Plasmonics Meets Perovskite Photovoltaics: Innovations and Challenges in Boosting Efficiency
Abstract
:1. Introduction
2. Mechanisms of Plasmonic Nanostructures for Enhanced Optical and Electrical Properties
2.1. Far–Field Scattering
2.2. Near–Field Enhancement
2.3. Hot–Electron Transfer
2.4. Plasmon Resonant Energy Transfer
3. Types of Plasmonic Materials in PSCs
3.1. Noble Metal Plasmonic Nanomaterials
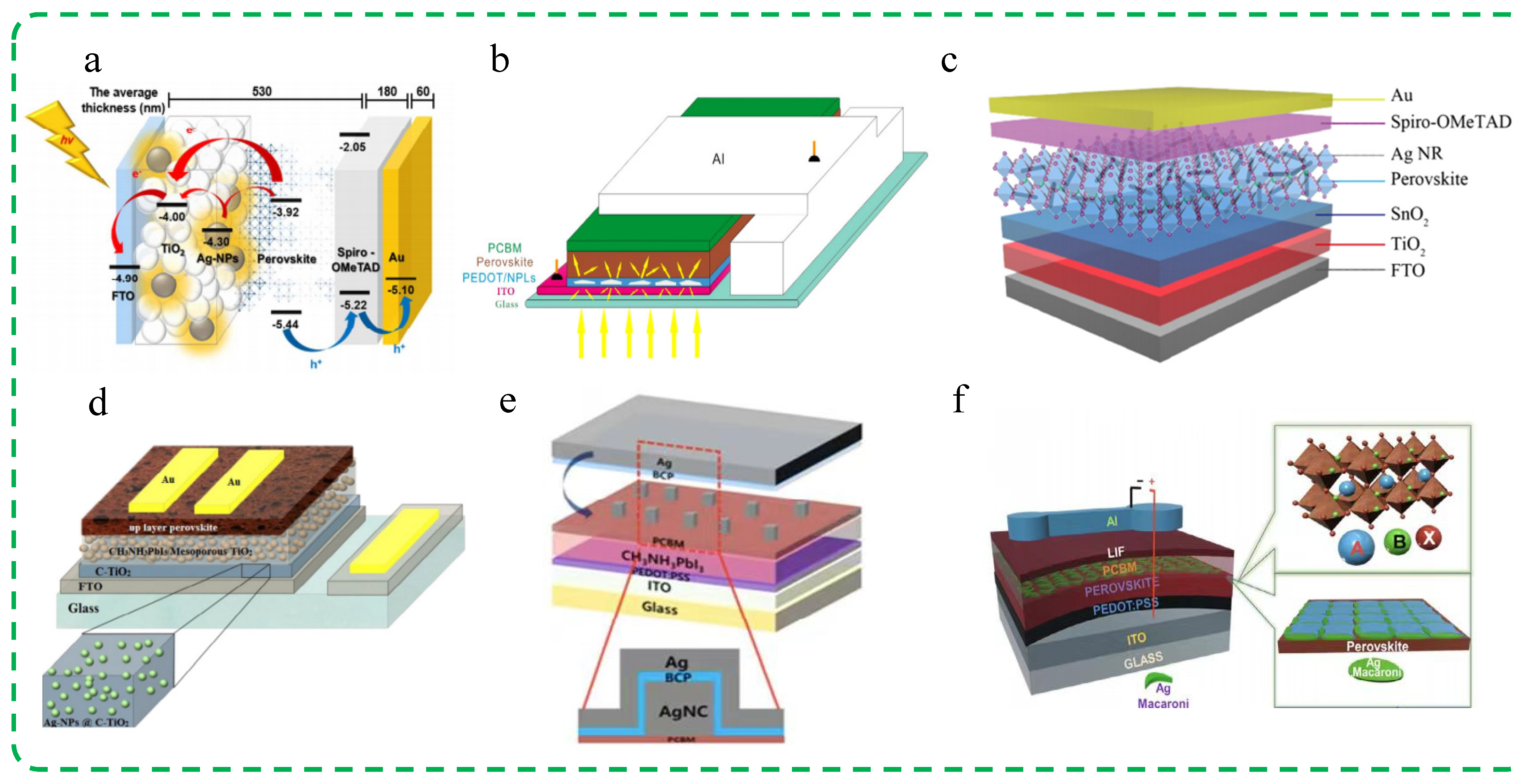
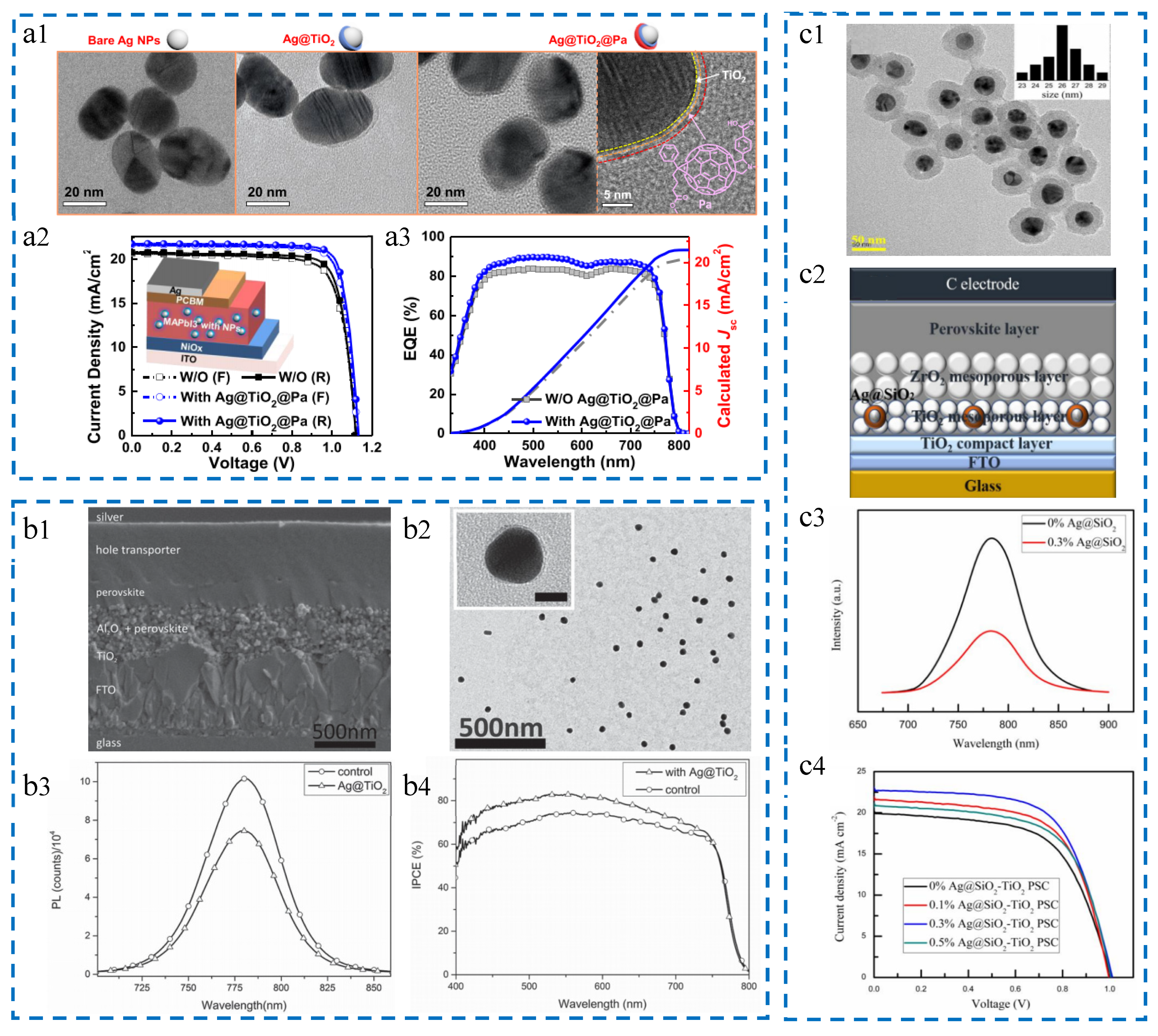
3.1.1. Ag Nanomaterials
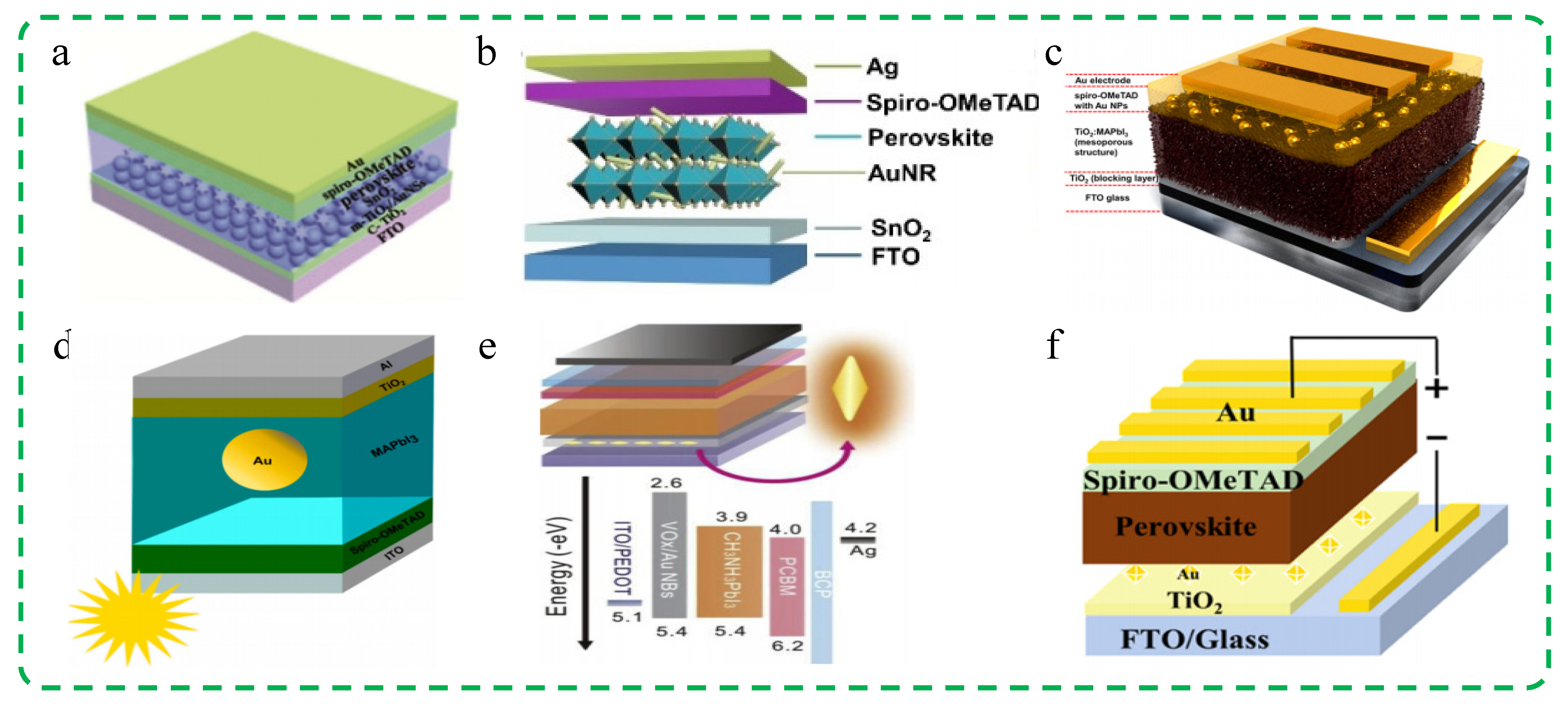
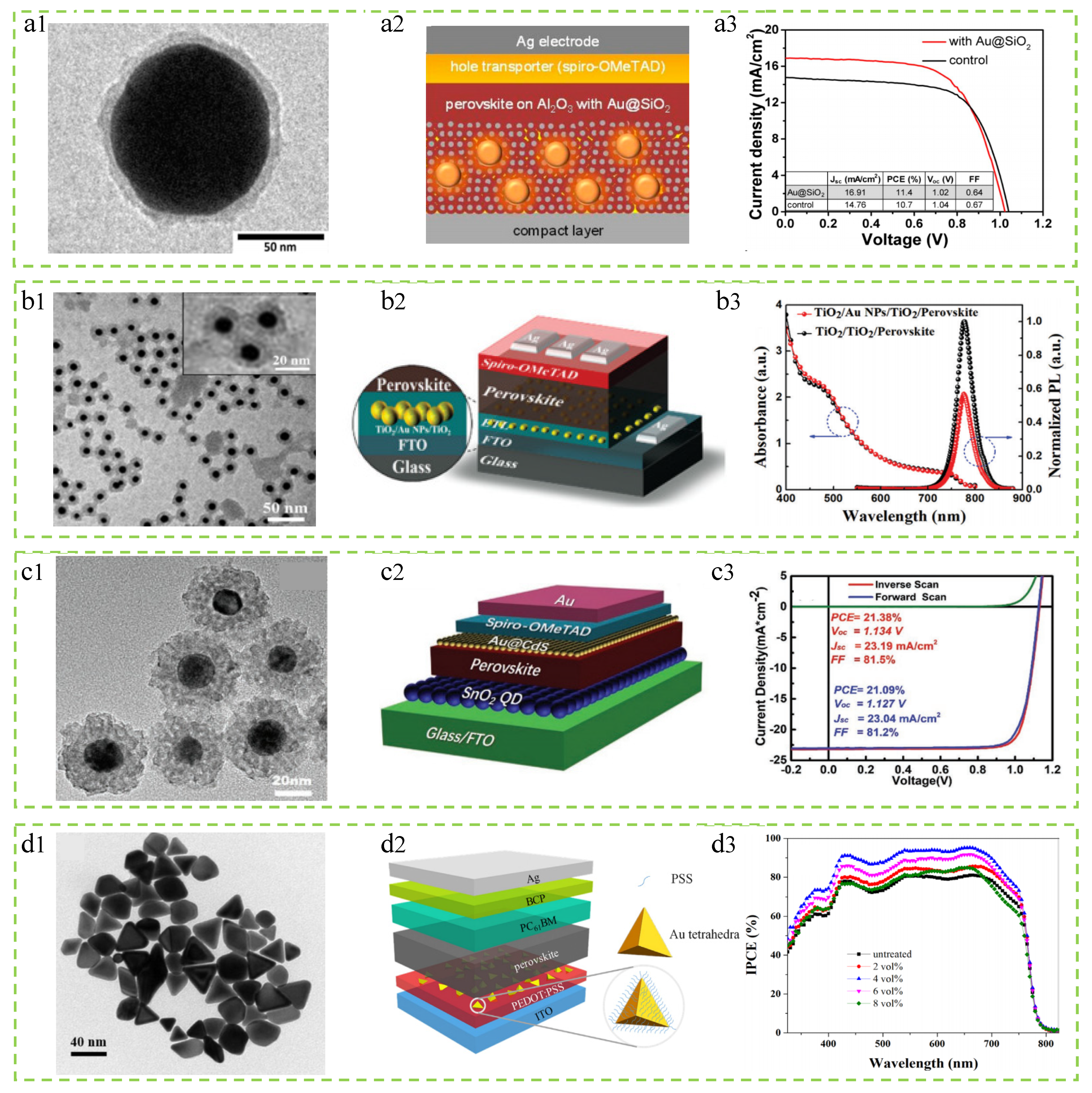
3.1.2. Au Nanomaterials
3.2. Other Metal Plasmonic Nanomaterials
3.2.1. Al Nanomaterials
3.2.2. Cu Nanomaterials
3.3. Alloy Plasmonic Nanomaterials
3.4. Other Novel Plasmonic Nanomaterials
4. Conclusions and Outlook
- (1)
- The selection of plasmonic materials that are both cost-effective and compatible with perovskite chemistry is crucial. At present, the primary materials employed are noble metals such as Au and Ag, which are both expensive and limited in supply. Consequently, there emerges an urgent imperative to scout and cultivate alternative materials that not only promise cost savings but are also abundant in nature. Al and Cu, albeit harboring potential as promising plasmonic materials, have been relatively neglected in the realm of research and application. Apart from these materials, we can develop a range of alternative plasmonic materials, such as transition metal materials, oxide materials, semiconductor materials, metal alloy materials, composite materials, and so on, to facilitate the further advancement of research on plasmonic solar cells. Moreover, the fabrication of plasmonic nanostructures encounters formidable challenges, encapsulated in substantial equipment expenditures, intricate manufacturing processes, substantial energy depletion, and the elusive pursuit of uniformity and stability. Therefore, the development of large-area, uniform, and scalable nanostructuring methods is essential. Techniques such as roll-to-roll nanoimprinting, nanosphere lithography, and colloidal self-assembly can enable the fabrication of plasmonic structures over large areas with high precision. Furthermore, research on hybrid plasmonic–dielectric structures can offer a balance between cost and performance.
