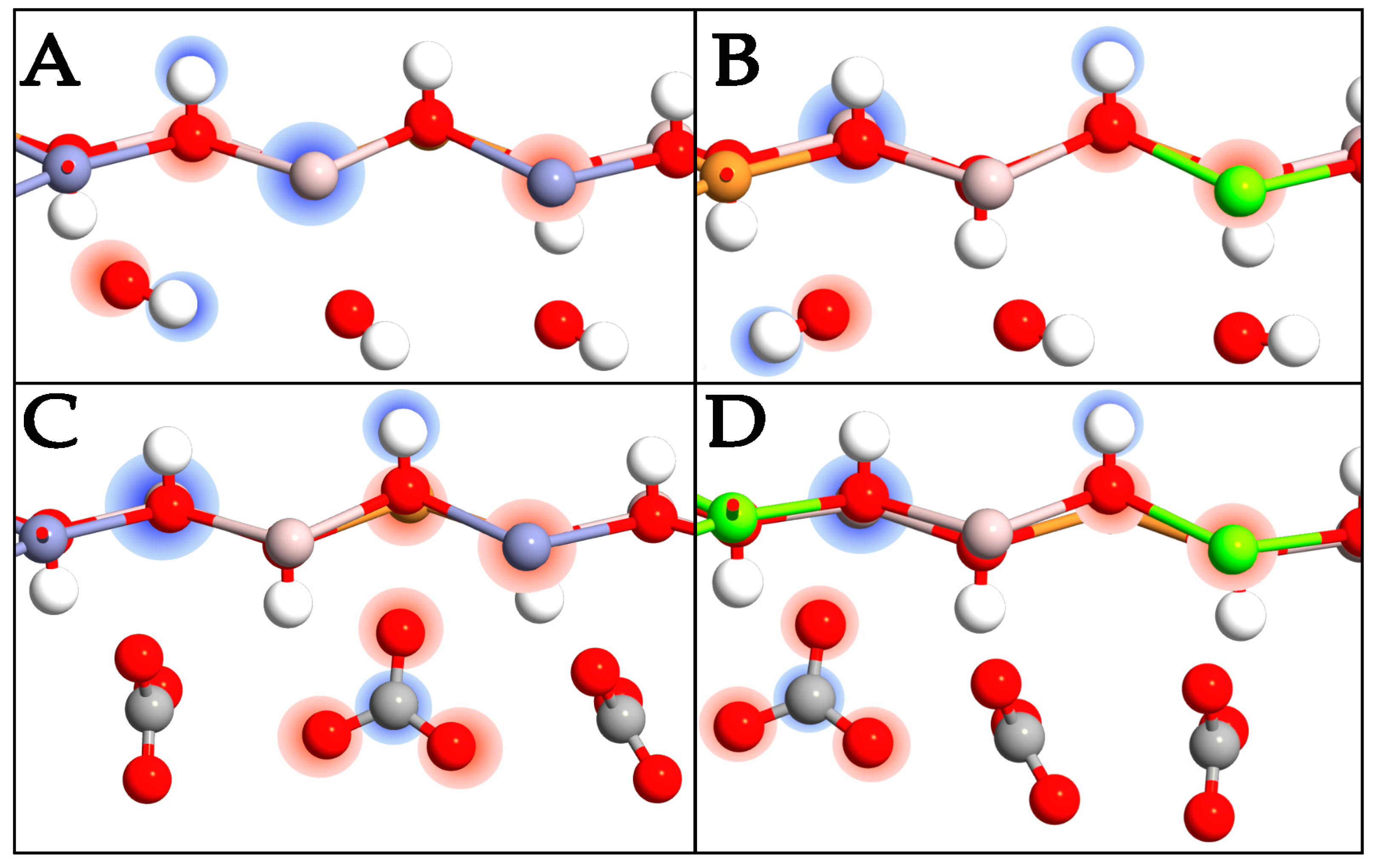
2.4. Charge Transfer
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhui, K.; Newbury, J.B.; Latham, R.M.; Ucci, M.; Nasir, Z.A.; Turner, B.; O’Leary, C.; Fisher, H.L.; Marczylo, E.; Douglas, P.; et al. Air Quality and Mental Health: Evidence, Challenges and Future Directions. BJPsych Open 2023, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, J.; Leonova, N. Global Air Pollution Exposure and Poverty. Nat. Commun. 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans–International Agency for Research on Cancer. Available online: https://monographs.iarc.who.int/ (accessed on 25 September 2024).