- (2)
- A major hurdle that hinders the large-scale deployment of PSCs is their limited stability. Plasmonic nanostructures, while effective in boosting optical absorption and electron extraction, can also introduce additional interfaces and materials that can compromise the device’s longevity. The degradation of perovskite materials under illumination, humidity, and temperature fluctuations is further exacerbated by the presence of plasmonic particles. Developing encapsulation techniques that effectively isolate the plasmonic–perovskite interface from environmental stressors is crucial. Researchers can also explore the use of more stable plasmonic materials or hybrid nanostructures that combine the benefits of multiple materials. Additionally, optimizing the perovskite composition and interface engineering can further improve the stability of PSCs.
- (3)
- There are potentially detrimental aspects of plasmonic nanomaterials for the performance of solar cells. Although plasmonic materials have shown remarkable advantages in improving the performance of solar cells, they can also bring some harmful effects, including the introduction of more defects, an increase in recombination centers, the generation of hotspots, the induction of material degradation, and environmental pollution. To fully leverage their advantages while mitigating the harmful impacts, continuous technological research and innovation, as well as the optimization of production processes and environmental protection measures, are essential for achieving sustainable development. By optimizing the synthesis methods of nanomaterials, controlling plasma treatment parameters, and improving device structures, we can maximize the benefits of plasma nanomaterials while minimizing their adverse effects on solar cell performance.
- (4)
- Most research on plasmonic-enhanced PSCs has focused on laboratory-scale devices with small active areas. As the device size increases, challenges arise due to non-uniform distribution of light, plasmonic hotspots, and increased resistance losses, resulting in a significant drop in the overall efficiency. Future research should focus on integrating plasmonic-enhanced PSCs into larger photovoltaic systems, considering factors such as module design, interconnection strategies, and overall system optimization. This includes developing methods to mitigate efficiency losses in large-area devices and optimizing the integration of PSCs with other photovoltaic technologies, such as silicon-based cells, in tandem or multi-junction configurations.
- (5)
- We could consider integrating plasmonic technology with cutting-edge innovative technologies such as artificial intelligence (AI) and machine learning [154]. For instance, by leveraging big data analysis and machine learning algorithms, we can swiftly screen and predict potential novel materials in plasmonic technology [155]; with the assistance of AI’s optimization algorithms, we can fine-tune the plasmonic process parameters to achieve customized material properties [156]; AI’s formidable data processing capabilities enable it to explore material combinations and process conditions that are difficult to reach with traditional methods [157,158]. In this way, we can not only enhance material research and development efficiency, optimize material performance, and facilitate the discovery of new materials but also improve equipment performance, achieve intelligent control, and optimize process flows, thereby accelerating technological innovation, boosting industrial competitiveness, and promoting sustainable development.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koroneos, C.; Spachos, T.; Moussiopoulos, N. Exergy analysis of renewable energy sources. Renew. Energy 2003, 28, 295–310. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Yuan, S.-Y.; Zhao, H.; Wang, Y.-T.; Li, Z.-Z.; Wang, X.-D.; Cao, W.-P. Research status of all-inorganic perovskite solar cells: A review. J. Solid State Chem. 2023, 328, 124299. [Google Scholar] [CrossRef]
- Barker, P.P.; Bing, J.M. Advances in solar photovoltaic technology: An applications perspective. In Proceedings of the IEEE Power Engineering Society General Meeting, San Francisco, CA, USA, 16 June 2005; pp. 1955–1960. [Google Scholar]
- Hu, Y.; Schlipf, J.; Wussler, M.; Petrus, M.L.; Jaegermann, W.; Bein, T.; Müller-Buschbaum, P.; Docampo, P. Hybrid Perovskite/Perovskite Heterojunction Solar Cells. ACS Nano 2016, 10, 5999–6007. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Lal, N.N.; Dkhissi, Y.; Li, W.; Hou, Q.; Cheng, Y.B.; Bach, U. Perovskite Tandem Solar Cells. Adv. Energy Mater. 2017, 7, 1602761. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, B.; Zhang, W.; Yang, Z.; Li, M.; Ren, F.; Imran, T.; Sun, Z.; Zhang, S.; Zhang, Y.; et al. Solvent engineering towards scalable fabrication of high-quality perovskite films for efficient solar modules. J. Energy Chem. 2023, 80, 689–710. [Google Scholar] [CrossRef]
- Raza, H.; Imran, T.; Gao, Y.; Azeem, M.; Younis, M.; Wang, J.; Liu, S.; Yang, Z.; Liu, Z.; Chen, W. Potential-induced degradation: A challenge in the commercialization of perovskite solar cells. Energy Environ. Sci. 2024, 17, 1819–1853. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Pan, M.; Ren, F.-M.; Chen, R.; Sun, Z.-X.; Yang, Z.-C.; Liu, Z.-H.; Chen, W. Boosting stability of inverted perovskite solar cells with magnetron-sputtered molybdenum rear electrodes. Rare Met. 2023, 42, 3741–3754. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Kim, M.; Lee, S.-U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.-L.; Xu, M.; Feng, J.; Sun, H.-B. Flexible and efficient ITO-free semitransparent perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 157, 660–665. [Google Scholar] [CrossRef]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, J.; Segawa, H. Evolution of organometal halide solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2018, 35, 74–107. [Google Scholar] [CrossRef]
- Pérez-del-Rey, D.; Forgács, D.; Hutter, E.M.; Savenije, T.J.; Nordlund, D.; Schulz, P.; Berry, J.J.; Sessolo, M.; Bolink, H.J. Strontium Insertion in Methylammonium Lead Iodide: Long Charge Carrier Lifetime and High Fill-Factor Solar Cells. Adv. Mater. 2016, 28, 9839–9845. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Shi, S.; Li, Y.; Li, X.; Wang, H. Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites. Mater. Horiz. 2015, 2, 378–405. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.G. Causes and Solutions of Recombination in Perovskite Solar Cells. Adv. Mater. 2018, 31, 1803019. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Hodes, G.; Cahen, D. Perovskite cells roll forward. Nat. Photonics 2014, 8, 87–88. [Google Scholar] [CrossRef]
- Kim, H.-S.; Im, S.H.; Park, N.-G. Organolead Halide Perovskite: New Horizons in Solar Cell Research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- Roy, P.; Kumar Sinha, N.; Tiwari, S.; Khare, A. A review on perovskite solar cells: Evolution of architecture, fabrication techniques, commercialization issues and status. Sol. Energy 2020, 198, 665–688. [Google Scholar] [CrossRef]
- Liu, K.; Rafique, S.; Musolino, S.F.; Cai, Z.; Liu, F.; Li, X.; Yuan, Y.; Bao, Q.; Yang, Y.; Chu, J.; et al. Covalent bonding strategy to enable non-volatile organic cation perovskite for highly stable and efficient solar cells. Joule 2023, 7, 1033–1050. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, F.; Qu, Z.; Yu, S.; Shen, T.; Deng, H.-X.; Chu, X.; Peng, X.; Yuan, Y.; Zhang, X.; et al. Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells. Science 2022, 377, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Dong, J.; Xu, C.; Yao, Y.; You, J.; Bian, H.; Zeng, W.; Zhou, G.; He, X.; Wang, M.; et al. Fabrication of an ultrathin PEG-modified PEDOT:PSS HTL for high-efficiency Sn–Pb perovskite solar cells by an eco-friendly solvent etching technique. J. Mater. Chem. A 2023, 11, 7246–7255. [Google Scholar] [CrossRef]
- You, J.; Bian, H.; Wang, M.; Cai, X.; Li, C.; Zhou, G.; Lu, H.; Fang, C.; Huang, J.; Yao, Y.; et al. Eco-friendly glucose assisted structurally simplified high-efficiency tin-lead mixed perovskite solar cells. J. Energy Chem. 2023, 85, 83–90. [Google Scholar] [CrossRef]
- Shen, X.; Lin, X.; Peng, Y.; Zhang, Y.; Long, F.; Han, Q.; Wang, Y.; Han, L. Two-Dimensional Materials for Highly Efficient and Stable Perovskite Solar Cells. Nano-Micro Lett. 2024, 16, 201. [Google Scholar] [CrossRef]