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the Combustion of Solid Biomass Fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ristovski, Z.; Milic, A.; Gu, Y.; Islam, M.S.; Wang, S.; Hao, J.; Zhang, H.; He, C.; et al. A Review of Biomass Burning: Emissions and Impacts on Air Quality, Health and Climate in China. Sci. Total Environ. 2017, 579, 1000–1034. [Google Scholar] [CrossRef]
- Karanasiou, A.; Viana, M.; Querol, X.; Moreno, T.; de Leeuw, F. Assessment of Personal Exposure to Particulate Air Pollution during Commuting in European Cities—Recommendations and Policy Implications. Sci. Total Environ. 2014, 490, 785–797. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and Policies to Decarbonize Global Industry: Review and Assessment of Mitigation Drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Allen, O.; Knight, M.M.; Verbruggen, S.W. Air Pollution and Osteoporosis. Curr. Osteoporos. Rep. 2024, 1–9. [Google Scholar] [CrossRef]
- Sellaro, F.; Pernetti, R.; Oddone, E. Early Biological Effects in Outdoor Workers Exposed to Urban Air Pollution: A Systematic Review. Environ. Pollut. 2024, 362, 124985. [Google Scholar] [CrossRef]
- Liu, M.; Shang, F.; Lu, X.; Huang, X.; Song, Y.; Liu, B.; Zhang, Q.; Liu, X.; Cao, J.; Xu, T.; et al. Unexpected Response of Nitrogen Deposition to Nitrogen Oxide Controls and Implications for Land Carbon Sink. Nat Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L.; Tronconi, E. Encyclopedia of Catalysis; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. NH3–NO/NO2 Chemistry over V-Based Catalysts and Its Role in the Mechanism of the Fast SCR Reaction. Catal. Today 2006, 114, 3–12. [Google Scholar] [CrossRef]
- Biggeri, A.; Baccini, M.; Bellini, P.; Terracini, B. Meta-Analysis of the Italian Studies of Short-Term Effects of Air Pollution (MISA), 1990–1999. Int. J. Occup. Environ. Health 2005, 11, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, R.B. Sulfur Oxides. Compr. Toxicol. Second. Ed. 2010, 8, 277–290. [Google Scholar] [CrossRef]
- Maynard, D.; Coull, B.A.; Gryparis, A.; Schwartz, J. Mortality Risk Associated with Short-Term Exposure to Traffic Particles and Sulfates. Environ. Health Perspect. 2007, 115, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Feng, W.Y.; Broadwin, R.; Green, S.; Lipsett, M. The Effects of Components of Fine Particulate Air Pollution on Mortality in California: Results from CALFINE. Environ. Health Perspect. 2007, 115, 13–19. [Google Scholar] [CrossRef]