- Xing, J.; Sun, Y.; He, S.; Huang, X.; Li, Y.; Huang, Z.; Wang, B.; Zhou, R.; Li, Y.; Zhang, J.; et al. Triple-Cation Mixed-Halide Perovskite Single-Crystal Thin-Film for High-Performance Photodetector via Adjusting Lattice Strain and Mitigating Surface Defects. Adv. Funct. Mater. 2024, 2411619. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2013, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor–free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Wang, Q.; Phung, N.; Di Girolamo, D.; Vivo, P.; Abate, A. Enhancement in lifespan of halide perovskite solar cells. Energy Environ. Sci. 2019, 12, 865–886. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Chan, K.; Wright, M.; Elumalai, N.; Uddin, A.; Pillai, S. Plasmonics in Organic and Perovskite Solar Cells: Optical and Electrical Effects. Adv. Opt. Mater. 2016, 5, 1600698. [Google Scholar] [CrossRef]
- Siavash Moakhar, R.; Gholipour, S.; Masudy-Panah, S.; Seza, A.; Mehdikhani, A.; Riahi-Noori, N.; Tafazoli, S.; Timasi, N.; Lim, Y.F.; Saliba, M. Recent Advances in Plasmonic Perovskite Solar Cells. Adv. Sci. 2020, 7, 1902448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Kou, Z.-L.; Feng, J.; Sun, H.-B. Plasmon-enhanced organic and perovskite solar cells with metal nanoparticles. Nanophotonics 2020, 9, 3111–3133. [Google Scholar] [CrossRef]
- Atwater, H.A. The promise of plasmonics. Sci. Am. 2007, 296, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kasani, S.; Curtin, K.; Wu, N. A review of 2D and 3D plasmonic nanostructure array patterns: Fabrication, light management and sensing applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Ai, B.; Fan, Z.; Wong, Z.J. Plasmonic–perovskite solar cells, light emitters, and sensors. Microsyst. Nanoeng. 2022, 8, 5. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Liu, X.; Li, X.; Kang, L. One-step implementation of plasmon enhancement and solvent annealing effects for air-processed high-efficiency perovskite solar cells. J. Mater. Chem. A 2018, 6, 24036–24044. [Google Scholar] [CrossRef]
- Higgins, M.; Ely, F.; Nome, R.C.; Nome, R.A.; dos Santos, D.P.; Choi, H.; Nam, S.; Quevedo-Lopez, M. Enhanced reproducibility of planar perovskite solar cells by fullerene doping with silver nanoparticles. J. Appl. Phys. 2018, 124, 065306. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Alexaki, K.; Stratakis, E.; Kymakis, E. Efficiency and stability enhancement of inverted perovskite solar cells via the addition of metal nanoparticles in the hole transport layer. RSC Adv. 2017, 7, 12998–13002. [Google Scholar] [CrossRef]
- Hopper, E.R.; Boukouvala, C.; Asselin, J.; Biggins, J.S.; Ringe, E. Opportunities and Challenges for Alternative Nanoplasmonic Metals: Magnesium and Beyond. J. Phys. Chem. C 2022, 126, 10630–10643. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Alcaraz de la Osa, R.; Ortiz, D.; Saiz, J.; González, F.; Moreno, F. Plasmonics in the Ultraviolet with Aluminum, Gallium, Magnesium and Rhodium. Appl. Sci. 2018, 8, 64. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, P.; Lu, S.; Yang, S.; Peng, F.; Chang, W.-S.; Liu, K. Synthesis and Multipole Plasmon Resonances of Spherical Aluminum Nanoparticles. J. Phys. Chem. Lett. 2020, 11, 5836–5843. [Google Scholar] [CrossRef]
- Jacobson, C.R.; Solti, D.; Renard, D.; Yuan, L.; Lou, M.; Halas, N.J. Shining Light on Aluminum Nanoparticle Synthesis. Acc. Chem. Res. 2020, 53, 2020–2030. [Google Scholar] [CrossRef]
- Ringe, E. Shapes, Plasmonic Properties, and Reactivity of Magnesium Nanoparticles. J. Phys. Chem. C 2020, 124, 15665–15679. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kurouski, D. Plasmon-Driven Chemistry on Mono- and Bimetallic Nanostructures. Acc. Chem. Res. 2021, 54, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Lomonosov, V.; Yang, J.; Fan, Y.; Hofmann, S.; Ringe, E. Stability of Plasmonic Mg-MgO Core–Shell Nanoparticles in Gas-Phase Oxidative Environments. Nano Lett. 2024, 24, 7084–7090. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Petridis, K.; Kymakis, E. Recent advances in plasmonic metal and rare-earth-element upconversion nanoparticle doped perovskite solar cells. J. Mater. Chem. A 2017, 5, 21604–21624. [Google Scholar] [CrossRef]
- Ijaz, M.; Shoukat, A.; Ayub, A.; Tabassum, H.; Naseer, H.; Tanveer, R.; Islam, A.; Iqbal, T. Perovskite solar cells: Importance, challenges, and plasmonic enhancement. Int. J. Green Energy 2020, 17, 1022–1035. [Google Scholar] [CrossRef]
- Wang, H.; Brandl, D.W.; Nordlander, P.; Halas, N.J. Plasmonic Nanostructures: Artificial Molecules. Acc. Chem. Res. 2007, 40, 53–62. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, R.; Du, C.; Rong, W.; Li, X.; Shi, D. Enhanced photocurrent of perovskite solar cells by a layer of randomly-distributed-Ag-nanospheres. Phys. Lett. A 2021, 414, 127620. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, L.; Gu, S.; Zhu, W.; Wang, Y.; Xu, J.; Deng, Z.; Yu, T.; Lu, Z.; Zhu, J. Fine-tuning the metallic core-shell nanostructures for plasmonic perovskite solar cells. Appl. Phys. Lett. 2016, 109, 183901. [Google Scholar] [CrossRef]
- Hui, Y.; You, E.-M.; Luo, Q.-P.; Wang, T.; Nan, Z.-A.; Gu, Y.; Zhang, W.-H.; Cai, Z.-Y.; Chen, L.; Zhou, J.-Z.; et al. Efficient plasmon-enhanced perovskite solar cells by molecularly isolated gold nanorods. J. Energy Chem. 2022, 73, 60–67. [Google Scholar] [CrossRef]
- Batmunkh, M.; Macdonald, T.J.; Peveler, W.J.; Bati, A.S.R.; Carmalt, C.J.; Parkin, I.P.; Shapter, J.G. Plasmonic Gold Nanostars Incorporated into High-Efficiency Perovskite Solar Cells. ChemSusChem 2017, 10, 3750–3753. [Google Scholar] [CrossRef]
- Li, S.; Zhu, X.; Wang, B.; Qiao, Y.; Liu, W.; Yang, H.; Liu, N.; Chen, M.; Lu, H.; Yang, Y. Influence of Ag Nanoparticles with Different Sizes and Concentrations Embedded in a TiO2 Compact Layer on the Conversion Efficiency of Perovskite Solar Cells. Nanoscale Res. Lett. 2018, 13, 183901. [Google Scholar] [CrossRef]
- Wang, D.; Chan, K.H.; Elumalai, N.K.; Mahmud, M.A.; Upama, M.B.; Uddin, A.; Pillai, S. Interfacial engineering of hole transport layers with metal and dielectric nanoparticles for efficient perovskite solar cells. Phys. Chem. Chem. Phys. 2017, 19, 25016–25024. [Google Scholar] [CrossRef]
- Cui, J.; Chen, C.; Han, J.; Cao, K.; Zhang, W.; Shen, Y.; Wang, M. Surface Plasmon Resonance Effect in Inverted Perovskite Solar Cells. Adv. Sci. 2016, 3, 1500312. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jang, Y.J.; Kim, S.; Quan, L.N.; Chung, K.; Kim, D.H. Plasmonic Solar Cells: From Rational Design to Mechanism Overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef]
- Erwin, W.R.; Zarick, H.F.; Talbert, E.M.; Bardhan, R. Light trapping in mesoporous solar cells with plasmonic nanostructures. Energy Environ. Sci. 2016, 9, 1577–1601. [Google Scholar] [CrossRef]
- Akimov, Y.A.; Koh, W.S.; Sian, S.Y.; Ren, S. Nanoparticle-enhanced thin film solar cells: Metallic or dielectric nanoparticles? Appl. Phys. Lett. 2010, 96, 073111. [Google Scholar] [CrossRef]
- Thrithamarassery Gangadharan, D.; Xu, Z.; Liu, Y.; Izquierdo, R.; Ma, D. Recent advancements in plasmon-enhanced promising third-generation solar cells. Nanophotonics 2017, 6, 153–175. [Google Scholar] [CrossRef]
- Zayats, A.V.; Smolyaninov, I.I.; Maradudin, A.A. Nano-optics of surface plasmon polaritons. Phys. Rep. 2005, 408, 131–314. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, S.; Song, H. Photon management to reduce energy loss in perovskite solar cells. Chem. Soc. Rev. 2021, 50, 7250–7329. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Pillai, S. Harnessing plasmonics for solar cells. Nat. Photonics 2012, 6, 130–132. [Google Scholar] [CrossRef]
- Spurio, E.; Pelli Cresi, J.S.; Ammirati, G.; Pelatti, S.; Paladini, A.; D’Addato, S.; Turchini, S.; O’Keeffe, P.; Catone, D.; Luches, P. Injecting Electrons into CeO2 via Photoexcitation of Embedded Au Nanoparticles. ACS Photonics 2023, 10, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Wu, N. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2017, 118, 2927–2954. [Google Scholar] [CrossRef]
- Ferry, V.E.; Sweatlock, L.A.; Pacifici, D.; Atwater, H.A. Plasmonic Nanostructure Design for Efficient Light Coupling into Solar Cells. Nano Lett. 2008, 8, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Ferry, V.E.; Verschuuren, M.A.; Li, H.B.T.; Schropp, R.E.I.; Atwater, H.A.; Polman, A. Improved red-response in thin film a-Si:H solar cells with soft-imprinted plasmonic back reflectors. Appl. Phys. Lett. 2009, 95, 183503. [Google Scholar] [CrossRef]
- Tan, S.; Argondizzo, A.; Ren, J.; Liu, L.; Zhao, J.; Petek, H. Plasmonic coupling at a metal/semiconductor interface. Nat. Photonics 2017, 11, 806–812. [Google Scholar] [CrossRef]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hsu, C.-M.; Yu, Z.; Fan, S.; Cui, Y. Nanodome Solar Cells with Efficient Light Management and Self-Cleaning. Nano Lett. 2009, 10, 1979–1984. [Google Scholar] [CrossRef]
- Munday, J.N.; Atwater, H.A. Large Integrated Absorption Enhancement in Plasmonic Solar Cells by Combining Metallic Gratings and Antireflection Coatings. Nano Lett. 2010, 11, 2195–2201. [Google Scholar] [CrossRef]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express 2012, 2, 478–489. [Google Scholar] [CrossRef]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative Plasmonic Materials: Beyond Gold and Silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem.–A Eur. J. 2004, 11, 454–463. [Google Scholar] [CrossRef]
- Yun, J.; Hwang, S.H.; Jang, J. Fabrication of Au@Ag Core/Shell Nanoparticles Decorated TiO2 Hollow Structure for Efficient Light-Harvesting in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 2055–2063. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; El-Sayed, M.A. Dependence of the Enhanced Optical Scattering Efficiency Relative to That of Absorption for Gold Metal Nanorods on Aspect Ratio, Size, End-Cap Shape, and Medium Refractive Index. J. Phys. Chem. B 2005, 109, 20331–20338. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jang, J.G.; Lim, J.; Lee, J.-K.; Kim, S.H.; Hong, J.-I. Correlations of Optical Absorption, Charge Trapping, and Surface Roughness of TiO2 Photoanode Layer Loaded with Neat Ag-NPs for Efficient Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 21522–21530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-L.; Juang, T.-Y.; Chen, C.-P.; Hsieh, C.-M.; Yang, C.-C.; Huang, C.-L.; Jeng, R.-J. Enhanced efficiency of organic and perovskite photovoltaics from shape-dependent broadband plasmonic effects of silver nanoplates. Sol. Energy Mater. Sol. Cells 2015, 140, 224–231. [Google Scholar] [CrossRef]
- Liu, S.; Liang, L.; Meng, L.; Tian, X.; Zhang, Z.; Yu, Y.; Lan, Z.; Wu, J.; Zhang, J.; Gao, P. Synergy of plasmonic silver nanorod and water for enhanced planar perovskite photovoltaic devices. Sol. Rrl 2019, 4, 1900231. [Google Scholar] [CrossRef]
- Nourolahi, H.; Behjat, A.; Hosseini Zarch, S.M.M.; Bolorizadeh, M.A. Silver nanoparticle plasmonic effects on hole-transport material-free mesoporous heterojunction perovskite solar cells. Sol. Energy 2016, 139, 475–483. [Google Scholar] [CrossRef]
- Kim, G.M.; Tatsuma, T. Photocurrent Enhancement of Perovskite Solar Cells at the Absorption Edge by Electrode-Coupled Plasmons of Silver Nanocubes. J. Phys. Chem. C 2017, 121, 11693–11699. [Google Scholar] [CrossRef]
- Ali, A.; Kang, J.H.; Seo, J.H.; Walker, B. Effect of Plasmonic Ag Nanoparticles on the Performance of Inverted Perovskite Solar Cells. Adv. Eng. Mater. 2019, 22, 1900976. [Google Scholar] [CrossRef]
- Yao, K.; Zhong, H.; Liu, Z.; Xiong, M.; Leng, S.; Zhang, J.; Xu, Y.-X.; Wang, W.; Zhou, L.; Huang, H.; et al. Plasmonic Metal Nanoparticles with Core–Bishell Structure for High-Performance Organic and Perovskite Solar Cells. ACS Nano 2019, 13, 5397–5409. [Google Scholar] [CrossRef]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Johnston, M.B.; Goriely, A.; Wiesner, U.; Snaith, H.J. Plasmonic-Induced Photon Recycling in Metal Halide Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.; Li, S.; Chen, M.; Lu, H.; Yang, Y. Ag@SiO2 Core-shell Nanoparticles Embedded in a TiO2 Mesoporous Layer Substantially Improve the Performance of Perovskite Solar Cells. Nanomaterials 2018, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, C.; Wang, H.; Bi, W.; Song, Z.; Chen, X.; Jin, J.; Chen, X.; Xu, L.; Dai, Q.; et al. Toward ultra-thin and full functional perovskite solar cells by broadband light scattering management and efficient interfacial modification. Sol. Energy Mater. Sol. Cells 2020, 206, 110297. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Zhang, Z.; Li, Z.; Xiong, Q.; Deng, L.; Zhou, Q.; Meng, L.; Du, Y.; Zuo, T.; et al. Plasmon-Enhanced Perovskite Solar Cells with Efficiency Beyond 21 %: The Asynchronous Synergistic Effect of Water and Gold Nanorods. ChemPlusChem 2021, 86, 291–297. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, W.; Cha, B.G.; Kwon, J.; Kim, S.J.; Kim, M.; Kim, J.; Wang, D.H.; Park, J.H. Self-Position of Au NPs in Perovskite Solar Cells: Optical and Electrical Contribution. ACS Appl. Mater. Interfaces 2015, 8, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ghahremanirad, E.; Olyaee, S.; Hedayati, M. The Influence of Embedded Plasmonic Nanostructures on the Optical Absorption of Perovskite Solar Cells. Photonics 2019, 6, 37. [Google Scholar] [CrossRef]
- Dong, H.; Lei, T.; Yuan, F.; Xu, J.; Niu, Y.; Jiao, B.; Zhang, Z.; Ding, D.; Hou, X.; Wu, Z. Plasmonic enhancement for high efficient and stable perovskite solar cells by employing “hot spots” Au nanobipyramids. Org. Electron. 2018, 60, 1–8. [Google Scholar] [CrossRef]
- Juan, F.; Wu, Y.; Shi, B.; Wang, M.; Wang, M.; Xu, F.; Jia, J.; Wei, H.; Yang, T.; Cao, B. Plasmonic Au Nanooctahedrons Enhance Light Harvesting and Photocarrier Extraction in Perovskite Solar Cell. ACS Appl. Energy Mater. 2021, 4, 3201–3209. [Google Scholar] [CrossRef]