- Elliott, P.; Shaddick, G.; Wakefield, J.C.; De Hoogh, C.; Briggs, D.J. Long-term Associations of Outdoor Air Pollution with Mortality in Great Britain. Thorax 2007, 62, 1088. [Google Scholar] [CrossRef]
- Lippmann, M.; Ito, K. Separating the Effects of Temperature and Season on Daily Mortality from Those of Air Pollution in London: 1965–1972. Inhal. Toxicol. 1995, 7, 85–97. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A. Electrochemical Study of Bulk and Monolayer Copper in Alkaline Solution. J. Electrochem. Soc. 2016, 163, H252–H259. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Barucca, G.; Notarstefano, V.; Cantarini, M.; Armeni, T.; Mobbili, G. Encapsulation of a Neutral Molecule into a Cationic Clay Material: Structural Insight and Cytotoxicity of Resveratrol/Layered Double Hydroxide/BSA Nanocomposites. Nanomaterials 2019, 10, 33. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, J.; Zhang, P.; Li, Y.; Zhang, S.; Wang, Z.; Xu, L.; Guo, H.; Chen, K.; Wei, Y. Recent Progress on Density Functional Theory Calculations for Catalytic Control of Air Pollution. ACS ES&T Eng. 2024, 4, 47–65. [Google Scholar]
- Heimann, J.E.; Grimes, R.T.; Rosenzweig, Z.; Bennett, J.W. A Density Functional Theory (DFT) Investigation of How Small Molecules and Atmospheric Pollutants Relevant to Art Conservation Adsorb on Kaolinite. Appl. Clay Sci. 2021, 206, 106075. [Google Scholar] [CrossRef]
- Mohammadi, M.D.; Patsalidis, N.; Bhowmick, S.; Harmandaris, V.A.; Biskos, G. Adsorption of Air Pollutants onto Silver and Gold Atomic Clusters: DFT and PNO-LCCSD-F12 Calculations. RSC Adv. 2023, 13, 18014–18024. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Gramigni, D.; Pavoni, E.; Ripani, L.; Laudadio, E.; Mobbili, G.; Barucca, G.; Stipa, P.; Galeazzi, R.; Mengucci, P.; et al. Copper-Layered Double Hydroxide for Methanol Electrooxidation: A Combined DFT and Experimental Characterization. In Proceedings of the 2024 IEEE International Workshop on Metrology for Living Environment (MetroLivEnv), Chania, Greece, 12–14 June 2024; pp. 202–206. [Google Scholar] [CrossRef]
- Miyata, S. Anion-Exchange Properties of Hydrotalcite-Like Compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Liu, H.M.; Zhao, X.J.; Zhu, Y.Q.; Yan, H. DFT Study on MgAl-Layered Double Hydroxides with different Interlayer Anions: Structure, Anion Exchange, Host–Guest Interaction and Basic Sites. Phys. Chem. Chem. Phys. 2020, 22, 2521–2529. [Google Scholar] [CrossRef]
- Lebrun, N.; Mahe, F.; Lamiot, J.; Foulon, M.; Petit, J.C.; Prevost, D. Kinetic Behaviour Investigations and Crystal Structure of Nitric Acid Dihydrate. Acta Crystallogr. B 2001, 57, 27–35. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Liu, H.; Wang, Y.; Zhou, P. Effect of Monovalent Anions on Cationic Gemini Micro-Emulsion. Chin. J. Chem. Eng. 2018, 26, 2636–2640. [Google Scholar] [CrossRef]
- Sun, X.; Shi, L.; Huang, H.; Song, X.; Ma, T. Surface Engineered 2D Materials for Photocatalysis. Chem. Commun. 2020, 56, 11000–11013. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhao, Q.; Ma, T.; Wang, L. Thin-Layered Photocatalysts. Adv. Funct. Mater. 2020, 30, 1910005. [Google Scholar] [CrossRef]
- Zhao, Y.; Waterhouse, G.I.N.; Chen, G.; Xiong, X.; Wu, L.Z.; Tung, C.H.; Zhang, T. Two-Dimensional-Related Catalytic Materials for Solar-Driven Conversion of COx into Valuable Chemical Feedstocks. Chem. Soc. Rev. 2019, 48, 1972–2010. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, G.I.N.; Tang, J.; Zhang, T. Two-Dimensional Photocatalyst Design: A Critical Review of Recent Experimental and Computational Advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.Q.; Zulfiqar, S.; Shah, S.; Bahadur, I. An Overview of Semiconductors/Layered Double Hydroxides Composites: Properties, Synthesis, Photocatalytic and Photoelectrochemical Applications. J. Mol. Liq. 2019, 289, 111114. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered Double Hydroxide (LDH)-Based Materials: A Mini-Review on Strategies to Improve the Performance for Photocatalytic Water Splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Smidstrup, S.; Markussen, T.; Vancraeyveld, P.; Wellendorff, J.; Schneider, J.; Gunst, T.; Verstichel, B.; Stradi, D.; Khomyakov, P.A.; Vej-Hansen, U.G.; et al. QuantumATK: An Integrated Platform of Electronic and Atomic-Scale Modelling Tools. J. Phys. Condens. Matter 2019, 32, 015901. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Pavoni, E.; Mohebbi, E.; Stipa, P.; Pierantoni, L.; Mencarelli, D.; Dragoman, M.; Aldrigo, M.; Laudadio, E. First-Principles Investigation of Interface Phenomena in Hafnium-Based Metal–Insulator–Metal Diodes. Nanoscale Adv 2023, 5, 2748–2755. [Google Scholar] [CrossRef]
- Pavoni, E.; Mohebbi, E.; Stipa, P.; Mencarelli, D.; Pierantoni, L.; Laudadio, E. The Role of Zr on Monoclinic and Orthorhombic HfxZryO2 Systems: A First-Principles Study. Materials 2022, 15, 4175–4188. [Google Scholar] [CrossRef]
- van Setten, M.J.; Giantomassi, M.; Bousquet, E.; Verstraete, M.J.; Hamann, D.R.; Gonze, X.; Rignanese, G.-M. The PseudoDojo: Training and Grading a 85 Element Optimized Norm-Conserving Pseudopotential Table. Comput. Phys. Commun. 2018, 226, 39–54. [Google Scholar] [CrossRef]