- Zhang, W.; Saliba, M.; Stranks, S.D.; Sun, Y.; Shi, X.; Wiesner, U.; Snaith, H.J. Enhancement of Perovskite-Based Solar Cells Employing Core–Shell Metal Nanoparticles. Nano Lett. 2013, 13, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chen, Y.; Zhang, M.; Harn, Y.W.; Qi, J.; Gao, L.; Wang, Z.L.; Huang, J.; Yang, Y.; Lin, Z. Tailoring carrier dynamics in perovskite solar cells via precise dimension and architecture control and interfacial positioning of plasmonic nanoparticles. Energy Environ. Sci. 2020, 13, 1743–1752. [Google Scholar] [CrossRef]
- Qin, P.; Wu, T.; Wang, Z.; Xiao, L.; Ma, L.; Ye, F.; Xiong, L.; Chen, X.; Li, H.; Yu, X.; et al. Grain Boundary and Interface Passivation with Core–Shell Au@ CdS Nanospheres for High-Efficiency Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1908408. [Google Scholar] [CrossRef]
- Hao, H.; Wang, L.; Ma, X.; Cao, K.; Yu, H.; Wang, M.; Gu, W.; Zhu, R.; Anwar, M.S.; Chen, S.; et al. Improved efficiency of inverted Perovskite Solar Cells via surface plasmon resonance effect of Au@ PSS core-shell tetrahedra nanoparticles. Sol. Rrl 2018, 2, 1800061. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, X.; Dai, S.; Li, M.; Zheng, L.; Wen, Q.; Tang, B.; Yun, D.-Q.; Xiao, L. Broad-Band-Enhanced Plasmonic Perovskite Solar Cells with Irregular Silver Nanomaterials. ACS Appl. Mater. Interfaces 2022, 14, 16269–16278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, H.; Huang, T.; Song, Y.; Wang, Y.; Wang, F.; Fan, L.; Liu, X.; Yang, L.; Liu, H. Ag-LSPR and molecular additive: A collaborative approach to improve the photovoltaic performance of perovskite solar cells. Chem. Eng. J. 2024, 481, 148572. [Google Scholar] [CrossRef]
- Ma, X.; Ma, B.; Yu, T.; Xu, X.; Zhang, L.; Wang, W.; Cao, K.; Deng, L.; Chen, S.; Huang, W. Indepth Studies on Working Mechanism of Plasmon-Enhanced Inverted Perovskite Solar Cells Incorporated with Ag@SiO2 Core–Shell Nanocubes. ACS Appl. Energy Mater. 2019, 2, 3605–3613. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, S.; Li, H.; Gao, Y.; Yu, S.; Yu, Y.; Meng, L.; Liu, W.; Zhang, J.; Gao, P. Perovskite-loaded plasmonic gold nanorod composites enhanced solar cell performance. Adv. Compos. Hybrid Mater. 2023, 6, 55. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Kumar, G.; Chen, F.-C. Interfacial plasmonic effects of gold nanoparticle-decorated graphene oxides on the performance of perovskite photovoltaic devices. Sol. Energy 2020, 211, 822–830. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Chen, K.; Cui, Y.; Intemann, J.J.; Leng, S.; Cui, M.; Qin, C.; Fei, L.; Yao, K.; et al. Tailoring carrier dynamics in inverted mesoporous perovskite solar cells with interface-engineered plasmonics. J. Mater. Chem. A 2021, 9, 2394–2403. [Google Scholar] [CrossRef]
- Aeineh, N.; Barea, E.M.; Behjat, A.; Sharifi, N.; Mora-Seró, I. Inorganic Surface Engineering to Enhance Perovskite Solar Cell Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 13181–13187. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, C.; Deng, X.; Zhu, H.; Li, Z.; Wang, Z.; Chen, X.; Huang, S. Plasmonic Effects of Metallic Nanoparticles on Enhancing Performance of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 34821–34832. [Google Scholar] [CrossRef]
- Ullah, I.; Hossain, M.A.; Armghan, A.; Rana, M.S.; Al Asad, M.A. The optoelectronic enhancement in perovskite solar cells using plasmonic metal-dielectric core-shell and nanorod nanoparticles. Opt. Quantum Electron. 2023, 55, 1018. [Google Scholar] [CrossRef]
- Knight, M.W.; King, N.S.; Liu, L.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum for Plasmonics. ACS Nano 2014, 8, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; Liu, L.; Wang, Y.; Brown, L.; Mukherjee, S.; King, N.S.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum Plasmonic Nanoantennas. Nano Lett. 2012, 12, 6000–6004. [Google Scholar] [CrossRef] [PubMed]
- Hylton, N.P.; Li, X.F.; Giannini, V.; Lee, K.H.; Ekins-Daukes, N.J.; Loo, J.; Vercruysse, D.; Van Dorpe, P.; Sodabanlu, H.; Sugiyama, M.; et al. Loss mitigation in plasmonic solar cells: Aluminium nanoparticles for broadband photocurrent enhancements in GaAs photodiodes. Sci. Rep. 2013, 3, 2874. [Google Scholar] [CrossRef] [PubMed]
- Akimov, Y.A.; Koh, W.S. Design of Plasmonic Nanoparticles for Efficient Subwavelength Light Trapping in Thin-Film Solar Cells. Plasmonics 2010, 6, 155–161. [Google Scholar] [CrossRef]
- Kesavan, A.V.; Rao, A.D.; Ramamurthy, P.C. Tailoring optoelectronic properties of CH3NH3PbI3 perovskite photovoltaics using al nanoparticle modified PC61BM layer. Sol. Energy 2020, 201, 621–627. [Google Scholar] [CrossRef]
- Sahai, S.; Varshney, A. The Effect of Morphologies of Embedded Plasmonic Cu-nanoparticles on Solar Absorption of Perovskite Solar Cells. A Comprehensive Study. Opt. Spectrosc. 2021, 129, 1165–1172. [Google Scholar] [CrossRef]
- Bao, Z.; Fu, N.; Qin, Y.; Lv, J.; Wang, Y.; He, J.; Hou, Y.; Jiao, C.; Chen, D.; Wu, Y.; et al. Broadband Plasmonic Enhancement of High-Efficiency Dye-Sensitized Solar Cells by Incorporating Au@Ag@SiO2 Core–Shell Nanocuboids. ACS Appl. Mater. Interfaces 2019, 12, 538–545. [Google Scholar] [CrossRef]
- Borah, R.; Verbruggen, S.W. Silver–Gold Bimetallic Alloy versus Core–Shell Nanoparticles: Implications for Plasmonic Enhancement and Photothermal Applications. J. Phys. Chem. C 2020, 124, 12081–12094. [Google Scholar] [CrossRef]
- Pei, C.; Choi, M.S.; Yu, X.; Xue, H.; Xia, B.Y.; Park, H.S. Recent progress in emerging metal and covalent organic frameworks for electrochemical and functional capacitors. J. Mater. Chem. A 2021, 9, 8832–8869. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, J.; Huang, S.; Ou-Yang, W.; Xie, W.; Sun, Z.; Chen, X. Plasmon-enhanced perovskite solar cells using ultra-thin LiF spacer isolating AgAl and Au composite nanoparticles from metal electrode. Org. Electron. 2018, 59, 272–278. [Google Scholar] [CrossRef]
- Fu, N.; Bao, Z.Y.; Zhang, Y.-L.; Zhang, G.; Ke, S.; Lin, P.; Dai, J.; Huang, H.; Lei, D.Y. Panchromatic thin perovskite solar cells with broadband plasmonic absorption enhancement and efficient light scattering management by Au@ Ag core-shell nanocuboids. Nano Energy 2017, 41, 654–664. [Google Scholar] [CrossRef]
- Lu, Z.; Pan, X.; Ma, Y.; Li, Y.; Zheng, L.; Zhang, D.; Xu, Q.; Chen, Z.; Wang, S.; Qu, B.; et al. Plasmonic-enhanced perovskite solar cells using alloy popcorn nanoparticles. RSC Adv. 2015, 5, 11175–11179. [Google Scholar] [CrossRef]
- Sun, Z.; Xiahou, Y.; Cao, T.; Zhang, K.; Wang, Z.; Huang, P.; Zhu, K.; Yuan, L.; Zhou, Y.; Song, B.; et al. Enhanced p-i-n type perovskite solar cells by doping AuAg@ AuAg core-shell alloy nanocrystals into PEDOT:PSS layer. Org. Electron. 2018, 52, 309–316. [Google Scholar] [CrossRef]