- Pavoni, E.; Modreanu, M.G.; Mohebbi, E.; Mencarelli, D.; Stipa, P.; Laudadio, E.; Pierantoni, L. First-Principles Calculation of MoO2 and MoO3 Electronic and Optical Properties Compared with Experimental Data. Nanomaterials 2023, 13, 1319. [Google Scholar] [CrossRef]
- Wang, Y.; Wisesa, P.; Balasubramanian, A.; Dwaraknath, S.; Mueller, T. Rapid Generation of Optimal Generalized Monkhorst-Pack Grids. Comput. Mater. Sci. 2021, 187, 110100. [Google Scholar] [CrossRef]
- Xu, D.; Fu, G.; Li, Z.; Zhen, W.; Wang, H.; Liu, M.; Sun, J.; Zhang, J.; Yang, L. Functional Regulation of ZnAl-LDHs and Mechanism of Photocatalytic Reduction of CO2: A DFT Study. Molecules 2023, 28, 738. [Google Scholar] [CrossRef]
- Moraes, P.I.R.; Wypych, F.; Leitao, A.A. DFT Study of Layered Double Hydroxides with Cation Exchange Capacity: (A+(H2O)6)[M62+Al3(OH)18(SO4)2]·6H2O (M2+ = Mg, Zn and A+ = Na, K). J. Phys. Chem. C 2019, 123, 9838–9845. [Google Scholar] [CrossRef]
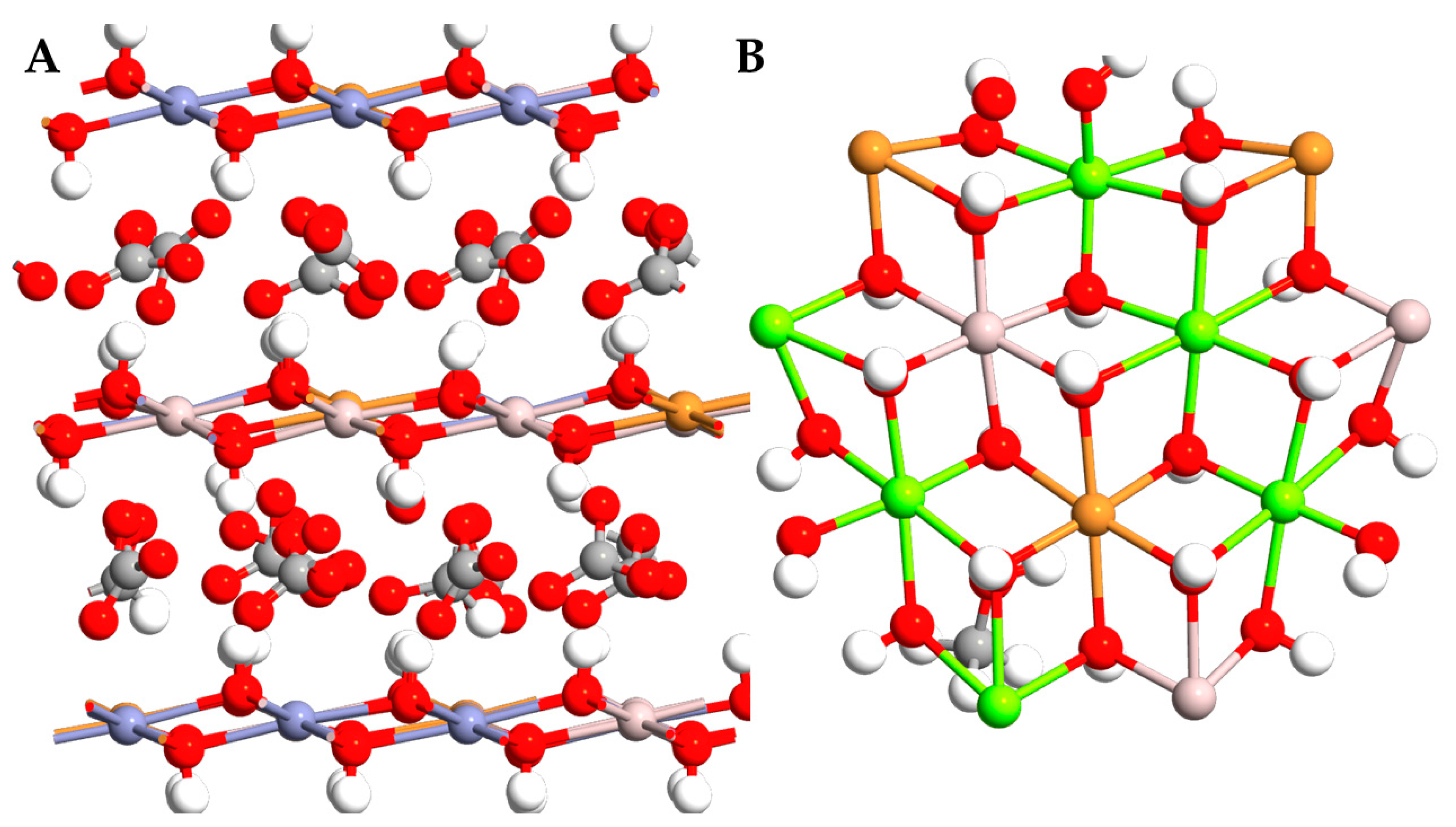


| CuZnAl-OH− | CuMgAl-OH− | CuZnAl-CO32− | CuMgAl-CO32− | |
|---|---|---|---|---|
| Cell parameters | x 3.062 Å y 3.062 Å | x 3.071 Å y 3.071 Å | x 3.062 Å y 3.062 Å | x 3.071 Å y 3.071 Å |
| Interlayer spacing | z 7.446 Å | z 7.446 Å | z 8.208 Å | z 8.208 Å |
| Formation energy | −9.35 eV | −6.92 eV | −23.23 eV | −21.46 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohebbi, E.; Minnelli, C.; Pavoni, E.; Sisti, L.; Laudadio, E.; Stipa, P. Layered Double Hydroxides as Systems for Capturing Small-Molecule Air Pollutants: A Density Functional Theory Study. Molecules 2024, 29, 4996. https://doi.org/10.3390/molecules29214996
Mohebbi E, Minnelli C, Pavoni E, Sisti L, Laudadio E, Stipa P. Layered Double Hydroxides as Systems for Capturing Small-Molecule Air Pollutants: A Density Functional Theory Study. Molecules. 2024; 29(21):4996. https://doi.org/10.3390/molecules29214996
Chicago/Turabian StyleMohebbi, Elaheh, Cristina Minnelli, Eleonora Pavoni, Laura Sisti, Emiliano Laudadio, and Pierluigi Stipa. 2024. "Layered Double Hydroxides as Systems for Capturing Small-Molecule Air Pollutants: A Density Functional Theory Study" Molecules 29, no. 21: 4996. https://doi.org/10.3390/molecules29214996
APA StyleMohebbi, E., Minnelli, C., Pavoni, E., Sisti, L., Laudadio, E., & Stipa, P. (2024). Layered Double Hydroxides as Systems for Capturing Small-Molecule Air Pollutants: A Density Functional Theory Study. Molecules, 29(21), 4996. https://doi.org/10.3390/molecules29214996