- Han, N.; Ji, T.; Wang, W.; Li, G.; Li, Z.; Hao, Y.; Wu, Y.; Cui, Y. Boosting the efficiency of quasi two-dimensional perovskite solar cells via an interfacial layer of metallic nanoparticles. Org. Electron. 2019, 74, 190–196. [Google Scholar] [CrossRef]
- Furasova, A.; Calabró, E.; Lamanna, E.; Tiguntseva, E.; Ushakova, E.; Ubyivovk, E.; Mikhailovskii, V.; Zakhidov, A.; Makarov, S.; Di Carlo, A. Resonant Silicon Nanoparticles for Enhanced Light Harvesting in Halide Perovskite Solar Cells. Adv. Opt. Mater. 2018, 6, 1800576. [Google Scholar] [CrossRef]
- Deng, W.; Yuan, Z.; Liu, S.; Yang, Z.; Li, J.; Wang, E.; Wang, X.; Li, J. Plasmonic enhancement for high-efficiency planar heterojunction perovskite solar cells. J. Power Sources 2019, 432, 112–118. [Google Scholar] [CrossRef]
- Yu, H.; Roh, J.; Yun, J.; Jang, J. Synergistic effects of three-dimensional orchid-like TiO2 nanowire networks and plasmonic nanoparticles for highly efficient mesoscopic perovskite solar cells. J. Mater. Chem. A 2016, 4, 7322–7329. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Q.; Shi, J.; Yue, L.; Wang, Z.; Chen, X.; Huang, S. Efficient perovskite solar cells by combination use of Au nanoparticles and insulating metal oxide. Nanoscale 2017, 9, 2852–2864. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, C.; Chen, X.; Jin, J.; Li, H.; Song, H.; Dai, Q. Considerably enhanced perovskite solar cells via the introduction of metallic nanostructures. J. Mater. Chem. A 2017, 5, 6515–6521. [Google Scholar] [CrossRef]
- Chen, X.; Gu, M. Hole Blocking Layer-Free Perovskite Solar Cells with High Efficiencies and Stabilities by Integrating Subwavelength-Sized Plasmonic Alloy Nanoparticles. ACS Appl. Energy Mater. 2019, 2, 2094–2103. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Yang, M.; Han, D.; Fan, L.; Sui, Y.; Yang, J.; Yang, L.; Wang, F. Hot-Carrier Injection Antennas with Hemispherical AgOx@Ag Architecture for Boosting the Efficiency of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 41446–41453. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Li, S.; Liu, Z.; Ying, Y.; Dvořák, P.; Fei, L.; Šikola, T.; Huang, H.; Nordlander, P.; Jen, A.K.Y.; et al. Plasmon-induced trap filling at grain boundaries in perovskite solar cells. Light Sci. Appl. 2021, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Zarick, H.F.; Boulesbaa, A.; Puretzky, A.A.; Talbert, E.M.; DeBra, Z.R.; Soetan, N.; Geohegan, D.B.; Bardhan, R. Ultrafast carrier dynamics in bimetallic nanostructure-enhanced methylammonium lead bromide perovskites. Nanoscale 2017, 9, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Qu, H.; Cao, J.; Tai, H.; Li, J.; Zheng, N. Light absorption enhancement by embedding submicron scattering TiO2 nanoparticles in perovskite solar cells. RSC Adv. 2016, 6, 24596–24602. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Kim, H.; Patil, P.S.; Hong, C.K. In situ processed gold nanoparticle-embedded TiO2 nanofibers enabling plasmonic perovskite solar cells to exceed 14% conversion efficiency. Nanoscale 2016, 8, 2664–2677. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, C.; Feng, Z.; Wu, Z.; Wang, Z.; Chen, X.; Huang, S. Synergetic Effect of Plasmonic Gold Nanorods and MgO for Perovskite Solar Cells. Nanomaterials 2020, 10, 1830. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Deng, X.; Yan, B.; Wang, Z.; Chen, X.; Sun, Z.; Huang, S. Enhancing photovoltaic performance of perovskite solar cells utilizing germanium nanoparticles. Sol. Energy 2019, 188, 839–848. [Google Scholar] [CrossRef]
- Li, F.; Lo, T.W.; Deng, X.; Li, S.; Fan, Y.; Lin, F.R.; Cheng, Y.; Zhu, Z.; Lei, D.; Jen, A.K.Y. Plasmonic Local Heating Induced Strain Modulation for Enhanced Efficiency and Stability of Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2200186. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, S.; Yin, Y.; Li, M.; Cotlet, M.; Nam, C.-Y.; Lee, J.-K. Near-Band-Edge Enhancement in Perovskite Solar Cells via Tunable Surface Plasmons. Adv. Opt. Mater. 2022, 10, 2201116. [Google Scholar] [CrossRef]
- Omelianovych, O.; Sandhu, S.; Ewusi, M.A.; Larina, L.; Kim, B.; Trinh, B.T.; Szaniel, A.; Yoon, I.; Lee, J.J.; Choi, H.S. Stable and Efficient Perovskite Solar Cells by Controlling the Crystal Growth via Introduction of Plasmonic TiN Nanoparticles. Adv. Funct. Mater. 2024, 2407343. [Google Scholar] [CrossRef]
- Talebi, H.; Emami, F. Broadband plasmonic absorption enhancement of perovskite solar cells with embedded Au@ SiO2@ graphene core–shell nanoparticles. Semicond. Sci. Technol. 2022, 37, 055002. [Google Scholar] [CrossRef]
- Taukeer Khan, M.; Khan, F. Enhancement in photovoltaic performance of perovskites solar cells through modifying the electron transport layer with reduced graphene oxide. Mater. Lett. 2022, 323, 132578. [Google Scholar] [CrossRef]
- Oliveira, O.N.; Beljonne, D.; Wong, S.S.; Schanze, K.S. Forum on Artificial Intelligence/Machine Learning for Design and Development of Applied Materials. ACS Appl. Mater. Interfaces 2021, 13, 53301–53302. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, A.S.; Sumpter, B.G. Deep Generative Models for Materials Discovery and Machine Learning-Accelerated Innovation. Front. Mater. 2022, 9, 865270. [Google Scholar] [CrossRef]
- Dean, J.; Scheffler, M.; Purcell, T.A.R.; Barabash, S.V.; Bhowmik, R.; Bazhirov, T. Interpretable machine learning for materials design. J. Mater. Res. 2023, 38, 4477–4496. [Google Scholar] [CrossRef]
- Wang, J.; Xu, P.; Ji, X.; Li, M.; Lu, W. Feature Selection in Machine Learning for Perovskite Materials Design and Discovery. Materials 2023, 16, 3134. [Google Scholar] [CrossRef]
- Thadson, K.; Sasivimolkul, S.; Suvarnaphaet, P.; Visitsattapongse, S.; Pechprasarn, S. Measurement precision enhancement of surface plasmon resonance based angular scanning detection using deep learning. Sci. Rep. 2022, 12, 2052. [Google Scholar] [CrossRef]



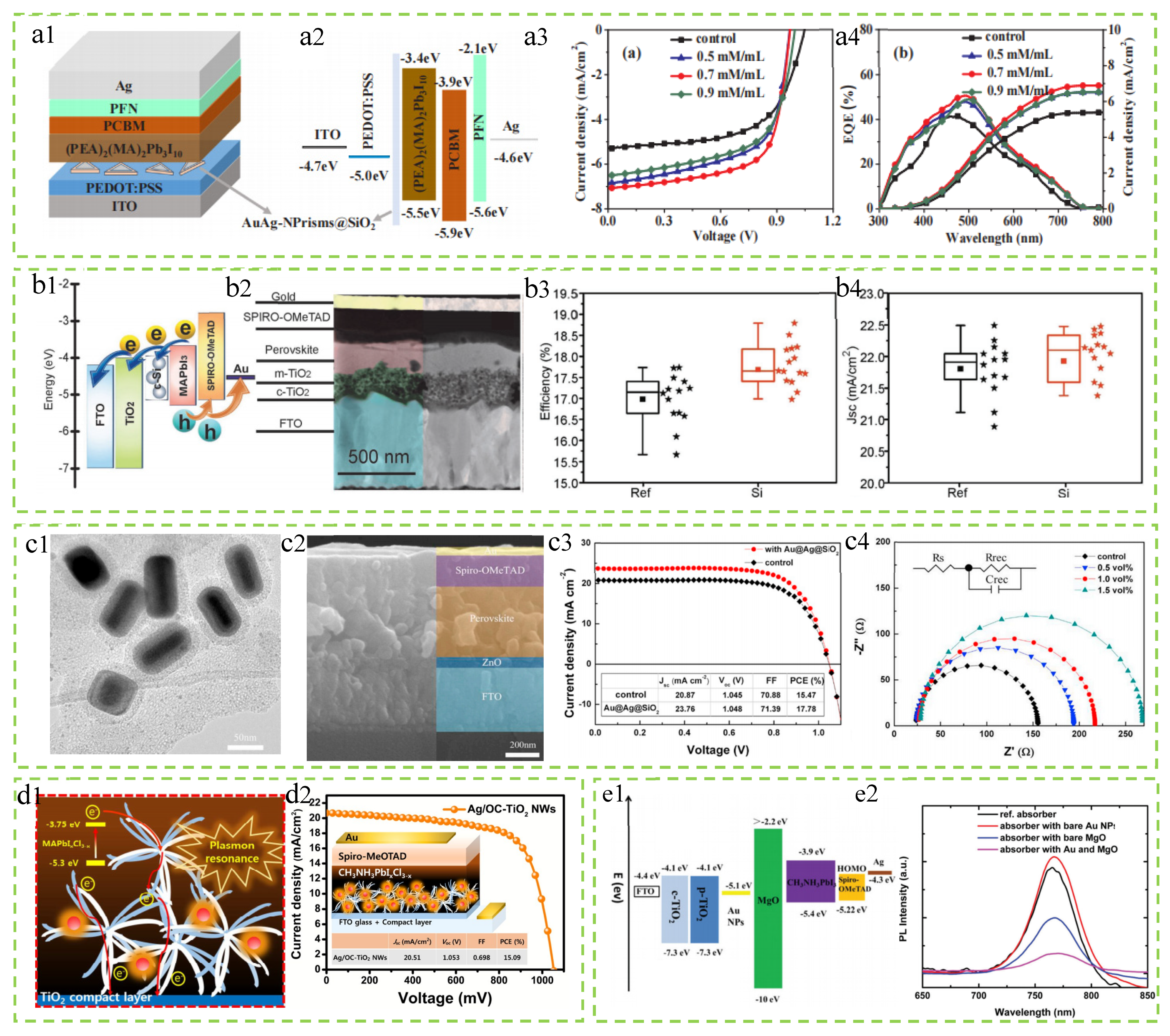
| Materials | Size | Position of NPs | JSC (mA/cm2) | VOC (V) | FF (%) | ∆FF (%) | PCE (%) | ∆PCE (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Ag nanoparticles | 25 nm | ETL (TiO2) | 18.91 (17.85) | 0.90 (0.88) | 70.20 (69.46) | 1.07 | 11.96 (10.96) | 9.12 | [94] |
| Ag nanomaterials | 10–100 nm | ETL | 1.13 (1.05) | 24.51 (24.67) | 80.93 (75.13) | 7.72 | 22.42 (19.52) | 14.86 | [113] |
| Ag NPLs | 70 ± 20 nm | HTL (PEDOT: PSS) | 15.40 (13.70) | 0.92 (0.88) | 68.20 (70.70) | −3.54 | 9.60 (8.50) | 12.94 | [95] |
| Ag NRs | 20 nm (200 nm) | Perovskite | 22.18 (21.08) | 1.12 (1.12) | 81.68 (78.36) | 4.24 | 20.29 (18.50) | 9.68 | [96] |
| Ag nanoparticles | 30 nm | ETL (TiO2) | 13.14 (10.96) | 0.78 (0.72) | 60.18 (58.42) | 3.01 | 6.15 (4.57) | 34.57 | [97] |
| Ag NCs | 70 nm | ETL (PCBM) | 21.40 (19.50) | 1.00 (1.00) | 62.00 (61.00) | 1.64 | 13.30 (11.86) | 12.14 | [98] |
| Ag NPLs | 79 ± 6 nm | Perovskite | 24.41 (19.89) | 0.85 (0.90) | 65.00 (65.00) | 0.00 | 13.46 (11.63) | 15.74 | [99] |
| Ag@TFP NPs | 10–15 nm | Between perovskite and HTL | 24.79 (23.52) | 1.19 (1.15) | 80.44 (78.96) | 1.87 | 23.86 (21.54) | 10.77 | [114] |
| Ag@TiO2@Pa | 28 nm | MAPbI3 | 21.69 (20.71) | 1.13 (1.12) | 83.00 (79.00) | 5.06 | 20.24 (18.37) | 10.18 | [100] |
| Ag@TiO2 | 42 nm | ETL | 22.00 (20.20) | 1.06 (1.03) | 69.00 (67.00) | 2.99 | 16.30 (14.50) | 12.41 | [101] |
| Ag@SiO2 | 40 nm | ETL (TiO2) | 23.04 (20.23) | 1.02 (1.00) | 62.17 (60.45) | 2.85 | 14.61 (12.23) | 19.46 | [102] |
| Ag@SiO2 | 50 nm | HTL (PEDOT: PSS) | 22.58 (20.21) | 1.01 (0.97) | 75.50 (74.40) | 1.48 | 17.22 (14.58) | 18.11 | [115] |
| Au NRs | 104.8 ± 6.8 nm (37.4 ± 1.8 nm) | Between perovskite and HTL | 24.82 (22.88) | 1.09 (1.08) | 81.18 (80.47) | 0.88 | 22.02 (19.96) | 10.32 | [64] |
| Au NRs | 50 nm (20 nm) | Perovskite | 21.60 (21.03) | 1.10 (1.09) | 81.92 (78.65) | 4.16 | 19.46 (18.02) | 7.99 | [116] |
| Au NSs | 40 nm | ETL(TiO2) | 22.30 (20.97) | 1.13 (1.11) | 80.00 (76.00) | 5.26 | 20.06 (17.64) | 13.72 | [103] |
| Au NRs | 17 nm (93 nm) | Perovskite | 23.72 (22.98) | 1.11 (1.11) | 82.47 (78.48) | 5.08 | 21.73 (20.12) | 8.00 | [104] |
| Au nanoparticles | 15 nm | HTL (Spiro-OMeTAD) | 20.04 (19.63) | 0.95 (0.96) | 66.96 (67.23) | −0.40 | 12.74 (12.66) | 0.63 | [105] |
| Au NBs | 45–50 nm (15–18 nm) | HTL(VOx) | 22.68 (—) | 1.08 (—) | 77.10 (—) | — | 18.84 (16.02) | 17.60 | [107] |
| Au NOs | 115 nm | ETL(TiO2) | 23.63 (22.29) | 1.08 (1.05) | 73.90 (72.38) | 2.10 | 19.05 (16.95) | 12.39 | [108] |
| Au@SiO2 NPs | 80 nm | ETL | 16.91 (14.76) | 1.02 (1.04) | 64.00 (67.00) | −4.48 | 11.40 (10.70) | 6.54 | [109] |
| Au@SiO2 NPs | 18 nm | ETL | 22.30 (20.90) | 1.07 (1.04) | 72.12 (—) | — | 19.42 (17.76) | 9.35 | [110] |
| Au@CdS NPs | 35 nm | Between perovskite and HTL | 23.14 (21.40) | 1.12 (1.09) | 79.90 (75.60) | 5.69 | 20.67 (17.71) | 16.71 | [111] |
| Au@PSS NPs | 54 nm | HTL (PEDOT: PSS) | 23.34 (20.41) | 1.06 (1.07) | 70.50 (67.30) | 4.75 | 16.53 (13.91) | 18.84 | [112] |
| Au@GO NPs | 28 nm | Between perovskite and HTL (PEDOT: PSS) | 18.56 (17.05) | 1.02 (0.99) | 74.00 (72.00) | 2.78 | 14.00 (12.17) | 15.04 | [117] |
| Au@SiO2 NPs | −25 nm | HTL (NiO) | 20.86 (20.40) | 1.12 (1.12) | 79.28 (79.72) | −0.55 | 18.52 (18.21) | 1.70 | [118] |
| Au@NiO NPs | −25 nm | HTL (NiO) | 21.75 (20.40) | 1.15 (1.12) | 82.42 (79.72) | 3.39 | 20.61 (18.21) | 13.18 | [118] |
| Au@SiO2 NPs | 14 nm | ETL (between c-TiO2 and m-TiO2) | 20.73 (19.86) | 1.08 (1.08) | 78.29 (75.71) | 3.41 | 17.55 (16.18) | 8.47 | [119] |
| Au@TiO2 NPs | 80 nm | Between porous TiO2 and MAPbI3 | 23.12 (17.40) | 1.04 (0.98) | 75.50 (73.70) | 2.44 | 18.24 (12.59) | 44.88 | [120] |
| Au@TiO2 NPs | 70 nm | Perovskite | 22.93 (18.70) | 0.97 (0.93) | 83.30 (82.28) | 1.24 | 18.47 (14.32) | 28.98 | [121] |
| Materials | Size | Position of NPs | JSC (mA/cm2) | VOC (V) | FF (%) | ∆FF (%) | PCE (%) | ∆PCE (%) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Al NPs | 20–70 nm | Between ETL (PCBM) and electrode | 18.15 (16.71) | 0.97 (0.96) | 67.40 (65.10) | 3.53 | 11.74 (10.54) | 11.39 | [126] |
| Au@Ag nanocuboids | 15 nm | ETL(m-TiO2) | 23.60 (20.68) | 1.04 (1.00) | 74.60 (73.00) | 2.19 | 18.31 (15.16) | 20.78 | [132] |
| Au-Ag alloy popcorns | 150 ± 50 nm | ETL(m-TiO2) | 16.46 (15.51) | 0.95 (0.92) | 66.00 (63.00) | 4.76 | 10.30 (8.90) | 15.73 | [133] |
| AuAg@AuAg core–shell alloy nanocrystals | 46 nm | HTL (PEDOT: PSS) | 21.89 (20.42) | 1.02 (0.95) | 77.20 (71.60) | 7.82 | 16.76 (13.14) | 27.55 | [134] |
| AuAgNPrisms@SiO2 | ~40 nm | Between perovskite and HTL | 7.08 (5.33) | 0.97 (1.05) | 67.00 (62.00) | 8.06 | 4.60 (3.43) | 34.11 | [135] |
| Si NPs | 140 nm | Between perovskite and ETL | 22.40 (22.00) | 1.06 (1.05) | 78.90 (77.00) | 2.47 | 18.80 (17.70) | 6.21 | [136] |
| Au@Ag@SiO2 core–shell nanocuboids | 50 nm | Perovskite | 23.76 (20.87) | 1.05 (1.05) | 71.39 (70.88) | 0.72 | 17.78 (15.47) | 14.93 | [137] |
| SiO2@Ag@OC-TiO2 NW | 400 nm | ETL(TiO2) | 20.51 (19.36) | 1.05 (1.00) | 69.80 (64.50) | 8.22 | 15.09 (12.54) | 20.33 | [138] |
| Au nanoparticles and MgO | 40 nm (400 nm) | Between perovskite and ETL | 21.76 (19.49) | 1.09 (0.94) | 68.00 (66.00) | 3.03 | 16.10 (12.00) | 34.17 | [139] |
| Au-Ag nanoalloy | 40 nm | ETL(TiO2) | 21.75 (20.38) | 1.02 (1.03) | 65.70 (63.90) | 2.82 | 14.42 (13.05) | 10.50 | [140] |
| Cu-Ag alloy NPs | 200 nm | Between perovskite and electrode | 22.96 (—) | 1.12 (—) | 73.20 (—) | — | 18.89 (13.68) | 38.08 | [141] |
| AgOx@Ag NPs | 40 nm | ETL(m-TiO2) | 23.35 (21.86) | 1.12 (1.09) | 77.75 (75.00) | 3.67 | 20.33 (17.87) | 13.77 | [142] |
| Au@PAT NPs | 22 nm | MAPBI3 | 21.71 (21.20) | 1.15 (1.12) | 82.17 (78.00) | 5.35 | 20.52 (18.59) | 10.38 | [143] |
| Au/Ag NSs | ~60 nm | ETL(m-TiO2) | — | — | — | — | 4.90 (3.90) | 25.64 | [144] |
| Submicron s-TiO2 NPs | 160 nm | ETL(m-TiO2) | 21.61 (20.28) | 1.05 (1.08) | 68.00 (67.00) | 1.49 | 16.72 (16.31) | 2.51 | [145] |
| Au@TiO2 nanofibers | 60 nm | ETL | 21.63 (19.14) | 0.99 (0.84) | 70.00 (58.00) | 20.69 | 14.92 (9.23) | 61.65 | [146] |
| Au nanorods@MgO | 12 nm (40 nm) | ETL (c-TiO2) | 22.35 (20.10) | 1.04 (1.02) | 75.00 (72.00) | 4.17 | 17.40 (14.70) | 18.37 | [147] |
| Ge NPs | 100 nm | ETL (m-TiO2) | 22.93 (21.10) | 1.06 (1.08) | 75.91 (71.42) | 6.29 | 18.59 (16.24) | 14.47 | [148] |
| GNR@SiO2 | 55 nm | Perovskite | 24.25 (24.16) | 1.08 (1.06) | 81.41 (79.24) | 2.74 | 23.26 (20.29) | 14.64 | [149] |
| SiO2@Ag@Ag2S NPs | 120 nm | Perovskite | 24.66 (22.17) | 1.06 (1.06) | 76.00 (74.30) | 2.29 | 19.88 (17.49) | 13.66 | [150] |
| TiN NPs | 20 nm | Perovskite | 26.45 (25.09) | 1.06 (1.05) | 76.07 (72.70) | 4.64 | 21.37 (19.07) | 12.06 | [151] |
| Au@SiO2@Graphene NPs | 17 nm | CH3NH3PbI3 | 28.17 (21.20) | 1.00 (0.98) | 71.00 (68.00) | 4.41 | 20.05 (14.21) | 41.10 | [152] |
| rGO NPs | — | ETL | 22.51 (21.01) | 1.06 (1.05) | 72.00 (72.00) | 0.00 | 17.08 (15.93) | 7.22 | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, X.; Luo, B.; Shi, X.; Shen, X. Plasmonics Meets Perovskite Photovoltaics: Innovations and Challenges in Boosting Efficiency. Molecules 2024, 29, 5091. https://doi.org/10.3390/molecules29215091
Wang C, Wang X, Luo B, Shi X, Shen X. Plasmonics Meets Perovskite Photovoltaics: Innovations and Challenges in Boosting Efficiency. Molecules. 2024; 29(21):5091. https://doi.org/10.3390/molecules29215091
Chicago/Turabian StyleWang, Chen, Xiaodan Wang, Bin Luo, Xiaohao Shi, and Xiangqian Shen. 2024. "Plasmonics Meets Perovskite Photovoltaics: Innovations and Challenges in Boosting Efficiency" Molecules 29, no. 21: 5091. https://doi.org/10.3390/molecules29215091
APA StyleWang, C., Wang, X., Luo, B., Shi, X., & Shen, X. (2024). Plasmonics Meets Perovskite Photovoltaics: Innovations and Challenges in Boosting Efficiency. Molecules, 29(21), 5091. https://doi.org/10.3390/molecules29215091





